Abstract
The rewiring of gene regulatory networks can generate phenotypic novelty. It remains an open question, however, how the large number of connections needed to form a novel network arise over evolutionary time. Here, we address this question using the network controlled by the fungal transcription regulator Ndt80. This conserved protein has undergone a dramatic switch in function—from an ancestral role regulating sporulation to a derived role regulating biofilm formation. This switch in function corresponded to a large-scale rewiring of the genes regulated by Ndt80. However, we demonstrate that the Ndt80-target gene connections were undergoing extensive rewiring prior to the switch in Ndt80’s regulatory function. We propose that extensive drift in the Ndt80 regulon allowed for the exploration of alternative network structures without a loss of ancestral function, thereby facilitating the formation of a network with a new function.
DOI: http://dx.doi.org/10.7554/eLife.23250.001
Research Organism: S. cerevisiae, Other
Introduction
The emergence of novel traits has long fascinated evolutionary biologists, with many intriguing examples observed across the tree of life (Pigliucci, 2008; Rieppel, 2001; Shubin et al., 2009; Wagner and Lynch, 2010). Although the rewiring of gene regulatory networks over evolutionary time is recognized as a key source of variation responsible for the modification of complex phenotypes (Carroll, 2005; Davidson and Erwin, 2006; Li and Johnson, 2010; Wray, 2007), we still lack an understanding of how new, large gene regulatory networks controlling novel phenotypes arise over evolutionary time. Typically, gene regulatory networks are composed of ‘master’ transcription regulators and many downstream ‘target’ genes, whose expression is controlled by these regulators in response to environmental or developmental signals. It has been proposed that new gene regulatory networks arise from a combination of de novo genes as well as conserved genes that have undergone changes in regulation (Wagner and Lynch, 2010). Yet, it remains unclear how a large number of genes (whether old or new) can be brought together to form a new network.
To address these questions, we examined the evolutionary history of Ndt80, a sequence-specific DNA-binding protein that is deeply conserved across a large group of fungal species encompassing approximately 300 million years of diversity. In most of these species, Ndt80 controls meiosis and sporulation, the coupled processes that form a portion of the fungal sexual cycle (Chu and Herskowitz, 1998; Xu et al., 1995). However, in the narrow lineage leading to the human fungal pathogen species Candida albicans, Ndt80 acquired a new role as a master regulator of the gene regulatory network that controls the formation of biofilms, multicellular communities of surface-associated cells (Figure 1A) (Nobile et al., 2012). This newly evolved trait enables C. albicans to persist on mucosal surfaces and on implanted medical devices (Bonhomme and d'Enfert, 2013; Kojic and Darouiche, 2004; Nobile et al., 2012) and is responsible for many of the disease-causing properties of C. albicans (Nobile and Johnson, 2015). The entire biofilm gene regulatory network in C. albicans is complex—Ndt80 alone controls the expression of hundreds of genes involved in biofilm formation—but it appears to have evolved relatively recently (Nobile et al., 2012). In this paper, we infer that approximately 20 to 100 million years ago (Taylor and Berbee, 2006), Ndt80’s role changed from regulating sporulation and meiosis (its ancestral role) to regulating biofilm formation. We rigorously exclude a model in which the Ndt80 network remained relatively static until a sudden change occurred in the Candida clade when Ndt80’s overall function changed from sporulation to biofilm formation. Instead, we found that the Ndt80 regulon was continuously undergoing significant rewiring in all lineages examined, even in those where the overall function of Ndt80 remained unchanged. Indeed, at the resolution of our experiments, the extent of Ndt80 rewiring was approximately the same whether its function had changed or not.
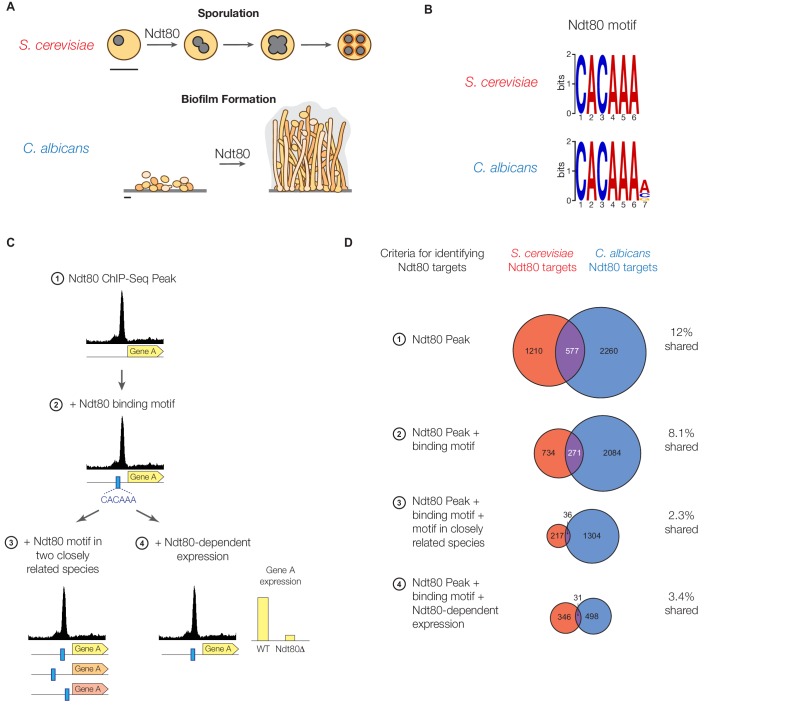
Figure 1. Ndt80 target genes differ between S. cerevisiae and C. albicans.
(A) Diagram of sporulation in S. cerevisiae and biofilm formation in C. albicans. Scale bars represent 5 µm. (B) The cis-regulatory motif most highly enriched at locations of Ndt80 ChIP binding in S. cerevisiae and C. albicans. Motifs were generated independently for each species using DREME. The Ndt80 motifs determined de novo in this study closely match those identified previously for Ndt80 (Chu and Herskowitz, 1998; Jolly et al., 2005; Nobile et al., 2012). (C) Diagram of the four criteria used to identify Ndt80 regulatory targets. Criteria 1: significant ChIP-Seq enrichment in the intergenic region upstream of a gene relative to untagged control experiments. Criteria 2: ChIP-Seq enrichment and the presence of an Ndt80 motif in the intergenic region. Criteria 3: ChIP-Seq enrichment with the Ndt80 motif present in the intergenic region and also in orthologous intergenic regions of two very closely related species, suggesting the motif has been maintained by selection. Criteria 4: ChIP-Seq enrichment with the Ndt80 motif present in the intergenic region and Ndt80-dependent expression of the nearby gene, indicating that expression of the gene is under Ndt80 control. (D) Overlap in targets of S. cerevisiae Ndt80 (red) and C. albicans Ndt80B (blue), using the four different criteria from (C) to identify targets, when Ndt80 is highly expressed in each species (Materials and methods). Venn diagrams are roughly area-proportional (Hulsen et al., 2008).
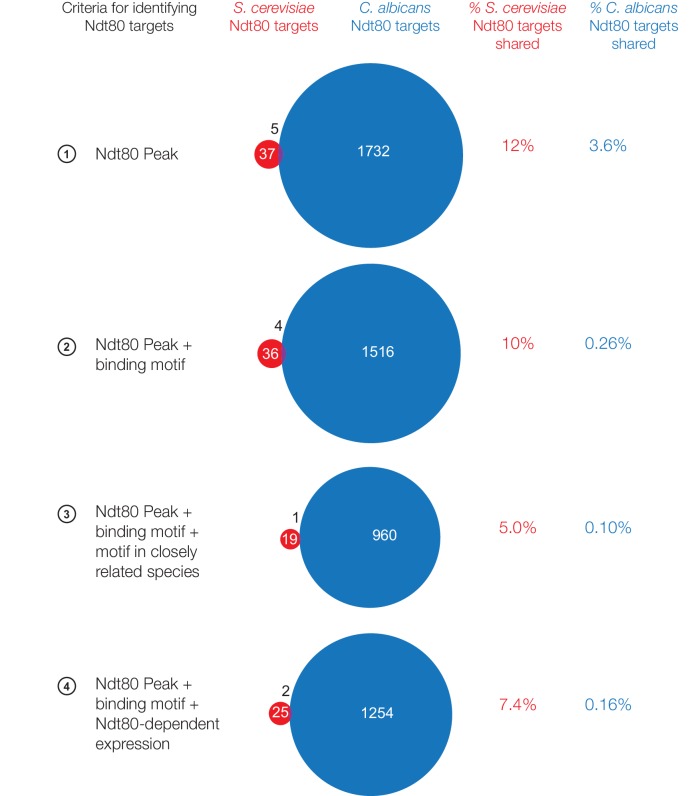
Figure 1—figure supplement 1. Ndt80 targets in S. cerevisiae and C. albicans when Ndt80 is endogenously expressed.
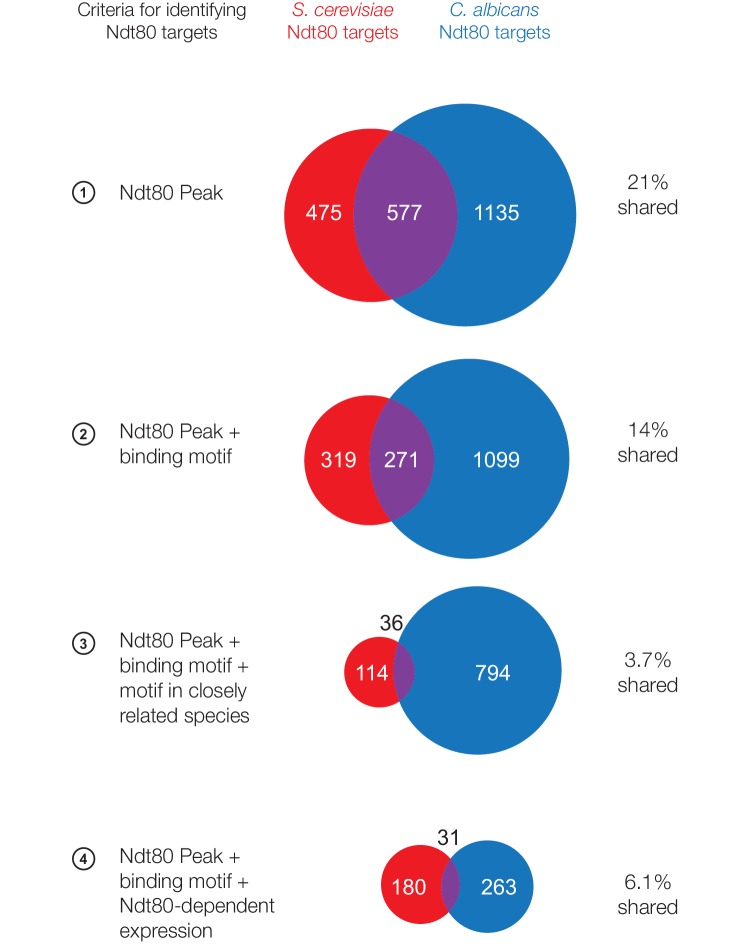
Figure 1—figure supplement 2. Ndt80 targets in S. cerevisiae and C. albicans considering only 1:1 orthologs between species.
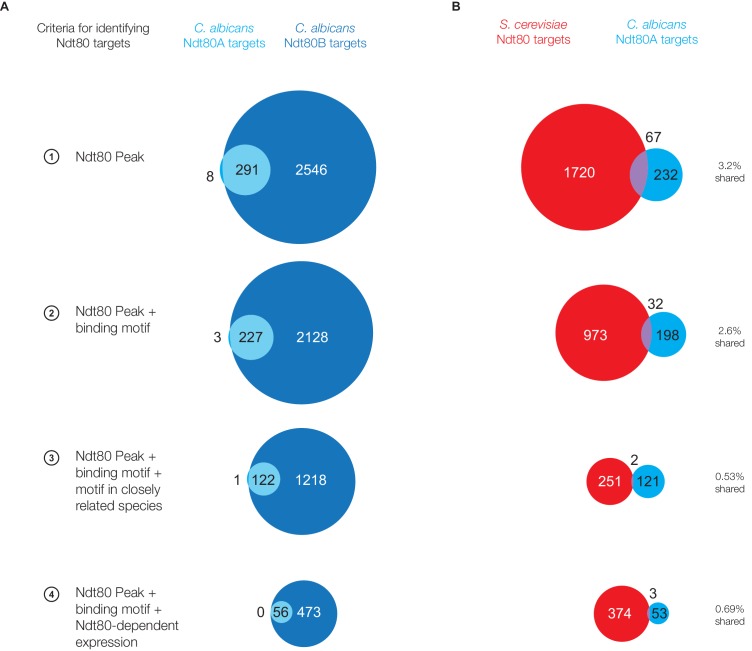
Figure 1—figure supplement 3. Ndt80 targets for paralogs Ndt80A and Ndt80B in C. albicans.
