Supplemental Digital Content is available in the text.
Key Words: socioeconomic factors, breast cancer, mortality, medicare
Abstract
Background:
Breast cancer patients exhibit survival disparities based on socioeconomic status (SES). Disparities may be attributable to access to expensive oral endocrine agents.
Objectives:
Define recent socioeconomic disparities in breast cancer survival and determine whether these improved after implementation of the Medicare Part D program.
Design:
Difference-in-difference natural experiment of women diagnosed and treated before or after implementation of Medicare Part D.
Subjects:
Female Medicare beneficiaries with early-stage breast cancer: 54,772 diagnosed in 2001 and 46,371 in 2007.
Measures:
SES was based on Medicaid enrollment and zip code per capita income, all-cause mortality from Medicare, and cause of death from National Death Index.
Results:
Among women diagnosed pre-Part D, 40.5% of poor beneficiaries had died within 5 years compared with 20.3% of high-income women (P<0.0001). Post-Part D, 33.6% of poor women and 18.4% of high-income women died by 5 years. After adjustment for potential confounders, improvement in all-cause mortality post-Part D was greater for poorer women compared with more affluent women (P=0.002). However, absolute improvement in breast cancer-specific mortality was 1.8%, 1.2%, and 0.8% (P=0.88 for difference in improvement by SES), respectively for poor, near-poor, and high-income women, whereas analogous improvement in mortality from other causes was 5.1%, 3.8%, and 0.9% (P=0.067 for difference in improvement by SES).
Conclusions:
Large survival disparities by SES exist among breast cancer patients. The Part D program successfully ameliorated SES disparities in all-cause mortality. However, improvement was concentrated in causes of death other than breast cancer, suggesting remaining gaps in care.
Traditionally breast cancer has been thought of as an illness subject to reverse socioeconomic disparities in outcome; that is, population incidence and mortality rates were both lower for women of lower socioeconomic status (SES). During the mid-1980s to early 1990s, mortality rates equalized across groups defined by census-based socioeconomic characteristics, and by the late 1990s mortality rates were higher among those residing in the poorer areas despite the fact that incidence rates remained lower in those same areas.1 Worse survival has also been demonstrated directly for those of lower SES, including those with Medicaid insurance and those with lesser educational attainment.2,3 Although breast cancer-specific mortality declined between 1987 and 2004, the decline was greater for women living in areas of more advantaged SES, compared with those living in less advantaged areas.1
One factor that has been proposed as a cause of socioeconomic disparities in breast cancer outcomes is the advent of oral adjuvant antiendocrine therapies, which occurred during the 1990s.1,4 These therapies are highly effective. For example, the administration of tamoxifen for 5 years was found in a meta-analysis of 55 trials to lead to a relative reduction in disease recurrence of 47% at 10 years after initiation of therapy.5 Published trials of aromatase inhibitors show that these agents lead to about 30%–50% lower breast cancer recurrence rates compared with the use of tamoxifen alone.6
Despite their effectiveness, breast cancer endocrine agents have historically been expensive, particularly the aromatase inhibitors, for which the Institute of Medicine reported an annual retail cost of about $2900 in 2004, before the Medicare Part D program.7 Clear socioeconomic disparities in the initiation of and adherence to these therapies have been documented.4,8,9 Until the advent of the Medicare Prescription Drug, Improvement and Modernization Act (Part D program) in 2006, these oral drugs were not covered by Medicare nor by most Medicare Supplemental insurance programs. A major goal of Medicare Part D was to reduce medication-related disparities in health outcomes among older Americans by enhancing access to oral medications.
The purpose of this study was to define the magnitude of socioeconomic disparities in breast cancer survival among more recent cohorts and to examine the extent to which these disparities were ameliorated after the implementation of the Part D Program. We have previously demonstrated that almost all Medicare Part D plans provided coverage for oral adjuvant endocrine agents.10 We expected that any improvement in survival post-Part D would be most prominent among the poor and near-poor groups, which presumably were the most likely to benefit from the increased access to costly oral medications. In contrast, Part D was hypothesized to have no substantial effect in improving the survival of wealthier women, who presumably were able to afford medications even in the absence of the program.
