Synopsis
Heart failure is the quintessential cardiovascular syndrome of aging that results from common cardiovascular conditions in older adults in conjunction with age-associated changes in cardiovascular structure and function. To a large extent, heart failure is a geriatric syndrome in much the same way that dementia, falls, and frailty are geriatric syndromes. The incidence and prevalence of heart failure increase strikingly with age and make heart failure the most common reason for hospitalization among older adults. While outcomes for older adults with heart failure have improved over time, mortality, hospitalization, and rehospitalization rates remain high.
Keywords: heart failure, elderly, epidemiology, pathophysiology, prognosis, mortality, hospitalization, rehospitalization
Introduction
Heart failure (HF) is the quintessential cardiovascular syndrome of aging that results from age-related cardiovascular conditions and age-associated changes in cardiovascular structure and function. The incidence and prevalence of HF increase strikingly with age and make HF the most common reason for hospitalization in older adults.1 While outcomes of HF have improved over time, mortality, hospitalization, and rehospitalization rates remain high. Accordingly, total costs of care for persons with HF exceed $30 billion annually and are expected to rise to more than $70 billion by the year 2030 due to population aging and growth.2
This review describes the epidemiology, pathophysiology, and prognosis of HF in older adults. We present data on the incidence and prevalence of HF, including changes over time. Where data exist, we provide estimates for HF with preserved ejection fraction (HFpEF), the most common form of HF in older adults. We then describe the pathophysiology of HF in the elderly, including the contributions of age-associated physiologic changes in cardiovascular and non-cardiovascular systems. Finally, we describe the prognosis of HF in older adults with regard to mortality, hospitalization, and rehospitalization.
Epidemiology
Identification of Heart Failure
American College of Cardiology/American Heart Association guidelines define HF as a “complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill or eject blood.”3 As HF is a clinical syndrome and not a disease, many epidemiologic studies have relied on clinical diagnostic criteria for its identification.4,5 These criteria include the Framingham criteria,6 Boston criteria,7 Gothenburg criteria,8 and Cardiovascular Health Study criteria,9 all of which have relatively similar performance characteristics for the detection of HF with high sensitivity compared to cardiologist evaluation.10 These criteria may be less accurate for identifying acute decompensated HF11 and do not differentiate between heart failure with reduced ejection fraction (HFrEF) and HFpEF. Ejection fraction criteria for distinguishing HFpEF from HFrEF have been highly variable across studies.4
Incidence of Heart Failure
New diagnoses of HF are common and strongly related to age. Data from the Atherosclerosis Risk in Communities Study have shown that approximately 915,000 new cases of HF occur each year in the United States.12 Incidence rates increase with age for patients of both sexes. For example, data from the Framingham Heart Study have shown that annual rates of new HF events per 1000 person-years is 9.2 for white men 65 to 74 years of age, 22.3 for white men 75 to 84 years of age, and 43.0 for white men ≥85 years of age. Corresponding rates among white women are 4.7, 14.8, and 30.7 per 1000 person-years, respectively.13 Similar findings relating HF incidence rates with age have also been noted among more ethnically and racially diverse populations.14
HF incidence varies by race, ethnicity, and socioeconomic factors. Data from the Multiethnic Study of Atherosclerosis have shown that HF incidence is highest among African-Americans, followed by Hispanic Americans, white Americans, and Chinese Americans (incidence rates 4.6, 3.5, 2.4, and 1.0 per 1000 person-years, respectively).12,15 Similar relationships were found in the Atherosclerosis Risk in Communities Study population, where HF incidence rates were highest for black men, followed by black women, white men, and white women.16 In both studies, the higher incidence of HF among African-Americans was largely explained by the greater prevalence of cardiovascular risk factors in this population. In addition, a systematic review of data from multiple countries including the United States, Sweden, Denmark, and Scotland found that income, educational attainment, and community factors suggestive of economic deprivation were all strongly associated with new onset HF.17
