Abstract
Stress plays an important role in major depressive disorder (MDD) and is one of the state dependent factors in suicidal behavior. A dysfunctional hypothalamic-pituitary-adrenal axis is a common feature in this disorder. The involvement of environmental factors has added additional complexity to understanding depression or suicidal behavior. In this regard, epigenetic regulation has been considered a mechanistic interface between environmental stress stimuli and altered functioning of underlying gene network that may increase susceptibility to depression or suicidal behavior. The present study examined whether epigenetic modifications of stress related genes are associated with MDD and whether there are differences in these epigenetic marks between depressed individuals with and without serious suicidal ideation. Using MeDIP analysis in genomic DNA isolated from peripheral blood mononuclear cells (PBMC) of healthy controls (n=20), MDD patients with (n=14) or without serious suicidal ideation (n=10), we studied methylation of the stress-associated genes Brain Derived Neurotrophic Factor (BDNF), Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1), FK506 Binding Protein 5 (FKBP5), Corticotropin Releasing Hormone Binding Protein (CRHBP), and Corticotropin Releasing Hormone Receptor 1 (CRHR1) . In addition, we determined their transcript levels in RNAs isolated from the same PBMC. We found that BDNF, FKBP5, CRHBP, and NR3C1 gene promoters were significantly hypermethylated in MDD patients with and without suicidal ideation. We also found concomitant reductions in expression of BDNF, FKBP5 transcript variants (1, 2 and 3), and NR3C1 genes in these patients, suggesting that promoter hypermethylation in these genes may functionally be associated with their observed downregulation in MDD patients. In a secondary analysis, methylation of these genes was compared between MDD patients with or without serious suicidal ideation and controls. The MDD with serious suicidal ideation were significantly different from controls while the MDD without were not, although MDD with or without suicidal ideation were not different from each other, likely owning to a relatively small sample size. Thus, our findings underline the importance of epigenetic modifications of stress-associated genes in depression and, possibly, suicidal behavior, which, in future, needs to be confirmed in a larger patient population.
Keywords: DNA methylation, depression, suicidal behavior, PBMC, stress, gene expression
INTRODUCTION
Suicide is a major public health concern. Approximately one million people commit suicide worldwide each year and 40,000 people commit suicide in the United States alone (CDC (Centers for Disease Control and Prevention), 2014; WHO (World Health Organization), 2012). In the U.S., the lifetime rate of suicidal ideation is estimated at 13.5%, suicidal plan at 3.9%, and suicide attempt at 4.6% (Kessler et al., 1999). Among adolescents, suicide is the 3rd leading cause of death after motor vehicle accidents and homicide (CDC (Centers for Disease Control and Prevention), 2013). Previously, most of the studies of suicidal behavior were focused on the role of psychosocial and sociocultural factors; however, these factors are of too little predictive value to be of clinically useful. Therefore, research on the biological perspective of suicide has gained momentum and is a promising approach for identifying biological risk factors associated with suicidal behavior (Dwivedi and Pandey, 2011).
The presence of psychopathology is a strong predictor of suicide; however, only a minority of people with such diagnoses commit suicide (Mann, 1998, 2002; Turecki, 2005). This is evident from studies suggesting that though MDD is a major risk factor in suicide, but only 9% of severely ill MDD patients commit suicide (Bostwick and Pankratz, 2000). Further, not all people who commit suicide are depressed. Thus, the existence of suicidal syndromes that are independent of psychiatric illnesses has been proposed (Mann, 2010; van Heeringen and Mann, 2014). According to this hypothesis, suicidal behavior is a function of the interplay between state-dependent factors, such as illness and life events, and trait-dependent factors, which include biological markers (Mann and Haghighi, 2010). One of the state-dependent factors in suicidal behavior is stress (Mann and Haghighi, 2010). Several clinical evidence show a strong association between hyperactive hypothalamic-pituitary-adrenal axis and suicidal behavior (Dumser et al., 1998; Lopez et al., 1992; Nemeroff et al., 1988; Szigethy et al., 1994). More recently, a number of environmental factors have been found to act as contributory factory in suicide vulnerability, including maternal stress, childhood maltreatment, traumatic events, and exposure to stress (Lopizzo et al., 2015). It has been argued that adverse life events such as early life stress or traumatic exposure can cause gene x environment interaction with a result in improper functioning of neuronal circuitry, which can lead to an early onset of MDD and can increase susceptibility to suicidal behavior (Brezo et al., 2008; Martin et al., 2004; Menke and Binder, 2014; Plunkett et al., 2001).
Because of the complex genetic composition and overlapping molecular cross-talk, the current understanding of gene x environment interaction has opened new approaches to better understand the mechanisms that affect multiple genes involved in an abnormal behavior (Lesch, 2004; Tsuang et al., 2004). In this regard, modulation in neuronal functions mediated by epigenomic changes, which cause alteration in chromatin architecture without affecting the nucleotide sequence arrangements in DNA, have been identified as one of the major risk factors in several psychiatric illnesses including depression and suicidal behavior (Haghighi et al., 2014; Higuchi et al., 2011; Keller et al., 2010; Maussion et al., 2014; Numata et al., 2015; Zhang et al., 2014; Zhang et al., 2015). Interestingly, in suicidal patients, a few studies show that these epigenetic modifications can be independent of psychiatric illnesses such as depression (Kang et al., 2013; Kim et al., 2014). The present study was undertaken to further examine whether epigenetic modifications of stress related genes play a role in suicidal behavior and whether these modifications are common to or independent of depression. More specifically, we examined whether: 1) genes associated with stress show altered DNA methylation in their promoter regions in MDD patients, 2) changes in DNA methylation are associated with suicidal behavior independent of MDD diagnosis, and 3) changes in DNA methylation are associated with their functional response at gene transcript level. For this, we determined DNA methylation of stress-related genes such as Brain Derived Neurotrophic Factor (BDNF), Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1), FK506 Binding Protein 5 (FKBP5), Corticotropin Releasing Hormone Binding Protein (CRHBP), and Corticotropin Releasing Hormone Receptor 1 (CRHR1) in PBMC of healthy controls and MDD patients with and without serious suicidal ideation. We also determined the expression of these genes to examine the functional response of altered DNA methylation.
MATERIALS AND METHODS
Subjects
The study was reviewed and approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB) and written informed consent was obtained from all subjects prior to the study. The study was performed in 20 healthy controls and 24 MDD patients. Of 24 MDD patients, 14 had recent clinically significant suicidal ideation and 10 did not. Patients were recruited from UAB Department of Psychiatry clinics and the UAB Medical Center Emergency Department. All participants were evaluated using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and the Structured Clinical Interview for DSM-IV TR (First, 2002). Depression severity was determined using the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and all participants had a MADRS total score >14. Depressed patients were divided into two groups: one with MDD without recent serious suicidal ideation (MDD-non-suicide) and a second with recent serious suicidal ideation (MDD-suicide) as determined by MADRS item 10 score of less than or greater than or equal to 4. All MDD participants were psychotropic drug free at least two weeks prior to blood sampling.
Patients were excluded with any of the following characteristics: history of bipolar disorder or any psychotic disorder; the use of lithium or an antipsychotic within the prior two weeks; substance use disorder within three months; uncontrolled clinically significant medical illnesses including the initiation of any new medications within 30 days; pregnancy or lactation. Healthy controls had no lifetime history of any mental disorder as determined by MINI or SCID and were psychotropic drug free.
Processing of blood samples
Isolation of PBMC from venous blood was carried out using Ficoll based density gradient centrifugation method. Briefly equal volume of freshly drawn blood and RPMI1640 media (Sigma-Aldrich, St. Louis, MO, USA) were mixed and layered on top of Ficoll and processed for centrifugation (833 x g for 20 minutes) at room temperature. Following the centrifugation, the PBMC layer lying underneath the plasma layer was carefully drawn and centrifuged at 425x g for 10 minutes following the dilution of separated PBMC layer with fresh RPMI1640 media. PBMC pellet was washed with RPMI1640 with a brief spinning (300 x g for 5 minutes) at room temperature. The washed PBMC pellet was processed for DNA and RNA isolation.
Isolation of PBMC DNA
Isolation of genomic DNA from PBMC pellet was carried out following the silica column based nucleic acid purification method using Qiagen DNeasy Blood and Tissue Kit (Qiagen Inc, Valencia, CA, USA). Only DNA samples showing 260/280 ratio >1.8 were used.