METHODS
Study Populations and Data Sources
Using a validated algorithm that employs Medicare administrative data to preferentially capture early-stage incident breast cancer,11 we identified cohorts of Medicare breast cancer patients with initial diagnosis and 5 years of follow-up in the pre-Part D era (2001, N=54,772) and with initial diagnosis in the post-Part D era (2007, N=46,371). Membership in both cohorts was restricted to females aged 66–90 years with available information on baseline comorbidity and SES. Data on overall and disease-specific survival were obtained from Medicare Vital Status and National Death Index sources, respectively. Zip code designations were used to determine neighborhood SES from the 2000 US Census. The study was approved by the Medical College of Wisconsin Institutional Review Board.
SES and Key Covariates
Individual-level indicators of enrollment in Medicaid or in a state buy-in program (Qualified Medicare Beneficiary, Specified Low-Income Medicare Beneficiary) and ecological data at the zip code level were used to classify patients according to SES as poor, near-poor, medium income, or high income. Specifically, women were classified as poor if they were enrolled in Medicaid or in a state buy-in program at the time of diagnosis. Those not enrolled in such a program were subsequently classified hierarchically as near-poor if they resided in a zip code ranking in the lowest quartile of per capita income within their state of residence or high income if they resided in a zip code ranking at the highest quartile of per capita income. The residual group of women living in neighborhoods characterized by per capita income ranking in the middle quartiles was classified as having medium income. Information about age at time of the breast cancer diagnosis and race/ethnicity (coded as African American/black, Hispanic, and other) was ascertained from Medicare enrollment files. Comorbidities were captured from Medicare claims during the 12-month period preceding the breast cancer diagnosis.12
Outcome Measures
Our primary study outcomes were all-cause mortality and breast cancer-specific mortality. For both pre-Part D and post-Part D cohorts, information on date of death from any cause was ascertained from Medicare Vital Status files. Medicare data do not include cause of death. Because of the large sample size, breast cancer-specific mortality was ascertained for a stratified random sample of women classified as poor, near-poor, or high income and was based on cause of death information obtained from the National Death Index. Cause of death was available for 96.7% of this sample.
Statistical Analyses
Cumulative incidence functions, by SES, were calculated to document pre-Part D and post-Part D survival for all-cause and breast cancer-specific mortality outcomes. Difference-in-differences logistic regressions were then applied to the pooled cohort of women diagnosed with breast cancer pre-Part D and post-Part D to estimate the impact of the program on mortality and SES disparities. By comparing the pre-post difference in mortality outcomes by SES, this strategy enabled us to identify the effect attributable to Part D after accounting for any likely secular trend over the 6-year study timeframe.
We included an indicator variable capturing exposure to the Part D program based on the year of breast cancer diagnosis (2001 vs. 2007), SES groups (poor, near-poor, medium income, high income), and an interaction term between these 2 variables (representing the differential effect of Part D on SES groups). For the stratified random sample of women with cause of death information, a competing risk multinomial logistic regression specification was used to consider breast cancer death and death from any other cause as separate outcomes, using surviving patients as a reference group.
In addition to the main factors of interest (Part D and SES), all regressions included covariates capturing the woman’s age, race/ethnicity, and comorbid illness. On the basis of coefficient estimates from these models, we calculated adjusted (predicted) probabilities that women would die from breast cancer, die from other causes, or survive over the 5-year period after incident breast cancer diagnosis, by SES and Part D exposure.
Survival analysis methods were also employed to analyze the relationship between SES, Part D status, and the outcome measures. The conclusions were similar to those reported here for the logistic regression analyses. We chose to present the logistic regression results due to ease of interpretation.