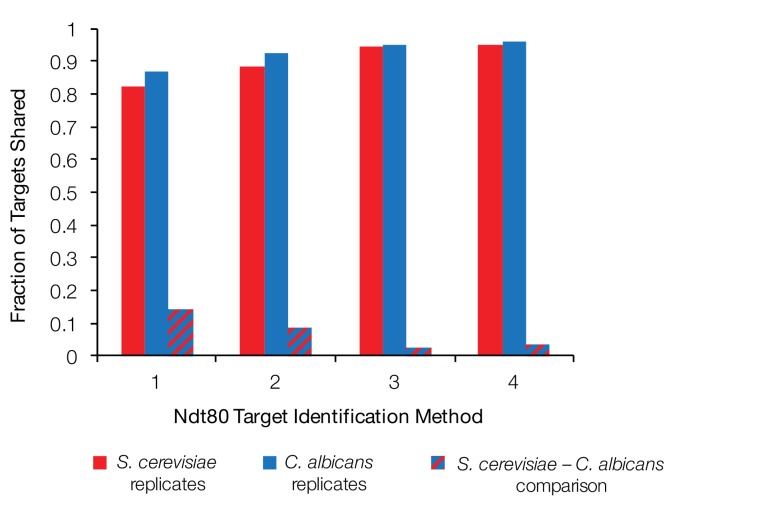
Figure 1—figure supplement 4. Fraction of Ndt80 targets shared between biological replicates and between S. cerevisiae and C. albicans.
We propose that the inherent flexibility of the Ndt80 regulon facilitated the exploration of new regulatory networks, allowing it to reach positions in ‘network space’ that allowed for the evolution of a novel phenotype. This idea is analogous to examples of protein evolution in which mutational space can be sampled by drift without compromising the ancestral function of the protein. Such neutral excursions can then be exploited if they provide, as a by-product, a new function, for example weak catalysis of an alternative reaction by an enzyme (Aharoni et al., 2005; Bridgham et al., 2006; Coyle et al., 2013; Khersonsky and Tawfik, 2010; Wagner, 2005a, 2005b). Our work provides an empirical, case study of an analogous exploitation of regulatory network plasticity in the evolution of novel transcription regulator function.
It has been well established that transcription regulator-target gene interactions change over evolutionary timescales. For example, the regulators controlling the ribosomal genes and the a-specific genes in fungi have changed over several hundred million years of evolution (Baker et al., 2012; Ihmels et al., 2005; Lavoie et al., 2010; Tanay et al., 2005; Tsong et al., 2006) (Sorrells et al., 2015). Likewise, transcription regulators (such as Mcm1 and Ste12) have been shown to undergo extensive rewiring of their regulatory targets over similar timescales (Borneman et al., 2007; Tuch et al., 2008). Such rewiring is not restricted to fungi; for example, enhancers in Drosophila have been shown to undergo rapid changes in the nature and arrangement of their cis-regulatory sequences (Frankel et al., 2012; Gompel et al., 2005; Ludwig et al., 2000; Swanson et al., 2011). This high degree of network plasticity seems to be a general feature of many regulatory networks. Based on the case study of Ndt80, we propose that this plasticity can be captured to produce new, complex regulatory networks.
Results
Ndt80 target genes differ markedly between S. cerevisiae and C. albicans
As described above, the transcription factor Ndt80 is required for sporulation and meiosis in S. cerevisiae and biofilm formation in C. albicans (Figure 1A). In principle, the difference in phenotype could be due to a difference in the identity of the genes regulated by Ndt80 between these species; alternatively, Ndt80 could regulate the same gene set in both species and the difference in phenotype could be due to something else, for example, Ndt80 target genes acquiring new enzymatic functions or Ndt80 itself being regulated differently in the two species.
To distinguish between these models, we identified the genes directly regulated by Ndt80 in both S. cerevisiae and C. albicans using chromatin immunoprecipitation of epitope-tagged Ndt80 followed by high-throughput sequencing (ChIP-Seq). To minimize the effect of species-specific differences in the levels of Ndt80 expression (and to capture as much of the network as possible), the promoters of Ndt80 in both species were replaced with high-expression constitutive promoters (Materials and methods). It has been well established that, even using proper controls, chromatin immunoprecipitation produces false positives in addition to valid instances of binding (Fan and Struhl, 2009; Teytelman et al., 2013). To eliminate spurious signals and identify bona fide instances of Ndt80 binding, we employed four different criteria of increasing stringency (Figure 1C). First, we simply identified genes with significant peaks of ChIP enrichment in their intergenic regions; this is our least stringent criteria. Second, we filtered this set to include only those ChIP enrichment peaks that contained an Ndt80 cis-regulatory motif in the intergenic region (this motif is discussed in more detail below). Third, of those ChIP peaks that contained an Ndt80 motif, we further refined the set of Ndt80 targets by requiring that the motif also be present in the orthologous intergenic region of two very closely related species, as this greatly increases the likelihood that the Ndt80-binding site was maintained by selection. (For S. cerevisiae, we used S. mikatae and S. kudriavzevii, for C. albicans we used C. tropicalis and C. dubliniensis [Byrne and Wolfe, 2005; Lohse et al., 2013]). Lastly, we used gene expression data to restrict Ndt80 targets to ChIP peaks with an Ndt80 motif where the gene also exhibited a significant change in mRNA expression when Ndt80 was deleted (Chu et al., 1998; Nobile et al., 2012).
Each of the four criteria were used to identify and compare the genes regulated by Ndt80 between S. cerevisiae and C. albicans. While the number of genes identified as Ndt80 targets differs depending on the criteria used, all four methods produce the same overall conclusion: Ndt80 regulates many genes in each species, with very little overlap between them (Figure 1D). By all methods, fewer than 13% of the targets of Ndt80 in these two species are shared; with the most stringent criteria this drops to 3.4% (Figure 1D). To ensure that constitutive overexpression of Ndt80 was not responsible for this large difference in Ndt80 targets, we also performed ChIP-Seq on Ndt80 in both species under the control of the endogenous promoter. While many fewer regions are bound than with constitutive Ndt80 expression, we similarly find very little overlap in the Ndt80 targets in S. cerevisiae and C. albicans (less than 12%, Figure 1—figure supplement 1). We note that experimental noise is not sufficient to explain the difference in targets, as the differences we observe between biological replicates accounts for only a small fraction of the difference observed between S. cerevisiae and C. albicans (Figure 1—figure supplement 4).
These results all lead to the conclusion that Ndt80 regulates distinct sets of genes in S. cerevisiae and C. albicans. Most of these genes have 1:1 orthologs in both species, although there are also a smaller number of species-specific genes. If we exclude the species-specific genes from our analysis, we still find relatively little overlap in Ndt80 targets (4–21% targets shared, depending on target identification criteria used, Figure 1—figure supplement 2), showing that gene gains and losses alone cannot account for the change in Ndt80 targets between S. cerevisiae and C. albicans. Despite the significant differences in Ndt80 targets, however, the cis-regulatory sequence bound by Ndt80 in both species is highly conserved ([Jolly et al., 2005; Nobile et al., 2012], Figure 1B). This observation indicates that the Ndt80 regulon has been significantly rewired between S. cerevisiae and C. albicans without a change in the DNA-binding specificity of Ndt80.
Divergence in Ndt80 targets not a result of Ndt80 gene duplication
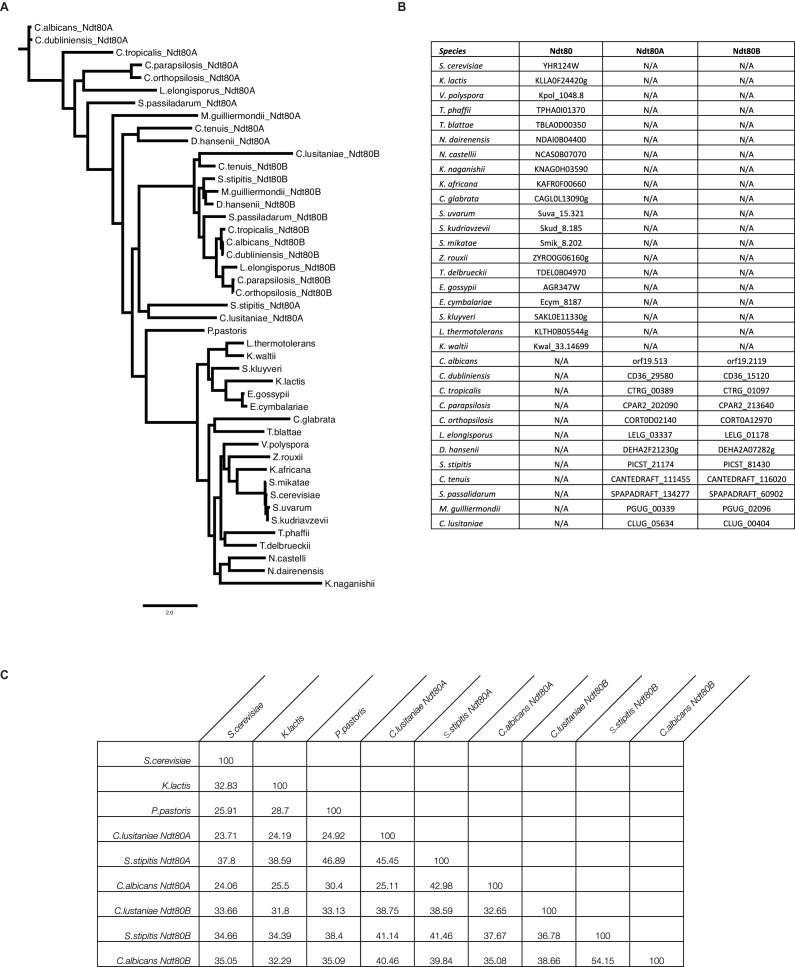
While the NDT80 genes in S. cerevisiae and C. albicans discussed above are homologous, C. albicans has an additional paralog of Ndt80, resulting from a gene duplication (Figure 2A, [Sellam et al., 2010]). The Ndt80 homolog whose targets we identified above will be referred to as Ndt80B, while the additional paralog will be referred to as Ndt80A (Figure 2—figure supplement 1). While Ndt80B has been shown to be required for biofilm formation in C. albicans (Nobile et al., 2012), the regulatory function of Ndt80A is unknown (deletion of Ndt80A has no effect on biofilm formation (Figure 4H, [Nobile et al., 2012]) or any other phenotype tested). To test the possibility that the difference in targets between Ndt80 in S. cerevisiae and Ndt80B in C. albicans is simply a consequence of this gene duplication, we identified the regulatory targets of Ndt80A in C. albicans by ChIP-Seq under control of the same constitutive promoter used for Ndt80B. We found that the targets regulated by Ndt80A represent a small subset of the targets of Ndt80B (9–11%, Figure 1—figure supplement 3); that is, Ndt80A binds to many fewer genomic regions, but all these regions are also bound by Ndt80B. If we compare the targets of Ndt80 in S. cerevisiae to the targets of Ndt80A in C. albicans, we find that less than 4% of the overall targets are shared, regardless of target identification criteria (Figure 1—figure supplement 3), demonstrating that Ndt80A’s targets are no more similar to the targets of Ndt80 in S. cerevisiae than that of Ndt80B. These experiments demonstrate that both of the Ndt80 paralogs in C. albicans have undergone significant rewiring since S. cerevisiae and C. albicans diverged, indicating that the Ndt80 gene duplication was not directly responsible for the divergence in targets between these two species.
