Abstract
Reductions in hydrogen sulfide (H2S) production have been implicated in the pathogenesis of vascular dysfunction in animal models of hypertension; however, no studies have examined a functional role for H2S contributing to microvascular dysfunction in hypertensive (HTN) adults. We hypothesized that endogenous production of H2S would be reduced, impaired endothelium-dependent vasodilation would be mediated by reductions in H2S-dependent vasodilation, and vascular responsiveness to exogenous H2S (Na2S) would be attenuated in HTN compared to normotensive (NTN) adults. Fifteen NTN [51±2 yrs; blood pressure (BP) 116±3/76±3 mmHg] and 14 HTN adults (57±2 yrs; BP 140±3/89±2 mmHg) participated. H2S biosynthetic enzyme expression (Western blot) and substrate-dependent H2S production (amperometric probe) were measured in cutaneous tissue homogenates. Red cell flux (laser Doppler flowmetry) was measured during graded perfusions of acetylcholine (ACh; 10−6 –10−1 mol∙L−1) and Na2S (10−5–101 mol∙L−1) using intradermal microdialysis; the functional role of H2S was determined using pharmacological inhibition with aminooxyacetic acid (AOAA; 0.5 mmol∙L−1). H2S biosynthetic enzyme expression and substrate-dependent H2S production were reduced in HTN adults (all p<0.05). ACh-induced endothelium-dependent vasodilation was blunted in HTN compared to NTN adults (p=0.012). AOAA attenuated ACh-induced vasodilation in NTN adults (ACh: 1.31±0.13 vs. ACh+AOAA: 1.07±0.09 flux∙mmHg−1; P=0.025) but had no effect on vasodilation in HTN adults (ACh: 1.16±0.10 v. ACh+AOAA: 1.37±0.11 flux∙mmHg−1; p=0.47). Na2S-induced vasodilation was not different between groups. Collectively, these findings indicate that while the microvasculature maintains the ability to vasodilate in response to exogenous H2S, reductions in endogenous synthesis and H2S-dependent vasodilation contribute to endothelial dysfunction in human hypertension.
Keywords: endothelium-dependent dilation, microdialysis, nitric oxide, endothelial dysfunction, cystathionine γ-lyase, 3-mercaptopyruvate sulphurtransferase
INTRODUCTION
Hydrogen sulfide (H2S) is one of three gasotransmitters, along with nitric oxide (NO) and carbon monoxide, critical for cardiovascular homeostasis1. It is increasingly apparent that dysregulation of the enzymatic production and function of H2S plays an important role in the pathogenesis of hypertension-associated vascular dysfunction2–4. In the vasculature, H2S is enzymatically synthesized by cystathionine γ-lyase (CSE)5, and 3-mercaptopyruvate sulphurtransferase (3-MPST)6. Mice lacking endogenous CSE exhibit reduced cholinergic vasorelaxation and hyperpolarization5, 7 resulting in the development of pronounced hypertension5, comparable to that observed in endothelial NO synthase (NOS)-deleted mice8. These data suggest that impairments in H2S-mediated regulation of vascular function contribute to elevations in blood pressure.
H2S elicits vasodilation by directly hyperpolarizing vascular smooth muscle cells, predominately through ATP-sensitive and calcium-dependent potassium channels (KATP and KCa, respectively)7, 9. H2S also modulates vascular function via extensive “crosstalk” with the NO signaling pathway at multiple regulatory points1 and multiple lines of evidence suggest that the vascular effects of H2S and NO are interdependent. In this regard, H2S administration increases vascular NO10, and at low concentrations, potentiates the vasodilatory effect of NO11. Further, CSE silencing attenuates NO-dependent vasodilation11, and, conversely, inhibition of NO synthase attenuates the vasodilatory response to H2S9, 11. Taken together, these data demonstrate a functional synergistic interaction between the H2S and NO signaling pathways in the control of vascular function and suggest that each is obligatory for the maintenance of vascular homeostasis.
In animal models of hypertension, expression and activity of H2S-synthesizing enzymes, as well as plasma H2S concentrations, are reduced and associated with impairments in endothelium-dependent dilation4, 12, 13. Further, systemic treatment with NaHS improves endothelial function, at least in part, via increased NO bioavailability12, 14, 15. In humans, plasma H2S concentration appears to be negatively correlated with blood pressure16. However, despite the compelling pre-clinical evidence, no studies have mechanistically examined the role of H2S in contributing to vascular dysfunction in hypertensive (HTN) adults.
The aim of the present study was to examine a functional role for H2S in contributing to cutaneous microvascular dysfunction in middle-aged HTN adults. The human cutaneous circulation is a validated model to assess mechanisms underlying microvascular dysfunction17, 18, as the pathogenesis of hypertension-associated microvascular dysfunction occurs simultaneously in multiple vascular beds17, 19, 20. We hypothesized that CSE and 3-MPST expression, as well as endogenous production of H2S, would be reduced in HTN compared to normotensive (NTN) adults. We additionally hypothesized that H2S-mediated endothelium-dependent vasodilation would be attenuated in HTN adults. Finally, we hypothesized that vascular responsiveness to exogenous H2S would be blunted in HTN adults.
