Abstract
Purpose
In immune-mediated rheumatic diseases (IMRDs), persistence to treatment may be used as a surrogate marker for long-term treatment success. In previous comparisons of persistence to tumor necrosis factor α inhibitors (TNFis), a paucity of data for subcutaneous (SC) golimumab was identified. The aim of this study was to conduct a systematic review of persistence to SC golimumab in clinical practice and contextualize these data with five-year persistence estimates from long-term open-label extension (OLE) trials of SC TNFis in IMRDs.
Patients and methods
PubMed, Embase, MEDLINE, and conference proceedings from European League Against Rheumatism (EULAR), American College of Rheumatology (ACR), and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) were searched. All studies on patients treated with SC golimumab for IMRD were included if they reported data on the persistence to golimumab.
Results
Of 376 available references identified through the searches, 12 studies with a total of 4,910 patients met the inclusion criteria. Furthermore, nine OLE trials were available. Among the included studies from clinical practice, at six months, one year, two years, and three years, the proportion of patients persistent to treatment ranged from 63% to 91%, 47% to 80%, 40% to 77%, and 32% to 67%, respectively. In the four studies that included comparisons to other biologics, golimumab was either statistically noninferior or statistically superior to other treatments, an observation that was supported by indirect comparisons of unadjusted point estimates of OLE trials.
Conclusion
The data reviewed in this study indicate that golimumab may have higher persistence than other TNFis, a notion that is supported by indirect comparisons of persistence data from OLEs of randomized controlled trials (RCTs). Furthermore, the study suggests that persistence may be lower in biologic-experienced compared with biologic-naive patients and higher in axial spondyloarthritis compared with rheumatoid arthritis and psoriatic arthritis.
Keywords: golimumab, Simponi, Treatment persistence, drug survival, retention rates, real-world evidence (RWE)
Introduction
Medication taking behavior can be described in terms of adherence (also called compliance) and persistence.1 Adherence refers to the degree of conformity between prescribed instructions and actual medication taking behavior.1 Persistence to therapy is defined as “the duration of time from initiation to discontinuation of therapy”1 and may be employed as a surrogate marker of long-term treatment success given that it reflects clinical effectiveness, absence of significant adverse events, and treatment satisfaction.2–4
Axial Spondyloarthritis (axial SpA), psoriatic arthritis (PsA), and rheumatoid arthritis (RA) are immune-mediated rheumatic diseases (IMRDs).5 These progressive disorders can lead to severe pain, joint damage, loss of function,6–8 and result in substantial humanistic and economic burdens.9,10
Biologic therapy has revolutionized the treatment of IMRD, and subcutaneous (SC) tumor necrosis factor α inhibitors (TNFis) are the most frequently prescribed biologic treatment class in IMRD. The first SC TNFi introduced was etanercept (Enbrel®, Amgen Inc., Thousand Oaks, CA, USA), which was approved by the European Medicines Agency (EMA) in 2000, followed by adalimumab (Humira®, Abbvie Inc, North Chicago, IL, USA), certolizumab pegol (Cimzia®, UCB, Inc., Brussels, Belgium), and golimumab (Simponi®, Janssen Biotech, Inc., Horsham, PA, USA).11
Golimumab is a human monoclonal immunoglobulin G (IgG)1κ that binds to TNF-α with a high affinity.12 It is the first SC TNFi with monthly administration in Europe and the US, and other regions. It was approved in 2009 for RA, Ankylosing Spondylitis (AS), and PsA.11 It has also since been approved for patients with ulcerative colitis (UC) in 2013 and nonradiographic axial spondyloarthritis (nr-axial SpA) in 2015. The efficacy of golimumab in rheumatology indications has been proven by several randomized controlled trials (RCTs), including the GO-FORWARD in RA,13 GO-RAISE in AS,14 GO-REVEAL in PsA,15 and GO-AHEAD in nr-axial SpA16 trials. The safety profile has been shown to be similar to that of other SC TNFis.12 In the rheumatology indications, according to the EMA and the United States (US) Food and Drug Administration (FDA) labels,17,18 golimumab should be administered subcutaneously as 50 mg injection once per month, on the same day each month.19 In Japan, a 100 mg dose with the same schedule is also approved.20 Furthermore, in the US, golimumab has also been approved as an intravenous infusion for RA.21
Data on long-term persistence to SC TNFi can be obtained from open-label extension (OLE) studies of RCTs or from clinical practice. OLE studies provide long-term persistence data in well-defined populations with extensive follow-up.22 However, patients participating in RCTs are typically carefully selected on comorbidities, comedications, and disease activity, limiting their representativeness for patients in clinical practice.23 Furthermore, patients who participate in RCTs may alter their behavior to comply with study instructions,23 potentially affecting their persistence to treatment. Data from clinical practice may be obtained from registers or health care databases.24 These data may be more generalizable than data from OLE studies. However, patients in these types of data may be less well described and more prone to loss-to-follow-up. Therefore, data on persistence to treatment from OLE studies and clinical practice can be complementary, and assessing data from both sources may allow clinicians, patients, and payers to form more accurate expectations of long-term treatment outcomes.
Persistence to TNFi in clinical practice has been studied, and a recent systematic review and meta-analysis of persistence to biologic therapy in RA identified 98 studies with real-world data on the subject.24 However, the authors of the review identified a paucity of data for SC golimumab, reflecting that only one study with persistence data for SC golimumab was identified.24 Therefore, our aim was to conduct a systematic review of persistence to SC golimumab in clinical practice and contextualize these data with persistence results presented for five years’ follow-ups from long-term OLE studies of SC TNFis in IMRD.