RESULTS
Table 1 describes the characteristics of women at time of incident breast cancer diagnosis for the full and cause-of-death samples, by Part D exposure. Our full sample included 101,143 subjects: 54,772 women treated pre-Part D and 46,371 women treated post-Part D. The median age at diagnosis was 75 years for each cohort. In each cohort, about 90% of women were of white race, 7% were black, and the remainder had Hispanic ethnicity or were members of other racial groups. Among the pre-Part D cohort, about 60% of women had no significant comorbid illness, 25% had 1 comorbidity, and 15% had 2 or more comorbid conditions. Among the post-Part D cohort, a slightly smaller percentage had no comorbid illness. In each cohort, about 11% were categorized as poor, 9% as near-poor, 41% as medium income, and 39% as high income.
TABLE 1.
Patient Characteristics at Time of Breast Cancer Diagnosis
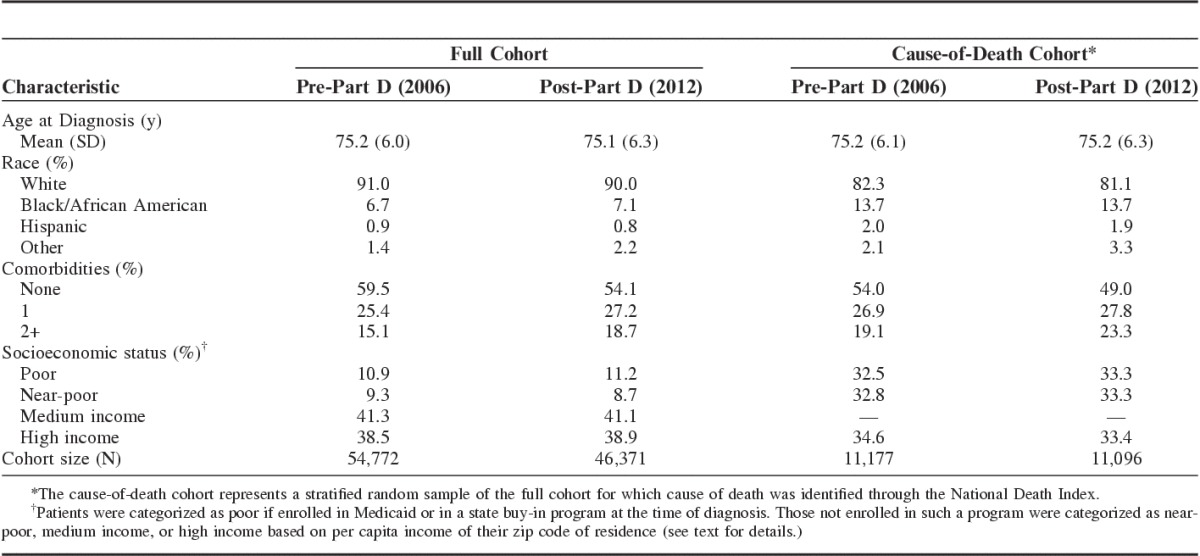
The cause-of-death group, a stratified random sample of the full sample, included 11,177 women in the pre-Part D era and 11,096 in the post-Part D era. To ensure adequate representation of poor and near-poor women, the stratified sample included only poor, near-poor, and high-income women. Compared with the full cohort, the cause-of-death cohort included a greater representation of poor, near-poor, and minority subjects.
Five-Year Mortality Trends
Cumulative incidence curves illustrate the relative patterns of all-cause and breast cancer-specific mortality among the patients of different SES (Figs. 1, 2). There were marked differences in all-cause mortality by SES and also between the pre-Part D and post-Part D cohorts. Among poor women not exposed to the Part D program, roughly 40% had died within 5 years after their breast cancer diagnosis compared with about 20% of the most affluent women. Post-Part D gains in survival for the full sample are evident at 1 year after breast cancer diagnosis, although become more prominent later in time. Improvements in survival in the post-Part D era appear to be the most marked for the poor and near-poor groups, and relatively smaller for the medium and high-income groups. The curves for breast cancer-specific mortality generally demonstrate similar trends as the overall mortality curves.
