Abstract
Background
The human immunodeficiency virus (HIV) is associated with cognitive impairment, and loneliness is associated with cognitive decline in old age. Older Black adults with HIV may be at particular risk for loneliness due to stigma and lack of social resources.
Objective
We tested the hypotheses that (1) older Black adults with HIV would show greater loneliness than older White adults with HIV, and (2) greater loneliness among older Black adults with HIV would be associated with poorer cognitive function.
Methods
Three hundred and seventy participants (177 with HIV, 193 without HIV, age mean=58.8, s.d.=6.2; education mean=13.4, s.d.=2.9; 73.9% male, 68.9% Black) in a community-based cross-sectional study of the Rush Center of Excellence on Disparities in HIV and Aging (CEDHA) completed a five-item self-report scale used to measure emotional loneliness and a battery of cognitive measures.
Results
Contrary to our expectations, older Black adults indicated less overall loneliness than White adults (B=−0.3893; SE=0.1466, p=0.0087) in models controlling for the effects of age, education, sex, global cognition, and income. However, in models with cognitive function as the outcome, an interaction between race and loneliness was observed such that older Black adults who indicated greater loneliness showed poorer cognitive function relative to White adults (B=−0.2736, SE=0.1138, p=0.0174).
Conclusion
Older Black adults with HIV reported less loneliness than older White adults; however, the inverse association between loneliness and cognitive function was stronger in Black than White older adults. Additional work is needed to elucidate the mechanisms underlying this interaction.
Keywords: social isolation, race, HIV, cognition
Introduction
The human immunodeficiency syndrome (HIV) affects approximately 1.2 million persons in the U.S. [1] and an estimated 35 million worldwide [2]. The introduction of combination antiretroviral therapies (cART) in 1996 has resulted in dramatic improvements in the quality of life, CD4 cell counts, and survival rates of those with HIV [3]. However, despite these dramatic improvements in major health and well being outcomes, HIV-associated neurocognitive disorders (HAND) remain a substantial problem, and some reports suggest rates of cognitive impairment have increased among those who are not immunosuppressed [4, 5]. The success of cART therapies has resulted in an increasing number of persons with HIV living into old age. In 2013, over a quarter of all persons with HIV were over the age of 50, and the CDC estimates by 2015, more than half of all persons with HIV would be over 50 years old [1]. Adults living with HIV into old age are at greater risk for cognitive impairment [6], however the factors associated with this are not well understood.
One potentially important factor is loneliness. Feelings of loneliness generally increase with age among older adults, and this has been associated with a number of negative health and psychological outcomes [7]. We previously demonstrated that loneliness is associated with cognitive decline and the development of Alzheimer’s dementia [8]. Loneliness may be a particular problem in the context of aging with HIV given the well-documented social stigma and prejudice that HIV infection confers [9], and this seems particularly deleterious among older persons [10, 11]. The net effect of increased feelings of loneliness due to aging and long-term HIV infection may place neurocognitive abilities at heightened risk for decline or impairment in old age.
Black Americans have the most severe burden of HIV of all racial/ethnic groups in the U.S., specifically accounting for higher proportions of new HIV infections, of those living with HIV, and of those ever diagnosed with AIDS [12]. Black adults with HIV report a particularly high level of social stigma [13], which has been associated with social isolation [14], and in general, older Black adults have fewer social resources, namely smaller social networks and less social engagement [15], than older White adults. For these reasons, older Black adults might experience loneliness differentially and more drastically than older White adults. A theoretical model of racial/ethnic HIV health disparities has been proposed [16] that suggests minorities with HIV often possess multiple intersecting stigmas at the structural level (e.g., segregation) as well as the individual level (e.g., perceived discrimination). Older Black adults with HIV not only have to contend with the negative historical consequences of institutionalized racism, but also the social stigma associated with HIV infection, and these may interact to produce downstream effects upon health outcomes such as cognition. Since loneliness has been associated with poorer cognitive functioning in old age [8], this in turn may have an effect of exacerbating any negative effects of the HIV virus on cognitive functioning in old age among Black adults.
We tested the hypotheses that (1) older Black adults with HIV would experience greater loneliness than older White adults with HIV, and (2) the association of greater loneliness with lower cognitive function would be stronger in Black adults with HIV than White adults with HIV. Participants came from the Research Core of the Rush Center of Excellence on Disparities in HIV and Aging (CEDHA), a community-based cohort study of older Black and White adults aging with and at risk of HIV, based in the Chicago metropolitan area. In regression models that adjusted for the effects of age, education, income, and sex, we additionally explored whether our findings were specific to those older adults with HIV by including control participants who were HIV negative.