Material and methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see S1 PRISMA checklist).25 A short form protocol is provided in S2 Short Form protocol. Study eligibility criteria for the review were defined in terms of Patients, Interventions, Comparators, Outcomes, and Study (PICOS) approach. PICOS is a structured method for identifying studies that meet predefined eligibility criteria. The predefined criteria used in the study are detailed below:
Patients
Studies on all IMRDs, ie, RA, axial SpA, and PsA, were included in the systematic review. Studies in which data were presented for groups of fewer than 20 subjects receiving golimumab were excluded.
Interventions
Studies on treatment with SC golimumab were included. Studies on biologic treatment from which data on golimumab could not be isolated, and studies on intravenous (IV) golimumab, or where the method of administration could not be ascertained, were excluded. Therefore, studies conducted in countries where IV golimumab is available (such as the US and Canada) were excluded unless it was explicitly stated that the study presented data for SC goli-mumab in isolation.
Comparators
No inclusion or exclusion criteria for comparators were implemented.
Outcomes
Studies reporting 1) the proportion of patients remaining on treatment at given time points; 2) mean or median time to discontinuation over a given time period; or 3) mean or median time until 50% of patients were no longer persistent were included. Studies reporting other outcomes and studies in which persistence data were not presented numerically were excluded.
Study design
Studies reporting original data from clinical practice were included. Literature reviews, editorials, guidelines, and RCTs were excluded.
Only one study on the same (or partially the same) patients from a specific data source was included in the systematic review. In cases where there were two or more studies on the same (or partially the same) patients from the same data source, studies including evaluation of other biologics, in addition to golimumab, were preferentially selected to allow for contextualization of golimumab persistence estimates. In cases where there was still more than one candidate study, the study reporting persistence estimates for the largest number of golimumab patients was included.
Studies were identified by searching PubMed, and Embase and MEDLINE via Ovid up to June 2016. The search string used was the following: golimumab AND (persistence OR adherence OR compliance OR “retention” OR “time to discontinuation” OR “drug survival”). Following a previous systematic review on persistence to biologics,24 the two largest rheumatology conferences worldwide, the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), were searched in the respective electronic databases for relevant abstracts. In addition, the largest pharmacoeconomic and outcomes research conference, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) conference, was included in the search for relevant abstracts. Owing to limitations in search options, six different searches were run for EULAR and ACR: golimumab AND persistence, golimumab AND drug survival, golimumab AND adherence, golimumab AND compliance, golimumab AND retention, and golimumab AND time to discontinuation. For the ISPOR scientific presentations database, it was not possible to combine search terms, so only the keyword golimumab was searched for.
All studies identified in the search were electronically stored to facilitate reviewing and tracking of included and excluded studies. The study selection was conducted by two independent reviewers on two levels (title/abstract and full text) in sequence. Disagreements between the two reviewers were solved by discussion and consensus on which studies to include or exclude.
A Data Abstraction Table (DAT) was developed, pilot-tested and amended accordingly. The data were extracted by one reviewer and verified by a second reviewer (see S3 Data extraction).
Information extracted for each included study covered the following: 1) bibliographic details (author, publication type, year, title, journal, volume, issue, pages, and abstract); 2) population characteristics (country, year of data collection, number of subjects on golimumab, indication, sex, age, disease duration, prior biologic-exposure status, and proportion of patients treated concomitantly with traditional disease-modifying antirheumatic drugs [DMARDs]); and 3) persistence data (proportion of persistent patients at a reported follow-up time, mean or median persistence, mean or median time to 50% of the cohort being nonpersistent, and results of comparisons to other biologics).
As recommended by the Cochrane collaboration,26 the bias and quality of the included studies were assessed using the Newcastle–Ottawa Scale (NOS) for cohort studies.27 The NOS consists of three domains (selection, comparability, and outcome), and the results of the assessment of individual studies are provided in S4 Bias assessment. Given that no statistical meta-analysis was conducted, systematic bias across studies – such as publication bias – was not conducted.
Results
Results from search of electronic databases and conference proceedings
The searches in PubMed, MEDLINE, and Embase yielded 374 hits. The searches in EULAR, ISPOR, and ACR abstract databases resulted in 129, 140, and 79 included conference proceedings abstracts, respectively. After de-duplication, 376 titles and abstracts were included for title or abstract review. After title or abstract and full text review, data from 17 manuscripts and abstracts were considered. Subsequently, five studies were excluded on the basis that they presented persistence estimates from the same, or partially the same, patients from the same data source as other studies.28–32 Therefore, a total of twelve references were included in the review.20,33–43 Figure 1 provides a description of the flow of information during the selection process. Among the twelve references, all presented proportions of patients on golimumab at reported follow-up(s), and one reported median time to 50% of patients being nonpersistent.33 Five studies compared persistence to golimumab with persistence to other TNFis.20,33,34,36,38
Figure 1.
Selection flow chart for studies identified in the systematic review.
Study and patient characteristics
Of the twelve references, two were manuscripts,20,33 and the rest were abstracts presented at conferences.34–43 Eight studies were conducted in Europe,33,35–39,41,42 three in Japan,20,40,43 and one in Canada.34 The years of data collection ranged from 2009 to 2015. Three studies did not report the time period of data collection.40,42,43
The two most frequently reported follow-up time points were 12 and 24 months with data on 21 cohorts in seven studies33–35,39–41,43 and 26 cohorts in seven studies,33,35–37,39,40,42 respectively. Four studies reported data for several time points,33,35,39,40 while the other eight studies reported data for one time point only.20,34,36–38,41–43 The time periods for follow-up ranged from six months to 36 months.
In total, 4,910 patients with IMRDs on golimumab treatment were included in the twelve studies.20,33–43 Eight studies reported the sex distribution of patients for one or more cohorts.33,35–41 In those cohorts, the proportion of women ranged from 29% to 89%. Half or more of the patients were female in all indications except for axial SpA.35,37,39 Nine studies reported mean or median age at treatment initiation,20,33,35–41 ranging from 40 to 62 years of age across the different studies and cohorts.
