Abstract
The locus coeruleus (LC) is a key node of the sympathetic nervous system and suppresses parasympathetic activity that would otherwise increase heart rate variability. In the current study, we examined whether LC-MRI contrast reflecting neuromelanin accumulation in the LC was associated with high-frequency heart rate variability (HF-HRV), a measure reflecting parasympathetic influences on the heart. Recent evidence indicates that neuromelanin, a byproduct of catecholamine metabolism, accumulates in the LC through young and mid adulthood, suggesting that LC-MRI contrast may be a useful biomarker of individual differences in habitual LC activation. We found that, across younger and older adults, greater LC-MRI contrast was negatively associated with HF-HRV during fear conditioning and spatial detection tasks. This correlation was not accounted for by individual differences in age or anxiety. These findings indicate that individual differences in LC structure relate to key cardiovascular parameters.
Keywords: Locus coeruleus, Neuromelanin, Anxiety, Stress, HRV, Parasympathetic system
Introduction
Despite having its own pacemaker cells, the heart does not usually beat at a steady rate. Sympathetic and parasympathetic inputs from the brain modulate the timing of the heart’s sinoatrial node impulses and increase variability. These sympathetic and parasympathetic inputs have interacting and often opposing effects over heart rate variability (HRV), with parasympathetic input providing the dominant influence (Reyes del Paso, et al., 2013; Uijtdehaage and Thayer, 2000). Parasympathetic vagus nerve input influences both high- and low-frequency oscillations in heart rate, whereas sympathetic input directly influences only low-frequency oscillations (Berntson, et al., 1993; Laitio, et al., 2007), in part because sympathetic influences on the heart are slower due to different neurokinetics compared with para-sympathetic influences (e.g., Saul, 1990).
HRV can be assessed in both the time and the frequency domains. Time domain indices of high-frequency variability such as the root mean square of successive differences (RMSSD) act as a high pass filter and thus reflect primarily parasympathetic influences. In the frequency domain, high-frequency HRV (HF-HRV) has been shown to correlate highly with activity of the vagus nerve and thus also reflects primarily parasympathetic influences on the heart (Kuo, et al., 2005). RMSSD and HF-HRV are highly correlated with each other and both can be used to index vagally-mediated HRV (Thayer, et al., 2010).
Recent neuroimaging work in humans has mapped out various ways in which individual differences in brain structure and function relate to HRV. A meta-analysis of this work showed that activations in the medial prefrontal cortex, anterior cingulate, putamen and amygdala were most consistently associated with HRV across studies (Thayer, et al., 2012). For instance, greater baseline RMSSD is associated with greater functional connectivity between amygdala and medial prefrontal cortex (Sakaki et al., 2016). Furthermore, across individuals, greater structural thickness in the anterior cingulate and prefrontal regions was associated with greater HF-HRV/RMSSD across several samples and among both younger and older adults (Winkelmann et al., 2016; Yoo et al., In preparation). These findings provide important insights into the cortical control of HF-HRV. However, evidence from patients and animal research indicate that various brainstem nuclei also exert key influences over HRV (George et al., 2004; Korpelainen, et al., 1996), and HRV has even been proposed as a way to assess brainstem function in order to discriminate between coma and brain death (Baillard et al., 2002; Schwarz, et al., 1987). Despite these linkages, little imaging work in healthy humans has addressed the relationships between brainstem and HRV, mainly due to the technical challenges of imaging small brainstem nuclei.
In the current study, we focus on the relationship between individual differences in HF-HRV and structure of the locus coeruleus, a brainstem nucleus that influences heart rate via multiple pathways. In addition to activating the sympathetic nervous system, LC activity inhibits parasympathetic cardiac vagal neurons (Wang, et al., 2014), including those in the dorsal motor nucleus of the vagus and the nucleus ambiguus, both vagal nuclei that influence heart rate (Samuels and Szabadi, 2008). Firing of these vagal nuclei reduces heart rate, whereas inhibiting them disrupts these reductions in heart rate (Samuels and Szabadi, 2008). Consistent with these LC noradrenergic suppressive influences over vagal nerve activity, administration of ß-adrenoceptor blocker drugs reduce heart rate and increase heart rate variability, effects that are attenuated when vagus nerve activity is blocked (Bittiner and Smith, 1986; for review see Grossman and Taylor, 2007).
In living humans, it has been difficult to obtain measures of LC structure, as it is small and has no visible markers on typical magnetic resonance imaging (MRI) scans. However, LC neurons contain neuromelanin, a pigmented polymer produced during oxidation of catecholamines, including norepinephrine in the LC (Wakamatsu et al., 2015). Recently developed MRI sequences optimize contrast for detecting neuromelanin and reveal the LC as bright white spots (Keren, et al., 2009; Shibata et al., 2006). Furthermore, by comparing the LC with a nearby control region, the neuromelanin signal intensity can be measured, and this LC-MRI contrast measure has been corroborated in post-mortem brains by comparing it to histological analyses of neuromelanin in LC brain tissue (Keren et al., 2015). In-vivo scans of a group of adults between ages 23 and 80 suggest that, during early and middle adulthood, neuromelanin builds up in the LC, presumably reflecting the accumulated effects of catecholamine metabolism over time, but then show a decreasing trend in the 70’s (Shibata et al., 2006). The decreasing LC-MRI contrast values in late life are consistent with postmortem findings that the number of neuromelanin containing cells in the LC are lower in late life (German et al., 1988; Tomlinson, et al., 1981; Vijayashankar and Brody, 1979; Wree, et al., 1980). Thus, LC-MRI contrast provides a trait measure of LC structure that, based on hypothesized mechanisms of LC neuromelanin deposition (Wakamatsu et al., 2015), should be influenced by previous levels of LC activity through young and mid adulthood, as well as by late-life LC structural integrity.
