Abstract
Background
G-protein-coupled bile acid receptor 1 (GPBAR1, also known as TGR5) has been shown to participate in glucose homeostasis. In animal models, a TGR5 agonist increases incretin secretion to reduce hyperglycemia. Many agonists have been developed for clinical use. However, the effects of TGR5 blockade have not been studied extensively, with the exception of studies using TGR5 knockout mice. Therefore, we investigated the potential effect of triamterene on TGR5.
Methods
We transfected the TGR5 gene into cultured Chinese hamster ovary cells (CHO-K1 cells) to express TGR5. Then, we applied a fluorescent indicator to examine the glucose uptake of these transfected cells. In addition, NCI-H716 cells that secrete incretin were also evaluated. Fura-2, a fluorescence indicator, was applied to determine the changes in calcium concentrations. The levels of cyclic adenosine monophosphate (cAMP) and glucagon-like peptide (GLP-1) were estimated using enzyme-linked immunosorbent assay kits. Moreover, rats with streptozotocin (STZ)-induced type 1-like diabetes were used to investigate the effects in vivo.
Results
Triamterene dose dependently inhibits the increase in glucose uptake induced by TGR5 agonists in CHO-K1 cells expressing the TGR5 gene. In cultured NCI-H716 cells, TGR5 activation also increases GLP-1 secretion by increasing calcium levels. Triamterene inhibits the increased calcium levels by TGR5 activation through competitive antagonism. Moreover, the GLP-1 secretion and increased cAMP levels induced by TGR5 activation are both dose dependently reduced by triamterene. However, treatment with KB-R7943 at a dose sufficient to block the Na+/Ca2+ exchanger (NCX) failed to modify the responses to TGR5 activation in NCI-H716 cells or CHO-K1 cells expressing TGR5. Therefore, the inhibitory effects of triamterene on TGR5 activation do not appear to be related to NCX inhibition. Blockade of TGR5 activation by triamterene was further characterized in vivo using the STZ-induced diabetic rats.
Conclusion
Based on the obtained data, we identified triamterene as a reliable inhibitor of TGR5. Therefore, triamterene can be developed as a clinical inhibitor of TGR5 activation in future studies.
Keywords: triamterene, CHO-K1 cells, TGR5, transfection, sitagliptin
Introduction
G-protein-coupled bile acid receptor 1 (GPBAR1), also known as Takeda G-protein-coupled receptor 5 (TGR5), GPR131, or M-BAR, is a member of the membrane-bound G-protein-coupled receptor family.1–3 TGR5 is expressed throughout the body; it is expressed at high levels in the placenta and spleen but is expressed at lower levels in other tissues, such as the heart, liver, lung, stomach, kidney, gallbladder, intestine, brown adipose tissue, and endocrine glands.1,2 The wide distribution of TGR5 indicates that bile acids may have many unknown functions throughout the body. Regarding signaling, TGR5 activation may induce cyclic adenosine monophosphate (cAMP) to activate protein kinase A, which can induce the transduction of downstream signaling.1,4 Therefore, TGR5 is known to regulate the lipid and glucose metabolism, energy homeostasis, and inflammation. TGR5 activation has also been reported to induce glucagon-like peptide-1 (GLP-1) secretion from cultured cells, including mouse enteroendocrine stanniocalcin-1 cells and human intestinal NCI-H716 cells.5 GLP-1 may reduce glucagon secretion and increase insulin secretion to decrease hyperglycemia through a glucose-dependent mechanism. Therefore, TGR5 agonists improve metabolic disorders in obese and insulin-resistant mice.6 Moreover, in addition to its role as a gastrointestinal and metabolic regulator, TGR5 also has important roles in the immune system.7 Therefore, many studies have focused on the applications of TGR5 agonists.8
However, the pleiotropic effects of TGR5 activation may result in some adverse reactions, such as pruritus9 and inappropriate gallbladder filling.10 Moreover, TGR5 activation may promote cholangiocyte proliferation to increase the risk of cholangiocarcinoma.11 Recently, TGR5 was shown to be expressed in human gastric cancer,12 although this finding was controversial compared to data from a previous report.13 Therefore, questions remain regarding the essential role of TGR5 inhibition in clinical applications. However, TGR5 antagonists have not been developed, with the exception of DFN406, a recently described antagonist.14
Triamterene (6-phenyl-2,4,7-pteridinotriamine) is a widely used, mild diuretic that reduces potassium ion secretion to decrease the reabsorption of chloride ions in distal tubular cells. Blockade of Na+ channels with triamterene may hyperpolarize the luminal membrane to reduce the K+, H+, Ca2+, and Mg2+ excretion rates.15 Therefore, triamterene is applied as a K+-sparing diuretic in the clinic.16 In addition, triamterene is known to block the epithelial sodium channel on the lumenal side of the collecting tubule in kidney.17 However, triamterene has been shown to have other effects in addition to its K+-sparing effect as a treatment for hypertension.18 Triamterene has also been described to be an inhibitor of vascular endothelial growth factor binding.19 Recently, a TGR5 agonist was found to relax the urinary bladder by inhibiting the opening of the Na+/Ca2+ exchanger (NCX).20 Therefore, we aimed to understand the effect of triamterene on TGR5.