The lifetime risk of developing HF is high. Data from the predominantly white Framingham Heart Study found that one in five men and women without HF at age 40 develop HF during their lifetimes.18 A subsequent report from a more diverse study population derived from the Chicago Heart Association Detection Project and the Cardiovascular Health Study found that at age 45, lifetime risks for HF are 30% to 42% in white men, 20% to 29% in black men, 32% to 39% in white women, and 24% to 46% in black women, respectively.19 The lower lifetime risks of HF in black men were largely due to higher competing risks for non-cardiovascular death from renal failure, homicide, and other causes. Data from the international context confirm that elevated lifetime risk for HF is not restricted to the United States.20
With time, the incidence of HF may be declining in both North America and Europe. An examination of medical record data from Olmstead County, Minnesota, found that the age- and sex-adjusted incidence of HF declined from 315.8 per 100,000 persons in 2000 to 219.3 per 100,000 persons in 2010.21 Similarly, an analysis of administrative data from a nationally representative sample of Medicare beneficiaries in the United States found that HF incidence declined from 32 per 1000 person-years in 1994 to 29 per 1000 person-years in 2003.22 Both absolute and relative declines were greatest for Medicare beneficiaries aged 80 to 84 years (HF incidence declined from 57.5 to 48.4 per 1000 person-years). Similar declines in HF incidence have also been identified in Canada23, Scotland,24, and Sweden.25
Prevalence of Heart Failure
The prevalence of HF is high and increasing over time. Recent data from the National Health and Nutrition Examination Survey (NHANES) demonstrated that approximately 5.7 million Americans have HF.12 This number is expected to rise to at least 8 million by the year 2030. Factors driving the increase in HF include aging of the population, increased prevalence of specific risk factors for HF including diabetes and obesity,26,27 improvements in the treatment of concomitant cardiovascular conditions, and better treatment for HF itself.2,5 Similar findings have also been noted in the international context, where population aging and population growth continue to drive the increased prevalence of cardiovascular disease.28
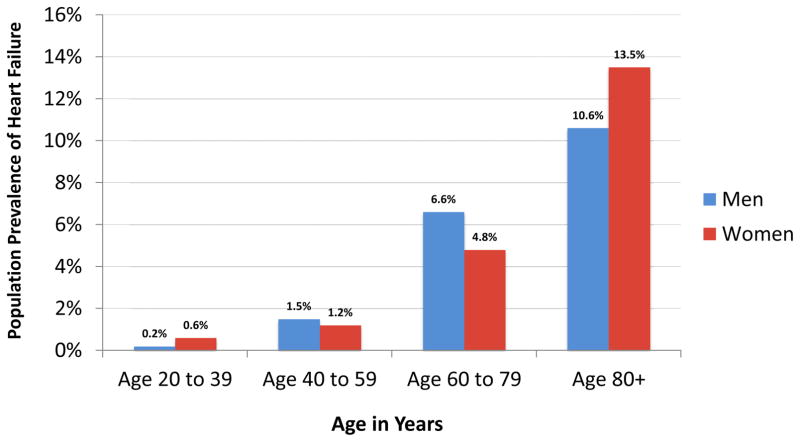
As with HF incidence rates, the prevalence of HF increases sharply with age. Data from NHANES have shown that the proportion of adults with HF is 1.5% for men aged 40 to 59 years, 6.6% for men aged 60 to 79 years, and 10.6% for men aged ≥80 years.12 Corresponding proportions among women are 1.2%, 4.8%, and 13.5%, respectively (Figure 1). These data demonstrate that HF prevalence among women surpasses that of men in the oldest-old.
Figure 1. Prevalence of Heart Failure in the United States by Age and Sex.
Adapted from Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133(4):e38–360; with permission.
Heart Failure with Preserved Ejection Fraction
With time, there has been increasing interest in identifying persons with HFpEF, the predominant form of HF in older adults.29 To date, however, there has been relatively little written about the incidence and prevalence of HFpEF. Diagnostic criteria for HFpEF, especially pertaining to left ventricular ejection fraction, have varied across studies.5,21,29–31 In addition, administrative data are often unable to differentiate persons with HFpEF from those with HFrEF.
A recent study, however, applied validated clinical criteria6 and specialty society definitions of HFpEF3 to electronic health record data from Olmstead County and found that the proportion of incident HF cases due to HFpEF increased from 47.8% in 2000 to 2003 to 56.9% in 2004 to 2007 and to 52.3% in 2008 to 2010.21 With time, there was an increase in prevalence of hypertension, diabetes mellitus, hyperlipidemia, and multiple chronic conditions at the time of HF diagnosis among persons with HFpEF. Notably, the overall incidence of both HFrEF and HFpEF decreased over time, but the decline was greater for HFrEF.