Methods
Participants
Participants were recruited as part of the Research Core of the Rush Center of Excellence on Disparities in HIV and Aging (CEDHA), which is a collaboration among the Rush Alzheimer’s Disease Center of Rush University Medical Center, the Ruth M. Rothstein Core Center of Stroger Hospital, and the Community Outreach Intervention Projects within the School of Public Health at the University of Illinois Chicago. This study, which began in 2012 and has on-going longitudinal data collection, enrolls participants who are 50 years of age and older, identify as either Black or White, and are HIV seropositive or seronegative. HIV seropositive participants were eligible for the study if they had CD4+ > 200 cells/mm3 on cART or CD4+ > 500 cART naïve, and viral loads ranging from undetectable up to 50,000 copies. All participants undergo assessment of risk factors and annual clinical evaluations. The clinical evaluation is comprised of a medical history, cognitive and motor function testing, and assessment of risk factors, including a questionnaire to assess loneliness. To more fully capture variation in socioeconomic status, income was measured using a show card methodology. Participants were shown a card with the following 10 possible categories and asked to choose the option that represents their household annual income: (1) USD 0–4,999, (2) USD 5,000–9,999, (3) USD 10,000–14,999, (4) USD 15,000–19,999, (5) USD 20,000–24,999, (6) USD 25,000–29,999, (7) USD 30,000–34,999, (8) USD 35,000–49,999, (9) USD 50,000–74,999, (10) USD>75,000. The CEDHA study is independent from other studies currently being administered through the Rush Alzheimer’s Disease Center and there is no overlap in study participants.
In order to further test the robustness of our loneliness findings in the CEDHA cohort, we also conducted additional loneliness analyses on a separate group of 1,180 participants without dementia or HIV, including 590 White participants of the Memory and Aging Project (MAP; [17]), and 590 Black participants of the Minority Aging Research Study (MARS; [18]). MAP and MARS are both community-based, longitudinal epidemiologic studies of aging and dementia administered through the Rush Alzheimer’s Disease Center. Both MAP and MARS focus on adults over the age of 65, but MARS is exclusively older Blacks, while MAP includes all racial and ethnic groups. CEDHA, in contrast, includes only Blacks and Whites, and has a focus on midlife adults (age 50 and older) who are either infected with HIV or at risk of HIV. Importantly, all three cohort studies have a common core of data collection allowing the studies to be compared on the key variables of interest in this paper. Demographic and other information on the samples used from these three cohorts is presented in Table 1.
Table 1.
Characteristics of CEDHA, MAP, and MARS subsample participants
| Factor | CEDHA (N=370) | MAP (N=590) | MARS (N=590) |
|---|---|---|---|
| Mean Age (SD) | 58.79 (6.19) | 74.88 (6.89) | 74.81 (6.89) |
| Mean Education (SD) | 13.38 (2.88) | 14.43 (3.53) | 14.48 (3.39) |
| Sex (M/F) | 272/96 | 125/146 | 140/450 |
| Mean MMSE (SD) | 27.60 (2.22) | 28.38 (1.94) | 27.51 (2.54) |
| Black Race | 31.08% | 0% | 100% |
| Number of Medical Conditions | 2.18 (1.58) | 1.38 (1.03) | 1.59 (0.99) |
CEDHA=Center for Excellence on Disparities in HIV and Aging; MAP=Memory and Aging Project; MARS=Minority Aging Research Study; Number of Medical Conditions refers to total number endorsed of the following: Hypertension, Diabetes, Heart disease, Cancer, Thyroid disease, Head injury with loss of consciousness, Stroke
Loneliness Assessment
We assessed loneliness at each evaluation with a modified version of the de Jong-Gierveld Loneliness Scale [19, 20]. Three modifications were made to the original scale. First, we eliminated 5 items assessing social loneliness because emotional loneliness was the intended focus of our interests. Second, we combined 2 similar items and made minor wording changes to another item to improve clarity of the scale for older adults. Third, we asked participants to rate agreement with each item on a 5-point scale (rather than dichotomously) to improve the sensitivity of the scale. The following 5 items were employed: “I experience a general sense of emptiness”, “I miss having people around”, “I feel like I don’t have enough friends”, “I often feel abandoned”, and “I miss having a really good friend.” An average of the item scores yielded a total score that ranged from 1 to 5, with higher values indicating greater loneliness [8].
Assessment of Cognition
A battery of 21 cognitive measures was administered by technicians trained by a board-certified clinical neuropsychologist. Measures of cognitive function assessed a wide and diverse range of cognitive functions and are identical in all essential details with the Minority Aging Research Study (MARS; [18]). Two of the 21 tests, the Complex Ideational Material and the Mini-Mental Status Examination (MMSE), are only used for diagnosis of cognitive impairment and description. Raw scores on the remaining 19 tests were converted to z-scores using the mean and standard deviation. A global cognition score was calculated by averaging the z-scores across these 19 measures of cognitive function as previously described [21]. These 19 measures included the Word List Memory, Word List Recall and Word List Recognition from the procedures established by the CERAD; immediate and delayed recall of Logical Memory Story A and the East Boston Story; Verbal Fluency, Boston Naming, the National Adult Reading Test, the Digit Span subtests (forward and backward) of the Wechsler Memory Scale-Revised, Digit Ordering, the oral version of the Symbol Digit Modalities Test, Number Comparison, Stroop Color Naming, Stroop Word Reading, Judgment of Line Orientation, and Standard Progressive Matrices.