In the current study, we propose that triamterene inhibits TGR5. CHO-K1 cells transfected with the TGR5 gene were used to investigate the effect of triamterene on glucose uptake. The effects of triamterene on calcium influx and signals induced by TGR5 activation were also examined in NCI-H716 cells. Furthermore, the effects of triamterene on the blood glucose and GLP-1 levels were further characterized in streptozotocin (STZ)-induced diabetic rats administered with the TGR5 agonist.
Materials and methods
Materials
Triamterene, lithocholic acid (LCA) (Sigma–Aldrich Chemical Co., St Louis, MO, USA), betulinic acid (Tokyo Chemical Institute, Tokyo, Japan), and KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea) mesylate (Cayman, Ann Arbor, MI, USA) were dissolved in dimethyl sulfoxide (Merck, Cramlington, Northumberland, UK) to prepare stock solutions. In addition, sitagliptin phosphate (Merck), a dipeptidyl peptidase-4 (DPP-4) inhibitor, was dissolved in aqueous solution. Protein concentrations were determined using the BCA assay kit (Thermo Scientific, Rockford, IL, USA).
Animals
Male Sprague–Dawley (SD) rats weighing 240–270 g were obtained from the National Laboratory Animal Center (Taipei, Taiwan) and maintained at the animal center of Chi Mei Medical Center. The animal experiments were approved by the Institutional Animal Ethics Committee (No 103120201) of Chi Mei Medical Center. Animal studies were performed under anesthesia with sodium pentobarbital (35 mg/kg, ip) to minimize the animals’ suffering. All experiments conformed to the Guide for the Care and Use of Laboratory Animals and the guidelines of the Animal Welfare Act.
Induction of diabetes in rats
A single intravenous (i.v.) injection of 65 mg/kg STZ (Sigma–Aldrich) was administered to rats to induce type 1-like diabetes.21 Animals were considered diabetic once their plasma glucose level reached ≥320 mg/dL in addition to presenting diabetic features. Studies were then started 2 weeks after the successful induction of diabetes.
Determination of blood glucose and GLP-1 levels in diabetic rats
Diabetic rats were orally administered 5 mg/kg/day sitagliptin (an inhibitor of DPP-4) or a vehicle for 14 days before treatment with the test substance. Blood samples were obtained from the femoral vein of rats.21 The plasma glucose concentration was then measured using a glucose kit and an automatic analyzer (Quik-Lab, Ames; Miles, Inc., Elkhart, IN, USA). The plasma GLP-1 level was estimated using an enzyme-linked immunosorbent assay (ELISA) kit (EZGLP1T-36K; EMD Millipore Co., Billerica, MA, USA), as previously described.22
Cell cultures
The human NCI-H716 cells and CHO-K1 cells were purchased from the Culture Collection and Research Center (BCRC) of the Food Industry Institute (Hsin-Chiu, Taiwan). Similar to our previous report,22 human NCI-H716 cells (BCRC No CCL-251) were cultured in RPMI 1640 medium containing 10% (v/v) fetal bovine serum (FBS) and 2 mM L-glutamine in the presence of 5% CO2. CHO-K1 cells (BCRC No CCL-61) were cultured in F-12K growth medium containing 10% FBS. The cells were subcultured according to our previous report.22
Transfection of TGR5 in CHO-K1 cells
The CHO-K1 cells were transfected with the human GPBAR1 gene that had been cloned into an expression vector (pCMV6-Entry; OriGene, Rockville, MD, USA).22 One day later, successful transfection was confirmed using Western blotting using the method described in our previous study.22 The bands for TGR5 (32 kDa) denoted TGR5 expression, and β-actin (43 kDa) was used as an internal reference. Next, the TGR5–CHO-K1 cells were treated with the indicated concentrations of agonists, LCA or betulinic acid. The effectiveness of triamterene was also investigated using a 30 min pretreatment.
