Abstract
The validation of laboratory paradigms that reliably induce a stress response [including hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) activation], is critical for understanding how children’s stress-response systems support emotional and cognitive function. Early childhood research to date is markedly limited, given the difficulty in establishing paradigms that reliably induce a cortisol response. Furthermore, research to date has not included a control condition or examined concurrent ANS reactivity. We addressed these limitations by characterizing the extent to which a modified matching task stressor paradigm induces HPA and ANS activation, beyond a closely matched control condition. Modifications include an unfamiliar and unfriendly assessor to increase the stressful nature of the task.
Results validate the matching task as a laboratory stressor, with significant differences in HPA and ANS responsivity between conditions. The Stressor group exhibited a cortisol increase post-stressor, while the Control group was stable over time. Children in both conditions exhibited reduced parasympathetic activity to the first-half of the task, but in the second-half, only children in the Stressor condition, who were experiencing exaggerated signals of failure, exhibited further parasympathetic decline. The Stressor condition induced higher sympathetic activity (versus Control) throughout the task, with exaggerated second-half differences. Within the Stressor condition, responsivity was convergent across systems, with greater cortisol reactivity correlated with the magnitude of parasympathetic withdrawal and sympathetic engagement. Future research employing the matching task will facilitate understanding the role of HPA and ANS function in development.
Keywords: Acute Stress, Cortisol, Autonomic Nervous System, Child
Research on children’s acute stress-response has grown markedly, with function of both the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) linked to numerous health and well-being domains. Such work has been facilitated by standardized laboratory paradigms, which are key to establishing how flexible biological engagement supports emotional and cognitive function (Gunnar et al., 2009). A paradigm’s effectiveness in eliciting temporal cortisol increases, consistent with HPA activation, has been considered a benchmark for stressor validation. Utilizing such benchmark measures is important to ensure engagement across systems when examining the interplay between biological markers of stress-reactivity.
Though numerous paradigms induce frustration or anxiety, few reliably elicit mean elevations in cortisol. There is a dearth of well-validated laboratory paradigms for early childhood (~3-6 years), during which children undergo rapid development and important transitions such as school-entry (Blair, 2002). Following a comprehensive review, Gunnar and colleagues (2009) identified only two promising paradigms in this age range.1 One has limited practical utility given an 80-minute implementation time and complex task battery (van Goozen et al., 1998). The second, which employed both success and failure trials during a cognitively-demanding ‘matching task,’ only induced a cortisol response in children exhibiting embarrassment or shame (Lewis & Ramsay, 2002). After modifying this paradigm with blocked reward and repeated failure, Kryski and colleagues (2011) demonstrated a mean cortisol response, when assessed in home. Tolep and Dougherty (2014) modified this in the laboratory using an assessor familiar to the child, but did not elicit a mean cortisol response (though small increases were noted, adjusting for individual differences in highest cortisol measurement 20–50 minutes post-stressor).
Although this matching task paradigm is promising, research to date has not included a control condition to demonstrate that the matching task engages the HPA-axis significantly more than other non-stressful tasks, which is critical given that cognitively-demanding tasks engage the HPA-axis for some children (e.g. Piccolo et al., 2016). Research in adults emphasizes the need for control conditions matched for cognitive and motor demands to understand the effects of psychosocial stress, as opposed to cognitive load or fatigue, on subsequent behavior (Het et al., 2009).
Furthermore, prior matching task research has not characterized ANS responsivity, perhaps due to the relative focus on cortisol within the stressor literature (Kryski et al., 2011; Tolep & Dougherty, 2014; Gunnar et al., 2009). Given that the ANS is critical for the short-term mobilization of the ‘fight or flight’ response as well as arousal regulation, we argue that ANS activation should be on par with HPA activation as a benchmark for laboratory stressors. Here, we used a modified matching task to assess children’s stress-reactivity, as measured by both HPA-axis and ANS (parasympathetic and sympathetic) function, versus a closely controlled non-stressful condition. Prior matching task research conducted in-home has utilized some of the key cortisol-inducing task features (i.e. unpredictability, uncontrollability, social-evaluative threat; Dickerson & Kenny, 2004) through techniques such as blocking the child’s ability to succeed and providing feedback about the child’s failure to earn a preferred toy. However, when this task was employed in-lab, it failed to produce a robust cortisol response (Tolep & Dougherty, 2014). Consistent with social-evaluative threat paradigms in older samples (i.e. the Trier Social Stress Task; Kudielka, Hellhammer, Kirschbaum, Harmon-Jones, & Winkielman, 2007), we hypothesized that employing an unfamiliar, unfriendly assessor who used non-reinforcing language and stern, flat affect would be essential to inducing a cortisol response signal over and above the HPA-activating demands of entering a novel lab environment. We also modified the task by providing repeated feedback, throughout the task, about the child’s failure to meet task demands to constrain individual differences in children’s perceptions about their own performance. Results lend insight into both HPA-axis and ANS responsivity to a laboratory stressor in early childhood and will facilitate future examinations of acute stress effects on behavior.
