Abstract
Macroautophagy allows for bulk degradation of cytosolic components in lysosomes. Overexpression of GFP/RFP‐LC3/GABARAP is commonly used to monitor autophagosomes, a hallmark of autophagy, despite artifacts related to their overexpression. Here, we developed new sensors that detect endogenous LC3/GABARAP proteins at the autophagosome using an LC3‐interacting region (LIR) and a short hydrophobic domain (HyD). Among HyD‐LIR‐GFP sensors harboring LIR motifs of 34 known LC3‐binding proteins, HyD‐LIR(TP)‐GFP using the LIR motif from TP53INP2 allowed detection of all LC3/GABARAPs‐positive autophagosomes. However, HyD‐LIR(TP)‐GFP preferentially localized to GABARAP/GABARAPL1‐positive autophagosomes in a LIR‐dependent manner. In contrast, HyD‐LIR(Fy)‐GFP using the LIR motif from FYCO1 specifically detected LC3A/B‐positive autophagosomes. HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP efficiently localized to autophagosomes in the presence of endogenous LC3/GABARAP levels and without affecting autophagic flux. Both sensors also efficiently localized to MitoTracker‐positive damaged mitochondria upon mitophagy induction. HyD‐LIR(TP)‐GFP allowed live‐imaging of dynamic autophagosomes upon autophagy induction. These novel autophagosome sensors can thus be widely used in autophagy research.
Keywords: autophagosome sensor, autophagy, hydrophobic domain, LC3‐interacting region motif
Subject Categories: Autophagy & Cell Death
Introduction
Macroautophagy, hereafter referred to as autophagy, is an evolutionarily conserved lysosomal degradation pathway for long‐lived proteins, organelles, or certain cytosolic components, such as RNA or lipids (Feng et al, 2014). Although autophagy is primarily considered to be a type of cellular defense mechanism to provide nutrients via self‐digestion and protection of cells during starvation (Pfeifer, 1973; Klionsky, 2007), increasing evidence suggests that autophagy has important roles in many other physiological processes, such as the regulation of cellular homeostasis, differentiation, or metabolism (Klionsky & Codogno, 2013). Furthermore, dysfunction of autophagy is strongly associated with many human diseases, such as neurodegenerative diseases, lysosomal storage disorders, liver diseases, some cancers, myopathy, and certain infectious diseases (Mizushima et al, 2008; Jiang & Mizushima, 2014; Schneider & Cuervo, 2014).
Autophagy, which is constitutively activated depending on the cell type, can be effectively induced under various physiological or pathological conditions (Yang & Klionsky, 2010). Once autophagy is activated, the preautophagosomal membrane (also known as the isolation membrane or phagophore) becomes nucleated and expands to form a double‐membrane‐bound structure, the autophagosome, which sequesters cytosolic cargo components and is a morphological hallmark of autophagy. Subsequently, the autophagosome can be fused with endosomes for its maturation or with lysosomes for cellular degradation. The multistep process is tightly regulated by core autophagy machinery composed of several AuTophaGy (ATG)‐related proteins (Feng et al, 2014). Extensive genetic studies in yeast and mammals have identified several autophagy‐related genes (~40 genes in yeast) with specific roles in the autophagy pathway (Feng et al, 2014; Zhang & Baehrecke, 2015).
Core ATG‐related proteins are classified into functional groups based on their roles in different processes such as autophagy induction, formation/maturation of autophagosomes, or degradation of cargo. Among the core autophagy proteins, there is one Atg8 in yeast and several homologs in mammals, which are subdivided into the light chain 3 (LC3) and GABARAP family. Mammalian LC3/GABARAP family proteins are involved in autophagosome formation, cargo recognition, and recruitment of cargos into the autophagosomal membrane (Kalvari et al, 2014). Although autophagy is considered a non‐selective degradation pathway, recent evidence has revealed several forms of selective autophagy (mitophagy, aggrephagy, pexophagy, or xenophagy) that preferentially select cargo via binding to LC3 and selective autophagy receptors (Birgisdottir et al, 2013; Kalvari et al, 2014). ATG8/LC3/GABARAP proteins are the most widely used autophagosome markers. Eight human homologs comprise the microtubule‐associated protein 1 light chain (MAP1LC3) family (MAP1LC3A [LC3A], MAP1LC3B [LC3B], MAP1LC3B2 [LC3B2], and MAP1LC3C [LC3C], collectively referred to as “LC3”) and the γ‐aminobutyric acid (GABA) receptor‐associated proteins (GABARAP, GABARAPL1, GABARAPL2/GATE‐16, and GABARAPL3) (Wild et al, 2014; Lee & Lee, 2016). Although the specific functions of each isoform of mammalian LC3/GABARAP protein remain unknown, among these homologs, LC3B, an interacting partner of microtubule‐associated proteins 1A and 1B (MAP1A/1B), has been extensively studied and characterized as a component essential for autophagosome biogenesis. During autophagy, LC3B, as a ubiquitin‐like protein, is known to be directly conjugated to phosphatidylethanolamine (PE) by ubiquitin‐like conjugation systems (Wild et al, 2014). Briefly, LC3B is processed into its cytosolic form, LC3‐I, by exposure of the C‐terminal Gly to the action of ATG4B. LC3‐I is then transformed into its membrane‐bound form, LC3‐II, which is generated by conjugation of PE to the carboxyl group of its glycine residue. Because LC3‐II specifically localizes to autophagosomes and is degraded in the autolysosome (Tanida et al, 2004), autophagic flux may be analyzed in an LC3 turnover assay (a biochemical method) (Klionsky et al, 2012). Moreover, to monitor autophagosomes and track their dynamic changes at the cellular level in live or fixed cells, overexpressed fluorescently labeled LC3 (green/red fluorescent protein or GFP/RFP‐LC3) has been widely used as a specific marker of autophagy (Kabeya et al, 2000, 2004). More recently, Kaizuka et al (2016) developed an autophagic flux probe (GFP‐LC3‐RFP‐LC3ΔG), in which GFP‐LC3 is degraded by autophagy, while RFP‐LC3ΔG remains in the cytosol, serving as an internal control (Kaizuka et al, 2016). During the revision of our study, Stolz et al (2017) reported fluorescence‐based ATG8 sensors for monitoring the localization and function of LC3/GABARAP proteins (Stolz et al, 2017).
Although the GFP/RFP‐LC3 reporter has been widely used in autophagy research in vivo and in vitro, its applications are inescapably accompanied by artifacts resulting from LC3 overexpression (Klionsky et al, 2012). One concern is that autophagy‐deficient cells occasionally show GFP‐LC3‐positive aggregates. Furthermore, highly expressed GFP‐LC3 can associate with ubiquitin‐positive protein aggregates in the presence of aggregate‐prone proteins in autophagy‐deficient cells. Higher expression of LC3 also results in its strong nuclear localization (Klionsky et al, 2012). In addition, in polarized neurons, overexpression of LC3 sometimes causes abnormal neurite branching because LC3 associates with MAP, so excess LC3 may affect the microtubule structure in axons and dendrites (Appendix Fig S1). Overexpression of GFP‐LC3 may increase basal autophagosome levels even in the presence of nutrients. Although some researchers have proposed that endogenous LC3 can be detected immunocytochemically with an anti‐LC3 antibody (Klionsky et al, 2012; Ktistakis, 2015), this approach is not always feasible because of either lower antibody specificity or lower LC3 expression in certain types of cells under some conditions (Buckingham et al, 2014). An even more important limitation is that it is impossible to detect endogenous LC3‐positive autophagosomes and track their dynamics using live‐imaging. Therefore, we attempted to monitor endogenous LC3/GABARAP‐positive autophagosomes in living cells. To develop new sensors for detecting endogenous LC3/GABRARAP‐positive autophagosomes, we constructed sensors containing a short hydrophobic LC3‐interacting motif (LIR). Many LC3‐interacting proteins, such as selective autophagy receptors including p62, contain a basic hydrophobic LIR motif with the core consensus sequence (W/F/Y)XX(L/I/V) (Ichimura et al, 2008; Birgisdottir et al, 2013; Kalvari et al, 2014; Wild et al, 2014). The LIR motif electrostatically interacts with basic residues in the N‐terminal extension and Ubl domain of LC3 (Pankiv et al, 2007; Shvets et al, 2008). Therefore, many types of LIR motifs may interact with LC3/GABARAP proteins with varying affinities. Additionally, during detection of LC3/GABARAP proteins anchored in the autophagosome, enhanced subcellular localization of a LIR sensor on the autophagosome must be considered. Many membrane‐associated proteins typically require several motifs for their membrane localization, for example, a specific protein interaction motif, specific lipid‐binding domain, or hydrophobic domain (Stahelin, 2009). In our previous studies, we identified an N‐terminal moderate hydrophobic domain (HyD) that enhanced the membrane association of Aplysia phosphodiesterase 4 (ApPDE4) (Kim et al, 2014). HyD alone was not sufficient for membrane targeting, but if combined with other motifs such as a basic‐rich and UCR1‐2 domain, it facilitated efficient localization of the reporter protein to the plasma membrane.
Therefore, we hypothesized that a sensor protein combining a LIR motif and HyD could selectively detect endogenous LC3/GABARAP proteins in the autophagosome. We developed new sensors by fusing a LIR motif with HyD for reproducible detection of LC3/GABARAP proteins in the autophagosomal membrane (Fig 1A). To do this, we generated and tested 34 different HyD‐LIR sensors. As a result, we found that HyD‐LIR(TP)‐GFP using the LIR motif from TP53INP2 was the most efficient for detecting all LC3/GABARAP‐positive autophagosomes and preferentially localized to GABARAPL1‐positive autophagosomes upon autophagy induction. HyD‐LIR(Fy)‐GFP using a LIR motif from FYCO1 was more specifically localized to LC3A/B‐positive autophagosomes in wild‐type (WT) mouse embryonic fibroblasts (MEFs), but not in Atg5 −/− or Atg7 −/− MEFs. Autophagosome association was LIR motif dependent and did not affect autophagic flux. Moreover, our new sensors (HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP) efficiently co‐localized with MitoTracker‐positive damaged mitochondria upon mitophagy induction. HyD‐LIR(TP)‐GFP faithfully reflected autophagosome dynamics upon autophagy induction in living cells in the absence of LC3/GABARAP protein overexpression.
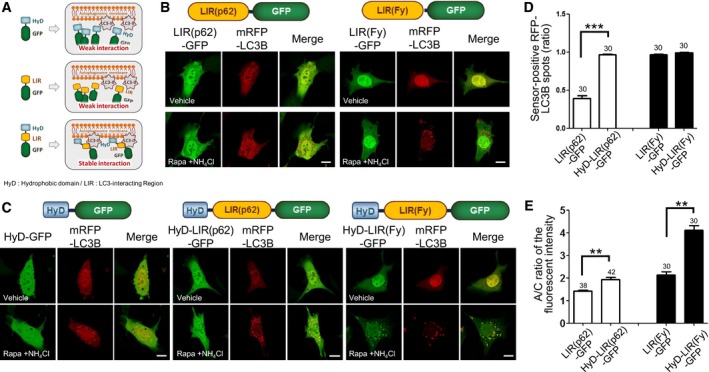
Figure 1. Efficient localization of HyD‐LIRs‐GFP to mRFP‐LC3B‐positive autophagosomes.
-
ASchematic model of the development of new autophagosome sensors. LIR: LC3‐interacting region, HyD: hydrophobic domain.
-
B, CConfocal images showing cellular localization of various LIR‐GFP constructs (HyD‐GFP, LIR(p62)‐GFP, HyD‐LIR(p62)‐GFP, LIR(Fy)‐GFP, or HyD‐LIR(Fy)‐GFP) together with mRFP‐LC3B in MEFs incubated with 100 nM rapamycin (Rapa) + 10 mM NH4Cl for 4 h. Scale bar: 10 μm.
-
D, EThe bar graphs illustrate the ratios of sensor‐positive mRFP‐LC3B spots (D) and ratios of autophagosomal/cytosol (A/C) fluorescence intensity (E) of each tested GFP sensor construct. The values are presented as the mean ± SEM. ***P < 0.001, two‐tailed unpaired Student's t‐test. **P < 0.001, two‐tailed Mann–Whitney U‐test. The numbers on the bars indicate the number of cells used for the experiment.