Uptake of 2-NBDG into TGR5–CHO-K1 cells
Glucose uptake into TGR5–CHO-K1 cells was examined using the fluorescent indicator 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG). The assay was performed using the method described in our previous study.22 The fluorescent intensity indicated the uptake of 2-NBDG in cells that were treated with the indicated concentrations of agonists, betulinic acid or LCA. The effectiveness of triamterene was also investigated using a 30 min pretreatment.
Determination of the calcium level
We applied the fluorescent probe Fura-2 to examine the changes in intracellular calcium concentrations ([Ca2+]i). Briefly, NCI-H716 cells were incubated with 5 μmol/L Fura-2 before the treatment with the indicated concentrations of agonist. Moreover, in some experiments, the cells were incubated with triamterene for 30 min prior to the agonist treatment using the method shown in the Glucose uptake section. [Ca2+]i was then determined using the method described in our previous report.23
Assessment of GLP-1 secretion from NCI-H716 cells
NCI-H716 cells were incubated with the indicated concentrations of agonist for 1 h. In some experiments, cells were incubated with triamterene for 30 min prior to the agonist treatment.22 The GLP-1 level was determined using a commercial ELISA kit (EMD Millipore Co.) according to the manufacturer’s instructions.
Determination of intracellular cAMP levels
The NCI-H716 cells were treated with the indicated concentrations of agonist for 1 h.22 In some experiments, cells were incubated with triamterene for 30 min prior to the agonist treatment. Intracellular cAMP levels were measured with a commercial ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer’s instructions.
Statistical analysis
All results are presented as the means ± SEM from the number of samples (n) in each group. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to analyze the differences, and the data sets from two groups were analyzed with an independent t-test using SPSS for Windows, version 17 (Chicago, IL, USA). A P-value ≤0.05 was considered significant.
Results
Effect of triamterene on TGR5 activation in TGR5–CHO-K1 cells
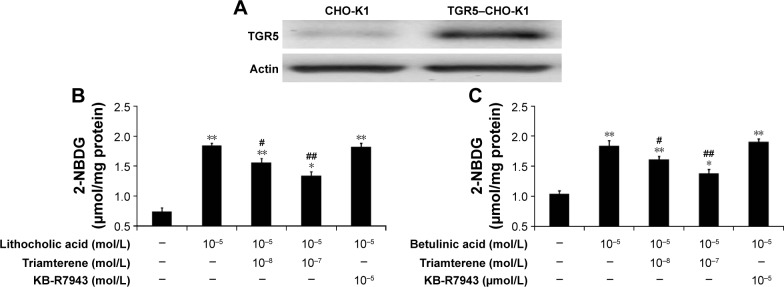
We used Western blots to confirm that the TGR5 protein was expressed in CHO-K1 cells (Figure 1A). The TGR5 protein expressed in TGR5–CHO-K1 cells was functional, as described in our previous report.22
Figure 1.
Inhibitory effect of triamterene on glucose uptake induced by TGR5 agonists in TGR5-transfected cells.
Notes: The successful transfection of CHO-K1 cells with TGR5 is shown in (A). The lithocholic acid (LCA)-induced increased in glucose uptake in TGR5-transfected CHO-K1 cells (TGR5–CHO-K1 cells) compared with cells transfected with empty vector (CHO-K1 cells) shown in the first column is reduced by triamterene in a dose-dependent manner (B). The effects of betulinic acid blockade by triamterene are shown in (C). In addition, treatment with KB-R7943 at a dose sufficient to block the Na+/Ca2+ exchanger was used as a negative control. Values (means ± SE) were obtained from eight determinations in each group. *P<0.05 and **P<0.01 compared with the vehicle-treated group are given in the first column. #P<0.05 and ##P<0.01 compared with the vehicle-treated, agonist-stimulated group are given in the second column.