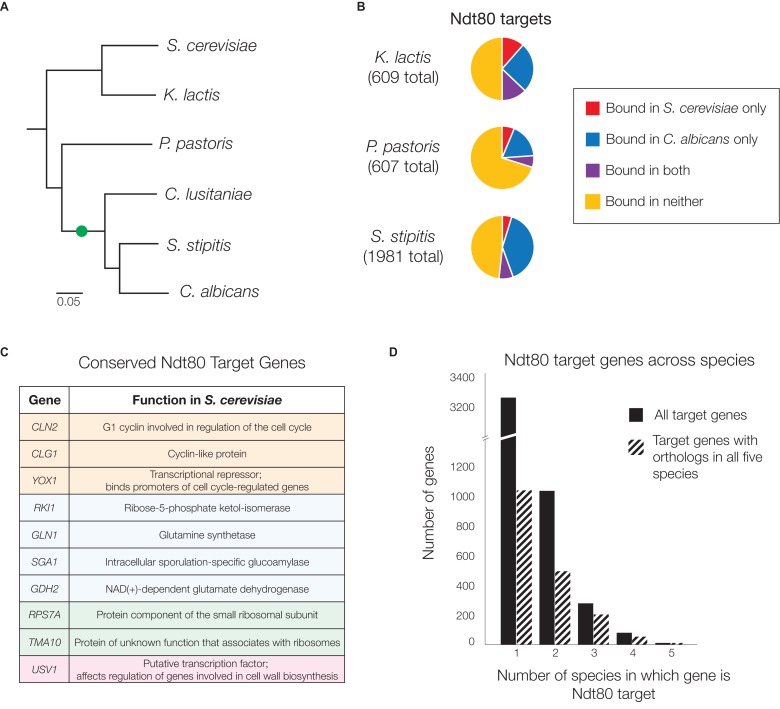
Figure 2. Ndt80 target genes differ across species descending from the S. cerevisiae - C. albicans common ancestor.
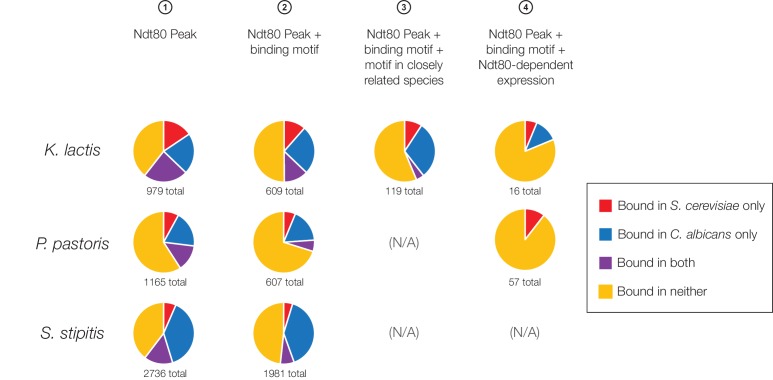
(A) Phylogenetic tree of the species investigated, inferred from protein sequences of 73 highly conserved genes (Lohse et al., 2013) with the scale representing the number of substitutions per site. The likely position of the NDT80 gene duplication is indicated with a green circle. Ndt80 protein tree shown in Figure 2—figure supplement 1. (B–D) Ndt80 targets identified by Criteria 2 (Figure 1C). (B) The proportion of Ndt80 targets in K. lactis, P. pastoris, and S. stipitis that are shared with the Ndt80 targets in S. cerevisiae, C. albicans, or both. The proportion of targets shared, using all four different criteria for identifying targets (Figure 1C) shown in Figure 2—figure supplement 2. (C) List of genes bound by Ndt80 in all five species tested with functional annotations (color-coded) from S. cerevisiae, as described in the text (SGD, [Cherry et al., 2012]). (D) Histogram of all Ndt80 targets in the five species tested (S. cerevisiae, K. lactis, P. pastoris, S. stipitis, and C. albicans) according to the number of species in which that gene is a target. All target genes (black) and those with 1:1 orthologs across all five species (dashed) are shown. For (B–D), the union of Ndt80A and Ndt80B bound genes were used for S. stipitis and C. albicans. The cis-regulatory site bound by Ndt80 in each species shown in Figure 2—figure supplement 4.
Figure 2—figure supplement 1. Similarity of Ndt80 protein sequences across many yeast species.
Figure 2—figure supplement 2. Ndt80 targets in K. lactis, P. pastoris, and S. stipitis categorized according to overlap with S. cerevisiae and C. albicans, using all four methods of target identification.
Figure 2—figure supplement 3. Histogram of simulated overlap in Ndt80 targets across five species.
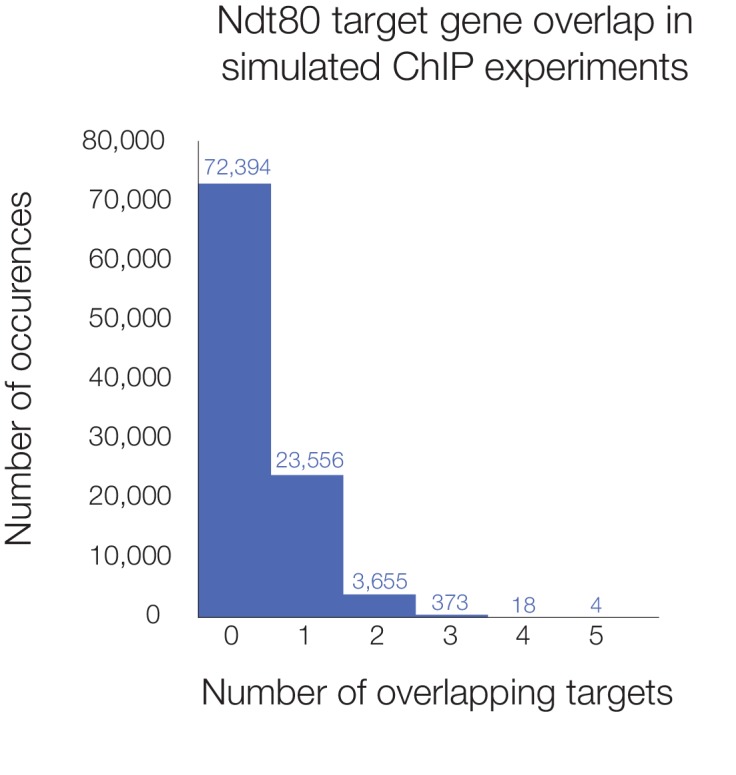
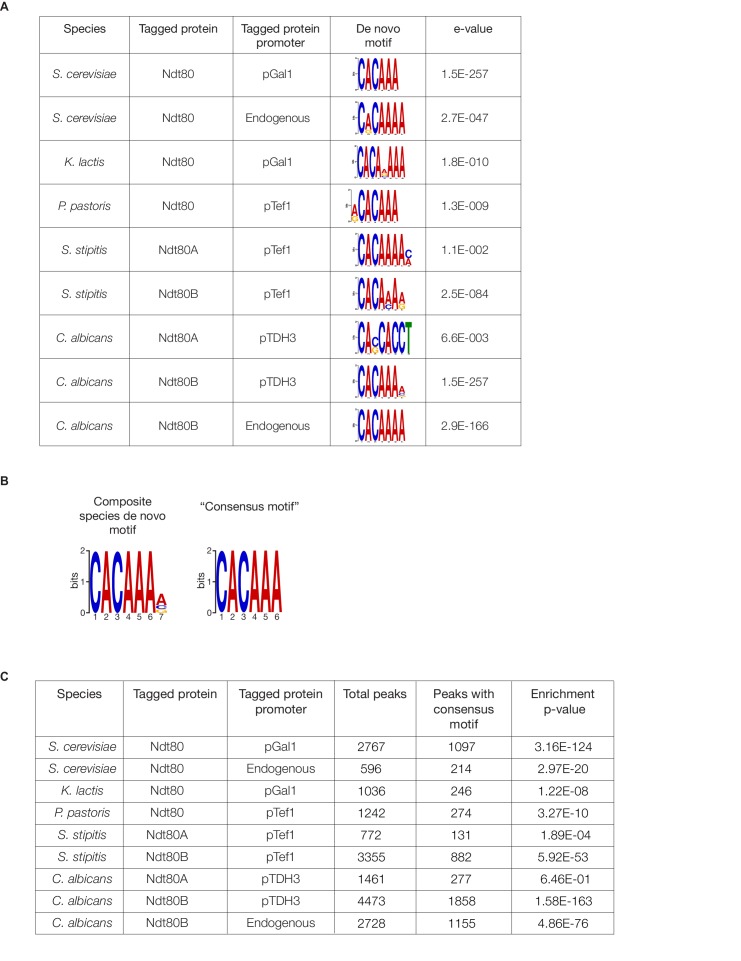
Figure 2—figure supplement 4. Ndt80 cis-regulatory motifs identified in ChIP-Seq experiments.
Ndt80 target genes also differ between S. cerevisiae, K. lactis, P. pastoris, S. stipitis, and C. albicans
To reconstruct a timeline of the evolution of the Ndt80 gene regulatory network, we conducted ChIP-Seq experiments in three additional species that branch from the common ancestor of S. cerevisiae and C. albicans at highly informative points: Kluyveromyces lactis, Pichia pastoris, and Scheffersomyces stipitis (Figure 2A). In each case, we used the strategy of expressing Ndt80 under the control of a strong constitutive promoter to minimize expression differences between species. In C. albicans and S. stipitis, targets were identified for the two Ndt80 paralogs separately, and found to be highly overlapping (Figure 1—figure supplement 3, Supplementary file 1). Thus, the union of Ndt80 paralog targets were taken to represent all Ndt80 targets in those species.
We first investigated whether the regulatory targets of Ndt80 in each of these species more closely resemble the targets in S. cerevisiae or the targets in C. albicans. Ndt80 targets were identified in all species based on the criteria of ChIP enrichment plus Ndt80 motif presence (Figure 1C), and compared to the targets of Ndt80 in S. cerevisiae and C. albicans. Counter to our initial expectation, we found that a very large number of Ndt80 targets in each species are not targets of Ndt80 in either S. cerevisiae or C. albicans (50% of all targets in K. lactis, 70% in P. pastoris, and 48% in S. stipitis, Figure 2B). Thus, the Ndt80 regulons in these three species do not closely resemble those of either S. cerevisiae or C. albicans; instead, each have acquired a distinctive set of Ndt80 target genes. If we repeat this analysis using alternative criteria for identifying Ndt80 targets (Figure 1C), this conclusion still holds (Figure 2—figure supplement 2), indicating that it is robust to the stringency of Ndt80 target identification.
Consistent with the idea that Ndt80 has acquired a unique set of targets in each species, only ten genes are targets of Ndt80 in all five species tested (Figure 2C). While this is more than expected strictly by chance considering the number of regions bound in each species (p<10−5, Figure 2—figure supplement 3), this number pales in comparison to the 3261 genes that are Ndt80 targets in just one of the five species (Figure 2D). Even if we consider only genes with 1:1 orthologs across all five species, there are more targets bound in only one species (1041) than bound in two or more species (772) (Figure 2D). In short, the Ndt80 regulon differs extensively between each of the five species tested.
Ndt80-binding differences are largely determined by the gain and loss of cis-regulatory sites
Because the Ndt80-target gene connections differ significantly between species, we considered whether these differences were due primarily to changes in the Ndt80 protein itself, or to changes in the distribution of its cis-regulatory motif across the different genomes. Although the cis-regulatory sequence recognized by Ndt80 is conserved across these species (Figure 2—figure supplement 4), changes in the Ndt80 protein itself that alter, for example, protein-protein interactions with other transcription regulators, could account for many of the differences between species. The sequence of the Ndt80 protein does differ considerably across the species tested, with only 55% similarity in the DNA-binding domain between S. cerevisiae and C. albicans (Sellam et al., 2010), and 35% similarity in the proteins overall (Figure 2—figure supplement 1).