FIGURE 1.
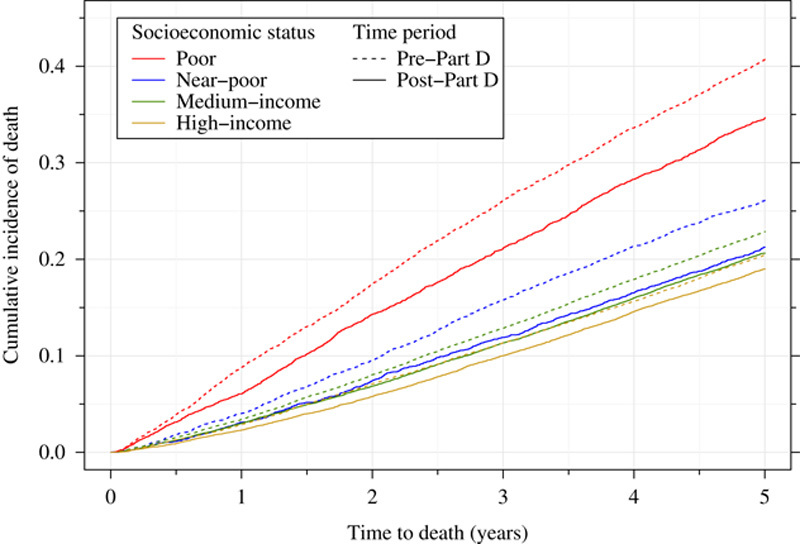
All-cause mortality among women with incident early-stage breast cancer, by Part D exposure and socioeconomic status.
FIGURE 2.
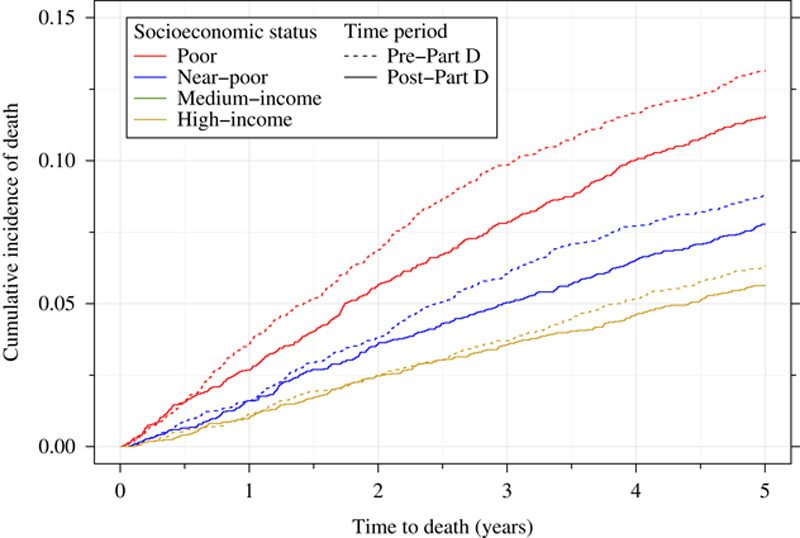
Breast cancer-specific mortality among women with incident early-stage breast cancer, by Part D exposure and socioeconomic status.
Table 2 shows unadjusted mortality rates for the full and cause-of-death samples by Part D exposure. All-cause 5-year mortality among women with incident early-stage breast cancer decreased from 24% to 20.9% (P<0.0001) for cohorts diagnosed and treated before and after Part D implementation, respectively. Although there were large decreases in all-cause mortality among poor (from 40.5% to 33.6%) and near-poor (from 25.9% to 20.5%) women, post-Part D improvement in survival was substantially smaller among more affluent women (22.7% to 19.9% for middle income subjects and 20.3% to 18.4% for high-income women).
TABLE 2.
