Abstract
Background
Percutaneous ablation is a common treatment for colorectal liver metastases (CLM). However, the effect of RAS mutation on outcome after ablation of CLMs is unclear.
Methods
Patients who underwent image-guided percutaneous ablation of CLMs from 2004 through 2015 and had known Rat sarcoma viral oncogene homolog (RAS mutation status were analyzed. Patients were evaluated for local tumor progression as observed on imaging at CLM treated with ablation. Multivariable Cox regression analysis was performed to determine factors associated with local tumour progression-free survival.
Results
The study included 92 patients who underwent ablation of 137 CLMs. Thirty-six patients (39%) had mutant RAS. Rates of local tumour progression were 14% (8/56) for patients with wild-type RAS and 39% (14/36) for patients with mutant RAS (p=0·007). Actuarial local tumour progression-free survival after percutaneous ablation were worse in patients with mutant RAS than wild-type RAS (3-year local tumour progression-free survival rate: 35% vs. 71%, p=0.001). In multivariable analysis, negative predictors of local tumour progression-free survival were minimal ablation margin <5 mm (hazard ratio [HR] 2·48, 95% confidence interval [CI] 1·31–4·72; p=0·006) and mutant RAS (HR 3·01, 95% CI 1·60–5·77; p=0·001).
Conclusion
Mutant RAS is associated with an earlier and higher rate of local tumour progression in patients undergoing ablation of CLM.
INTRODUCTION
Liver ablation is an effective treatment modality for patients with limited colorectal liver metastases (CLM)1–7. Current series demonstrate 5-year overall survival rates after ablation of CLMs ranging from 21% to 47·8%. 1–4 To achieve optimal results following liver ablation, local tumor progression should be minimized.1, 2 Small size of CLMs, limited number of CLMs,4–8 and adequate ablation margins2, 9, 10 have been positively correlated with low rates of LTP. Similarly, the existing surgical literature demonstrates that resection of CLMs with negative margins is associated with improved rates of overall survival11, 12, whereas resection with positive margins is strongly associated with a worse prognosis even with the use of modern preoperative chemotherapy regimens.13 Despite the importance of adequate ablation and resection margins on the local outcomes of the treated CLMs, the biologic factors associated with poor local tumor control following local therapies for CLMs remains unclear, and further investigations are needed.
Rat sarcoma viral oncogene homolog (RAS) mutations are found in up to 40% of patients with colorectal cancer and have been associated with reduced survival after resection of primary colorectal cancer and CLMs.14–17 It has been reported that mutant RAS is associated with inferior response to preoperative chemotherapy and worse survival in patients with resectable CLMs18 and with a more invasive and migratory tumour biology.19 Additionally, a recent study demonstrated that mutant RAS was associated with positive and narrow resection margins in patients undergoing resection of CLMs.20
On the basis of these findings, it was hypothesized that the local tumour progression rate after ablation of CLMs is higher in patients with mutant RAS than in patients with wild-type RAS.
PATIENTS AND METHODS
Study population
This single-institution retrospective study was compliant with the Health Insurance Portability and Accountability Act and approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center with a waiver of informed consent (IRB protocol PA15-0566). The institution’s retrospectively compiled liver ablation database maintained by the Department of Interventional Radiology was searched to identify patients who underwent percutaneous ablation of CLMs from 2004 through 2015, had known RAS status, did not undergo combined transarterial therapies or subsequent surgical resection, and had imaging follow-up to at least 6 months after percutaneous ablation.
Variables extracted from the database or updated by review of electronic medical records for each patient included sex, age, location of primary tumour, lymph node status of primary tumour, number and type of pre-ablation chemotherapy regimens (if any), disease-free survival time between diagnosis of primary cancer and appearance of CLM, pre-ablation carcinoembryonic antigen level, presence of other sites of metastasis at the time of percutaneous ablation of CLM, clinical score defined by Fong et al,11, time between hepatic resection and CLM ablation, RAS mutation status, and site of any recurrence after ablation. Variables extracted for each ablated CLM were number of ablation sessions, largest diameter of the lesion at first ablation session, time from CLM discovery to first ablation session, ablation modality (radiofrequency or microwave), minimal ablation margin (<5 mm, 5–10 mm, or >10 mm), CLM location in relation to the liver capsule (subcapsular [within 1 cm of the liver capsule] or non-subcapsular), CLM located adjacent to major vessel (vessel >3 mm in diameter) (yes or no), and presence or absence of local tumour progression. In patients treated with pre-ablation chemotherapy, change in lesion size with chemotherapy was assessed by pre- and post-chemotherapy cross-sectional imaging.