Results
LIR motifs from FYCO1 or p62 incorporating the N‐terminal hydrophobic domain of ApPDE4 efficiently localized to mRFP‐LC3B‐positive autophagosomes
To generate new sensors for monitoring endogenous LC3/GABARAP proteins localized to autophagosomes, we used LIR motifs of well‐known LC3‐binding proteins (Birgisdottir et al, 2013). The LIR motif of p62 (LIR(p62)) (Johansen & Lamark, 2011), which was the first LIR motif identified, or the LIR motif of FYCO1 (LIR(Fy)), which is an LC3‐, Rab7‐, and PI3P‐interacting protein (Pankiv et al, 2010), were first examined for their ability to detect LC3‐positive autophagosomes during autophagy induction (Appendix Table S1). GFP was fused to the C‐terminus of the LIR motifs from FYCO1 and p62 to generate LIR(Fy)‐GFP and LIR(p62)‐GFP, respectively (Fig 1A and B). Either LIR(Fy)‐GFP or LIR(p62)‐GFP together with mRFP‐LC3B, a general marker for autophagosomes, was expressed for 24 h in MEFs. Next, the cells were incubated with rapamycin (100 nM, 4 h), a general inducer of autophagy, under conditions that inhibited lysosomal degradation: NH4Cl (10 mM, 4 h), a lysosomotrophic reagent, facilitates the detection of autophagosomes by maximizing their accumulation (Klionsky et al, 2008, 2012). As expected, mRFP‐LC3B was localized to autophagosomes in MEFs treated with rapamycin and NH4Cl, while it hardly localized to autophagosomes in non‐induced cells (Fig 1B). However, LIR(Fy)‐GFP and LIR(p62)‐GFP were mostly diffused in the cytosol and hardly localized to mRFP‐LC3B‐positive autophagosomes in cells incubated with rapamycin and NH4Cl (Fig 1B). These results showed that the LIR motif itself was not sufficient for targeting to mRFP‐LC3B‐positive autophagosomes.
In general, if a single domain is not sufficient for specific targeting, multiple domains in the same protein are required for efficient and stable localization of cellular membrane targeting proteins to their specific intracellular destinations (Lemmon, 2008). Therefore, we explored whether the addition of another motif or domain assisting membrane association would enhance targeting the LIR motif to LC3‐positive autophagosomes. We previously showed that the N‐terminal moderate HyD of Aplysia phosphodiesterase 4 (ApPDE4) short‐form was not sufficient to mediate membrane association (Kim et al, 2014). However, if combined with a flanking basic‐rich domain, which can electrostatically interact with acidic phospholipids, N‐terminal moderate HyD of ApPDE4 could stably localize to the plasma membrane (Kim et al, 2014). Therefore, we evaluated whether HyD, that is, N‐terminal 20 amino acids of the ApPDE4 short‐form, could enhance the localization of LIR(Fy)‐GFP or LIR(p62)‐GFP to the autophagosome (Fig 1A and C). As a control, we showed that HyD‐GFP alone did not localize to mRFP‐LC3B‐positive autophagosomes (Fig 1C). However, interestingly, HyD‐LIR(Fy)‐GFP and HyD‐LIR(p62)‐GFP both localized to mRFP‐LC3B‐positive autophagosomes in MEFs treated with rapamycin and NH4Cl with greater efficiency than did LIR(Fy)‐GFP or LIR(p62)‐GFP (Fig 1C). Interestingly, localization of HyD‐LIR(Fy)‐GFP to autophagosomes was more efficient and the sensor signal was less diffuse in the cytosol than that of HyD‐LIR(p62)‐GFP. To examine the efficiency of HyD‐LIR‐based autophagosome sensors, we quantified the ratio of sensor‐positive mRFP‐LC3B spots and autophagosome/cytosol (A/C) ratio of the sensor fluorescence intensities. As shown in Fig 1D, the ratio of sensor‐positive mRFP‐LC3B spots significantly increased in HyD‐LIR(p62)‐GFP‐expressing cells compared to that in LIR(p62)‐GFP‐expressing cells, whereas the ratio was similar in cells expressing either LIR(Fy)‐GFP or HyD‐LIR(Fy)‐GFP. There was no significant difference between the ratios of sensor‐positive mRFP‐LC3B spots in cells expressing HyD‐LIR(Fy)‐GFP and HyD‐LIR(p62)‐GFP. However, the A/C ratios of the sensor fluorescence intensities were higher in cells expressing either HyD‐LIR(p62)‐GFP or HyD‐LIR(Fy)‐GFP compared to those of LIR(p62)‐GFP or LIR(Fy)‐GFP without HyD (Fig 1E). Taken together, these findings suggest that each LIR motif has a different binding efficacy to LC3B and that a LIR motif fused with a hydrophobic domain enhanced its localization to autophagosomes.
HyD‐LIR(TP)‐GFP is the most efficient sensor for detecting all LC3/GABARAP‐positive autophagosomes, while HyD‐LIR(Fy)‐GFP is selective for LC3A/B‐positive autophagosomes
To identify more efficient autophagosome sensors, we generated and tested 32 new HyD‐LIR‐GFP candidate sensors using LIR motifs from proteins known to interact with LC3/GABARAP proteins in MEFs upon autophagy induction (Appendix Table S1) (Birgisdottir et al, 2013). To this end, each HyD‐LIR‐GFP sensor was first transfected together with mRFP‐LC3B, a general marker of autophagosomes, into MEFs. At 24 h after transfection, the cells were treated with rapamycin + NH4Cl (100 nM rapamycin and 10 mM NH4Cl for 4 h). Of the set of 34 HyD‐LIR‐GFP sensors tested, which included HyD‐LIR(Fy)‐GFP and HyD‐LIR(p62)‐GFP, 26 HyD‐LIR‐GFP sensors comprising LIRs from proteins such as NBR1, NDP52, and others only minimally or weakly localized to mRFP‐LC3B‐positive autophagosomes in MEFs upon autophagy induction (Fig EV1). In contrast, as shown in Fig 2A–C, the other eight HyD‐LIR‐GFP sensors containing LIR motifs from ULK2, ATG13, Bnip3, FUNDC1, ScATG3, TBC1D25, FYCO1, or TP53INP2 were localized to mRFP‐LC3B‐positive autophagosomes in MEFs to different extents (Fig 2A). Thus, we selected eight candidate HyD‐LIR‐GFP sensors for further analysis.
Figure EV1. Cellular localization of HyD‐LIR(X)‐GFP in MEFs expressing mRFP‐LC3 upon autophagy induction.
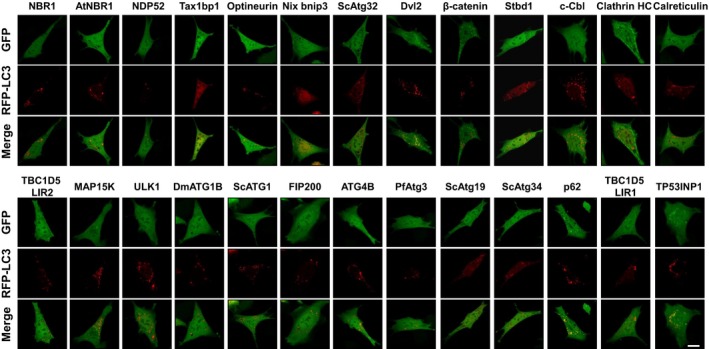
Confocal images showing cellular localization of various HyD‐LIR(X)‐GFP incorporating LIR motifs of different LC3‐binding proteins together with mRFP‐LC3B in MEFs upon autophagy induction (100 nM rapamycin + 10 mM NH4Cl, 4 h). Scale bar, 10 μm. Each X (LC3‐binding proteins) indicates HyD‐LIR(X)‐GFP.
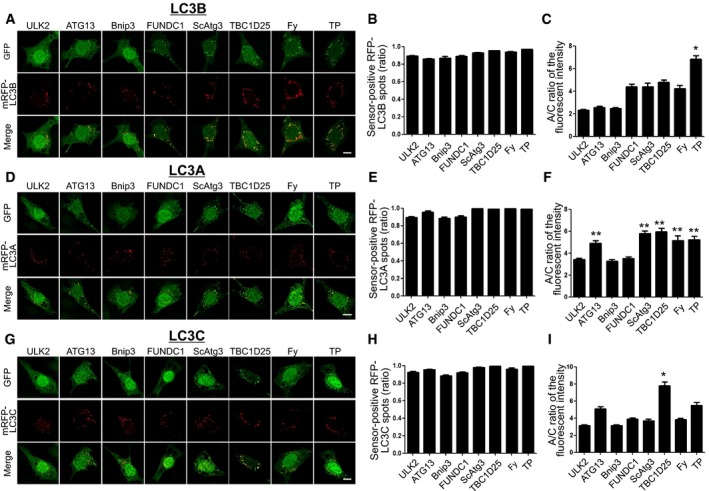
Figure 2. Cellular localization of HyD‐LIR(X)‐GFP to mRFP‐LC3(A, B, C)‐positive autophagosomes in MEFs upon autophagy induction.
-
A–IConfocal images showing cellular localization of various HyD‐LIR(X)‐GFP constructs together with either mRFP‐LC3B (A), mRFP‐LC3A (D), or mRFP‐LC3C (G) in MEFs upon autophagy induction (100 nM rapamycin + 10 mM NH4Cl, 4 h). X: LC3/GABARAP‐binding proteins (ULK2, ATG13, Bnip3, FUNDC1, ScAtg3, TBC1D25, FY (FYCO1), or TP (TP53INP2)). Scale bar, 10 μm. The bar graphs illustrate the ratios of sensor‐positive mRFP‐LC3B (B), mRFP‐LC3A (E), or mRFP‐LC3C (H) and A/C ratios of the fluorescent intensity (C, F, I) of each tested GFP sensor construct. *P < 0.05 (comparison with all other groups by Kruskal–Wallis test followed by Dunn's multiple comparison test), **P < 0.01 (comparison with ULK2, Bnip3, and FUNDC1 by Kruskal–Wallis test followed by Dunn's multiple comparison test). The values are presented as the mean ± SEM. For the quantitative analysis of the A/C ratio, 20 randomly selected cells in each group were used.
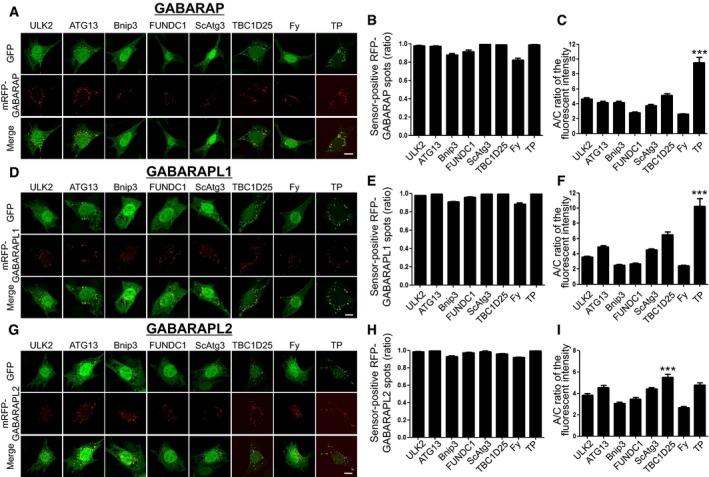
In order to compare the efficiency of eight candidate HyD‐LIR‐GFP sensors for cellular localization to other individual LC3/GABARAP‐positive autophagosomes, we further tested five different LC3/GABARAP family proteins (LC3A, LC3C, GABARAP, GABARAPL1, and GABARAPL2) with eight candidate HyD‐LIR‐GFP sensors. For this, each mRFP‐LC3(A or C) or mRFP‐GABARAP/GABARAP(L1 or L2) was transfected together with each candidate HyD‐LIR‐GFP sensor into MEFs upon autophagy induction (Figs 2 and 3). To examine the efficiency of each candidate HyD‐LIR‐GFP sensor in controlling the cellular localization to LC3/GABARAP‐positive autophagosomes, we quantified the ratio of sensor‐positive mRFP‐LC3/GABARAP spots and the A/C ratio of fluorescence intensity for each HyD‐LIR‐GFP sensor (Figs 2 and 3). Based on the quantification of the cellular localization and intensity of each sensor to autophagosomes, we summarized the efficiency of each sensor for detecting mRFP‐LC3/GABARAP‐positive autophagosomes in Table 1. We selected efficient HyD‐LIR‐GFP sensors (indicated by shaded box) for LC3/GABARAP‐positive autophagosomes if the A/C ratio of the sensor was above 5 and if the ratio of sensor‐positive mRFP‐LC3/GABARAP‐positive spots was above 0.95 (Table 1). According to our criteria for efficient HyD‐LIR‐GFP sensors, HyD‐LIR(ATG13)‐GFP was efficient for LC3C‐positive autophagosomes and either HyD‐LIR(ScAtg3)‐GFP or HyD‐LIR(Fy)‐GFP was better for LC3A‐positive autophagosomes. For either HyD‐LIR(TBC1D25)‐GFP or HyD‐LIR(TP)‐GFP, both sensors were efficiently localized to nearly all LC3/GABARAP‐positive autophagosomes with either similar or varying efficiencies (Figs 2 and 3, and Table 1). Interestingly, among these sensors, the A/C ratio of the HyD‐LIR(TP)‐GFP sensor on GABARAP or GABARAPL1‐positive autophagosomes showed the highest value (nearly 10) compared to that of the HyD‐LIR(TP)‐GFP sensor on LC3(A, B, C)/GABRAPL2‐positive autophagosomes. We also checked that HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP was efficiently localized to mRFP‐LC3/GABARAP‐positive autophagosomes or mRFP‐LC3(A, B)‐positive autophagosomes in HEK293T cells upon serum starvation (Fig EV2).