Unadjusted 5-Year Mortality, by Part D Exposure and Socioeconomic Status*
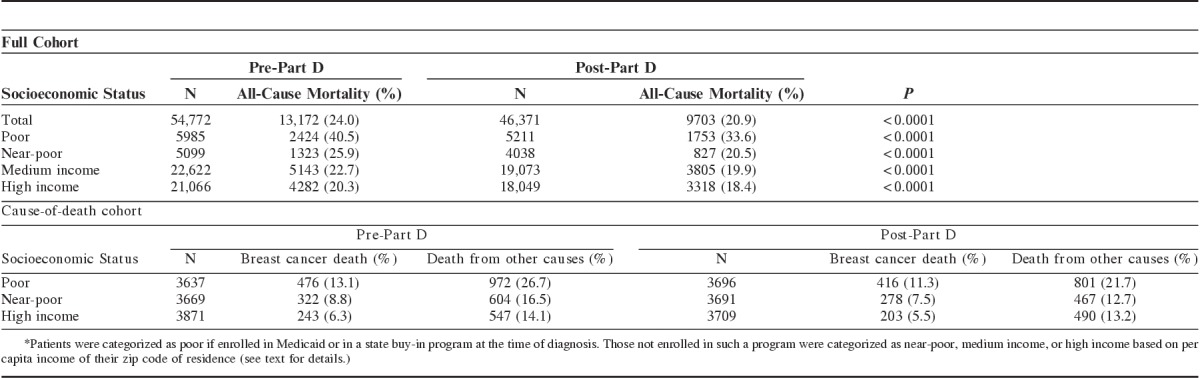
Breast cancer-specific mortality also decreased in the post-Part D era. However, the declines were smaller in magnitude and generally more uniform across socioeconomic groups. Among the pre-Part D sample, 9.3% had died of breast cancer within 5 years of initial diagnosis, ranging from 13.1% of the poor women to 6.3% of the high-income women. Among the post-Part D sample, 8.1% had died of breast cancer by 5 years after diagnosis, ranging from 11.3% of the poor women to 5.5% of the high-income women.
Multivariable Results
All-Cause Mortality
The first panel of Table 3 depicts the odds ratios and adjusted change in 5-year mortality that occurred between the pre-D and post-D cohorts. After adjusting for secular trends and beneficiaries’ age, race/ethnicity, and level of comorbidity, there was a significant decrease in all-cause mortality among all SES groups. Odds ratios ranged from 0.71 (95% confidence interval, 0.66–0.77) for the poor group to 0.82 (95% confidence interval, 0.78–0.87) for the high-income group. The alternative analysis employing survival analysis methods demonstrated similar results to the logistic regression (eTable 1, Supplemental Digital Content 1, http://links.lww.com/MLR/B327).
TABLE 3.
ORs and Average-predicted Mortality Differences by SES* and Part D Exposure
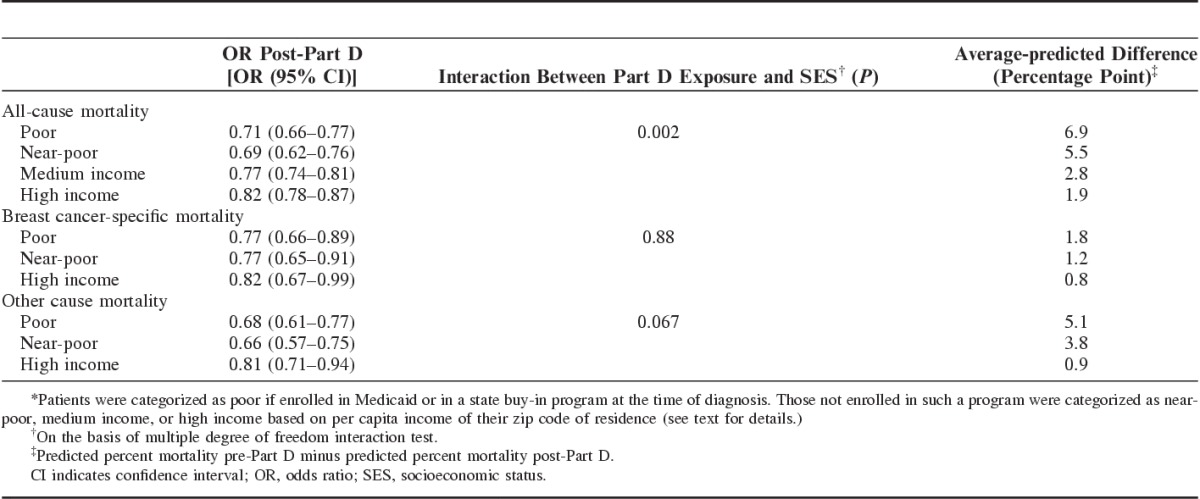
Consistent with our hypothesis, there were significant reductions in SES disparities in overall mortality among elderly breast cancer patients after implementation of the Part D program. The change in all-cause mortality, as hypothesized, was not uniform across socioeconomic groups (P=0.002, Table 3). Differences in the predicted probability of death from any cause across the pre-Part D and post-Part D cohorts ranged from 6.9 and 5.5 percentage points for poor and near-poor women to 1.9 percentage points for the high-income group. The relative post-Part D improvements in survival among poor and near-poor women relative to high income were both substantial (nearly 4-fold and 3-fold) and statistically significant (P<0.004).
Disease-specific Mortality
Although breast cancer-specific mortality was lower across all SES groups for the post-Part D cohort, there was no significant reduction in SES disparities across the 2 cohorts (Table 3). In contrast to the situation with all-cause mortality, the difference-in-difference coefficient was small and not statistically significant (P=0.88), indicating that socioeconomic disparities in breast cancer-specific mortality were not reduced post-Part D. Instead, the differential reduction in all-cause mortality across socioeconomic groups in this subset was completely explained by the reduction in socioeconomic disparities in mortality due to causes other than breast cancer (P=0.067). The observed breast cancer mortality (Table 2) declined by 1.8 percentage points (from 13.1% to 11.3%), 1.3 percentage points (from 8.8% to 7.5%) and 0.8 percentage points (from 6.3% to 5.5%) for poor, near-poor, and high-income women, respectively. Differences in average model-based predicted probabilities are similar (Table 3). For death due to other causes, the differences between pre-Part D and post-Part D ranged from 5.1 percentage points for the poor group to 0.9 percentage points for the high-income group.