METHODS
A complete description of the Materials and Methods is provided in the Online Supplement.
Subjects
The Institutional Review Board at The Pennsylvania State University approved all experimental procedures. Verbal and written consent were obtained voluntarily from all subjects prior to participation and according to guidelines set forth by the Declaration of Helsinki. Fifteen adults with normal BP and 14 adults with untreated stage I essential hypertension participated. All subjects underwent a complete medical screening, including a resting 12-lead electrocardiogram, physical examination, 24-hour blood pressure (BP) monitoring, and 12-h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA). Consistent with JNC7 guidelines21, and assessed in accordance with American Heart Association standards22, HTN adults had a resting seated systolic BP >140 mmHg or a diastolic BP >90 mmHg, and NTN adults had a resting seated systolic BP <120 mmHg or a diastolic BP <80 mmHg. Ambulatory BP monitoring (Ambulo 2400; Mortara Instrument Inc., Milwaukee, WI, USA) was used to confirm the diagnosis of hypertension. Subjects were non-obese (body mass index < 30 kg∙m−2), did not use tobacco products, were recreationally active, and were not taking any over-the-counter or prescription medications with primary or secondary cardiovascular effects (e.g., statins, anticoagulants, antidepressants, etc.).
Protocol 1: assessment of endogenous expression and activity of H2S biosynthetic enzymes
In a subset of participants (NTN n=5; HTN n=7), cutaneous tissue samples were obtained via punch biopsy23, 24. Using sterile technique, two 3 mm diameter samples were obtained after anesthetization (2% lidocaine without epinephrine), immediately snap frozen in liquid nitrogen, and stored at −80°C until analysis. CSE and 3-MPST expression were determined by Western blot and H2S production was measured by amperometry (Apollo 4000 Free Radical Analyzer detector with a 3 mm H2S-selective electrode; World Precision Instruments, Sarasota, FL, USA), as previously described24, 25 (Online Supplement).
Protocol 2: assessment of H2S-dependent cutaneous vasodilatory responsiveness
Four intradermal microdialysis probes (CMA Linear 30 probe, 6 kDa; Harvard Apparatus, Holliston, MA, USA) were inserted into the forearm skin23, 24, for the local delivery of pharmacological agents: lactated Ringer solution (control), 20 mmol∙L−1 NG-nitro-L-arginine methyl ester (L-NAME; Calbiochem, EMD Millipore, Billerica, MA, USA) to non-selectively inhibit NOS, 0.5 mmol∙L−1 aminooxyacetic acid (AOAA; Sigma-Aldrich Corp., St. Louis, MO, USA) to inhibit H2S biosynthesis26, or 20 mmol∙L−1 L-NAME + 0.5 mmol∙L−1AOAA to inhibit both NO and H2S vasodilatory pathways concurrently. Pilot studies using an in vitro preparation confirmed the efficacy of this concentration of AOAA to inhibit H2S production (Online Supplement). After microdialysis fiber insertion, 60-90 minutes were allowed for hyperemia resolution, during which site-specific pharmacological solutions were perfused (2 μmol∙L−1∙min; Hive controller and microinfusion pumps; BASi, West Lafayette, IN, USA). Thereafter, progressively increasing concentrations of acetylcholine (ACh; 10−6 – 10−1 mol∙L−1; USP, Rockville, MD, USA) were co-perfused with the site-specific pharmacological agent. Following the ACh dose-response protocol, 28 mmol∙L−1 sodium nitroprusside (USP, Rockville, MD, USA) was perfused and local temperature increased to 43°C to elicit maximal dilation23, 24. Cutaneous red blood cell flux, an index of cutaneous blood flow, was continually measured directly over each microdialysis site with an integrated laser Doppler flowmetry probe placed in a local heating unit (Temperature Monitor SH02; Moor Instruments, Devon, UK). Brachial BP (Cardiocap; GE Healthcare, Milwaukee, WI, USA) was measured every 5 min during the protocol.
Protocol 3: assessment of cutaneous vascular sensitivity to exogenous H2S
As described above, two intradermal microdialysis probes were inserted into the forearm skin. Sites were perfused with lactated Ringer solution or 20 mmol∙L−1 L-NAME (Calbiochem) for 60-90 min following probe placement. An index of cutaneous blood flow was obtained directly over the microdialysis site during perfusion (2 μl∙min−1) of progressively increasing doses of the H2S donor sodium sulfide (Na2S; 10−5 – 101 mol∙L−1; Sigma-Aldrich Corp.)24. Utilizing this model, we have previously demonstrated no difference in cutaneous vasodilation between the two commonly used H2S donors NaHS and Na2S24. Na2S was dissolved in lactated Ringer solution and titrated with hydrochloric acid (0.5 mol∙L−1 HCl) to pH=7.0. Solutions were mixed immediately before use, wrapped in foil to prevent photodegradation, and sealed from air to maintain pH=7.0 throughout the experiment. At the conclusion of the protocol, maximal dilation was obtained as detailed above. Brachial BP (Cardiocap; GE Healthcare) was measured every 5 min. A Food and Drug Administration Investigational New Drug (no. 105,572) was obtained for the in vivo utilization of all pharmacological agents.