Isolation of PBMC RNA
Total RNA from PBMC sample was isolated using TRIzol® method (Invitrogen, Grandsland, NY, USA). Only RNA samples showing absorbance ratio of 260/280 >1.8 and intact integrity determined by agarose gel electrophoresis were used. RNA samples were reverse transcribed to prepare mRNA specific 1st strand cDNA based on oligo dT priming.
Promoter MeDIP assay
Genomic DNA isolated from PBMC was used to immunoprecipitate the 5-methyl cytosine enriched fragments regardless of their genomic location. Briefly, 2 μg of fragmented and denatured genomic DNA were pulled down using anti 5mC monoclonal (clone 10G4) antibody (Zymo Research, Irvine, CA). Immunoprecipitated DNA samples were eluted and reverse cross-linked for uncoupling the antibody-DNA complex. The dissociated complex was used for phenol: chloroform: isoamylalcohol (25:24:1 v/v) based DNA purification.
Relative methylation enrichment of specific genomic loci was quantified following qPCR based amplification. Primers for amplification were designed based on the prior identification of CpG islands for corresponding gene promoter (Supplementary Table 1). Relative fold changes of immunocaptured loci in patients over healthy controls were determined after input normalization. The data are represented as percent of control.
qPCR-based gene expression quantification
Relative transcript abundance of BDNF, NR3C1, FKBP5, CRHBP and CRHR1 were determined using real time PCR as detailed earlier (Dwivedi et al., 2015). The primer sequences are provided in Supplementary Table 1. Since FKBP5 has four different transcript variants (TV1–4), we examined the expression of all of these variants by using two primer sets: FKBP5 I recognizing transcript variants 1–3 and FKBP5 II recognizing transcript variant 4. For BDNF, transcript quantification a pair of primers was designed to include all the transcript variants reported in NCBI RefSeq database (Supplementary Table 1). Relative gene expression levels were quantified following ΔΔCT method after normalization with two different normalizers: GAPDH and β-Actin (ACTB). Comparative gene expression data showed that CT values for BDNF, FKBP5, and NR3C1 were in the range of 24–25, whereas for CRHBP, the CT values were in the range of 31–32, indicating that expression of CRHBP was slightly lower than the other genes.
Statistical analyses
All data were analyzed using Statistical Package for the Social Sciences, version 23 (IBM, Chicago, IL, USA). The data are reported as the mean ± SEM. Our primary hypothesis was that BDNF, FKBP5, CRHBP, CRHR1, and NR3C1 would show promoter hypermethylation in MDD subjects compared with healthy controls. Secondary hypotheses were that the methylation would be significantly different between MDD with serious suicidal ideation but not MDD without suicidal ideation and healthy controls. To test the primary hypothesis, MDD (MDD-suicide + MDD non-suicide) group was compared with the healthy control group using independent sample t-tests. To test the secondary hypotheses, a series of independent sample t-tests were done comparing healthy controls, MDD with suicidal ideation, and MDD without suicidal ideation. The correlation between promoter methylation and gene expression as well as age and race were determined by Pearson product-moment correlation analysis. The effect of gender was analyzed by comparing males versus females using independent samples t-tests. An α level ≤0.05 was considered statistically significant. Effect sizes of the between-groups comparisons were calculated using Cohen’s d based on mean and SD of group (Cohen, 1992). Thresholds for effect sizes were 0.3 = small, 0.5 = medium, and 0.8 = large (Cohen, 1988).
RESULTS
Demographic and clinical characteristics of MDD and healthy control subjects
The demographic and clinical characteristics of subjects are provided in Table 1. There was no significant difference in age between MDD patients and healthy controls (F(2,41) = 0.06, p = 0.94). The MDD-suicide group had a significantly higher MADRS score than the MDD-non-suicide group (t = 4.16, p<0.001). This remained significant even after removing the suicide score from the MADRS total (t=3.24, p<0.005). The mean ± SEM of the MADRS in the MDD group was 36.3 ± 8.5, whereas in the MDD non-suicidal group, it was 21.0 ± 6.9 (Table 1). All MDD suicidal patients had a MADRS item 10 score >4. There were 6 males and 14 females in the control group and 9 males and 15 females in the MDD group (chi-square=0.029, p=.86). When MDD subjects were subdivided, there were 6 males and 8 females in MDD-suicide group and 3 males and 7 females in the MDD-non-suicide group. There were 7 Caucasian and 13 African-American subjects in the control group and 12 Caucasian and 12 African-American in the MDD group (chi-square=0.74, p=.39). Within the MDD-suicide group, there were 6 Caucasian and 8 African-American subjects, whereas in the MDD-non-suicide group, there were 6 Caucasian and 4 African-American subjects.
Table 1.
Demographic and Clinical Characteristics of Subjects
| Healthy Controls (N=20) | MDD (N = 24) | ||
|---|---|---|---|
| MDD-non-suicide (N = 10) | MDD-suicide (N = 14) | ||
| Age (Years)* | 44.06 ± 2.12 | 43.15 ± 2.59 | 42.90 ± 2.99 |
| Gender | |||
| Males | 6 | 3 | 6 |
| Females | 14 | 7 | 8 |
| Race | |||
| Caucasian | 7 | 6 | 6 |
| African-American | 13 | 4 | 8 |
| MADRS | N/A | 21.0 ± 6.9 | 36.3 ± 8.5** |
Data are the mean ± SEM.
F(2,41) = 0.06, p = 0.94
t = 4.16, p<0.001
In silico mapping and analysis of CpG islands on regulatory regions of FKBP5, CRHR1, CRHBP, NR3C1 and BDNF genes
Supplementary Figure S1A demonstrates the identification of CpG island near transcription start site of transcript variants 1, 3, and 4 of FKBP5 gene on chromosome 6 (UCSC genome browser (Human Genome Assembly (Dec., 2013), which spanned around 1245 bps (Supplementary Figure S2A). This CpG bearing locus was mapped to be located in the middle of intron 2 of FKBP5 transcript variant 2 (Supplementary Figure S1A). MethPrimer based in silico CpG site prediction algorithm (Li and Dahiya, 2002), indicated more than 60% GC content for the same predicted CpG island on intron 2 of FKBP5 (Supplementary Figure S3A). For CRHR1 gene, a CpG island harboring genomic locus around the transcription start site on chromosome 17 (Supplementary Figure S1B) was mapped and found to be 2244 bp long (Supplementary Figure S2B). This high GC rich locus was identified with five CpG islands with overall 50% GC content (Supplementary Figure S3B). Mapping of GC rich genomic sequence around the transcription start site of CRHBP gene on chromosome 5 (Supplementary Figure S1C) identified a 1093 bp long locus (GC content > 60%) (Supplementary Figure S2C) distributed into three CpG islands (Supplementary Figure S3C). NR3C1 gene was found to harbor a genomic locus at the promoter area close to transcription start site of transcript variants 1, 4, 6, 7, and 8 on chromosome 5 (Supplementary Figure S1D). This was found to be the longest stretch of genomic sequence (3000bp) (Supplementary Figure S2D) with more than 65% GC content and 5 CpG islands proximal to the promoter area including the 1st introns of transcript variants 2 and 3 (Supplementary Figure S3D). Similarly, the BDNF gene promoter was also found to be populated within a GC rich area on chromosome 11 close to the transcription start site of transcript variants 7, 9, 8, 3, and 2 (Supplementary Figure S1E). The GC rich area is expanded for 1092 bases (Supplementary Figure S2E) and analyzed to have four individual CpG islands with an average of 55–65% GC content (Supplementary Figure S3E).
MeDIP analysis for genes in total MDD vs. healthy controls
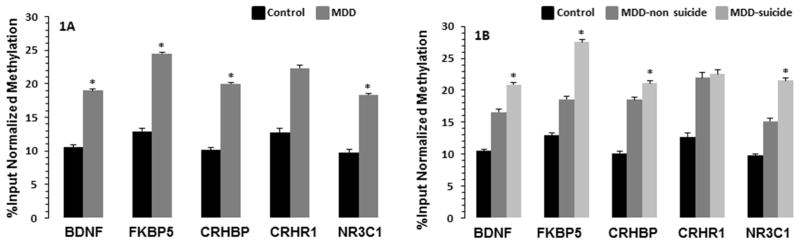
Figure 1A demonstrates the DNA methylation status of CpG islands located close to the transcription start site of BDNF, FKBP5, CRHBP, CRHR1, and NR3C1 genes in PBMC of the total MDD and control samples. Statistical analysis indicated significant promoter CpG sites immune-enrichment for all the genes except CRHR1. Individually, BDNF gene CpG methylation was significantly upregulated in MDD patients compared with healthy controls (p = 0.038; Cohen’s d = 0.63). Similarly, methylation of NR3C1 (p = 0.03; d = 0.65), FKBP5 (p = 0.044; d = 0.62), and CRHBP (p = 0.04; d = 0.62) genes were also significantly increased in MDD patient compared with healthy controls. There was no significant change in the methylation of CRHR1 gene (p = 0.28; d = 0.32) between MDD patients and healthy controls.