DISCUSSION
Among Medicare beneficiaries diagnosed with breast cancer, substantial differences in 5-year mortality by SES were present before and after the advent of the Part D pharmaceutical insurance program. Among those diagnosed after the initiation of the Part D program, fewer women in each socioeconomic class had died at 5 years after diagnosis compared with before Part D. Nonetheless, SES disparities persisted and were substantial in magnitude. Although women in the poorer SES groups experienced a larger mortality improvement than did women in the higher SES categories, this improvement could not be attributed to better mortality due to breast cancer, and was associated with mortality from other causes. Therefore, while the Part D program was associated with differentially improved mortality for women in lower socioeconomic classes, the improvement appeared attributable to general health improvements (perhaps due, in part, to better overall access to medications) and not necessarily due to better treatment of the breast cancer per se.
It is possible that factors other than Part D could have led to the observed reductions in mortality among breast cancer patients after the advent of the Part D program. For example, differential changes in incidence of late stage disease by SES could have led to differential changes in mortality among breast cancer patients. This would most likely have been due to changes in the use of mammography by SES. However, the overall use of mammography changed little between 2000 and 2013.13 Although socioeconomic disparities in use of mammography are present among the US women,13 the socioeconomic disparities in receipt of mammography have also been remarkably stable, at least between 2003 and 2013.13–15 The incidence of stage IV disease was similar by SES for women in this age group pre-Part D and post-Part D. For example, pre-Part D, 3.2% of wealthy women and 7.0% of poor women were diagnosed at stage IV; post-Part D, the corresponding percentages were 4.2% and 7.8% (authors’ calculations based on Surveillance, Epidemiology, and End Results -Medicare Linked Data). Therefore, changes in the incidence of late stage disease are unlikely to have biased these results. A number of differences in breast cancer treatment also occurred during the study period. However, treatment advances are usually adopted earlier by individuals of higher SES compared with individuals of lower SES.16 Because of our difference-in-difference study design, women in the most affluent group essentially represented a control group reflecting temporal improvements in breast cancer treatment.