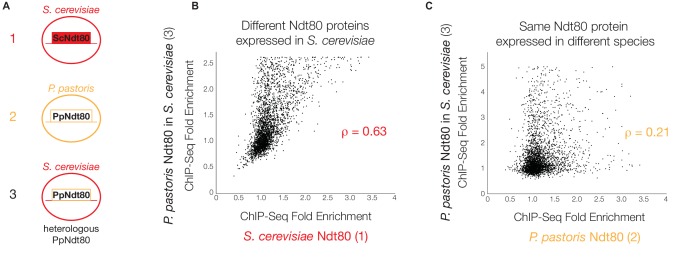
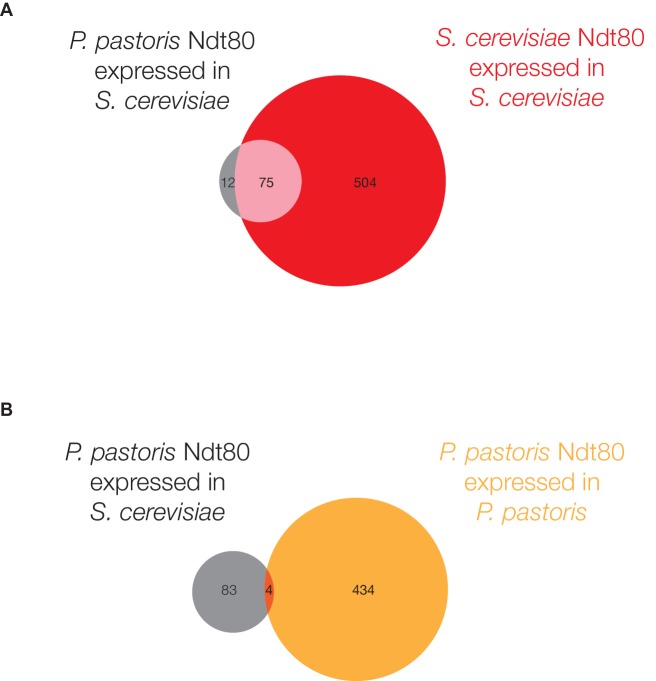
To test whether these changes in protein sequence were primarily responsible for the observed differences in Ndt80 target genes between species, we expressed the P. pastoris NDT80 gene in S. cerevisiae under the control of the same constitutive promoter used for ChIP-Seq of the endogenous S. cerevisiae NDT80, and carried out ChIP-Seq on the P. pastoris NDT80 (Figure 3A). If we compare Ndt80 ChIP enrichment genome-wide in this experiment to Ndt80 enrichment for the native Ndt80 in each species, we find a much stronger correlation between genomic binding of the heterologous P. pastoris Ndt80 and the native S. cerevisiae Ndt80 (Figure 3B) than between the heterologous P. pastoris Ndt80 and the native P. pastoris Ndt80 (Figure 3C). Similarly, we find more shared target genes between the different Ndt80 proteins expressed in S. cerevisiae (75 genes, Figure 3—figure supplement 1) than between the same P. pastoris Ndt80 protein expressed in different species (4 genes, Figure 3—figure supplement 1). These results demonstrate that changes in the Ndt80 protein sequence across species have had only a minor effect on the Ndt80-target gene connections; instead, the connections are predominantly determined by the distribution of cis-regulatory sequences across the different genomes.
Figure 3. P. pastoris Ndt80 binds to regions bound by S. cerevisiae Ndt80 when expressed in S. cerevisiae.
(A) Diagram of strains used to generate data in (B) and (C): (1) tagged native Ndt80 in S. cerevisiae, (2) tagged native Ndt80 in P. pastoris, and (3) tagged heterologous P. pastoris Ndt80 expressed in S. cerevisiae. (B and C) Comparisons of the maximum ChIP-Seq fold enrichment for the intergenic regions of all genes with 1:1 orthologs in S. cerevisiae and P. pastoris, with Spearman’s rank correlation coefficient shown (p-value=7.2×10−117 for (B), 1.02 × 10−296 for (C)). Numbers in axis labels correspond to simplified diagrams in (A). (B) S. cerevisiae Ndt80 vs. P. pastoris Ndt80 expressed in S. cerevisiae. (C) P. pastoris Ndt80 expressed in P. pastoris vs. P. pastoris Ndt80 expressed in S. cerevisiae. A comparison of Ndt80 targets identified in each experiment using Criteria 1 shown in Figure 3—figure supplement 1.
Figure 3—figure supplement 1. Heterologous P. pastoris Ndt80 targets compared to native P. pastoris and S. cerevisiae Ndt80 targets.
Although it seems likely that gains and losses of Ndt80 cis-regulatory sequences are largely responsible, we cannot formally exclude the contributions of changes in cis-regulatory sequences for other (thus far, unidentified) proteins that might help recruit Ndt80 to DNA. These observations on the key role of cis-regulatory change do not mean that changes in the protein are unimportant; indeed, the heterologous P. pastoris Ndt80 does not complement the sporulation defect observed in an ndt80 deletion in S. cerevisiae (data not shown), nor does it occupy all the genome positions characteristic of the S. cerevisiae protein. Our results do show, however, that the heterologous P. pastoris protein does occupy a substantial fraction of the S. cerevisiae-specific positions along the genome. This observation strongly supports the conclusion that the majority of the differences in the Ndt80 regulons across species are due to gains and losses of cis-regulatory sequences.
Ndt80 is required for sporulation in K. lactis, P. pastoris, and C. lusitaniae
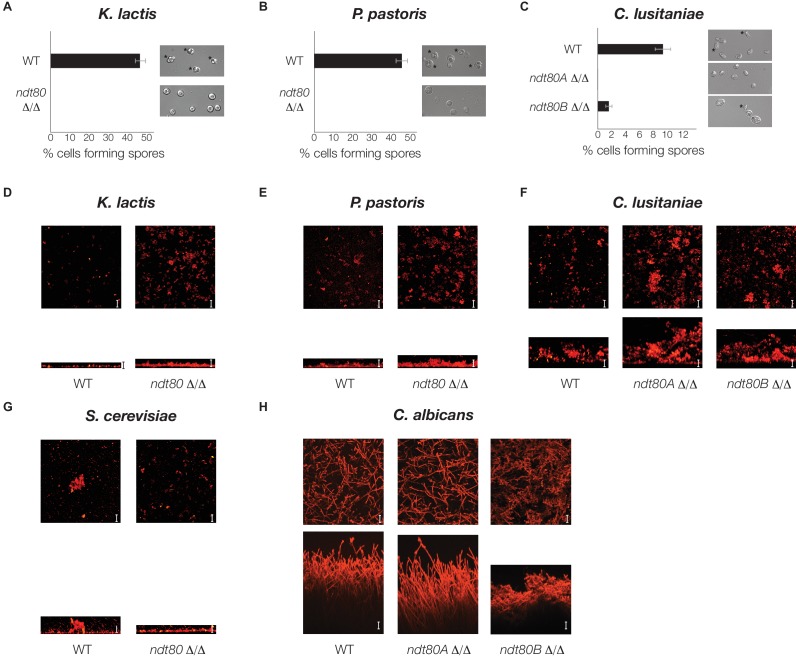
To determine whether the rewiring of the Ndt80 gene regulatory network corresponds to the changes in the overall function of Ndt80, we determined the phenotype of an ndt80 deletion mutant in three species that branch between S. cerevisiae and C. albicans: K. lactis, P. pastoris, and C. lusitaniae (Figure 2A). We first tested whether this deletion had an effect on sporulation and found, in K. lactis and P. pastoris, that the ndt80 deletion mutant is completely deficient in its ability to sporulate (Figure 4A and B). C. lusitaniae, like C. albicans, has two paralogs of Ndt80. Deletion of one paralog (Ndt80A) results in a complete deficiency in sporulation, while deletion of the other paralog (Ndt80B) results in a significant reduction in sporulation efficiency (Figure 4C). In contrast to these four species, C. albicans has never been observed to undergo sporulation and meiosis, relying instead on an alternate parasexual cycle (Bennett and Johnson, 2003; Butler et al., 2009). Given that Ndt80 is required for sporulation in S. cerevisiae (Chu and Herskowitz, 1998; Xu et al., 1995), K. lactis, P. pastoris, and C. lusitaniae, and given the phylogenetic relationship between these species (Figure 2A), the most parsimonious model is that, in the ancestor of these species, Ndt80 regulated sporulation.
Figure 4. Ndt80 is required for sporulation but is dispensable for biofilm formation, in K. lactis, P. pastoris, and C. lusitaniae.
(A–C) Light microscope images of genetically matched wild-type and ndt80 deletion strains (Stars indicate diploid cells that have undergone sporulation) and quantification of the percent of cells exhibiting spores, as measured by microscopy (200 cells counted for each strain). (D–H) Confocal scanning laser microscopy images of biofilm formation for genetically matched wild-type and ndt80 deletion strains. Top view of biofilm shown above side view for each, with scale bars representing 25 µm.
We next investigated the requirement for Ndt80 in biofilm formation in these species. In C. albicans, biofilms consist of thick structures of different cell types (yeast-form and hyphae) that can form both in vitro and in vivo [Andes et al., 2004]). Deletion of NDT80B in C. albicans causes a severe defect in surface adherence and biofilm formation (Figure 4H). In contrast, in S. cerevisiae, K. lactis, P. pastoris, and C. lusitaniae, the ndt80 mutants and wild-type are comparable in their ability to adhere to a solid surface (Figure 4D–G). It is worth noting that S. cerevisiae, K. lactis and P. pastoris form only a thin layer of yeast-form cells on the solid surface rather than the type of thick, multicellular biofilms characteristic of those formed in C. albicans, while C. lusitaniae forms a biofilm somewhat thinner than that of C. albicans. These results highlight the specialized type of biofilm produced by C. albicans and the pivotal role played by Ndt80 in its evolution.
Taken together, these results indicate that Ndt80 regulated sporulation in the shared ancestor of S. cerevisiae and C. albicans, and that it gained a role in biofilm formation along the C. albicans lineage, after it branched off from C. lusitaniae (Figure 2A). We believe this scenario is more likely than that of the next most parsimonious model, which holds that Ndt80 regulated both sporulation and biofilm formation in the shared ancestor, as this would require at least three independent losses of biofilm regulation. Given that Ndt80 regulates hundreds of genes in each species, a single gain of Ndt80 function seem more plausible than three independent losses.
Combining this phenotypic data with the Ndt80 target identification previously discussed, we can begin to pinpoint the importance of individual genes in the overall switch in Ndt80 function. Only 10 genes are targets of Ndt80 in all five species tested (S. cerevisiae, K. lactis, P. pastoris, S. stipitis, and C. albicans) (Figure 2C). This suggests that a very limited set of genes may have been repurposed from sporulation to biofilm formation. Notably, five of these genes (CLN2, CLG1, GLN1, GDH2, RPS7a) are strongly upregulated in biofilm formation (Fox et al., 2015). If we examine the 10 shared genes based on their known functions in S. cerevisiae, three are involved in cell cycle regulation (CLG1, CLN2, and YOX1), four are metabolic enzymes (GDH2, GLN1, RKI1, and SGA1), two are proteins associated with the ribosome (RPS7a and TMA10), and one is involved in regulation of cell wall biosynthesis (USV1) (Figure 2C). The protein Usv1 is particularly intriguing, as its ortholog in C. albicans, Bcr1, is one of the other master regulators of biofilm formation (Nobile et al., 2012; Nobile and Mitchell, 2005). This observation suggests that two of the master regulators of biofilm formation, Ndt80 and Bcr1, may have had an ancestral regulatory relationship that was co-opted in the evolution of the biofilm regulatory network. In summary, while there are small vestiges of the sporulation network present in the C. albicans biofilm network, the great majority of connections are new.
