Bacterial lipopolysaccharide activates Toll-like receptor 4 (TLR4) and triggers proinflammatory reactions of macrophages. TLR4 signaling is negatively regulated by Lyn tyrosine kinase, provided the kinase accumulates in membrane rafts as a result of palmitoylation, the catalytic activity, and SH2- and SH3-mediated intermolecular interactions.
Abstract
Lipopolysaccharide (LPS) is the component of Gram-negative bacteria that activates Toll-like receptor 4 (TLR4) to trigger proinflammatory responses. We examined the involvement of Lyn tyrosine kinase in TLR4 signaling of macrophages, distinguishing its catalytic activity and intermolecular interactions. For this, a series of Lyn-GFP constructs bearing point mutations in particular domains of Lyn were overexpressed in RAW264 macrophage-like cells or murine peritoneal macrophages, and their influence on LPS-induced responses was analyzed. Overproduction of wild-type or constitutively active Lyn inhibited production of TNF-α and CCL5/RANTES cytokines and down-regulated the activity of NFκB and IRF3 transcription factors in RAW264 cells. The negative influence of Lyn was nullified by point mutations of Lyn catalytic domain or Src homology 2 (SH2) or SH3 domains or of the cysteine residue that undergoes LPS-induced palmitoylation. Depending on the cell type, overproduction of those mutant forms of Lyn could even up-regulate LPS-induced responses, and this effect was reproduced by silencing of endogenous Lyn expression. Simultaneously, the Lyn mutations blocked its LPS-induced accumulation in the raft fraction of RAW264 cells. These data indicate that palmitoylation, SH2- and SH3-mediated intermolecular interactions, and the catalytic activity of Lyn are required for its accumulation in rafts, thereby determining the negative regulation of TLR4 signaling.
INTRODUCTION
Pattern recognition receptors recognize evolutionarily conserved molecules of pathogens and initiate immune responses. A major group of those receptors is made up of Toll-like receptors (TLRs), among which TLR4 is activated by lipopolysaccharide (LPS, endotoxin), the main component of the outer membrane of Gram-negative bacteria (Poltorak et al., 1998). On activation, TLR4 triggers proinflammatory reactions directed at eradication of the bacteria; however, excessive responses to LPS can lead to a systemic inflammatory condition called sepsis (Seymour et al., 2016). The activation of TLR4 is preceded by a series of events initiated by recognition of LPS aggregates in the blood serum by LPS-binding protein followed by the binding of LPS monomers by CD14, a glycosylphosphatidylinositol (GPI)-linked protein expressed at a high level in the plasma membrane of monocytes, macrophages, and dendritic cells. CD14, in turn, transfers the LPS to MD-2 protein, which is associated with the extracellular fragment of TLR4 (Da Silva Correia et al., 2001; Gioannini et al., 2005; Park et al., 2009). Once activated, TLR4 recruits TIRAP and MyD88 adaptor proteins to the intracellular TIR domain, initiating the assembly of a signaling complex named myddosome (Horng et al., 2002; Kagan and Medzhitov, 2006; Motshwene et al., 2009). The triggered signaling cascade leads to the activation of NFκB transcription factor and mitogen-activated protein kinases (MAPKs), which control the expression of genes encoding proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α; Kawai and Akira, 2011). The LPS-activated TLR4 also undergoes CD14-dependent internalization and, when in endosomes, recruits the second pair of adaptor proteins, TRAM and TRIF (Husebye et al., 2006; Kagan et al., 2008; Zanoni et al., 2011). The following signaling cascade leads to the activation of IRF3/7 transcription factors and production of type I interferons, expression of interferon-dependent genes, and late-phase activation of NFκB and MAPKs (Kawai and Akira, 2011).
Although the TLR4 signaling relies on cascades of protein serine/threonine phosphorylation and polyubiquitination, it also requires the activity of several tyrosine kinases (Stefanova et al., 1993; Horwood et al., 2003; Medvedev et al., 2007). Among those, kinases of the Src family have been identified as key players in LPS-induced signaling, based on disturbances of this signaling and the reduction of cytokine production after inhibition of these kinases (Stefanova et al., 1993; Medvedev et al., 2007; Smolinska et al., 2008) and results of recent studies using gene-knockout mice, small interfering RNA (siRNA)–mediated gene silencing, or adenoviral expression of distinct enzymes of the Src family (Keck et al., 2010; Smolinska et al., 2011; Avila et al., 2012). Six members of the Src family—Src, Lyn, Hck, Fgr, Fyn, and Yes—are expressed in macrophages (Smolinska et al., 2008), and LPS has been found to stimulate most of these kinases in macrophages and other cells (Stefanova et al., 1993; Smolinska et al., 2008, 2011; Ko et al., 2015). Of note, the activated Lyn kinase coimmunoprecipitated with CD14 (Stefanova et al., 1993), which probably reflected joint accumulation of CD14 and palmitoylated and miristoylated kinases of the Src family in plasma membrane nanodomains called rafts (Lingwood and Simons, 2010). Rafts are envisioned as sites of TLR4 activation due to accommodation of the activated receptor, CD14, and several other proteins and lipids involved in LPS-induced signaling (Plociennikowska et al., 2015a). However, to the best of our knowledge, the importance of the raft localization of Lyn or any other kinase of this family for TLR4-triggered signaling has not been addressed.
All kinases of the Src family have a similar organization and comprise an N-terminal SH4 domain that is myristoylated and most often also palmitoylated, guiding the kinases to rafts. They also contain SH3 and SH2 domains, a proline-rich region, a catalytic SH1 domain, and a short C-terminal tail crucial for controlling the conformation and hence enzymatic activity of the kinases. Depending on the phosphorylation status of a C-terminal tyrosine residue, the kinases adopt either a “closed,” kinase-inactive or an “open,” active conformation. In the latter, the SH2 and SH3 domains are exposed for binding of, respectively, phosphotyrosine-containing and proline-rich motifs of other proteins, and therefore possible scaffolding functions of Lyn kinase should be taken into consideration (Ingley, 2008, 2012).
Fyn and Hck kinases can phosphorylate TLR4 and/or up-regulate its distinct downstream effectors (English et al., 1997; Smolinska et al., 2011; Ko et al., 2015). Among the Src family members, Lyn kinase is unique in being able to both positively and negatively affect signaling pathways of a distinct receptor, as found for Fcε receptor I and B-cell receptor (BCR), thus preventing the deleterious consequences of receptor hyperactivation (Harder et al., 2001; Xu et al., 2005, 2012; Xiao et al., 2005). In accord with this, macrophages isolated from Lyn-/- mice and stimulated with the rough chemotype of LPS (LPS devoid of its polysaccharide moiety) produced more TNF-α, interleukin-6 (IL-6), and type I interferons than their wild-type counterparts, and the activity of phosphatidylinositol 3-kinase I (PI3-kinase) associated with Lyn was found to underlie this suppressory effect (Keck et al., 2010). On the other hand, Lyn kinase has been shown to affect positively TLR4-triggered signaling. The kinase could phosphorylate the TIR domain of TLR4 (Medvedev et al., 2007). Moreover, Lyn was found to bind, via its SH2 domain, a scaffolding protein, Themis-2. The Lyn-Themis2-Vav axis up-regulated selectively the phosphorylation of ERK and p38 MAPKs and affected positively TNF-α production (Peirce et al., 2010). In mast cells, Lyn kinase ensured proper dynamics of the association/dissociation of TRAF6/TAK-1 required for NFκB activity and MAPK phosphorylation (Avila et al., 2012). These data indicate that Lyn can affect distinct events of TLR4-induced signaling and suggest both a positive and negative effect of Lyn on this process.
To reveal the mechanisms governing the involvement of Lyn in LPS-induced signaling pathways, we analyzed the contribution of the catalytic activity of Lyn and its intermolecular interactions to TLR4 signaling in macrophages. Our results emphasize the role of the SH2 and SH3 domains of Lyn and its palmitoylation in the negative regulation of proinflammatory signaling of TLR4 via their influence on the association of Lyn with rafts.
RESULTS
Silencing of Lyn up-regulates LPS-induced cytokine production
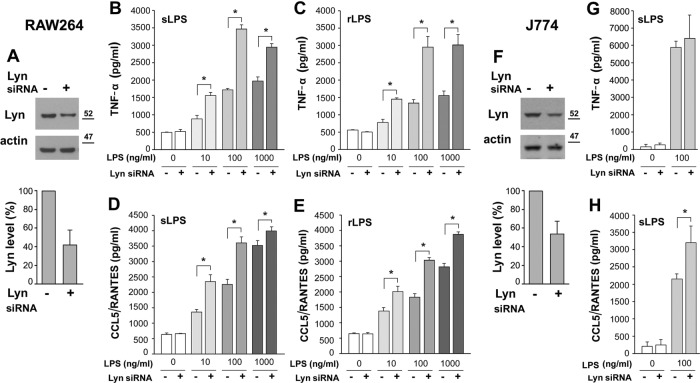
In view of the dual—positive or negative—influence of Lyn on LPS-induced signaling (Keck et al., 2010; Peirce et al., 2010, Avila et al., 2012), we assessed cytokine production in RAW264 macrophage-like cells after silencing the gene encoding Lyn. The amount of Lyn was reduced by ∼60% by a specific siRNA, judging from the level of the Lyn A isoform (Figure 1A). As seen in Figure 1, B–E, the down-regulation of Lyn led to a significant increase of the LPS-induced production of TNF-α and C-C motif chemokine ligand 5 (CCL5, also known as regulated upon activation, normal T-cell expressed and secreted [RANTES]), used respectively to gauge the MyD88- and TRIF-dependent signaling pathways of TLR4 (Bjorkbacka et al., 2004). After Lyn silencing, TNF-α release increased 1.5- to 2-fold and that of CCL5/RANTES increased 1.1- to 1.6-fold in cells stimulated with 10–1000 ng/ml smooth LPS (Figure 1, B and D). Similar effects pointing to negative regulation of LPS-induced cytokine production by Lyn were detected in RAW264 cells stimulated with 10–1000 ng/ml of the rough chemotype of LPS after silencing of the expression of the Lyn gene (Figure 1, C and E). Furthermore, we silenced Lyn in J744 cells of another macrophage-like cell line before stimulating them with 100 ng/ml smooth LPS. Reduction of the Lyn level by nearly 50% did not affect significantly the LPS-induced production of TNF-α in these cells, but it up-regulated production of CCL5/RANTES 1.5-fold, resembling the positive effect of Lyn silencing in RAW264 cells (Figure 1, F–H). The Lyn gene gives rise to Lyn A and B, which differ by the presence of a 21 amino acid–long insert in the unique domain of Lyn A. The functions of the two Lyn isoforms can vary (Alvarez-Errico et al., 2010), and Lyn A was recently reported as a checkpoint controlling inflammatory responses of macrophages (Freedman et al., 2015). Therefore, in our further studies, we focused on this Lyn isoform, using the smooth chemotype of LPS for stimulation of cells.