Ten studies20,34–37,39–43 presented persistence estimates by indication. All ten studies included at least one RA cohort, whereas PsA and axial SpA specific cohorts were included in three studies each.35,37,42 In total, approximately 51% of patients across the studies were defined as having RA. Disease duration was reported in seven studies20,35–37,39,40,42 and ranged from 2.9 years to 13.2 years across the cohorts in the different studies.
Two studies included biologic-naive patients only,33,41 seven studies reported persistence irrespective of prior biologic-exposure status,20,34,38–40,42,43 and three studies stratified analysis by prior biologic-exposure status.35–37 Concomitant medication with DMARDs was recorded in six studies,20,33,35,39,40,43 with the proportion treated with a concomitant DMARD ranging from 25% to 96% across the different cohorts. Key patient characteristics are displayed in Table 1.
Table 1.
Key patient characteristics
| References | Country | Type | Indication | Diagnosis
|
Number of patients on golimumab | Gender
|
Age
|
Disease duration in years
|
Biologic-naive
|
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Female, n (%) | Mean (SD) | Mean (SD) | n (%) | |||||
| Khalil and Tahami34 2012 | Canada | Admin | RA | 146 (100) | 146 | NR | NR | NR | NR |
| Dalén et al,33 2016 | Sweden | Admin | AS | 205 (27.2) | 754 | 440 (58.4) | 49.8 (15.6) | NR | 440 (100) |
| PsA | 155 (20.6) | ||||||||
| RA | 352 (46.7) | ||||||||
| Other | 44 (5.5) | ||||||||
| Hirano et al,40 2015 | Japan | Register | RA | 111 (100) | 111 | 93 (89.4) | 61.9 | 13.2 | NR (53.2) |
| Sato et al,20 2015 | Japan | Chart review | RA | 77 (100) | 77 | NR | 50.7 (14.4) | 11.0 (9.7) | NR (50.7) |
| Aaltonen et al,38 2016 | Finland | Register | RA | 195 (5.8) | 195 | NR (74a) | 55a,b | NR | NR |
| Saevarsdottir et al,35 2014 | Sweden | Register | RA | 849 (40) | 2,106 | NR (78) | 54b | 2.9–6.9c | NR (48) |
| PsA | 454 (22) | NR (50) | 48b | 6.1–7.0c | NR (46) | ||||
| AS | 303 (14) | NR (29) | 42b | 7.8–15.1c | NR (42) | ||||
| SpA | 242 (12) | NR (55) | 40b | 6.3–13.1c | NR (40) | ||||
| Other | 258 (12) | NR | NR | NR | NR | ||||
| Favalli et al,36 2016 | Italy | Register | RA | 677 (100) | 136 | NR (79.7a) | 54.3 (13.5)a | 8.9 (8.8)a | 85 (62.5) |
| Hayashi et al,43 2016 | Japan | Register | RA | 152 (100) | 152 | NR | NR | NR | 80 (52.6) |
| Manara et al,37 2016 | Italy | Register | RA | 180 (43.9) | 180 | NR (81.6) | 54.6 (13.6) | 9.5 (9.3) | 85 (47.2) |
| PsA | 110 (26.8) | 110 | NR (50.0) | 47.9 (12.8) | 7.9 (6.7) | 47 (42.7) | |||
| AS | 120 (29.3) | 120 | NR (43.3) | 45.2 (12.2) | 9.7 (10.0) | 51 (42.5) | |||
| Mourão et al,41 2016 | Portugal | Register | RA | 109 (100) | 109 | NR (86.3) | 55.5 (13.2) | 10 | 109 (100) |
| Rotar and Tomšič,39 2016 | Slovenia | Register | RA | 103 (34.2) | 103 | NR (76) | 59b | 6.8b | NR (79) |
| PsA | 76 (25.2) | 76 | NR (51) | 44b | 8.0b | NR (70) | |||
| AS | 122 (40.5) | 122 | NR (37) | 49b | 4.8b | NR (67) | |||
| Santo et al,42 2016 | Italy | Cohort | RA | 89 (21.4) | 89 | NR | NR | 8.1 (8) | 171 (41) |
| PsA | 180 (43.3) | 180 | NR | NR | 6.9 (6) | ||||
| Axial SpA | 147 (35.3) | 147 | NR | NR | 7.4 (7) |
Notes:
Multicenter prospective observational study;
Median;
Range across indications.
Abbreviations: AS, ankylosing spondylitis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; Axial SpA, axial spondyloarthritis; SD, standard deviation; NR, not reported.
Persistence to golimumab compared with other TNFis
Five studies compared persistence to golimumab with persistence to other biologic agents, two of which addressed confounding factors at baseline,33,38 and three of which reported results of nonparametric tests.20,34,36 The findings from the studies are summarized in Figure 2.
Figure 2.
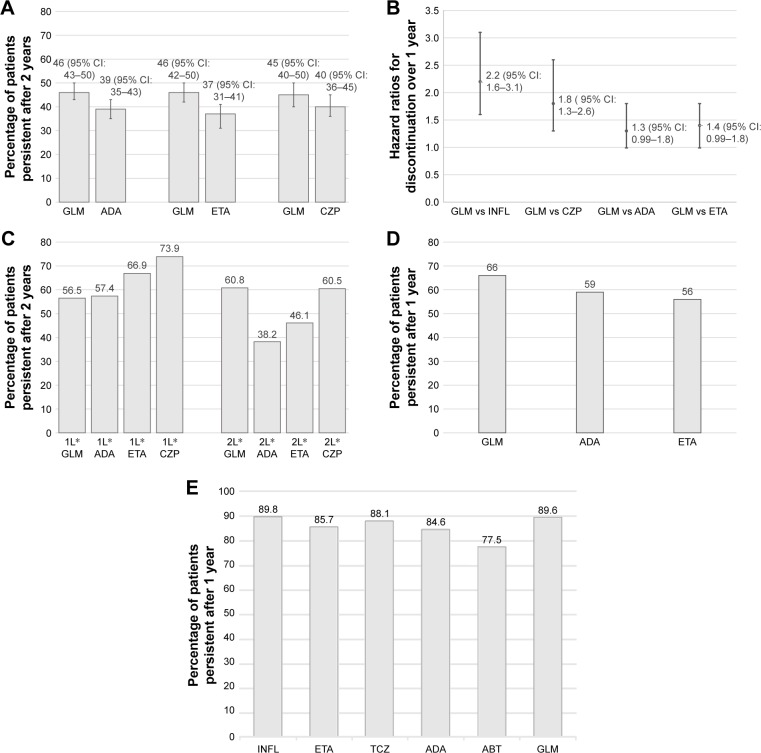
Selection flow chart for studies identified in the systematic review.