Insofar as LC activity suppresses parasympathetic influences that increase HF-HRV (Wang et al., 2014) and leads to catecholamine activity with neuromelanin as a byproduct (Wakamatsu et al., 2015), people with higher levels of LC activity on a daily basis should have both higher levels of neuromelanin and lower HF-HRV. In turn, in late life, loss of LC neuromelanin neurons should reduce LC suppression of parasympathetic influences. However, to our knowledge, no previous studies have examined the relationship between LC-MRI contrast and HRV. In the current study, we tested the prediction that higher levels of LC neuromelanin are associated with lower HF-HRV using electrocardiogram (ECG) derived measures of heart rate in both younger and older adults while they completed a couple of different tasks. These participants also completed structural MRI scans that included one targeting LC neuromelanin.
In this study we also examined and accounted for the role of age and trait anxiety in contributing to the relationship between LC-MRI contrast and HRV, as both aging and anxiety have been linked with the LC-NE system and HRV. For instance, in aging, LC structure changes (for review see Mather and Harley, 2016), and HRV declines (Bonnemeier et al., 2003; Lipsitz, et al., 1990; De Meersman and Stein, 2007; Umetani, et al., 1998). Likewise, anxiety has been linked with the LC-norepinephrine system (Redmond and Huang, 1979; Sullivan, et al., 1999; Tanaka, et al., 2000; Weiss et al., 1994) and is associated with lower HRV (for reviews see, Chalmers, et al., 2014; Friedman, 2007). We predicted that higher levels of LC-MRI neuromelanin contrast would be associated with lower HF-HRV even when controlling for age and trait anxiety. We used HF-HRV as our primary measure of vagally-mediated HRV. RMSSD and LF-HRV are included in the analyses to provide convergent and discriminant validity as RMSSD reflects primarily parasympathetic influences and so should yield similar results as HF-HRV, whereas LF-HRV reflects both sympathetic and parasympathetic influences and so is likely to show different results (Thayer et al., 2010).
Methods
Participants
Participants in this study were drawn from a larger set of participants recruited to complete a study with both MRI and HRV measures (see Clewett et al., 2016). For the current study, we included all those who had both an LC-MRI contrast measure and measures of heart rate variability during the experimental task runs. This yielded 27 younger adults (M age=23.63 years, SD=4.79, age range=18–34; 10 female) and 18 older adults (M age=67.89 years, SD=4.84, age range=60–75; 8 female). All participants were healthy, had normal or corrected-to-normal visual acuity, and were either students or community dwelling. Older adults were screened for dementia using the Telephone Instrument for Cognitive Status (TICS) over the phone before being scheduled (Brandt, et al., 1988), with a score of 31 or above required to participate. There were no significant differences between the two age groups in years of education (Myounger=16.3; Mo1der=16.5) or Wechsler Test of Adult Reading scores (Myounger=42.3; Mo1der=41.3). Participants provided written informed consent approved by the University of Southern California (USC) Institutional Review Board and were paid for their participation.
Procedure
Participants completed a series of structural and functional MRI scans acquired with a 3-T Siemans Magnetrom Trio scanner at the USC Dana & David Dornsife Cognitive Neuroscience Imaging Center. As part of this, a neuromelanin-sensitive-weighted MRI (LC-MRI) scan was conducted using a T1-weighted fast spin-echo sequence (repetition time=750 ms, echo time=12 ms, flip angle=120°, 11 axial slices, field of view=220 mm, bandwidth=220 Hz/Px, slice thickness=2.5 mm, slice gap=3.5 mm, in-plane resolution=0.429×0.429 mm2, and scan duration=1 min and 53 s).
Heart rate was measured using ECG during an initial fear-conditioning learning task (about 6 min) and five task blocks of a spatial detection task (about 5 min for each block). For our main analyses, we averaged across the six different measurement occasions for each person, as aggregating across different measurement occasions increases the proportion of variance accounted for by trait rather than situation effects (Bertsch, et al., 2012). During the fear-conditioning task, either a low- or high-pitched tone was paired with mild electric shock. Participants were presented with one of the CS tones for 0.7 s. A shock was delivered for 0.5 s on half of the trials for the tone assigned to the CS+ condition. On the CS− tone trials there was no shock. During the spatial detection task, the CS+ or CS− tone played for 0.7 s, and then a place-object image pair appeared and participants were asked to identify the location of the salient image by pressing a left or right button. In each block, 16 CS+ trials and 16 CS− trials (all 32 without associated shock) were randomly intermixed during this phase with three additional “booster” CS+ trials with shock.
Recording of heart rate
The experimenters attached electrodes to monitor participants’ heart rates. The ECG was obtained by a BIOPAC MP150 data acquisition system at the University of Southern California Dana and David Dornsife Neuroimaging Center. A LEAD108 setup with EL508 MRI-compatible/radio translucent electrodes was used for recording. The raw signal was amplified using an ECG100C amplifier. The signal was continuously digitized at a sampling rate of 10,000 Hz.