Methods
Participants and Procedure
Eighty-four children and their mothers volunteered to participate through community recruitment. Participants were randomly assigned to a Control (N = 26, 14 female) or Stressor condition (N = 58, 33 female), with more children in the Stressor condition to permit examination of within-condition individual differences. Participants (age 4.20 – 6.71 years, M = 5.38, SD =.65) represented a wide range of household incomes (median = $25,000-$29,999; range <$4,999 - $100,000+) and maternal education (median = some college or associate’s degree; range < high school - graduate or professional degree), with no significant sociodemographic (age, sex, household income, maternal education) differences between conditions (all ps > .05).
Participants completed one 2-hour laboratory visit (start times 9am – 3pm; with children awakening a least 1 hour prior and not eating for 1 hour prior). Visits included mother and child watching a 5-minute peaceful ocean video to assess baseline ANS (45 minutes post lab-entry; Piferi et al., 2000). The mother then left the room, and children completed one cognitive task (a Go/NoGo Task, not reported here) followed by the matching task (60 minutes post-lab-entry) and additional assessments (Go/NoGo Task, ocean video, mother-child interaction task; not reported here). Saliva samples were collected following consent and immediately, 20, 40, and 50 minutes post-matching task. Notably, with the exception of the matching task, all laboratory procedures were identical regarding assessments and children’s interactions with their mothers and assessors.
Matching task
In both Stressor and Control conditions, children played a game in which they were instructed to match colored stickers to transportation types on a worksheet with 30 squares using a key. Children performed this task on three consecutive two-minute trials. (Two minutes is insufficient for children of this age to complete the worksheet). Thus, cognitive and motor demands were equated between conditions.
Control
This condition was facilitated by a friendly, familiar assessor. After two minutes, the assessor told the children it was time to work on the next worksheet. There was no mention of prizes, winning, or losing.
Stressor
The stressor was based on a previous study (Kryski et al., 2011). Children picked a desired prize to win for successfully completing a worksheet. The assessor operated a stoplight that was set to green (90 seconds), to yellow (30 seconds), and finally to red, accompanied with a loud beep, signaling the end of a trial. Modifications included use of an unfamiliar assessor who used stern, flat affect (non-smiling, non-encouraging) and negative feedback twice each trial (e.g. you’re not going fast enough). After three failures, children were told they failed to earn their prize. Next, the assessor left the room and a friendly, familiar assessor returned. Children received the desired prize at the end of the visit (60 minutes post-stressor).
Cortisol
Salivettes were used to collect saliva (Sarstedt, Inc., Newton, NC), with samples frozen (-20°C) and sent to University of Trier to be assayed in duplicate [coefficients of variance for inter-assay (7.1-9.0%) and intra-assay (4.0-6.7%)]. Time-from-awakening was calculated as the difference between children’s first cortisol sample and the time they awoke (M = 5.28 hours; SD = 2.91; Range 1.17 – 10.00 hours). Ten participants (4 Control, 6 Stressor) were excluded due to refusal (N=5), eating/drinking < 60 mins prior to lab visit (N=4), and oral infection (N=1). Extreme values (> 3SDs condition mean; N=10 (of 370 total) were winsorized.