Pathophysiology
The frequent development of HF in older adults relates in large part to the high prevalence of traditional cardiovascular risk factors in this population. Data from the Cardiovascular Health Study, a prospective population-based study of 5,888 older adults, demonstrated that the population-attributable risk for the development of HF was 13.1% for coronary heart disease and 12.8% for a systolic blood pressure greater than 140 mm Hg.32 These findings were confirmed in the Atherosclerosis Risk in Communities Study26 and the National Health and Nutrition Examination Survey,27 both of which also found that diabetes and obesity are responsible for a significant proportion of HF incidence. Even a modest reduction in these risk factors could result in large reductions in the number of persons with HF.33
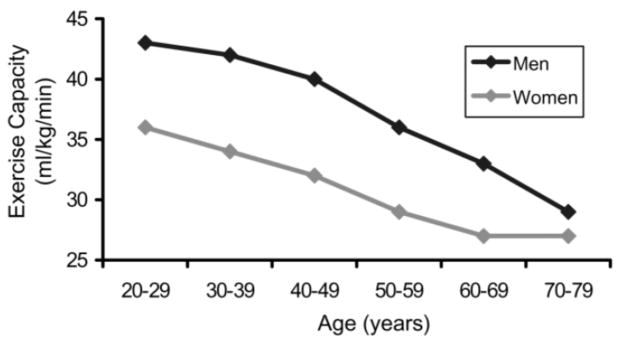
The higher prevalence of HF in the elderly also relates to common age-associated changes in cardiovascular structure and function. These changes diminish chronotropic and inotropic responses, raise intra-cardiac pressures with ventricular filling, and increase afterload. As a result, the ability of the heart to respond to stress is impaired, whether that stress is physiologic (e.g. exercise) or pathologic (e.g. myocardial ischemia or sepsis). This decline in cardiovascular reserve is reflected in age-related reductions in peak oxygen consumption (Figure 2).34 Box 1 summarizes age-associated cardiovascular changes, three of which are particularly important.
Figure 2. Association of Maximum Exercise Capacity with Age.
Maximum exercise capacity usually begins to decline between the ages of 20 and 30 years and falls approximately 10% per decade. Exercise capacity is described in terms of ml O2/kg/min.
From Oxenham H, Sharpe N. Cardiovascular aging and heart failure. Eur J Heart Fail 2003;5(4):427–34; with permission.
Box 1. Cardiovascular Changes with Aging.
Cardiac changes
Diminished responsiveness to beta-adrenergic stimulation
Myocyte hypertrophy
Increased interstitial deposition of collagen, amyloid, and lipofuscin
Impaired calcium release from contractile proteins and reuptake into the sarcoplasmic reticulum during diastole
Insufficient mitochondrial production of adenosine triphosphate in response to stress
Vascular changes
Diminished responsiveness to beta-adrenergic stimulation
Increased collagen deposition and cross-linking in arterial media
Fragmentation of arterial elastin
Diminished endothelium-mediated vasodilatation
First, age is associated with reduced responsiveness to beta-adrenergic stimulation. Deficits in intracellular signaling may be related to impaired G-protein coupling of receptors to adenyl cyclase, as well as to reductions in the amount and/or activation of adenyl cyclase.35,36 These changes impair the ability of the older heart to augment cAMP in response to beta-receptor stimulation.35 As a result, maximum heart rate (HR) declines almost linearly with age, often denoted by the formula: maximum HR = 220-age; peak contractility also declines with age.
Second, aging alters left ventricular diastolic filling. During diastolic isovolumic relaxation and the early rapid filling period, efficiency of filling is highly dependent on active myocardial relaxation, an energy dependent process. Aging is associated with impaired calcium release from contractile proteins and reuptake into the sarcoplasmic reticulum,37 thereby prolonging the heart’s contractile period. In contrast, efficient diastolic filling during mid and late diastole is highly dependent on the passive compliance properties of the heart. Aging is associated with myocyte hypertrophy38 and increased interstitial deposition of collagen, amyloid, and lipofuscin, all of which increase myocardial stiffness and reduce compliance.39,40 As a result, cardiac filling may be impaired when most needed, as in the presence of rapid atrial fibrillation or myocardial ischemia.