The transcription network that controls meiosis and sporulation changes extensively
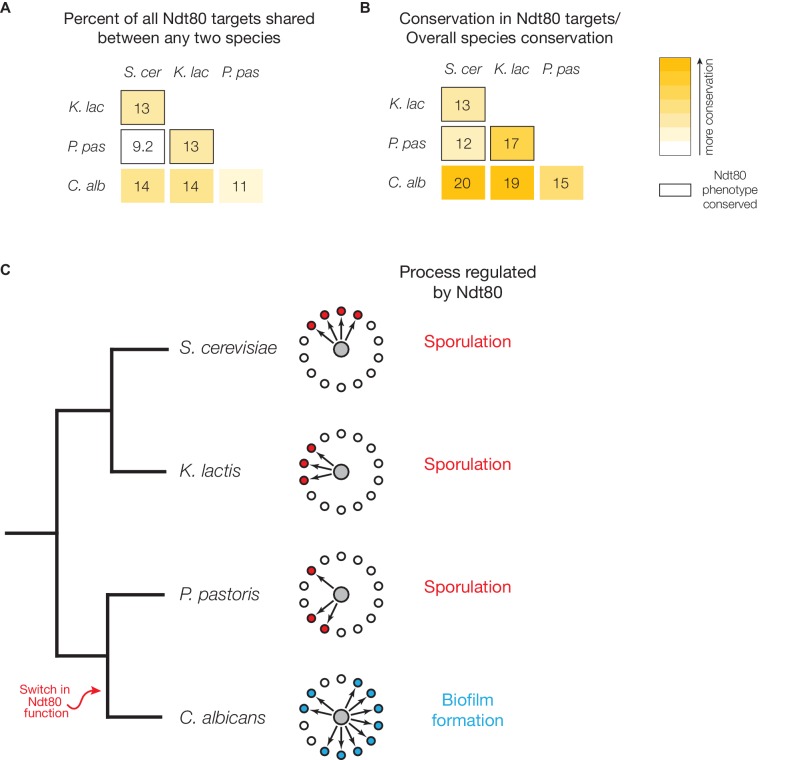
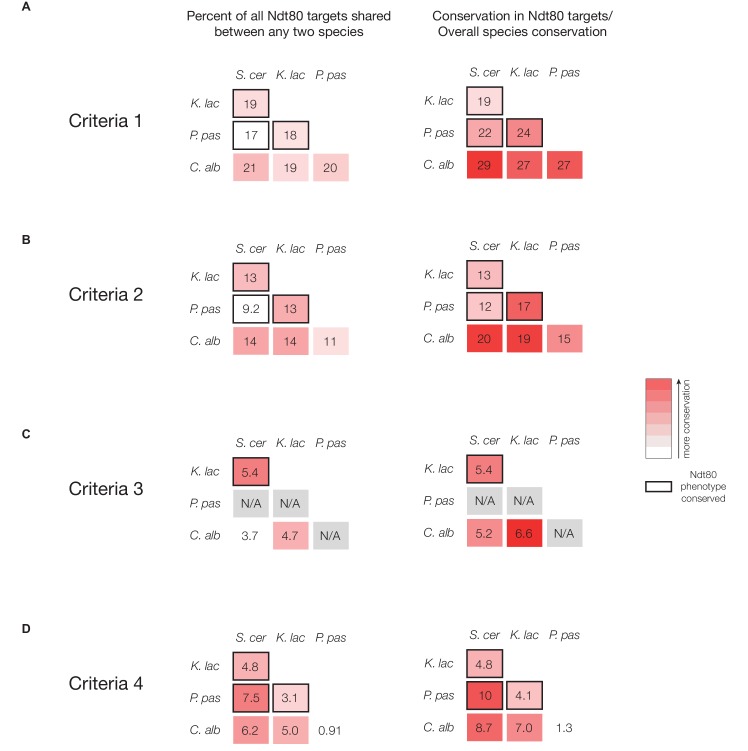
Because the targets of Ndt80 appear to have rewired significantly during the switch from sporulation regulation to biofilm regulation, we tested whether Ndt80 targets are more similar among species with a conserved Ndt80 phenotype than among species with a diverged Ndt80 phenotype. If so, this would suggest that the dramatic rewiring of targets was associated with the switch in Ndt80 regulatory function. We focused on the four species for which we know both the Ndt80 phenotype and the binding targets: S. cerevisiae, K. lactis, P. pastoris, and C. albicans. For every two-species pair, we compared the Ndt80 targets, considering only genes with 1:1 orthologs for both species. We find relatively little overlap in Ndt80 targets for any of these comparisons (9–14%), but more importantly, we do not find a correlation between target overlap and conservation of overall Ndt80 function (Figure 5A). For example, a larger fraction of targets (14%) are shared between S. cerevisiae and C. albicans than between S. cerevisiae and P. pastoris (9.2%). If we take into account the different divergence times for each of these two-species comparisons (normalizing to the divergence time between S. cerevisiae and K. lactis), we still find that conservation of overall Ndt80 function does not correlate with increased conservation of Ndt80 targets (Figure 5B). This pattern also holds if we use more stringent criteria to identify Ndt80 targets in each species (Figure 5—figure supplement 1). Overall, these results show that the targets of Ndt80 change extensively, even while Ndt80’s conserved role in regulating sporulation is maintained (Figure 5C). While we cannot entirely rule out the possibility that Ndt80 has another, unknown regulatory function in one or more of these species that accounts for the significant difference in Ndt80 targets, we know that S. cerevisiae Ndt80 is produced specifically during sporulation (Chu and Herskowitz, 1998). Similarly, by performing transcription profiling, we find that in K. lactis and P. pastoris, Ndt80 is not expressed in mitotic cells (Supplementary file 1), consistent with the idea that Ndt80 is primarily needed during sporulation and is unlikely to have additional regulatory roles.
Figure 5. The targets of Ndt80 differ among species even with a conserved Ndt80 phenotype.
(A) Percent of all Ndt80-bound genes (identified using Criteria 2) shared between any two species tested; for this analysis, only 1:1 orthologs in each two-species comparison were considered. (B) Percent of all Ndt80-bound genes shared between any two species, normalized to the genome-wide substitutions per site (S. cerevisiae – K. lactis set to 1). Species comparisons between two species with a conserved Ndt80 phenotype shown with black outline for (A) and (B). Percent overlap and normalized percent overlap for targets identified using all four criteria (Figure 1C) shown in Figure 5—figure supplement 1. (C) Cladogram of species for which Ndt80 phenotype and target genes have been identified, with simplified cartoons representing Ndt80 ChIP-Seq targets in each species. The grey circle in the center represents Ndt80, while the smaller circles represent genes present in all four species, with each small circle representing ~100 genes. The arrows indicate that Ndt80 binds to those genes in that species.
Figure 5—figure supplement 1. Percent overlap in Ndt80 targets across different species, using all four methods of target identification.
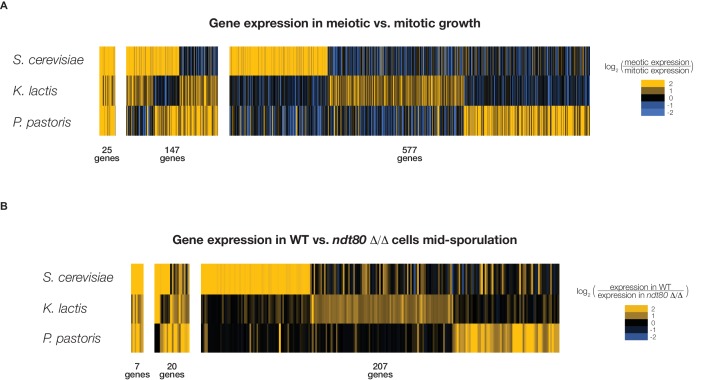
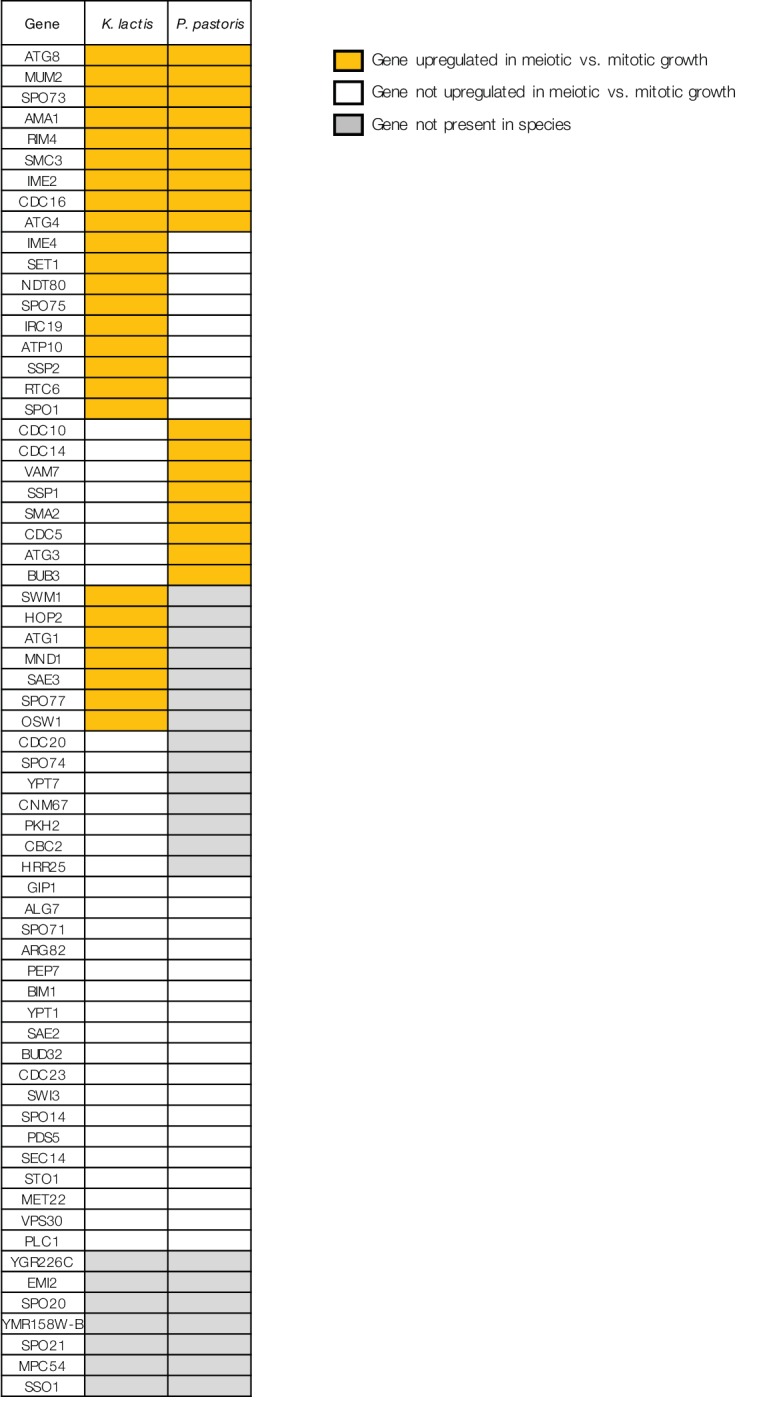
The rewiring of Ndt80 targets across species in which Ndt80 is known to regulate sporulation indicates that only a small number of Ndt80 targets must be maintained in order for the sporulation network to be functional. Although there are several possible explanations for how Ndt80 could have maintained a role in sporulation despite the near complete turnover of its target genes, we tested perhaps the most intriguing hypothesis: the genes required for sporulation and meiosis are themselves changing across species. To test this hypothesis, we measured global gene expression during sporulation and mitotic growth in K. lactis and P. pastoris (Materials and methods). We compared the genes upregulated during sporulation in these species to those upregulated during sporulation in S. cerevisiae (Chu et al., 1998) and found extensive differences in the genes activated during sporulation across these species (Figure 6A). Only 25 genes show sporulation-activation across S. cerevisiae, K. lactis, and P. pastoris, while 577 genes are activated uniquely in only one of the three species. Of the 65 genes known to be required for sporulation in S. cerevisiae that also exhibit sporulation-specific activation, only nine are activated during sporulation in both K. lactis and P. pastoris, with 21 of these genes missing altogether from the genomes of one or both of these species (Figure 6—figure supplement 2). We also measured global gene expression in an ndt80 deletion mutant in both K. lactis and P. pastoris under sporulation conditions, and found that the genes whose expression depends on Ndt80 during sporulation also differ significantly across species, with only seven genes showing Ndt80-dependent expression in all three species (Figure 6B, Figure 6—figure supplement 1). We conclude that there are significant differences in sporulation-specific gene expression among S. cerevisiae, K. lactis, and P. pastoris, and that this contributes significantly to the large differences observed in Ndt80 targets across species.
Figure 6. Genes induced during sporulation differ significantly across S. cerevisiae, K. lactis, and P. pastoris.
(A) Gene expression in meiotic growth compared to mitotic growth. Genes with significant upregulation in meiosis in at least one species shown. (B) Gene expression in a wild-type strain compared with an ndt80 △/△ strain for all three species. Genes with significant upregulation in wild-type in at least one species shown. For (A) and (B), each line represents a single gene with the color of the line representing the ratio of expression in the two conditions. Numbers below represent the number of genes shared in all three species (left), in two species (middle), or in just one species (right). Data for S. cerevisiae from Chu et al. (1998), data for K. lactis and P. pastoris generated in this study. A comparison of the 25 shared sporulation genes (A) and the seven shared Ndt80-dependent genes (B) shown in Figure 6—figure supplement 1.