Autonomic Physiology
An 11-electrode configuration was used for assessment of respiratory sinus arrhythmia [RSA; parasympathetic activity (PNS)] and pre-ejection period [PEP; sympathetic activity (SNS)]. Three electrodes in a lead II arrangement assessed electrocardiogram (ECG). An 8-electrode tetrapolar montage recorded cardiovascular impedance. Data were acquired via Biopac wireless transmitters (Biopac Systems Inc, Goleta, CA). RSA was derived from natural log-transformed values of high frequency (.24-1.04 Hz) ECG spectral power. PEP was calculated from the first-order derivative of impedance as the Q-to-B interval (Berntson et al., 2004).
Data processing was performed using Mindware software (Gahanna, OH). ECG signals were inspected to confirm heart beats in 30 second epochs. PEP processing involved visual inspection to verify Q and B placement. Epochs were averaged across baseline, first-half matching task, and second-half matching task for RSA and PEP. First-half and second-half matching task were examined separately because stress-inducing qualities (i.e. repeated failure) of the matching task were exaggerated in the second-half. Of 84 participants, 4 refused electrodes, with 12 ECG files and 10 additional Z0 files excluded for technical problems (< 50% artifact free for each of baseline, first-half matching task, and second-half matching task). This resulted in 68 participants (47 Stressor; 21 Control) with RSA data and 58 participants (44 Stressor, 14 Control) with PEP data.
Results
Repeated measures ANOVAs assessed the impact of condition (Stressor versus Control) on cortisol and ANS reactivity, controlling for age and sex. Paired samples t-tests characterized reactivity within each condition. Descriptives for biological measures across conditions are presented in Table 1, with patterns demonstrated in Figure 1.
Table 1.
Descriptive Statistics for Biological Measures Across Time.
| Total M(SD) | Stressor M(SD) | Control M(SD) | |
|---|---|---|---|
| Cortisol (nmol/L) | |||
| Lab entry | 2.48 (1.37) | 2.42 (1.11) | 2.64 (1.86) |
| 0 minutes post MT | 1.76 (.99) | 1.72 (1.01) | 1.85 (.95) |
| 20 minutes post MT | 2.22 (1.76) | 2.40 (2.00) | 1.78 (.87) |
| 40 minutes post MT | 2.08 (1.43) | 2.15 (1.53) | 1.93 (1.17) |
| 50 minutes post MT | 1.97 (1.25) | 2.00 (1.31) | 1.90 (1.09) |
| RSA (power) | |||
| Baseline | 6.86 (1.17) | 6.63 (1.12) | 7.36 (1.15) |
| MT first-half | 6.06 (1.09) | 5.92 (1.08) | 6.40 (1.07) |
| MT second-half | 5.83 (1.25) | 5.57 (1.20) | 6.43 (1.20) |
| PEP (ms) | |||
| Baseline | 90.93 (9.52) | 90.65 (10.12) | 91.85 (7.59) |
| MT first-half | 91.58 (10.51) | 90.25 (10.60) | 95.78 (9.38) |
| MT second-half | 89.28 (10.67) | 87.48 (10.41) | 94.95 (9.75) |
Note: nmol/L = nanomole per liter; ms = milliseconds;
(47 Stressor; 21 Control) with RSA data and 58 participants (44 Stressor, 14 Control)
Figure 1. Effects of Acute Stress on Biological Measures Across Time.