Statistical Analyses
Descriptive and bivariate statistics were calculated to characterize the sample. Chi-square tests were used for categorical variables and t-tests were used for continuous variables. Linear regression models were then performed to examine the associations between race and loneliness in persons with HIV. All models included terms to control for the potentially confounding effects of age, education, and sex. Subsequent models investigating the association of race with loneliness also controlled for the potentially confounding effects of global cognition and income. Next, models investigating the interaction between race and loneliness were conducted with global cognition as the outcome in persons with HIV. Finally, models investigating the interaction between race and loneliness were conducted in participants who were HIV negative. Analyses were programmed in SAS version 9.3.
Results
Descriptive Statistics of Participants with HIV
CEDHA HIV positive participants (N=177) included 124 Black adults and 53 White adults. The mean age of the total sample was 58.71 (s.d.=5.46), the mean education in years was 13.19 (s.d.=2.83), 134 identified as male, 42 identified as female, and 1 person identified as transgendered. Descriptive statistics characterizing the sample are presented in Table 2. Black and White participants differed with respect to age, education, income, MMSE total score, and global cognition. Black participants were older, had fewer years of education, had lower income, scored slightly lower on the MMSE, and performed lower on the global measure of cognition than White participants. No race difference in CD4 count was observed.
Table 2.
Characteristics of the HIV positive CEDHA participants
| Factor | HIV positive (N=177) | Black (N=124) | White (N=53) | t or X2 | p-value |
|---|---|---|---|---|---|
| Mean Age (SD) | 58.71 (5.46) | 59.28 (5.49) | 57.37 (5.18) | −2.15 | 0.033 |
| Mean Education (SD) | 13.19 (2.83) | 12.60 (2.39) | 14.55 (3.30) | 4.40 | <0.001 |
| Sex (M/F) | 134/42 | 92/31* | 42/11 | 0.40 | 0.525 |
| Mean MMSE (SD) | 28.42 (1.31) | 28.22 (1.25) | 28.91 (1.32) | 3.29 | 0.001 |
| Mean CD4 count (SD) | 617.9 (282.7) | 609.5 (264.0) | 637.3 (324.3) | 0.60 | 0.551 |
| Mean Income (SD) | 3.60 (2.32) | 3.07 (1.86) | 4.81 (2.81) | 4.14 | <0.001 |
| Mean Loneliness (SD) | 2.51 (0.77) | 2.44 (0.77) | 2.68 (0.77) | 1.88 | 0.061 |
| Mean Global Cognition (SD) | 0.10 (0.53) | −0.05 (0.51) | 0.46 (0.40) | 7.22 | <0.001 |
Values are presented as means and standard deviations in parentheses unless otherwise noted. Between-group statistic refers to difference between Black and White participants with HIV.
One person is transgendered.
Racial Differences in Loneliness Among Participants with HIV
We hypothesized that older Black adults might endorse higher levels of loneliness than older White adults. In models adjusted for the effects of age, education, sex, income, and global cognition, we observed the opposite finding in CEDHA participants. In this model, older Black participants reported significantly lower levels of loneliness than older White participants (Table 3), though the difference was notably small (Cohen’s d=−0.312). In additional post-doc analyses (Supplementary Table 3), older Whites endorsed not having enough friends more than older Blacks, suggesting that perceptions regarding friendships might play a role in this race difference.
Table 3.