FIGURE 1:
Lyn gene silencing up-regulates production of cytokines in cells stimulated with LPS. RAW264 (A–E) and J774 (F–H) cells were transfected with Lyn siRNA or scrambled siRNA, and the level of Lyn protein in the cells was analyzed by immunoblotting (A, F, top) and densitometry after normalization against actin content (A, F, bottom). Lyn A is the isoform preferably recognized by the anti-Lyn antibody used. Production of TNF-α (B, C, G) and CCL5/RANTES (D, E, H) in cells stimulated for 4 or 6 h, respectively, with 10–1000 ng/ml LPS of either smooth (B, D, G, H) or rough (C, E) LPS chemotype. Results (mean ± SD) of two or three experiments run in triplicate. *Data significantly different at p ≤ 0.05.
Stimulation of RAW64 cells with LPS increases cellular level and activity of overproduced Lyn–green fluorescent protein
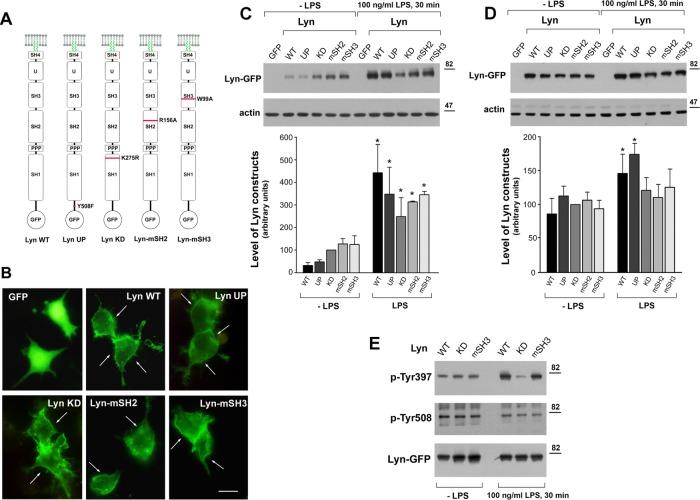
To assess the importance of Lyn A catalytic activity and/or its interactions with other proteins for the LPS-induced signaling, we prepared green fluorescent protein (GFP)–fused constructs of wild-type Lyn A (Lyn WT) and Lyn bearing point mutations in distinct domains (Figure 2A). To obtain a constitutively active kinase, Lyn UP, we substituted the C-terminal tyrosine residue 508 with alanine, and substitution of lysine 275 with arginine in the catalytic domain gave rise to a kinase-dead Lyn, Lyn KD (Yoshida et al., 1999; Harder et al., 2001). The substitutions of arginine 156 with alanine in the SH2 domain in Lyn-mSH2 and of tryptophan 99 with alanine in the SH3 domain in Lyn-mSH3 were analogous to the mutations of corresponding conserved arginine and tryptophan residues in Src kinase, which abolished the SH2-phosphotyrosine and SH3-polyproline interactions, respectively (Shvartsman et al., 2007; Hammond et al., 2009; Ikeda et al., 2009). After expression in RAW264 cells, all of the Lyn-GFP constructs located under the plasma membrane and in the cytoplasm, which contrasted with the cytoplasmic and nuclear localization of GFP (Figure 2B). Immunoblotting analysis revealed that the cellular level of Lyn WT and Lyn UP was significantly lower (by ∼55–70%) than that of Lyn KD, Lyn-mSH2, and Lyn-mSH3 (Figure 2C). Subsequent stimulation of cells with 100 ng/ml LPS for 30 min induced an elevation of all Lyn constructs. However, whereas the amounts of Lyn KD, Lyn-mSH2, and Lyn-mSH3 increased by up to 2.5- to 3.0-fold, Lyn WT and Lyn UP were up-regulated as much as 13- and 7-fold from their low basic levels (Figure 2C). We found that a 2-h preincubation of cells in medium containing 2% fetal bovine serum (FBS) made it possible to increase and equalize the amounts of all Lyn constructs in cells before their stimulation with LPS (Figure 2D), and so we performed further studies in these conditions. When the cells were stimulated with LPS, increases of Lyn WT and Lyn UP level were again observed (Figure 2D). LPS also induced activation of Lyn WT and Lyn-mSH3, as revealed by intense phosphorylation of tyrosine 397, located in the catalytic center of Lyn (Figure 2E), indicating that the enzyme activation is an event of TLR4 signaling. Simultaneous dephosphorylation of the C-terminal tyrosine 508 was also detectable but less pronounced (Figure 2E). As expected, no changes in the phosphorylation level of Lyn KD were detected in any conditions (Figure 2E). In summary, the data indicate that various forms of Lyn-GFP can be reproducibly overexpressed in RAW264 cells, allowing studies on their involvement in LPS-induced signaling.
FIGURE 2:
LPS up-regulates cellular level of Lyn expressed in RAW264 cells. (A) Lyn-GFP constructs studied, with sites of mutations indicated. (B) Cellular distribution of indicated Lyn-GFP constructs. Arrows indicate submembraneous Lyn. Bar, 10 μm. (C, D) Lyn protein level in transfected RAW264 cells, as analyzed by immunoblotting and densitometry. Cells were cultured in 10% FBS (C) or shifted for 2 h to 2% FBS (D) before a 30-min stimulation with 100 ng/ml LPS. The level of Lyn variants in cell lysates was examined by immunoblotting with anti-Lyn antibody, normalized against actin content, and expressed in histograms relative to that of Lyn KD in unstimulated cells. Results are mean ± SD of three or four experiments. *Significantly different from unstimulated cells at p ≤ 0.05. (E) LPS-induced activation of Lyn revealed by immunoprecipitation of Lyn-GFP constructs and analysis of immunoprecipitates with antibodies directed against phosphotyrosine 397 (p-Tyr307) or phosphotyrosine 508 of Lyn (p-Tyr508). Efficiency of immunoprecipitation determined by blotting with anti-GFP antibody.
The kinase activity and the SH2 and SH3 domains of Lyn determine its involvement in LPS-induced cytokine production
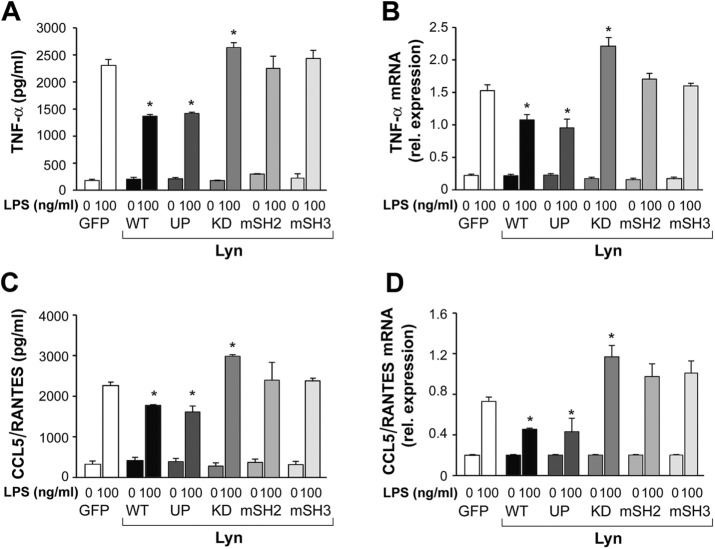
To assess the role of individual domains of Lyn and its kinase activity in LPS-induced signaling, we examined the influence of the expression of Lyn WT and its mutated forms on LPS-induced cytokine production in RAW264 cells. Overexpression of Lyn WT or Lyn UP reduced the production of TNF-α by ∼44% and CCL5/RANTES production by ∼15% (Figure 3, A and C), which was correlated with a significant down-regulation of TNF-α and CCL5/RANTES mRNA level (Figure 3, B and D). In contrast, cells expressing Lyn KD produced more TNF-α and CCL5/RANTES, by ∼11 and 42%, respectively (Figure 3, A and C), and had increased amounts of TNF-α and CCL5/RANTES mRNA than the GFP-expressing counterparts (Figure 3, B and D). Of note, the mRNA and protein levels of the cytokines in cells expressing Lyn-mSH2 or Lyn-mSH3 were equal to those in control cells (Figure 3, A–D), indicating that disabling of the SH2 or SH3 domain of Lyn affected its ability to modulate LPS-induced signaling.
FIGURE 3:
Wild-type and constitutively active Lyn inhibit and kinase-dead Lyn up-regulates LPS-induced production of TNF-α and CCL5/RANTES in RAW264 cell. Cells transfected with indicated constructs of Lyn-GFP or GFP alone were stimulated with 100 ng/ml LPS for 4 or 6 h for TNF-α and CCL5/RANTES measurements, respectively. Concentration of TNF-α (A) and CCL5/RANTES (C) in culture supernatants was measured with ELISA and expression of respective genes (B, D) analyzed using real-time quantitative PCR. Results are mean ± SD from one experiment run in triplicate and are representative of two or three independent experiments. *Significantly different from LPS-stimulated cells transfected with GFP alone at p ≤ 0.05.
