Abstract
ERK signaling and Akt signaling are inversely correlated in some cancers. Yet, the precise molecular mechanism for cross‐inhibition remains unclear. In this issue of The EMBO Journal, Pan et al (2017) show that when Akt is on, its phosphorylated cytoplasmic substrate FOXO1 turns off ERK activity by reshaping the Ras‐ERK scaffold IQGAP1.
Subject Categories: Cancer, Signal Transduction
The Ras‐extracellular signal‐regulated kinase (ERK) and phosphoinositide 3‐kinase (PI3K)‐Akt signaling pathways are key mechanisms for controlling cell survival, proliferation, differentiation, metabolism, motility, stemness, and are often deregulated in human cancers (Mendoza et al, 2011). In response to agonist activation of receptors, these two pathways crosstalk to regulate common downstream functions. In principle, mechanisms of the crosstalk are either activating or inhibiting. A well‐studied activating mechanism is that Ras directly binds and allosterically activates PI3K (Burke & Williams, 2015). The Ras‐ERK and PI3K‐Akt pathways can also attenuate each other. This cross‐inhibition is often manifested when one pathway is blocked. For example, pharmacological inhibition of components of one pathway leads to activation of the other pathway in many different cancer types (Mendoza et al, 2011). Acquired activation of one pathway by inhibition of the other has been recognized as a major resistance mechanism of chemotherapy in cancers (Chandarlapaty et al, 2011; Mendoza et al, 2011). However, molecular details of cross‐inhibition are not fully understood. In a study published in this issue of The EMBO Journal, Pan et al (2017) demonstrate that in prostate cancer cells, Akt phosphorylates O‐class forkhead factors (FOXO), especially FOXO1 among other members. Phosphorylated FOXO1 localized in the cytoplasm binds to IQGAP1, a scaffold protein for the Ras‐ERK pathway, and this disrupts scaffold assembly leading to inhibition of ERK activity (Fig 1). This novel mechanism of cross‐inhibition of the Ras‐ERK and PI3K‐Akt pathways is further supported clinically by showing that FOXO1 expression is inversely correlated with ERK activation in human prostate cancer biopsies. Moreover, the authors show that a small FOXO1‐derived phosphorylation‐mimetic peptide has therapeutic effect to overcome chemoresistance in cancers (Pan et al, 2017).
Figure 1. IQGAP1 scaffolds both the Ras‐ERK and PI3K‐Akt pathways.
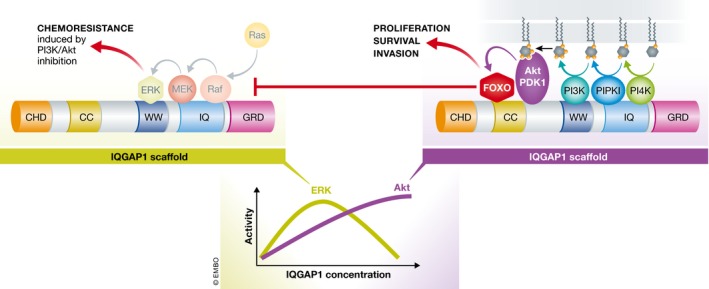
The binding of all the components of the pathways to IQGAP1 facilitates sequential phosphorylation, leading to ERK or Akt activation. Pan et al (2017) reveal that an Akt target FOXO1 also binds to IQGAP1. The binding of phosphorylated FOXO1 induces conformation changes, which in turn promote dissociation of Ras‐ERK pathway components from IQGAP1 scaffold. Cellular concentration of IQGAP1 appears to modulate the pathway switch. At higher concentration, IQGAP1 largely scaffolds the PI3K‐Akt pathway, whereas at lower concentration, IQGAP1 scaffold is utilized for the Ras‐ERK pathway. CHD, calponin homology domain; CC, coiled‐coil; WW, WW domain; IQ, IQ domain; GRD, GTPase‐activating protein‐related domain; PI4K, phosphatidylinositol 4‐kinase.
IQ motif containing GTPase‐activating protein 1 (IQGAP1) is a multidomain protein that scaffolds multiple signaling pathways (Smith et al, 2015). The scaffold role of IQGAP1 is emerging as key in the Ras‐ERK, Wnt, integrin, and PI3K‐Akt pathways. For example, in the Ras‐ERK pathway, Raf, MEK, and ERK interact with IQGAP1, which physically links the kinases in close proximity to facilitate their sequential phosphorylation (Ren et al, 2007). Recently, a scaffold role for IQGAP1 in the PI3K‐Akt pathway has been revealed (Choi et al, 2016). In response to receptor activation, PI4KIIIα, PIPKIα, and PI3K are assembled by IQGAP1 and this results in sequential generation of phosphatidylinositol‐3,4,5‐trisphosphate (PI3,4,5P3) from phosphatidylinositol. PI3,4,5P3 effectors, phosphoinositide‐dependent kinase 1 (PDK1), and Akt also assemble in the IQGAP1 complex. Pan et al (2017) now further show that a downstream target of Akt, FOXO1, also is in the IQGAP1 complex. FOXO1 acts as a transcription factor in the nucleus, but is degraded in the cytoplasm upon Akt‐mediated phosphorylation. The study by Pan et al (2017) attributes a role to cytoplasmic, phosphorylated FOXO1 in the context of signaling, as p‐FOXO1 binds IQGAP1, thereby promoting inhibition of Ras‐ERK signaling. This suggests that the IQGAP1 multienzyme complex streamlines the synthesis of the lipid signal PI3,4,5P3 and that PI3,4,5P3 is then directly handed off to Akt and FOXO1 leading to their activation (Fig 1). In the phosphoinositide signal transduction pathways, the synthesis of lipid signals are tightly linked to its usage as the lipid signals rapidly diffuse in cellular membranes (Choi et al, 2015). Binding of FOXO1 to the IQGAP1‐Akt complex might be another example of such proximity‐dependent mechanism.