Data and Statistical Analysis
Subject characteristics, CSE and 3-MPST expression and H2S production were compared using unpaired Student’s t-tests. Functional data were collected at 40 Hz (Windaq; DataQ Instruments, Akron, OH, USA). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux (perfusion units; PU) divided by mean arterial pressure. CVC was averaged during 5 minutes of baseline and during the plateau of each ACh or Na2S dose. Data were analyzed using three-way (group ×. dose ×. pharmacological treatment) mixed model repeated-measures ANOVA (SAS v. 9.1.3; Cary, NC, USA). When appropriate, post hoc Bonferroni corrections were applied to correct for multiple comparisons. Results are presented as means ± SEM, and significance was set at α < 0.05.
RESULTS
Subject characteristics are presented in Table 1. There were no statistically significant differences between groups in age, anthropometric characteristics, or blood biochemistry. By design, resting screening BP, as well as 24-hr BP, were significantly elevated in HTN adults (all P<0.01). BP did not change significantly during the course of the experiment in either group (NTN P=0.35; HTN P=0.54).
Table 1.
Subject Characteristics.
| Baseline Characteristic | NTN | HTN |
|---|---|---|
| N (M/F) | 15 (5/10) | 14 (6/8) |
| Age (yr) | 51 ± 2 | 57 ± 2 |
| Height (cm) | 169 ± 2 | 165 ± 2 |
| Mass (kg) | 75 ± 3 | 78 ± 3 |
| BMI (kg/m2) | 26.3 ± 0.8 | 28.3 ± 0.9 |
| Waist Circumference (cm) | 36.8 ± 1.3 | 36.5 ± 0.7 |
| Screening Systolic BP (mmHg) | 116 ± 3 | 140 ± 3* |
| Screening Diastolic BP (mmHg) | 76 ± 3 | 89 ± 2* |
| 24-hr Systolic BP (mmHg) | 107 ± 2 | 140 ± 2* |
| 24-hr Diastolic BP (mmHg) | 70 ± 1 | 87 ± 1* |
| Heart Rate (bpm) | 64 ± 2 | 63 ± 2 |
| Blood Biochemistry | ||
| HbA1c (%) | 5.5 ± 0.1 | 5.4 ± 0.1 |
| Fasting Glucose (mg/dl) | 88.5 ± 2.0 | 91.2 ± 2.2 |
| Fasting Total Cholesterol (mg/dl) | 187 ± 7 | 194 ± 9 |
| Fasting HDL (mg/dl) | 60 ± 5 | 56 ± 4 |
| Fasting LDL (mg/dl) | 109 ± 6 | 116 ± 7 |
| Fasting Triglycerides (mg/dl) | 91 ± 11 | 127 ± 23 |
NTN, normotensive; HTN, hypertensive; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Values are mean ± SE.
P<0.05 v. NTN.
Activity of H2S-producing enzymes is reduced in HTN adults (in vitro)
In HTN adults, CSE and 3-MPST expression in cutaneous tissue homogenates were markedly reduced (Fig. 1A, B). In addition, both CSE- and 3-MPST-mediated H2S production were attenuated in HTN adults (Fig. 1C, D).
Figure 1.
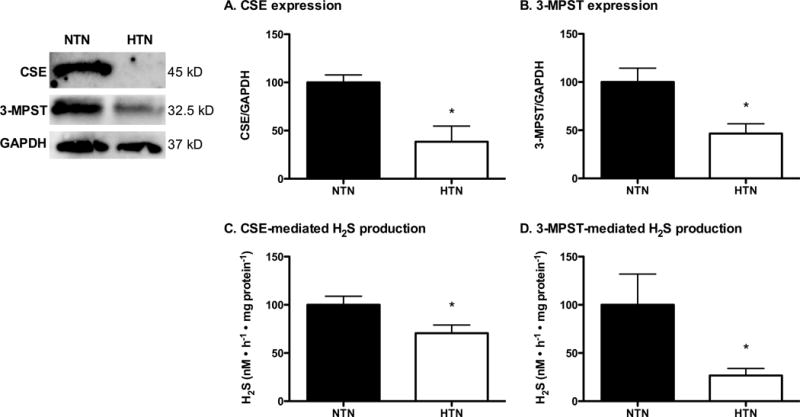
Expression of cystathionine γ-lyase (CSE; Panel A) and 3-mercaptopyruvate sulphurtransferase (3-MPST; Panel B), as well as CSE-mediated (Panel C) and 3-MPST-mediated H2S production, in normotensive (NTN; filled bars) and hypertensive adults (HTN; open bars). A representative blot is shown in the first panel. *P<0.01 vs. NTN.