Fig. 1.
Methylation enrichment of CpG islands in promoter regions of stress related genes in PBMC of MDD-suicide, MDD-non-suicide, and healthy control groups. Figure 1A demonstrates the input normalized methylation enrichment of CpG islands in promoter areas of BDNF, FKBP5, CRHBP, CRHR1 and NR3C1 genes. The levels of significance were determined with independent sample t-test and represented as the mean ± SEM from 20 healthy controls and 24 MDD patients. *p <0.05 compared with healthy controls. The significance levels of individual genes were as follows: BDNF: t = 2.14, df = 42, p = 0.38; FKBP5: t = 2.08, df = 42, p = 0.04; CRHBP: t = 2.1, df = 42, p = 0.04; CRHR1: t = 1.08, df = 42, p = 0.28; NR3C1: t = 2.12, df = 42, p = 0.03. Figure 1B exhibits CpG island methylation enrichment of selected five genes in healthy control, MDD non-suicide and MDD suicide groups. Data are the mean ± SEM from 20 healthy controls, 14 MDD-suicide, and 10 MDD-non-suicide patients. Data were analyzed using a series of independent sample t-tests. MDD non-suicide and MDD suicide groups were compared with healthy control group whereas MDD non-suicide group was compared with MDD suicide group. *p <0.05 compared with healthy controls. No significant differences were noted in any of the genes between MDD-non-suicide and MDD-suicide groups (BDNF: p = 0.31; FKBP5: p = 0.13; CRHBP: p = 0.72; CRHR1: p = 0.96; NR3C1: p = 0.15) as well as between MDD-non-suicide and healthy control groups (BDNF: p = 0.16; FKBP5: p = 0.30; CRHBP1: p = 0.18; CRHR1: p = 0.37; NR3C1: p = 0.17). On the other hand, BDNF (p = 0.039), FKBP5 (p = 0.035), CRHBP (p = 0.039), and NR3C1 (p = 0.025) but not CRHR1 (p = 0.37) were significantly different between MDD-suicide and healthy control groups.
MeDIP Analysis for Genes in MDD-suicide versus MDD-no suicide and Control Groups
We performed a series of independent sample “t” test among healthy controls, MDD-non-suicide, and MDD-suicide groups. The analysis showed significant hypermethylation of CpG sites in the MDD-suicide group compared with healthy control group for BDNF (p = 0.039), FKBP (p = 0.035), NR3C1 (p = 0.025), and CRHBP (p = 0.039) but not CRHR1 (0.36) (Figure 1B). On the other hand, there were no significant differences in methylation of BDNF (p = 0.31), FKBP5 (p = 0.12), NR3C1 (p = 0.15), CRHBP (p = 0.72), and CRHR1 (p = 0.96) genes between MDD-suicide and MDD-non-suicide groups. Similarly, no significant differences in methylation of BDNF (p = 0.16), FKBP5 (p = 0.30), NR3C1 (p = 0.17), CRHBP (p = 0.18), and CRHR1 (p = 0.36) genes were noted between MDD-non-suicide versus healthy controls. The effect sizes (Cohen’s d) of the significant contrasts were as follows: BDNF: MDD-suicide vs. control: 0.69; MDD-no suicide vs. control: 0.45; MDD-suicide vs. MDD-no suicide: 0.41. FKBP5: MDD-suicide vs. control: 0.68; MDD-no suicide vs. control: 0.32, MDD-suicide vs. MDD-no suicide: 0.64. NR3C1: MDD-suicide vs. control: 0.74; MDD-no suicide vs. control: 0.41; MDD-suicide vs. MDD-no suicide: 0.60.
Relative transcript expression of BDNF, FKBP5, CRHBP, CRHR1 and NR3C1 genes in MDD-suicide and control groups
When GAPDH was used as normalizer for qPCR based quantification, we found ~66% downregulation in the expression of BDNF gene in the MDD-suicide group compared with the healthy controls, which was statistically significant (t = 2.09, df = 27, p = 0.04). The NR3C1 gene expression was also significantly downregulated by ~32% in the MDD-suicide group (t = 2.56, df = 27, p = 0.01). Of the four FKBP5 transcript variants, the expression of FKBP5 1–3 transcript variant (represented as FKBP5 I) was decreased by ~25% (t = 2.21, df = 27, p = 0.03) in the MDD-suicide group without any significant change in the expression of FKBP5 transcript variant 4 (represented with FKBP5 II) (t = 1.71, df = 27, p = 0.09). The expression of CRHBP was not significantly different in the MDD-suicide group compared with healthy control group (t = 0.77, df = 27, p = 0.44) (Figure 2). The expression levels of CRHR1 was determined in PBMC but was found to be too low (CT values > 35) for a meaningful analysis.
Fig. 2.
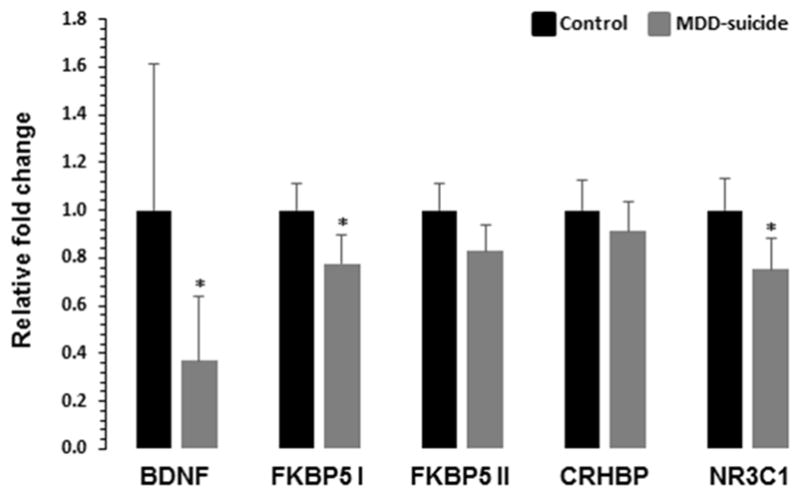
qRT-PCR expression profile of BDNF, FKBP5, CRHBP and NR3C1 genes in PBMC of MDD-suicide and healthy control groups. The bar diagram represents the GAPDH normalized relative transcript abundance of BDNF, FKBP5, CRHBP and NR3C1 genes in MDD-suicide patients (n = 14) as compared to healthy control group (n = 15). Data are represented as the mean ± SEM. The levels of significance were determined with independent Student’s t-test. *p < 0.05 compared with healthy controls. Four different transcript variants of human FKBP5 gene were amplified with two sets of primers and represented as FKBP5 I for transcript variant 1–3 and FKBP5 II for transcript variants 4. The significance levels of these genes were as follows: BDNF: t = 2.09, df = 27, p = 0.04; NR3C1: t = 2.56, df = 27, p = 0.16; FKBP5 I: t = 2.21, df = 27, p = 0.03; FKBP5 II: t = 1.71, df = 27, p = 0.09; CRHBP: t = 0.77, df = 27, p = 0.44.
We also used ACTB as normalizer and quantified expression of all the genes mentioned above. We found similar changes in the expression of BDNF (t = 3.26, df = 27, p = 0.003), NR3C1 (t = 2.44, df = 27, p = 0.02), and FKBP5 I (t = 2.17, df = 27, p = 0.03) in MDD suicide group compared with healthy controls. No significant changes were observed in CRHBP (t = 0.23, df = 27, p = 0.81) gene and FKBP5 transcript variant 4 (FKBP5 II) (t = 1.56, df = 27, p = 0.12).