Predictors of loneliness among participants with HIV (N=177)
| Variable | Model 1 | Model 2 |
|---|---|---|
| Adjusted R2 | 0.0619 | 0.0544 |
| B (SE, p-value) | ||
| Age | −0.0124 (0.0107, 0.2479) | −0.0126 (0.0111, 0.2574) |
| Male Sex | −0.0843 (0.1354, 0.5343) | −0.0690 (0.1391, 0.6207) |
| Education | −0.0560 (0.0230, 0.0160) | −0.0554 (0.0236, 0.0201) |
| Black Race | −0.3894 (0.1403, 0.0061) | −0.3893 (0.1466, 0.0087) |
| Global Cognition | −0.1088 (0.1260, 0.3889) | −0.1106 (0.1280, 0.3887) |
| Income | −0.0007 (0.0274, 0.9801) | |
Loneliness and Cognitive Function Among Participants with HIV
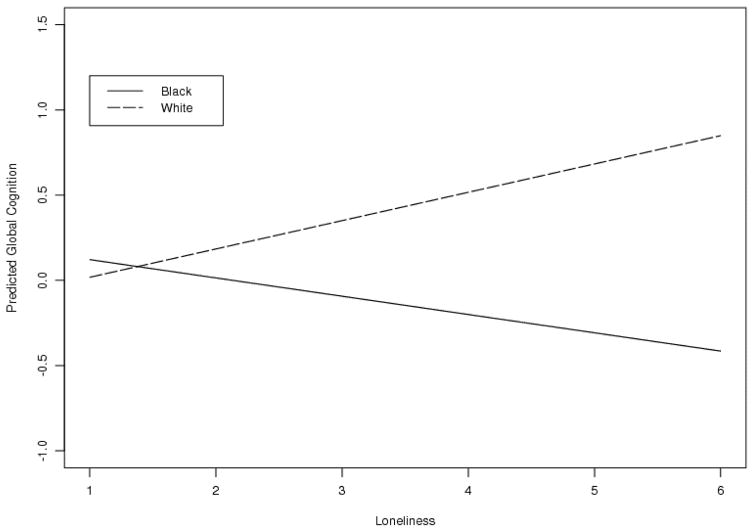
In order to investigate the association of loneliness with global cognition, we conducted linear regression models adjusting for the effects of age, education, sex, income, and race in CEDHA participants. There was no significant association between loneliness and global cognition. However, results revealed an interaction between race and loneliness such that in older Black adults, greater loneliness was associated with lower global cognition (Table 4). This interaction is visualized in Figure 1. Notably, we calculated Pearson and Spearman correlation coefficients for loneliness by global cognition stratified by race (Black, White) among HIV positive persons. For Blacks, loneliness was significantly correlated with global cognition (Pearson r=−0.24128, p=0.0069; Spearman rho=−0.20833, p=0.0202); however, for Whites, loneliness was not significantly correlated with global cognition (Pearson r=0.19706, p=0.1573; Spearman rho=0.03300, p=0.8146).
Table 4.
Associations of loneliness with global cognition, 177 participants with HIV
| Factor | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Adjusted R2 | 0.2791 | 0.2780 | 0.3018 |
| B (SE, p-value) | |||
| Age | −0.0110 (0.0066, 0.1013) | −0.0114 (0.0067, 0.0890) | −0.0152 (0.0078, 0.0523) |
| Male Sex | −0.0234 (0.0841, 0.7809) | −0.0261 (0.0842, 0.7568) | −0.1280 (0.1134, 0.2610) |
| Education | 0.0597 (0.0135, <0.0001) | 0.0571 (0.0138, 0.0001) | 0.0564 (0.0168, 0.0010) |
| Income | 0.0222 (0.0165, 0.1795) | 0.0221 (0.0165, 0.1823) | 0.0189 (0.0206, 0.3618) |
| Black Race | −0.3359 (0.0847, 0.0001) | −0.3501 (0.0864, 0.0001) | −0.1697 (0.1094, 0.1227) |
| Loneliness | −0.0405 (0.0468, 0.3887) | 0.0369 (0.1761, 0.8344) | |
| Loneliness by age | −0.0010 (0.0086, 0.9063) | ||
| Loneliness by sex | 0.1278 (0.1297, 0.3259) | ||
| Loneliness by education | 0.0073 (0.0198, 0.7123) | ||
| Loneliness by income | 0.0005 (0.0222, 0.9811) | ||
| Loneliness by race | −0.2736 (0.1138, 0.0174) | ||
Figure 1.
Global Cognition Versus Loneliness by Race, 177 Participants with HIV
Race, Loneliness, and Cognitive Function in those without HIV
Although we demonstrated that older Black adults with HIV who indicated greater loneliness also had lower cognitive function, we wanted to investigate whether this pattern was due to race or to HIV infection by considering Black adults without HIV in the CEDHA cohort. We hypothesized that HIV status in particular would be associated with negative health consequences given its well-documented associations with neurological impairment, poorer psychological wellbeing, and mortality (e.g., [4–6]), among others. We therefore investigated whether older Black adults showed greater loneliness than older White adults in subsequent analyses of HIV negative participants who were recruited as part of the same study as a control group. Differences in sample characteristics between HIV positive and HIV negative participants are described in Table 5. By design, HIV positive and HIV negative groups did not differ with respect to age, education, sex, or race. They also did not differ on scores of loneliness or income in bivariate comparisons. Interestingly, differences in MMSE and global cognition were observed such that HIV positive persons exhibited higher scores than HIV negative persons; the differences were about 1.5 points on the MMSE and 0.20 z-score on the measure of global cognition. These differences, although significant, are notably small and may reflect the fact that the HIV positive participants were more likely to be linked to medical care than the HIV negative participants. This greater access to medical care may have addressed comorbidities that could have an effect upon cognition.