Endogenous H2S-mediated vasodilation is functionally absent in HTN adults
Maximal SNP-induced cutaneous vasodilation was reduced in HTN adults (Fig. 2; P=0.039). As expected, ACh-induced endothelium-dependent vasodilation was attenuated in HTN adults (Fig. 2). ACh-induced vasodilation at each pharmacological treatment site is presented in Figure 3. NOS inhibition attenuated ACh-induced vasodilation in both subject groups (Fig. 3); however, there was no group difference in the vasodilatory response in the presence of NOS inhibition (NTN: 0.88±0.09 vs. HTN: 0.089±0.07 flux∙mmHg−1; P=0.92), reflecting hypertension-induced reductions in vascular NO bioavailability. AOAA attenuated ACh-induced vasodilation in NTN adults (Fig. 3B), but had no effect in HTN adults (Fig. 3E), suggesting a functional lack of endogenous H2S-mediated vasodilation. Combined pharmacological inhibition of both NO synthase and H2S-producing enzymes attenuated ACh-induced vasodilation in NTN adults; however, the attenuation in cutaneous vasodilation was not statistically different from that during L-NAME treatment alone (P>0.05). In HTN adults, combined treatment with L-NAME+AOAA had minimal effect on vasodilatory responsiveness to exogenous ACh, such that there was no difference between the combined site and the control site.
Figure 2.
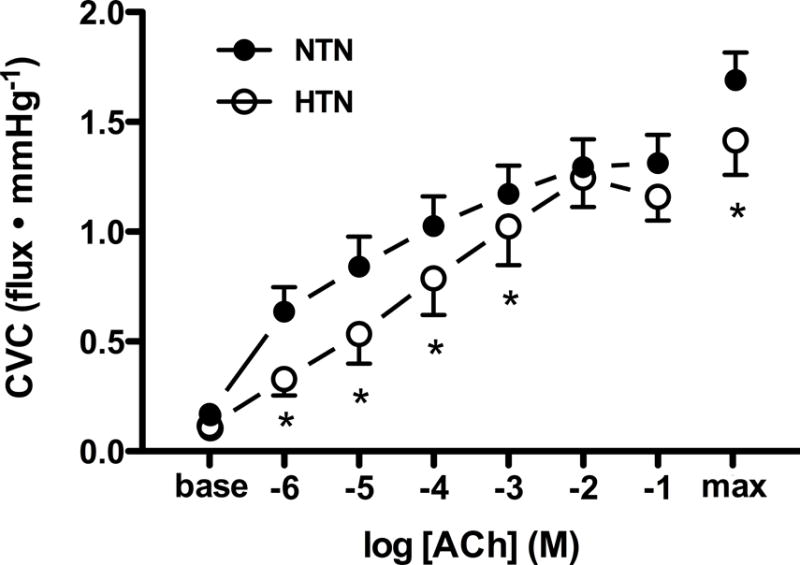
Cutaneous vascular conductance (CVC) in response to increasing doses of acetylcholine (ACh) in normotensive (NTN; filled symbols) and hypertensive adults (HTN; open symbols). Maximal CVC (max) was elicited by perfusion of sodium nitroprusside during local heating to 43°C at the conclusion of the ACh dose-response protocol. ACh-induced vasodilation was blunted in HTN adults. *P<0.05 vs. NTN.
Figure 3.
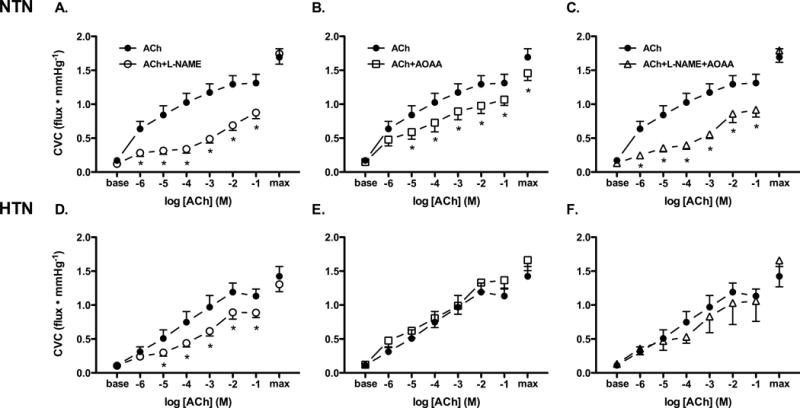
Cutaneous vasodilation (cutaneous vascular conductance; CVC) in response to increasing doses of exogenous acetylcholine alone (ACh; filled circles), during nitric oxide synthase inhibition (L-NAME; open circles), during inhibition of H2S-producing enzymes (AOAA; open squares), and during combined nitric oxide and H2S inhibition (L-NAME+AOAA; open triangles) in normotensive (NTN; Panels A-C) and hypertensive adults (HTN; Panels D-F). Maximal CVC (max) was elicited by perfusion of sodium nitroprusside during local heating to 43°C at the conclusion of the ACh dose-response protocol. *P<0.05 vs. ACh alone.