Effect of confounding variables on DNA methylation and expression of various genes
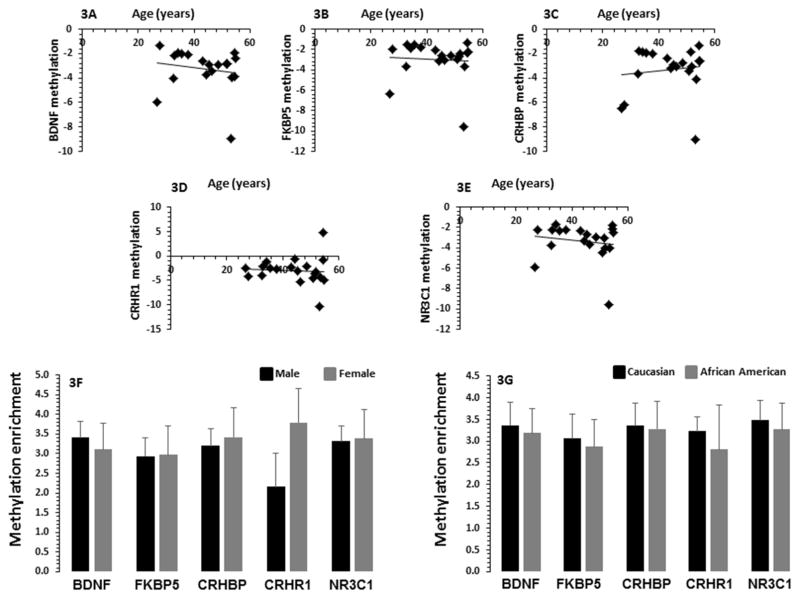
Age did not have significant correlation with methylation enrichment for CpG sites analyzed for BDNF (r = 0.16, p = 0.45), FKBP5 (r = 0.19, p = 0.39), CRHBP (r = 0.26, p = 0.22), CRHR1 (r = 0.13, p = 0.55) or NR3C1 (r = 0.18, p = 0.42) genes (Figures 3A–E). In order to examine the effect of gender on methylation status of the CpG sites, Student’s t-test was performed in 9 males and 15 females in the MDD group for all five genes tested in this study. No significant differences were observed in relative methylation enrichment for BDNF (p = 0.12), FKBP5 (p = 0.06), CRHBP (p = 0.79), CRHR1 (p = 0.99), and NR3C1 (p = 0.12) (Figure 3F). There were 12 African-American and 12 Caucasian patients in the MDD group. We found no significant effect of race on CpG methylation of BDNF (p = 0.75), FKBP5 (p = 0.83), CRHBP (p = 0.39), CRHR1 (p = 0.90) or NR3C1 (p = 0.79) (Figure 3G) gene.
Fig. 3.
Effect of age, gender and race on methylation enrichment five selected stressor genes in PBMC of MDD patients with suicidal behavior. In Fig. 3A–E, the scatterplot demonstrates the correlation between the methylation enrichment of BDNF, FKBP5, CRHBP, CRHR1 and NR3C1 promoters in PBMC of MDD-suicide patients (BDNF, r = 0.063, p = 0.0689, n = 43; FKBP5, r = 0.101, p = 0.515, n = 43; CRHBP, r = 0.175, p = 0.255, n= 43; CRHR1, r = 0.098, p = 0.527, n = 43; NR3C1, r = 0.037, p = 0.811, n = 42). Fig. 3F represents the bar diagram showing the effect of gender on CpG methylation of BDNF, FKBP5, CRHBP, CRHR1 and NR3C1 promoters in PBMC of MDD-suicide patients. The levels of significance determined for each gene are as follows: BDNF, p = 0.719; FKBP5, p = 0.978; CRHBP p = 0.804; CRHR1, p = 0.194; and NR3C1, p = 0.933. In Fig. 3G the bar diagram demonstrates the effect of race on CpG methylation of BDNF, FKBP5, CRHBP, CRHR1 and NR3C1 promoter in PBMC of MDD-suicide patients. There were 6 Caucasian and 8 Black patients in the MDD-suicide group. The levels of significance determined for each gene are as follows: BDNF, p = 0.840; FKBP5, p = 0.836; CRHBP p = 0.935; CRHR1, p = 0.746; and NR3C1, p = 0.804.
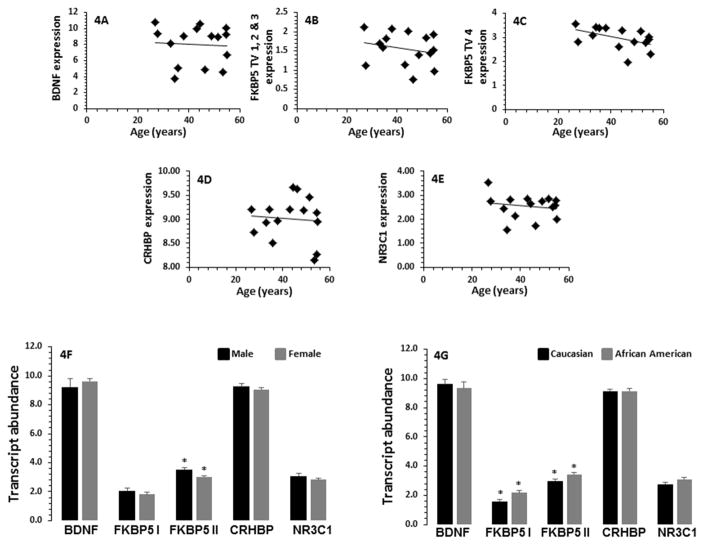
No significant effects of age were noted on mRNA expression of BDNF (r = 0.03, p = 0.92), FKBP5 I (r = 0.26, p = 0.35), FKBP5 II (r = 0.10, p = 0.72), CRHBP (r = 0.14, p = 0.53), and NR3C1 (r = 0.36, p = 0.39) genes (Figure 4A–E). Comparison of males and females also showed no significant differences in mRNA expression of BDNF (p = 0.49), CRHBP (p = 0.41), and NR3C1 (p = 0.18) (Figure 4F). Whereas FKBP5 I was not significantly affected (p = 0.31), but FKBP5 II showed significantly lower expression in males compared with females (p = 0.01) in the MDD-suicide group (Figure 4F). There was no significant effect of race on the expression of BDNF (p = 0.64), CRHBP (p = 0.98), and NR3C1 (p = 0.09) (Figure 4G), however, race had significant effect on expression of both variants of FKBP5 in MDD-suicide patients such that their expression levels were lower in the African-American patients compared with Caucasian patients (FKBP5 I: p = 0.013; FKBP5 I1: p = 0.036) (Figure 4G).
Fig. 4.
Effect of age, gender and race on mRNA expression levels of BDNF, FKBP5, CRHBP and NR3C1 genes in PBMC of MDD patients with suicidal behavior. In Fig. 4A–E, the scatterplot demonstrates the correlation between the transcript abundance of BDNF, FKBP5, CRHBP, and NR3C1 genes in PBMC of 14 MDD-suicide patients (BDNF, r = −0.030, p = 0.918; FKBP5 I, r = 0.268, p = 0.355; FKBP5 II, r = 0.104, p = 0.724; CRHBP, r = 0.139, p = 0.636; NR3C1, r = 0.362, p = 0.203). Fig. 4F represents the bar diagram showing the effect of gender on expression of BDNF, NR3C1, FKBP5 I, FKBP II and CRHBP genes. There were 6 males and 8 females in the MDD-suicide group. The levels of significance for each gene are as follows: BDNF, p = 0.489; NR3C1, p = 0.178; FKBP5 I, p = 0.314; FKBP5 II, *p = 0.014; and CRHBP p = 0.410. Fig. 4G represents the effect of race on expression of BDNF, NR3C1, FKBP5 I, FKBP II and CRHBP. The levels of significance for each gene are as follows: BDNF, p = 0.639; NR3C1, p = 0.089; FKBP5 I, *p = 0.013; FKBP5 II, *p = 0.036; and CRHBP, p = 0.988.
Correlation of DNA methylation with MADRS
We correlated MADRS with DNA methylation of stress related genes individually in total MDD group, MDD without suicidal ideation group, and MDD with suicidal ideation group. There was no significant correlation of MADRS with DNA methylation of BDNF (r = 0.06, p = 0.76), FKBP5 (r = 0.13, p = 0.51), NR3C1 (r = 0.09, p = 0.67), CRHBP (r = 0.10, p = 0.62) genes in total MDD group. Correlation between MADRS and DNA methylation of BDNF (r = 0.49, p = 0.07), FKBP5 (r = 0.44, p = 0.11), and CRHBP (r = 0.22, p = 0.45) genes were not significant in MDD with suicidal ideation group except NR3C1 gene which showed significant correlation with MADRS (r = 0.56, p = 0.05). Correlation between MADRS and DNA methylation of stress related genes in MDD without suicidal ideation group were also not significant (BDNF: r = 0.29, p = 0.41; FKBP5: r = 0.12, p = 0.73; NR3C1: r = 0.22, p = 0.54; CRHBP: r = 0.36, p = 0.30).