Notes: (A) Proportion of patients persistent after two years, data from Dalen et al33 from propensity score matching analysis; (B) Hazard ratios for discontinuation at one year vs golimumab, data from Aaltonen et al;38 (C) Proportion of patients persistent after two years stratified by treatment line and agent, data from Favalli et al;36 (D) Proportion of patients persistent after one year, data from Khalil and Tahami;34 (E) Proportion of patients persistent after six months, data from Sato et al.20 *1L denotes first line and 2L denotes second line. In D, persistence rates calculated as 1-discontinuation rates at one year.
Abbreviations: GLM, golimumab; CZP, certolizumab; INFL, infliximab; ADA, adalimumab; ETA, etanercept; ABT, abatacept; TCZ, tocilizumab.
Dalen et al33 found that golimumab had significantly higher persistence than etanercept and adalimumab at the 5% level, and a trend (not significant) to higher persistence compared with certolizumab pegol over a maximum of approximately 42 months (Figure 2A). The analyses were conducted using propensity score matching, thereby addressing differences between the groups observed at start of the treatment.
Aaltonen et al38 found that golimumab had significantly higher persistence than certolizumab pegol and infliximab at the 5% level, and trends (nonsignificant) to higher persistence compared with adalimumab and etanercept (Figure 2B). The analyses were conducted using semiparametric time-to-event regression models adjusted for confounders.
In first line patients, Favalli et al36 did not observe any significant difference at the 5% level in drug survival between golimumab and adalimumab, etanercept, or certolizumab pegol in biologic-naive patients, although numerically golimumab exhibited the lowest persistence among the biologics. Conversely, in second-line patients, golimumab exhibited significantly higher persistence at the 5% level compared with adalimumab, and showed numerically higher persistence than etanercept and certolizumab pegol over two years (Figure 2C).
Khalil and Tahami34 observed trends toward higher persistence to golimumab compared with adalimumab and etanercept for a period of one year, although the differences were not statistically significant (Figure 2D).
Sato et al20 did not find a significant difference in persistence at six months in patients treated with infliximab, etanercept, tocilizumab, adalimumab, abatacept, or golimumab within one year of launch of the respective agent (P=0.33) (Figure 2E).
Reported persistence to golimumab overall, by prior biologic-exposure status and by indication
The observed persistence rates across the cohorts and follow-up time points ranged from 88% (at 12 months)20 to 40% (at 36 months).33 At six months, one year, two years, and three years, the reported persistence estimates ranged from 63% to 90%,20,33,35,39 47% to 80%,33–35,39–41,43 32% to 77%,20,33,35–37,39,40,42 and 40%,33 respectively.
Three studies presented persistence estimates stratified by prior biologic treatment.35–37 Saevarsdottir et al35 showed that for patients with RA and axial SpA, persistence was significantly higher in biologic-naive patients compared with biologic-experienced patients. A similar trend (not statistically significant) was observed for PsA, but not for SpA. The comparisons were conducted using nonparametric methods and did not adjust for differences between biologic-naive and biologic-experienced patients. Favalli et al36 noted a significant difference in first- and second-line persistence for all patients on TNFis. However, for golimumab, the proportion of biologic-experienced patients persistent after two years was estimated at 61% compared with 57% for biologic-naive patients. Manara et al37 did not find a statistically significant difference in persistence between biologic-naive and biologic-experienced patients treated with golimumab for axial SpA (P=0.127), PsA (P=0.333), or RA (P=0.724). Similarly, Rotar and Tomšič 39 did not find a statistically significant difference in persistence between biologic-naive and biologic-experienced patients treated with golimumab, although no numeric estimates were presented in the study.
Four studies stratified persistence estimates with golimumab by indication.35,37,39,42 Three of those studies did not conduct formal comparisons of persistence across the indications. However, among the 14 out of 17 possible combinations of measurement time-points and treatment lines, patients with axial SpA exhibited higher persistence than patients with RA or PsA. The fourth study, Santo et al,29 found that in a mix of biologic-naive and biologic-experienced patients, patients with axial SpA had numerically higher crude persistence than patients with RA and PsA, although the difference was not statistically significant when tested in a semiparametric model controlling for confounders.
Real-world persistence rates with golimumab in the context of long-term persistence from RCTs
Long-term persistence data from clinical trials are presented in Table 2.
Table 2.