Vascular responsiveness to exogenous Na2S is preserved in HTN adults
Maximal CVC was not different between groups or treatment sites (P>0.05 for all). There was no difference in cutaneous vascular responsiveness to exogenous Na2S between NTN and HTN adults (Fig. 4; 10−1 mM Na2S: 1.31±0.19 NTN v. 1.34±0.24 HTN flux∙mmHg−1; P=0.80). NO synthase inhibition blunted Na2S-induced vasodilation at the highest dose in both subject groups (Fig. 4; P<0.05).
Figure 4.
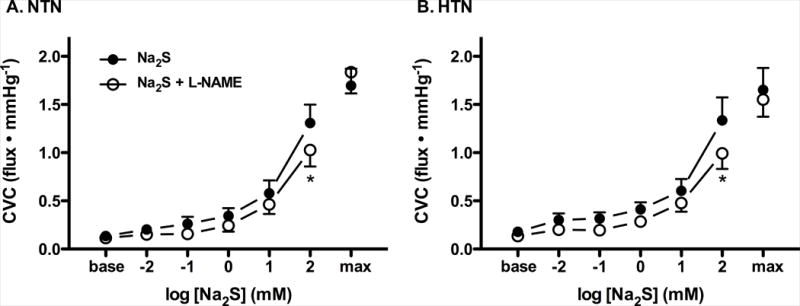
Cutaneous vasodilation (cutaneous vascular conductance; CVC) in response to increasing doses of the exogenous H2S donor sodium sulfide (Na2S) alone (ACh; filled circles) and during nitric oxide synthase inhibition (L-NAME; open circles) in normotensive (NTN; Panel A) and hypertensive adults (HTN; Panel B). Maximal CVC (max) was elicited by perfusion of sodium nitroprusside during local heating to 43°C at the conclusion of the Na2S dose-response protocol. *P<0.05 vs. Na2S alone.
DISCUSSION
The primary novel findings of the present study are that expression and substrate-dependent activity of H2S biosynthetic enzymes are reduced and H2S-dependent ACh-induced cutaneous vasodilation is impaired in HTN adults. Contrary to our hypothesis, vascular responsiveness to exogenous H2S was preserved in HTN adults, further implicating reductions in H2S bioavailability, and not alterations in downstream vascular smooth muscle sensitivity, in contributing to hypertension-associated vascular dysfunction. Moreover, we are the first to demonstrate an interaction between the H2S and NO signaling pathways in the regulation of microvascular function in humans. Taken together, these findings suggest that reductions in both H2S- and NO-dependent mechanisms contribute to endothelial dysfunction in the microcirculation of HTN adults.
Impaired endothelium-dependent vasodilation is a well-established contributing factor to hypertensive pathology27 and is evident in the cutaneous microcirculation of HTN adults23, 28–30. In the present study, HTN adults exhibited endothelial dysfunction, evidenced by impaired ACh-induced vasodilation. Inhibition of NOS attenuated vasodilation by ~30% in response to a cholinergic stimulus in NTN adults, reflecting a robust contribution of NO to ACh-induced vasodilation23, 31, 32 but this was substantially reduced in HTN adults, confirming that reductions in vascular NO bioavailability contribute to the endothelial dysfunction characteristic of hypertension27–30, 33–35.
While the roles of endothelium-derived relaxing and constricting factors, including NO and cyclooxygenase-derived metabolites, have been extensively characterized in hypertension, no studies have investigated potential alterations in H2S metabolism in human hypertension. CSE and 3-MPST are the enzymes largely responsible for endogenous endothelial-derived H2S synthesis5, 6. In young adults, these enzymes are present in the cutaneous microvasculature24 and synthesize H2S, which induces vasodilation, in part, by activating intermediate calcium-dependent potassium channels24. CSE and 3-MPST expression were markedly reduced in cutaneous tissue homogenates from HTN adults, consistent with previous reports36. In addition, H2S production was detected in each group, suggesting that the intra-cellular source of H2S has a cytosolic and mitochondrial component37. The present results demonstrate marked reductions in enzymatic H2S production through both CSE and 3-MPST biosynthetic pathways in HTN adults. These in vitro amperometric H2S measurements were performed in cutaneous tissue, and thus directly relate to our functional assessments of vascular regulation. Because of the difficulty in accurately measuring H2S concentration and the ambiguity of data interpretation from gross measurements of bioactive sulfide metabolites, relatively few studies have examined H2S production in humans. Although low plasma H2S concentration is associated with cardiovascular mortality38, and plasma H2S concentration appears negatively correlated with blood pressure16, to our knowledge, only one previous study has measured H2S production in untreated HTN adults36. Interestingly, the authors reported a greater serum H2S concentration, measured via a sulfide sensitive electrode, in HTN adults, despite lower expression of 3-MPST. We did not measure serum H2S in the present study due to the aforementioned methodological difficulties and instead favored measurement of tissue-specific H2S production from the substrates for CSE and 3-MPST, L-cysteine and 3-MP, respectively. Our findings are consistent with those observed in rodent models of hypertension, in which expression and activity of H2S-producing enzymes are reduced and contribute to vascular dysfunction4, 12, 13, 39.