HRV analysis
The recorded data were analyzed with Acqknowledge software (BIOPAC Systems Inc., USA) for noise reduction. Because the ECG was recorded during MRI scanning, noise from the scanner can affect the ECG signal. Noise removal was performed through three steps. First, low pass filtering was applied at 40 Hz. Second, high pass filtering was applied at 0.5 Hz. In the last step, a comb band stop was applied to filter out the noise frequency from the scanner. HRV calculations were performed on the corrected data using Kubios version 2.2 (Tarvainen et al., 2014). Spectral analysis using a fast Fourier transform was used to generate the heart period power spectrum (Task-Force-of-the-European-Society-of-Cardiology, 1996). The high frequency (HF) component (0.15–0.40 Hz) was used as our primary estimate of parasympathetic modulation. The root mean square of the successive difference (RMSSD) is a measure of rapid heart rate changes that, like HF-HRV, is predominantly vagally mediated (Sztajzel, 2004) and is used as another measure of parasympathetic influence over heart rate (e.g., Hansen, et al., 2003). Thus, to facilitate future cross-study comparisons we included it among our measures. We also included a measure of low frequency (LF) HRV (0.04–0.15 Hz) for comparison with HF-HRV. Six separate measures of each of these metrics were obtained for each subject from the six scans. To provide an index of individual differences we calculated the average HF-HRV, LF-HRV and RMSSD across all scans for each participant that had sufficiently good quality recordings (as detailed below). HF-HRV and LF-HRV indices were natural log transformed prior to averaging because their distributions were highly skewed, which yielded normal distributions for all HF-HRV and LF-HRV indices used in analyses (including those conducted separately by age group), as assessed by one-sample Kolmogorov Smirnov tests. We excluded data from scans where all three of the following criteria were met: 1) The beat-to-beat (RR) interval was below 500 ms and so was faster than would be expected (Umetani et al., 1998); 2) RMSSD was below 10, which would be more regular than expected for a normal heart rate (Umetani et al., 1998); 3) and the R peaks could not be identified via visual inspection, due to noise. In addition, we excluded all data from one scan that had an RMMSD more than 3 SD higher than the average RMMSD across all participant scans. Based on these criteria, 10 older adults had data from one or more of the six scans excluded (3, 2, 3, and 2 participants with 1, 2, 3, or 4 scans excluded, respectively) and eight younger adults had data excluded (3, 3, 1, and 1 participants with 1, 2, 3, or 4 scans excluded, respectively).
Anxiety questionnaire
Participants completed the 40-question State-Trait anxiety inventory (STAI; Spielberger, 1983) before their MRI scan. We computed trait anxiety score from 20 of the questions.4
LC-MRI signal intensity
As outlined in Clewett et al., (2016), left and right LC regions of interest were hand-drawn on each participant’s LC-MRI image by two researchers who were blind to all other participant information. The intraclass correlations between ratings were .81 and .88, for right and left LC regions respectively. Left and right LC regions of interest (ROIs) were defined in the axial slice 7 mm below the inferior boundary of the inferior colliculus (e.g., Shibata et al., 2006) using a cross that was ~1.29 mm (or 3 voxels) wide and high to cover the 1–2mm distribution of LC neurons in this slice (German et al., 1988). The centers of these ROIs were manually placed on the left and right voxels with the highest signal intensity within locations anatomically consistent with the LC (Fig. 1). In cases in which the peak voxel was right next to the fourth ventricle, the center of the voxel was placed one voxel further away from the ventricle (such that the LC ROI still included the peak voxel) to avoid inclusion of ventricle voxels in the ROI, which could lead to underestimation of signal intensity. To control for scan differences in signal noise, a reference ROI was drawn in the dorsal pontine tegmentum (PT) using a 10×10 voxel square (18.4 mm2) equidistant between the left and right LC ROIs. To obtain our individual difference measure of LC-MRI contrast, we averaged the mean signal intensities from the left and right LC ROIs and used this average together with the PT value to compute the LC contrast-to-noise ratio using this formula: (LCintensity−PTintensity)/PTintensity (Sasaki et al., 2006; Shibata et al., 2006).
Fig. 1.
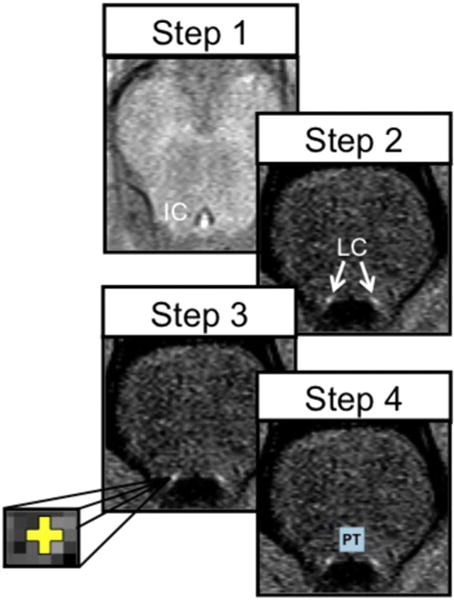
The steps used to delineate the LC regions of interest (ROIs). Step 1: In the axial plane, we identified the most inferior slice of the inferior colliculus (sagittal slices were also referenced to help identify the inferior-most portion of the inferior colliculus); Step 2: We then moved down 7 mm (2 slices) to a slice where LC should be most visible based on previous findings; Step 3: We drew a cross that was three voxels wide and three voxels tall and centered on the peak intensities of the left and right LC (each voxel was 0.429×0.429 mm2); Step 4: Six voxels above the more ventral of the two LC ROIs and equidistantly between them, we drew a reference 10×10 voxel square ROI in the dorsal pontine tegmentum to allow us to normalize the LC based on noise in the image. Figure reprinted from Clewett et al. (2016).