The major expected improvement of the Medicare Part D program for breast cancer treatment would have been better accessibility to adjuvant endocrine agents, particularly the more expensive aromatase inhibitor agents, for the large majority of women in this age group who have hormone receptor-positive disease, and therefore are eligible for these agents.17 However, as we have previously demonstrated, the out-of-pocket annual drug costs for aromatase inhibitors approximately doubled during the first 5 years of the Part D program, with out-of-pocket costs falling only after the introduction of generic agents in 2010–2011.10,18 One reason that out-of-pocket costs rose so dramatically was the Part D coverage gap (also known as “donut hole”), which was reached after about $2500–3100 of out-of-pocket drug costs in a calendar year.10 Once they reach the coverage gap, patients essentially bear the full cost of medications until the “catastrophic” level is reached. Because of the combination of increased monthly costs after deductibles and the coverage gap, it is possible that the women of lower SES in our post-Part D cohort did not experience a sufficient improvement in access to aromatase inhibitor agents to lead to a major improvement in their disease-specific mortality. That being said, the improvement in overall mortality could have been attributable to better access to other medications, such as those used for cardiovascular risk factors including hypertension or diabetes. This possibility would support the hypothesis that some of the observed worse survival for low SES breast cancer patients compared with high SES breast cancer patients may be attributed to treatment of illnesses other than the breast cancer itself. The fact that a substantial portion of the overall mortality improvement was attributable to causes of death other than breast cancer would support this hypothesis.
Our findings have several limitations that lead to important yet unanswered questions. First, although we used subjects’ zip code-linked census-level data to measure SES, we were unable to identify subjects’ individual-level poverty status except for those enrolled in Medicaid or a state buy-in program, and thus could not provide a more granular measure of SES by which to stratify the sample for our analyses. As patients enrolled in a Medicaid program before Part D typically had coverage for aromatase inhibitors (as well as many other drugs), one might have expected a greater improvement in outcomes post-Part D among the near-poor group than among the poor group. Yet this was not observed, with the greatest improvement occurring among the poorest group, and neither poor nor near-poor groups demonstrated significant improvement in breast cancer-specific mortality post-Part D. Medicare Advantage patients could not be included due to insufficient claims data to accurately identify incident cancer. The follow-up period for the post-Part D sample ended in 2012, shortly after the introduction of generic aromatase inhibitors. Future studies of even more recent cohorts may determine whether Part D has had a differential impact on reducing socioeconomic disparities in survival among breast cancer patients after introduction of generic aromatase inhibitors, which reduced out-of-pocket cost to patients by a much greater extent than the Part D program itself.10,18
This study was structured according to an “intention-to-treat” analytic plan. Although information was available for the post-Part D cohort at an individual level regarding Part D coverage, we did not employ this data element in our analysis. It has been previously demonstrated that actual enrollment in Part D was subject to selection bias19–21; credibly controlling for this bias through administrative data would have been challenging, if not impossible. Our approach, based on an intent-to-treat, difference-in-difference estimation, takes advantage of the natural experiment afforded by the advent of Part D. By providing estimates of the program’s effect on SES disparities that are not conditional on participation, these results are more generalizable to the population targeted by the program.