Table 5.
Characteristics by HIV Status of CEDHA participants
| Factor | HIV positive (N=177) | HIV negative (N=193) | t or X2 | p-value |
|---|---|---|---|---|
| Race (B/W) | 124/53 | 131/62 | 0.21 | 0.651 |
| Mean Age (SD) | 58.71 (5.46) | 58.86 (6.80) | 0.24 | 0.807 |
| Mean Education (SD) | 13.19 (2.83) | 13.56 (2.91) | 1.26 | 0.210 |
| Sex (M/F) | 134/42 | 138/54 | 0.86 | 0.352 |
| Mean MMSE (SD) | 28.42 (1.31) | 26.84 (2.59) | −7.51 | <0.001 |
| Mean Income (SD) | 3.60 (2.32) | 3.51 (2.55) | −0.33 | 0.74 |
| Mean Loneliness (SD) | 2.51 (0.77) | 2.38 (0.73) | −1.74 | 0.083 |
| Mean Global Cognition (SD) | 0.10 (0.53) | −0.10 (0.56) | −3.44 | <0.001 |
Values are presented as means and standard deviations in parentheses unless otherwise noted.
In a regression model that adjusted for the effects of age, education, sex, income, and global cognitive function, older Black CEDHA participants who were HIV negative also indicated less loneliness than older White adults (B=−0.4652, SE=0.1265, p<0.001; Supplementary Table 6). However, in regression models investigating the association between loneliness, race, and cognitive function, the interaction was not significant in HIV negative persons (Supplementary Table 7). The three-way interaction between race, loneliness, and HIV status in the combined group of HIV negative and positive participants was significant (B=−0.3381, SE=0.1495, p=0.0244). This suggests that higher loneliness among older Black adults with HIV is associated with lower cognitive function.
We found that older Black adults reported less loneliness than older White adults in the CEDHA cohort, regardless of HIV status, and this was contrary to our initial hypothesis. In order to test the generalizability of this finding, we conducted additional analyses on a separate group of 1,180 older participants without dementia and without HIV, including 590 White participants of the Memory and Aging Project (MAP; [17]), and 590 Black participants of the Minority Aging Research Study (MARS; [18]). Participants for this subsequent analysis were different than those in previous CEDHA analyses, and were demographically matched according to age, education, and sex using propensity score matching methods. In regression models controlling for the effects of age, education, and sex, older Black adults reported less loneliness than older White adults in this separate larger group. In additional models further adjusting for global cognition and income, this finding remained significant.
Discussion
Contrary to our first hypothesis, we observed that older Black adults with HIV reported less overall loneliness than older White adults with HIV in the CEDHA cohort. However, consistent with our second hypothesis, the association between greater loneliness and lower cognitive function was stronger in Black adults with HIV than White adults with HIV. As a comparison reference, we tested the generalizability of this finding in two separate populations – in the control CEDHA subjects (similar in demographics but without HIV) and in a group of adults 15–20 years older without dementia or HIV. We observed that older Black adults without HIV indicated less overall loneliness than older White adults without HIV in the CEDHA cohort; but there was no race difference in the association of loneliness and cognitive function among those without HIV. However, in a three-way model of the combined cohort of positives and negatives, we observed a race difference in the association of loneliness and cognition by HIV status. We also found in the cohort of older adults without dementia or HIV that older Blacks reported less loneliness than older Whites. Altogether these results suggest that older Blacks tend to report less loneliness than older Whites, but when they are lonely, it has a particularly negative association with cognitive function, at least among older Black adults with HIV.
Over a quarter of persons with HIV are over the age of 50, and since the advent of cART, a rapidly increasing number of adults with HIV are living into old age [1]. Black adults account for a higher proportion of new and current HIV infections than other racial groups [12]. Our results support a difference by race in overall loneliness experienced among older adults living with HIV; however, the exact mechanisms for this are unclear. Research has suggested that HIV status among older black adults is associated with significant stigma and prejudice, and this may consequently result in depression and social isolation [22]. For these reasons, we hypothesized that older Black adults might report greater overall rates of loneliness than older White adults; however, we found the opposite to be true.