Percutaneous ablation eligibility criteria and technique
Patients were eligible for percutaneous ablation of CLMs if they had no more than five CLMs measuring ideally no more than 5 cm each.21 All procedures were performed with the goal of completely ablating each CLM, but during the period of study accrual, there was no consensus regarding the acceptable minimal ablation margins. All percutaneous hepatic ablation procedures were performed by one of four interventional radiologists (B.C.O, 7 years of experience; S.Y.H., 6 years of experience; K.A., 15 years of experience; and S.G., 17 years of experience) with the patient under general anesthesia and with continuous hemodynamic monitoring by an anesthesiologist. CT imaging guidance was utilized. CT fluoroscopy or ultrasonography was utilized if real-time imaging was deemed appropriate. Ablations were performed with radiofrequency (82 sessions in 77 CLMs) (Cool-tip ablation system, Covidien, Boulder, CO, USA) or microwave (61 sessions in 60 CLMs) (Certus probe, Certus 140 2·4-GHz ablation system, Neuwave, Madison, WI, USA) according to the operator’s choice. Patients were discharged home within 24 hours after the procedure.
Imaging follow-up and assessment of response
Imaging assessment was independently performed by two readers (B.C.O., an interventional radiologist with 7 years of experience; S.Y., a hepatobiliary research fellow with 7 years of experience in hepatobiliary surgery), and discrepancies in interpretation were resolved by consensus. All available pre-ablation contrast-enhanced CT and magnetic resonance or positron emission tomography(PET)–CT studies were reviewed to identify the date of diagnosis of each CLM. If a CLM was present on the first cross-sectional imaging study available in the electronic medical record, the date of this study was considered the date of diagnosis of that particular CLM.
The initial post-ablation cross-sectional imaging assessment of the efficacy of ablation was performed within 4 to 8 weeks after ablation. The width of the minimal ablation margin was assessed by comparing the distances of the index tumor on the baseline cross-sectional imaging and the ablation zone on the initial post-ablation cross-sectional imaging from intrahepatic landmarks on portal venous phase CT images as previously described.2 After the initial post-ablation imaging assessment, further imaging assessments were performed at 2- to 4-month intervals until patient death or loss to follow-up.
To describe ablation endpoints, the standardized terminology and reporting criteria initially described by Goldberg et al22 and later updated by Ahmed et al23 were utilized. Residual unablated tumour was defined as irregular peripheral or nodular enhancement within 1 cm of the ablated area on the initial post-ablation cross-sectional imaging study. LTP was defined as the appearance of tumour foci within 1 cm of the edge of the ablation zone on contrast-enhanced CT or magnetic resonance images after at least one contrast-enhanced post-ablation follow-up study had documented adequate ablation and an absence of viable tissue in the target tumour and surrounding ablation margin.
RAS mutation profiling
RAS mutation profiling was performed as previously described.20 In brief, DNA from the primary tumour or from a CLM was subjected to a routine polymerase chain reaction-based primer extension assay. Screening for mutations in KRAS codons 12 and 13 was performed in all patients, and screening for mutations in KRAS codons 61 and 146 and NRAS codons 12, 13, and 61 was performed in the majority of patients in the most recent years of the study period. The lower detection limit of the assay was approximately one mutant allele in the background of nine wild-type alleles. Single mutations in the various codons of KRAS and NRAS were analyzed together and reported as RAS mutations.