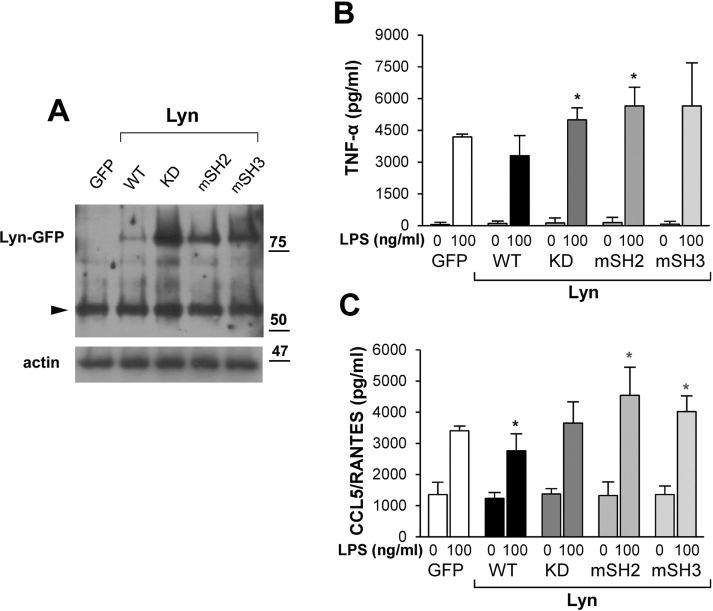
To verify our findings on the roles of individual Lyn domains in LPS-stimulated primary macrophages, we used nucleofection to introduce DNA encoding Lyn WT and its mutated forms in thioglycolate-elicited murine peritoneal macrophages. In a striking resemblance to RAW264 cells, Lyn WT was expressed in macrophages in lower amounts than Lyn KD, Lyn-mSH2, or Lyn-mSH3 (Figure 4A). Expression of Lyn UP was as low as that of Lyn WT, and incubation of cells in the presence of 2% FBS did not equalize the level of the Lyn variants in primary macrophages, suggesting that in these cells, Lyn level was regulated even more strictly than in RAW264 cells (unpublished data). Nevertheless, we observed the ability of Lyn WT to down-regulate cytokine production in LPS-stimulated macrophages, and, in the case of CCL5/RANTES production, the inhibition was significant (Figure 4B). In contrast to Lyn WT, expression of Lyn KD, Lyn-mSH2, or Lyn-mSH3 either moderately up-regulated or did not affect the production of TNF-α and CCL5/RANTES. The positive influence on cytokine production was most evident for Lyn-mSH2 (Figure 4B), strengthening the assumption that it is not only the catalytic activity but also the intermolecular interactions of Lyn that determine its negative influence on LPS-induced signaling.
FIGURE 4:
Mutations of specific domains of Lyn affect its influence on TNF-α and CCL5/RANTES production in murine peritoneal macrophages. Cells nucleofected with indicated Lyn constructs or GFP alone were analyzed by immunoblotting with anti-Lyn antibody (A) or stimulated with 100 ng/ml LPS to measure the amount of released TNF-α and CCL5/RANTES by ELISA (B, C). In A, arrowhead points to endogenous Lyn. In B and C, results are mean ± SD from three experiments run in triplicate. *Significantly different from LPS-stimulated cells transfected with GFP alone at p ≤ 0.05.
Association of Lyn WT and Lyn UP with raft-enriched fraction is enhanced by LPS
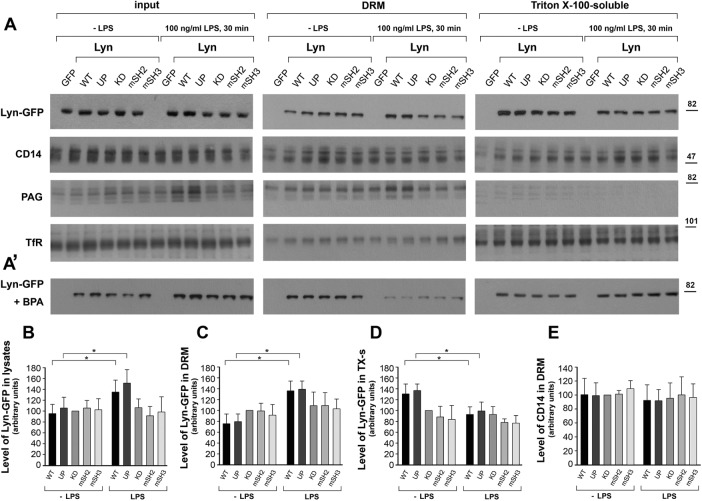
The foregoing data indicate that the SH2 and SH3 domains of Lyn are key to its ability to down-regulate LPS-induced cytokine expression and release. Therefore we asked whether these domains and/or the catalytic activity of Lyn affect its association with rafts—the nanodomains of the plasma membrane considered to be sites of TLR4 activation (Plociennikowska et al., 2015a). To address this question, we examined the distribution of the Lyn constructs in the Triton X-100–soluble fraction and Triton X-100–insoluble (also known as detergent-resistant membrane [DRM]), raft-enriched fraction of RAW264 cells (Plociennikowska et al., 2015a). Of all the variants of Lyn tested, LPS significantly affected the distribution of Lyn WT and Lyn UP between the Triton X-100–soluble and –insoluble fractions (Figure 5, A–D). In unstimulated cells, Lyn WT and Lyn UP prevailed in the Triton X-100–soluble fraction, whereas after 30 min of stimulation with LPS, those two forms of Lyn accumulated in the DRM fraction at the expense of the Triton X-100–soluble fraction (Figure 5, A, C, and D). This suggests that the enrichment of Lyn WT and Lyn UP in the DRM fraction resulted not only from local incorporation of the new pool of Lyn appearing in LPS-stimulated cells but also from a redistribution of preexisting Lyn between the two fractions. On the other hand, stimulation of cells with LPS did not induce a comparable redistribution of Lyn KD, Lyn-mSH2, or Lyn-mSH3 (Figure 5, A–D). The DRM fraction accommodated a substantial pool of CD14, a canonical raft-associated protein, both before and after stimulation of transfected cells with LPS (Figure 5, A and E). On the other hand, the DRM fraction contained only small amounts of transferrin receptor, a marker nonraft protein of the plasma membrane (Figure 5A). In addition to Lyn WT, Lyn UP, and CD14, phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG; also known as Cbp), a transmembrane adaptor protein that is a substrate of Lyn and binds the kinase (Hrdinka and Horejsi, 2014), also was enriched in the DRM fraction. In cells expressing Lyn WT or Lyn UP, the level of raft PAG was up-regulated by LPS (Figure 5A). The data indicate that LPS induces accumulation of wild-type and constitutively active Lyn in rafts, as inferred from their accumulation in the DRM fraction. A lack of the catalytic activity or a disabled SH2 or SH3 domain of Lyn precludes its translocation to this raft-enriched fraction.
FIGURE 5:
LPS induces association of Lyn WT and Lyn UP with the DRM fraction of RAW264 cells. Cells transfected with indicated constructs of Lyn-GFP or with GFP alone were preincubated without (A, B–E) or with 125 µM BPA (A′) and stimulated with 100 ng/ml LPS for 30 min. Cells were fractionated into the DRM fraction and Triton X-100–soluble fractions, equal volumes of both fractions were loaded onto a gel, and amount of Lyn-GFP, CD14, PAG, or transferrin receptor (TfR) was analyzed by immunoblotting and densitometry. The levels of Lyn constructs (B–D) and CD14 (E) in histograms correspond to blots in A (top) and are expressed relative to that of Lyn KD or CD14 in unstimulated cells. Mean ± SD of four or five experiments. *Data significantly different at p ≤ 0.05.
Palmitoylation of Lyn is required for its translocation to the DRM fraction in LPS-stimulated cells
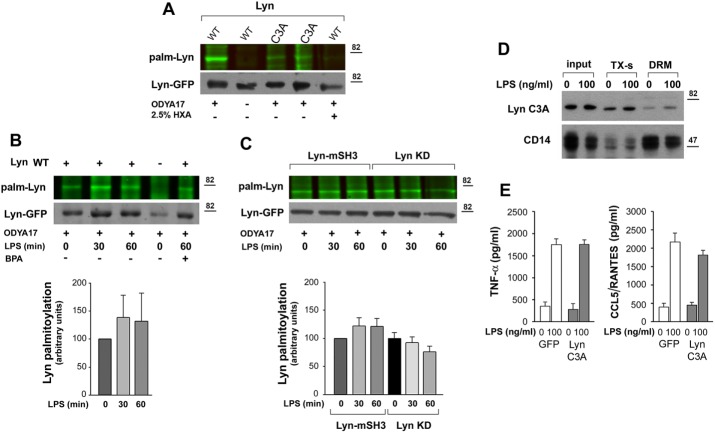
The foregoing data indicate that the accumulation of Lyn WT and Lyn UP in rafts correlates with their ability to regulate negatively TLR4-induced cytokine production. Because palmitoylation of cysteine residue(s) located in the SH4 domain was found earlier to affect the association of Src kinases with plasma membrane rafts (Webb et al., 2000; Pyenta et al., 2001; Kwiatkowska et al., 2003), we analyzed the role of palmitoylation of Lyn in LPS-induced signaling. Exposure of RAW264 cells to 2-bromopalmitic acid (BPA), an inhibitor of protein palmitoylation (Davda et al., 2013), and their subsequent stimulation with LPS led to an almost complete depletion of the DRM fraction of all of the Lyn constructs examined (Figure 5A’), suggesting that LPS accelerates the turnover of Lyn palmitoylation. In the presence of BPA, the process is inhibited, and as a result, none of the Lyn constructs could associate with the DRM fraction. We applied biorthogonal labeling of cells with a palmitic acid analogue, 17-octadecynoic acid (ODYA17), followed by click chemistry reaction to reveal the dynamics of Lyn palmitoylation in LPS-exposed RAW264 cells. ODYA17 did label Lyn WT and only faintly Lyn C3A mutated in the palmitoylation site; furthermore, no labeling of Lyn WT was detected in samples pretreated with 2.5% hydroxylamine (Figure 6A). Both of the last-named results confirmed that ODYA17 was incorporated predominantly at the palmitoylation site of Lyn. Fractionation of ODYA17-labeled and LPS-stimulated cells revealed that the content of palmitoylated Lyn WT found in the DRM fraction increased after 30–60 min of the stimulation. Palmitoylation was required for the association of Lyn with the DRM fraction, given that the accumulation of the ODYA17 label paralleled the accumulation of Lyn protein. On the other hand, pretreatment of cells with BPA reduced the amount of labeled Lyn WT in the DRM fraction of LPS-stimulated cells (Figure 6B). The stimulation did not increase the level of palmitoylated Lyn-mSH3 or Lyn KD in the DRM fraction (Figure 6C), reflecting a lack of LPS-induced accumulation of these kinase mutants in this fraction. In accord with these data, the nonpalmitoylated Lyn C3A was predominantly found in the Triton X-100–soluble fraction (Figure 6D). Of note, Lyn C3A failed to affect significantly the LPS-induced production of TNF-α and CCL5/RANTES (Figure 6E). Taken together, the data indicate that those Lyn constructs that failed to accumulate in the raft-enriched fraction of LPS-stimulated cells were also unable to inhibit cytokine production.