Abbreviations: CHO-K, Chinese Hamster ovary cells; NBDG, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose; SE, standard error; TGR5, Takeda G-protein-coupled receptor 5.
The direct effect of triamterene on the TGR5 was then investigated. After incubation with the agonists LCA (Figure 1B) or betulinic acid (Figure 1C), the 2-NBDG concentration was significantly increased in TGR5–CHO-K1 cells. Moreover, the increased intracellular 2-NBDG concentration was markedly reduced by triamterene in a dose-dependent manner. However, a 30 min pretreatment with KB-R7943 at a dose sufficient to block the NCX24 did not modify the increase in the 2-NBDG levels induced by the agonists LCA or betulinic acid.
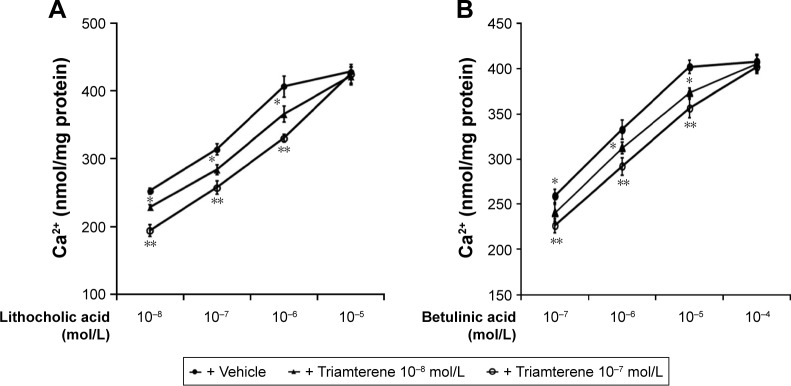
Effects of triamterene on calcium influx induced by TGR5 activation in cultured NCI-H716 cells
In general, the cultured NCI-H716 intestinal cell line has been widely used to study GLP-1 secretion. A TGR5 agonist-induced increase in GLP-1 secretion from NCI-H716 cells via calcium influx has been previously reported.22 NCI-H716 cells exhibited a dose-dependent increase in the calcium concentrations following treatment with the TGR5 agonists LCA and betulinic acid. Treatment with triamterene competitively inhibited the action of LCA in NCI-H716 cells (Figure 2A). Triamterene induced a similar competitive inhibition of the betulinic acid-induced changes in calcium concentrations in NCI-H716 cells (Figure 2B).
Figure 2.
Inhibitory effects of triamterene on TGR5 agonist-induced calcium influx in NCI-H716 cells.
Notes: LCA induced dose-dependent increases in cellular calcium (Ca2+) levels in NCI-H716 cells that were markedly inhibited by triamterene, as shown in (A). Similar effects of triamterene on betulinic acid-induced Ca2+ mobilization are shown in (B). Values (means ± SE) were obtained from eight determinations in each group. *P<0.05 and **P<0.01 compared with the vehicle-treated group at the same concentration.
Abbreviations: LCA, lithocholic acid; SE, standard error; TGR5, Takeda G-protein-coupled receptor 5.
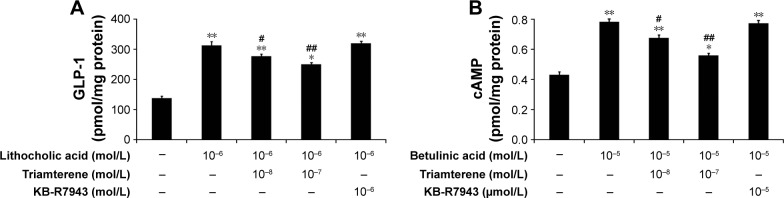
Effects of triamterene on signals induced by TGR5 activation in cultured NCI-H716 cells
NCI-H716 cells expressing TGR5 have previously been characterized.25 Therefore, we used NCI-H716 cells to identify the effects of the agonists, LCA or betulinic acid, on receptor-coupled signals via TGR5 activation.22 In the presence of triamterene, the LCA-induced release of GLP-1 was reduced (Figure 3A). Moreover, the betulinic acid-induced increase in the cAMP concentration (Figure 3B) was also dose-dependently attenuated by triamterene. However, a 30 min pretreatment with KB-R7943 at a dose sufficient to block the NCX24 did not affect GLP-1 secretion or the increased cAMP concentrations induced by TGR5 agonists. Taken together, these results further support the inhibitory effect of triamterene on TGR5 in vitro.