Pan et al (2017) clearly show that depletion of IQGAP1 reduces both ERK and Akt activation, further supporting that IQGAP1 scaffolds both the Ras‐ERK and PI3K‐Akt pathways. Of note, binding sites of each pathway components on IQGAP1 are overlapping. For example, ERK and PI3K both bind to the WW domain, and MEK and PIPKIα both bind to the IQ domain (Hedman et al, 2015; Choi et al, 2016). This supports that the two pathway components compete for binding to IQGAP1, and that IQGAP1‐dependent ERK or Akt activation is mutually exclusive. In agreement, Pan et al (2017) demonstrate that binding of Akt‐phosphorylated FOXO1 to IQGAP1's coiled‐coil (CC) domain induces conformational changes, facilitating dissociation of Raf, MEK, and ERK from the IQGAP1 scaffold (Fig 1).
It is surprising that cancer cells adapt to use the same IQGAP1 scaffold for the Ras‐ERK, PI3K‐Akt, and other key signaling pathways. During cancer progression, cancer cells acquire multiple genetic and epigenetic alterations that drive sustained proliferation and survival. At the same time, these alterations cause constant genetic, epigenetic, and metabolic stress. To overcome this, cancer cells often appear to depend on one or a few oncogenic pathways for their proliferation and survival (Luo et al, 2009). This so‐called oncogene addiction hypothesis provides a theoretical basis for the targeted cancer therapy. However, in many instances, targeting an oncogenic pathway is hampered by activation of other pathways. Studies by Pan et al (2017) and others (Choi et al, 2016) demonstrate how cancer cells would efficiently turn on other pathways when the addicted oncogenic pathway is inhibited. For example, in cancer cells with inactive phosphatase and tensin homolog (PTEN), IQGAP1 would scaffold all the components of the PI3K‐Akt pathway leading to FOXO1 phosphorylation and this would drive proliferation and survival. Once the PI3K‐Akt pathway is inhibited, cancer cells now would use the IQGAP1 platform for scaffolding Ras‐Raf‐MEK‐ERK components to overcome the detrimental conditions.
The level of IQGAP1 expression is critical for deciding which pathway it would scaffold. Both knockdown and overexpression of IQGAP1 diminish ERK activation (Roy et al, 2005), whereas overexpression of IQGAP1 enhances Akt activation (Choi et al, 2016). We envision that in cancer cells expressing high level of IQGAP1, IQGAP1 largely scaffolds the PI3K‐Akt pathway (Fig 1). In agreement, in some IQGAP1 overexpressing breast cancer cells, no IQGAP1 association with Ras‐ERK pathway components is detected (Choi et al, 2016). However, the precise mechanism of scaffolding pathway change by IQGAP1 expression level remains to be elucidated. As an IQGAP1 homolog, IQGAP3 is suggested to also scaffold the Ras‐ERK pathway leading to ERK activation, while IQGAP2 has inhibitory functions. Dynamic expression levels of the IQGAPs would determine the scaffold switch of IQGAP1. Further, the IQGAPs appear to be present in cells at much higher stoichiometry than either Ras‐ERK or PI3K‐Akt components. This illustrates the complexity in understanding how FOXO1 likely at lower stoichiometry than IQGAP1 can modify scaffolding for other pathways. In addition, the assembly of PI3K or ERK pathway components must be highly dynamic both spatially and temporally and likely receptor driven, suggesting that the stoichiometric issue is defined at the receptor level. Clearly, there remains much to understand the fundamental roles of IQGAPs in the Ras‐ERK and PI3K‐Akt signaling pathways.
See also: C-W Pan et al (April 2017)
References
- Burke JE, Williams RL (2015) Synergy in activating class I PI3Ks. Trends Biochem Sci 40: 88–100 [DOI] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik‐Outmezguine V, Grbovic‐Huezo O, Serra V, Majumder PK, Baselga J, Rosen N (2011) AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19: 58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Thapa N, Tan X, Hedman AC, Anderson RA (2015) PIP kinases define PI4,5P(2)signaling specificity by association with effectors. Biochim Biophys Acta 1851: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Hedman AC, Sayedyahossein S, Thapa N, Sacks DB, Anderson RA (2016) Agonist‐stimulated phosphatidylinositol‐3,4,5‐trisphosphate generation by scaffolded phosphoinositide kinases. Nat Cell Biol 18: 1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman AC, Smith JM, Sacks DB (2015) The biology of IQGAP proteins: beyond the cytoskeleton. EMBO Rep 16: 427–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non‐oncogene addiction. Cell 136: 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J (2011) The Ras‐ERK and PI3K‐mTOR pathways: cross‐talk and compensation. Trends Biochem Sci 36: 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CW, Jin X, Zhao Y, Pan Y, Yang J, Karnes RJ, Zhang J, Wang L, Huang H (2017) AKT‐phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1‐MAPK interaction. EMBO J 36: 995–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JG, Li Z, Sacks DB (2007) IQGAP1 modulates activation of B‐Raf. Proc Natl Acad Sci USA 104: 10465–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB (2005) IQGAP1 is a scaffold for mitogen‐activated protein kinase signaling. Mol Cell Biol 25: 7940–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Hedman AC, Sacks DB (2015) IQGAPs choreograph cellular signaling from the membrane to the nucleus. Trends Cell Biol 25: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]