FIGURE 6:
Palmitoylated wild-type Lyn accumulates in the DRM fraction of RAW264 cells. (A–C) Palmitoylation of Lyn revealed by click chemistry. RAW264 transfectants were subjected to metabolic labeling with palmitic acid analogue ODYA17 or its carrier DMSO and left unstimulated or stimulated with 100 ng/ml LPS for 30–60 min. Cells were either lysed (A) or fractionated to obtain the DRM fraction (B, C). Subsequently Lyn-GFP was immunoprecipitated from the lysates or the DRM fraction and subjected to click chemistry with the fluorescent dye IRDye 800CW azide to reveal palmitoylation (top). The efficiency of immunoprecipitation was determined by blotting with anti-GFP antibody (bottom). (A) Labeling of Lyn WT and Lyn C3A with ODYA17 in unstimulated cells. HXA, samples treated with 2.5% hydroxylamine before electrophoresis. (B) Labeling of Lyn WT present in the DRM fraction during stimulation of cells with 100 ng/ml LPS. Control cells expressing GFP (–) and cells exposed to 125 μM BPA before labeling with ODYA17 and stimulation with LPS. (C) Labeling of Lyn-mSH3 and Lyn KD present in the DRM fraction during stimulation of cells with 100 ng/ml LPS. The level of Lyn palmitoylation was normalized against Lyn-GFP content in samples and is expressed in histograms relative to that in unstimulated cells. Mean ± SD of four or five experiments. (D) Distribution of Lyn C3A between Triton X-100–soluble (TX-s) and DRM fractions in unstimulated cells and cells exposed to 100 ng/ml LPS for 30 min. (E) Concentration of TNF-α and CCL5/RANTES in culture supernatants of GFP- or Lyn C3A–expressing cells measured with ELISA after 4 or 6 h of stimulation with 100 ng/ml LPS, respectively. Mean ± SD of two experiments run in triplicate.
Lyn WT and Lyn UP negatively regulate activity of NFκB and IRF3
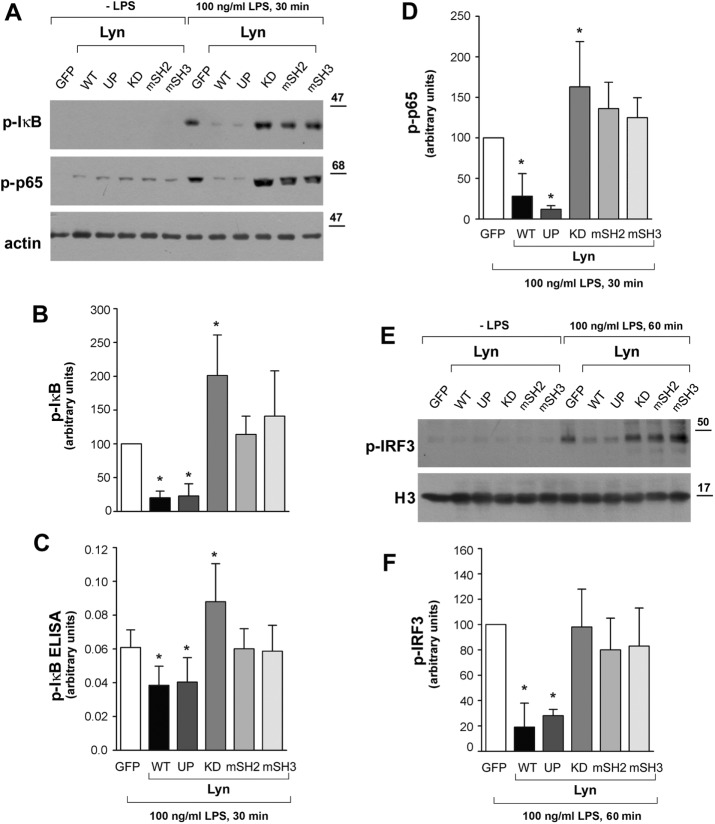
To reveal the TLR4 signaling pathways affected by Lyn, we analyzed how the overproduction of the Lyn constructs modulated the activity of NFκB and IRF3, the transcription factors controlling the MyD88- and TRIF-dependent expression of proinflammatory mediators. In RAW264 cells expressing Lyn WT or Lyn UP, the activity of NFκB was significantly lower than with control cells expressing GFP alone, as indicated by reduced phosphorylation of IκBα and p65 found by both immunoblotting (Figure 7, A, B, and D) and phospho-IκBα enzyme-linked immunosorbent assay (ELISA; Figure 7C). On the other hand, expression of Lyn KD led to a significant up-regulation of IκBα and p65 phosphorylation, whereas expression of Lyn with the SH2 or SH3 domain disabled allowed the phosphorylation of IκBα and p65 at a level comparable to that seen in control cells (Figure 7, A–D). Of note, the changes of NFκB activity caused by expression of the various forms of Lyn kinase in LPS-stimulated cells closely correlated with the changes of the production of cytokines in those cells (Figure 3), suggesting that the latter are caused mainly by Lyn affecting this major proinflammatory transcription factor. Moreover, Lyn WT and Lyn UP also evoked strong inhibition of phosphorylation of IRF3 (Figure 7, E and F), the transcription factor activated exclusively in the TRIF-dependent signaling pathway of TLR4 leading to CCL5/RANTES production. Similarly to the NFκB activity, the effect of Lyn-mSH2 and Lyn-mSH3 on IRF3 phosphorylation was negligible. Lyn KD also failed to up-regulate the IRF3 activity (Figure 7, E and F). Taken together, these data suggest that the intermolecular interactions of Lyn mediated by its SH2 and SH3 domains, in addition to the catalytic activity of the kinase, determine its ability to down-regulate the NFκB and IRF3 activities.
FIGURE 7:
Disabling of SH2 or SH3 domains or lack of enzymatic activity of Lyn reduces its ability to inhibit the activity of NFκB and IRF3 in RAW264 cells. Cells were transfected with indicated forms of Lyn-GFP or with GFP alone, stimulated with 100 ng/ml LPS, lysed, and analyzed for the presence of phospho-IκBα (p-IκB) and phospho-NFκB p65 (p-p65) by immunoblotting (A) and densitometry (B, D). (C) Level of IκB phosphorylation analyzed by ELISA. (E, F) The presence of phospho-IRF3 (p-IRF3) in nuclear fraction of cells. H3, histone H3. Histograms display mean ± SD from three experiments. In B, D, and F, the values of IκBα, p65, or IRF3 phosphorylation are normalized against actin or H3 content and expressed relative to those in cells transfected with GFP alone. *Significantly different from LPS-stimulated cells transfected with GFP alone at p ≤ 0.05.
Lyn controls nuclear translocation of p38
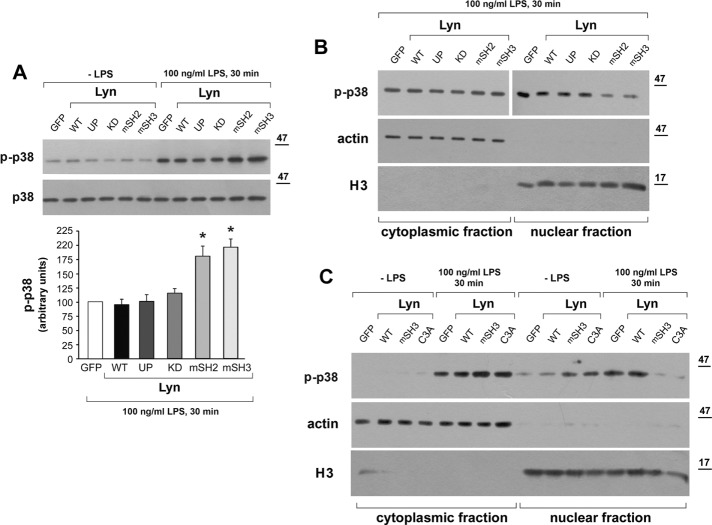
Among the Lyn constructs tested, Lyn KD, Lyn-mSH2, and Lyn-mSH3 failed to accumulate in the raft-enriched fraction, yet only Lyn KD up-regulated both TNF-α and CCL5/RANTES production in RAW264 cells. To address this discrepancy, we analyzed the influence of the Lyn construct cells on the activity of p38, a MAPK, yet another key effector controlling the expression of proinflammatory cytokines in LPS-stimulated cells (Kawai and Akira, 2011). In LPS-simulated RAW264 cells transfected with GFP as a control, p38 kinase underwent rapid phosphorylation that peaked at 30 min (Figure 8A). In distinct contrast to their effects on IκBα and IRF3, neither expression of Lyn WT nor that of Lyn UP reduced, nor did Lyn KD up-regulate, the level of p38 phosphorylation. Moreover, the phosphorylation of this MAPK was substantially intensified in cells expressing Lyn-mSH2 or Lyn-mSH3 (Figure 8, A and B), which indicates the complex nature of the Lyn involvement in TLR4 signaling.