Figure 3.
Inhibitory effects of triamterene on TGR5 agonist-induced changes in cAMP or GLP-1 levels in CHO-K1 cells.
Notes: Triamterene dose-dependently inhibited the LCA-induced increase in GLP-1 secretion (A) or the betulinic acid-induced increase in cAMP levels (B) in NCI-H716 cells. In addition, treatment with KB-R7943 at a dose sufficient to block the Na+/Ca2+ exchanger was used as a negative control. Values (means ± SE) were obtained from eight determinations in each group. *P<0.05 and **P<0.01 compared with the vehicle-treated group (first column). #P<0.05 and ##P<0.01 compared with the vehicle-treated, agonist-stimulated group (second column).
Abbreviations: cAMP, cyclic adenosine monophosphate; CHO-K1, Chinese Hamster ovary cells; GLP, glucagon-like peptide; LCA, lithocholic acid; SE, standard error; TGR5, Takeda G-protein-coupled receptor 5.
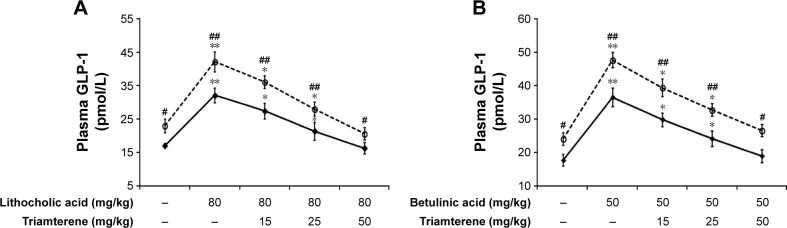
Effect of triamterene on plasma GLP-1 levels in diabetic rats
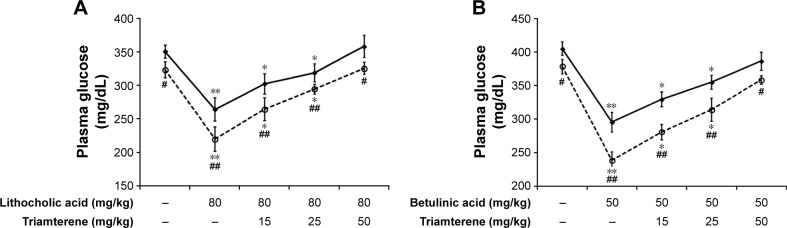
Diabetic rats were orally administered 5 mg/kg/day sitagliptin (an inhibitor of DPP-4) or a vehicle for 2 weeks before treatment with the testing substance. In STZ-induced diabetic rats, TGR5 agonists further increased the plasma GLP-1 levels induced by sitagliptin at a dose sufficient to block DPP-4, as shown in Figure 4A and B. Triamterene dose-dependently attenuated the TGR5 agonist-induced increase in the GLP-1 levels (Figure 4A and B). Furthermore, TGR5 activation markedly attenuated the hyperglycemia in diabetic rats, and this action was also potentiated by the DPP-4 inhibitor sitagliptin (Figure 5A and B). Therefore, the effects of TGR5 agonists on GLP-1 secretion to decrease hyperglycemia in type 1-like diabetic rats seem to be markedly regulated by DPP-4, the GLP-1-inactivating enzyme. In addition, triamterene produced a dose-dependent reversion of these effects induced by TGR5 agonists. However, triamterene (50 mg/kg) alone did not modify the plasma glucose or GLP-1 levels in diabetic rats. Moreover, the plasma insulin levels in type 1-like diabetic rats (0.57±0.11 ng/L; n=8) were not modified (P>0.05) by LCA (0.56±0.08 ng/L; n=8) or betulinic acid (0.55±0.12 ng/L; n=8). Triamterene (50 mg/kg) alone did not modify the plasma insulin levels (0.58±0.08 ng/L; n=8) in these diabetic rats. Therefore, the TGR5 agonists do not appear to induce changes in insulin levels in type 1-like diabetic rats.
Figure 4.