Proportion of patients on treatment at reported follow-up times (in months) in all study arms
| References | Indication | Line | Proportion on treatment at × months
|
||||
|---|---|---|---|---|---|---|---|
| 6 | 12 | 24 | 36 | 60 | |||
| Real-world studies identified in systematic review | |||||||
| Khalil and Tahami,34 2012 | RA | 1st | 76% | ||||
| Dalén et al,33 2016 | Mix | 1st | 76% | 58% | 46% | 40% | |
| Hirano et al,40 2015 | RA | Mix | 79% | 77% | |||
| Sato et al,20 2015 | RA | Mix | 90% | ||||
| Aaltonen et al,38 2016 | RA | Mix | 80% | ||||
| Saevarsdottir et al,35 2014 | RA | 1st | 81% | 67% | 63% | 60% | |
| PsA | 1st | 85% | 73% | 63% | 61% | ||
| AS | 1st | 85% | 80% | 68% | 64% | ||
| SpA | 1st | 81% | 75% | 67% | 67% | ||
| RA | 2nd | 77% | 63% | 56% | 54% | ||
| PsA | 2nd | 77% | 62% | 57% | 53% | ||
| AS | 2nd | 80% | 70% | 63% | 60% | ||
| SpA | 2nd | 78% | 62% | 54% | 52% | ||
| RA | 3rd+ | 65% | 47% | 40% | 32% | ||
| PsA | 3rd+ | 72% | 54% | 45% | 45% | ||
| AS | 3rd+ | 71% | 52% | 47% | 47% | ||
| SpA | 3rd+ | 63% | 60% | 54% | 60% | ||
| Favalli et al,36 2016 | RA | 1st | 57% | ||||
| RA | 2nd | 61% | |||||
| Hayashi et al,43 2016 | RA | Mix | 72% | ||||
| Manara et al,37 2016 | RA | 1st | 49% | ||||
| RA | 2nd | 53% | |||||
| PsA | 1st | 66% | |||||
| PsA | 2nd | 54% | |||||
| AS | 1st | 57% | |||||
| AS | 2nd | 76% | |||||
| Mourão et al,41 2016 | RA | 1st | 75% | ||||
| Rotar and Tomšič,39 2016 | RA | Mix | 82% | 65% | 56% | ||
| PsA | Mix | 83% | 75% | 57% | |||
| AS | Mix | 91% | 83% | 73% | |||
| Santo et al,42 2016 | Axial SpA | Mix | 75% | ||||
| RA | Mix | 63% | |||||
| PsA | Mix | 64% | |||||
| Randomized controlled clinical trials with five years follow-up data | |||||||
| Keystone et al,13 2016, GO-FORWARD study on golimumab | RA | 1st | 71% | ||||
| Smolen et al,44 2015, GO-AFTER study on golimumab | RA | 2nd | 40% | ||||
| Emery et al,45 2016, GO-BEFORE study on golimumab | RA | 1st | 66% | ||||
| Klareskog et al,46 2011, study on etanercept | RA | 1st | 56% | ||||
| Keystone et al,48 2014, RAPID 1 study on certolizumab pegol | RA | 1st | 55% | ||||
| Keystone et al,47 2011, DE019 study on adalimumab | RA | 1st | 55% | ||||
| Deodhar et al,14 2015, GO-RAISE study on golimumab | AS | 1st | 72% | ||||
| Sieper et al,49 2012, ATLAS study on adalimumab | AS | 1st | 65% | ||||
| Kavanaugh et al,15 2014, GO-REVEAL study on golimumab | PsA | 1st | 69% | ||||
Abbreviations: AS, ankylosing spondylitis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; Axial SpA, axial spondyloarthritis.
In RA, three OLE studies13,44,45 presented data on per sistence to golimumab after 256 weeks (approximately five years). In biologic-naive patients, GO-BEFORE enrolled methotrexate-naive patients;45 and GO-FORWARD enrolled patients with inadequate response to methotrexate.13 The proportions of patients who remained in the trials – and consequently were persistent to golimumab treatment – after 256 weeks were 65.8% in GO-BEFORE and 70.5% in GO-FORWARD. For etanercept, adalimumab, and certolizumab pegol, the five-year persistence rates from extension studies of RCTs in TNFi-naive RA patients were 56%,46 49%,47 and 55%,48 respectively. The persistence estimate with the longest follow-up (36 months) from clinical practice in biologic-naive RA patients was 60%.35 In TNFi-experienced patients with RA, one OLE study presented data on persistence to golimumab after 256 weeks:44 In the GO-AFTER study, 40% of patients remained persistent to golimumab until study completion. For adalimumab, etanercept, and certolizumab pegol, no RCT has been conducted in this patient population. The corresponding persistence estimates from clinical practice in cohorts containing biologic-experienced RA patients treated with golimumab ranged from 32% to 56% after 36 months.35,39
In axial SpA, one OLE study presented data on persistence to golimumab after 256 weeks:14 In the GO-RAISE study, conducted in AS biologic-naive NonSteroidal Inflammatory Agent (NSAID) inadequate responders, 72% of patients remained persistent to golimumab until study completion. For adalimumab, the OLE of the ATLAS study was conducted in AS biologic-naive NSAID inadequate responders, and 65% remained persistent to adalimumab until the completion of the study after five years.49 For etanercept and certolizumab pegol, five-year data from OLE studies have not been presented, and the longest follow-up presented for etanercept and certolizumab pegol was 3.7 years50 and two years,51 respectively. Persistence estimates from clinical practice in biologic-naive or a mix of biologic-naive and biologic-experienced axial SpA patients treated with golimumab ranged between 60%35 and 73%39 after 36 months.
In PsA, one OLE study presented data on persistence to golimumab after 256 weeks.15 In the GO-REVEAL study, conducted in biologic-naive DMARD inadequate responders, 69% of patients remained persistent to golimumab until study completion. For adalimumab, etanercept, and certolizumab pegol, persistence has not been reported beyond two years.52–54 Persistence rates in clinical practice on biologic-naive or a mix of biologic-naive and biologic-experienced PsA patients ranged between 45% and 61%35 after 36 months.
Discussion
This systematic review reports data on real-world persistence to golimumab in IMRD. The persistence data presented in the studies were heterogeneous with respect to data sources, follow-up, and definition of patient populations. Furthermore, ten of the twelve identified studies were available only as conference abstracts and therefore provided limited information.34–43 These factors render synthesis of the data difficult, although some observations can be made.