FIGURE 8:
Lyn mutated in the SH2 or SH3 domain or in the palmitoylation site enhances phosphorylation of p38 and prevents its nuclear translocation in RAW264 cells. Cells expressing indicated constructs of Lyn-GFP or GFP alone as a control were stimulated with 100 ng/ml LPS for 30 min. (A) Cell lysates were analyzed for the presence of phospho-p38 (p-p38) by immunoblotting and densitometry. Histogram shows mean ± SD from three experiments. The values of p38 phosphorylation were normalized against p38 content in samples and are expressed relative to those in cells transfected with GFP alone. *Significantly different from LPS-stimulated cells transfected with GFP alone at p ≤ 0.05. (B, C) Cytoplasmic and nuclear proteins were analyzed for the presence of p-p38, actin, or histone H3. Results of one experiment representative of two to four.
The enhanced phosphorylation of p38 induced by Lyn-mSH2 and Lyn-mSH3 did not result in an increased expression of proinflammatory cytokines in RAW264 cells (Figure 3). We therefore examined whether Lyn controls the nuclear translocation of phosphorylated p38. In control GFP-expressing cells and cells expressing Lyn WT, Lyn UP, or Lyn KD, phospho-p38 translocated to the nucleus efficiently, whereas overexpression of Lyn mutated in the SH2 or SH3 domains kept phospho-p38 in the cytoplasm (Figure 8B). Thus the intense phosphorylation of p38 induced by those mutant forms of Lyn correlated with disturbances in its nuclear translocation.
A common feature of Lyn-mSH2 and Lyn-mSH3 is that they did not enrich in the DRM fraction of LPS-stimulated cells despite retaining the enzymatic activity. Therefore we examined whether Lyn C3A mutated in the palmitoylation site and sharing the other characteristics with Lyn-mSH2 and Lyn-SH3 also affected the nuclear translocation of phospho-p38 in RAW 264 cells. As seen in Figure 8C, in cells overexpressing Lyn C3A, increased phosphorylation of p38 was detected in the cytoplasmic fraction, and the phospho-p38 did not redistribute to the nucleus.
DISCUSSION
An exaggerated “cytokine storm” triggered by TLR4 during bacterial infection leads to potentially fatal severe sepsis and septic shock (Angus and van der Poll, 2013). In addition, gut bacterial flora altered by a high-fat diet can activate TLR4, inducing a prolonged subclinical inflammation that underlies the development of several metabolic diseases (Moreira et al., 2012). In addition, TLR4 can be activated by endogenous components such as oxidized low-density lipoprotein (Stewart et al., 2010) and has also been implicated in the development of atherosclerosis (Chavez-Sanchez et al., 2014). Therefore factors that can down-regulate the proinflammatory signaling of TLR4 have drawn special attention, and among such factors, Lyn kinase has been considered (Keck et al., 2010). Indeed, we found that depletion of RAW264 macrophage-like cells of Lyn enhanced the MyD88- and TRIF-dependent production of TNF-α and CCL5/RANTES that was induced by the smooth or rough form of LPS. Production of CCL5/RANTES was also increased in J774 cells depleted of Lyn (Figure 1). Conversely, overexpression of wild-type or constitutively active Lyn in RAW264 cells inhibited the expression and production of both of these cytokines. A tendency to down-regulate production of cytokines was also found for wild-type Lyn overexpressed in primary murine macrophages (Figures 3 and 4). These data are in agreement with earlier studies performed on bone marrow–derived murine macrophages (Keck et al., 2010) and taken together indicate that Lyn inhibits the LPS-induced production of cytokines in murine macrophages, although the magnitude of the inhibition depends on the particular type of cell.
Our data indicate that in LPS-stimulated cells, Lyn exerts its inhibitory effect on TLR4 signaling, provided it accumulates in rafts. This conclusion is based on studies performed on RAW264 cells, which were relatively susceptible to transfection and expressed all Lyn constructs, allowing both an analysis of LPS-induced signaling and fractionation of the cells. Thus fractionation of detergent lysates of RAW264 cells indicated that the LPS-induced accumulation of Lyn in the raft-enriched DRM fraction requires its palmitoylation, intermolecular interactions driven by SH2 and SH3 domains, and the kinase activity. The data agree with earlier reports showing that the aforementioned factors were also required for sustained interaction of Lyn with activated Fcε receptor I in rafts (Kovarova et al., 2001; Hammond et al., 2009). In our hands, the Lyn constructs mutated in the palmitoylation site (Lyn C3A) or in the SH2 or SH3 domain (Lyn-mSH2, Lyn-mSH3) and the kinase-dead one (Lyn KD) failed to redistribute to the DRM fraction and concomitantly had lost the ability to inhibit LPS-induced cytokine production, as well as NFκB and IRF3 activation. In fact, Lyn KD in RAW264 cells and Lyn-mSH2 and Lyn-mSH3 in primary macrophages even up-regulated production of TNF-α and RANTES. Among the factors controlling the association of Lyn with rafts in LPS-stimulated cells, palmitoylation seems very important. Inhibition of protein palmitoylation with BPA displaced almost completely the kinase from the DRM fraction. Dynamic palmitoylation of Lck, another kinase of the Src family, has been described during T-cell receptor or Fas receptor activation (Zhang et al., 2010; Akimzhanov and Boehning, 2015). These results suggest that LPS-induced palmitoylation of Lyn can be catalyzed by one of the plasma membrane–localized S-palmitoyl transferases (Ohno et al., 2006), facilitating translocation of Lyn to the rafts. Rafts are implicated in LPS-induced signaling due to local accumulation of CD14 and other proteins involved in TLR4 activation and signaling (Plociennikowska et al., 2015a). We found previously that binding of LPS to CD14 induces clustering of the protein in the plasma membrane, which triggers generation of phosphatidylinositol 4,5-bisphosphate, which is required for maximal production of TNF-α and CCL5/RANTES (Plociennikowska et al., 2015b). Clustering of rafts is a well-known event facilitating activation of immunoreceptors, which rely on the activity of Src tyrosine kinases for signal transduction, and Lyn kinase facilitates clustering of the GPI-anchored raft receptor CD59 (Suzuki et al., 2007). We assume that the LPS-induced clustering of CD14-bearing rafts induces palmitoylation of Lyn and its redistribution in the plane of the plasma membrane. When in rafts, interactions of Lyn with other proteins of the forming signaling complex of TLR4 mediated by the SH2 or SH3 domains of the kinase can facilitate its local accumulation and allow its subsequent involvement in TLR4 signaling as a negative regulator of NFκB and IRF3 transcription factors.
Previous studies linked the inhibitory effect of Lyn on TLR4 signaling in macrophages with the activation of PI3-kinase. This supposition was based on the facts that the knockout of Lyn or incubation of macrophages with wortmannin, a PI3-kinase inhibitor, up-regulated the production of IL-6 and TNF-α in response to rough LPS, whereas depletion of the cells of the phosphatidylinositol-3,4,5-trisphosphate phosphatase SHIP1 had the contrary effect (Keck et al., 2010). In addition, in LPS-stimulated dendritic cells, PI3-kinase down-regulated the production of IL-12 by acting through two signaling pathways involving mammalian target of rapamycin and glycogen synthase kinase 3 (Ohtani et al., 2008). A possible link between the Lyn kinase and PI3-kinase activities in LPS-stimulated macrophages was found in studies on the adaptor protein named B-cell adaptor for PI3-kinase (BCAP; Ni et al., 2012, Troutman et al., 2012b). Once phosphorylated on tyrosine residue(s), BCAP provides sites for binding of p85 subunit of PI3-kinase and contributes to its activation (Okada et al., 2000; Inabe and Kurosaki, 2002). BCAP-mediated activation of PI3-kinase was subsequently shown to be involved in down-regulation of TNF-α, IL-6, and IL-12 production in macrophages (Ni et al., 2012; Troutman et al., 2012b). Furthermore, a pool of BCAP was found to undergo tyrosine phosphorylation during stimulation of macrophages with LPS (Ni et al., 2012). Lyn kinase, which can interact with BCAP via the SH3 domain, was proposed to catalyze this process, as found during CD19 ligation (Inabe and Kurosaki, 2002; Troutman et al., 2012a). Because BCAP has a cryptic TIR domain, it can also interact directly with MyD88 or TIRAP and affect negatively the MyD88-dependent signaling of TLR4 by bringing PI3-kinase into the vicinity of the LPS-activated receptor (Troutman et al., 2012a,b). The Lyn–BCAP–PI3-kinase axis is likely to contribute to the inhibition of NFκB activity by Lyn WT and Lyn UP found in our studies, which, in turn, controls the expression of most of the proinflammatory cytokines induced by LPS (Kawai and Akira, 2011). In agreement with this, we found pronounced activity of PI3-kinase in immunoprecipitates of Lyn WT and Lyn UP (unpublished data). Note, however, that the 110δ isoform of PI3-kinase promotes the TRIF-dependent pathway of TLR4 at the expense of the MyD88-dependent one (Aksoy et al., 2012). This suggests that the interaction of Lyn with this PI3-kinase is unlikely to account for the inhibition of the TRIF-dependent IRF3 phosphorylation and CCL5/RANTES production found in our studies. It is plausible that Lyn can exert its negative regulatory function by phosphorylating proteins of the TLR4 signaling cascades other than BCAP. Lyn has an almost unique ability among the Src family kinases to phosphorylate the immunoreceptor tyrosine-based inhibitory motif of plasma membrane receptors that recruit protein and lipid phosphatases likely to inhibit TLR4 signaling. A less conventional cross-inhibition of TLR4 by receptors bearing immunoreceptor tyrosine-based activatory motifs has also been considered (Lowell, 2011). Among the raft proteins collaborating with Lyn in macrophages, PAG deserves special attention. PAG is a substrate of Src family kinases, including Lyn. After phosphorylation, PAG interacts with the SH2 domain of Lyn (in addition to the interaction with the SH3 domain of the kinase), and it also binds Csk kinase, which subsequently phosphorylates tyrosine 508 of Src family kinases, forcing their “closed” conformation and inactivation. This cascade of events can contribute to the negative regulation of Fcε receptor I signaling by Lyn (Hrdinka and Horejsi, 2014) and plausibly also occurs during TLR4 signaling.