Statistical analysis
Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the χ2 test. Local tumour progression -free survival was measured in months from the date of last ablation session to the date when local tumour progression was detected on cross-sectional imaging or last follow-up. Recurrence-free survival was measured in months from the date of ablation to the date of detection of any organ recurrence on cross-sectional imaging or last follow-up. Overall survival was measured in months from the date of ablation to the date of death or last follow-up. Survival curves were generated using the Kaplan-Meier method, and differences between curves were evaluated with the log-rank test. Univariablee and multivariblre analyses to identify predictors of local tumour progression -free survival were performed by Cox proportional hazards regression models. Variables with p<0·1 in univariate analysis were entered into each multivariate analysis. p<0·05 was considered statistically significant in all analyses. Statistical analysis was performed with JMP software (version 12.1.0; SAS Institute Inc, Cary, NC).
RESULTS
Patients
A total of 149 patients with CLMs underwent percutaneous liver ablation during the study period. Of these, 57 patients were excluded from the analysis because of undetermined RAS mutation status (n=38), use of cryoablation (n=8), use of transarterial chemoembolization (n=4) or surgical resection (n=3) of the ablated lesion, or lack of cross-sectional imaging after ablation (n=4). After these exclusions, 92 patients who underwent percutaneous ablation of 137 CLMs were eligible for the analysis. Of the 137 CLMs, 135 (98·5%) were successfully eradicated after the initial ablation procedure; two CLMs had residual unablated tumour detected on the first cross-sectional imaging study following percutaneous ablation and were successfully ablated after an additional ablation session.
Local tumour progresison
Of the 92 patients in the study, 22 (23·9%) experienced local tumour progression. Local tumour progression occurred at 25 (18·2%) of the 137 ablated CLMs. Among 22 patients with local tumour progression, 17 patients concurrently progressed with other sites of intra- or extra-hepatic metastases and were treated with systemic therapy. The remaining 5 patients had local tumour progression that was deemed unsafe for repeat ablation due to tumour size and/or proximity to critical structures.
Patient, tumour, and treatment characteristics by RAS mutation status
Mutant RAS was detected in 36 patients (39%). Patient, tumour, and treatment characteristics by RAS status are summarized in Table 1. The median age was 59 years (range 28–92), and there were 62 men. The primary tumour was located in the colon in 76 patients (83%) and in the rectum in 16 patients (17%). Positive lymph nodes at diagnosis of the primary tumour were noted in 64 patients (70%).
Table 1.
Patient, tumour, and treatment characteristics according to RAS mutantion status *
| Characteristic | Total | Wild-type RAS | Mutant RAS | p value † |
|---|---|---|---|---|
| All patients | 92 | 56 | 36 | |
| Sex, M: F | 62: 30 | 40: 16 | 22: 14 | 0·303 |
| Age at CLM ablation, median (range), yrs | 59 (28–92) | 59 (33–92) | 58 (28–78) | 0·782‡ |
| Primary tumour | ||||
| Colon: rectum | 76: 16 | 46: 10 | 30: 6 | 0·883 |
| Lymph node metastases | 64 (70) | 37 (66) | 27 (75) | 0·364 |
| Time between date of diagnosis of primary cancer and date of CLM discovery treated with ablation (range), months | 16 (0–295) | 19 (0–295) | 15 (0–93) | 0·076‡ |
| History of hepatic resection before ablation | 55 (60) | 37 (66) | 18 (50) | 0·125 |
| Time between last hepatic resection and ablation, median (range), months | 11 (0·4–125) | 14 (0·4–125) | 9·0 (0·9–22) | 0·088‡ |
| Pre-ablation chemotherapy | 59 (64) | 36 (64) | 23 (64) | 0·969 |