Third, aging leads to increased vascular stiffness. Arterial wall media in large- and medium-sized arteries undergo structural changes due to increased collagen content, non-enzymatic collagen cross linking to form advanced glycation end products, and breakage of elastin fibers.41,42 In addition, endothelium-dependent vasodilation is compromised due to diminished secretion of endothelial nitric oxide and increased signaling of angiotensin II, a potent vasopressor and mitogen.41,43 These changes heighten afterload through increased impedance to left ventricular ejection and increase the prevalence of isolated systolic hypertension in older adults.
Cardiovascular disease and aging inevitably occur in the context of diseases and aging of other organ systems. Many of these non-cardiovascular conditions and age-related changes directly contribute to the development of heart failure or its worsening. For example, chronic kidney disease and age-associated declines in glomerular filtration rate and renal sodium and potassium handling can increase the likelihood of acute decompensated heart failure and adverse effects from drug treatment including dehydration and electrolyte abnormalities.44–46 Similarly, concomitant chronic lung disease, sleep-disordered breathing, and age-associated changes in lung function can contribute to pulmonary hypertension, diminished biventricular filling, and increased sensation of dyspnea.47–49 More recently, it has been recognized that a large number of commonly used medications may directly contribute to the development of both chronic and acute heart failure.50 The likelihood of drug-disease interactions increases with age due to the frequent presence of concomitant medical conditions and altered pharmacokinetics and pharmacodynamics associated with aging.51–54
Prognosis
Mortality
A new diagnosis of HF is associated with a high mortality rate that exceeds that associated with many cancers.55 A recent study of persons with new onset HF from 2000 to 2010 in Olmstead County found mortality rates of 20.2% and 52.6% at one and five years after diagnosis, respectively.21 One- and five-year mortality rates increased significantly with age and were 7.4% and 24.4% for 60 year-olds and 19.5% and 54.4% for 80 year-olds, respectively. Rates of mortality were similar for persons with HFpEF and HFrEF in fully adjusted models. These data are consistent with previous research from the Framingham Heart Study,56 a commercially managed population,14 Medicare beneficiaries22, and earlier cohorts from Olmstead County57 that also demonstrated high rates of mortality after HF diagnosis. These studies also demonstrated modest survival gains over time that largely relate to the increased use of evidence-based treatments for HFrEF.12,58 These survival improvements may have lessoned over time, however.21
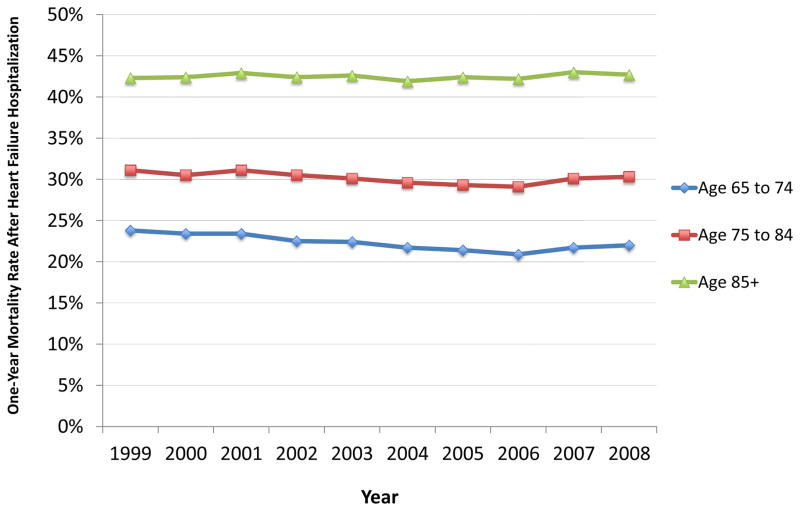
Prognosis is worse for persons hospitalized with HF. Among US Medicare beneficiaries hospitalized with HF in 2006, mortality within 30 days and 1 year of admission was 10.8%59 and 30.7%,60 respectively. Mortality outcomes at one year demonstrate a clear relationship with age (Figure 3). For example, rates of one-year mortality in 2008 were 22.0%, 30.3%, and 42.7% for persons aged 65 to 74 years, 75 to 84 years, and 85 years and older, respectively. This figure also demonstrates that mortality outcomes after hospitalization have not improved significantly in recent years.60 While data describing five-year mortality rates after hospitalization are not available for Medicare beneficiaries, research from Olmstead County found mortality rates in excess of 65% within five years of hospitalization.61 Rates of mortality after hospitalization are slightly higher for patients with HFrEF compared with HFpEF 61,62 and much higher for patients discharged to a skilled nursing facility, in whom 30-day and one-year mortality rates may exceed 14% and 50%, respectively.63
Figure 3. Mortality within One Year of Hospitalization for Heart Failure by Age in the United States, 1999 to 2008.