Our data indicate that, besides the role of Lyn in down-regulation of the activity of NFκB and IRF3 transcription factors, its influence on p38 MAPK should not be neglected. In contrast to IκBα or IRF3, LPS-induced phosphorylation of p38 in RAW264 cells was not affected by overexpression of Lyn WT or Lyn UP. On the other hand, it increased in cells expressing Lyn mutated in the SH2 or SH3 domains and the nonpalmitoylatable Lyn C3A. However, concomitantly, translocation of phosphorylated p38 to the nucleus was inhibited. This can explain why, in RAW264 cells, those Lyn constructs did not increase cytokine production despite up-regulating p38 phosphorylation. The mechanisms governing translocation of p38 to the nucleus for regulation of gene expression are slowly being revealed (Plotnikov et al., 2011). The p38 kinase has no identifiable nuclear localization signal, and its accumulation in the nucleus is regulated by interaction of phospho-p38 with a nuclear shuttle and/or release of p38 from proteins trapping it in the cytoplasm. One such protein is TAK-1–binding protein (TAB-1), which binds p38 and favors its autophosphorylation. TAB-1–driven autophosphorylation antagonizes the downstream activity induced by the canonical phosphorylation of p38 catalyzed by MAPK kinases, thereby inhibiting inflammatory gene induction in cardiomyocytes (Lu et al., 2006). It seems possible that Lyn kinase that is activated by LPS but cannot translocate to rafts due to a lack of palmitoylation or the disability of its SH2 or SH3 domains disturbs the dynamics of the TRAF6/TAK-1 complex, including TAB-1 in RAW264 cells. As a result, TAB-1 can be prone to binding p38 and catalyzing its autophosphorylation, thereby trapping it in the cytoplasm. From this point of view, the translocation of Lyn to rafts induced by LPS would indirectly aid the nuclear localization of phospho-p38. Note that, in contrast to RAW264 cells, primary murine peritoneal macrophages responded to Lyn-mSH2 expression by increased production of TNF-α and CCL5/RANTES. These data underline the importance of the cellular context of the Lyn influence on LPS-induced responses and may indicate that in primary macrophages, the trapping of p38 in the cytoplasm does not shape substantially the final outcome of Lyn activity. On the other hand, in murine macrophages, PAG is more abundant than in RAW264 cells, and in both cell types, PAG migrates differently in SDS–PAGE (unpublished data), which reflects probably different patterns of its phosphorylation Tauzin et al., 2008). Of note, a nonpalmitoylated mutant form of PAG that is excluded from rafts can either be neutral or exert a positive effect on membrane receptor signaling, depending on which of its downstream effectors are involved (Posevitz-Fejfar et al., 2008). Although purely hypothetical, there is a possibility that in macrophages, the Lyn-PAG inhibitory axis can affect LPS-induced cytokine production to a higher extent than in RAW264 cells, and its disabling contributes to increased cytokine production in macrophages expressing mutant forms of Lyn.
Taken together, the presented data indicate that LPS induces translocation of Lyn kinase to rafts of macrophages. When in rafts, interactions of Lyn with other proteins of the forming signaling complex of TLR4 mediated via the SH2 or SH3 domains of the kinase can facilitate its local accumulation. This, in turn, enables the subsequent involvement of Lyn in TLR4 signaling as a negative regulator of the NFκB and IRF3 activity. Lyn is likely to act via various downstream effectors, and their identity can depend on the specific cellular context of macrophages.
MATERIALS AND METHODS
Cell culture and stimulation
RAW264 and J774 cells were cultured in DMEM containing 10% FBS at 5% CO2 and 37°C. Before experiments, the medium was exchanged for fresh DMEM containing 10 or 2% FBS (ThermoFisher Scientific, Waltham, MA). Cells were stimulated with ultrapure smooth LPS from Escherichia coli O111:B4 (List Biological Laboratories, Campbell, CA) in the presence of 10 or 2% FBS, respectively, at 37°C. In a series of experiments, cells were stimulated with rough LPS from E. coli, serotype 515, Re mutant (Enzo Life Sciences, Warsaw, Poland) in the medium without FBS after a 2-h preincubation in these conditions. When indicated, cells were preincubated for 2 h at 37°C in the presence of BPA in complex with BSA. For preparation of the BPA/BSA complexes, 4 mg of BPA was dissolved in 0.25 ml of chloroform:methanol (1:1 [vol:vol]) and mixed with 20 mg of Celite (Sigma-Aldrich, Poznan, Poland). After 10 min (25°C), solvents were dried in N2, and Celite was supplemented with 66 mg of defatted BSA and agitated for 1 h. Celite was pelleted (15,000 × g, 10 min, 25°C), and the supernatant was filtered and added to the medium at the final concentration of BPA of 125 µM. As a control, cells were exposed to BSA alone and processed as described. The concentration of the drugs was reduced by half during subsequent stimulation of cells with LPS.
Macrophages were isolated from 8- to 14-wk-old male C57BL/6 mice (Center of Experimental Medicine, Bialystok, Poland) injected intraperitoneally with 1 ml of 3% thioglycolate (Sigma-Aldrich). Four days after injection, animals were killed by inhalation of isoflurane (Baxter, Deerfield, IL) followed by cervical dislocation. Peritoneal inflammatory cells were washed out with phosphate-buffered saline (PBS) and suspended in RPMI containing 2 mM Ultraglutamine (Lonza, Basel, Switzerland), 20% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin and plated (8 × 106) in 10-cm nontreated culture dishes. The procedure was reviewed and approved by the Local Animal Ethics Committee (Permission No. 696/2015). After overnight culturing, nonadherent cells were removed with PBS, and macrophages were detached with 2 ml of Accutase (ThermoFisher Scientific) and subjected to nucleofection as described later. Transfected cells were plated at 0.3 × 106/well in 96-well plates in RPMI/20% FBS and cultured for 6 h, and the culture medium was exchanged. After 10 h, the medium was changed for RPMI containing 2% FBS, and 2 h later, the cells were stimulated with 100 ng/ml LPS in 200 μl of fresh RPMI/2% FBS.
Expression constructs of Lyn
Plasmids expressing various forms of murine Lyn A fused with GFP at the C-terminus were generated from the pcDNA3-Lyn A template kindly provided by Kiyotsugu Yoshida (Medical and Dental University, Tokyo, Japan). The Lyn cDNA was amplified by PCR using primers 5′GCCGCCGAATTCACCATGGGATGTATAAAATC3′ and 5′CAATACCAGCAGCAGCCTAAGGATCCACCG 3′, which contained BamHI and EcoRI restriction sites (underlined), respectively. The amplified and purified DNA fragment was digested with respective enzymes and ligated with the pEGFP-N1 vector (Takara-Clontech, Kyoto, Japan). Using the obtained pEGFP-N1-Lyn WT as a template, we used the following primers for the construction of 1) constitutively active Lyn UP with the Tyr508Phe substitution (Harder et al., 2001), 5′GCCACGGAAGGGCAATTCCAGCAGCAGCCTAAG3′ and 5′CTTAGGCTGCTGCTGGAATTGCCCTTCCGTGGC3′; 2) Lyn KD with the Lys275Arg substitution (Yoshida et al., 1999), 5′CAACAGTACCAAGGTGGCTGTGAGAACCCTGAAGC3′ and 5′GCTTCAGGGTTCTCACAGCCACCTTGGTACTGTTG3′; 3) Lyn-mSH2 with the Arg156Ala substitution (Shvartsman et al., 2007; Hammond et al., 2009; Ikeda et al., 2009), 5′GCTGGAGCTTTCCTTATTGCCGAAAGTGAAACATTAAAAGGAAGC3′ and 5′GCTTCCTTTTAATGTTTCACTTTCGGCAATAAGGAAAGCTCCAGC3′; 4) Lyn-mSH3 with the Trp99Ala substitution (Shvartsman et al., 2007; Hammond et al., 2009; Ikeda et al., 2009), 5′GAGGAGCATGGAGAAGCCTGGAAAGCAAAGTCC3′ and 5′GGACTTTGCTTTCCAGGCTTCTCCATGCTCCTC3′; and 5) Lyn C3A with the Cys3Ala substitution (Kovarova et al., 2001), 5′CGAATTCACCATGGGAGCTATAAAATCAAAAGGGAAAGACAGC3′ and 5′GCTGTCTTTCCCTTTTGATTTTATAGCTCCCATGGTGAATTCG3′. Phusion High Fidelity DNA polymerase (ThermoFisher Scientific) was used for PCR. The constructs were verified by sequencing. Plasmids were introduced into E. coli DH5α, purified using GenElute Endotoxin-free Plasmid HP Midiprep (Sigma-Aldrich), and used for transfection of cells.