| ≤6 cycles | 30 (51) | 15 (42) | 15 (65) | 0·078 |
| ≥2 regimens | 14 (24) | 10 (28) | 4 (17) | 0·360 |
| Fluorouracil-based chemotherapy regimen | ||||
| Oxaliplatin | 33 (56) | 18 (50) | 15 (65) | 0·251 |
| Irinotecan | 29 (49) | 20 (56) | 9 (39) | 0·218 |
| Use of bevacizumab | 37 (63) | 22 (61) | 15 (65) | 0·750 |
| Use of anti-EGFR agent | 11 (19) | 9 (25) | 2 (8·7) | 0·117 |
| Change in lesion size | ||||
| Decrease: Stable: Increase | 27: 26: 6 | 17: 16: 3 | 10: 10: 3 | 0·839 |
| Time between last chemotherapy and ablation, median (range), days | 34 (6–3674) | 32 (6–3674) | 36 (6–520) | 0·376‡ |
| Interval between CLM discovery and ablation, median (range), days | 144 (4–1397) | 142 (6–828) | 144 (4–1397) | 0·873‡ |
| Indication for ablation | ||||
| Recurrence after resection | 53 (58) | 35 (63) | 18 (50) | 0·236 |
| Not candidate for surgery | 36 (39) | 21 (38) | 15 (42) | 0·689 |
| CEA level at ablation, median (range), ng/mL | 3·6 (0·6–328) | 3·8 (1·0–186) | 3·3 (0·6–328) | 0·943‡ |
| Clinical risk score¶ | ||||
| 0/1: ≥2 | 59: 33 | 37: 19 | 22: 14 | 0·628 |
| Ablation modality | ||||
| RFA: microwave | 50: 42 | 30: 26 | 20: 16 | 0·852 |
| Ablation sessions | ||||
| 1: ≥2 | 89: 3 | 53: 3 | 36: 0 | 0·158 |
| Ablation margin | ||||
| < 5 mm: 5–10 mm: > 10 mm | 36: 30: 26 | 20: 20: 16 | 16: 10: 10 | 0·652 |
| Ablated lesion adjacent to major vessel(s)© | 21 (23) | 13 (23) | 8 (22) | 0·912 |
| Liver metastases | ||||
| Synchronous: metachronous | 35: 57 | 18: 38 | 17: 19 | 0·146 |
| Largest tumour size at ablation, median (range), cm | 1·6 (0·4–4·0) | 1·6 (0·4–4·0) | 1·6 (0·7–4·0) | 0·700‡ |
| Tumour number (solitary: multiple) | 70: 22 | 46: 10 | 24: 12 | 0·089 |
| Subcapsular lesion | 51 (55) | 32 (57) | 19 (53) | 0·681 |
| Concomitant extrahepatic disease | 23 (25) | 13 (23) | 10 (28) | 0·622 |
| Post-ablation chemotherapy | 46 (50) | 25 (45) | 21 (58) | 0·200 |
| Local tumour progression | 22 (24) | 8 (14) | 14 (39) | 0·007 |
Values in table are number of patients (percentage) unless indicated otherwise.
wild-type RAS vs. mutant RAS ; χ2 test, unless indicated otherwise.
Wilcoxon rank-sum test.
Clinical risk score was defined by a disease-free interval from primary to liver metastasis ≤12 months, more than one liver tumor, largest hepatic metastasis ≥5 cm, carcinoembryonic antigen level >200 ng/mL, and the presence of extrahepatic disease.21
A major vessel meant a vessel >3 mm in diameter.
CLM, colorectal liver metastases; EGFR, epidermal growth factor receptor; CEA, carcinoembryonic antigen; RFA, radiofrequency ablation.
Patients with mutant RAS were associated with higher rate of LTP (14% in patients with wild-type RAS versus 39% in patients with mutant RAS; p=0·007). Patients with mutant and wild-type RAS did not differ with respect to other demographic, clinical, or imaging characteristics, including sex, age, history of hepatic resection, location of the primary tumor, lymph node status of the primary tumor, or ablation modality.
Pre-ablation chemotherapy was utilized in 59 patients (64%), and patients with wild-type and mutant RAS did not differ significantly in terms of the frequency of use of pre-ablation chemotherapy, number of cycles and regimens of pre-ablation chemotherapy, and types of pre-ablation chemotherapy. Finally, patients with wild-type and mutant RAS did not differ with respect to time between CLM discovery and first ablation session, number, location, and size of CLMs treated with ablation (Table 1).
Influence of RAS mutation status on local tumour progression-free survival, recurrence-free survival, and overall survival
The median (range) follow-up period was not significantly different between patients with wild-type RAS and those with mutant RAS: 35 (6·1–138) months and 28 (6·0–133) months, respectively (p=0·63).