Correlation of DNA methylation between groups
A significant association in the extent of methylation was found for several genes (Tables 2 and 3 and). The strongest associations were among BDNF, FKPB5, and CRHR1 (ranging from r = 0.95–0.97), which held for the total sample, controls, MDD with and without suicidal ideation (Table 2). Most other associations were similar between groups, with the exception for the associations between CRHR1 versus CRHBP and NRC31, which were lower in the MDD without suicidal ideation than the other groups (r = 0.13–0.25). Interestingly, methylation enrichment in the promoter regions of FKBP5, NR3C1 and BDNF genes were highly correlated (r2 = 0.93, df = 2,39, p < 0.0001) (Figure 5).
Table 2.
Correlation of DNA methylation between genes, by group (Pearson product moment r).
| A. Controls | ||||
|---|---|---|---|---|
|
| ||||
| NR3C1 | CRHR1 | CRHBP | FKBP5 | |
|
| ||||
| BDNF | .9483 | .4534 | .7835 | .9725 |
| p<.001 | p=.051 | p<.001 | p<.001 | |
|
| ||||
| FKBP5 | .9731 | .5827 | .8731 | |
| p<.001 | p=.009 | p<.001 | ||
|
| ||||
| CRHBP | .8502 | .5860 | ||
| p<.001 | p=.008 | |||
|
| ||||
| CRHR1 | .6609 | |||
| p=.002 | ||||
|
| ||||
| B. MDD with suicidal ideation | ||||
|---|---|---|---|---|
| NR3C1 | CRHR1 | CRHBP | FKBP5 | |
|
| ||||
| BDNF | .9118 | .2477 | .9449 | .9725 |
|
| ||||
| p<.001 | p=.415 | p<.001 | p<.001 | |
|
| ||||
| FKBP5 | .9271 | .2625 | .9556 | |
|
| ||||
| p<.001 | p=.386 | p<.001 | ||
|
| ||||
| CRHBP | .8769 | .1291 | ||
|
| ||||
| p<.001 | p=.674 | |||
|
| ||||
| CRHR1 | .5511 | |||
|
| ||||
| p=.051 | ||||
|
| ||||
| C. MDD without suicidal ideation | ||||
|---|---|---|---|---|
|
| ||||
| NR3C1 | CRHR1 | CRHBP | FKBP5 | |
|
| ||||
| BDNF | .9245 | .3470 | .5899 | .7948 |
| p<.001 | p=.326 | p=.073 | p=.006 | |
|
| ||||
| FKBP5 | .7955 | .4967 | .5274 | |
| p=.006 | p=.144 | p=.117 | ||
|
| ||||
| CRHBP | .6311 | .1801 | ||
| p=.050 | p=.619 | |||
|
| ||||
| CRHR1 | .2447 | |||
| p=.496 | ||||
|
| ||||
Table 3.
Correlation of DNA methylation between genes, total sample (Pearson product moment r).
| NR3C1 | CRHR1 | CRHBP | FKBP5 | |
|---|---|---|---|---|
|
| ||||
| BDNF | .9461 | .4086 | .7871 | .9620 |
| p<0.01 | p=.007 | p<.001 | p<0.01 | |
|
| ||||
| FKBP5 | .9592 | .5042 | .8375 | |
| p<0.01 | p=.001 | p<.001 | ||
|
| ||||
| CRHBP | .8246 | .4402 | ||
| p<.001 | p=.004 | |||
|
| ||||
| CRHR1 | .5888 | |||
| p<.001 | ||||
|
| ||||
Fig. 5.
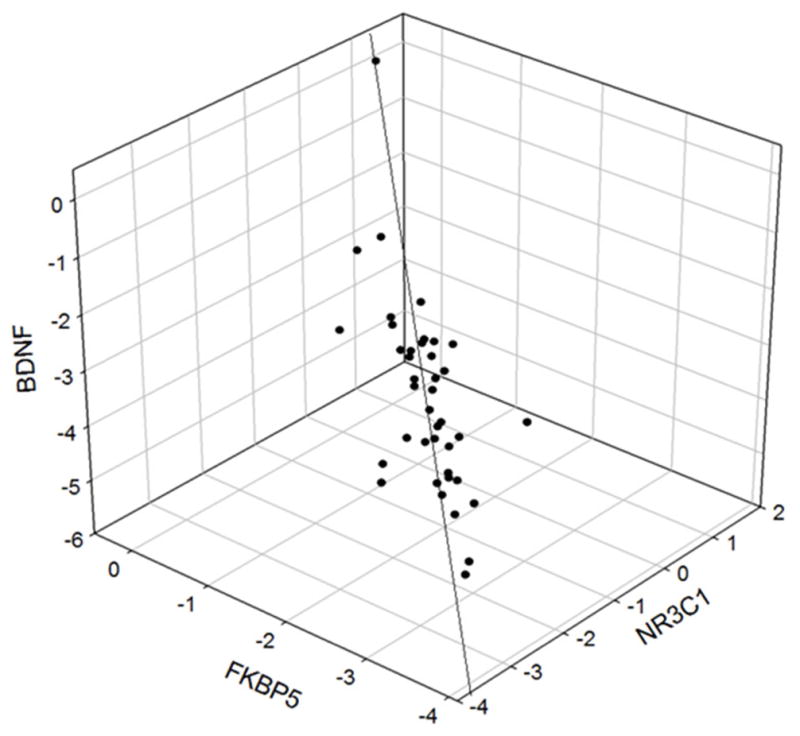
3D plot of the multiple correlation of the methylation level of BDNF versus FKBP5 and NR3C1 (Multiple R2 = 0.93, df = 2,39, p<0.0001).
DISCUSSION
The primary objective of the study was to examine whether MDD was associated with epigenetic modifications in the genes that are critical in stress response. The exploratory objective was to test whether these modifications were specifically associated with serious suicidal ideation or whether they represented a general feature of MDD. Our data show a significant increase in DNA methylation of stress related genes including BDNF, NR3C1, FKBP5, and CRHBP in PBMC of total MDD patients (with and without suicidal ideation) compared with healthy controls. Further exploratory analyses suggest that methylation of these genes may only be in MDD-suicide patients and not in the MDD-non-suicide group compared with healthy controls. A concomitant decrease in expression of BDNF, NR3C1, and FKBP5 transcript variant 1, 2, and 3 but not 4 were also noted in PBMC of MDD-suicide patients compared with healthy controls.
Epigenetic regulation of gene transcription is an important transducer of environmental inputs that shape the phenotypic characteristics of mammalian systems (Roth and Sweatt, 2011). The significance of epigenetic modifications is more obvious in psychiatric illnesses in which phenotypes are dependent on gene x environment interactions (Klengel and Binder, 2015). Given the increased risk of MDD by earlier trauma and precipitation of MDD episodes by recent stressors, both stable and transient epigenetic marks in the form of DNA methylation or histone modification and concomitant changes in gene expression are likely to be involved in the etiopathogenesis of MDD (Oh et al., 2015; Sabunciyan et al., 2012; Tsankova et al., 2006; Wilkinson et al., 2009). In this study, we selected five genes: BDNF, FKBP5, CRHBP, CRHR1 and NR3C1, whose roles have been well established in stress (Hornung and Heim, 2014; Lee and Sawa, 2014; Zannas et al., 2016). Our study shows that promoter regions of BDNF, NR3C1, FKBP5 and CRHBP genes were hypermethylated in PBMC of MDD patients without any change in methylation of CRHR1 gene. Further exploratory analysis showed greater hypermethylation of BDNF, FKBP5, NR3C1, and CRHBP gene promoters in MDD patients with serious suicidal ideation relative to healthy controls with medium to large effect sizes (Cohen’s d = 0.34–0.85). However, there were no significant differences in the promoter methylation of these genes between the MDD patients without suicidal ideation versus healthy controls. The effect sizes of the contrasts suggest that methylation of these genes should be compared in healthy controls, MDD-suicide, and MDD-no suicide groups in larger samples since the contrasts between the control and MDD-suicide groups were large (ES = 0.68–0.74) and between MDD-suicide and MDD-non-suicide were in the medium to large range (ES = 0.41–0.64). It should be noted, however, that the depression severity was greater in the MDD-suicide group compared with MDD-no suicide group. Thus, these differences may be accounted for by severity of depression. Interestingly, we did not find any significant correlation between promoter DNA methylation and MADRS scores in total MDD patients (with and without suicidal ideation) or MDD patients without suicidal ideation. Similarly, except for NR3C1, we did not find significant correlation between MADRS and promoter methylation for any other gene in the MDD suicide group. This suggests that the observed methylation changes could also be associated with serious suicidal ideation. Since the present study was conducted in a small sample size, further large scale studies needed to be carried out to further test these findings.