A key stated goal of the Medicare Part D program was to enhance access to oral medications, thereby reducing medication cost-related disparities in health outcomes. Our findings suggest that the program failed to fulfill its promise for breast cancer patients, likely due to a combination of marked increases in drug costs after deductibles and to the Medicare Part D coverage gap (donut hole). Nonetheless, the likelihood of dying of breast cancer was notably lower among all socioeconomic groups during the post-Part D era compared with the pre-Part D era. Of course, Part D did not remove all financial barriers to high-quality care. It is plausible that we were setting the bar too high to require a greater improvement in mortality among the poorer groups. Given the substantial disparities in survival existing before the advent of the Part D program, observing a similar mortality reduction among all socioeconomic groups may represent worthwhile progress.
The Medicare Part D Prescription Drug Benefit program has led to significant restructuring of pharmaceutical financing and improved access for many beneficiaries.22–24 Nonetheless, our results indicate that, although the Part D program was successful in ameliorating SES disparities in all-cause mortality and survival among breast cancer patients, gains were concentrated in causes of death other than breast cancer. This suggests remaining gaps in access to effective screening, initial therapy, and/or follow-up breast cancer care.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.lww-medicalcare.com.
Footnotes
Present address: Jianing Li, PhD, Merck & Co. Pharmaceutical, North Wales, PA.
Supported by the National Cancer Institute (R01CA170945) and the American Cancer Society (RSG-13-070-01-CPHPS).
The authors declare no conflict of interest.
REFERENCES
- 1.Harper S, Lynch J, Meersman SC, et al. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121–131. [DOI] [PubMed] [Google Scholar]
- 2.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. [DOI] [PubMed] [Google Scholar]
- 3.Sprague BL, Trentham-Dietz A, Gangnon RE, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Translation. Washington, DC: The National Academies Press; 2006. doi:10.17226/11468. [Google Scholar]
- 8.Pezzin LE, O’Niel MB, Nattinger AB. The economic consequences of breast cancer adjuvant hormonal treatments. J Gen Intern Med. 2009;24:446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen TW, Czypinski LK, Sparapani RA, et al. Socioeconomic factors associated with adjuvant hormone therapy use in older breast cancer survivors. Cancer. 2011;117:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nattinger AB, Pezzin LE, McGinley EL, et al. Patient costs of breast cancer endocrine therapy agents under Medicare Part D vs with generic formulations [published online February 3, 2015]. Springerplus. doi:10.1186/s40064-015-0827-8. [DOI] [PMC free article] [PubMed]
- 11.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. Breast Cancer: Mammography Statistics 2015. http://m.cancer.org/research/infographicgallery/mammography-statistics. Accessed November 28, 2015.
- 14.American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2006. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 15.American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 16.Schmidt C. What drives diffusion of new cancer therapies? J Natl Cancer Inst. 2015;107:2–3. [DOI] [PubMed] [Google Scholar]
- 17.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare Part D. J Natl Cancer Inst. 2015;107:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abaluck J, Gruber J. Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Am Econ Rev. 2011;101:1180–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duggan M, Healy P, Morton FS. Providing prescription drug coverage to the elderly: America’s experiment with Medicare Part D. J Econ Perspect. 2008;22:69–92. [DOI] [PubMed] [Google Scholar]
- 21.Heiss F, McFadden D, Winter J. Who failed to enroll in Medicare Part D, and why? Early results”. Health Aff. 2006;25:w344–w354. [DOI] [PubMed] [Google Scholar]
- 22.Yin W, Basu A, Zhang J, et al. The impact of the Medicare Part D prescription drug benefit on drug utilization and expenditures. Ann Intern Med. 2008;148:169–177. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Patrick AR, Pedan A, et al. The effect of Medicare Part D coverage on drug use and cost sharing among seniors without prior drug benefits. Health Aff. 2009;28:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polinski JM, Kilabuk E, Schneeweiss S, et al. Changes in drug use and out-of-pocket costs associated with Medicare Part D implementation: a systematic review. J Am Geriatr Soc. 2010;58:1764–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.lww-medicalcare.com.