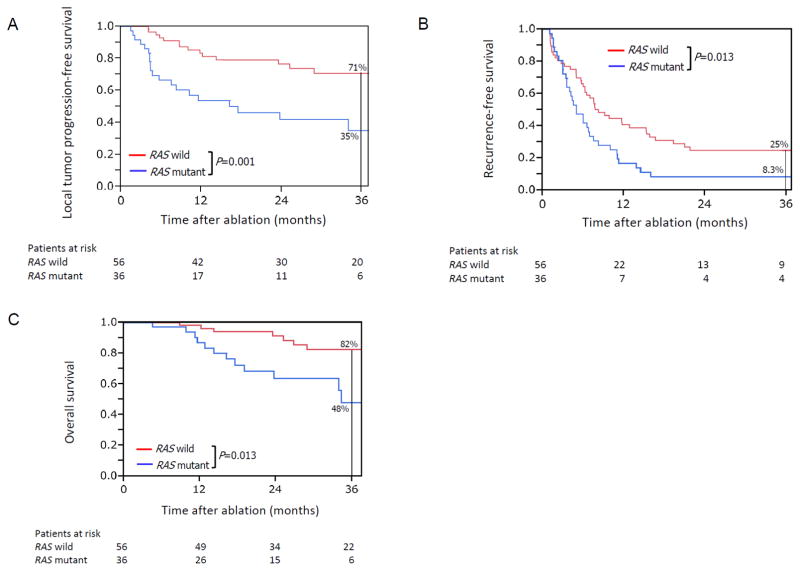
Kaplan-Meier plots of local tumour progression-free survival, recurrence-free survival, and overall survival by RAS mutation status are shown in Figure 1. Among the patients with mutant RAS, 58% experienced local tumour progression within 2 years (Figure 1A). The 3-year LTP-free survival, recurrence-free survival, and overall survival rates were significantly worse in the patients with mutant RAS than in the patients with wild-type RAS (Figure 1). Among the 56 patients with wild-type RAS, 24 patients (43%) developed one or more new CLMs on the liver distant from the ablated lesion(s) on imaging follow-up, and four (17%) of these 24 patients presented with local tumour progression at previously ablated CLMs. Among the 36 patients with mutant RAS, 20 patients (56%) developed one or more new CLMs distant from the ablated lesion(s) on imaging follow-up, and 12 (60%) of these 20 patients also had local tumour progression at previously ablated CLMs.
Figure 1.
Kaplan-Meier plots of (A) local tumor progression–free survival, (B) recurrence-free survival, and (C) overall survival after ablation of CLM among patients with wild-type RAS (red lines) and mutant RAS (blue lines) status.
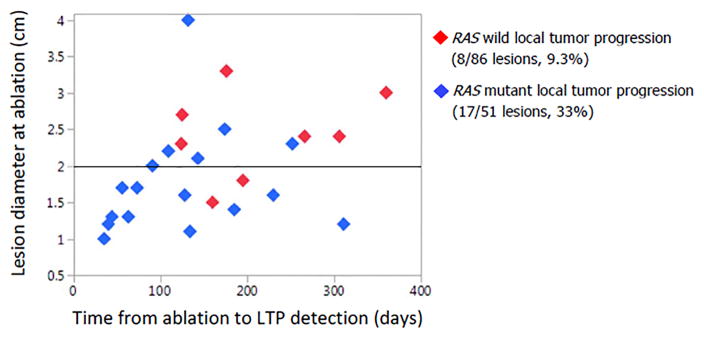
Analysis of the 25 ablated CLMs with local tumour progression demonstrated that local tumour progression occurred earlier in patients with mutant RAS than in patients with wild-type RAS and that the size of the CLMs where local tumour progression occurred were smaller in patients with mutant RAS than in patients with wild-type RAS (Figure 2).
Figure 2.
Graphic representation of the 25 ablated CLMs that showed LTP according to time from ablation to detection of LTP on imaging follow-up, maximum diameter of the ablated CLM at ablation, and RAS status (wild-type RAS: red diamonds; mutant RAS: blue diamonds).