Cell transfection
RAW264 cells were plated at 1.5 × 105/well in 24-well plates in DMEM/10% FBS 24 h before transfection. The medium was replaced with 0.8 ml of DMEM/10%FBS for 45 min, and then 0.2 ml of the DNA/TrueFect complex was added. The complex was prepared by mixing 2 µg of DNA with 6 µl of TrueFect (United Biosystems, Herndon, VA) in 0.2 ml of serum-free DMEM. Cells were cultured for 24 h, subsequently plated in 96- or 48-well plates (0.5 × 105/well in 0.2 ml of DMEM/10% FBS or 1 × 105/well in 300 µl of the medium, respectively), cultured for 20 h, and used for experiments. When required, cells plated at 5 × 105/5-cm plate were transfected with 5 µg of DNA in the presence of 15 µl of TrueFect. Transfection efficiency was estimated based on GFP fluorescence under a Nikon Eclipse TS100 inverted microscope equipped with a DXM 1200C digital camera and by flow cytometry using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ). It reached 35.4 ± 1.3% for controls expressing GFP alone.
Macrophages were transfected with DNA by nucleofection. For this purpose, cells detached from the substratum with Accutase were washed with PBS by centrifugation (200 × g, 10 min), suspended at 2 × 106 in 100 μl of Amaxa Mouse Macrophage Nucleofector solution (Lonza), and transferred to a tube containing 5 μg of DNA. After gentle mixing, suspended cells were transferred into a cuvette and submitted to nucleofection using a Nucleofector II device (Lonza) and Y-001 program according to the manufacturer’s instruction. Immediately after that, 1.8 ml of RPMI/20% FBS medium was added to the cell suspension, and cells were seeded in 96-well culture-treated plates.
Gene silencing
Silencing of Lyn gene was performed using siRNA essentially as described (Borzecka et al., 2013). In brief, 2 × 105 RAW264 cells were suspended in 1 ml of RPMI containing 5% FBS and mixed with 1 ml of serum-free RPMI containing 200 pmol of either Lyn siRNA or scrambled siRNA (Qiagen, Hilden, Germany) and 20 µl of TrueFect-Lipo (United BioSystems). For silencing of Lyn in J774 cells (8 × 105/sample), 260 pmol of siRNA and 20 μl of Truefect-Lipo were used. Cells were seeded and after 12 h, the medium was exchanged for DMEM/10% FBS, and cells were cultured for 24 h.
Fractionation of cells
RAW264 cells (1 × 106/sample) were collected by centrifugation (4 min, 300 × g, 4°C) in PD buffer (125 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 1 mM KH2PO4, 10 mM glucose, 1 mM MgCl2, 1 mM CaCl2, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], pH 7.4) and lysed for 30 min at 4°C with 50 μl of 0.05% Triton X-100 in buffer A (100 mM NaCl, 2 mM EDTA, 2 mM ethylene glycol tetraacetic acid [EGTA], 30 mM HEPES, pH 7.4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM Na3VO4, 50 µM phenylarsine oxide, 10 mM p-nitrophenyl phosphate). After centrifugation (5 min, 10,000 × g), supernatant (Triton X-100–soluble fraction) was collected while pellet was resuspended in 50 μl of 1.8% octyl-β-d-glucoside in buffer A (30 min, 4°C). After centrifugation, supernatant was collected as the DRM fraction (Brown, 2002). Equal volumes of fractions were subjected to SDS–PAGE analysis. Extracts of cytoplasmic and nuclear proteins were obtained from 1 × 106 cells/sample according to Prus and Filipek (2010). Proteins were loaded onto SDS–PAGE gels at 3.5 and 0.6 µg/lane, respectively.
Immunoprecipitation
RAW264 cells (2 × 106/sample) were collected by centrifugation in PD buffer and lysed in 0.2 ml of a lysis buffer containing 0.5% Nonidet P-40, 150 mM NaCl, 0.5 mM EDTA, 20 mM Tris, pH 7.4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM PMSF, 1 mM Na3VO4, 50 μM phenylarsine oxide, and 20 mM p-nitrophenyl phosphate (30 min, 4°C). The lysate was centrifuged (10 min, 20 000 × g, 4°C), diluted 2.5-fold in the lysis buffer devoid of Nonidet P-40, and supplemented with 20 µl of GFP-trap magnetic beads (ChromoTec, Planegg-Martinsried, Germany). After a 3-h incubation (4°C), beads were washed three times with the lysis buffer containing 0.05% Nonidet P-40 and once without the detergent, boiled in 12 µl of 2× SDS-sample buffer, and subjected to 10% SDS–PAGE. After separation, proteins were analyzed by immunoblotting as described later.
Analysis of Lyn palmitoylation
RAW264 transfectants (6 × 106/sample) were subjected to labeling with 50 µM ODYA17 (Cayman Chemicals, Tallinn, Estonia) or 0.05% dimethyl sulfoxide (DMSO; solvent) in DMEM containing 2% charcoal-stripped FBS (ThermoFisher Scientific) and 30 mM HEPES, pH 7.4. After 4 h (37°C), cells were either left unstimulated or were stimulated with 100 ng/ml LPS and collected, and after being washed with ice-cold PBS, they were either lysed in 400 μl of 50 mM phosphate buffer, pH 7.4, containing 0.5% Nonidet P-40, 100 mM NaCl, protease inhibitors (10 μg/ml aprotinin, leupeptin, and pepstatin each, and 1 mM PMSF), phosphatase inhibitors (1 mM Na3VO4, 50 µM phenylarsine oxide, and 10 mM p-nitrophenyl phosphate), and palmitoyl transferase inhibitors (10 µM palmostatin [Merck, Warsaw, Poland] and 0.2 mM 1-hexadecanesulfonyl fluoride [Cayman]) or fractionated into Triton X-100–soluble and DRM fractions as described earlier, with supplementation of the detergent buffers with 10 µM palmostatin and 0.2 mM 1-hexadecanesulfonyl fluoride. Lyn-GFP was immunoprecipitated from cell lysates or from the DRM fraction, as described, in buffers devoid of EDTA, and the immunoprecipitates were suspended in 44 µl of PBS containing EDTA-free protease inhibitor cocktail (Roche, Warsaw, Poland) and 1 mM PMSF. For the click reaction, the mixture was supplemented with 1 mM Tris(2-carboxyethyl)phosphine, 1 mM CuSO4, 100 µM Tris (benzyltriazolylmethyl)amine, and 10 µM of fluorescent reagent IRDye 800CW azide (LI-COR, Lincoln, NE). The final volume of the reaction mixture was 49 μl. The reaction was carried out for 1 h at room temperature in the dark with gentle rotation. Subsequently the samples were washed as after immunoprecipitation, then washed once in PBS, suspended in 20 µl of SDS-sample buffer, and heated for 5 min at 95°C with shaking. In a series of experiments, proteins eluted with SDS-sample buffer were supplemented with 2.5% hydroxylamine, incubated for 10 min at room temperature, and heated again for 5 min at 95°C. Proteins were separated by 10% SDS–PAGE, transferred onto nitrocellulose (1 h, 0.4 A), and analyzed in an Odyssey CLx Imager (LI-COR) or subjected to immunoblotting for the presence of Lyn-GFP.
Immunoblotting
Cell lysates (3.5 μg of protein/lane), immunoprecipitates, or indicated quantities of cell fractions were subjected to 10% SDS–PAGE. Separated proteins were transferred onto nitrocellulose and immunoblotted with rabbit anti-Lyn (Cell Signaling Technology, Leiden, Netherlands), rabbit anti–phosphotyrosine 397, or rabbit anti–phosphotyrosine 508 of Lyn (both Abcam, Cambridge, United Kingdom), rabbit anti-PAG (EXBIO, Vestec, Czech Republic), rabbit anti-GFP, rat anti-CD14 (BD Bioscience), mouse anti–transferrin receptor (Invitrogen, Warsaw, Poland), rabbit anti-p38, rabbit anti-p38 phosphorylated at Thr-180 and Tyr-182, rabbit anti-IκBα phosphorylated at Ser-32, rabbit anti-IRF3 phosphorylated at Ser-396, rabbit anti-NFκB p65 phosphorylated at Ser-536 (all from Cell Signaling Technology), rabbit anti–histone H3 (Sigma-Aldrich), or mouse anti-actin (MP Biomedicals, Warsaw, Poland) antibodies. They were followed by anti-rabbit, anti-mouse, or anti-rat immunoglobulin G conjugated with peroxidase (Rockland, Limerick, PA; Jackson ImmunoResearch, West Grove, PA). Immunoblotting with the anti-Lyn antibody required dephosphorylation of proteins with 2 mg/ml alkaline phosphatase (Sigma-Aldrich) for 45 min (37°C). The anti-Lyn antibody recognized preferably the Lyn A isoform. Immunoreactive bands were visualized with chemiluminescence using SuperSignal West Pico substrate (ThermoFisher Scientific) and analyzed densitometrically using ImageJ. Prestained molecular mass standards were from BioRad (Puchheim, Germany).
Analysis of IκB phosphorylation by ELISA
RAW264 cells (1 × 106/sample) were lysed in 0.2 ml of a buffer composed of 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM Tris, pH 7.4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM Na3VO4, and 10 mM p-nitrophenyl phosphate. After centrifugation (10 min, 14,000 × g, 4°C) supernatants were analyzed for the presence of phospho-IκBα by immunoblotting as described or with application of PathScan Phospho-IκBα (Ser-32) Sandwich ELISA (Cell Signaling Technology) according to the manufacturer’s instructions. Samples were run in triplicate and normalized against protein content estimated with the Bradford ULTRA kit (Expedeon, Swavesey, United Kingdom).