Figure 6—figure supplement 1. Comparing sporulation-induced genes and Ndt80-dependent genes across S. cerevisiae, K. lactis, and P. pastoris.
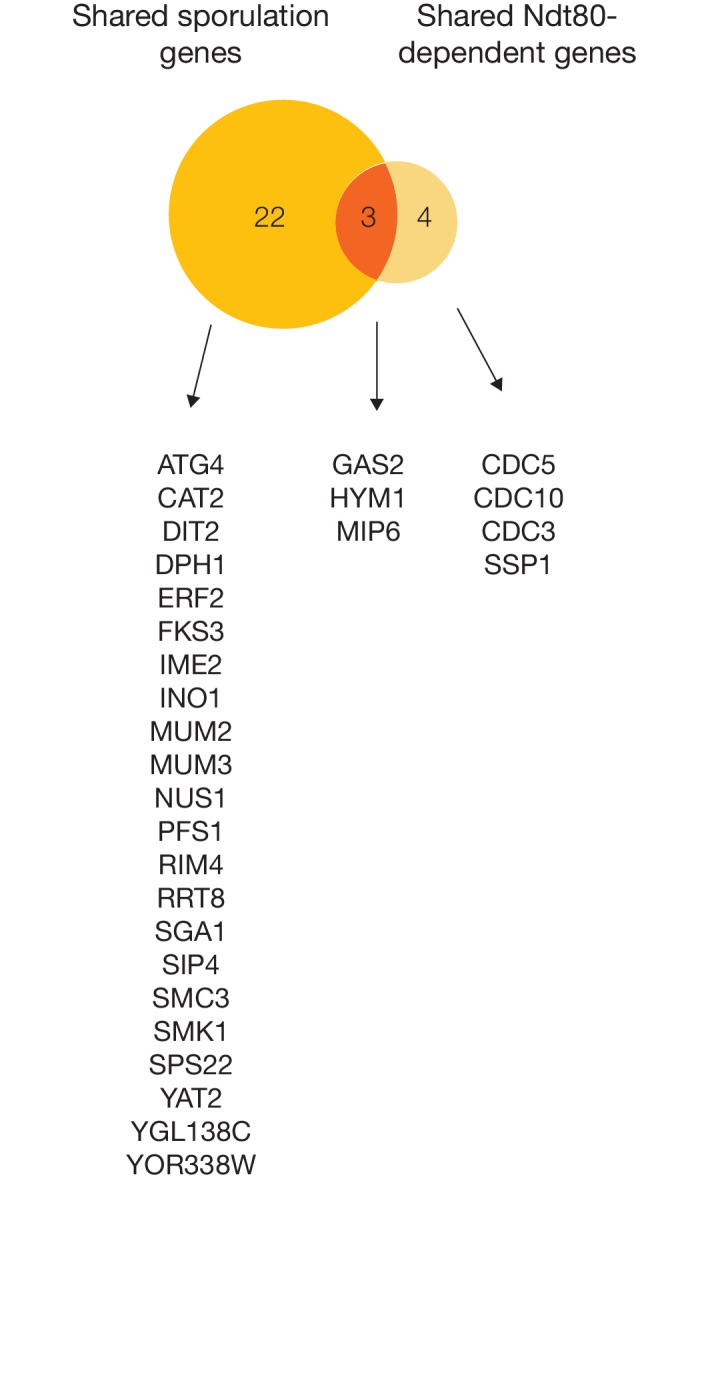
Figure 6—figure supplement 2. Comparing patterns of meiotic expression for genes required for sporulation in S. cerevisiae.
Discussion
Using experiments performed in six different extant yeast species, we have deduced that the transcription regulator Ndt80 underwent extensive rewiring of its regulatory connections before it became incorporated into a newly evolving regulatory network. This incorporation, which occurred on the lineage leading to C. albicans, resulted in an overall switch in Ndt80’s function from an ancestral role in regulating sporulation and meiosis to a derived role in regulating biofilm formation. We have shown that this switch in function was not accompanied by a change in the DNA-binding specificity of Ndt80 (Figure 1B, Figure 2—figure supplement 4); rather, a change in the distribution of the conserved Ndt80 cis-regulatory sequence across hundreds of genes in the genome largely accounts for the different functions of Ndt80 in S. cerevisiae and C. albicans.
A priori, we considered two likely explanations for the change in Ndt80 function. First, we hypothesized that a duplication of Ndt80 in the C. albicans clade could have allowed the ancestral function (sporulation) to be conserved in one paralog, while the other acquired a new function (biofilm regulation). However, the similarity in function of Ndt80 paralogs in C. lusitaniae (Figure 4C and F) as well as the similarity in targets of Ndt80 paralogs in C. albicans (Figure 1—figure supplement 3) rule out this model. A second plausible model held that a sudden change in Ndt80-target gene connections occurred along the C. albicans lineage, perhaps triggered by the loss of meiosis in this clade, relaxing constraints on Ndt80 target gene connections and allowing old connections to rapidly break and new connections to rapidly form. However, we also ruled out this model by showing that similarly high rates of Ndt80 rewiring occurred in all phylogenetic branches examined, even those where the function of Ndt80 remains conserved in sporulation (Figure 5A and B). Rather than a sudden shift in regulation, our results indicate continuous flexibility in the regulon of Ndt80, allowing it to sample many configurations even while retaining its ancestral function. In support of this idea, we showed that genes upregulated during sporulation (by Ndt80 and other regulators of sporulation) vary considerably across different species (Figure 6), indicating that many different network configurations can support sporulation. Consistent with this idea, it has previously been noted that several key genes required for meiosis and sporulation in S. cerevisiae (IME1, ZIP2, SPO13) are missing from the genomes of species such as C. lusitaniae that are known to undergo meiosis and sporulation (Bennett and Johnson, 2003; Sherwood and Bennett, 2009). These observations, taken together, indicate that there is no ‘universal’ solution to meiosis and sporulation in terms of both the genes required for this process and the regulatory network itself. Instead, many different networks can apparently orchestrate this ancient process.
This work illustrates how a transcription regulator with hundreds of ancestral connections can, nonetheless, completely shift its cellular function. We propose that the continuous exploration of regulatory connections available to Ndt80—while still maintaining its role in sporulation—facilitated it reaching a network configuration that supported a new role in biofilm regulation. Computational models had previously led to this view, namely that gene regulatory networks are inherently flexible, allowing for the exploration of many configurations that maintain an overall function (Barve and Wagner, 2013; Wagner, 2005a). This exploration allows networks to sample many points in ‘network space’, some of which may be only a few changes away from generating a novel output. We believe that Ndt80 represents a tangible example of this idea: we propose that it is only through the inherent flexibility of the Ndt80 connections that the network—while still maintaining sporulation and meiosis—could reach a position where the initial transition to regulating biofilm formation would be a simple one.
Although other transcription regulators may be subject to stronger evolutionary constraints than Ndt80, there is ample evidence that many transcription networks, like that of Ndt80, can drift through new configurations while preserving their transcriptional output (Baker et al., 2012; Hare et al., 2008; Lavoie et al., 2010; Swanson et al., 2011; Tanay et al., 2005; Tsong et al., 2006; Villar et al., 2015). Although the overall rate of network rewiring has not been extensively documented, it seems likely that the rewiring of the Ndt80 regulon is not unusually rapid when compared to at least some other fungal regulators (Borneman et al., 2007; Tuch et al., 2008). While much regulatory rewiring likely occurs through drift with no obvious shift in phenotype, we propose that continuous rewiring also predisposes regulators to a shift in function. Such extreme, inherent flexibility in gene regulatory networks, such as that exhibited by Ndt80, seems likely to be a general model to explain how complex regulatory networks can evolve to produce novel phenotypes.
Materials and methods
Media
All strains were grown in YPD at 30°C unless otherwise noted. For galactose induction, strains were grown in SRaffinose +1.7% galactose. For sporulation, S. cerevisiae strains were grown in liquid YPA (2% peptone, 2% potassium acetate, 1% yeast extract) and incubated at room temperature for 20–30 hr. For K. lactis, saturated liquid cultures in YPD were spotted onto SPO plates (1% potassium acetate, 2% agar + amino acids) and incubated at room temperature for 3 days. For P. pastoris, cells growing on YPD plates were patched onto 0.5% sodium acetate, 1% potassium chloride, 1% glucose, 2% agar plates and incubated at room temperature for 3 days. For C. lusitaniae, cells growing on YPD plates were patched onto PDA plates (0.37% potato dextrose +1.45% agar) and incubated at room temperature for 4 days. To quantify sporulation efficiency, three technical replicates (the same strain, grown up and plated on sporulation media independently) were performed and 200 cells were counted for each sample by DIC microscopy. In addition, a biological replicate (an independently-generated deletion strain) was assayed to confirm the phenotype of the first biological replicate. Nuclear staining using DAPI (K. lactis, P. pastoris) and Hoecsht (C. lusitaniae) was used to verify spore formation in each experiment.
Strain construction
Strains used in this study are listed in Supplementary file 2A and primers used for strain construction are listed in Supplementary file 2B. Gene disruption cassettes for Ndt80 deletions were constructed by fusion PCR. For K. lactis, two cassettes were constructed, one with a URA3 marker, one with a KanMX marker, each with 700 bp homology flanking the markers on either side. These constructs were transformed, sequentially, into a wild-type diploid strain. For P. pastoris and C. lusitaniae, a split marker approach was used and two constructs were generated for each gene disruption. For P. pastoris, a Hygromycin resistance marker and 700 bp flanking homology were used, and the split markers were transformed into two haploid strains of complementary mating types. These strains were then mated to form a diploid ndt80 deletion (ploidy was verified by FACS). For C. lusitaniae, a NAT resistance marker was used along with 1 kb of flanking homology. The split markers were transformed into a wild-type α-cell, that was then mated with a wild-type a-cell, sporulated, and ndt80 deletion a-cells were picked and verified by PCR. These were then mated with the ndt80 deletion α-cell to generate a diploid ndt80 deletion strain.
Tagged strains for ChIP were generated using a 13x C-terminal Myc tag (Longtine et al., 1998) inserted into the genome at the C terminus of the endogenous Ndt80 protein sequence. For S. cerevisiae, long primers were used to amplify the Myc tag fused to a KAN marker from pFA6a-13Myc- kanMX6 (Longtine et al., 1998), and this construct was integrated into a wild-type W303 strain. For K. lactis, split marker constructs were generated with 700 bp flanking homology fused to the Myc-KAN cassette and transformed into a wild-type diploid strain. For P. pastoris, split marker constructs were generated with 700 bp flanking homology fused to the Myc-NAT construct amplified from pADH34 (Hernday et al., 2010). In S. stipitis, homologous recombination was found to be very inefficient, and thus, constructs were randomly integrated into the genome. Constructs containing a NAT marker upstream of an E. gossypii Tef1 promoter upstream of S. stipitis Ndt80 fused to a 13x C-terminal Myc tag with a Sat1 terminator were generated by PCR for both Ndt80A and Ndt80B and transformed into a wild-type strain. Two independent isolates were generated for each paralog and tested independently by ChIP-Seq to verify that the location of integration did not affect Ndt80 function or genomic binding. To test binding of P. pastoris Ndt80 in S. cerevisiae, fusion PCR was used to generate a construct with a Hygromycin resistance marker fused to the Gal1 promoter from S. cerevisiae upstream of P. pastoris Ndt80 fused to a 13x C-terminal Myc tag with a Sat1 terminator. This was fused to homology to URA3 and integrated in an S. cerevisiae wild-type at the URA3 locus. All Myc-tagged Ndt80 strains in S. cerevisiae, K. lactis, and P. pastoris, were tested for their ability to sporulate to ensure the tag did not interfere with endogenous Ndt80 function.
To generate strains with highly expressed Ndt80, different constitutive promoters were used, and constructs generated by fusion PCR to integrate these upstream of Ndt80. In S. cerevisiae, pGal1 from S. cerevisiae was used; in K. lactis pGal1 from K. lactis was used; in P. pastoris and S. stipitis pTef1 from E. gossypii was used; in C. albicans pTDH3 from C. albicans was used. In all cases, RT-qPCR was performed on the resulting strains as well as a wild-type strain to ensure that the constitutive promoters were indeed driving high Ndt80 expression.