Figure 3. Cellular localization of HyD‐LIR(X)‐GFP to mRFP‐GABARAP/GABARPL(1, 2)‐positive autophagosomes in MEFs upon autophagy induction.
-
A–IConfocal images showing cellular localization of various HyD‐LIR(X)‐GFP constructs together with either mRFP‐GABARAP (A), mRFP‐GABARAPL1 (D), or mRFP‐ GABARAPL2 (G) in MEFs upon autophagy induction (100 nM rapamycin + 10 mM NH4Cl, for 4 h). X: LC3/GABARAP‐binding proteins (ULK2, ATG13, Bnip3, FUNDC1, ScAtg3, TBC1D25, FY (Fyco1), or TP (TP53INP2)). Scale bar, 10 μm. The bar graphs illustrate the ratios of sensor‐positive mRFP‐GABARAP (B), mRFP‐GABARAPL1 (E), or mRFP‐GABARAPL2 (H) and A/C ratios of the fluorescence intensity (C, F, I) of each tested GFP sensor construct. ***P < 0.001 (comparison with all other groups by Kruskal–Wallis test followed by Dunn's multiple comparison test). The values are presented as the mean ± SEM. For the quantitative analysis of the A/C ratio, 20 randomly selected cells in each group were used.
Table 1.
Summary on efficiency of HyD‐LIR(X)‐GFP for detecting LC3/GABARAP‐positive autophagosomes in MEFs upon autophagy induction
| Autophagosome/cytosol ratio of the fluorescent intensity [sensor‐positive mRFP‐LC3 spots (ratio)] | ||||||
|---|---|---|---|---|---|---|
| LIR protein | LC3A | LC3B | LC3C | GABARAP | GABARAL1 | GABARAPL2 |
| ULK2 | 3.41 ± 0.15 (0.89 ± 0.01) | 2.30 ± 0.09 (0.89 ± 0.01) | 3.12 ± 0.12 (0.92 ± 0.01) | 4.67 ± 0.17 (0.98 ± 0.01) | 3.59 ± 0.12 (0.98 ± 0.00) | 3.81 ± 0.17 (0.98 ± 0.00) |
| ATG13 | 4.89 ± 0.26 (0.96 ± 0.01) | 2.56 ± 0.11 (0.86 ± 0.01) | 5.09 ± 0.25 (0.95 ± 0.01) | 4.21 ± 0.14 (0.98 ± 0.00) | 4.88 ± 0.22 (0.99 ± 0.00) | 4.54 ± 0.18 (0.99 ± 0.00) |
| Bnip3 | 3.28 ± 0.13 (0.88 ± 0.02) | 2.45 ± 0.12 (0.87 ± 0.02) | 3.12 ± 0.13 (0.88 ± 0.11) | 4.21 ± 0.14 (0.88 ± 0.01) | 2.50 ± 0.10 (0.91 ± 0.01) | 3.05 ± 0.12 (0.93 ± 0.01) |
| FUNDC1 | 3.49 ± 0.17 (0.90 ± 0.01) | 4.39 ± 0.24 (0.89 ± 0.01) | 3.88 ± 0.14 (0.92 ± 0.01) | 2.78 ± 0.15 (0.91 ± 0.02) | 2.69 ± 0.10 (0.96 ± 0.01) | 3.48 ± 0.16 (0.98 ± 0.00) |
| ScAtg3 | 5.79 ± 0.25 (0.99 ± 0.00) | 4.40 ± 0.33 (0.93 ± 0.01) | 3.70 ± 0.18 (0.98 ± 0.00) | 3.73 ± 0.22 (1.00 ± 0.00) | 4.52 ± 0.13 (0.99 ± 0.00) | 4.42 ± 0.14 (0.99 ± 0.01) |
| TBC1D25 | 5.93 ± 0.34 (0.99 ± 0.00) | 4.77 ± 0.23 (0.95 ± 0.00) | 7.79 ± 0.50 (0.99 ± 0.00) | 5.14 ± 0.25 (0.99 ± 0.00) | 6.50 ± 0.37 (0.99 ± 0.00) | 5.52 ± 0.28 (0.96 ± 0.01) |
| FY | 5.17 ± 0.41 (0.99 ± 0.00) | 4.24 ± 0.26 (0.94 ± 0.00) | 3.83 ± 0.15 (0.96 ± 0.15) | 2.60 ± 0.11 (0.82 ± 0.02) | 2.42 ± 0.08 (0.88 ± 0.02) | 2.68 ± 0.09 (0.92 ± 0.01) |
| TP | 5.24 ± 0.32 (0.99 ± 0.00) | 6.83 ± 0.32 (0.97 ± 0.00) | 5.50 ± 0.34 (0.99 ± 0.00) | 9.61 ± 0.05 (0.99 ± 0.00) | 10.26 ± 0.97 (1.00 ± 0.00) | 4.79 ± 0.20 (0.99 ± 0.00) |
Row presents each LC3/GABARAP protein and column indicates the LIR from each LC/GABARAP‐binding protein. HyD‐LIR‐GFP sensors (indicated by shaded boxes) for LC3/GABARAP‐positive autophagosomes were considered efficient if the A/C ratio of the sensor was above 5 and the ratio of sensor‐positive mRFP‐LC3/GABARAP‐positive spots was above 0.95.
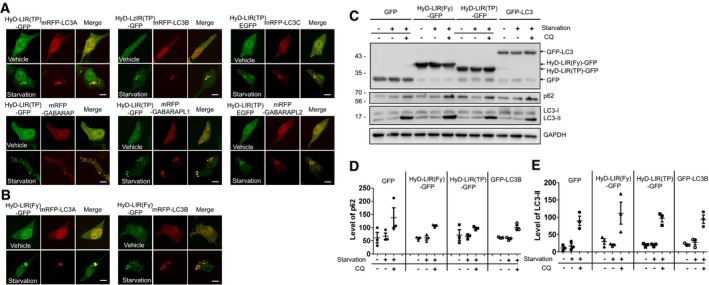
Figure EV2. Efficient localization of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP to mRFP‐LC3/GABARAP‐positive or mRFP‐LC3A/B‐positive autophagosomes in HEK293T cells upon serum starvation and effects of HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP expression on the autophagic flux.
-
A, BConfocal images showing cellular localization of HyD‐LIR(TP)‐GFP (A) or HyD‐LIR(Fy)‐GFP(B) into individual mRFP‐LC3/GABARAP‐positive autophagosome or mRFP‐LC3A/B‐positive autophagosome in HEK293T cells upon autophagy induction (serum starvation, 8 h).
-
C–EHEK293T cells were transfected with a vector encoding GFP, GFP‐LC3, HyD‐LIR(Fy)‐GFP, or HyD‐LIR(TP)‐GFP. Twenty‐four hours after transfection, the cells were deprived of serum in the presence or absence of chloroquine (CQ) for 8 h. The cell lysates were then subjected to Western blot analyses (C) with an anti‐GFP, anti‐LC3, anti‐p62, or anti‐GAPDH antibody. Autophagic flux indicates differences in the levels of p62 (D) or LC3‐II (E) in the presence and absence of CQ. The levels of LC3‐II and p62 in the GFP‐, GFP‐LC3, or HyD‐LIR(Fy/TP)‐GFP‐expressing cells were normalized to that of GAPDH in cells expressing GFP or one of the GFP‐tagged sensors. The data are presented as the mean ± SEM of three independent experiments. Starvation, serum starvation.
Source data are available online for this figure.
To further analyze the binding preference of HyD‐LIR‐GFP sensors for each LC3/GABARAP protein, we performed co‐immunoprecipitation (co‐IP) experiments with an anti‐GFP antibody using HEK293T cell lysates expressing either HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP together with each mRFP‐fused LC3(A, B, C)/GABARAP/GABRAP(L1, L2) 24 h after transfection. We used HEK293T cells to get reliable expression of the GFP sensors because of the higher efficiency of transient transfection in these cells. Western blot analysis was performed using an anti‐GFP, anti‐LC3A, anti‐LC3B, anti‐LC3C, anti‐GABARAP, anti‐GABARAPL1, or anti‐GABARAPL2 antibody. As shown in Fig 4A1, HyD‐LIR(Fy)‐GFP showed limited associations with either GABARAP family protein (GABARAP, GABARAPL1) or LC3C, while it bound to LC3A/B, or GABARAPL2. On the other hand, HyD‐LIR(TP)‐GFP showed good binding to all six LC3/GABARAP proteins (LC3A/B/C, GABARAP, and GABARAPL1/L2) (Fig 4D1). Our co‐IP experiments support the result regarding the A/C ratio of the fluorescence intensities of our sensors in LC3/GABARAP‐positive autophagosomes in MEFs (Figs 2 and 3). However, in the case of HyD‐LIR(Fy)‐GFP, this sensor binds to GABARAPL2 in pull‐down assay, but was hardly localized to GABAPAPL2‐positive autophagosomes upon autophagy induction.
Figure 4. Binding of HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP with each LC3/GABARAP protein and their cellular localization to mRFP‐LC3/GABARAP‐positive autophagosomes in MEFs upon serum starvation in a LIR‐dependent manner.
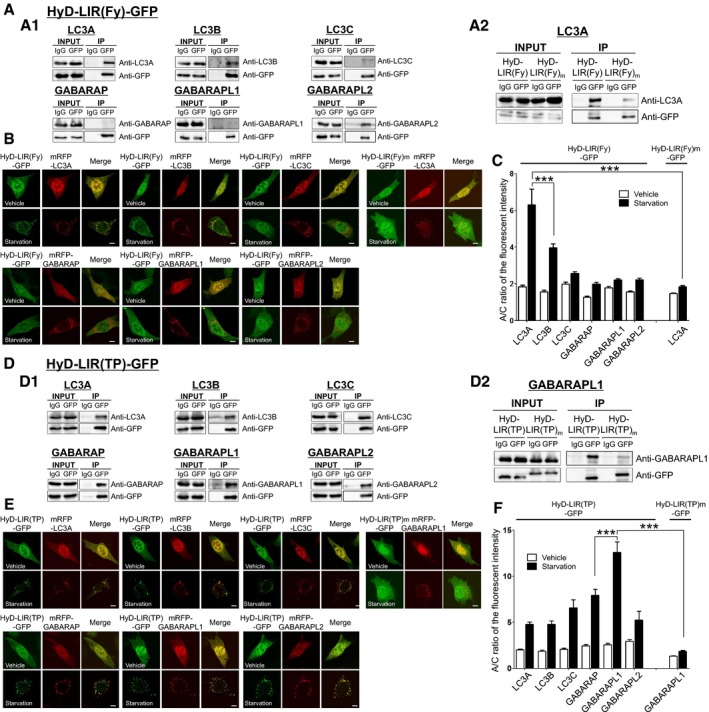
- Pull‐down assay with anti‐GFP antibody using HEK293T cell lysates expressing either HyD‐LIR(Fy)‐GFP (A1) or mutant LIR HyD‐LIR(Fy)m‐GFP (A2) together with either mRFP‐LC3A, mRFP‐LC3B, mRFP‐LC3C, mRFP‐GABARAP, mRFP‐GABARAPL1, or mRFP‐GABARAPL2. IgG (mouse IgG) was used as a negative control. Western blotting was performed using anti‐LC3(A, B, C), GABARAP, GABARAPL1, or GABARAPL2 antibody or anti‐GFP antibody.