Predictors of local tumour progression-free survival
On multivariable analysis of factors associated with LTP-free survival, independent predictors of worse outcome were minimal ablation margin <5 mm (hazard ratio [HR] 2·48, 95% confidence interval [CI] 1·31–4·72; p=0·006) and mutant RAS (HR 3·01, 95% CI 1·60–5·77; p=0·001) (Table 2).
Table 2.
Univariable and multivariable analyses for local tumour progression-free survival
| N | Local tumour progression-free survival rate, %* | Univariable P value† | Hazard ratio (95% CI) | Multivariable P value‡ | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 year | 3 years | |||||
| All patients | 92 | 72 | 57 | - | - | - |
| Sex | ||||||
| M | 62 | 76 | 60 | 0·189 | - | - |
| F | 30 | 61 | 51 | |||
| Age, years | ||||||
| ≥60 | 43 | 76 | 59 | 0·450 | - | - |
| <60 | 49 | 67 | 55 | |||
| Primary tumour | ||||||
| Colon | 76 | 73 | 61 | 0·204 | - | - |
| Rectum | 16 | 68 | 41 | |||
| Positive metastatic node of primary tumour | ||||||
| Yes | 64 | 75 | 66 | 0·254 | - | - |
| No | 28 | 64 | 42 | |||
| Pre-ablation chemotherapy§ | ||||||
| Yes | 59 | 70 | 46 | 0·083 | 1·64 (0·82–3·48) | 0·164 |
| No | 33 | 78 | 78 | |||
| Pre-ablation chemotherapy cycles | ||||||
| >6 | 29 | 68 | 50 | 0·256 | - | - |
| ≤6 | 63 | 74 | 61 | |||
| Fluorouracil-based chemotherapy regimen | ||||||
| Oxaliplatin | ||||||
| Yes | 33 | 71 | 42 | 0·139 | - | - |
| No | 59 | 72 | 67 | |||
| Irinotecan | ||||||
| Yes | 29 | 71 | 48 | 0·491 | - | - |
| No | 63 | 72 | 62 | |||
| Use of bevacizumab | ||||||
| Yes | 37 | 66 | 41 | 0·107 | - | - |
| No | 55 | 76 | 68 | |||
| Use of anti-EGFR agent | ||||||
| Yes | 11 | 73 | 64 | 0·395 | - | - |
| No | 81 | 72 | 56 | |||
| Time between CLM discovery and ablation. Days§ | ||||||
| >120 | 52 | 65 | 34 | 0·097 | 1·28 (0·64–2·63) | 0·488 |
| ≤120 | 40 | 80 | 62 | |||
| Ablation type | ||||||
| RFA | 50 | 76 | 59 | 0·520 | - | - |
| Microwave | 42 | 67 | 57 | |||
| Minimal ablation margin, mm§ | ||||||
| <5 | 36 | 57 | 38 | 0·005 | 2·48 (1·31–4·72) | 0·006 |
| ≥5 | 56 | 81 | 69 | |||
| Lesion location | ||||||
| Subcapsular | 51 | 61 | 50 | 0·117 | - | - |
| Non-subcapsular | 41 | 85 | 67 | |||
| CEA level at ablation, ng/mL | ||||||
| ≥5 | 36 | 64 | 50 | 0·356 | - | - |
| <5 | 56 | 77 | 63 | |||
| Maximum CLM diameter at ablation, cm§ | ||||||
| ≥2 | 28 | 55 | 40 | 0·020 | 1·88 (0·95–3·65) | 0·069 |
| <2 | 64 | 79 | 64 | |||
| Number of liver metastases | ||||||
| Solitary | 70 | 72 | 66 | 0·210 | - | - |
| Multiple | 22 | 72 | 35 | |||
| RAS status§ | ||||||
| Mutant | 36 | 54 | 35 | 0·001 | 3·01 (1·60–5·77) | 0·001 |
| Wild-type | 56 | 83 | 71 | |||
| Post-ablation chemotherapy | ||||||
| Yes | 46 | 71 | 59 | 0·835 | - | - |
| No | 46 | 73 | 56 | |||
Kaplan-Meier analysis.
Log-rank test.
Cox regression model.
Variables entered into the Cox regression model.