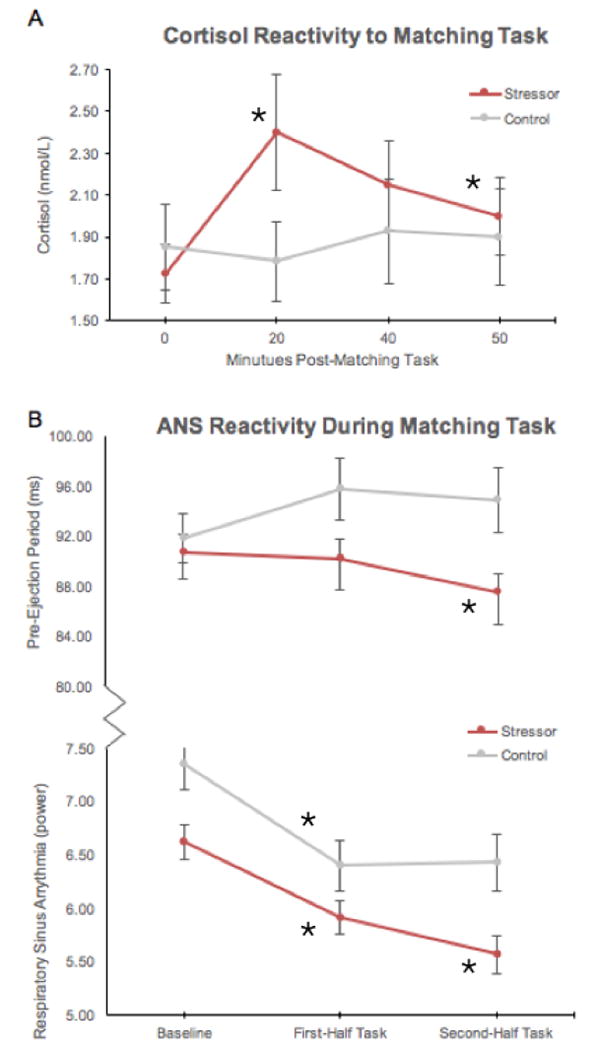
Line graphs illustrating changes in Cortisol, PEP, and RSA for the Stressor and Control groups. A) Stressor group showed a significant rise in Cortisol from 0 to 20 minutes, and significant recovery at 50 minutes relative to 20 minutes. B) Stressor group showed a significant shortening of PEP in time-half of the matching task. C) Both groups showed reduction in RSA from baseline to first-half of the matching task, but only the Stressor group showed a further decrease in RSA during the second-half of the matching task. nmol/L = nanomole per liter; ms = milliseconds.
Cortisol reactivity was characterized based on time points immediately following the matching task, controlling for lab-entry cortisol, time-from-wakening, and total time-in-lab. Analyses indicated a significant main effect of time-from-wakening [F(1,67) = 12.60, p =.001] and a significant time×condition interaction [F(2.32,201) = 3.13, p =.04; Greenhouse-Geisser corrected], with contrasts identifying different quadratic [F(1,67) = 5.21, p =.03] and cubic [F(1,67) = 5.00, p =.03] trends between groups. Within-group paired sample t-tests indicated that the Stressor group experienced a cortisol rise from 0 to 20 minutes post-stressor [t(51) = -3.26, p = .002], and decrease from 20 to 50 minutes post-stressor [t(51) = 2.30, p =.03]; the Control group did not (all ps > .05).
RSA analyses identified a main effect of condition on RSA [F(1,64) = 6.20, p = .02], reflecting higher RSA overall in the Control group (Table 1; Figure 2B), and a non-significant time×condition interaction [F(1.73, 128) = 2.76, p = .08; Greenhouse-Geisser corrected], with contrasts identifying a significantly different quadratic trend between groups [F(1, 64) = 7.34, p = .009]. Within-group paired sample t-tests indicated significant RSA withdrawal between baseline and first-half matching task for Stressor [t(46) = 7.18, p <.001] and Control conditions [t(20) = 8.26, p <.001], but only significant withdrawal from first-half to second-half matching task RSA for the Stressor condition [t(46) = 4.22, p <.001].
PEP analyses indicated no significant main effects and a significant time×condition interaction [F(1.235, 108) = 4.07, p =.04; Greenhouse-Geisser corrected], with contrasts identifying a different linear trend between groups [F(1, 54) = 5.40, p =.02]. Within-group paired sample t-tests indicated no significant change in PEP from baseline for the Stressor or Control conditions. First-half and second-half matching task paired sample t-tests indicated significant shortening of PEP for the Stressor condition [t(43) = 5.00, p <.001].
Cortisol, PEP, and RSA reactivity were calculated in the Stressor condition by subtracting baseline from group-level peak reactivity (i.e., highest cortisol, 20 minutes post-stressor; shortest PEP and lowest RSA, second-half matching task). Correlations were conducted to examine coherence in activation across systems. Higher cortisol was associated with shorter PEP [r(41) = -.45, p = .003] and lower RSA [r(44) = -.31, p =.04]. Shorter PEP was associated with lower RSA [r(39) = .40, p =.009].
Effect size calculations were also conducted at peak reactivity time points for both between-group independent samples t-test and within-stressor repeated measures t-test analyses to facilitate power analyses for future research. For cortisol, the between-group effect size from 0 to 20 minutes post-stressor was large (d = .80), while the within-stressor effect size was moderate (d = .57). For ANS reactivity, the between-groups effect size of second-half matching task reactivity from baseline for RSA was small (d = .16), with a large between-groups effect size for PEP reactivity (d = .72). The within-group stressor RSA reactivity effect was very large (d =1.42) and for PEP reactivity was low to moderate (d = .35).