The two studies that compared persistence to golimumab with persistence to other TNFis using statistical methods to address confounding factors showed that golimumab had numerically higher persistence compared with other TNFis.33,38 In terms of the statistical significance of the observed differences, Dalen et al33 found that golimumab exhibited statistically significantly higher persistence than adalimumab and etanercept, whereas Aaltonen et al38 found that golimumab showed statistically significantly higher persistence than certolizumab pegol and infliximab. In this context – cognizant of the limitations of comparing long-term persistence rates across RCTs – it may be noted that among the SC TNFis with published five-year extension RCT data, golimumab reported higher five-year persistence in biologic-naive patients than other SC TNFis in RA (70.5% for golimumab, 56.0% for etanercept, 55.3% for certolizumab pegol, and 49.1% for adalimumab) and axial SpA (71.5% for golimumab, 64.1% for adalimumab), whereas no comparable five-year data exist for PsA (Table 2).
The identified studies did not explore the reasons for the observed differences in persistence between golimumab and other TNFis. One potential explanation is the sustained clinical efficacy and safety observed in the long-term extension of clinical trials.13–15,45 Another reason may be the monthly administration frequency;55 this point was also reflected by a retrospective multicenter observational study conducted in Spain, in which it was found that adherence was higher with a monthly administration of SC biologics compared with fortnightly or weekly administration.56 In addition, a study in axial SpA found that combination therapy of TNFis and DMARDs affects drug retention,57 while a study in RA identified the reason for persistence with biologics in RA to be multifactorial, including age, comorbidities, and patient type.58 Moreover, patient satisfaction regarding the administration of SC TNFis plays an important role, although study findings are inconsistent so far: one study found that there was a high degree of patient satisfaction with the golimumab autoinjector,55 while another study identified injection experience as the second most important reason for patients to discontinue treatment.59
Persistence to golimumab varied across studies. For example, at 24 months after treatment initiation, the estimated proportion of patients who remained persistent to golimumab ranged from 40%33 to 68%35. The divergence in persistence rates may partially be explained by systematic differences between studies in terms of diagnoses and prior biologic exposure. Previous studies have shown that persistence may be lower to the second TNFi compared with the first TNFi, a notion that is largely supported by the data identified in this review.60–64 Furthermore, the reported persistence rates for biologic-experienced patients were lower in the golimumab RCT on biologic-experienced patients compared with the studies on biologic-naive patients (Table 2).
This study has a number of limitations. First, the review pertained to golimumab only and therefore provides limited scope for comparisons with other biologics. In this context, it is notable that the search strings were targeted to identify studies including golimumab instead of TNFis in general, thereby risking missing relevant studies. However, the probability of identifying further studies with a broader search string may be small as a recent systematic review on real-world persistence to biologics in RA with a very broad search string identified only one study with data on golimumab,24 and that study was also identified in this review. Furthermore, data presented in this review were heterogeneous, and ten of twelve studies identified were conference abstracts providing limited information, rendering assessment of comparability across studies difficult. Therefore, it may be difficult to compare persistence estimates across studies given observed and unobserved differences in patient populations in terms of sex, age, concomitant DMARD medication, baseline disease activity, and geography-specific reimbursement restrictions and practice patterns. Furthermore, differences in types of data sources and statistical methodology across studies may also affect comparability across studies. In this context, it should be noted that comparisons of persistence data from extensions of RCTs to real-world data are hampered by differences in patient populations and treatment settings.65 In RA, it has been shown that a high proportion of patients in clinical practice would not be eligible for RCTs of biologic treatments.66,67 Furthermore, on average, patients in RCTs have higher disease activity and lower prevalence of comorbidities than patients in clinical practice.66 Importantly, the monitoring in RCTs may also affect patient behavior,65 and therefore potentially increase persistence rates.
The study also has some strengths. In terms of methodology, the study employed a systematic design, which followed the PRISMA guideline;25 EMBASE, Medline, and PubMed were searched without language restrictions; abstracts from the ACR, EULAR, and ISPOR congresses were included in the searches; and eligibility assessment and data extraction were conducted by two independent researchers. In terms of results, the total number of patients treated with golimumab in clinical practice across the included studies was large, almost 5,000. Furthermore, the findings from clinical practice identified in the study were compared and contrasted to data from OLEs of RCTs, providing additional context to the findings.
In terms of further research, new studies on real-world persistence of golimumab are needed. Areas of particular interest include register studies comparing golimumab with other SC TNFi agents stratified by indication and positions in the treatment pathway. Furthermore, studies combining administrative and register data may promote interpretability of the results by facilitating analysis of two measures of persistence in the same patients.
Conclusion
In conclusion, the data reviewed in this study indicate that golimumab may have higher persistence than other TNFis, a notion that is supported by indirect comparisons of persistence data from long-term OLEs of RCTs. Furthermore, the study suggests that persistence may be lower in biologic-experienced compared with biologic-naive patients and higher in axial SpA compared with RA and PsA.