Previous reports investigating persons of different cultures using this loneliness measure have found greater loneliness endorsed among minority and immigrant groups [23, 24]. Our results are surprising as they are discrepant from these reports. First, it should be noted that many studies of loneliness among older adults have not focused on older Blacks, and it is possible that Black adults as a group have different experiences from other minority or immigrant populations. Second, our measure of loneliness was slightly modified in that the items reflecting social loneliness were dropped and only the items reflecting emotional loneliness were retained. This difference in could also be a possible explanation for our discrepant findings as social loneliness may reflect the objective absence of others whereas emotional loneliness may reflect a subjective perception of loneliness. Third, although racial differences in loneliness were significant, the overall differences were small. However, in order to test the direction and strength of the finding, we conducted additional analyses on a separate group of 1,180 nondemented participants from the MAP and MARS cohorts. As stated previously, older Black adults in this separate larger group also reported less loneliness than older White adults, supporting our loneliness by race finding. Some work has suggested that Black individuals may show greater resilience to psychosocial stressors [25]. Consistent with this notion, previous work has suggested that among older Black and White persons with HIV experiencing comparable levels of distress, older Black adults engage in more adaptive coping strategies [26, 27]. The engagement of more adaptive coping strategies might explain our overall observation of less reported feelings of loneliness among older Black adults. It is also possible that older Black adults may show greater resilience to loneliness due to the necessary development of social or emotional coping strategies to deal with the well documented and longstanding institutionalized racism and systemic injustice [28]. More work is needed to clarify the mechanisms involved in this finding.
Although older Black adults might report less loneliness overall, it is possible that they might experience loneliness differentially and more drastically than older White adults for multiple reasons. For example, older Black adults, regardless of HIV status, have been found to have fewer social resources (smaller social networks and less engagement in social activities) than older White adults [15], and therefore may be more prone to the negative health effects of loneliness after learning of an HIV infection. Older Black adults with HIV not only have to contend with the negative historical consequences of institutionalized racism, but also the social stigma associated with HIV infection, and these may interact to produce downstream effects upon health outcomes such as cognition. For these reasons, we secondarily hypothesized that loneliness experienced among older Black adults with HIV would be associated with poorer cognitive functioning in the context of an infection believed to result in cognitive impairment. Our finding of higher loneliness associated with lower cognitive function among older Black adults with HIV is striking and suggests that race and loneliness may be important linked factors to consider for assessing risk of cognitive impairment in the context of aging with HIV. However, the exact mechanisms for this are again unclear. From a neurobiological perspective, loneliness has been associated with decreased dendritic arborization in the hippocampal and prefrontal cortices in animal models [29], and dendritic injury has been described as a pathological substrate for HIV-related cognitive impairment [30], but it is unclear how these associations may be influenced by race. Again, one possible explanation may lie in the association between loneliness and stress in old age. Older adults who report experiencing a greater number of chronic social stressors also report greater loneliness [31]. Chronic stress induces the release of glucocorticoids, which in turn induces dysfunctional changes in glutamate neurotransmission in the hippocampus and prefrontal cortex [32]. Older Black adults with HIV may experience greater stress from multiple intersecting stigmas compared to White adults, and the association of loneliness with lower cognition among Black adults may be an indicator of increased race-specific stress from multiple sources acting upon neurobiological functioning. Older Black adults may also be more resilient to loneliness than White adults due to the development of necessary coping strategies in response to longstanding institutionalized racism. If this is true, then this may explain why Blacks report less loneliness overall. Furthermore, any loneliness endorsed among older Black adults may represent a more serious breakdown in social or other resources than among White adults, and this in turn may be associated with worse cognitive function. Future research is needed to examine the neurobiological and psychosocial mechanisms underlying racial differences in the association of loneliness with cognitive functioning. Future research is also needed to explore the effects of stigma upon neurobiological functions, and to determine what resources might be useful as intervention strategies to increase resilience.
The present study had some limitations. First, temporal ordering and causal inferences cannot be based on cross-sectional data. It is currently unknown how the present findings represent the possible time courses of loneliness, cognition, and HIV status. It is plausible that Black adults with HIV who have poorer cognition might experience more loneliness. Longitudinal studies would greatly assist in investigating causal links in our results. A final limitation is the relatively small number of Whites in the study which may have reduced power to detect an association between loneliness and cognition in this population.
This study also has multiple strengths. These include the use of participants from a community-based sample, a large sample size, the controlling of multiple demographic characteristics that could confound results, and the use of an HIV-negative control group that was similar in age, sex, education, income, and race to elucidate the specificity of results to HIV. Our findings suggest that loneliness among older Black adults with HIV may be associated with worse cognitive functioning relative to White adults with HIV. This suggests the development of interventions aimed at reducing the loneliness of older Black adults with HIV might have a consequent protective benefit upon cognitive functioning. Future work is needed to examine this and the neurobiological or psychosocial mechanisms underlying these associations.
Supplementary Material
Table 6.