EGFR, epidermal growth factor receptor; CLM, colorectal liver metastases; RFA, radiofrequency ablation; CEA, carcinoembryonic antigen.
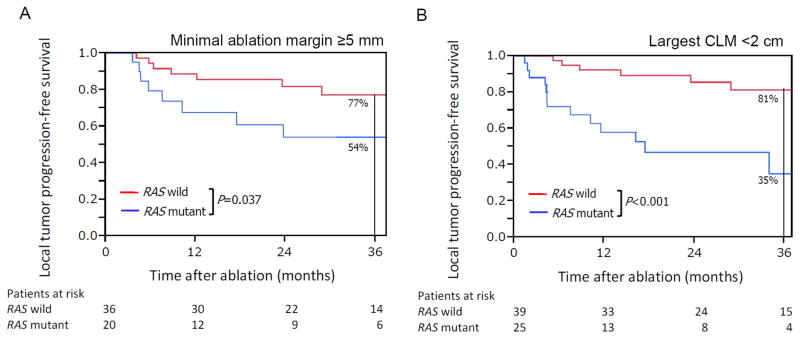
In the subgroup of patients treated with minimal ablation margin ≥5 mm (n=56), actuarial 3-year local tumour progression-free survival with mutant RAS was 54%, compared to 77% with RAS wild-type (p=0.037, Figure 3A). In the subgroup of patients undergoing ablation of CLM <2 cm (n=64), 3-year local tumour progression-free survival was significantly worse in patients with mutant RAS (35%, mutant vs. 81%, wild-type, p<0.001, Figure 3B).
Figure 3.
Kaplan-Meier plots of (A) local tumor progression–free survival in patients treated with minimal ablation margin ≥5 mm and (B) local tumor progression–free survival in patients undergoing ablation of largest CLM <2 cm.
DISCUSSION
This study shows that local tumour progression-free survival following percutaneous ablation of CLMs was worse in patients with mutant RAS than in patients with wild-type RAS. In patients with mutant RAS, the actuarial local tumour progression rate at 2 years was 58%, which was significantly higher than that in patients with wild-type RAS (24%).
RAS mutation status is increasingly being recognized as a biologic predictor of patterns of response and recurrence after chemotherapy and liver resection of CLMs, but to our knowledge, this is the first study to report worse outcomes for patients with RAS mutation after liver ablation.15, 18, 24 In the present study, there were no significant differences between patients with wild-type RAS and patients with mutant RAS with respect to ablation modality, pre-ablation chemotherapy regimen, and extent of primary colorectal cancer and CLMs. Importantly, all ablations were performed without consideration of RAS status. In agreement with the present study, a recent study showed a higher rate of positive margins following resection of CLMs among patients with mutant RAS when compared to patients with wild-type RAS, suggesting different pathologic and phenotypic features in patients with mutant and wild-type RAS.20 Although analysis of KRAS codons 12 and 13 was performed in all patients, analysis of KRAS codons 61 and 146 and NRAS codons 12, 13, and 61 was performed during the latter years of the study period. However, mutations outside KRAS codons 12 and 13 comprise less than 10% of RAS mutations, and their inclusion would likely increase the observed differences in survival between groups20, 25.
This study identified minimum ablation margin < 5 mm as the one local factor independently associated with LTP-free survival after CLM ablation. Size of ablated CLM ≥2 cm was associated with worse local tumour progression-free survival on univariablee but not multivariable analysis. In addition to those local factors, this study showed that RAS mutation status, a biological factor, was an independent predictor of local tumour progression-free survival after CLM ablation. Previous reports have shown the prognostic values of lesion size and ablation margin, but to the best of our knowledge, this is the first study to report an association between RAS mutation status and local tumour progression-free survival after ablation of CLMs.2, 9, 10, 26 Notably, the present multivariable analysis suggests that factors such as nodal status of primary colorectal cancer, pre-ablation carcinoembryonic antigen level, and metachronous/synchronous CLMs, which were traditionally reported to be associated with oncologic outcome, are less significant predictors of local tumour progression-free survival after CLM ablation.27
This study has several limitations. First, the retrospective selection of patients in whom RAS status had been determined might have created a selection bias. However, percutaneous ablation and imaging assessment for LTP were performed without consideration of RAS status. Most patients (60%) had a history of surgical resection, which creates the potential for selection bias. However, rates of post-resection ablation were in keeping with those available in the literature.2 Most patients (64%) underwent pre-ablation chemotherapy with various regimens. Despite the heterogeneity of chemotherapy, there were no differences between the patients with wild-type RAS and those with mutant RAS type in terms of presence/absence of chemotherapy and type of regimen. Finally, the minimum follow-up was 6 months, and a few patients with local recurrences beyond 6 months may not be captured by our results. However, the median length of follow-up was over 2 years for both RAS mutated and wild-type groups.