Discussion
Results validate the matching task paradigm as a laboratory stressor that induces both HPA-axis and ANS responses in young children relative to a closely-matched control condition. By characterizing both HPA-axis and ANS activity, we suggest that this paradigm may be used to elucidate the symphonic engagement of multiple biological systems involved in stress-reactivity.
Cortisol results highlight different reactivity patterns across conditions. The Stressor condition produced a steep rise ~20 minutes post-matching task followed by recovery ~50 minutes post-matching task, similar to previous research conducted in-home using the matching task, and consistent with the expected response to psychosocial stressors (Kryski et al., 2011; Gunnar et al., 2009). The Control condition resulted in minimal post-matching task cortisol change. Given the identical laboratory protocols across conditions, with the exception of the matching task, and closely-matched cognitive and motor demands within the matching task, results may be attributed to the stressor’s social-evaluative threat qualities, as opposed to other task demand characteristics.
The cortisol results reported here, using the matching-task paradigm, address previously noted challenges about the difficulty of eliciting in-laboratory cortisol responses in young children (Tolep & Dougherty, 2014). We suggest that amplifying the stressful nature of the task through the use of an unfamiliar and unfriendly assessor, in addition to providing repeated negative feedback, may be important to successfully induce an in-lab cortisol response. In the future, it will be important to determine how much assessor social support and familiarity influence the stress-eliciting potency of the task (Hostinar et al., 2014).
An additional methodological difference in the present study is the hour (versus 30 minutes; Tolep & Dougherty, 2014) of in-lab acclimation time during which children were engaged in non-stressful activities prior to the short stressor and our ‘baseline’ sample, taken 0 minutes post-stressor. We note that a sample 0 minutes post-stressor does not reflect a typical ‘baseline’ in adults, which is often taken at laboratory entry and almost always before stressor onset. However, consistent with other research in children, laboratory entry cortisol samples in the present sample were high and are theorized to reflect the anticipatory excitement (or anxiety) of entering the laboratory environment and, thus, do not represent an appropriate baseline (Gunnar & Talge, 2008; Tolep & Dougherty, 2014). Instead, we determined the cortisol measurement 0 minutes post-stressor to be the most appropriate baseline given both the time course of the salivary cortisol response (i.e. peaking ~20 minutes post stressor) and our inclusion of a control condition, which allowed us to demonstrate nearly identical cortisol values between conditions at this ‘baseline’ time point. For future acute stress research, we highlight the utility of including a control condition with which to compare the cortisol trajectories of stressor conditions. We also suggest that an hour of in-lab acclimation time may be useful for obtaining a pre-stressor baseline for cortisol and would advocate for the inclusion of an additional cortisol sample immediately prior to the stressor, more consistent with adult and older adolescent research, which was not collected in the present study.
ANS results reveal the effectiveness of the Stressor condition in activating the ANS, relative to Control. Regarding RSA, for the first-half of the matching task, children in both conditions lifted the vagal brake (lower RSA) to support the effort of task engagement. By the second-half, children in the Stressor condition, who were now experiencing exaggerated signals of failure, further lifted the vagal brake, resulting in even lower RSA, and showed an increase in SNS activity (shortening of PEP), while those in the Control condition remained stable. This observed diminution of RSA and PEP with acute stress exposure is consistent with what has been reported repeatedly in adults (Cacioppo et al., 1998).
Results are, notably, convergent across systems, suggesting that the matching task is appropriate for studying stress-reactivity of both the ANS and HPA-axis. Within the Stressor condition, higher cortisol peaks were linked to greater RSA withdrawal and PEP shortening during the matching task second-half. Thus, the degree to which anticipation of threat increased HPA-axis output was associated with the degree of PNS deactivation and SNS engagement. This characterization of stress-reactivity across systems is highly consistent with the Research Domain Criteria (RDoC; Insel, 2014) initiative to identify and validate transdiagnostic markers of mental health risk and thus makes an important contribution to the field through validation of an RDoC-consistent measurement of stress-reactivity.