Effects of triamterene on plasma GLP-1 levels induced by TGR5 agonists in type 1-like diabetic rats.
Notes: (A) Plasma GLP-1 levels were determined 1 h after the administration of 80 mg/kg LCA (solid line) or sitagliptin (5 mg/kg/day orally for 14 days; broken line) to the diabetic rats. Both groups were pretreated with the indicated dose of triamterene for 30 min. (B) Plasma GLP-1 levels were determined 1 h after the administration of 50 mg/kg betulinic acid (solid line) or sitagliptin (5 mg/kg/day orally for 14 days; broken line) to the diabetic rats. Both groups were pretreated with the indicated dose of triamterene for 30 min. Values are expressed as the means ± SE obtained from eight determinations per group. *P<0.05 and **P<0.01 compared with the vehicle-treated group. #P<0.05 and ##P<0.01 compared with the basal group without sitagliptin treatment.
Abbreviations: GLP, glucagon-like peptide; LCA, lithocholic acid; SE, standard error; TGR5, Takeda G-protein-coupled receptor 5.
Figure 5.
Effects of triamterene on plasma glucose levels induced by TGR5 agonists in type 1-like diabetic rats.
Notes: (A) Plasma glucose levels were determined 1 h after the administration of 80 mg/kg LCA (solid line) or sitagliptin (5 mg/kg/day orally for 14 days; broken line) to the diabetic rats. Both groups were pretreated with the indicated dose of triamterene for 30 min. (B) Plasma glucose levels were determined 1 h after the administration of 50 mg/kg betulinic acid (solid line) or sitagliptin (5 mg/kg/day orally for 14 days; broken line) to the diabetic rats. Both groups were pretreated with the indicated dose of triamterene for 30 min. Values are expressed as the means ± SE obtained from eight determinations per group. *P<0.05 and **P<0.01 compared with the vehicle-treated group. #P<0.05 and ##P<0.01 compared with the basal group without sitagliptin treatment.
Abbreviations: LCA, lithocholic acid; SE, standard error; TGR5, Takeda G-protein-coupled receptor 5.
Discussion
As shown in the present study, triamterene inhibits TGR5. A direct effect of triamterene on the TGR5 has also been observed in CHO-K1 cells transfected with the TGR5 gene. Moreover, the actions of TGR5 agonists in cultured cells were also competitively inhibited by triamterene. Furthermore, the TGR5 agonist-induced increase in plasma GLP-1 levels in diabetic rats was markedly reduced by triamterene. Therefore, triamterene may be a useful TGR5 inhibitor in vitro and in vivo.
TGR5 has been shown to play important roles in regulating inflammation, glucose metabolism, and energy metabolism.26 Therefore, previous studies have focused on the development of TGR5 agonists.8 However, TGR5 has recently been shown to be the physiological mediator of pruritus.9,27 Therefore, TGR5 inhibition may be a rational strategy to avoid side effects and might have utility as a treatment for some cholestatic disorders.14 A TGR5 antagonist is still not available commercially; however, one compound, DFN406, has recently been identified.14 Studies investigating the role of TGR5 antagonists in metabolism would be meaningful. We showed the inhibitory effect of triamterene on the TGR5, a new finding that has not been reported previously.
In the present study, the administration of TGR5 agonists, betulinic acid or LCA, to CHO-K1 cells transfected with the TGR5 gene induced a significant increase in glucose uptake, consistent with previous studies.22,28 Triamterene treatment exhibited dose-dependent inhibition of the increase of glucose uptake induced by LCA or betulinic acid. Nevertheless, the administration of KB-R7943 at a dose sufficient to block the NCX24 failed to affect the glucose uptake induced by both agonists. Therefore, the mechanism underlying the inhibitory action of triamterene appears to be NCX independent.
The competitive antagonism-like action of triamterene was further confirmed in NCI-H716 cells. NCI-H716 cells are widely used as a murine and human model of GLP-1 secretion.29 Also, TGR5 activation induces GLP-1 secretion by affecting cAMP signaling and increasing calcium influx in NCI-H716 cells.22 The triamterene pretreatment inhibited the increased calcium influx induced by betulinic acid or LCA in NCI-H716 cells in a dose-dependent manner. Thus, the effect of triamterene on decreasing calcium influx in NCI-H716 cells mainly occurs through TGR5 inhibition. The possible mechanisms might be that the competitive antagonist triamterene binds to TGR5 to decrease its activation by betulinic acid or LCA. However, a radioligand-binding study would be useful to clarify this hypothesis in the future.