Data reflects the national population of Medicare fee-for-service beneficiaries in the United States. Mortality rates were calculated for one year from the date of admission.
Data from Chen J, Normand SL, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011;306(15):1669–78.
Hospitalization and Rehospitalization
Hospitalizations are common in patients with HF. Among those with incident HF in Olmstead County, 83% were hospitalized at least once over a mean follow-up of 4.7 years.64 In addition, 66.9%, 53.6%, and 42.6% of patients were hospitalized at least 2, 3, and 4 times, respectively. Interestingly, only 16.5% of hospitalizations were for HF. The majority of hospitalizations (61.9%) were for non-cardiovascular conditions, suggesting that multimorbidity is a key driver of risk in patients with HF, rather than HF itself.52
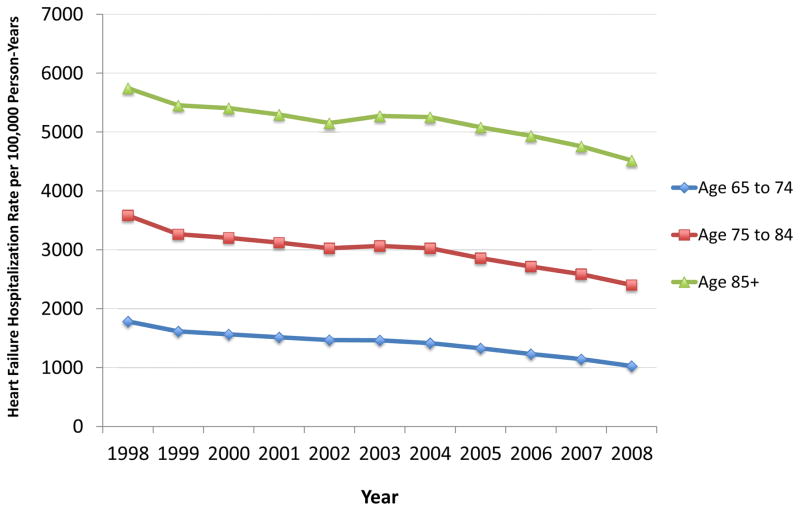
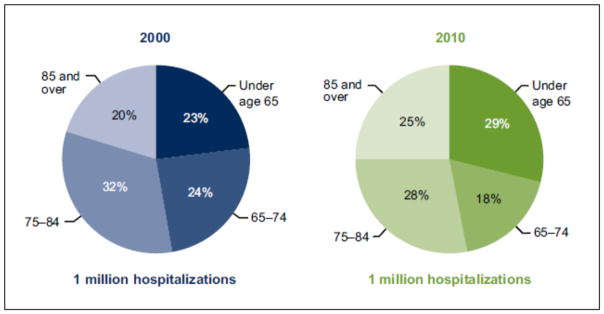
The risk of hospitalization for acute decompensated HF, in particular, has declined over time. Data from Medicare beneficiaries have shown that hospitalization rates for HF have decreased for older adults across age categories (Figure 4). These findings have been confirmed in other data sets, including the National Inpatient Sample,65,66 a nationally representative database of inpatient hospital stays in the United States. Despite these declines, hospitalization for HF continues to predominantly affect older adults (Figure 5). In 2010, more than 70% of hospitalizations for HF were among adults aged 65 years and older.
Figure 4. Heart Failure Hospitalization Rates for Older Adults in the United States, 1998 to 2008.
Data reflects the national population of Medicare fee-for-service beneficiaries in the United States. Hospitalization rates were calculated as the observed heart failure hospitalization rate per 100,000 person-years at risk among persons aged 65 to 74 years, 75 to 84 years, and 85+ years.
Data from Chen J, Normand SL, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011;306(15):1669–78.
Figure 5. Distribution of Hospitalizations for Heart Failure by Age in the United States, 2000 and 2010.
Data from National Center for Health Statistics, Data Brief No. 108, October, 2012.