To examine associations between LC-MRI contrast and HRV we calculated Pearson correlations (we report the correlation 95% confidence intervals in brackets). The Fisher z-test was used to calculate differences between correlations.
Results
Age group mean differences
Means and statistical comparisons across age groups for all variables assessed are shown in Table 1. As expected, older adults had significantly higher LC-MRI contrast than younger adults. In addition, older adults had significantly less HF-HRV power and more LF-HRV power than younger adults. RMSSD was marginally significantly greater among younger than older adults. Trait anxiety was significantly lower in older adults than in younger adults, consistent with declines in anxiety typically seen across middle adulthood (Gum, et al., 2009; Teachman, 2006).
Table 1.
Mean values of all measures by age group and t-tests comparing average values across age groups.
| Measure | Younger Adult Group Mean (and SD) | Older Adult Group Mean (and SD) | Independent Means T-test t and p values (df = 43) |
|---|---|---|---|
| LC-MRI Contrast | 0.15 (.04) | 0.18 (.05) | t=−2.20, p=.03 |
| HF-HRV | 5.31 (.56) | 4.77 (.57) | t=3.14, p=.003 |
| LF-HRV | 5.76 (.46) | 6.08 (.57) | t=−2.09, p=.04 |
| RMSSD | 24.88 (4.73) | 21.43 (6.73) | t=2.02, p=.05 |
| Trait Anxiety | 36.19 (9.96) | 27.00 (6.97) | t=3.39, p=.002 |
| Age | 23.63 (4.79) | 67.89 (4.84) | t=−30.25, p<.001 |
Individual differences in LC-MRI correlate with HF-HRV
For all correlation coefficients, we provide 95% confidence intervals in brackets. Across all participants, LC-MRI contrast correlated negatively with HF-HRV, r(43)=−.52 [−.71, −.27], p < .001 (Fig. 2), but did not correlate significantly with LF-HRV, r(43)=.13 [−.17, .41], p >.3 (Table 2).5 The negative correlation with HF-HRV remained significant when age was included as a covariate, r(42)=−.45 [−.65, −.17], p=.002 (Table 3). Similar results were obtained for RMSSD as for HF-HRV. Likewise, including participant sex as a covariate did not change the nature of any of these correlations.
Fig. 2.
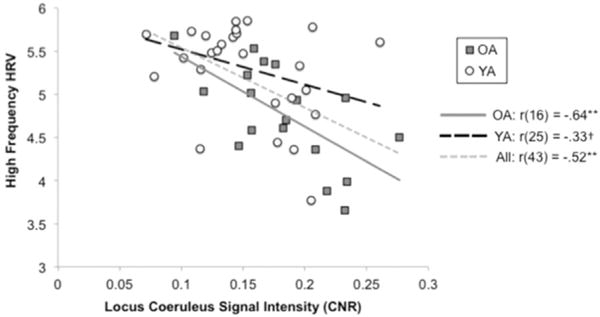
The correlation between the locus coeruleus signal intensity based on the contrast-to-noise (CNR) ratio and high frequency heart rate variability (HRV) for younger adults (plotted using circles) and for older adults (plotted using squares).
Table 2.
Bivariate correlations across all participants (N=45), with 95% confidence intervals in brackets.
| Measure | LC-MRI | HF-HRV | LF-HRV | RMSSD | Trait Anxiety | Age |
|---|---|---|---|---|---|---|
| LC-MRI Contrast | – | |||||
| HF-HRV | −.52** [−.71, −.27] |
– | ||||
| LF-HRV | .13 [−.17, .41] |
−.17 [−.44, .13] |
– | |||
| RMSSD | −.41** [−.63, −.13] |
.76** [.60, .86] |
−.10 [−.38, .20] |
– | ||
| Trait Anxiety | −.29† [−.54, .004] |
.22 [−.08, .48] |
−.34* [−.58, −.05] |
.29† [−.004, .54] |
– | |
| Age | .33* [.04, .57] |
−.44** [−.65, −.17] |
.31* [.02, .55] |
−.30* [−.55, −.007] |
−.49** [−.69, −.23] |
– |
p <.1.
p <.01.
p <.05.
Table 3.
Partial correlations controlling for age (N=45), with 95% confidence intervals in brackets.
| Measure | LC-MRI | HF-HRV | LF-HRV | RMSSD | Trait Anxiety |
|---|---|---|---|---|---|
| LC-MRI Contrast | – | ||||
| HF-HRV | −.45** [−.65, −.17] |
– | |||
| LF-HRV | .03 [−.27, .32] |
−.04 [−.33, .26] |
– | ||
| RMSSD | −.35* [−.53, −.06] |
.74** [.57, .85] |
−.01 [−.30, .28] |
– | |
| Trait Anxiety | −.16 [−.43, .14] |
.01 [−.28, .30] |
−.23 [−.49, .07] |
.17 [−.13, .44] |
– |
p <.1.
p <.01.
p < .05.