Associations of Race, Loneliness, HIV Status with Global Cognition
| Factor | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| Adjusted R2 | 0.2161 | 0.2267 | 0.2307 | 0.2283 | 0.2273 | 0.2279 | 0.2406 |
| B (SE, p-value) | |||||||
| Age | −0.0071 (0.0044, 0.1076) | −0.0080 (0.0044, 0.0715) | −0.0083 (0.0044, 0.0598) | −0.0077 (0.0044, 0.0803) | −0.0063 (0.0046, 0.1783) | −0.0064 (0.0046, 0.1713) | −0.0078 (0.0046, 0.0930) |
| Male Sex | −0.0815 (0.0604, 0.1779) | −0.0746 (0.0600, 0.2149) | −0.0770 (0.0599, 0.1995) | −0.0654 (0.0604, 0.2794) | −0.0793 (0.0602, 0.1885) | −0.0703 (0.0606, 0.2467) | −0.0860 (0.0605, 0.1558) |
| Education | 0.0589 (0.0100, <0.0001) | 0.0551 (0.0101, <0.0001) | 0.0546 (0.0100, <0.0001) | 0.0556 (0.0101, <0.0001) | 0.0546 (0.0101, <0.0001) | 0.0552 (0.0101, <0.0001) | 0.0558 (0.0100, <0.0001) |
| Income | −0.0059 (0.0115, 0.6115) | −0.0049 (0.0115, 0.6669) | −0.0044 (0.0114, 0.6993) | −0.0048 (0.0114, 0.6731) | −0.0064 (0.0115, 0.5765) | −0.0061 (0.0115, 0.5983) | −0.0066 (0.0115, 0.5675) |
| Black Race | −0.3283 (0.0599, <0.0001) | −0.3593 (0.0609, <0.0001) | −0.2917 (0.0727, 0.0001) | −0.3600 (0.0608, <0.0001) | −0.2928 (0.0848, 0.0006) | −0.3056 (0.0855, 0.0004) | −0.3335 (0.0989, 0.0008) |
| Loneliness | −0.0862 (0.0356, 0.0160) | −0.0017 (0.0614, 0.9778) | −0.1331 (0.0503, 0.0085) | −0.0844 (0.0356, 0.0184) | −0.1259 (0.0510, 0.0140) | −0.1548 (0.0849, 0.0689) | |
| HIV positive | 0.2208 (0.0523, <0.0001) | 0.2289 (0.0521, <0.0001) | 0.2301 (0.0519, <0.0001) | 0.1876 (0.0607, 0.0022) | 0.3222 (0.0979, 0.0011) | 0.2689 (0.1085, 0.0137) | 0.1092 (0.1257, 0.3853) |
| Race*Loneliness | −0.1256 (0.0744, 0.0923) | 0.0450 (0.1051, 0.6686) | |||||
| HIV*Loneliness | 0.0917 (0.0695, 0.1876) | 0.0803 (0.0706, 0.2562) | 0.3119 (0.1223, 0.0112) | ||||
| Race*HIV | −0.1345 (0.1195, 0.2613) | −0.1098 (0.1214, 0.3664) | 0.0973 (0.1469, 0.5081) | ||||
| Race*Loneliness*HIV | −0.3381 (0.1495, 0.0244) | ||||||
Table 7.
Loneliness by Race in a Separate Sample of 1180 Participants Without Dementia (590 Black, 590 White)
| Factor | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Adjusted R2 | 0.0741 | 0.0846 | 0.0964 | 0.1237 |
| Beta (SE, p-value) | ||||
| Age | 0.0109 (0.0026, <0.0001) | 0.0111 (0.0026, <0.0001) | 0.0071 (0.0028, 0.0115) | 0.0076 (0.0028, 0.0059) |
| Male Sex | 0.0442 (0.0432, 0.3056) | 0.0483 (0.0429, 0.2609) | 0.0336 (0.0428, 0.4327) | 0.0790 (0.0432, 0.0680) |
| Education | −0.0419 (0.0052, <0.0001) | −0.0418 (0.0052, <0.0001) | −0.0310 (0.0058, <0.0001) | −0.0179 (0.0062, 0.0038) |
| Black Race | −0.1336 (0.0361, 0.0002) | −0.1931 (0.0388, <0.0001) | −0.2158 (0.0387, <0.0001) | |
| Global Cognition | −0.1544 (0.0391, 0.0001) | −0.1201 (0.0398, 0.0026) | ||
| Income | −0.0452 (0.0078, <0.0001) | |||
Acknowledgments
This research was supported by National Institute on Minority Health and Health Disparities grant P20MD6886 (LLB), RF1AG022018 (LLB), R01AG017917 (DAB), and a National Institute on Aging health disparities administrative supplement to grant K23AG040625 (SDH). The authors gratefully thank the CEDHA, MARS, and MAP participants and staff.