S. cerevisiae and C. albicans were transformed using standard lithium acetate protocols. Electroporation protocols were used for K. lactis (Booth et al., 2010), P. pastoris (Cregg et al., 2009), and S. stipitis. The protocol for S. stipitis was adapted from Cregg et al. (2009), with cells harvested at an OD of 1.3–1.5.
Genome sequences, gene annotations, and orthology mapping
Genome sequences and gene annotations for S. mikatae, S. kudriavzevii, K. lactis, E. gossypii, and E. cymbalariae were downloaded from the Yeast Gene Order Browser (YGOB) (Byrne and Wolfe, 2005). Genome sequences and gene annotations for S. cerevisiae, C. lusitaniae, S. stipitis, C. dubliniensis, C. tropicalis, and C. albicans were downloaded from the Candida Gene Order Browser (CGOB) (Maguire et al., 2013). The genome sequence of P. pastoris strain CB7435 (Küberl et al., 2011) was downloaded from NCBI (accession number: PRJEA62483), and gene annotations were generated using the Yeast Genome Annotation Pipeline (Proux-Wéra et al., 2012). Gene annotations for YGOB, CGOB, and YGAP also include synteny-based orthology mapping, which was used to compare genes across species.
Chromatin immunoprecipitation and high-throughput sequencing
For all ChIP experiments with endogenous or pTef1 promoters driving Ndt80 expression, samples were isolated from log-phase cultures grown in YPD and chromatin immunoprecipitation was performed as previously described (Hernday et al., 2010) using an anti-Myc monoclonal antibody (RRID: AB_2536303). Libraries were prepared using NEBNext Multiplex Kit for Illumina as previously described (Sorrells et al., 2015) and sequenced on an Illumina HiSeq 4000. For the mid-sporulation ChIP in S. cerevisiae, cells were induced to sporulate as previously described (Chu and Herskowitz, 1998; Hepworth et al., 1998), and samples were isolated after 16 hr in sporulation media. For ChIP of strains with pGal1 driving Ndt80 expression, strains were grown overnight in SRaffinose, diluted back and grown until log-phase in SRaffinose, then grown in SRaffinose +1.7% galactose for 5 hr.
For each ChIP-Seq experiment, a control experiment was performed using a matched strain missing the C-terminal Myc tag. Two biological replicates (independently grown single-colonies of the same strain) were performed for both the tagged strain and the control untagged strain to identify regions bound by Ndt80 for that strain and condition.
RNA sequencing in P. pastoris design and analysis
RNA expression was measured in P. pastoris using RNA-Seq. Samples were isolated from log-phase cultures in YPD or from mid-sporulation cultures after 30 hr. Total RNA was isolated using the Ambion RiboPure kit, and mRNA was isolated using the Oligotex mRNA Mini kit. Purified mRNA was then concentrated using the Zymo RNA Clean and Concentrator kit. Sequencing libraries were prepared using the NEBNext Ultra Directional RNA Library Prep Kit and sequenced on an Illumina HiSeq 4000. Three technical replicates (independently-grown single colonies of the same strain) were performed for each strain.
Reads were aligned to the genome using TopHat (Trapnell et al., 2009). Transcripts were then assembled, abundance was estimated, and differential expression was detected using Cufflinks (Trapnell et al., 2013).
Full genome expression microarray in K. lactis
Custom-designed K. lactis microarrays were used to measure differential expression of genes in sporulation vs. mitotic growth and in an ndt80 deletion vs. wild-type in sporulation media as previously described (Booth et al., 2010). Samples were isolated from log-phase cultures in YPD or from mid-sporulation cultures after 21 hr. Two technical replicates (independently-grown single colonies of the same strain) of each experiment were performed and the average of these replicates was taken.
Comparing gene expression across S. cerevisiae, K. lactis, and P. pastoris
To identify genes upregulated in sporulation (Figure 6—figure supplement 1), different thresholds were used to compare gene expression in a wild-type strain grown in YPD to a wild-type strain grown in sporulation media. For S. cerevisiae, this threshold was a 2.5-fold expression increase in sporulation vs. mitotic growth, in a published dataset (Chu et al., 1998). For K. lactis, the threshold was a 1.5-fold expression increase in sporulation vs. mitotic growth in expression microarrays (described in detail above). For P. pastoris, the threshold was a statistically significant increase in sporulation vs. mitotic growth, as determined by analysis using Cuffdiff (Trapnell et al., 2013).
Similarly, to identify genes exhibiting Ndt80-specific expression in sporulation (Figure 6—figure supplement 1), expression was compared between a wild-type strain grown in sporulation media and an ndt80 deletion strain grown in sporulation media. Different thresholds were applied to identify genes showing Ndt80-dependent expression: at least two-fold expression increase in WT vs. ndt80 mutant for S. cerevisiae, at least 1.5-fold expression increase in in WT vs. ndt80 mutant for K. lactis, and a statistically significant increase (Trapnell et al., 2013) in P. pastoris.
Biofilm experiments
Biofilms were grown and imaged by confocal scanning laser microscopy (CSLM) similar to previously described (Mancera et al., 2015). In brief, silicon squares were pre-incubated overnight in bovine serum at 30°C, washed with phosphate-buffered saline (PBS), and then submerged in Spider media containing 1% glucose instead of mannitol. Then cells from a culture grown overnight in YEPD at 30°C were added to the silicone squares upon reaching an OD600 of 0.5. The squares were incubated for 90 min at 30°C shaking at 200 rpm for cell adherence. After adherence the squares were washed with PBS and transferred to fresh Spider 1% glucose and incubated for 48 hr at 30°C shaking at 200 rpm. For CSLM, the biofilms grown on the silicon squares were stained with concanavalin A Alexa Fluor 594 conjugate (50 μg/ml) and visualized using a Nikon Eclipse C1si upright spectral imaging confocal microscope and a 40x/0.80W Nikon objective.
Identifying regions of Ndt80 binding
Sequencing reads were aligned to the genome using Bowtie (Langmead et al., 2009). Alignments were converted for visualization using SAMtools (Li et al., 2009). Peaks and fold enrichment values were generated using MACS2 (Zhang et al., 2008) with peak shift sizes generated using SPP (Kharchenko et al., 2008). Peaks were assigned to genes if any portion of the peak overlapped with the intergenic region upstream of that gene using MochiView (Homann and Johnson, 2010). For the heterologous P. pastoris Ndt80 in S. cerevisiae ChIP, fold enrichment values were mapped to genes by taking the maximum fold enrichment value for each intergenic region and mapping that to neighboring genes, also using MochiView.
Two biological replicates were performed for each ChIP-Seq experiment. Genes with Ndt80 peaks were identified for each replicate, and only genes with peaks in both replicates were considered as bona fide targets. For fold enrichment per gene calculations, the average of the maximum fold enrichment per intergenic region in each replicate was taken as the fold enrichment for neighboring genes.
Ndt80 targets were identified using several different criteria (Figure 1C). For Criteria 1, a gene was considered to be bound by Ndt80 if a peak was present in the intergenic upstream of that gene. For Criteria 2, a gene was considered to be bound by Ndt80 if a peak was present in upstream intergenic region and a consensus motif (CACAAA) was also present in that intergenic region (we did not require the motif to overlap the peak location). For Criteria 3, a gene was considered to be bound by Ndt80 if a peak was present in the upstream intergenic region, and a consensus motif was present in the intergenic of that species as well as intergenic regions upstream of orthologous genes in two closely related species. For S. cerevisiae, S. mikatae and S. kudriavzevii were used in this analysis; for K. lactis, E. gossypii and E. cymbalariae were used; and for C. albicans, C. tropicalis and C. dubliniensis were used (Figure 2—figure supplement 1). For Criteria 4, a gene was considered to be bound by Ndt80 if a peak was present in the upstream intergenic region, a consensus motif was also present in the upstream intergenic region, and the gene exhibited Ndt80-dependent expression. In S. cerevisiae, this was defined as at least a twofold change in expression in an ndt80 deletion strain (up or down) compared with a wild-type strain during middle-meiosis (Chu et al., 1998). In K. lactis, this was defined as at least a 1.5-fold change in expression in the average of two replicates of an ndt80 deletion compared to wild-type, as measured by expression array. In P. pastoris, this was defined as a statistically significant change in expression, as measured by Cufflinks (Trapnell et al., 2013) between three replicates of an ndt80 deletion strain and three replicates of a wild-type strain. In C. albicans, this was defined as at least a 1.5-fold change in expression between an ndt80 deletion and wild-type (Nobile et al., 2012).
DNA cis-regulatory sequence motif discovery and enrichment
Ndt80 motifs were generated de novo for each ChIP-Seq experiment using DREME (Bailey, 2011). The union of peak locations in two biological replicates for each experiment were submitted to DREME, with a random set of genomic sequences of the same length as a negative control. The motif most closely matching the known Ndt80 binding site from S. cerevisiae (Jolly et al., 2005) and C. albicans (Nobile et al., 2012) was recorded along with the accompanying e-value (Figure 2—figure supplement 4).
A consensus Ndt80 motif across all species was generated using the union of replicate peak locations for all experiments performed with highly-expressed Ndt80. For C. albicans and S. stipitis, the intersection of replicate peaks was taken for each Ndt80 paralog, and then the union of paralog peak locations were taken to represent Ndt80 binding in that species. Peak locations for all five species tested were submitted to DREME, using a shuffled set of the same sequences as a negative control. The resulting motif (Figure 2—figure supplement 4) was trimmed by one base pair to give the high-confidence ‘consensus motif’ using for identifying high-confidence Ndt80 binding: CACAAA.
To determine enrichment of the consensus motif in binding locations for each ChIP-Seq experiment, compared to a random genomic background, a Fisher’s one-tailed exact test was performed. The number of motifs in the peak locations for an experiment was compared to the number of motifs in a set of randomly generated genomic sequences of the same length to generate a p-value representing enrichment (Figure 2—figure supplement 4).
Phylogenetic tree building
A phylogenetic tree of relevant species was constructed as previously described (Lohse et al., 2013). Protein sequences for 73 orthologs present in a single copy in all species were concatenated and aligned using MUSCLE (Edgar, 2004) and a tree was constructed using PHYML (Guindon et al., 2010). Two outgroup species, N. crassa and A. nidulans were used in the building of the tree to improve root placement, but were omitted from the tree image (Figure 2A) for simplicity.
A phylogenetic tree of Ndt80 protein sequences (Figure 2—figure supplement 1) was similarly constructed by aligning all Ndt80 protein sequences using MUSCLE (Edgar, 2004) and using PHYML to build a tree (Guindon et al., 2010). Similarity matrix of protein sequences (Figure 2—figure supplement 1) was generated by MUSCLE.
Estimating sequence conservation between species
To determine overall sequence conservation between two species (Figure 5B, Figure 5—figure supplement 1), branch lengths from the phylogenetic species tree were used (Figure 2A). The additive branch lengths separating two species from the most recent common ancestor were calculated for each two-species comparison, and then this was normalized to the value for S. cerevisiae and K. lactis (arbitrarily set to 1). This number was then multiplied by the fraction of Ndt80 targets conserved for any given two-species comparison to get a divergence time-corrected value.
Simulation of overlap in Ndt80 ChIP-Seq targets across all species
In order to determine whether the 10 genes shown to be Ndt80 targets in all five species represented more than expected by chance (based on the number of targets in each species), we performed 100,000 simulations. In each simulation, genes were randomly selected from the pool of 3171 genes with orthologs in all five species, to represent the Ndt80 targets in all five species. For S. cerevisiae, 424 genes were selected, for K. lactis 269, for P. pastoris 300, for S. stipitis 958, and for C. albicans 989, as these are the number of mappable targets of Ndt80 in each species according to Criteria 2 (Figure 1C). For 100,000 simulations, the median number of genes found in all five sets was zero, and the maximum was five. Ten or more genes were not found in any round of the simulation (p-value<0.00001).