When each age group was considered separately, the correlation between LC-MRI and HF-HRV did not quite attain significance for younger adults, r(25)=−.33, p=.098 (Table 4) but was significant for older adults, r(16)=−.64, p=.004 (Table 5). These correlations did not differ significantly from each other, Fisher’s z=1.26, p=0.21.
Table 4.
Bivariate correlations among younger adults only (N=27), with 95% confidence intervals in brackets.
| Measure | LC-MRI | HF-HRV | LF-HRV | RMSSD | Trait Anxiety |
|---|---|---|---|---|---|
| LC-MRI Contrast | – | ||||
| HF-HRV | −.33† [−.63, .06] |
– | |||
| LF-HRV | .02 [−.36, .40] |
.18 [−.21, .52] |
– | ||
| RMSSD | −.32‡ [−.62, .07] |
.78** [.57, .90] |
.33† [−.06, 63] |
– | |
| Trait Anxiety | −.11 [−.47, .28] |
−.11 [−.47, .28] |
−.12 [−.48, .27] |
.04 [−.35, .41] |
– |
p <.1.
p=.1.
p < .01.
Table 5.
Bivariate correlations among older adults only (N=18), with 95% confidence intervals in brackets.
| Measure | LC-MRI | HF-HRV | LF-HRV | RMSSD | Trait Anxiety |
|---|---|---|---|---|---|
| LC-MRI Contrast | – | ||||
| HF-HRV | −.64** [−.85, −.25] |
– | |||
| LF-HRV | .06 [−.42, .51] |
−.31 [−.67, .18] |
– | ||
| RMSSD | −.39 [−.73, .09] |
.72** [.38, .89] |
−.31 [−.67, .18] |
– | |
| Trait Anxiety | −.32 [−.69, .17] |
.32 [−.17, .67] |
−.48* [−.76, −.02] |
.42† [−.06, .74] |
– |
p <.1.
p < .01;
p < .05;
We note that if we included only those participants who only had clean HRV data from four or more scans (and thus were those for whom we could compute the most stable estimate of trait HRV), the correlation between HF-HRV and LC-MRI became significant for younger adults, r(23)=−.41 [−.69, −.02], p=04, and the other significant correlations between HF-HRV and LC-MRI measures remained significant both at the whole group level and for older adults (see Supplementary Tables 1–4).
In summary, both younger and older adults showed a negative relationship between LC-MRI contrast and HF-HRV (although the relationship was stronger for older than for younger adults the correlations were not significantly different) and the negative correlation at the group level could not be accounted for by age differences in these variables.
As trait anxiety also differed across age groups and could potentially be a factor contributing to LC-MRI contrast, we also examined whether the correlation between LC-MRI contrast and HF-HRV could be accounted for by individual differences in trait anxiety. However, including trait anxiety as a covariate did not eliminate the negative correlation between LC-MRI and HF-HRV, r(42)=−.49 [−.68, −.23], p=.001. There was a significant negative correlation between trait anxiety and LF-HRV at the group level, r(43)=−.34 [−.58, −.05], p=.02 (Table 2), that was no longer significant for the overall group when age was included as a covariate, r(42)=−.23 [−.49, .07], p=.13 (Table 3). This same correlation was significant within the older group, r(16) =−.48 [−.76, −.02], p=.04 (Table 5), although not when just those with 4 or more useable HRV estimates were included (Supplementary Table 4). Thus, there may be a weak relationship such that those with higher trait anxiety have lower LF-HRV. However, trait anxiety was not significantly correlated with HF-HRV in either the whole group or within the age subgroups (all p > .1; see Tables 2, 4 and 5), and so is unlikely to be driving the relationships we found between HF-HRVand LC-MRI.
LC-MRI contrast and trait anxiety showed a weak negative relationship
As shown in Table 2, across all participants, there was a marginally significant correlation between LC-MRI contrast and trait anxiety. However, this correlation was no longer significant when age was included as a covariate (Table 3), and was not significant in either age group considered separately, nor after those with fewer HRV estimates were excluded (Supplementary Tables 1–4).
Conclusions
LC activity influences HRV in part by inhibiting parasympathetic modulation of the heart (Samuels and Szabadi, 2008). Because, via the vagus nerve, parasympathetic inputs increase HF-HRV (Laitio et al., 2007), we predicted that individuals who have higher LC-MRI neuromelanin contrast should also have lower HF-HRV. Neuromelanin is a by-product of catecholamine activity (Wakamatsu et al., 2015). In the LC, it increases across young and mid-adulthood, then decreases in late life (Shibata et al., 2006), suggesting that neuromelanin may index habitual activation of the LC as well as its integrity in late life. We found that, across our cross-sectional sample that included younger and older adults, greater LC-MRI contrast was associated with lower HF-HRV during a couple of different tasks. This correlation is consistent with the LC’s role inhibiting parasympathetic modulation of the heart (Samuels and Szabadi, 2008).