Footnotes
Disclosure
The study was funded by Merck & Co, Inc., Kenilworth, NJ, USA. Ahmed Khalifa and Sumesh Kachroo are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, SA, and hold stock and options. Marinella Govoni is an employee of MSD Italy and holds stocks and restricted stock units of Merck & Co., Inc. Axel Svedbom and Chiara Storck are employees of Mapi. Mapi were paid consultants to Merck & Co., Inc., Kenilworth, NJ, USA, in conjunction with the development of this manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Marcum SB, Callahan LF. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol. 1992;19(12):1885–1894. [PubMed] [Google Scholar]
- 3.Fries JF. Effectiveness and toxicity considerations in outcome directed therapy in rheumatoid arthritis. J Rheumatol Suppl. 1996;44:102–106. [PubMed] [Google Scholar]
- 4.Aletaha D, Smolen JS. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J Rheumatol. 2002;29(8):1631–1638. [PubMed] [Google Scholar]
- 5.Silman AJ, Hochberg MC. Epidemiology of the Rheumatic Diseases. Oxford: Oxford University Press; 2001. [Google Scholar]
- 6.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 8.Drossaers-Bakker KW, de Buck M, van Zeben D, Zwinderman AH, Breedveld FC, Hazes JM. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum. 1999;42(9):1854–1860. doi: 10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65(9):1175–1183. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency Human medicines, 2016. [Accessed March 1, 2016]. Available from: http://www.ema.europa.eu/ema/
- 12.Rossini M, Viapiana O, Orsolini G, et al. Why golimumab in the treatment of psoriatic arthritis, ankylosing spondylitis and rheumatoid arthritis? Reumatismo. 2015;66(4):285–303. doi: 10.4081/reumatismo.2014.799. [DOI] [PubMed] [Google Scholar]
- 13.Keystone EC, Genovese MC, Hall S, et al. Safety and efficacy of subcutaneous golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: Final 5-year results of the GO-FORWARD trial. J Rheumatol. 2016;43(2):298–306. doi: 10.3899/jrheum.150712. [DOI] [PubMed] [Google Scholar]
- 14.Deodhar A, Braun J, Inman RD, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis. 2015;74(4):757–761. doi: 10.1136/annrheumdis-2014-205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study) Ann Rheum Dis. 2014;73(9):1689–1694. doi: 10.1136/annrheumdis-2013-204902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015;67(10):2702–2712. doi: 10.1002/art.39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency . Simponi®: EPAR Product Characteristics. London: European Medicines Agency; 2016. [Google Scholar]
- 18.Food and Drug Administration . Simponi Label Information. London: Food and Drug Administration; 2016. [Google Scholar]
- 19.European Medicines Agency . EPAR summary for the public – Simponi (golimumab) London: European Medicines Agency; 2016. [Google Scholar]
- 20.Sato E, Tanaka E, Ochiai M, et al. Chronological changes in baseline disease activity of patients with rheumatoid arthritis who received biologic DMARDs between 2003 and 2012. Mod Rheumatol. 2015;25(3):350–357. doi: 10.3109/14397595.2014.958274. [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration Drugs, 2016. [Accessed April 1, 2016]. Available from: http://www.fda.gov/default.htm.
- 22.Klareskog L, Gaubitz M, Rodriguez-Valverde V, et al. A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs. Ann Rheum Dis. 2006;65(12):1578–1584. doi: 10.1136/ard.2005.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koncz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther. 2010;10(9):1367–1378. doi: 10.1517/14712598.2010.510508. [DOI] [PubMed] [Google Scholar]
- 24.Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford) 2016;55(3):523–534. doi: 10.1093/rheumatology/kev374. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. The Cochrane Collaboration; 2011. Tools for assessing methodological quality or risk of bias in non-randomized studies. updated March 2011. [Google Scholar]
- 27.Wells G, Shea B, O’Connell D. New Castle-Ottawa Quality Assessment Scale – Cohort Studies, 2012. [Accessed 15 June, 2012]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 28.Saevarsdottir S, Santacatterina M, Stawiarz L, et al. Drug survival in patients receiving golimumab treatment 2010–2013. Results from the Swedish Rheumatology Quality Register. Arthritis Rheum. 2013;(65):S641–S642. [Google Scholar]
- 29.Santo L, Semeraro A, Zuccaro C, et al. Two-years survival of golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis and predictors thereof in real-life settings. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 30.Grosso V, Gorla R, Sarzi-Puttini P, et al. Golimumab therapy retention rates in patients with rheumatoid arthritis and seronegative spondyloarthritis: data from the Italian lorhen registry. Arthritis Rheumatol. 2014;66:S1097–S1098. [Google Scholar]
- 31.Monfared A, Khalil H. Real-life treatment persistence with golimumab (GLM), etanercept (ETA), and adalimumab (ADA) in patients with rheumatoid arthritis in Canada. Pharmacoepidemiol Drug Safety. 2012;21:327. [Google Scholar]
- 32.Giuseppe Di D, Frisell T, Askling J, group A Effectiveness and survival-on-drug of golimumab across rheumatic disease indications in clinical practice: results from the national Swedish register; Paper presented at: EULAR; London. 2016. [Google Scholar]
- 33.Dalén J, Svedbom A, Black CM, et al. Treatment persistence among patients with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int. 2016;36(7):987–995. doi: 10.1007/s00296-016-3423-5. [DOI] [PubMed] [Google Scholar]
- 34.Khalil H, Tahami A. Golimumab drug utilization patterns in Canada-higher retention rate in golimumab treated rheumatoid arthritis patients compared to etanercept and adalimumab. Arthritis Rheum. 2012;64:S218. [Google Scholar]
- 35.Saevarsdottir S, Santacatterina M, Turesson C, Forsblad H, Jacobsson L, Lindblad S. Clinical characteristics and outcome of golimumab treatment differs between bio-naive and patients previously exposed to biologicals. Nationwide results on Rheumatoid Arthritis (RA), Psoriatic Arthritis (PSA), ankylosing spondylitis (AS) and Other Spondylarthritidies (SPA) Arthritis Rheumatol. 2014;66:S705. [Google Scholar]
- 36.Favalli E, Caporali R, Monti S, et al. Two-year retention rate of subcutaneous anti-tumor necrosis factor agents for rheumatoid arthritis: a retrospective analysis of the LORHEN registry; Paper presented at: EULAR; London. 2016. [Google Scholar]