This study showed that RAS mutations are associated with worse LTP-free survival after percutaneous ablation of CLMs. Although larger ablation margins might be associated with lower local tumour progression rates following percutaneous ablation of CLMs in patients with mutant RAS, no specific recommendations can be made regarding the optimal ablation margin for such CLMs and further investigation of this matter is warranted.
Acknowledgments
The authors thank Stephanie Deming, an employee of the Department of Scientific Publications, MD Anderson Cancer Center, for copyediting the manuscript, and Ruth J. Haynes, an employee of the Department of Surgical Oncology, MD Anderson Cancer Center, for secretarial assistance in the preparation of the manuscript. This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
References
- 1.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 2.Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes-A 10-year Experience at a Single Center. Radiology. 2016;278:601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 4.Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30:567–574. doi: 10.1007/s11604-012-0089-0. [DOI] [PubMed] [Google Scholar]
- 5.Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007;33:67–71. doi: 10.1016/j.ejso.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Liang P, Dong B, Yu X, et al. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol. 2003;181:1319–1325. doi: 10.2214/ajr.181.5.1811319. [DOI] [PubMed] [Google Scholar]
- 7.Yan DB, Clingan P, Morris DL. Hepatic cryotherapy and regional chemotherapy with or without resection for liver metastases from colorectal carcinoma: how many are too many? Cancer. 2003;98:320–330. doi: 10.1002/cncr.11498. [DOI] [PubMed] [Google Scholar]
- 8.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins Can be Used to Predict Oncologic Outcome. Radiology. 2016:151005. doi: 10.1148/radiol.2016151005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadot E, Groot Koerkamp B, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–485. doi: 10.1097/SLA.0000000000001427. discussion 483–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 15.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–626. doi: 10.1097/SLA.0b013e3182a5025a. discussion 626–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taieb J, Zaanan A, Le Malicot K, et al. Prognostic Effect of BRAF and KRAS Mutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab: A Post Hoc Analysis of the PETACC-8 Trial. JAMA Oncol. 2016:1–11. doi: 10.1001/jamaoncol.2015.5225. [DOI] [PubMed] [Google Scholar]
- 17.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175–1183. doi: 10.1002/bjs.9870. [DOI] [PubMed] [Google Scholar]
- 18.Mise Y, Zimmitti G, Shindoh J, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22:834–842. doi: 10.1245/s10434-014-4042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schramm K, Krause K, Bittroff-Leben A, Goldin-Lang P, Thiel E, Kreuser ED. Activated K-ras is involved in regulation of integrin expression in human colon carcinoma cells. Int J Cancer. 2000;87:155–164. doi: 10.1002/1097-0215(20000715)87:2<155::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Brudvik KW, Mise Y, Chung MH, et al. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol. 2015;25:3438–3454. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg SN, Charboneau JW, Dodd GD, 3rd, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passot G, Chun YS, Kopetz SE, et al. Predictors of Safety and Efficacy of 2-Stage Hepatectomy for Bilateral Colorectal Liver Metastases. J Am Coll Surg. 2016;223:99–108. doi: 10.1016/j.jamcollsurg.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung U, Kuk D, D’Angelica MI, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102:85–91. doi: 10.1002/bjs.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cucchetti A, Ferrero A, Cescon M, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2015;22:1908–1914. doi: 10.1245/s10434-014-4234-0. [DOI] [PubMed] [Google Scholar]