- Confocal images showing cellular localization of either HyD‐LIR(Fy)‐GFP (or HyD‐LIR(Fy)m‐GFP) into mRFP‐LC3/GABARAP‐positive autophagosomes in MEFs upon autophagy induction (serum starvation for 4 h). Scale bar: 10 μm.
- The bar graphs illustrate A/C ratios of the fluorescent intensity (B) of HyD‐LIR(Fy)(or LIR(Fy)m)‐GFP. ***P < 0.001 according to Kruskal–Wallis test followed by Dunn's multiple comparison test. For the quantitative analysis of the A/C ratio, 20 randomly selected cells in each group were used. The values are presented as the mean ± SEM.
- Pull‐down assay with anti‐GFP antibody using HEK293T cell lysates expressing either HyD‐LIR(TP)‐GFP (D1) or HyD‐LIR(TP)m‐GFP (D2) together with either mRFP‐LC3A, mRFP‐LC3B, mRFP‐LC3C, mRFP‐GABARAP, mRFP‐GABARAPL1, or mRFP‐GABARAPL2. IgG (mouse IgG) was used as a negative control. Western blotting was performed using anti‐LC3(A, B, C), GABARAP, GABARAPL1, or GABARAPL2 antibody or anti‐GFP antibody.
- Confocal images showing cellular localization of either HyD‐LIR(TP)‐GFP (or HyD‐LIR(TP)m‐GFP) into mRFP‐LC3/GABARAP‐positive autophagosomes in MEFs upon autophagy induction (serum starvation for 4 h). Scale bar: 10 μm.
- The bar graph illustrates A/C ratios of the fluorescent intensity (E) of HyD‐LIR(TP)(or LIR(TP)m)‐GFP. ***P < 0.001 according to Kruskal–Wallis test followed by Dunn's multiple comparison test. For the quantitative analysis of the A/C ratio, 20 randomly selected cells in each group were used. The values are presented as the mean ± SEM.
Source data are available online for this figure.
Next, we further confirmed the cellular localization and A/C ratio of the fluorescence intensities of HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP at LC3/GABARAP‐positive autophagosomes upon serum starvation for 4 h in MEFs. As shown in Fig 4B and C, HyD‐LIR(Fy)‐GFP was most efficiently localized to LC3A‐ or LC3B‐positive autophagosomes compared to LC3C/GABARAP/GABARAP(L1, L2)‐positive autophagosomes. On the other hand, HyD‐LIR(TP)‐GFP was very efficiently localized to mRFP‐LC3/GABARAP‐positive autophagosomes, but preferentially localized to GABARAPL1‐positive autophagosomes (Fig 4E and F). These results are similar with those observed for rapamycin + NH4Cl‐induced autophagy in MEFs (Figs 2 and 3). Overall, both the pull‐down assay and cellular localization data strongly support the potential use of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP as efficient sensors for detecting all LC3/GABARAP‐positive autophagosomes or LC3A/B‐positive autophagosomes, respectively.
To further confirm whether localization of HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP to autophagosomes was LIR domain‐specific, we generated LIR mutants, HyD‐LIR(TP)m‐GFP and HyD‐LIR(Fy)m‐GFP, in which some amino acids in the consensus sequence of the TP53INP2 or FYCO1 LIR motif ((W/F/Y)‐X‐X‐(I/L/V)) were point‐mutated to alanine (TP53NP2: VDGWLIIDLP ‐> VDGALIADLP; FYCO1: AVFDIITD ‐> AVFDIATD) (Birgisdottir et al, 2013). Next, we transfected MEFs with mRFP‐GABARAPL1 or mRFP‐LC3A as the most efficient binding partner of each sensor and one of the following: HyD‐LIR(TP)m‐GFP or HyD‐LIR(Fy)m‐GFP. We performed a co‐IP experiment using an anti‐GFP antibody with HEK293T cell lysates expressing either HyD‐LIR(TP)m‐GFP or HyD‐LIR(Fy)m‐GFP together with mRFP‐GABARAPL1 or mRFP‐LC3A 24 h after transfection. As shown in Fig 4A2 and D2, HyD‐LIR(Fy)m‐GFP and HyD‐LIR(TP)m‐GFP showed reduced association with mRFP‐LC3A or mRFP‐GABARAPL1, respectively, indicating that the HyD‐LIR‐GFP sensor binds to LC3/GABARAP in a LIR‐dependent manner. Next, we further examined whether HyD‐LIR(Fy)m‐GFP and HyD‐LIR(TP)m‐GFP localized to LC3A‐ or GABARAPL1‐positive autophagosomes. HyD‐LIR(Fy)m‐GFP and HyD‐LIR(TP)m‐GFP, which harbored the mutant LIR motifs, were diffusely expressed in the cytoplasm of MEFs (Fig 4B and E). As shown in Fig 4C and F, the A/C ratios of the fluorescence intensity of HyD‐LIR(Fy)m‐GFP and HyD‐LIR(TP)m‐GFP were significantly lower than those of HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP. In conclusion, the autophagosome localization of the HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP sensors upon autophagy induction was LIR‐dependent.
HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP efficiently localize to endogenous GABARAPL1‐ or LC3A/B‐positive autophagosomes
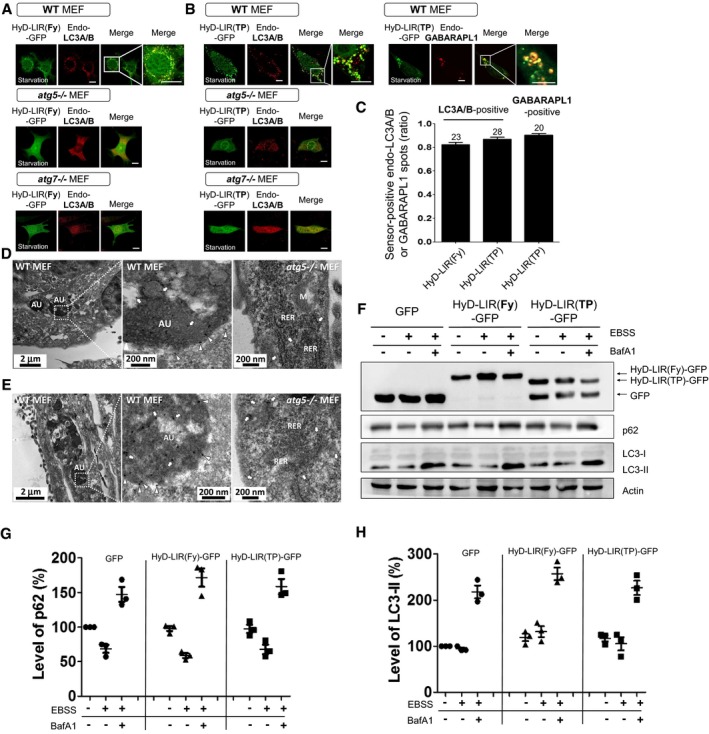
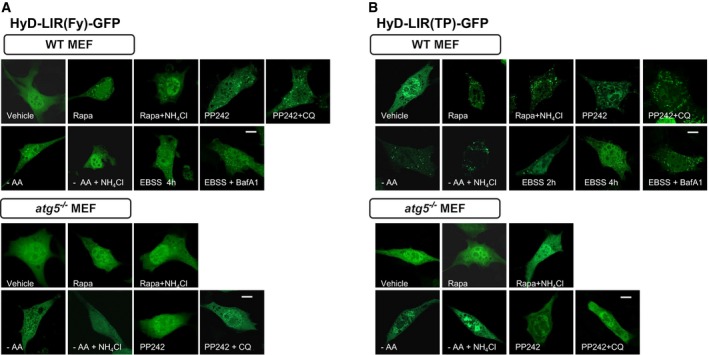
Next, to examine whether the sensors could efficiently detect endogenous LC3/GABARAP proteins at the autophagosome, we selected HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP as the most efficient candidate sensors based on the results above (Figs 2, 3, 4). First, to test the specificity of these sensors in relation to autophagy, HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP was expressed in WT, Atg5 −/−, or Atg7 −/− MEFs in which autophagy was induced by serum starvation. The cells were fixed and immunostained with anti‐LC3A/B or anti‐GABARAPL1 antibodies to detect endogenous LC3A/B‐ or GABARAPL1‐positive autophagosomes, respectively (Fig 5A and B). We also quantified the ratio of sensor‐positive endogenous LC3A/B or GABARAPL1 spots (Fig 5C). As shown in Fig 5A–C, HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP efficiently co‐localized with endogenous LC3A/B‐ or GABARAPL1‐positive autophagosomes upon serum starvation in WT MEFs, but not in Atg5 −/− or Atg7 −/− MEFs. We further checked that HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP efficiently localized to autophagosomes in WT MEFs, but not in Atg5 −/− MEFs upon autophagy induction by amino acid starvation, Earle's balanced salt solution (EBSS) starvation, rapamycin or PP242 (Fig EV3A and B). Our new sensors also efficiently localized to autophagosomes in HeLa cells upon EBSS starvation or rapamycin treatment (Appendix Fig S3).
Figure 5. Effective localization of HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP to endogenous LC3/GABARAP‐positive autophagosomes during autophagy induction with serum starvation in WT MEFs, but not in autophagy‐deficient MEFs.
-
A, BConfocal images show cellular localization of HyD‐LIR(Fy)‐GFP (A) or HyD‐LIR(TP)‐GFP (B) to endogenous LC3A/B‐positive autophagosomes in wild‐type (WT), Atg5 −/−, or Atg7 −/− MEFs upon serum starvation (8 h). Confocal images show cellular localization of HyD‐LIR(TP)‐GFP (B) to endogenous GABARAPL1‐positive autophagosomes in wild‐type (WT) MEFs upon serum starvation (8 h). The merged image was enlarged. Scale bar: 10 μm.
-
CThe bar graph illustrates the ratios of sensor‐positive LC3A/B or GABARAPL1 spots. The values are presented as the mean ± SEM. The numbers on the bars indicate the number of cells used for the experiment.
-
D, EElectron microscopy images show immunogold labeling of HyD‐LIR(Fy)‐GFP (D) or HyD‐LIR(TP)‐GFP (E) in autophagic vacuoles (AU) in WT (left) and Atg5 −/− MEFs (right) during serum starvation (8 h). Arrows: immunogold labeling of LC3/GABARAP‐bound GFP inside the autophagosomes. Arrowheads: immunogold labeling of LC3/GABARAP‐bound GFP in the autophagosomal membrane. R: region, M: mitochondrion, RER: rough endoplasmic reticulum.
-
FAutophagic flux assay in MEFs expressing GFP, HyD‐LIR(Fy)‐GFP, or HyD‐LIR(TP)‐GFP upon starvation with EBSS (in the presence or absence of bafilomycin A1 (BafA1) for 4 h). The cell lysates were then subjected to Western blot analyses with an anti‐GFP, anti‐LC3, anti‐p62, or anti‐actin antibody.
-
G, HAutophagic flux indicates differences in the levels of p62 (G) or LC3‐II (H) in the presence and absence of CQ. The bar graphs illustrate the percentage of level of p62 or LC3‐II. The levels of LC3‐II or p62 in the GFP‐ or HyD‐LIR(Fy/TP)‐GFP‐expressing cells were normalized to that of actin in cells expressing GFP or one of the GFP‐tagged sensors. The data are presented as the mean ± SEM of three independent experiments.