References
- 1.Centers for Disease Control. HIV Surveillance Report. 2015;25 [Google Scholar]
- 2.World Health Organization. [Accessed 1 August 2015];Global Health Observatory (GHO) Data – HIV/AIDS. 2013 http://www.who.int/gho/hiv/en/
- 3.Powderly WG. Sorting through confusing messages: the art of HAART. Journal of Acquired Immune Deficiency Syndromes. 2002;31:S1–S9. doi: 10.1097/00126334-200209011-00002. [DOI] [PubMed] [Google Scholar]
- 4.Grant I, Sacktor N, McArthur JC. HIV neurocognitive disorders. In: Gendelman HE, Grant I, Everall I, Lipton SA, Swindells S, editors. The neurology of AIDS. 2. New York: Oxford University Press; 2005. pp. 359–373. [Google Scholar]
- 5.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimer’s Research and Therapy. 2015;7:37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkley LC, Cacioppo JT. Aging and loneliness: Downhill quickly? Current Directions in Psychological Science. 2007;16:187–191. [Google Scholar]
- 8.Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer’s disease. Archives of General Psychiatry. 2007;64(2):234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 9.Herek GM, Capitanio JP, Widaman KF. HIV-related stigma and knowledge in the United States: Prevalence and trends, 1991–199. American Journal of Public Health. 2002;92:371–377. doi: 10.2105/ajph.92.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emlet CA. “You’re awfully old to have this disease”: Experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. The Gerontologist. 2006;46:781–790. doi: 10.1093/geront/46.6.781. [DOI] [PubMed] [Google Scholar]
- 11.Greysen SR, Horwitz LI, Covinsky KE, et al. Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? JAGS. 2013;61:1456–1463. doi: 10.1111/jgs.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17(4) [Google Scholar]
- 13.Shacham E, Rosenberg N, Onen NF, Donovan MF, Overton ET. Persisting HIV-related stigma among an outpatient US clinic population. J STD AIDS. 2015;26:243–250. doi: 10.1177/0956462414533318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichenstein B, Laska MK, Clair JM. Chronic sorrow in the HIV-positive patient: Issues of race, gender, and social support. AIDS Patient Care and STDs. 2002;16:27–37. doi: 10.1089/108729102753429370. [DOI] [PubMed] [Google Scholar]
- 15.Barnes LL, Mendes de Leon CF, Bienias JL, Evans DE. A longitudinal studies of Black-White differences in social resources. Journal of Gerontology—Social Sciences. 2004;59B:S146–S153. doi: 10.1093/geronb/59.3.s146. [DOI] [PubMed] [Google Scholar]
- 16.Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: Moving toward resilience. Am Psychol. 2013;68:225–236. doi: 10.1037/a0032705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alz Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong-Gierveld J, Kamphuis F. The development of a Rasch-type loneliness scale. Appl Psychol Measurement. 1985;9:289–299. [Google Scholar]
- 20.de Jong-Gierveld J. Developing and testing a model of loneliness. J Pers Soc Psychol. 1987;53:119–128. doi: 10.1037//0022-3514.53.1.119. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Psychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 22.Flowers P, Davis M, Hart G, Rosengarten M, Frankis J, Imrie J. Diagnosis and stigma and identity amongst HIV positive Black Africans living in the U. K Psychology and Health. 2006;21:109–122. [Google Scholar]
- 23.De Jong Gierveld J, Van der Pas S, Keating N. Loneliness of older immigrant groups in Canada: Effects of ethic-cultural background. J Cross Cult Gerontol. 2015;30:251–268. doi: 10.1007/s10823-015-9265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin X, Bryant C, Boldero J, Dow B. Psychological wellbeing of older Chinese immigrants living in Australia: a comparison with older Caucasians. International Psychogeriatrics. 2016:9. doi: 10.1017/S1041610216001010. http://dx.doi.org/10.1017/S1041610216001010. [DOI] [PubMed]
- 25.Brondolo E. Race and ethnic disparities in health: Examining the contexts that shape resilience and risk. Psychosomatic Medicine. 2015;77:2–5. doi: 10.1097/PSY.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 26.Heckman TG, Kochman A, Sikkema KJ, Kalichman SC, Masten J, Goodkin K. Late middle-aged and older men living with HIV/AIDS: Race differences in coping, social support, and psychological distress. J Natl Med Assoc. 2000;92:436–444. [PMC free article] [PubMed] [Google Scholar]
- 27.Heckman TG. Psychosocial differences between whites and African Americans living with HIV/AIDS in rural areas of 13 U.S. States. J Rural Health. 2009;22:131–139. doi: 10.1111/j.1748-0361.2006.00021.x. [DOI] [PubMed] [Google Scholar]
- 28.Barnes LL, Wilson RS, Hebert LE, et al. Racial differences in the association of education with physical and cognitive function in older Blacks and Whites. Journal of Gerontology, Social Sciences. 2011;66:354–363. doi: 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 30.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus—related cognitive disorders. Annals of Neurology. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 31.Hawkley LC, Hughes ME, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. From social structural factors to perceptions of relationship quality and loneliness: The Chicago Health, Aging, and Social Relations Study. J Gerontol B Psychol Sci Soc. 2008;63:S375–S384. doi: 10.1093/geronb/63.6.s375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.