Moreover, triamterene inhibits TGR5 activation-induced GLP-1 secretion from NCI-H716 cells. Additionally, the increase in cAMP levels induced by TGR5 activation was also reduced by triamterene in a dose-dependent manner. However, treatment with KB-R7943 at a dose sufficient to block the NCX24 did not modify either effect. Although triamterene is known as a K+-sparing diuretic agent,16 researchers have challenged that triamterene has additional functions to its role as a K+-sparing drug.18 The dose of KB-R7943 used in this study has been shown to block ATP-sensitive potassium currents.30 Therefore, our results are consistent with the findings showing that triamterene exhibits a pleiotropic effect in addition to a K+-sparing effect. Thus, functional blockade of TGR5 by triamterene can be identified in vitro.
GLP-1 plays an important role in the regulation of glucose homeostasis via actions including increasing glucose-dependent insulin secretion, proinsulin gene expression, and β-cell proliferation and anti-apoptotic signaling.31 Therefore, we investigated the in vivo effects of triamterene on STZ-induced diabetic rats. In this model, the role of endogenous insulin is negligible, as previously described.21 Similar to patients with diabetes,32 activation of the TGR5 induces GLP-1 secretion. The increase in plasma GLP-1 levels induced by TGR5 agonists in type 1-like diabetic rats was dose-dependently reduced by triamterene. Moreover, TGR5 agonists also reduced hyperglycemia, and this action was blocked by triamterene in a similar manner. However, the inhibition of DPP-4, the GLP-1-inactivating enzyme, by sitagliptin markedly increased the plasma GLP-1 levels in diabetic rats. Sitagliptin is a clinically used antidiabetic drug that inhibits the degradation of incretins, mainly GLP-1, as previously described.33 The reduction of hyperglycemia induced by TGR5 activation was also promoted in the presence of sitagliptin at a dose sufficient to block DPP-4. Changes in plasma GLP-1 and glucose levels induced by TGR5 agonists in the presence of the DPP-4 blockade were inhibited by triamterene in a similar manner. Therefore, we propose that triamterene inhibits GLP-1 secretion in diabetic rats by inhibiting TGR5.
In conclusion, we provide evidence showing that triamterene inhibits human TGR5. Triamterene attenuated the increase in GLP-1 secretion induced by TGR5 activation in cells and animals. Therefore, triamterene is suitable for development as a TGR5 inhibitor and could be applied from bench to bedside in the future.
Acknowledgments
We thank Ya-Pin Lin and Yi-Zhi Chen for their kind assistance with the experiments. We also thank American Journal Experts (AJE) for editing the manuscript. The present study was partially supported by a grant obtained from the Ministry of Science and Technology (MOST 104-2320-B-384-004-MY3) in Taiwan, Republic of China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 3.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54(6):1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 5.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diab Rep. 2011;11(3):160–166. doi: 10.1007/s11892-011-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copple BL, Li T. Pharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal S, Patil A, Aware U, et al. Discovery of a potent and orally efficacious TGR5 receptor agonist. ACS Med Chem Lett. 2016;7(1):51–55. doi: 10.1021/acsmedchemlett.5b00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123(4):1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briere DA, Ruan X, Cheng CC, et al. Novel Small Molecule Agonist of TGR5 Possesses Anti-Diabetic Effects but Causes Gallbladder Filling in Mice. PLoS One. 2015;10(8):e0136873. doi: 10.1371/journal.pone.0136873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reich M, Deutschmann K, Sommerfeld A, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65(3):487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 12.Carino A, Graziosi L, D’Amore C, et al. The bile acid receptor GPBAR1 (TGR5) is expressed in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines. Oncotarget. 2016;7(38):61021–61035. doi: 10.18632/oncotarget.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Su J, Li Z, et al. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell proliferation and migration through antagonizing STAT3 signaling pathway. Oncotarget. 2015;6(33):34402–34413. doi: 10.18632/oncotarget.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepe V, Renga B, Festa C, et al. Investigation on bile acid receptor regulators. Discovery of cholanoic acid derivatives with dual G-protein coupled bile acid receptor 1 (GPBAR1) antagonistic and farnesoid X receptor (FXR) modulatory activity. Steroids. 2016;105:59–67. doi: 10.1016/j.steroids.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Luis ML, Fraga JM, Jimenez AI, Jimenez F, Hernandez O, Arias JJ. Application of PLS regression to fluorimetric data for the determination of furosemide and triamterene in pharmaceutical preparations and triamterene in urine. Talanta. 2004;62(2):307–316. doi: 10.1016/j.talanta.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Horisberger JD, Giebisch G. Potassium-sparing diuretics. Ren Physiol. 1987;10(3–4):198–220. doi: 10.1159/000173130. [DOI] [PubMed] [Google Scholar]
- 17.Busch AE, Suessbrich H, Kunzelmann K, et al. Blockade of epithelial Na+ channels by triamterenes – underlying mechanisms and molecular basis. Pflugers Arch. 1996;432(5):760–766. doi: 10.1007/s004240050196. [DOI] [PubMed] [Google Scholar]
- 18.Smetana GW. Triamterene in the treatment of hypertension: more than just potassium sparing? J Gen Intern Med. 2016;31(1):7–8. doi: 10.1007/s11606-015-3515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong KW, Lee JY, Lee SA, et al. Dynamics of a heparin-binding domain of VEGF(165) complexed with its inhibitor triamterene. Biochemistry. 2011;50(22):4843–4854. doi: 10.1021/bi2000752. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Dong X, Liu Q, et al. Hydrophobic bile acids relax rat detru-sor contraction via inhibiting the opening of the Na+/Ca2+ exchanger. Sci Rep. 2016;6:21358. doi: 10.1038/srep21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JT, Liu IM, Chi TC, Tzeng TF, Lu FH, Chang CJ. Plasma glucose-lowering effect of tramadol in streptozotocin-induced diabetic rats. Diabetes. 2001;50(12):2815–2821. doi: 10.2337/diabetes.50.12.2815. [DOI] [PubMed] [Google Scholar]
- 22.Lo SH, Cheng KC, Li YX, Chang CH, Cheng JT, Lee KS. Development of betulinic acid as an agonist of TGR5 receptor using a new in vitro assay. Drug Des Devel Ther. 2016;10:2669–2676. doi: 10.2147/DDDT.S113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng KC, Li YX, Asakawa A, et al. Characterization of preptin-induced insulin secretion in pancreatic beta-cells. J Endocrinol. 2012;215(1):43–49. doi: 10.1530/JOE-12-0176. [DOI] [PubMed] [Google Scholar]
- 24.Amran MS, Homma N, Hashimoto K. Pharmacology of KB-R7943: a Na+-Ca2+ exchange inhibitor. Cardiovasc Drug Rev. 2003;21(4):255–276. doi: 10.1111/j.1527-3466.2003.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang XY, Zhang SY, Li J, Liu HN, Xie X, Nan FJ. Highly lipophilic 3-epi-betulinic acid derivatives as potent and selective TGR5 agonists with improved cellular efficacy. Acta Pharmacol Sin. 2014;35(11):1463–1472. doi: 10.1038/aps.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Lou G, Meng Z, Huang W. TGR5: a novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:853501. doi: 10.1155/2011/853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieu T, Jayaweera G, Zhao P, et al. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147(6):1417–1428. doi: 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fickert P, Fuchsbichler A, Marschall HU, et al. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168(2):410–422. doi: 10.2353/ajpath.2006.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abramochkin DV, Vornanen M. Inhibition of the cardiac ATP-dependent potassium current by KB-R7943. Comp Biochem Physiol A Mol Integr Physiol. 2014;175:38–45. doi: 10.1016/j.cbpa.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Kuhre RE, Holst JJ, Kappe C. The regulation of function, growth and survival of GLP-1-producing L-cells. Clin Sci (Lond) 2016;130(2):79–91. doi: 10.1042/CS20150154. [DOI] [PubMed] [Google Scholar]
- 32.Hansen M, Scheltema MJ, Sonne DP, et al. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes Obes Metab. 2016;18(6):571–580. doi: 10.1111/dom.12648. [DOI] [PubMed] [Google Scholar]
- 33.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]