Source data are available online for this figure.
Figure EV3. Efficient localization of HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP to autophagic vacuoles in WT and Atg5 −/− MEFs upon various conditions of autophagy induction.
-
A, BConfocal images showing cellular localization of HyD‐LIR(Fy)‐GFP (A) and HyD‐LIR(TP) (B) in WT and Atg5 −/− MEFs upon autophagy induction with amino acid deprivation (8 h), PP242 (20 μM, 4 h), EBSS starvation (2 or 4 h), or rapamycin (100 nM, 4 h) in the presence or absence of CQ (50 μM), BafA1 (100 nM) or NH4Cl (10 mM). Scale bar: 10 μm.
Next, to directly test whether the cellular localization of HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP on autophagosomes could be observed at the ultrastructural level, we used an anti‐GFP antibody and performed immunogold electron microscopy analysis of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP‐expressing WT or Atg5 −/− MEFs after autophagy induction by serum starvation. As shown in Fig 5D and E, HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP mostly localized to autophagic vesicles in WT but not in Atg5 −/− MEFs. Moreover, immunogold‐labeled GFP in WT MEFs expressing HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP was detected inside the autophagosome (indicated by arrows), at the region of the autophagosomal membrane (indicated by arrowheads), and, occasionally, in the cytoplasm (Fig 5D and E). In contrast, the GFP sensor labeled with gold particles was detected only in the cytoplasm of Atg5 −/− MEFs (indicated by arrows; Fig 5D and E). Taken together, these data clearly show that HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP preferentially localized to autophagic vesicles in MEFs during autophagy induction, further supporting the applicability of HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP as new LC3/GABARAP‐positive autophagosome sensors.
HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP do not affect autophagic flux
Because HyD‐LIR‐GFP sensors bind to the LC3/GABARAP proteins, they may also affect autophagic flux via expression of the exogenous LIR motif. The latter process may stimulate sequestration of endogenous LC3 and/or modify its functions. Therefore, we tested whether HyD‐LIR(Fy)‐GFP and HyD‐LIR(TP)‐GFP expression itself affect autophagic flux in MEFs and HEK293T cells (Figs 5F–H and EV2C–E). To measure autophagic flux, the levels of autophagic substrates, such as LC3‐II or p62, in MEFs (EBSS starvation) or HEK293T cells (serum starvation) expressing GFP, HyD‐LIR(Fy)‐GFP, or HyD‐LIR(TP)‐GFP in the presence or absence of 100 nM bafilomycin A1 (BafA1, in MEFs) or 50 μM chloroquine (CQ, in HEK293T cells) were quantified by Western blotting (Klionsky et al, 2012; Chittaranjan et al, 2015). As shown in Figs 5F–H and EV2C–E, the levels of substrate proteins, which indicate the autophagic flux rate, in MEFs or HEK293T cells expressing either HyD‐LIR(Fy)‐GFP or HyD‐LIR(TP)‐GFP sensors were similar to that in control cells expressing either GFP or GFP‐LC3B. This suggests that the expression of HyD‐LIR‐GFP sensors did not affect autophagic flux in turnover assays for LC3 or p62.
HyD‐LIR(TP)‐mRFP detects DFCP1‐positive omegasomes, STX17‐positive autophagosomes, and autolysosomes
To further validate HyD‐LIR(TP)‐GFP/mRFP as the most efficient sensor for autophagosomes, we examined which kind of autophagic vesicles are detected by the HyD‐LIR(TP)‐mRFP sensor after autophagy induction. To this end, MEFs were transfected with HyD‐LIR(TP)‐mRFP or mRFP‐LC3B together with either GFP‐fused double FYVE domain‐containing protein 1 (GFP‐DFCP1), an omegasome marker, or GFP‐fused syntaxin 17 (GFP‐STX17), a marker for fully matured autophagosomes. At 24 h after transfection, the cells were starved by serum deprivation in the presence or absence of CQ. DFCP1 is translocated to subdomains of the endoplasmic reticulum called omegasomes, which are in close proximity to LC3 puncta (Axe et al, 2008). It has been reported that although most DFCP1‐positive puncta are LC3B‐positive, DFCP1 is absent from the mature autophagosome, which in turn co‐localizes with STX17 (Itakura et al, 2012). As shown in Fig 6A–D, HyD‐LIR(TP)‐mRFP and mRFP‐LC3B detected more GFP‐DFCP1‐positive and GFP‐STX17‐positive autophagosomes after serum starvation compared to the number of autophagosomes detected under vehicle‐treated conditions. These results indicate that HyD‐LIR(TP)‐GFP/mRFP can also detect DFCP1‐positive omegasomes and fully matured autophagosomes.
Figure 6. Efficient localization of HyD‐LIR(TP)‐mRFP to GFP‐DFCP1‐positive, GFP‐STX17‐positive, or LysoTracker‐positive autophagic vacuoles in MEFs upon autophagy activation by serum starvation.
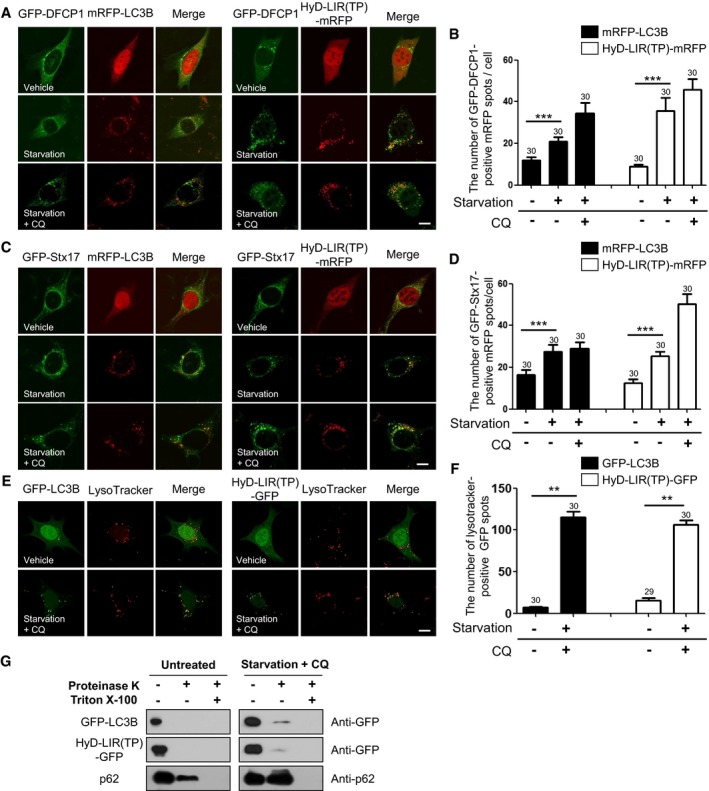
-
A–FConfocal images showing cellular localization of either mRFP‐LC3B (or GFP‐LC3B) or HyD‐LIR(TP)‐mRFP together with GFP‐DFCP1 (A), GFP‐STX17 (C), or Lysotracker (E) in MEFs upon serum starvation (in the absence or presence of CQ). The bar graphs illustrate the ratios of GFP‐DFCP1‐positive (B), GFP‐STX17‐positive mRFP dots per cell (D), and ratios of LysoTracker‐positive GFP spots (F). The data are presented as the mean ± SEM of three independent experiments. ***P < 0.001, according to one‐way ANOVA followed by Tukey's post hoc test. **P = 0.013, according two‐tailed Mann–Whitney U‐test. The numbers on the bars indicate the number of cells used for the experiment.
-
GProteinase K protection assay in HEK293T cells expressing HyD‐LIR(TP)‐EGFP upon serum starvation (in the absence or presence of CQ, 8 h) with (or without) proteinase K (25 μg/ml) or Triton X‐100 (0.5%). Western blot analysis was performed using anti‐GFP or anti‐p62 antibodies.
Source data are available online for this figure.
Next, we examined whether HyD‐LIR(TP)‐GFP could detect autolysosomes by using LysoTracker. For this purpose, HyD‐LIR(TP)‐GFP was expressed in MEFs for 24 h, and then, the cells were starved by serum deprivation for 8 h in the presence of CQ. LysoTracker was added to detect acidic components in starved MEFs expressing HyD‐LIR(TP)‐GFP. As shown in Fig 6E and F, HyD‐LIR(TP)‐GFP was localized to LysoTracker‐positive autolysosomes, which accumulated in the presence of CQ, suggesting that HyD‐LIR(TP)‐RFP were associated with autolysosomes. These data provide further support of the potential use of our newly developed HyD‐LIR(TP)‐fluorescent sensor for detecting LC3/GABARAP‐positive autophagosomes/autolysosomes.
Because we showed that HyD‐LIR(TP)‐GFP was localized to sealed (mature) autophagosomes/autolysosomes, we next evaluated whether HyD‐LIR(TP)‐GFP was localized to the inner membrane and the inside of autophagosomes. To test this, we performed a proteinase K protection assay in HEK293T cells expressing HyD‐LIR(TP)‐GFP (McEwan et al, 2015). To do this, we overexpressed GFP‐LC3B or HyD‐LIR(TP)‐GFP in HEK293T cells and induced autophagy by serum starvation in the presence of CQ. As a control, we monitored endogenous p62. p62 was increased by serum starvation + CQ treatment, indicating that p62 accumulated within autophagosomes in HEK293T cells (Fig 6G). Similarly, HyD‐LIR(TP)‐GFP or GFP‐LC3B levels increased under conditions of serum starvation + CQ treatment, indicating that HyD‐LIR(TP)‐GFP and GFP‐LC3B are located within sealed autophagosomes (Fig 6G). Considering that GFP‐LC3B is known to be localized to the inner membrane and inside of mature autophagosomes, our results indicate that HyD‐LIR(TP)‐GFP was also localized to the inner membranes and inside of autophagosomes similar to GFP‐LC3B.
HyD‐LIR(TP)‐GFP allows live‐imaging of dynamic autophagosomes
To further compare the effectiveness of HyD‐LIR(TP)‐GFP as a sensor for endogenous LC3/GABARAP‐positive autophagosomes with that of GFP‐LC3B, both reporter constructs were transfected into MEFs starved by serum deprivation (8 h) 24 h after transfection. As shown in Fig 7A and B, the number of LIR sensor‐positive autophagosomes in MEFs expressing HyD‐LIR(TP)‐GFP was significantly increased following serum starvation compared to that of vehicle‐treated cells, as was the case for the GFP‐LC3B reporter. Therefore, our data suggest that HyD‐LIR(TP)‐GFP is an effective autophagosome sensor after autophagy induction under conditions that do not require LC3/GABARAP overexpression.
Figure 7. Detection of dynamic autophagosomes in live MEFs upon serum starvation without LC3/GABARAPs overexpression by using HyD‐LIR(TP)‐GFP .
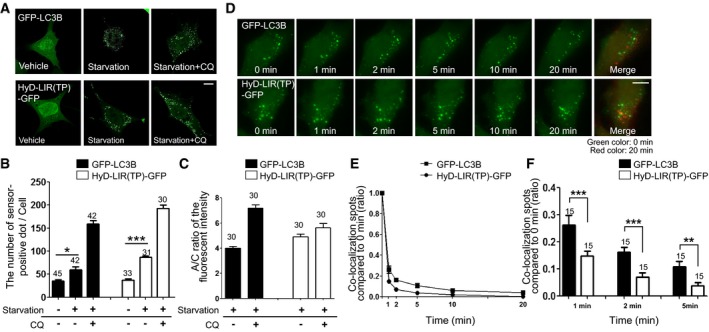
-
AConfocal images showing cellular localization of GFP‐LC3B or HyD‐LIR(TP)‐GFP in MEFs upon serum starvation (8 h). Scale bar: 10 μm.
-
B, CThe bar graphs illustrate (B) the number of GFP‐LC3B‐ or HyD‐LIR(TP)‐GFP‐positive spots and (C) A/C ratio of fluorescence intensity of GFP‐fused sensors. The data are presented as the mean ± SEM of three independent experiments. *P = 0.02, according to Kruskal–Wallis test followed by Dunn's multiple comparison test; ***P < 0.001, according to one‐way ANOVA followed by Tukey's post hoc test. The numbers on the bars indicate the number of cells used for the experiment.