Data deposition
ChIP-Seq and RNA-Seq data has been deposited to the NCBI Gene Expression Omnibus (GEO) repository under accession numbers GSE90660 (S. cerevisiae mitotic ChIP-Seq), GSE90661 (S. cerevisiae meiotic ChIP-Seq), GSE90662 (K. lactis ChIP-Seq), GSE90663 (P. pastoris ChIP-Seq), GSE90664 (S. stipitis ChIP-Seq), GSE90665 (C. albicans ChIP-Seq), and GSE92766 (RNA-Seq).
Acknowledgements
We thank C Dalal, S Coyle, C Baker, C Britton, S Singh-Babak, and L Pack for valuable comments on the manuscript; T Sorrells and C Nobile for important technical contributions and comments on the manuscript; E Chow and the UCSF CAT for expert advice; and R Bennett and R Sherwood for strains and protocols. This work was supported by grant R01 GM037049 from the National Institutes of Health. EM was supported by the Human Frontier Science Program and UC-MEXUS.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
National Institutes of Health R01 GM037049 to Isabel Nocedal, Eugenio Mancera, Alexander D Johnson.
Human Frontier Science Program to Eugenio Mancera.
UC-MEXUS to Eugenio Mancera.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
IN, Conceptualization, Formal analysis, Investigation, Writing—original draft, Writing—review and editing.
EM, Formal analysis, Investigation.
ADJ, Conceptualization, Formal analysis, Supervision, Writing—review and editing.
Additional files
Rows correspond to groups of orthologous genes. Columns show results of all genomic experiments discussed here, colors correspond to experiments in different species. Cell value of ‘N/A’ indicates no orthologous gene for that orthogroup in that species.
(A) Strains used in this study. (B) Primers used in this study.
Major datasets
The following previously published dataset was used:
Chu S,DeRisi J,Eisen M,Mulholland J,Botstein D,Brown PO,Herskowitz I,1998,The transcriptional program of sporulation in budding yeast,https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS104,Also available at the NCBI Gene Expression Omnibus (accession no: GDS104)
References
- Aharoni A, Gaidukov L, Khersonsky O, McQ Gould S, Roodveldt C, Tawfik DS. The 'evolvability' of promiscuous protein functions. Nature Genetics. 2005;37:1–4. doi: 10.1038/ng1482. [DOI] [PubMed] [Google Scholar]
- Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter candida albicans biofilm model. Infection and Immunity. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL. DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics. 2011;27:1653–1659. doi: 10.1093/bioinformatics/btr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CR, Booth LN, Sorrells TR, Johnson AD. Protein modularity, cooperative binding, and hybrid regulatory states underlie transcriptional network diversification. Cell. 2012;151:80–95. doi: 10.1016/j.cell.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve A, Wagner A. A latent capacity for evolutionary innovation through exaptation in metabolic systems. Nature. 2013;500:203–206. doi: 10.1038/nature12301. [DOI] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. Completion of a parasexual cycle in candida albicans by induced chromosome loss in tetraploid strains. The EMBO Journal. 2003;22:2505–2515. doi: 10.1093/emboj/cdg235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme J, d'Enfert C. Candida Albicans biofilms: building a heterogeneous, drug-tolerant environment. Current Opinion in Microbiology. 2013;16:398–403. doi: 10.1016/j.mib.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Booth LN, Tuch BB, Johnson AD. Intercalation of a new tier of transcription regulation into an ancient circuit. Nature. 2010;468:959–963. doi: 10.1038/nature09560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman AR, Gianoulis TA, Zhang ZD, Yu H, Rozowsky J, Seringhaus MR, Wang LY, Gerstein M, Snyder M. Divergence of transcription factor binding sites across related yeast species. Science. 2007;317:815–819. doi: 10.1126/science.1140748. [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. Evolution of pathogenicity and sexual reproduction in eight candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne KP, Wolfe KH. The yeast gene order browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Research. 2005;15:1456–1461. doi: 10.1101/gr.3672305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evolution at two levels: on genes and form. PLoS Biology. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M, Weng S, Wong ED. Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Research. 2012;40:D700–D705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Herskowitz I. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Molecular Cell. 1998;1:685–696. doi: 10.1016/S1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- Coyle SM, Flores J, Lim WA. Exploitation of latent allostery enables the evolution of new modes of MAP kinase regulation. Cell. 2013;154:875–887. doi: 10.1016/j.cell.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, Tolstorukov I, Kusari A, Sunga J, Madden K, Chappell T. Expression in the yeast pichia pastoris. Methods in Enzymology. 2009;463:169–189. doi: 10.1016/S0076-6879(09)63013-5. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311:796–800. doi: 10.1126/science.1113832. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Struhl K. Where does mediator bind in vivo? PLoS One. 2009;4:e5029–6. doi: 10.1371/journal.pone.0005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EP, Bui CK, Nett JE, Hartooni N, Mui MC, Andes DR, Nobile CJ, Johnson AD. An expanded regulatory network temporally controls candida albicans biofilm formation. Molecular Microbiology. 2015;96:1226–1239. doi: 10.1111/mmi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel N, Wang S, Stern DL. Conserved regulatory architecture underlies parallel genetic changes and convergent phenotypic evolution. PNAS. 2012;109:20975–20979. doi: 10.1073/pnas.1207715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompel N, Prud'homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature. 2005;433:481–487. doi: 10.1038/nature03235. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hare EE, Peterson BK, Iyer VN, Meier R, Eisen MB. Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genetics. 2008;4:e1000106–1000113. doi: 10.1371/journal.pgen.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Friesen H, Segall J. NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in saccharomyces cerevisiae. Molecular and Cellular Biology. 1998;18:5750–5761. doi: 10.1128/MCB.18.10.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernday AD, Noble SM, Mitrovich QM, Johnson AD. Genetics and molecular biology in candida albicans. Methods in Enzymology. 2010;470:737–758. doi: 10.1016/S0076-6879(10)70031-8. [DOI] [PubMed] [Google Scholar]
- Homann OR, Johnson AD. MochiView: versatile software for genome browsing and DNA motif analysis. BMC Biology. 2010;8:49. doi: 10.1186/1741-7007-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional venn diagrams. BMC Genomics. 2008;9:488–6. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihmels J, Bergmann S, Gerami-Nejad M, Yanai I, McClellan M, Berman J, Barkai N. Rewiring of the yeast transcriptional network through the evolution of motif usage. Science. 2005;309:938–940. doi: 10.1126/science.1113833. [DOI] [PubMed] [Google Scholar]
- Jolly ER, Chin CS, Herskowitz I, Li H. Genome-wide identification of the regulatory targets of a transcription factor using biochemical characterization and computational genomic analysis. BMC Bioinformatics. 2005;6:275. doi: 10.1186/1471-2105-6-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko PV, Tolstorukov MY, Park PJ. Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nature Biotechnology. 2008;26:1351–1359. doi: 10.1038/nbt.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annual Review of Biochemistry. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clinical Microbiology Reviews. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küberl A, Schneider J, Thallinger GG, Anderl I, Wibberg D, Hajek T, Jaenicke S, Brinkrolf K, Goesmann A, Szczepanowski R, Pühler A, Schwab H, Glieder A, Pichler H. High-quality genome sequence of pichia pastoris CBS7435. Journal of Biotechnology. 2011;154:312–320. doi: 10.1016/j.jbiotec.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H, Hogues H, Mallick J, Sellam A, Nantel A, Whiteway M. Evolutionary tinkering with conserved components of a transcriptional regulatory network. PLoS Biology. 2010;8:e1000329. doi: 10.1371/journal.pbio.1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup The sequence alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Johnson AD. Evolution of transcription networks--lessons from yeasts. Current Biology. 2010;20:R746–R753. doi: 10.1016/j.cub.2010.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MB, Hernday AD, Fordyce PM, Noiman L, Sorrells TR, Hanson-Smith V, Nobile CJ, DeRisi JL, Johnson AD. Identification and characterization of a previously undescribed family of sequence-specific DNA-binding domains. PNAS. 2013;110:7660–7665. doi: 10.1073/pnas.1221734110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Ludwig MZ, Bergman C, Patel NH, Kreitman M. Evidence for stabilizing selection in a eukaryotic enhancer element. Nature. 2000;403:564–567. doi: 10.1038/35000615. [DOI] [PubMed] [Google Scholar]
- Maguire SL, ÓhÉigeartaigh SS, Byrne KP, Schröder MS, O'Gaora P, Wolfe KH, Butler G. Comparative genome analysis and gene finding in candida species using CGOB. Molecular Biology and Evolution. 2013;30:1281–1291. doi: 10.1093/molbev/mst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera E, Porman AM, Cuomo CA, Bennett RJ, Johnson AD. Finding a missing gene: efg1 regulates morphogenesis in candida tropicalis. G3: Genes|Genomes|Genetics. 2015;5:849–856. doi: 10.1534/g3.115.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Current Biology. 2005;15:1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Johnson AD. Candida Albicans biofilms and human disease. Annual Review of Microbiology. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M. What, if anything, is an evolutionary novelty? Philosophy of Science. 2008;75:887–898. doi: 10.1086/594532. [DOI] [Google Scholar]
- Proux-Wéra E, Armisén D, Byrne KP, Wolfe KH. A pipeline for automated annotation of yeast genome sequences by a conserved-synteny approach. BMC Bioinformatics. 2012;13:237. doi: 10.1186/1471-2105-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieppel O. Turtles as hopeful monsters. BioEssays. 2001;23:987–991. doi: 10.1002/bies.1143. [DOI] [PubMed] [Google Scholar]
- Sellam A, Askew C, Epp E, Tebbji F, Mullick A, Whiteway M, Nantel A. Role of transcription factor CaNdt80p in cell separation, hyphal growth, and virulence in candida albicans. Eukaryotic Cell. 2010;9:634–644. doi: 10.1128/EC.00325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RK, Bennett RJ. Fungal meiosis and parasexual reproduction--lessons from pathogenic yeast. Current Opinion in Microbiology. 2009;12:599–607. doi: 10.1016/j.mib.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- Sorrells TR, Booth LN, Tuch BB, Johnson AD. Intersecting transcription networks constrain gene regulatory evolution. Nature. 2015;523:361–365. doi: 10.1038/nature14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CI, Schwimmer DB, Barolo S. Rapid evolutionary rewiring of a structurally constrained eye enhancer. Current Biology. 2011;21:1186–1196. doi: 10.1016/j.cub.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanay A, Regev A, Shamir R. Conservation and evolvability in regulatory networks: the evolution of ribosomal regulation in yeast. PNAS. 2005;102:7203–7208. doi: 10.1073/pnas.0502521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Berbee ML. Dating divergences in the fungal tree of life: review and new analyses. Mycologia. 2006;98:838–849. doi: 10.3852/mycologia.98.6.838. [DOI] [PubMed] [Google Scholar]
- Teytelman L, Thurtle DM, Rine J, van Oudenaarden A. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. PNAS. 2013;110:18602–18607. doi: 10.1073/pnas.1316064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature Biotechnology. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong AE, Tuch BB, Li H, Johnson AD. Evolution of alternative transcriptional circuits with identical logic. Nature. 2006;443:415–420. doi: 10.1038/nature05099. [DOI] [PubMed] [Google Scholar]
- Tuch BB, Galgoczy DJ, Hernday AD, Li H, Johnson AD. The evolution of combinatorial gene regulation in fungi. PLoS Biology. 2008;6:e38. doi: 10.1371/journal.pbio.0060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar D, Berthelot C, Aldridge S, Rayner TF, Lukk M, Pignatelli M, Park TJ, Deaville R, Erichsen JT, Jasinska AJ, Turner JM, Bertelsen MF, Murchison EP, Flicek P, Odom DT. Enhancer evolution across 20 mammalian species. Cell. 2015;160:554–566. doi: 10.1016/j.cell.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. Robustness, evolvability, and neutrality. FEBS Letters. 2005a;579:1772–1778. doi: 10.1016/j.febslet.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Wagner A. Robustness and Evolvability in Living Systems. Princeton University Press; 2005b. pp. 1–13. [Google Scholar]
- Wagner GP, Lynch VJ. Evolutionary novelties. Current Biology. 2010;20:R48–R52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wray GA. The evolutionary significance of cis-regulatory mutations. Nature Reviews Genetics. 2007;8:206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- Xu L, Ajimura M, Padmore R, Klein C, Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in saccharomyces cerevisiae. Molecular and Cellular Biology. 1995;15:6572–6581. doi: 10.1128/MCB.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome Biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]