- 37.Manara M, Favalli E, Caporali R, Sarzi Puttini P, Marchesoni A, Sinigaglia L. Two-year retention rate of golimumab for rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: a retrospective analysis of the Italian LORHEN registry; Paper presented at: EULAR; London. 2016. [Google Scholar]
- 38.Aaltonen K, Joensuu J, Pirilä L, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis in Finland; Paper presented at: EULAR; London. p. 2016. [Google Scholar]
- 39.Rotar Ž, Tomšič M, Slovenian Rheumatologists The persistence of golimumab in daily clinical practice for the treatment of rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: analysis of prospectively collected data in the Slovenian National On-line Registry of patients treated with biologics BioRx.si; Poster presented at: ISPOR 21st Annual International Meeting; May, 2016; Washington, DC. [Google Scholar]
- 40.Hirano Y, Hayashi M, Hirabara S, et al. Predictors of effectiveness in golimumab treatment and efficacy of dose-escalation of golimumab in patients with rheumatoid arthritis-a multicenter registry study TBCR. Ann Rheum Dis. 2015;74(Suppl 2):719. [Google Scholar]
- 41.Mourão A, Ribeiro C, Borges J, et al. Real-life effectiveness of golimumab in biologic-naïve rheumatoid arthritis patients – Data from rheuma.pt, a Portuguese registry; Paper presented at: EULAR; London. 2016. [PubMed] [Google Scholar]
- 42.Santo L, Semeraro A, Zuccaro C, et al. Two-years survival of golimumab in 400 patients with rheumatoid arthritis, psoriatic arthrithitis, and axial spondyloarthritis in real-life world; Paper presented at: EULAR; London. 2016. [Google Scholar]
- 43.Hayashi M, Kanamono T, Matsubara H, et al. Drug survival of golimumab on Japanese patients with rheumatoid arthritis is independent of methotrexate and prednisolone concomitance: results from the multicenter biologics registry; Paper presented at: EULAR; London. 2016. [Google Scholar]
- 44.Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14. doi: 10.1186/s13075-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emery P, Fleischmann R, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthrit Care Res. 2016;68(6):744–752. doi: 10.1002/acr.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klareskog L, Gaubitz M, Rodriguez-Valverde V, et al. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(2):238–247. [PubMed] [Google Scholar]
- 47.Keystone EC, Kavanaugh A, Weinblatt ME, Patra K, Pangan AL. Clinical consequences of delayed addition of adalimumab to methotrexate therapy over 5 years in patients with rheumatoid arthritis. J Rheumatol. 2011;38(5):855–862. doi: 10.3899/jrheum.100752. [DOI] [PubMed] [Google Scholar]
- 48.Keystone E, Landewe R, van Vollenhoven R, et al. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis. 2014;73(12):2094–2100. doi: 10.1136/annrheumdis-2013-203695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie F, Pangan AL. Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(5):700–706. doi: 10.1136/annrheumdis-2011-200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis JC, Jr, van der Heijde DM, Braun J, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis. 2008;67(3):346–352. doi: 10.1136/ard.2007.078139. [DOI] [PubMed] [Google Scholar]
- 51.Sieper J, Landewe R, Rudwaleit M, et al. Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol. 2015;67(3):668–677. doi: 10.1002/art.38973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mease PJ, Kivitz AJ, Burch FX, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol. 2006;33(4):712–721. [PubMed] [Google Scholar]
- 53.Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT) Ann Rheum Dis. 2009;68(5):702–709. doi: 10.1136/ard.2008.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mease P, Deodhar A, Fleischmann R, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open. 2015;1(1):e000119. doi: 10.1136/rmdopen-2015-000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulze-Koops H, Giacomelli R, Samborski W, et al. Factors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(2):201–208. [PubMed] [Google Scholar]
- 56.Calvo-Alen J, Monteagudo I, Salvador G, et al. Non-adherence to subcutaneous biological medication in patients with rheumatoid arthritis: a multicentre, non-interventional study. Clin Exp Rheumatol. 2016 Dec 28; Epub. [PubMed] [Google Scholar]
- 57.Nissen MJ, Ciurea A, Bernhard J, et al. The effect of comedication with a conventional synthetic disease-modifying antirheumatic drug on drug retention and clinical effectiveness of anti-tumor necrosis factor therapy in patients with axial spondyloarthritis. Arthritis Rheumatol. 2016;68(9):2141–2150. doi: 10.1002/art.39691. [DOI] [PubMed] [Google Scholar]
- 58.Mahlich J, Sruamsiri R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence. 2016;10:1509–1519. doi: 10.2147/PPA.S110147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121–131. doi: 10.2147/PPA.S70834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatzidionysiou K, Kristensen LE, Eriksson J, Askling J, van Vollenhoven R, Group A. Effectiveness and survival-on-drug of certolizumab pegol in rheumatoid arthritis in clinical practice: results from the national Swedish register. Scand J Rheumatol. 2015;44(6):431–437. doi: 10.3109/03009742.2015.1026840. [DOI] [PubMed] [Google Scholar]
- 61.Fagerli KM, Lie E, van der Heijde D, et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis. 2013;72(11):1840–1844. doi: 10.1136/annrheumdis-2012-203018. [DOI] [PubMed] [Google Scholar]
- 62.Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65(5):1213–1223. doi: 10.1002/art.37876. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Reino J, Carmona L, BIOBADASER Group Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):1–7. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti–tumor necrosis factor α agent to a second anti–tumor necrosis factor α agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi: 10.1002/art.22331. [DOI] [PubMed] [Google Scholar]
- 65.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 66.Sokka T, Pincus T. Eligibility of patients in routine care for major clinical trials of anti-tumor necrosis factor alpha agents in rheumatoid arthritis. Arthritis Rheum. 2003;48(2):313–318. doi: 10.1002/art.10817. [DOI] [PubMed] [Google Scholar]
- 67.Gogus F, Yazici Y, Yazici H. Inclusion criteria as widely used for rheumatoid arthritis clinical trials: patient eligibility in a Turkish cohort. Clin Exp Rheumatol. 2005;23(5):681–684. [PubMed] [Google Scholar]