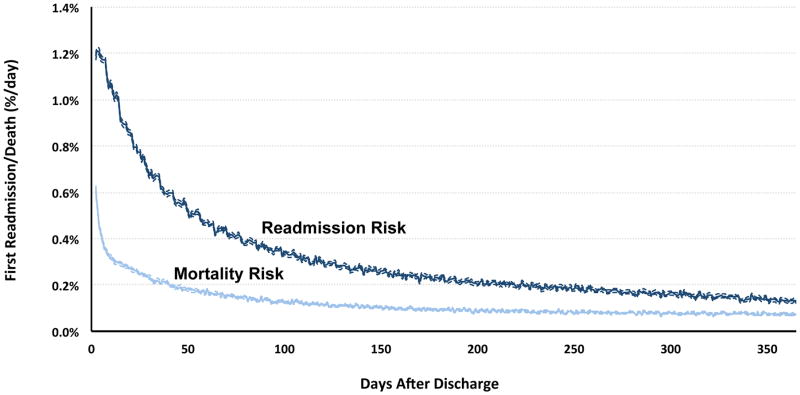
Rehospitalizations are a considerable source of morbidity. Almost 25% of older adults with HF are rehospitalized within one month of discharge; almost 70% are rehospitalized within one year.67,68 As with admissions to the hospital, readmissions after hospitalization for HF are usually not for HF and are often for non-cardiovascular conditions.67 In addition, patients remain vulnerable to major adverse events for a prolonged period of time after hospital discharge.68,69 For example, it takes nearly seven weeks for the daily risk of readmission to decline by 50% (Figure 6). This period of extended vulnerability after hospitalization is not associated with increasing age, 70,71 suggesting that readmission may more strongly relate to age-independent variables such as the quality of transitional care and complex social factors.
Figure 6. Daily Risk of Readmission and Death Among Older Adults in the Year After Hospitalization for Heart Failure.
Risk was calculated using hazard rates for the national population of older Medicare fee-for-service beneficiaries discharged after hospitalization for heart failure between 2008 and 2010. The risk of hospital readmission was calculated after incorporating the competing risk of death after hospital discharge.
From Dharmarajan K. Comprehensive strategies to reduce readmissions in older patients with cardiovascular disease. Can J Cardiol 2016;32(11):1306–14; with permission.
Conclusions
Heart failure is a common condition in older adults that results from the complex interplay of age-related diseases and age-associated physiologic changes. The societal burden of heart failure will continue to rise due to population aging, population growth, and improved treatment of heart failure and other cardiovascular disorders. As a result, we will be increasingly challenged to develop treatment plans and care systems that reduce the high levels of morbidity and mortality experienced by these patients, both from their heart failure and concomitant cardiovascular and non-cardiovascular conditions.
Key points.
Heart failure is a common condition in older adults that results from the complex interplay of age-related diseases and age-associated physiologic changes.
Despite recent declines in the age-adjusted incidence of heart failure, the prevalence of heart failure continues to rise due to population aging and improved treatment of both heart failure and concomitant cardiovascular conditions.
Outcomes for older adults with heart failure have improved over time; however, mortality, hospitalization, and rehospitalization rates remain high.
Acknowledgments
Funding/support: Dr. Dharmarajan is supported by grant K23AG048331 from the National Institute on Aging and the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. He is also supported by grant P30AG021342 via the Yale Claude D. Pepper Older Americans Independence Center.
Footnotes
This is an updated version of an article that appeared in Heart Failure Clinics, Volume 3, Issue 4.