H2S regulates vascular tone via its direct effects on vascular smooth muscle cells as well as by extensive interactions with other endothelium-dependent signaling pathways1, 7, 9, 40. H2S and NO are mutually dependent and multiple potential points of functional interaction exist, both upstream in the endothelium and downstream in the vascular smooth muscle11. In this regard, NaHS-induced dilation is blunted in the presence of L-NAME in blood vessels isolated from both rodents and humans11, 41 as well as in vessels harvested from eNOS−/− mice11, suggesting that the vasodilatory action of H2S requires the presence of endogenously produced NO. In the current study, there was a modest, but significant, attenuation in Na2S-induced vasodilation during NOS inhibition in NTN adults. Given that the vasodilatory effect of H2S was only modestly blunted by NOS inhibition, H2S-induced vasodilation likely involves parallel signaling pathways, such as KATP and KCa7, 9, 24.
Contrary to our hypothesis, we found that perfusion of an exogenous H2S donor elicited significant vasodilation in HTN adults that was not different from that observed in NTN adults, suggesting vascular responsiveness to exogenous H2S is maintained in human hypertension. In addition, we noted an attenuation in the vasodilatory response to the highest dose of Na2S during concurrent NOS inhibition in HTN adults, the magnitude of which was not different from that in NTN adults, providing evidence that the NO contribution to exogenous H2S-mediated vasodilation remains intact in HTN adults. In hypertensive CSE−/− mice, relaxation in response to H2S was augmented compared to that in wild-type mice, a response indicative of supersensitivity associated with the diminished endogenous synthesis of H2S5. Thus, it appears that vascular sensitivity to exogenous H2S, as well as its synergistic interaction with NO, is preserved in hypertension-induced vascular dysfunction. Because bioavailability of both H2S and NO is reduced in hypertension, exogenous delivery of a H2S donor may be beneficial for improving vascular function through both signaling pathways, a potentially important clinical implication given the development of novel pharmacological agents with slow-release H2S moieties for the treatment of hypertension42. This area of research warrants future investigation.
Despite preserved vascular sensitivity to exogenous H2S, reductions in the endogenous production and function of H2S clearly contribute to the pathogenesis of hypertension-associated vascular dysfunction4, 12, 13. Moreover, long-term systemic treatment with a H2S donor improves endothelial function in hypertensive rodents, in part, via increased NO bioavailability12, 14, 15, 39, suggesting that the loss of redundancy and synergism between the NO and H2S signaling pathways contributes to vascular dysfunction in hypertension5, 11, 43. In this regard, endogenous H2S appears to be required for the full vasodilatory actions of NO. siRNA-mediated silencing of CSE attenuates, but does not completely block, dilation in response to a NO donor or to the endothelium-dependent agonist ACh11, and methacholine-induced vasodilation is attenuated in CSE−/− mice5.
In the current study, inhibition of CSE with AOAA blunted ACh-induced endothelium-dependent vasodilation in NTN adults, providing the first direct evidence in humans that H2S mediates a portion of the vasodilatory response to ACh. Mechanistically, it has been suggested that ACh, via endothelial calcium mobilization, may stimulate H2S release from endothelial cells, resulting in prolonged phosphodiesterase type 5 inhibition and subsequent relaxation11. It is plausible to suggest that the reductions in endogenous H2S production in HTN adults may contribute to altered regulation of vascular responsiveness to ACh in hypertension. Consistent with this hypothesis, inhibition of CSE did not effect ACh-induced vasodilation in HTN adults, indicating that the H2S-mediated component of cholinergic vasodilation is functionally absent in human hypertension. Interestingly, concurrent antagonism of NOS and CSE did not further attenuate ACh-induced vasodilation in either NTN or HTN adults compared to either pharmacological agent alone, a finding consistent with the notion that other parallel signaling pathways are compensatory in response to cholinergic stimulation. Collectively, the results of the present study suggest that alterations in the production and function of endogenous H2S likely contribute to the endothelial dysfunction in human hypertension.
Additional studies are necessary to determine the potential for alterations in H2S-dependent mechanisms to contribute to vascular dysfunction in HTN adults with additional major cardiovascular risk factors. In the present study every attempt was made to match NTN and HTN groups for all clinical characteristics aside from resting BP. Although not statistically different, and still within normal limits, HTN adults tended to be older and have a greater body mass index. Because aging and overweight/obesity are independently associated with impairments in endothelial function44–46 it is possible that these non-statistically significant trends in cardiovascular risk may have contributed to the observed functional differences.