TNF-α and CCL5/RANTES assays
TNF-α and CCL5/RANTES levels were determined in supernatants of cells after 4 or 6 h of stimulation, respectively, with application of appropriate murine ELISA kits (BioLegend, Katowice, Poland; R&D Systems, Abingdon, United Kingdom) according to the manufacturer’s instructions, using a Sunrise plate reader (Tecan Group, Zurich, Switzerland). Real-time quantitative PCR analysis was performed essentially as described by Plociennikowska et al. (2015b).
Data analysis
The significance of differences between groups was calculated using Student’s t test. p ≤ 0.05 was considered statistically significant.
Acknowledgments
We are grateful to Andrzej Sobota for critical discussion of the results. This work was supported by the National Science Centre, Poland, through Grants DEC-2013/08/A/NZ3/00850 to K.K. and 2014/13/N/NZ3/00884 to K.B.-S.
Abbreviations used:
- BCAP
B-cell adaptor for PI3-kinase
- BCR
B-cell receptor
- BPA
2-bromopalmitic acid
- CCL5
C-C motif chemokine ligand 5
- DRM
detergent-resistant membrane
- FBS
fetal bovine serum
- GPI
glycosylphosphatidylinositol
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- ODYA17
17-octadecynoic acid
- PAG
phosphoprotein associated with glycosphingolipid-enriched microdomains
- PI3-kinase
phosphatidylinositol 3-kinase I
- RANTES
regulated upon activation, normal T-cell expressed and secreted
- SH
Src homology
- TAB-1
TAK-1–binding protein
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-09-0632) on February 22, 2017.
REFERENCES
- Akimzhanov AM, Boehning D. Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci USA. 2015;112:11876–11880. doi: 10.1073/pnas.1509929112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13:1045–1054. doi: 10.1038/ni.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Errico D, Yamashita Y, Suzuki R, Odom S, Furumoto Y, Yamashita T, Rivera J. Functional analysis of Lyn kinase A and B isoforms reveals redundant and distinct roles in Fc epsilon RI-dependent mast cell activation. J Immunol. 2010;184:5000–5008. doi: 10.4049/jimmunol.0904064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- Avila M, Martinez-Juarez A, Ibarra-Sanchez A, Gonzalez-Espinosa C. Lyn kinase controls TLR4-dependent IKK and MAPK activation modulating the activity of TRAF-6/TAK-1 protein complex in mast cells. Innate Immun. 2012;18:648–660. doi: 10.1177/1753425911435265. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Fitzgerald KA, Huet F, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- Borzecka K, Plociennikowska A, Bjorkelund H, Sobota A, Kwiatkowska K. CD14 mediates binding of high doses of LPS but is dispensable for TNF-α production. Mediators Inflamm. 2013;2013:824919. doi: 10.1155/2013/824919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Isolation and use of rafts. Curr Protoc Immunol. 2002 doi: 10.1002/0471142735.im1110s51. Chapter 11, Unit 11.10. [DOI] [PubMed] [Google Scholar]
- Chavez-Sanchez L, Garza-Reyes MG, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Blanco-Favela F. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol. 2014;75:322–329. doi: 10.1016/j.humimm.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- Davda D, El Azzouny MA, Tom CT, Hernandez J L, Majmudar JD, Kennedy RT, Martin BR. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem Biol. 2013;8:1912–1917. doi: 10.1021/cb400380s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English BK, Orlicek SL, Mei Z, Meals EA. Bacterial LPS and IFN-γ trigger the tyrosine phosphorylation of vav in macrophages: evidence for involvement of the hck tyrosine kinase. J Leukoc Biol. 1997;62:859–864. doi: 10.1002/jlb.62.6.859. [DOI] [PubMed] [Google Scholar]
- Freedman TS, Tan YX, Skrzypczynska KM, Manz BN, Sjaastad FV, Goodridge HS, Lowell CA, Weiss A. Lyn A regulates an inflammation-sensitive signaling checkpoint in macrophages. Elife. 2015;4:e09183. doi: 10.7554/eLife.09183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–123. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- Hammond S, Wagenknecht-Wiesner A, Veatch SL, Holowka D, Baird B. Roles for SH2 and SH3 domains in Lyn kinase association with activated FcεRI in RBL mast cells revealed by patterned surface analysis. J Struct Biol. 2009;168:161–167. doi: 10.1016/j.jsb.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Mahon T, McDaid JP, Campbell J, Mano H, Brennan FM, Webster D, Foxwell BM. Bruton’s tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor α production. J Exp Med. 2003;197:1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdinka M, Horejsi V. PAG-a multipurpose transmembrane adaptor protein. Oncogene. 2014;33:4881–4892. doi: 10.1038/onc.2013.485. [DOI] [PubMed] [Google Scholar]
- Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Nakayama Y, Ishii M, Obata Y, Kasahara K, Fukumoto Y, Yamaguchi N. Requirement of the SH4 and tyrosine-kinase domains but not the kinase activity of Lyn for its biosynthetic targeting to caveolin-positive Golgi membranes. Biochim Biophys Acta. 2009;1790:1345–1352. doi: 10.1016/j.bbagen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Inabe K, Kurosaki T. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood. 2002;99:584–589. doi: 10.1182/blood.v99.2.584. [DOI] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun Signal. 2012;10:21. doi: 10.1186/1478-811X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-Like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Keck S, Freudenberg M, Huber M. Activation of murine macrophages via TLR2 and TLR4 is negatively regulated by a Lyn/PI3K module and promoted by SHIP1. J Immunol. 2010;184:5809–5818. doi: 10.4049/jimmunol.0901423. [DOI] [PubMed] [Google Scholar]
- Ko HM, Lee SH, Kim KC, Joo SH, Choi WS, Shin CY. The role of TLR4 and Fyn interaction on lipopolysaccharide-stimulated PAI-1 expression in astrocytes. Mol Neurobiol. 2015;52:8–25. doi: 10.1007/s12035-014-8837-z. [DOI] [PubMed] [Google Scholar]
- Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcε receptor I aggregation. Mol Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska K, Frey J, Sobota A. Phosphorylation of FcγRIIA is required for the receptor-induced actin rearrangement and capping, the role of membrane rafts. J Cell Sci. 2003;116:537–550. doi: 10.1242/jcs.00254. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3:a002352. doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Kang YJ, Han J, Herschman HR, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281:6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock A M, Carol V, Robinson C, Latz E, Gay NJ. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284:25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, MacFarlane AW, 4th, Toft M, Lowell CA, Campbell KS, Hamerman JA. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proc Natl Acad Sci USA. 2012;109:267–272. doi: 10.1073/pnas.1111957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Peirce MJ, Brook M, Morrice N, Snelgrove R, Begum S, Lanfrancotti A, Notley C, Hussell T, Cope AP, Wait R. Themis2/ICB1 is a signaling scaffold that selectively regulates macrophage Toll-like receptor signaling and cytokine production. PLoS One. 2010;3:e11465. doi: 10.1371/journal.pone.0011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plociennikowska A, Hromada-Judycka A, Borze˛cka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015a;72:557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plociennikowska A, Zdioruk MI, Traczyk G, Swia˛tkowska A, Kwiatkowska K. LPS-induced clustering of CD14 triggers generation of PI(4,5)P2. J Cell Sci. 2015b;128:4096–4111. doi: 10.1242/jcs.173104. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Posevitz-Fejfar A, Smida M, Kliche S, Hartig R, Schraven B, Lindquist JA. A displaced PAG enhances proximal signaling and SDF-1-induced T cell migration. Eur J Immunol. 2008;38:250–259. doi: 10.1002/eji.200636664. [DOI] [PubMed] [Google Scholar]
- Prus W, Filipek A. S100A6 mediates nuclear translocation of Sgt1: a heat shock-regulated protein. Amino Acids. 2010;41:781–787. doi: 10.1007/s00726-010-0526-2. [DOI] [PubMed] [Google Scholar]
- Pyenta PS, Holowka D, Baird B. Cross-correlation analysis of inner-leaflet-anchored green fluorescent protein co-redistributed with IgE receptors and outer leaflet lipid raft components. Biophys J. 2001;80:2120–2132. doi: 10.1016/S0006-3495(01)76185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvartsman DE, Donaldson JC, Diaz B, Gutman O, Martin GS, Henis YI. Src kinase activity and SH2 domain regulate the dynamics of Src association with lipid and protein targets. J Cell Biol. 2007;178:675–686. doi: 10.1083/jcb.200701133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Mol Immunol. 2008;45:990–1000. doi: 10.1016/j.molimm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Smolinska MJ, Page TH, Urbaniak AM, Mutch BE, Horwood NJ. Hck tyrosine kinase regulates TLR4-induced TNF and IL-6 production via AP-1. J Immunol. 2011;187:6043–6051. doi: 10.4049/jimmunol.1100967. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and Gα for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin S, Ding H, Khatib K, Ahmad I, Burdevet D, van Echten-Deckert G, Lindquist JA, Schraven B, Din NU, Borisch B, Hoessli DC. Oncogenic association of the Cbp/PAG adaptor protein with the Lyn tyrosine kinase in human B-NHL rafts. Blood. 2008;111:2310–2320. doi: 10.1182/blood-2007-05-090985. [DOI] [PubMed] [Google Scholar]
- Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012a;11:3559–67. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, Pasare C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci USA. 2012b;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda- Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Huntington ND, Harder KW, Nandurkar H, Hibbs ML, Tarlinton DM. Phosphatidylinositol-3 kinase activity in B cells is negatively regulated by Lyn tyrosine kinase. Immunol Cell Biol. 2012;90:903–911. doi: 10.1038/icb.2012.31. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kharbanda S, Kufe D. Functional interaction between SHPTP1 and the Lyn tyrosine kinase in the apoptotic response to DNA damage. J Biol Chem. 1999;274:34663–34668. doi: 10.1074/jbc.274.49.34663. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Tsou LK, Charron G, Raghavan AS, Hang HC. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci USA. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]