-
DConfocal images in live cells showing HyD‐LIR(TP)‐GFP‐ or GFP‐LC3B‐positive autophagosomes (dots) at 0, 1, 2, 5, 10, or 20 min after autophagy induction with rapamycin (100 nM) for 20 min. Cell images between 0 and 20 min (pseudo‐red image) after autophagy induction were merged. Scale bar: 10 μm.
-
E, FQuantification of the ratio of the number of co‐localized dots in images at the indicated time to that in the image at time 0 min in cells expressing HyD‐LIR(TP)‐GFP or GFP‐LC3B (E). Bar graph illustrates the ratio of the number of co‐localized dots in images at 1, 2, and 5 min compared to that of co‐localized dots at 0 min (F). The data are presented as the mean ± SEM of three independent experiments. **P = 0.007, ***P < 0.001, according to two‐tailed unpaired t‐test. The numbers on the bars indicate the number of cells used for the experiment.
Next, we examined whether HyD‐LIR(TP)‐GFP could be used to monitor autophagosome dynamics via live‐imaging. We performed real‐time live cell imaging in GFP‐LC3B or HyD‐LIR(TP)‐GFP‐transfected MEFs at 1‐min intervals for 20 min after treatment with rapamycin. As shown in Fig 7D, and Movies EV1 and EV2, HyD‐LIR(TP)‐GFP, and GFP‐LC3B‐positive vesicles were dynamically formed and moving. To quantify the dynamics of sensor‐positive spots, we counted the stationary spots in the images taken at 1, 2, 5, 10, or 20 min and compared them with the stationary spots on the image taken at 0 min. As shown in Fig 7E, the ratio of co‐localization of GFP sensor‐positive dots at 0 min and at the indicated time points (1, 2, 5, 10, 20 min) in cells expressing GFP‐LC3B or HyD‐LIR(TP)‐GFP upon autophagy induction was significantly reduced, indicating that the mobility of sensor‐positive autophagosomes is dynamic during autophagy induction. Interestingly, the ratio of co‐localization spots (compared to 0 min) of HyD‐LIR(TP)‐GFP was significantly lower than that of GFP‐LC3B (Fig 7F). These results suggest that HyD‐LIR(TP)‐GFP‐positive autophagosomes are more dynamic than GFP‐LC3B‐positive autophagosomes. Collectively, these data suggest that our newly developed sensor HyD‐LIR(TP)‐GFP is a useful marker of dynamic LC3/GABARAP‐positive autophagosomes formed after autophagy induction in the absence of LC3/GABARAP overexpression in living cells.
HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP allow monitoring of mitophagy
To test whether our sensors could also detect mitophagy as a selective autophagic process, we used two different mitophagy inducers.
First, we examined whether our sensors could detect CCCP‐induced mitophagy in MEFs using MitoTracker (red). CCCP is known as a potent mitochondrial uncoupling agent that can induce mitophagy for lysosomal removal of depolarized mitochondria (Georgakopoulos et al, 2017). Thus, mitophagy was induced by CCCP treatment (20 μM for 3 h) in MEFs expressing GFP, GFP‐LC3B, HyD‐LIR(TP)‐GFP, or HyD‐LIR(Fy)‐GFP in the presence and absence of CQ. As shown in Fig 8A and B, HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP was efficiently localized to MitoTracker‐positive spots (damaged mitochondria) in MEFs, as seen for GFP‐LC3B. To examine the efficiency of detecting mitophagy using the sensor, we counted both GFP and MitoTracker‐positive spots in vehicle‐, CCCP‐, or CCCP+CQ‐treated cells. As shown in Fig 8B, the number of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP‐positive dots co‐localized with MitoTracker increased upon mitophagy induction by CCCP treatment and was further increased by CCCP+CQ treatment, which was similar to the results obtained for GFP‐LC3B.
Figure 8. Co‐localization of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP with MitoTracker‐positive spots in mitophagy induced by CCCP or phenanthroline treatment.
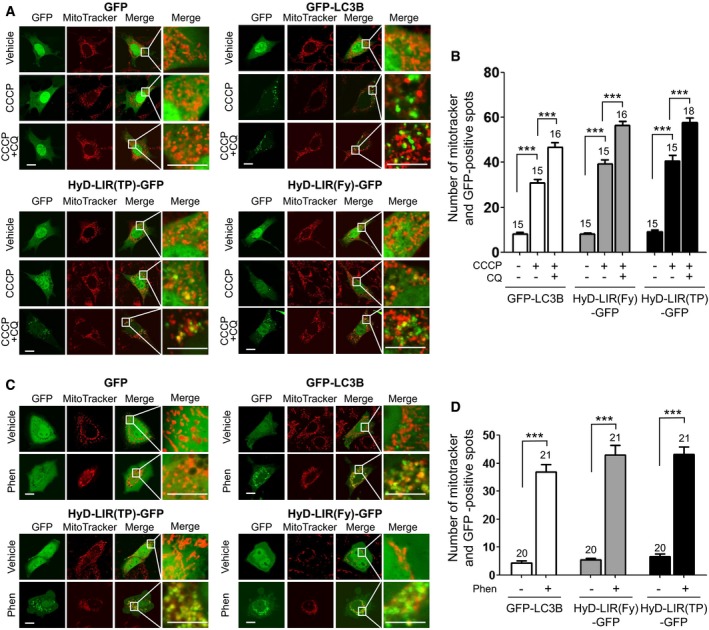
-
A–DConfocal images showing cellular localization of GFP, GFP‐LC3B, HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP together with MitoTracker in MEFs (A) upon mitophagy induction (CCCP (20 μM) or CCCP+CQ (50 μM)) and in HeLa cells (C) upon mitophagy induction (50 μM phenanthroline). Scale bar, 10 μm. The bar graphs (B, D) illustrate the number of co‐localized spots (GFP and MitoTracker‐positive‐spots). ***P < 0.001, according to one‐way ANOVA followed by Tukey's post hoc test. The data are presented as the mean ± SEM. The numbers on the bars indicate the number of cells used for the experiment.
Second, mitophagy was induced in HeLa cells expressing GFP, GFP‐LC3B, HyD‐LIR(TP)‐GFP, or HyD‐LIR(Fy)‐GFP with 50 μM phenanthroline for 48 h, and the cells were stained with MitoTracker (Park et al, 2012). As shown in Fig 8C, GFP‐LC3B was co‐localized with MitoTracker‐positive spots. We found that either HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP also localized to MitoTracker‐positive spots in HeLa cells upon mitophagy induction after 48 h of phenanthroline treatment. As shown in Fig 8D, there was increased co‐localization of HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP with MitoTracker‐positive spots. Taken together, our newly developed sensors either HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP efficiently co‐localized with MitoTracker‐positive damaged mitochondria showing their potential application for research on mitophagy.
Discussion
Standardized conditions and criteria for monitoring autophagy are important issues in autophagy research (Klionsky, 2007; Zhou & Huang, 2010; Klionsky et al, 2016; Ktistakis & Tooze, 2016). Regardless of the type of induction signals, the most striking phenomenon during autophagy is the formation of double‐membrane vesicles, autophagosomes, which is the hallmark of autophagic processes (Klionsky et al, 2012; Feng et al, 2014; Carlsson & Simonsen, 2015).
The LC3/GABARAP family (ATG8 in yeast) is the best‐known autophagy marker, which specifically localizes to autophagic vesicles during autophagy in various organisms from yeast to mammals (Kabeya et al, 2000; Tanida et al, 2004; Klionsky et al, 2016). Among the methods suggested for monitoring autophagy, microscopy analysis of GFP/RFP‐tagged LC3 has been most widely used to study dynamic changes in autophagosome functions in cells in vitro and in vivo (Klionsky et al, 2007, 2012, 2016; Kaizuka et al, 2016). However, this approach is accompanied by some artifacts resulting from the overexpression of GFP/RFP‐LC3, as well as by practical limitations of detecting and tracking endogenous LC3/GABARAP proteins in the autophagosome in living cells.
To overcome these limitations, in the present study, we developed fluorescent sensors for monitoring endogenous LC3/GABARAP‐positive autophagosomes and their dynamic changes upon autophagy induction. These sensors incorporate a LIR motif and HyD, which enhances the association of the sensor with the membrane in living cells. According to our analysis, the LIR motif, which can interact with LC3/GABARAP proteins, and HyD separately fail to be strongly localized to the autophagosome during autophagy induction. In contrast, when HyD, which enhances membrane association, is fused with the LIR motif from an LC3/GABARAP‐binding protein, the resulting HyD‐LIR sensor can efficiently detect endogenous LC3/GABARAP‐positive autophagosomes upon autophagy induction (Fig 1).
To date, several LIR motifs have been predicted and identified in various LC3/GABARAP‐binding proteins in eukaryotes (Johansen & Lamark, 2011; Birgisdottir et al, 2013; Kalvari et al, 2014; Jacomin et al, 2016). Although most LIR motifs contain a core consensus sequence (W/F/Y)xx(L/I/V), where “x” can be any amino acid, LIR motifs with different sequences have distinct affinities to LC3/GABARAP family proteins. Our analysis of the ratios of sensor‐positive LC3/GABARAP spots and A/C ratios of fluorescence intensity of these HyD‐LIR sensors clearly demonstrate the reliable LC3/GABARAP‐binding properties of each LIR motif of the sensors. However, HyD‐LIR(Fy)‐GFP associates with GABARAPL2 based on our pull‐down assay, but it was inefficiently localized to GABARAPL2‐positive autophagosomes, raising the possibility that HyD‐LIR(Fy)‐GFP is not efficiently localized to GABARAPL2‐positive autophagosomal membrane. HyD‐LIR(p62)‐GFP, the LIR motif from p62, weakly associated with LC3B‐positive autophagosomes (Fig 1). In line with this result, it is known that the p62 LIR motif binds LC3/GABARAP proteins with low affinity, indicating that the binding affinity of each LIR motif to LC3/GABARAP proteins impacts localization to the autophagosome (Maruyama et al, 2014). Although further biochemical and structural studies are needed to characterize the interaction of the LIR motifs with LC3/GABARAP proteins, analysis of the A/C ratio of fluorescence intensity of the sensors with co‐expression of each LC3/GABARAP protein is a reliable method for identifying the relative binding properties of LIR motifs.
In mammalian cells, several homologs of yeast Atg8 such as LC3 (A, B, C), GABARAP, and GABARAPL1/2 have been identified. Specific roles of LC3/GABARAP proteins using each LC3/GABARAP‐specific knockout HeLa cells have been recently suggested (Nguyen et al, 2016), but their exact roles in autophagosome biogenesis and autophagy pathways remain unclear. Several LIR motifs are also known to bind the homologs of GABARAP or GABARAPL1/2, as well as LC3(A, B, C), with different affinities. Considering our results and those shown in Appendix Table S1, HyD‐LIR(Fy)‐GFP could be specific sensor for LC3A/B‐positive autophagosomes, while HyD‐LIR(TP)‐GFP can be used to detect all LC3/GABARAP‐positive autophagosomes. However, HyD‐LIR(TP)‐GFP appears to be more preferentially localized to GABARAP/GABARAPL1. Therefore, it would be interesting to compare autophagosome biogenesis and dynamics by utilizing HyD‐LIR sensors containing different LIR motifs, for example, those specifically targeting LC3(A, B, C), GABARAP, or GABARAPL1/2. We also showed that our sensors co‐localized with MitoTracker‐positive damaged mitochondria upon mitophagy induction, raising their potential application for mitophagy research (Fig 8). During the revision of our work, Stolz et al reported the development of fluorescence‐based LC3/GABARAP‐specific sensors using engineered peptides by multiplication, charge distribution, and fusion with a FYVE or oligomerization (PB) domain (Stolz et al, 2017). Autophagy research using our new developed HyD‐LIR‐based LC3/GABARAP sensors as well as the fluorescence‐based LC3/GABARAP‐specific sensors from Stolz and colleagues will improve the understanding of the specific roles of LC3/GABARAP proteins and detailed mechanism underlying this dynamic interaction during macroautophagy/selective autophagy under physiological or pathological conditions.