Limitations
Because of the complexity of H2S metabolism47, it is methodologically challenging to determine the specific bioactive H2S-derived intermediate mediating the observed vasodilatory responses. Given these technical limitations, we instead measured substrate-dependent H2S production as a surrogate for the activity of the H2S biosynthetic enzymes CSE and 3-MPST. We acknowledge the inability of the amperometric probe to determine the specific H2S-derivative species mediating the observed biological responses48. Nevertheless, because reductions in both H2S biosynthetic enzyme expression and substrate-mediated H2S production are evident in HTN adults, the corresponding signal for eliciting a physiological response is therefore also reduced, regardless of the specific biochemical intermediate pathway, ultimately resulting in a blunted contribution to vasodilation.
We also recognize the potential limitation of the use of AOAA to inhibit endogenous production of H2S; however, AOAA is currently the only CSE inhibitor available for use in humans. Importantly, AOAA has recently been demonstrated to be a more specific inhibitor of CSE than cystathionine-β-synthase (CBS: neuronal enzymatic source of H2S) in the vasculature26. In addition, the inhibition of CSE by AOAA was verified by measurement of H2S production using the CSE-specific substrate L-cysteine (Supplement Fig. S1A). Moreover, we were not able to delineate the relative contribution of 3-MPST inhibition to the observed responses. Although AOAA may indirectly inhibit 3-MPST via cysteine aminotransferase49, it is not specific for 3-MPST, and thus we cannot determine the direct extent to which H2S production from 3-MPST contributes to vascular dysfunction in HTN adults. Additional studies are necessary once more specific inhibitors for the enzymatic sources of H2S are available in order to fully elucidate the contribution of H2S synthesized from MPST to vascular control in humans.
Perspectives
Endothelial dysfunction is thought to be the primary causative event in the development of atherosclerosis, occurs before angiographic evidence of disease, and predicts future cardiovascular events and mortality50, 51. The pathogenesis of vascular dysfunction associated with hypertension is a complex, multifaceted process that is thought to manifest to a proportional extent in multiple tissue beds. In this regard, the progression of endothelial dysfunction in the cutaneous microvasculature mirrors that in the coronary and renal circulations17, 19, 20, making it an accessible and representative model to examine vascular function. Using this methodology, we are the first to demonstrate that reductions in the enzymatic production and function of endogenous H2S contribute to microvascular endothelial dysfunction in HTN adults. Additional studies are warranted to examine the systemic effects of H2S in targeted pharmacotherapy for hypertensive vascular pathology. Stage I clinical trials indicate that anti-hypertensive medications with H2S-releasing moieties demonstrate superior clinical benefit42; however, future investigations as to whether this is due to the independent vasodilatory effect of H2S or via its synergistic interaction with the NO signaling pathway or through alterations in the enzymatic production of other endothelium-dependent signaling molecules within the vasculature (e.g., prostanoids) are necessary.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
These findings show that the hydrogen sulfide (H2S)-dependent contribution to vasodilation is functionally absent in hypertensive adults, likely due to a reduction in the endogenous production of H2S within the vasculature.
Vascular responsiveness to exogenous H2S is preserved in hypertensive adults.
We additionally demonstrate an interaction between H2S and nitric oxide signaling pathways in the regulation of vascular function in humans.
What is relevant?
Given that rodent models of hypertension demonstrate reductions in the endogenous production and function of H2S clearly contribute to the pathogenesis of hypertension-associated vascular dysfunction in rodent models, translating these findings to human hypertension may provide novel insight into the mechanisms of endothelial dysfunction.
These findings are clinically relevant and additional studies are warranted to examine the systemic effects of H2S in targeted pharmacotherapy for hypertensive vascular pathology.
Summary
Reductions in both H2S- and nitric oxide-dependent mechanisms contribute to microvascular endothelial dysfunction in hypertensive adults.
Acknowledgments
We greatly appreciate the effort expended by the volunteer participants. We also thank Susan Slimak, R.N. and Jane Pierzga, M.S. for their laboratory assistance.
SOURCES OF FUNDING
This work was supported by National Institutes of Health grant HL093238 (LMA).