Our study was further extended to understand the functional contribution of the observed methylation changes at promoter regions in modulating downstream gene expression. A significant downregulation in the expression of BDNF, FKBP5, and NR3C1 genes in PBMC of MDD-suicide patients suggest that changes in methylation of these genes were functionally associated with downregulation of the observed expression of respective genes. The expression of CRHBP could not reach the significance level, which could be attributed to the overall low expression of this gene in PBMC.
The role of BDNF in plasticity related functions has been well documented (Leal et al., 2015; Lu et al., 2013). Several studies have pointed out the involvement of epigenetically regulated BDNF expression and their compromised functionality in MDD pathophysiology (Dwivedi, 2009; Yu and Chen, 2011), which could be reversed with antidepressant treatment (Warner-Schmidt and Duman, 2006). We reported in an earlier study that expression of BDNF is downregulated in postmortem brain of depressed individuals who died by suicide (Dwivedi et al., 2003). A recent report has also pointed out higher occurrence of hypermethylation in BDNF exon IV in Wernicke’s area of post-mortem brain of suicidal patients (Keller et al., 2010). Another study in blood cells of MDD patients suggests that suicide is strongly associated with impaired BDNF function due to its altered DNA methylation (Kim et al., 2014). Our current observation of altered BDNF methylation in PBMCs from MDD patients with serious suicidal ideation and a previous study (Dwivedi et al., 2003) showing decreased BDNF expression in post-mortem brain tissue from MDD patients who died by suicide support a strong relationship of DNA modification and reduced expression of BDNF with suicidal behavior in MDD patients. The lower expression of BDNF transcripts analyzed in the same group of patients suggests that impaired BDNF functioning may induce a maladaptive response toward stressful stimuli, which can increase the risk of suicidal ideation or behavior in MDD patients.
The roles of CRHR1 and CRHBP in suicidal behavior have earlier been assessed for their association with the maladaptive HPA axis response (Wasserman et al., 2010). For example, it has been shown that sequence variation in CRHR1 gene increases the susceptibility to suicidal behavior in MDD patients (Wasserman et al., 2008). The functional variability attributed towards altered receptor sensitivity and/or responsiveness for CRHR1 gene, however, is not well documented from epigenetic point of view except a few recent reports of DNA methylation marks and repressive histone modifications at the promoter region of CRHR1 gene that were associated with chronic stress-induced anxiety and depression in rodent models (Sotnikov et al., 2014). In the present study, although hypermethylation in CRHR1 promoter regions was greater in MDD patients, this did not reach to the statistical significance level and the effect size was also small (d = 0.32). Thus, whereas CRHR1 gene may be hypermethylated in MDD patients, the differences from healthy controls are likely to be small. Moreover, we found a negligible expression of CRHR1 gene in PBMC. Thus, the significance of this gene to MDD could not be ascertained at the functional level. On the other hand, CRHBP promoter was significantly hypermethylated in MDD patients (with and without suicidal ideation) compared with healthy controls. Although, the methylation of this gene was higher in MDD-suicide patients compared with MDD-non-suicide patients, it was only at a trend level (p = 0.057). Thus, although CRHBP appears to be of interest in relation to MDD suicidal patients, its contribution needs to be explored in a larger patient population.
NR3C1 is a gene which encodes glucocorticoid receptor (GR) and can function both as a transcription factor that binds to GR elements (GRE) in the promoters of glucocorticoid responsive genes (John et al., 2011; Phuc Le et al., 2005) and as a regulator of other transcription factors (Ratman et al., 2013). GR has been well studied for its role in potential contribution to stress-related disorders (Fries et al., 2015; Labonte et al., 2014). Recently, we reported that protein and gene expression of GR-α was significantly decreased in the prefrontal cortex and amygdala of teenage suicide victims compared with normal control subjects (Pandey et al., 2013). In addition, we found that mRNA levels of GR-inducible target gene GILZ was significantly decreased in these brain regions of teenage suicide victims, demonstrating altered functional response of GR. Interestingly, McGowan et al. (2009) showed decreased levels of GR mRNA as well as mRNA transcripts bearing the GR1F splice variant and increased cytosine methylation of an NR3C1 promoter in the post-mortem hippocampus of suicide victims with a history of childhood abuse (McGowan et al., 2009). More recently, (Na et al., 2014) compared the degree of NR3C1 promoter methylation in the peripheral blood of MDD patients and correlated with structural abnormalities in hippocampal subfield volumes (Na et al., 2014). They found that MDD patients had significantly lower methylation than healthy controls at 2 CpG sites of NR3C1 gene, which had positive correlations with the bilateral CA 2–3 and CA4-dentate gyrus subfields of hippocampus. This is quite interesting with the perspective of our present study where we found significant hypermethylation of NR3C1 promoters in MDD patients, which was specifically associated with suicidal behavior. Moreover, our findings of decreased expression of GR mRNA in MDD-suicide patients further indicates disruption of GR functionality possibly mediated by the hypermethylated NR3C1 promoter element, which could be critical for heightened stress response aggravating suicidal thoughts.
The activity of the GR is modulated by a large number of proteins. This includes the co-chaperone FK506 binding protein 51 (FKBP5). FKBP5 regulates the folding and trafficking of GR (Grad and Picard, 2007; Pratt et al., 1993) in such a way that not only it reduces GR binding (Denny et al., 2000) but also delays translocation of GR to the nucleus (Wochnik et al., 2005). Several lines of evidence indicate strong functional link between active GR and induced transcription of FKBP5 gene, which may act as feedback loop to regulate GR sensitivity (Vermeer et al., 2003). It has been reported that functional polymorphisms in FKBP5 gene may be associated with differences in GR function in MDD and PTSD patients (Zannas et al., 2016). Interestingly, Roy et al. (2010) found that childhood trauma and variants of the FKBP5 gene interact with each other to increase the risk for suicide attempt (Roy et al., 2010). More recently, the same group also found that CRHBP and FKBP5 interact with childhood trauma to increase the risk for suicidal behavior (Roy et al., 2012). Our present study shows that MDD suicidal patients have strong CpG hypermethylation in the close vicinity of transcript variant 1 for FKBP5, which encompasses transcription start site and partly extends within the coding region of transcript variant 2. Close examination of the genomic loci of FKBP5 shows that the unique CpG island of variant 1 is enriched with multiple GREs on human chromosome 6 (Paakinaho et al., 2010). This indicates that possible disruption of GR binding to these GREs, which may be due to presence of strong methylation marks on FKBP5 CpG islands in MDD suicidal patients. Moreover, lower level of NR3C1 transcription due to its CpG island methylation, as observed in the present study, further suggests that a feed forward regulation may exist between GR and FKBP5, which could be involved in suicidal behavior among MDD patients.
The observation of highly correlated methylation enrichment in the promoter regions of FKBP5, NR3C1 and BDNF genes indicate a strong possibility of being regulated in a coordinated fashion. This could be the result of a common intracellular mechanism that might be synchronized with environmental stimuli. In this context, members from de novo DNA methyl transferase family such as DNMT3a and DNMT3b could be proposed as possible intracellular targets of adverse environmental stimuli to orchestrate the methylation signature at genomic level. Considering context specific intermediate regulatory mechanisms including post transcriptional regulation by microRNAs, post translational modifications by acetylation, methylation, phosphorylation, and ubiquitination of DNMT enzymes may mechanistically connect the functional dynamics of observed DNA promoter hypermethylation for the three aforementioned genes. In fact, we recently identified DNMT3b as a strong predicted target of miR-148b whose expression is significantly upregulated in post-mortem brain of suicide subjects (Smalheiser et al., 2012).
While the results of this analysis suggest that genes involved in stress response mechanisms may be hypermethylated in MDD patients and that there may be differences specifically in MDD patients with significant suicidal ideation, the results should be interpreted cautiously. The sample size was relatively small and some of the analyses were conducted in an exploratory manner. Further, there were large differences between MDD-suicide and MDD-non-suicide groups in severity of depression as measured by MADRS. As a result, the differences between MDD-suicide and MDD non-suicide groups could be the result of severity of depression, however, because of the small sample size, it was difficult to perform ANCOVA or multivariate logistic regression adjustments for factors such as severity of depression. Further, the differences between MDD groups could represent the results of variations in recent stressors since suicidal ideation is typically stress sensitive. This would not invalidate the data but rather point to a possible causal mechanism. Finally, the assessment of suicidal ideation was cross-sectional and by self-reports. There could have been underreporting of ideation and some people who were not suicidal at the time of assessment could have been suicidal at other points in their lives. Therefore, the data require replication to establish validity.