We attempted to use the hydrophobic domain to enhance stable detection of endogenous LC3 at the autophagosome. Generally, membrane‐associated proteins targeting specific intracellular organelles require multivalent domains in the same protein, for example, a dimerization/oligomerization domain, hydrophobic motif for general membrane association, or a specific lipid/protein‐binding motif for specific intracellular membrane targeting (Lemmon, 2008). For example, FYCO1 binding to LC3 via a LIR motif is necessary but not sufficient for FYCO1 targeting to the autophagosome. Dimerization of the FYVE domain via the CC region is known to be important for the association of FYCO1 with the membrane (Pankiv & Johansen, 2010; Pankiv et al, 2010). Therefore, targeting of the autophagosome by FYCO1 is mediated by the cooperative action of multiple domains, including the LIR motif. Similarly, RavZ, which is known as a Legionella anti‐autophagy effector, targets the autophagosome through the N‐ and C‐terminal LIR motifs and PI3P binding/membrane association domain (Horenkamp et al, 2015; Kwon et al, 2016). Similarly, to improve the binding affinity of fluorescence‐based LC3/GABARAP‐specific sensors to the LC3/GABARAP protein, multiplication of the LIR motif itself or the addition of a PI3P binding motif to increase the membrane association or PB domain to induce oligomerization can be applied (Stolz et al, 2017). Consistent with other reports, we also showed that the addition of a flexible HyD to the N‐terminus of the LIR motif enhanced autophagosomal membrane targeting (Fig 1).
Compared to GFP/RFP‐LC3/GABARAP overexpression, our newly developed autophagy sensors have several advantages with respect to monitoring autophagy in living cells. The efficiency of their cellular localization to the autophagosome was comparable to that of GFP/RFP‐LC3. Most importantly, our HyD‐LIR(TP)‐GFP sensor allows for tracking endogenous LC3/GABARAP proteins in autophagic vesicles during autophagy in living cells without the need for LC3/GABARAP protein overexpression. The size, morphology, or number of autophagic vesicles marked by the HyD‐LIR(TP)‐GFP sensor reflect the actual changes during the autophagic process, and the sensor does not affect basal autophagic flux. Moreover, using our new sensor, we clearly observed an autophagic membrane surrounding an mRFP‐LC3‐positive vesicle in some cases (Appendix Fig S4). This finding indicates that our sensor preferentially detected endogenous LC3/GABARAP‐positive autophagosomes. Therefore, the new sensor was more reliable than GFP/RFP‐LC3B in our culture system. In polarized neurons, as described above, overexpression of RFP/GFP‐LC3B sometimes induces abnormal neurite morphology. However, expression of the new sensor did not affect the morphology of cultured cortical neurons in our experiments (Appendix Fig S1). Furthermore, the new sensor was successfully used to monitor dynamic changes in autophagic vesicles by tracking endogenous LC3/GABARAPs upon autophagy induction in living cells in a time‐dependent manner. The sensor‐positive autophagosomes showed more dynamic changes upon serum starvation compared to GFP‐LC3B‐positive autophagosomes during live cell imaging (Fig 8E and F, and Movies EV1 and EV2). Our sensor may reflect actual dynamic changes of autophagosomes in living cells because it detected endogenous LC3/GABARAPs in autophagosomes without the need for LC3/GABARAP overexpression. Such experiments using our sensor should improve the understanding of the autophagic process as well as its dysfunction (Carlsson & Simonsen, 2015). Furthermore, to date, the dynamics of autophagosomes by detecting endogenous LC3/GABARAPs could not be monitored in vivo. Therefore, in transgenic animal models generated using our new sensors, monitoring of dynamic changes and mobility of LC3/GABARAP‐specific autophagosomes will be possible at different developmental stages as well as during pathological changes caused by aging or stress. However, if highly expressed, our HyD‐LIR‐based sensors might compete with endogenous LC3/GABARAP‐binding proteins. Therefore, low expression of our sensors might be important for monitoring dynamics of autophagosomes at physiological level in stable cell line or in transgenic animal models. Moreover, if our sensor is applied to the development of sensors for specific types of autophagy, a variety of selective autophagy sensors may be generated by modifying or changing the LIR motif or specific lipid‐ or protein‐binding domain. Such approaches may expand autophagy research and extend its various practical applications in vitro and in vivo.
Materials and Methods
DNA constructs
All primers are described in Appendix Table S2. mRFP was amplified by PCR and inserted in place of GFP to generate the pmRFP‐C1, pmRFP‐C3, and pmRFP‐N3 vectors using the restriction enzymes NheI‐BglII and BamHI‐NotI. Additionally, mRFP in the pmRFP‐C3 vector was inserted using NheI and HindIII in the pcDNA3.1 vector to produce pcDNA3.1‐mRFP. The Aplysia PDE4 short‐form (N20) (SN20, HyD)‐GFP was generated by PCR amplification of the full‐length Aplysia PDE4 short‐form gene and inserted using NheI and HindIII in the pGFP‐N1, pGFP‐N3, and pmRFP‐N3 vectors. The LIR motifs (Appendix Table S1) were amplified by PCR using primers (Appendix Table S2) and inserted into the pGFP‐N3, pGFP‐N3‐HyD, or pmRFP‐N3‐HyD vectors with the restriction enzymes HindIII and KpnI. Detailed domain structures and amino acid sequences of HyD‐LIR(TP)‐GFP and HyD‐LIR(Fy)‐GFP were described in Appendix Fig S2. LIR motif mutants were generated using recombinant primers. GFP‐LC3B, mRFP‐LC3B, GST‐LC3A, GST‐GABARAP, GST‐GABARAPL1, GST‐GABARAL2, and pMXs‐puro GFP‐DFCP1 were obtained from Addgene (Cambridge, MA, USA). In addition, pMRXIP GFP‐STX17 WT was kindly provided by Dr. Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) (Itakura et al, 2012).
To generate mRFP‐fused LC3/GABARAPs subfamilies, GABARAP and GABARAPL2 were amplified by PCR and inserted into the pmRFP‐C3 vectors with the restriction enzymes HindIII and SalI. In the same manner, LC3A, LC3C, and GABARAPL1 were amplified by PCR and inserted into the pcDNA3.1‐mRFP vectors with the restriction enzymes BamHI and NotI.
Cell culture, transfection, drug treatment, and live cell imaging
Cell culture was performed as described previously (Jang et al, 2010, 2011; Ryu et al, 2014). HEK293T cells, MEFs, and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum (FBS) and penicillin/streptomycin in a humidified atmosphere containing 5% (v/v) of CO2 at 37°C. Primary neuronal cell cultures were grown as described previously (Lee & Gao, 2009). For serum starvation, MEFs or HeLa cells were incubated in cell culture medium (DMEM) without FBS for 8–10 h. For amino acid starvation, MEFs or HeLa cells were incubated in HBSS medium for 8 h. For nutrient starvation, MEFs or HeLa cells were incubated EBSS medium for 2–4 h (Munafo & Colombo, 2001). For mitophagy induction, HeLa cells were treated with phenanthroline (50 μM, 48 h) or CCCP (20 μM, 3 h) in the presence or absence of 50 μM chloroquine (CQ) (Park et al, 2012). The cells were transfected with a DNA construct using either the calcium phosphate method or Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) and incubated for 24 h.
Fluorescent images were acquired with a confocal laser‐scanning microscope (Radiance 2000, Zeiss, Oberkochen, Germany) and analyzed with NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA). During live observation, the cells were kept at 37°C in an atmosphere of 95% air and 5% (v/v) of CO2 in a temperature‐controlled incubator. Cell images were acquired by a DeltaVision fluorescence microscope system (GE Healthcare, Little Chalfont, UK) using a 40× oil immersion objective lens and deconvolved with softWoRx software.
To induce autophagy, WT, Atg5 −/−, and Atg7 −/− MEFs were incubated with 100 nM rapamycin for 2–4 h in the absence or presence of 10 mM NH4Cl for 4 h, 50 μM CQ for 4 h, or 20 μM PP242 for 4 h and starvation with amino acid deprivation for 8 h or starvation in EBSS medium for 2 or 4 h depending on the cell type and experiment. In addition, Atg5 −/− and Atg7 −/− MEFs were kindly provided by Dr. Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) (Kuma et al, 2004) and Dr. Lee (EWHA Women's University, Seoul, Korea), respectively.
Immunocytochemistry
To detect endogenous LC3/GABARAP, cells were rinsed in PBS and fixed in 4% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% Triton X‐100 for 5 min or with 0.25% saponin for 10 min and then blocked with 3% bovine serum albumin for 1 h at room temperature (20–22°C). A primary antibody was added to the blocking solution and incubated overnight at 4°C. The cells were washed three times with 1× PBS and then incubated with Cy3‐donkey‐anti‐rabbit secondary antibodies (Jackson Laboratory, Bar Harbor, ME, USA; 1:200, #711‐165‐152) for 1 h at room temperature. Next, the cells were washed three times with 1× PBS. The cells were mounted onto glass slides, and the preparations were analyzed using an LSM700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Pull‐down assay (co‐immunoprecipitation) and Western blotting
HEK293T cells expressing either HyD‐LIR(TP)‐GFP or HyD‐LIR(Fy)‐GFP together with each RFP‐LC3(A, B, C)/GABARAP/GABARAP(L1, L2) were prepared in immunoprecipitation lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% NP‐40, and protease and phosphatase inhibitors). The cell lysates were incubated with anti‐GFP antibodies (Sigma‐Aldrich, St. Louis, MO, USA, #F1804) or mouse IgG (EMD Millipore Cop. Temecula, CA, USA, #12‐371) overnight at 4°C. The following day, antibody‐protein complex cell lysates were incubated with protein G‐agarose beads for 7 h at 4°C before washing with immunoprecipitation lysis buffer twice at 4°C. For Western blot analysis, we used primary antibodies and horseradish peroxidase‐conjugated anti‐rat, anti‐mouse, anti‐goat, or anti‐rat secondary antibodies.
Quantitative analysis of the number of sensor‐ and MitoTracker‐positive dots, ratios of sensor‐positive LC3/GABARAPs autophagosome dots, and A/C ratio of fluorescence intensities of the sensor‐positive spots
For quantitative analysis of co‐localization spots including both sensor‐ and MitoTracker‐positive dots and sensor‐positive LC3/GABARAP autophagosome dots, at least 20 cells were randomly selected and excluded from selection if they were directly adjacent to a previously selected cell. The number of both sensor‐ and MitoTracker‐positive dots or sensor‐positive LC3/GABARAP autophagosomes recognized by the respective sensor in a valid cell was counted by overlaying the two channels (co‐localization of objects in confocal images) using ZEN imaging software (Carl Zeiss). Ratios of sensor‐positive LC3/GABARAP autophagosome dots were calculated as the fold of number of co‐localization events divided by total number of LC3/GABARAP‐positive spots. To determine the quantitative ratio of autophagosomal/cytosol (A/C) fluorescent intensities, an average value of autophagosomal or cytosol fluorescent intensities was measured for at least five randomly selected points on the autophagosome and cytosol in single cell using ZEN software. In the same manner, the quantitative A/C ratio of at least 20 randomly selected cells was analyzed. All statistical data were calculated and graphed using GraphPad Prism 5 (GraphPad Inc., La Jolla, CA, USA).
Autophagic flux assay and quantification of autophagic flux
The autophagic flux assay was performed as described previously (Khaminets et al, 2015). MEFs or HEK293T cells were incubated with EBSS (4 h) or starved with serum deprivation (8 h) in the presence and absence of bafilomycin A1 (BafA1, 100 nM) or CQ (50 μM). Gel intensity was analyzed using ImageJ software (NIH) to quantify autophagic flux from the Western blots. The levels of p62 and LC3‐II were normalized to actin or GAPDH.