Disclosures: Dr. Dharmarajan works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures and is a consultant and scientific advisory board member for Clover Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Healthcare Research and Quality. [on November 26, 2016];HCUP Statistical Brief 66: Medicare hospital stays: comparisons between the fee-for-service plan and alternative plans. 2006 Accessed at https://www.hcup-us.ahrq.gov/reports/statbriefs/sb66.jsp. [PubMed]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11(4):404–415. doi: 10.1007/s11897-014-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 7.Carlson KJ, Lee DC, Goroll AH, Leahy M, Johnson RA. An analysis of physicians’ reasons for prescribing long-term digitalis therapy in outpatients. J Chronic Dis. 1985;38(9):733–739. doi: 10.1016/0021-9681(85)90115-8. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 9.Schellenbaum GD, Rea TD, Heckbert SR, et al. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160(7):628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 10.Mosterd A, Deckers JW, Hoes AW, et al. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur J Epidemiol. 1997;13(5):491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 13.Incidence and prevalence: 2006 chart book on cardiovascular and lung diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 14.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113(6):799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 15.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101(7):1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail. 2012;14(2):138–146. doi: 10.1093/eurjhf/hfr168. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 19.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61(14):1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168(4):418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 23.Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. Cmaj. 2012;184(14):E765–773. doi: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119(4):515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 25.Zarrinkoub R, Wettermark B, Wandell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995–1002. doi: 10.1093/eurjhf/hft064. [DOI] [PubMed] [Google Scholar]
- 26.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2(1):11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 28.Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 31.West R, Liang L, Fonarow GC, et al. Characterization of heart failure patients with preserved ejection fraction: a comparison between ADHERE-US registry and ADHERE-International registry. Eur J Heart Fail. 2011;13(9):945–952. doi: 10.1093/eurjhf/hfr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 33.Avery CL, Loehr LR, Baggett C, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60(17):1640–1646. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxenham H, Sharpe N. Cardiovascular aging and heart failure. Eur J Heart Fail. 2003;5(4):427–434. doi: 10.1016/s1388-9842(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 35.Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17(4–5):545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakatta EG. Diminished beta-adrenergic modulation of cardiovascular function in advanced age. Cardiol Clin. 1986;4(2):185–200. [PubMed] [Google Scholar]
- 37.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115(1):97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 39.Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001;122(15):1739–1756. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 40.Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23(8):723–729. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- 41.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 42.Semba RD, Sun K, Schwartz AV, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens. 2015;33(4):797–803. doi: 10.1097/HJH.0000000000000460. discussion 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83(3):279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 44.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Ronco C, Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10(2):251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens. 2004;13(2):163–170. doi: 10.1097/00041552-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grau M, Barr RG, Lima JA, et al. Percent emphysema and right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-Lung and Multi-Ethnic Study of Atherosclerosis-Right Ventricle Studies. Chest. 2013;144(1):136–144. doi: 10.1378/chest.12-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis. 2009;51(5):363–370. doi: 10.1016/j.pcad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Page RL, 2nd, O’Bryant CL, Cheng D, et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134(6):e32–69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 51.Dharmarajan K, Strait KM, Lagu T, et al. Acute decompensated heart failure is routinely treated as a cardiopulmonary syndrome. PLoS One. 2013;8(10):e78222. doi: 10.1371/journal.pone.0078222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dharmarajan K, Dunlay SM. Multimorbidity in Older Adults with Heart Failure. Clin Geriatr Med. 2016;32(2):277–289. doi: 10.1016/j.cger.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dharmarajan K, Strait KM, Tinetti ME, et al. Treatment for Multiple Acute Cardiopulmonary Conditions in Older Adults Hospitalized with Pneumonia, Chronic Obstructive Pulmonary Disease, or Heart Failure. J Am Geriatr Soc. 2016;64(8):1574–1582. doi: 10.1111/jgs.14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 56.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 57.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. Jama. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 58.Merlo M, Pivetta A, Pinamonti B, et al. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail. 2014;16(3):317–324. doi: 10.1002/ejhf.16. [DOI] [PubMed] [Google Scholar]
- 59.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. Jama. 2010;303(21):2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. Jama. 2011;306(15):1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 62.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 63.Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4(3):293–300. doi: 10.1161/CIRCHEARTFAILURE.110.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54(18):1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61(10):1078–1088. doi: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agarwal SK, Wruck L, Quibrera M, et al. Temporal Trends in Hospitalization for Acute Decompensated Heart Failure in the United States, 1998–2011. Am J Epidemiol. 2016;183(5):462–470. doi: 10.1093/aje/kwv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. Jama. 2013;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dharmarajan K, Hsieh AF, Kulkarni VT, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. Bmj. 2015;350:h411. doi: 10.1136/bmj.h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dharmarajan K. Comprehensive Strategies to Reduce Readmissions in Older Patients With Cardiovascular Disease. Can J Cardiol. 2016;32(11):1306–1314. doi: 10.1016/j.cjca.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 70.Dharmarajan K, Hsieh A, Dreyer RP, Welsh J, Qin L, Krumholz HM. Relationship Between Age and Trajectories of Rehospitalization Risk in Older Adults. J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranasinghe I, Wang Y, Dharmarajan K, Hsieh AF, Bernheim SM, Krumholz HM. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: a retrospective observational cohort study. PLoS Med. 2014;11(9):e1001737. doi: 10.1371/journal.pmed.1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]