In conclusion, we found interesting results, which suggest that epigenetic modifications of stress-related genes such as BDNF, NR3C1, and FKBP5 are associated with MDD; however, these changes may be more prominent in MDD with clinically significant suicidal ideation. Our study is limited by a small sample size in both MDD-non-suicide and MDD-suicide groups. Nevertheless, even with this small sample size, we found strong association of suicidal behavior with epigenetic modifications of these genes. Further studies are needed to confirm the findings of this study.
Supplementary Material
Suppl. Fig. S1A-S1E. Mapping of CpG islands in the upstream regulatory regions of FKBP5, CRHR1, CRHBP, NR3C1 and BDNF genes relative to the chromosomal coordinates using UCSC genome browser human genome assembly GRCh38/hg38 (December, 2013). The selected set of islands for the current study indicated with arrow head in each figure. Mapping of GC density shown on top of loci aligned with chromosomal coordinates. TV= Transcript Variants of a gene.
Suppl. Fig. S2A. FKBP5 (Coordinate: Chromosome 6 _35655608-35656856, Chromosomal strand= Negative). Suppl. Fig. S2B. CRHR1 (Coordinate: Chromosome 17_43860685-43862928, Chromosomal strand= Positive). Suppl. Fig. S2C: CRHBP (Coordinate: Chromosome 5_76249436-76250528, Chromosomal strand= Positive) Suppl. Fig. S2D. NR3C1 (Coordinate: Chromosome 5_142782072-142785071, Chromosomal strand= Negative) Suppl. Fig. S2E. BDNF (Coordinate: Chromosome 11_27743473-27744564, Chromosomal strand= Negative).
Suppl. Fig. S3A. Schematic diagram represents the in silico analyzed data for FKBP5 on chromosome 6. MethPrimer based CpG site prediction algorithm identifies one CpG island with a 60% GC content. Suppl. Fig. S3B. Schematic diagram represents the in silico analyzed data for CRHR1 on chromosome 17. MethPrimer based CpG site prediction algorithm identifies five CpG islands. The overall GC content of five CpG islands is more than 50%. Suppl. Fig. S3C. Schematic diagram represents the in silico analyzed data for CRHBP on chromosome 5. MethPrimer based CpG site prediction algorithm identifies three CpG islands. The overall GC content of three CpG islands is more than 60%. Suppl. Fig. S3D. Schematic diagram represents the in silico analyzed data for NR3C1 on chromosome 5. MethPrimer based CpG site prediction algorithm identifies five CpG islands. The overall GC content of three CpG islands is more than 65%. Suppl. Fig. S3E. Schematic diagram represents the in silico analyzed data for BDNF on chromosome 11. MethPrimer based CpG site prediction algorithm identifies four CpG islands. The overall GC content of three CpG islands is more than 55–65%.
Highlights.
Stress associated genes are hypermethylated in MDD individuals.
The extent of hypermethylation of stress related genes are more prominent in MDD patients who show significant suicidal ideation.
The expression of stress related genes are concomitantly decreased, suggesting a function link between epigenetic modifications and abnormal gene functions in suicidal behavior.
These genes may serve as predictive biomarker of suicidal behavior in MDD patients.
Acknowledgments
We would like to thank Dr. Birgit Ludwig for valuable comments.
Footnotes
Contributors
YD designed the study. RC managed the clinical study. BR conducted the biological and statistical analyses. RC conducted the statistical analyses. BR, RC, and YD co-wrote the manuscript. All authors have approved the final article.
ROLE OF THE FUNDING SOURCE: The study was supported by grants from National Institute of Mental Health (R01MH082802; R21MH081099; 1R01MH101890; R01MH100616; 1R01MH107183), and American Foundation for Suicide Prevention (SRG-1-042-14) to Dr. Dwivedi. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. The American journal of psychiatry. 2000;157(12):1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Brezo J, Barker ED, Paris J, Hebert M, Vitaro F, Tremblay RE, Turecki G. Childhood trajectories of anxiousness and disruptiveness as predictors of suicide attempts. Archives of pediatrics & adolescent medicine. 2008;162(11):1015–1021. doi: 10.1001/archpedi.162.11.1015. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) [Accessed 11 March 2016];National Center for Health Statistics, Adoloscent Health, Leading Cause of Death. 2013 http://www.cdc.gov/nchs/fastats/adolescent-health.htm.
- CDC (Centers for Disease Control and Prevention) [Accessed 10 March 2016];National Centers for Injury Prevention and Control: Web-Based Injury Statistics Query and Reporting System (WISQARS) 2014 http://www.cdc.gov/violenceprevention/suicide/statistics/
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum and Associates; Mahwah, NJ: 1988. [Google Scholar]
- Cohen J. A power primer. Psychological bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141(11):4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Dumser T, Barocka A, Schubert E. Weight of adrenal glands may be increased in persons who commit suicide. The American journal of forensic medicine and pathology. 1998;19(1):72–76. doi: 10.1097/00000433-199803000-00014. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatric disease and treatment. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Elucidating biological risk factors in suicide: role of protein kinase A. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35(4):831–841. doi: 10.1016/j.pnpbp.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of general psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Roy B, Lugli G, Rizavi H, Zhang H, Smalheiser NR. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: relevance to depression pathophysiology. Translational psychiatry. 2015;5:e682. doi: 10.1038/tp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MS, RL, Gibbon M, Williams JBW. Biometric Research. New York State Psychiatric Institute; New York, NY: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient ed. [Google Scholar]
- Fries GR, Gassen NC, Schmidt U, Rein T. The FKBP51-Glucocorticoid Receptor Balance in Stress-Related Mental Disorders. Current molecular pharmacology. 2015;9(2):126–140. doi: 10.2174/1874467208666150519114435. [DOI] [PubMed] [Google Scholar]
- Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Molecular and cellular endocrinology. 2007;275(1–2):2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Haghighi F, Xin Y, Chanrion B, O'Donnell AH, Ge Y, Dwork AJ, Arango V, Mann JJ. Increased DNA methylation in the suicide brain. Dialogues in clinical neuroscience. 2014;16(3):430–438. doi: 10.31887/DCNS.2014.16.3/jmann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi F, Uchida S, Yamagata H, Otsuki K, Hobara T, Abe N, Shibata T, Watanabe Y. State-dependent changes in the expression of DNA methyltransferases in mood disorder patients. Journal of psychiatric research. 2011;45(10):1295–1300. doi: 10.1016/j.jpsychires.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Heim CM. Gene-environment interactions and intermediate phenotypes: early trauma and depression. Frontiers in endocrinology. 2014;5:14. doi: 10.3389/fendo.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nature genetics. 2011;43(3):264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kim JM, Lee JY, Kim SY, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. BDNF promoter methylation and suicidal behavior in depressive patients. J Affect Disord. 2013;151(2):679–685. doi: 10.1016/j.jad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Archives of general psychiatry. 2010;67(3):258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Archives of general psychiatry. 1999;56(7):617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kang HJ, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. Association of BDNF promoter methylation and genotype with suicidal ideation in elderly Koreans. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22(10):989–996. doi: 10.1016/j.jagp.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Klengel T, Binder EB. Epigenetics of Stress-Related Psychiatric Disorders and Gene x Environment Interactions. Neuron. 2015;86(6):1343–1357. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Labonte B, Azoulay N, Yerko V, Turecki G, Brunet A. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Translational psychiatry. 2014;4:e368. doi: 10.1038/tp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain research. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Lee RS, Sawa A. Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology. 2014;100(4):278–287. doi: 10.1159/000369585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP. Gene-environment interaction and the genetics of depression. Journal of psychiatry & neuroscience : JPN. 2004;29(3):174–184. [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Palkovits M, Arato M, Mansour A, Akil H, Watson SJ. Localization and quantification of pro-opiomelanocortin mRNA and glucocorticoid receptor mRNA in pituitaries of suicide victims. Neuroendocrinology. 1992;56(4):491–501. doi: 10.1159/000126266. [DOI] [PubMed] [Google Scholar]
- Lopizzo N, Bocchio Chiavetto L, Cattane N, Plazzotta G, Tarazi FI, Pariante CM, Riva MA, Cattaneo A. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Frontiers in psychiatry. 2015;6:68. doi: 10.3389/fpsyt.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature reviews Neuroscience. 2013;14(6):401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Mann JJ. The neurobiology of suicide. Nature medicine. 1998;4(1):25–30. doi: 10.1038/nm0198-025. [DOI] [PubMed] [Google Scholar]