Proteinase K protection assay
HEK293T cells were seeded into six‐well plates and left untreated or treated with 50 μM CQ in DMEM medium for 4 h. After treatment, the cells were suspended in homogenization buffer (10 mM HEPES, pH 7.4, 0.22 M mannitol, 0.07 M sucrose, and protease inhibitors) and homogenized with 10 strokes using a syringe with 27‐gauge needle. After centrifugation at 500 g at 4°C for 5 min, the post‐nuclear supernatant was collected and equally divided into three microcentrifuge tubes. One of the samples was left untreated, whereas the other two were incubated with 25 μg/ml proteinase K (PK) alone or both PK and 0.5% Triton X‐100 for 10 min on ice. PK activity was then inhibited by the addition of 1 mM PMSF and incubated for 10 min on ice. The samples were precipitated with 10% trichloroacetic acid, washed once with ice‐cold acetone, resuspended in SDS–PAGE sample buffer, immediately boiled, and analyzed by SDS–PAGE.
Immunogold electron microscopy
WT and Atg5 −/− MEFs were transfected using Lipofectamine 2000 and fixed for 1 h at 4°C in PBS containing 0.1% glutaraldehyde, 4% formaldehyde, and 3.5% sucrose. The cells were detached from the culture dish with a cell scraper and pelleted by centrifugation at 2,000 g for 2 min at 4°C. The cell pellets were resuspended in warm agar (1% in PBS) and re‐centrifuged. After three washes in PBS, the agar‐embedded cell pellets were embedded in an Epon 812 mixture after dehydration in a graded series of ethanol and propylene oxide solutions. For immunogold labeling experiments, ultrathin (80‐nm) sections that had been deposited on Formvar‐coated gold grids were allowed to float on drops of freshly prepared 3% sodium metaperiodate solution for 30 min. The immunogold labeling procedure followed the manufacturer's protocol (British Biocell International, Cardiff, UK) with some modifications. After etching and washing, we placed the grids on 50‐μl drops of solution A (PBS, pH 8.2, containing 4% normal goat serum, 1% bovine serum albumin, 0.1% Tween 20, and 0.1% sodium azide) and incubated the grids for 30 min. The grids were then incubated for 3 h at room temperature in a humidified chamber on 50 μl droplets of solution B (same as solution A but with 1% normal goat serum) containing an anti‐rabbit GFP monoclonal antibody (cat. #2956, Cell Signaling Technology, Danvers, MA, USA) and then rinsed in pure solution B. The grids were reacted with a 15‐nm gold‐conjugated goat anti‐rabbit IgG secondary antibody diluted in solution A. Controls for the specificity of the GFP immunogold labeling included (i) omission of the primary antibody and (ii) replacement of the primary antibody with preimmune serum. After washing with PBS and deionized water, the grids were counterstained with uranyl acetate (7 min) and lead citrate (2 min) and were viewed by cryo‐transmission electron microscopy (JEM‐1400 Plus, at 120 kV) and Bio‐high‐voltage electron microscopy (JEM‐1000BEF, at 1,000 kV) (JEOL, Tokyo, Japan).
Statistical analysis
Kolmogorov–Smirnov (KS) test was performed to check the Gaussian distribution of the group. Student's t‐test (two‐tailed unpaired t‐test) or Mann–Whitney U‐test (two‐tailed) was used for comparing two groups as a parametric or non‐parametric test, respectively. In case of comparing more than three groups, one‐way ANOVA in conjunction with Tukey's multiple comparison test or Kruskal–Wallis test followed by Dunn's multiple comparison test was carried out as a parametric or non‐parametric test, respectively.
Author contributions
All experiments were conducted by Y‐KL, Y‐WJ, and H‐EC. YHH performed immune‐EM analysis. D‐JJ, B‐KK, and J‐AL designed all experiments, analyzed the data, and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Source Data for Expanded View
Review Process File
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
This work was supported by the National Honor Scientist Program through a grant to B.‐K. K., by the National Research Foundation (grant #2014R1A1A4A01003859), Korea Health Technology R&D project grant (grant #HI14C1891) to J.‐A. L., and National Research Foundation (grant #2014‐R1A1A2012804) to D.‐J. J.
The EMBO Journal (2017) 36: 1100–1116
Contributor Information
Bong‐Kiun Kaang, Email: kaang@snu.ac.kr.
Deok‐Jin Jang, Email: jangdj@knu.ac.kr.
Jin‐A Lee, Email: leeja@hnu.kr.
References
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3‐phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgisdottir AB, Lamark T, Johansen T (2013) The LIR motif ‐ crucial for selective autophagy. J Cell Sci 126: 3237–3247 [DOI] [PubMed] [Google Scholar]
- Buckingham EM, Carpenter JE, Jackson W, Grose C (2014) Nuclear LC3‐positive puncta in stressed cells do not represent autophagosomes. Biotechniques 57: 241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson SR, Simonsen A (2015) Membrane dynamics in autophagosome biogenesis. J Cell Sci 128: 193–205 [DOI] [PubMed] [Google Scholar]
- Chittaranjan S, Bortnik S, Gorski SM (2015) Monitoring autophagic flux by using lysosomal inhibitors and western blotting of endogenous MAP1LC3B. Cold Spring Harb Protoc 2015: 743–750 [DOI] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24: 24–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos ND, Wells G, Campanella M (2017) The pharmacological regulation of cellular mitophagy. Nat Chem Biol 13: 136–146 [DOI] [PubMed] [Google Scholar]
- Horenkamp FA, Kauffman KJ, Kohler LJ, Sherwood RK, Krueger KP, Shteyn V, Roy CR, Melia TJ, Reinisch KM (2015) The Legionella anti‐autophagy effector RavZ targets the autophagosome via PI3P‐ and curvature‐sensing motifs. Dev Cell 34: 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 283: 22847–22857 [DOI] [PubMed] [Google Scholar]
- Itakura E, Kishi‐Itakura C, Mizushima N (2012) The hairpin‐type tail‐anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151: 1256–1269 [DOI] [PubMed] [Google Scholar]
- Jacomin AC, Samavedam S, Promponas V, Nezis IP (2016) iLIR database: a web resource for LIR motif‐containing proteins in eukaryotes. Autophagy 12: 1945–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DJ, Park SW, Lee JA, Lee C, Chae YS, Park H, Kim MJ, Choi SL, Lee N, Kim H, Kaang BK (2010) N termini of apPDE4 isoforms are responsible for targeting the isoforms to different cellular membranes. Learn Mem 17: 469–479 [DOI] [PubMed] [Google Scholar]
- Jang DJ, Ban B, Lee JA (2011) Characterization of novel calmodulin binding domains within IQ motifs of IQGAP1. Mol Cells 32: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Mizushima N (2014) Autophagy and human diseases. Cell Res 24: 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7: 279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani‐Okamoto S, Ohsumi Y, Yoshimori T (2004) LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form‐II formation. J Cell Sci 117: 2805–2812 [DOI] [PubMed] [Google Scholar]
- Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T, Mizushima N (2016) An autophagic flux probe that releases an internal control. Mol Cell 64: 835–849 [DOI] [PubMed] [Google Scholar]
- Kalvari I, Tsompanis S, Mulakkal NC, Osgood R, Johansen T, Nezis IP, Promponas VJ (2014) iLIR: A web resource for prediction of Atg8‐family interacting proteins. Autophagy 10: 913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, Mauthe M, Katona I, Qualmann B, Weis J, Reggiori F, Kurth I, Hubner CA, Dikic I (2015) Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522: 354–358 [DOI] [PubMed] [Google Scholar]
- Kim KH, Jun YW, Park Y, Lee JA, Suh BC, Lim CS, Lee YS, Kaang BK, Jang DJ (2014) Intracellular membrane association of the Aplysia cAMP phosphodiesterase long and short forms via different targeting mechanisms. J Biol Chem 289: 25797–25811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8: 931–937 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO (2007) Methods for monitoring autophagy from yeast to human. Autophagy 3: 181–206 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard‐Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4: 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo‐Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre‐Ghiso JA, Ahn HJ, Ait‐Mohamed O, Ait‐Si‐Ali S, Akematsu T, Akira S, Al‐Younes HM, Al‐Zeer MA, Albert ML, Albin RL, Alegre‐Abarrategui J et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Codogno P (2013) The mechanism and physiological function of macroautophagy. J Innate Immun 5: 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT (2015) Monitoring the localization of MAP1LC3B by indirect immunofluorescence. Cold Spring Harb Protoc 2015: 751–755 [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Tooze SA (2016) Digesting the expanding mechanisms of autophagy. Trends Cell Biol 26: 624–635 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre‐Ghiso J, Airoldi EM et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N (2004) The role of autophagy during the early neonatal starvation period. Nature 432: 1032–1036 [DOI] [PubMed] [Google Scholar]
- Kwon DH, Kim S, Jung YO, Roh KH, Kim L, Kim BW, Hong SB, Lee IY, Song JH, Lee WC, Choi EJ, Hwang KY, Song HK (2016) The 1:2 complex between RavZ and LC3 reveals a mechanism for deconjugation of LC3 on the phagophore membrane. Autophagy 13: 70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Gao FB (2009) Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT‐III in frontotemporal dementia. J Neurosci 29: 8506–8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Lee JA (2016) Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep 49: 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA (2008) Membrane recognition by phospholipid‐binding domains. Nat Rev Mol Cell Biol 9: 99–111 [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Sou YS, Kageyama S, Takahashi T, Ueno T, Tanaka K, Komatsu M, Ichimura Y (2014) LC3B is indispensable for selective autophagy of p62 but not basal autophagy. Biochem Biophys Res Commun 446: 309–315 [DOI] [PubMed] [Google Scholar]
- McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, Coxon FP, Miranda de Stegmann D, Bhogaraju S, Maddi K, Kirchof A, Gatti E, Helfrich MH, Wakatsuki S, Behrends C, Pierre P, Dikic I (2015) PLEKHM1 regulates autophagosome‐lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell 57: 39–54 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self‐digestion. Nature 451: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo DB, Colombo MI (2001) A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci 114: 3619–3629 [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M (2016) Atg8 family LC3/GABARAP proteins are crucial for autophagosome‐lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol 215: 857–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145 [DOI] [PubMed] [Google Scholar]
- Pankiv S, Johansen T (2010) FYCO1: linking autophagosomes to microtubule plus end‐directing molecular motors. Autophagy 6: 550–552 [DOI] [PubMed] [Google Scholar]
- Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, Johansen T (2010) FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end‐directed vesicle transport. J Cell Biol 188: 253–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Shin JH, Kim ES, Jo YK, Kim JH, Hwang JJ, Kim JC, Cho DH (2012) Mitochondrial fragmentation caused by phenanthroline promotes mitophagy. FEBS Lett 586: 4303–4310 [DOI] [PubMed] [Google Scholar]
- Pfeifer U (1973) Cellular autophagy and cell atrophy in the rat liver during long‐term starvation. A quantitative morphological study with regard to diurnal variations. Virchows Arch B Cell Pathol 12: 195–211 [DOI] [PubMed] [Google Scholar]
- Ryu HH, Jun MH, Min KJ, Jang DJ, Lee YS, Kim HK, Lee JA (2014) Autophagy regulates amyotrophic lateral sclerosis‐linked fused in sarcoma‐positive stress granules in neurons. Neurobiol Aging 35: 2822–2831 [DOI] [PubMed] [Google Scholar]
- Schneider JL, Cuervo AM (2014) Autophagy and human disease: emerging themes. Curr Opin Genet Dev 26: 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvets E, Fass E, Scherz‐Shouval R, Elazar Z (2008) The N‐terminus and Phe52 residue of LC3 recruit p62/SQSTM1 into autophagosomes. J Cell Sci 121: 2685–2695 [DOI] [PubMed] [Google Scholar]
- Stahelin RV (2009) Lipid binding domains: more than simple lipid effectors. J Lipid Res 50(Suppl): S299–S304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Putyrski M, Kutle I, Huber J, Wang C, Major V, Sidhu SS, Youle RJ, Rogov VV, Dotsch V, Ernst A, Dikic I (2017) Fluorescence‐based ATG8 sensors monitor localization and function of LC3/GABARAP proteins. EMBO J 36: 549–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36: 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, McEwan DG, Dikic I (2014) The LC3 interactome at a glance. J Cell Sci 127: 3–9 [DOI] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Baehrecke EH (2015) Eaten alive: novel insights into autophagy from multicellular model systems. Trends Cell Biol 25: 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang S (2010) The complexes of mammalian target of rapamycin. Curr Protein Pept Sci 11: 409–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Source Data for Expanded View
Review Process File
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6