Other recent findings in humans support the link between increased LC-NE system activity and decreased HF-HRV. In one study, inducing pain both increased LC activity and decreased HF-HRV (Sclocco et al., 2016). In another study (Bosch, et al., 2003), increases in salivary α-amylase (a marker of adrenergic activity; Granger, et al., 2007) were associated with decreases in RMSSD (which as mentioned is a measure of rapid heart rate changes that—like HF-HRV—is predominantly vagally mediated; Sztajzel, 2004). Our study extends these findings and provides initial support for the notion that LC-MRI contrast provides a trait measure reflecting individual differences in LC-NE system activity. Furthermore, it is important to note that measures of HF-HRV even during tasks reflect primarily stable individual difference or trait variance particularly when several HF-HRV measures are aggregated as done in the present study (Bertsch et al., 2012). Thus, the present results suggest an association between trait levels of LC-NE system activity and trait levels of HF-HRV.
With age, HRV decreases, especially in measures reflecting high frequency parasympathetic modulation such as RMSSD or HF-HRV (Bonnemeier et al., 2003; Lipsitz et al., 1990; De Meersman and Stein, 2007; Umetani et al., 1998). In addition, LC neuromelanin contrast increases across middle adulthood, leading to higher average LC neuromelanin contrast in those in their 60’s than those in their 20’s (Shibata et al., 2006). These expected age differences were seen within our sample. However, age did not account for the association between LC-MRI and HF-HRV, as even when age was included as a covariate, the correlation was significant. In addition, the negative correlations between LC-MRI contrast and HF-HRV did not appear to be driven by only one age group, as within each age subgroup, negative correlations were seen between LC-MRI contrast and HF-HRV.
These findings showing that LC-MRI neuromelanin contrast relates to parasympathetic system activity raise many interesting questions. One question is whether the similar relationships between these measures seen among younger and older adults might be driven by slightly different mechanisms. Among younger adults, LC-MRI neuromelanin contrast may reflect individual differences in the likelihood of activating LC, whereas among older adults LC-MRI neuromelanin contrast may also reflect the structural integrity of the LC (as reviewed in the Introduction, the number of neuromelanin-containing neurons in the LC decrease in late life), and therefore the capacity for activating the LC.
Another question raised by these findings is whether the relationship is driven by individual differences in anxiety affecting both HF-HRV and LC activity. Although no significant relationships between trait anxiety and HF-HRV were seen in our sample of healthy adults (see also Dishman et al., 2000; Virtanen et al., 2003), people with anxiety disorders tend to have lower HRV (for reviews see Chalmers et al., 2014; Friedman, 2007). There are also links between anxiety and the noradrenergic system. One common characterization of anxiety disorders is that they are caused by noradrenergic system hyperactivation (e.g., Buoli, et al., 2013). Consistent with this, the α2-adrenergic agonist clonidine that inhibits synaptic noradrenaline release is one pharmacological treatment for anxiety (Hoehn-Saric, et al., 1981), and the α2-adrenergic antagonist yohimbine increases anxiety (Charney, 2003). However, despite these links between anxiety and α2-adrenergic pathways, the role of the LC in anxiety is not clear. One group reported that ablation of the LC in monkeys decreased behaviors associated with anxiety in humans (Redmond and Huang, 1979), but these findings were only reported in a meeting abstract and not replicated since to our knowledge.
In contrast with these findings, ablation of the LC in rodents leads to an increase in fear responses to novelty (Delini-Stula, et al., 1984; Koob, et al., 1984; Itoi and Sugimoto, 2010; Mason and Fibiger, 1979), or no change in social contacts during novelty (Crow, et al., 1978), and increasing LC activation decreases anxious behavior in the open field test (Weiss et al., 1994). These findings suggest that LC activity may help reduce anxiety in at least some contexts. In our overall sample, LC-MRI contrast was marginally negatively correlated with trait anxiety and not significantly correlated when age was included as a covariate. However, if anxiety was a causal factor influencing both greater LC-MRI contrast and lower HF-HRV and leading the correlation between these variables, one would expect to see positive correlations between LC-MRI and anxiety, which were clearly not evident in our sample. Thus, it is unlikely that the relationships we found were due to individual differences in trait anxiety.
A related question for future research is what factors influence neuromelanin accumulation in the LC. To date, there has been little research on this topic. The links between LC and anxiety described above may have some bearing on this issue. Insofar as anxiety is associated with hyperactivated LC-NE system activity (e.g., Buoli et al., 2013), people suffering from higher trait anxiety should have on average higher tonic levels of LC activity, and therefore potentially higher long-term accumulation of neuromelanin in the LC. However, we did not see a positive relationship between anxiety and LC-MRI contrast; instead, trait anxiety showed a trend towards a negative correlation with LC-MRI contrast. This suggests that, at least within the range of anxiety experienced in a healthy sample, higher tonic levels of arousal induced by anxiety are not a particularly strong factor in increasing LC-MRI contrast. Instead, it may be the strength of the response of the LC-NE system to phasic arousing or engaging events that more strongly predicts individual differences in LC-MRI contrast. In studies stimulating the LC, burst stimulation yields more NE release per impulse than do evenly spaced stimuli (Aston-Jones, et al., 2010), which suggests the possibility that phasic LC responses stimulate more LC metabolism than tonic activity, and so might be more important for determining long-term accumulation of neuromelanin.