- Mann JJ. A current perspective of suicide and attempted suicide. Annals of internal medicine. 2002;136(4):302–311. doi: 10.7326/0003-4819-136-4-200202190-00010. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Clinical pleomorphism of major depression as a challenge to the study of its pathophysiology. World psychiatry : official journal of the World Psychiatric Association (WPA) 2010;9(3):167–168. doi: 10.1002/j.2051-5545.2010.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Haghighi F. Genes and environment: multiple pathways to psychopathology. Biological psychiatry. 2010;68(5):403–404. doi: 10.1016/j.biopsych.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Martin G, Bergen HA, Richardson AS, Roeger L, Allison S. Sexual abuse and suicidality: gender differences in a large community sample of adolescents. Child abuse & neglect. 2004;28(5):491–503. doi: 10.1016/j.chiabu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Maussion G, Yang J, Suderman M, Diallo A, Nagy C, Arnovitz M, Mechawar N, Turecki G. Functional DNA methylation in a transcript specific 3'UTR region of TrkB associates with suicide. Epigenetics. 2014;9(8):1061–1070. doi: 10.4161/epi.29068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Binder EB. Epigenetic alterations in depression and antidepressant treatment. Dialogues in clinical neuroscience. 2014;16(3):395–404. doi: 10.31887/DCNS.2014.16.3/amenke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS, Yoon HK, Kim YK, Joe SH, Jung IK, Lee MS, Ham BJ. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One. 2014;9(1):e85425. doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Archives of general psychiatry. 1988;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Numata S, Ishii K, Tajima A, Iga J, Kinoshita M, Watanabe S, Umehara H, Fuchikami M, Okada S, Boku S, Hishimoto A, Shimodera S, Imoto I, Morinobu S, Ohmori T. Blood diagnostic biomarkers for major depressive disorder using multiplex DNA methylation profiles: discovery and validation. Epigenetics. 2015;10(2):135–141. doi: 10.1080/15592294.2014.1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh G, Wang SC, Pal M, Chen ZF, Khare T, Tochigi M, Ng C, Yang YA, Kwan A, Kaminsky ZA, Mill J, Gunasinghe C, Tackett JL, Gottesman II, Willemsen G, de Geus EJ, Vink JM, Slagboom PE, Wray NR, Heath AC, Montgomery GW, Turecki G, Martin NG, Boomsma DI, McGuffin P, Kustra R, Petronis A. DNA modification study of major depressive disorder: beyond locus-by-locus comparisons. Biological psychiatry. 2015;77(3):246–255. doi: 10.1016/j.biopsych.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakinaho V, Makkonen H, Jaaskelainen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Molecular endocrinology (Baltimore, Md) 2010;24(3):511–525. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology. 2013;38(11):2628–2639. doi: 10.1016/j.psyneuen.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuc Le P, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH. Glucocorticoid receptor-dependent gene regulatory networks. PLoS genetics. 2005;1(2):e16. doi: 10.1371/journal.pgen.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett A, O'Toole B, Swanston H, Oates RK, Shrimpton S, Parkinson P. Suicide risk following child sexual abuse. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2001;1(5):262–266. doi: 10.1367/1539-4409(2001)001<0262:srfcsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Czar MJ, Stancato LF, Owens JK. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? The Journal of steroid biochemistry and molecular biology. 1993;46(3):269–279. doi: 10.1016/0960-0760(93)90216-j. [DOI] [PubMed] [Google Scholar]
- Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Molecular and cellular endocrinology. 2013;380(1–2):41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Annual Research Review: Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(4):398–408. doi: 10.1111/j.1469-7610.2010.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(8):1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of psychiatric research. 2012;46(1):72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabunciyan S, Aryee MJ, Irizarry RA, Rongione M, Webster MJ, Kaufman WE, Murakami P, Lessard A, Yolken RH, Feinberg AP, Potash JB. Genome-wide DNA methylation scan in major depressive disorder. PLoS One. 2012;7(4):e34451. doi: 10.1371/journal.pone.0034451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PloS one. 2012;7(3):e33201. doi: 10.1371/journal.pone.0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikov SV, Markt PO, Malik V, Chekmareva NY, Naik RR, Sah A, Singewald N, Holsboer F, Czibere L, Landgraf R. Bidirectional rescue of extreme genetic predispositions to anxiety: impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Translational psychiatry. 2014;4:e359. doi: 10.1038/tp.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biological psychiatry. 1994;36(6):374–380. doi: 10.1016/0006-3223(94)91212-2. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World psychiatry : official journal of the World Psychiatric Association (WPA) 2004;3(2):73–83. [PMC free article] [PubMed] [Google Scholar]
- Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. Journal of psychiatry & neuroscience : JPN. 2005;30(6):398–408. [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ. The neurobiology of suicide. The lancet Psychiatry. 2014;1(1):63–72. doi: 10.1016/S2215-0366(14)70220-2. [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. The Journal of clinical endocrinology and metabolism. 2003;88(1):277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes, brain, and behavior. 2008;7(1):14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Wasserman D, Wasserman J, Sokolowski M. Genetics of HPA-axis, depression and suicidality. European psychiatry : the journal of the Association of European Psychiatrists. 2010;25(5):278–280. doi: 10.1016/j.eurpsy.2009.12.016. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Suicide prevention (SUPRE) Geneva: World Health Organization; 2012. [Accessed 10 March 2016]. [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(24):7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. The Journal of biological chemistry. 2005;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta pharmacologica Sinica. 2011;32(1):3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(1):261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kusumo H, Sakharkar AJ, Pandey SC, Guizzetti M. Regulation of DNA methylation by ethanol induces tissue plasminogen activator expression in astrocytes. Journal of neurochemistry. 2014;128(3):344–349. doi: 10.1111/jnc.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chang Z, Chen J, Ling Y, Liu X, Feng Z, Chen C, Xia M, Zhao X, Ying W, Qing X, Li G, Zhang C. Methylation of the tryptophan hydroxylase2 gene is associated with mRNA expression in patients with major depression with suicide attempts. Molecular medicine reports. 2015;12(2):3184–3190. doi: 10.3892/mmr.2015.3748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. S1A-S1E. Mapping of CpG islands in the upstream regulatory regions of FKBP5, CRHR1, CRHBP, NR3C1 and BDNF genes relative to the chromosomal coordinates using UCSC genome browser human genome assembly GRCh38/hg38 (December, 2013). The selected set of islands for the current study indicated with arrow head in each figure. Mapping of GC density shown on top of loci aligned with chromosomal coordinates. TV= Transcript Variants of a gene.
Suppl. Fig. S2A. FKBP5 (Coordinate: Chromosome 6 _35655608-35656856, Chromosomal strand= Negative). Suppl. Fig. S2B. CRHR1 (Coordinate: Chromosome 17_43860685-43862928, Chromosomal strand= Positive). Suppl. Fig. S2C: CRHBP (Coordinate: Chromosome 5_76249436-76250528, Chromosomal strand= Positive) Suppl. Fig. S2D. NR3C1 (Coordinate: Chromosome 5_142782072-142785071, Chromosomal strand= Negative) Suppl. Fig. S2E. BDNF (Coordinate: Chromosome 11_27743473-27744564, Chromosomal strand= Negative).
Suppl. Fig. S3A. Schematic diagram represents the in silico analyzed data for FKBP5 on chromosome 6. MethPrimer based CpG site prediction algorithm identifies one CpG island with a 60% GC content. Suppl. Fig. S3B. Schematic diagram represents the in silico analyzed data for CRHR1 on chromosome 17. MethPrimer based CpG site prediction algorithm identifies five CpG islands. The overall GC content of five CpG islands is more than 50%. Suppl. Fig. S3C. Schematic diagram represents the in silico analyzed data for CRHBP on chromosome 5. MethPrimer based CpG site prediction algorithm identifies three CpG islands. The overall GC content of three CpG islands is more than 60%. Suppl. Fig. S3D. Schematic diagram represents the in silico analyzed data for NR3C1 on chromosome 5. MethPrimer based CpG site prediction algorithm identifies five CpG islands. The overall GC content of three CpG islands is more than 65%. Suppl. Fig. S3E. Schematic diagram represents the in silico analyzed data for BDNF on chromosome 11. MethPrimer based CpG site prediction algorithm identifies four CpG islands. The overall GC content of three CpG islands is more than 55–65%.