Footnotes
DISCLOSURES
None
References
- 1.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circulation Research. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang HL, Wu HC, Li ZL, Geng B, Tang CS. changes of the new gaseous transmitter h2s in patients with coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:951–954. [PubMed] [Google Scholar]
- 3.Taniguchi S, Kang L, Kimura T, Niki I. Hydrogen sulphide protects mouse pancreatic beta-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 2011;162:1171–1178. doi: 10.1111/j.1476-5381.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2s as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 7.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circulation Research. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of h(2)s as a novel endogenous gaseous k(atp) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med. 2013;17:879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue H, Zhou S, Xiao L, Guo Q, Liu S, Wu Y. Hydrogen sulfide improves the endothelial dysfunction in renovascular hypertensive rats. Physiol Res. 2015;64:663–672. doi: 10.33549/physiolres.932848. [DOI] [PubMed] [Google Scholar]
- 13.d’Emmanuele di Villa Bianca R, Mitidieri E, Donnarumma E, Tramontano T, Brancaleone V, Cirino G, Bucci M, Sorrentino R. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide. 2015;46:80–86. doi: 10.1016/j.niox.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Al-Magableh MR, Kemp-Harper BK, Hart JL. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin ii-induced hypertensive mice. Hypertens Res. 2015;38:13–20. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 15.Ji W, Liu S, Dai J, Yang T, Jiang X, Duan X, Wu Y. Hydrogen sulfide defends against the cardiovascular risk of nw-nitro-l-argininemethyl ester-induced hypertension in rats via the nitric oxide/endothelial nitric oxide synthase pathway. Chinese Medical Journal. 2014;127:3751–3757. [PubMed] [Google Scholar]
- 16.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 17.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, Henry RM, Koster A, Sep SJ, Dagnelie PC, Schaper NC, Schram MT, Stehouwer CD. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The maastricht study. Circulation. 2016;134:1339–1352. doi: 10.1161/CIRCULATIONAHA.116.023446. [DOI] [PubMed] [Google Scholar]
- 19.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser doppler flowmetry correlates with renal resistive index. Journal of Human Hypertension. 2012;26:56–63. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 20.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008;115:295–300. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ, Subcommittee of P, Public Education of the American Heart Association Council on High Blood Pressure R Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension. 2011;58:935–942. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutz JL, Greaney JL, Santhanam L, Alexander LM. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J Physiol. 2015;593:2121–2129. doi: 10.1113/JP270054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo MM, Kim DH, Jandu S, Bergman Y, Tan S, Wang H, Pandey DR, Abraham TP, Shoukas AA, Berkowitz DE, Santhanam L. Mpst but not cse is the primary regulator of hydrogen sulfide production and function in the coronary artery. American journal of physiology. Heart and circulatory physiology. 2016;310:H71–79. doi: 10.1152/ajpheart.00574.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (cbs) and cystathionine gamma lyase (cse) Br J Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: Possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010;12:267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holowatz LA, Kenney WL. Local ascorbate administration augments no- and non-no-dependent reflex cutaneous vasodilation in hypertensive humans. American journal of physiology. Heart and Circulatory Physiology. 2007;293:H1090–1096. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 29.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–872. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruning RS, Kenney WL, Alexander LM. Altered skin flowmotion in hypertensive humans. Microvasc Res. 2015;97:81–87. doi: 10.1016/j.mvr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol (1985) 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 33.Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens (Greenwich) 2012;14:198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelm M, Preik M, Hafner DJ, Strauer BE. Evidence for a multifactorial process involved in the impaired flow response to nitric oxide in hypertensive patients with endothelial dysfunction. Hypertension. 1996;27:346–353. doi: 10.1161/01.hyp.27.3.346. [DOI] [PubMed] [Google Scholar]
- 35.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circulation Research. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 36.Zheng M, Zeng Q, Shi XQ, Zhao J, Tang CS, Sun NL, Geng B. Erythrocytic or serum hydrogen sulfide association with hypertension development in untreated essential hypertension. Chinese Medical Journal. 2011;124:3693–3701. [PubMed] [Google Scholar]
- 37.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng SJ, Li H, Wang SX. Lower hydrogen sulfide is associated with cardiovascular mortality, which involves cpkcbetaii/akt pathway in chronic hemodialysis patients. Blood Purif. 2015;40:260–269. doi: 10.1159/000439580. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Huang Y, Zhang R, Chen Q, Chen J, Zong Y, Liu J, Feng S, Liu AD, Holmberg L, Liu D, Tang C, Du J, Jin H. Hydrogen sulfide upregulates katp channel expression in vascular smooth muscle cells of spontaneously hypertensive rats. J Mol Med (Berl) 2015;93:439–455. doi: 10.1007/s00109-014-1227-1. [DOI] [PubMed] [Google Scholar]
- 40.Hancock JT, Whiteman M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann N Y Acad Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- 41.Materazzi S, Zagli G, Nassini R, Bartolini I, Romagnoli S, Chelazzi C, Benemei S, Coratti A, De Gaudio AR, Patacchini R. Vasodilator activity of hydrogen sulfide (h2s) in human mesenteric arteries. Microvasc Res. 2016;109:38–44. doi: 10.1016/j.mvr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Bucci M, Vellecco V, Cantalupo A, Brancaleone V, Zhou Z, Evangelista S, Calderone V, Papapetropoulos A, Cirino G. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ace inhibition. Cardiovasc Res. 2014;102:138–147. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 43.Beltowski J, Jamroz-Wisniewska A, Tokarzewska D. Hydrogen sulfide and its modulation in arterial hypertension and atherosclerosis. Cardiovasc Hematol Agents Med Chem. 2010;8:173–186. doi: 10.2174/187152510792481207. [DOI] [PubMed] [Google Scholar]
- 44.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 45.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: Hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21:149–157. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Cuevasanta E, Moller MN, Alvarez B. Biological chemistry of hydrogen sulfide and persulfides. Arch Biochem Biophys. 2016 doi: 10.1016/j.abb.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Cortese-Krott MM, Kuhnle GG, Dyson A, et al. Key bioactive reaction products of the no/h2s interaction are s/n-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A. 2015;112:E4651–4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine beta-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 51.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