Limitations include a relatively small Control condition sample size. However, the statistical robustness of results (with effect sizes across systems generally in the medium range) suggests that the sample was sufficient for identifying stress-reactivity effects. Obtaining a cortisol sample immediately prior to the matching task would have been ideal as children likely experienced anticipatory stress, reflected by high lab-entry cortisol (Alink et al., 2008). Despite this, the absence of group differences immediately following the matching task suggests stress-reactivity results are not compromised.
Results indicate the matching task is both feasible and robust for assessing reactivity of the HPA-axis and ANS to a laboratory stressor in young children. The inclusion of a control condition demonstrates that engagement of both the HPA-axis and ANS is due to the paradigm’s social-evaluative threat qualities. Replication of this task will enable its use as a reliable assessment of stress-reactivity in young children, with significant implications for delineating the role of acute stress-response systems in development.
Highlights.
Research provides validation of a matching-task laboratory stressor for inducing ANS and HPA reactivity in young children
Stressor (vs. control) children exhibit greater cortisol reactivity, parasympathetic withdrawal, and sympathetic engagement
Stressor effects are exaggerated in second half of the task, in which children experience exaggerated signs of failure
Reactivity of children’s biological systems (HPA, ANS) is correlated, suggesting convergent reactivity to the stressor
Future research employing this paradigm may inform understanding of the role of HPA and ANS reactivity in development
Highlights.
Research provides validation of a laboratory stressor for inducing for autonomic and endocrine reactivity in young children
Novel investigation of concurrent hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) acute-stress reactivity in early childhood
Stressor-exposed children exhibit greater cortisol reactivity, parasympathetic withdrawal, and sympathetic engagement, compared to a Control condition
Biological systems exhibit convergent reactivity with higher cortisol peaks correlated with the magnitude of parasympathetic withdrawal and sympathetic engagement during the matching-task second-half.
Future matching-task investigations may facilitate understanding the importance of acute stress-responsivity to emotional and cognitive development.
Acknowledgments
Leslie E. Roos received support from HHS-2014- ACF-ACYF- CA-0803. Philip A. Fisher received support from HHS-2014- ACF-ACYF- CA-0803 and NIH grants R01 HD075716 and P50 DA035763.
Footnotes
Since this review, one paradigm was developed for 5-6 year old children that induced a cortisol response, but research did not include ANS measures or a control condition (de Weerth et al., 2013)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Blair C. School readiness: Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. American psychologist. 2002;57(2):111. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesisa. Annals of the New York Academy of Sciences. 1998;840(1):664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- De Weerth C, Zijlmans MAC, Mack S, Beijers R. Cortisol reactions to a social evaluative paradigm in 5-and 6-year-old children. Stress. 2013;16(1):65–72. doi: 10.3109/10253890.2012.684112. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York, NY, US: Cambridge University Press; 2008. pp. 343–364. [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 2009;34(7):1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. American Journal of Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, Hayden EP. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36(8):1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, Harmon-Jones E, Winkielman P. Ten years of research with the Trier Social Stress Test—revisited. Social neuroscience: Integrating biological and psychological explanations of social behavior. 2007:56–83. [Google Scholar]
- Lewis M, Ramsay D. Cortisol response to embarrassment and shame. Child development. 2002;73(4):1034–1045. doi: 10.1111/1467-8624.00455. [DOI] [PubMed] [Google Scholar]
- Piccolo LDR, Salles JFD, Falceto OG, Fernandes CL, Grassi-Oliveira R. Can reactivity to stress and family environment explain memory and executive function performance in early and middle childhood? Trends in psychiatry and psychotherapy. 2016;38(2):80–89. doi: 10.1590/2237-6089-2015-0085. [DOI] [PubMed] [Google Scholar]
- Piferi RL, Kline KA, Younger J, Lawler KA. An alternative approach for achieving cardiovascular baseline: viewing an aquatic video. International Journal of Psychophysiology. 2000;37(2):207–217. doi: 10.1016/s0167-8760(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Sherwood, et al. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27 doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Tolep MR, Dougherty LR. The conundrum of the laboratory: challenges of assessing preschool-age children’s salivary cortisol reactivity. Journal of Psychopathology and Behavioral Assessment. 2014;36(3):350–357. [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]