Some strengths of this study are that it is the first study to examine the relationship between HRV and any measure of LC structure, that it aggregated across multiple measures of HRV increasing trait stability of the measure (Bertsch et al., 2012), and that it included both younger and older adults. One weakness is that we did not have a measure of HRV during a non-task resting state. Additional research is needed to examine whether the findings generalize to HRV collected during other types of contexts.
Our finding that greater LC-MRI neuromelanin contrast is associated with lower HF-HRV is intriguing and consistent with the notion that LC exerts an influence over HRV via its suppression of parasympathetic activity (Samuels and Szabadi, 2008). Furthermore, these results suggest that LC-MRI neuromelanin contrast measures provide a biomarker reflecting individual differences in the neural regulation of the heart. Further research is needed to investigate the build-up of neuromelanin in the LC across the life span and its relationship to other physiological and brain factors.
Supplementary Material
Acknowledgments
This project was supported by the National Institutes of Health Grant number R01AG025340.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuroimage.2017.02.025.
Footnotes
As we were interested in examining the trait aspects of HRV rather than its situational influences, we focused on the trait component of the STAI, as well. However, results were highly similar if the state measure of the STAI were used. Older adults had lower state anxiety (M=27.0) than did younger adults (M=39.5), p<.001, and all reported correlations between trait anxiety and LC-MRI were similar in nature for state anxiety and LC-MRI.
For our main analyses, we used averages of HRV measurements across all scans in order to increase the trait stability of the measures (Bertsch et al., 2012). However, if we examined HRV from the fear conditioning session separately from the averaged HRV measures from the detection sessions, we found similar results across these two types of sessions, with significant correlations between HF-HRV and LC-MRI for both fear conditioning, r(35)=−.41, p=.01, and detection, r(43)=−.50, p < .001, significant correlations between RMSSD and LC-MRI for both fear conditioning, r(35)=−.36, p=.03, and detection, r(43)=−.40, p=.006, and non-significant correlations between LF-HRV and LC-MRI for both fear conditioning, r(35)=.20, p=.2, and detection, r(43)=.10, p=.5. Thus, the negative correlations between HF-HRV/RMSSD and LC-MRI were consistent regardless of the task scan type during HRV measurement.
References
- Aston-Jones G, Meijas-Aponte C, Waterhouse B. Norepinephrine: CNS pathways and neurophysiology. Stress Sci: Neuroendocr. 2010;388 [Google Scholar]
- Baillard C, Vivien B, Mansier P, Mangin L, Jasson S, Riou B, Swynghedauw B. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med. 2002;30(2):306–310. doi: 10.1097/00003246-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. http://dx.doi.org/10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Naumann E, Schächinger H, Schulz A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology. 2012;49(5):672–682. doi: 10.1111/j.1469-8986.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- Bittiner S, Smith S. Beta-adrenoceptor antagonists increase sinus arrhythmia, a vagotonic effect. Br J Clin Pharmacol. 1986;22(6):691–695. doi: 10.1111/j.1365-2125.1986.tb02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemeier H, Wiegand UK, Brandes A, Kluge N, Katus HA, Richardt G, Potratz J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects. J Cardiovasc Electrophysiol. 2003;14(8):791–799. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Amerongen AVN. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med. 2003;65(2):245–258. doi: 10.1097/01.psy.0000058376.50240.2d. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Cogn Behav Neurol. 1988;1(2):111–118. [Google Scholar]
- Buoli M, Caldiroli A, Caletti E, Paoli RA, Altamura AC. New approaches to the pharmacological management of generalized anxiety disorder. Expert Opin Pharmacother. 2013;14(2):175–184. doi: 10.1517/14656566.2013.759559. [DOI] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney D. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand. 2003;108(s417):s38–s50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Clewett D, Lee TH, Greening SG, Ponzio A, Margalit E, Mather M. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging. 2016;37:117–126. doi: 10.1016/j.neurobiolaging.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Deakin J, File S, Longden A, Wendlandt S. The locus coeruleus noradrenergic system—evidence against a role in attention, habituation, anxiety and motor activity. Brain Res. 1978;155(2):249–261. doi: 10.1016/0006-8993(78)91021-1. [DOI] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol. 2007;74(2):165–173. doi: 10.1016/j.biopsycho.2006.04.008. http://dx.doi.org/10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Mogilnicka E, Hunn C, Dooley D. Novelty-oriented behavior in the rat after selective damage of locus coeruleus projections by DSP-4, a new noradrenergic neurotoxin. Pharmacol Biochem Behav. 1984;20(4):613–618. doi: 10.1016/0091-3057(84)90312-5. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37(2):121–133. doi: 10.1016/s0167-8760(00)00085-4. http://dx.doi.org/10.1016/S0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. http://dx.doi.org/10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brain stem injury after asphyxia in preterm fetal sheep. Am J Physiol-Regul Integr Comp Physiol. 2004;287(4):R925–R933. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- German D, Walker B, Manaye K, Smith W, Woodward D, North A. The human locus coeruleus: computer reconstruction ofcellulardistribution. J Neurosci. 1988;8(5):1776–1788. doi: 10.1523/JNEUROSCI.08-05-01776.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research. Ann N Y Acad Sci. 2007;1098(1):122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. http://dx.doi.org/10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Merchant AF, Keyser ML. Effects of clonidine on anxiety disorders. Arch Gen Psychiatry. 1981;38(11):1278–1282. doi: 10.1001/archpsyc.1981.01780360094011. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocr. 2010;22(5):355–361. doi: 10.1111/j.1365-2826.2010.01988.x. http://dx.doi.org/10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47(4):1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. http://dx.doi.org/10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm ACE, Aston-Jones GS, Eckert MA. Histologic validation of locus coeruleus MRI contrast in postmortem tissue. Neuroimage. 2015;113:235–245. doi: 10.1016/j.neuroimage.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Thatcher-Britton K, Britton DR, Roberts DC, Bloom FE. Destruction of the locus coeruleus or the dorsal NE bundle does not alter the release of punished responding by ethanol and chlordiazepoxide. Physiol Behav. 1984;33(3):479–485. doi: 10.1016/0031-9384(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Korpelainen J, Huikuri H, Sotaniemi K, Myllylä V. Abnormal heart rate variability reflecting autonomic dysfunction in brainstem infarction. Acta Neurol Scand. 1996;94(5):337–342. doi: 10.1111/j.1600-0404.1996.tb07076.x. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lai CJ, Huang YT, Yang CC. Regression analysis between heart rate variability and baroreflex-related vagus nerve activity in rats. J Cardiovasc Electrophysiol. 2005;16(8):864–869. doi: 10.1111/j.1540-8167.2005.40656.x. [DOI] [PubMed] [Google Scholar]
- Laitio T, Jalonen J, Kuusela T, Scheinin H. The role of heart rate variability in risk stratification for adverse postoperative cardiac events. Anesthesia Analg. 2007;105(6):1548–1560. doi: 10.1213/01.ane.0000287654.49358.3a. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mietus J, Moody GB, Goldberger AL. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81(6):1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. I. Anxiety: the locus coeruleus disconnection. Life Sci. 1979;25(26):2141–2147. doi: 10.1016/0024-3205(79)90086-9. [DOI] [PubMed] [Google Scholar]
- Mather M, Harley CW. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 2016;20:214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond D, Huang Y. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25(26):2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJ, Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50(5):477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. http://dx.doi.org/10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. NeuroReport. 2006;17(11):1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- Saul JP. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology. 1990;5(1):32–37. [Google Scholar]
- Schwarz G, Pfurtscheller G, Litscher G, List WF. Quantification of autonomic activity in the brainstem in normal, comatose and brain dead subjects using heart rate variability. Funct Neurol. 1987;2(2):149–154. [PubMed] [Google Scholar]
- Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Barbieri R. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philos Trans R Soc A: Math Phys Eng Sci. 2016;374(2067) doi: 10.1098/rsta.2015.0189. http://dx.doi.org/10.1098/rsta.2015.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3T. Magn Reson Med Sci. 2006;5(4):197–200. doi: 10.2463/mrms.5.197. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46(9):1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405(1–3):397–406. doi: 10.1016/s0014-2999(00)00569-0. http://dx.doi.org/10.1016/S0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- Task-Force-of-the-European-Society-of-Cardiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Teachman BA. Aging and negative affect: the rise and fall and rise of anxiety and depression symptoms. Psychol Aging. 2006;21(1):201. doi: 10.1037/0882-7974.21.1.201. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Johnsen BH. The Non-invasive Assessment of Autonomic Influences on the Heart Using Impedance Cardiography and Heart Rate Variability Handbook of Behavioral Medicine. Springer; New York: 2010. pp. 723–740. [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heartrate variability and neuroimaging studies: implications for heartrate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tomlinson B, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci. 1981;49(3):419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SHJ, Thayer JF. Accentuated antagonism in the control of human heart rate. Clin Auton Res. 2000;10(3):107–110. doi: 10.1007/BF02278013. http://dx.doi.org/10.1007/bf02278013. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. http://dx.doi.org/10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. Quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979;38(5):490–497. doi: 10.1097/00005072-197909000-00004. http://dx.doi.org/10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Jula A, Salminen JK, Voipio-Pulkki LM, Helenius H, Kuusela T, Airaksinen J. Anxiety and hostility are associated with reduced baroreflex sensitivity and increased beat-to-beat blood pressure variability. Psychosom Med. 2003;65(5):751–756. doi: 10.1097/01.psy.0000088760.65046.cf. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J Neurochem. 2015;135:768–776. doi: 10.1111/jnc.13237. http://dx.doi.org/10.1111/jnc.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Piñol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem α1 and β1 receptors. J Neurosci. 2014;34(18):6182–6189. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull. 1994;35(5–6):561–572. doi: 10.1016/0361-9230(94)90170-8. http://dx.doi.org/10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Winkelmann T, Thayer JF, Pohlack S, Nees F, Grimm O, Flor H. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct Funct. 2016:1–8. doi: 10.1007/s00429-016-1185-1. [DOI] [PubMed] [Google Scholar]
- Wree A, Braak H, Schleicher A, Zilles K. Biomathematical analysis of the neuronal loss in the aging human brain of both sexes, demonstrated in pigment preparations of the pars cerebellaris loci coerulei. Anat Embryol. 1980;160(1):105–119. doi: 10.1007/BF00315653. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Thayer JF, Greening SG, Lee TH, Ponzio A, Min J, Koenig J. Brain Structural Concomitants of Resting State Heart Rate Variability in the Young and Old: Evidence from Two Independent Samples. 2017 doi: 10.1007/s00429-017-1519-7. (In preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


