Abstract
Antibiotic resistance is one of the biggest public concerns in the 21st century. Host-defense peptides (HDPs) can potentially mitigate the problem through bacterial membrane disruption; however, they suffer from moderate activity and low stability. We recently developed a new class of peptidomimetics termed “AApeptides”. This class of peptidomimetics can mimic the mechanism of action of HDPs, and effectively arrest the growth of multidrug resistant Gram-positive and Gram-negative bacteria. As they are built on unnatural backbone, they are resistant to proteolytic degradation. In this review, we summarize the development of this class of antimicrobial peptidomimetics, and discuss the future perspective on how they can move forward on combating antibiotic resistance.
Keywords: Antibiotic resistance, Host-defense peptides (HDPs), AApeptides, peptidomimetics, Antimicrobial activity
INTRODUCTION
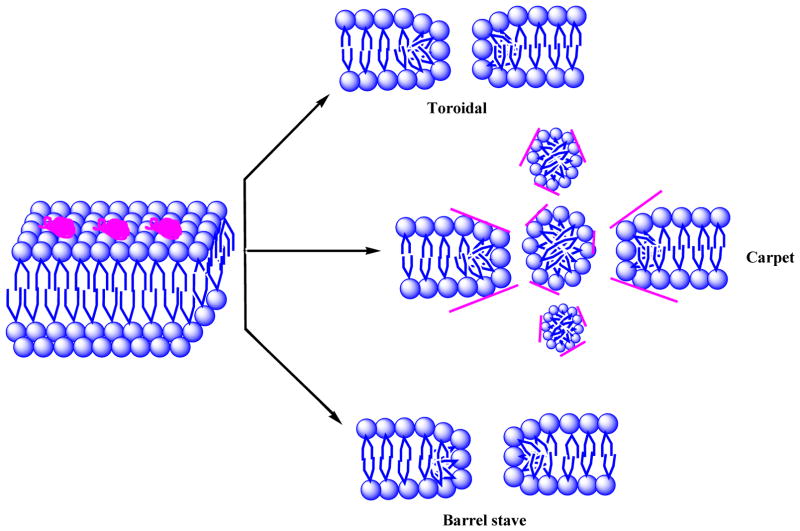
Although conventional antibiotics are widely used in clinical applications to treat bacterial infections, antibiotic resistance due to the over use of antibiotics is a major threat to human health [1–3]. Severe infections, caused by multi-drug resistant bacterial strains such as vancomycin–resistant enterococci (VRE), methicillin–resistant staphylococcus aureus (MRSA), and methicillin – resistant staphylococcus epidermidis (MRSE), occur more frequently, as most conventional antibiotics are not active towards these strains [4–6]. Thus, it is of considerable significance to develop the new generation of antibiotics combating drug resistance. Host-defense peptides (HDPs), frequently referred as antimicrobial peptides (AMPs), are natural cationic amphiphilic peptides found in virtually all life forms [2–3], and act as the first line of defense to prevent organisms from microbial infection [2–3, 7]. Although there is long-standing dispute over bactericidal mechanisms of HDPs [4], it is widely recognized that HDPs could adopt globally amphipathic structures when associating with bacterial membranes, in which their cationic and hydrophobic side chains form two separate domains (Fig. 1). The cationic patch is believed to be at least partially responsible for the selectivity of HDPs, as it interacts with negatively charged components in bacterial envelope, such as teichoic acids and lipo teichoic acids present on the surface of Gram-positive bacteria and lipopolysaccharides in outer membranes of Gram-negative bacteria [8].
Fig. 1.
The possible mechanism of HDPs on bacterial membranes.
As contrary, the surface of eukaryotic cell membranes is composed of zwitterionic phospholipids including phosphatidylcholine, cholesterol, and sphingomyelin [9], and therefore they only interact with cationic HDPs weakly. This is why HDPs have good selectivity for bacteria. After initial electrostatic interaction with bacterial membranes, the hydrophobic side chains in HDPs insert into membranes through hydrophobic interactions with the fatty acyl tails of membrane phospholipids. This interaction causes the bacterial membrane depolarization and subsequent cell death [4]. As such, unlike most of conventional antibiotics which are bacteriostatic, HDPs are generally bactericidal. Since HDPs lack well-defined membrane targets, HDPs could be diverse in sequences and folding structures. Indeed, α-helices, β-sheets, and other conformations of peptides all have been found to exhibit antibacterial activity [3]. To summarize, antimicrobial activity of HDPs relies on the biophysical interaction with bacterial cell membranes, which lacks defined biomolecular targets. Although resistance development is inevitable toward any therapeutic agents, HDPs may have relatively low tendency to induce antibiotic resistance than conventional antibiotics [4].
Despite considerable enthusiasm, it remains a challenge to develop HDPs as antibiotics. For instance, their intrinsic susceptibility to proteolytic degradation is one of the concerns. In addition, their antimicrobial activity is normally moderate; however, optimization is not straightforward [2]. To circumvent these drawbacks, non-natural peptidomimetics were employed recently as an alternative approach to mimic the structure and function of HDPs, in the hope to achieve similar antimicrobial activities while enhancing their stability. The examples of antimicrobial peptidomimetics include β-peptides [10–12], peptoids [9, 13–14], arylamide oligomers [4, 15–16], β-turn mimetics [17–18], and others [19]. Extensive reviews involving these antimicrobial agents have been summarized by many groups [2–3, 5]. The research findings on these antimicrobial peptidomimetics reveal that antimicrobial activity is not tightly related to pre-organized secondary structures HDPs and peptidomimetics [20–24]. It seems that the potent antimicrobial activity and good selectivity may actually require certain flexible, or even random coiled backbones, where side groups are capable of segregating into globally amphipathic patches upon interaction with bacterial membranes. Recently, our group has designed a new class of peptidomimetics “AApeptides” [26–27]. In the following content, we will summarize the recent development of “AApeptides” as a new class of antimicrobial agents by mimicking the global structure, function and mechanism of HDPs.
DESIGN AND SYNTHESIS OF AAPEPTIDES
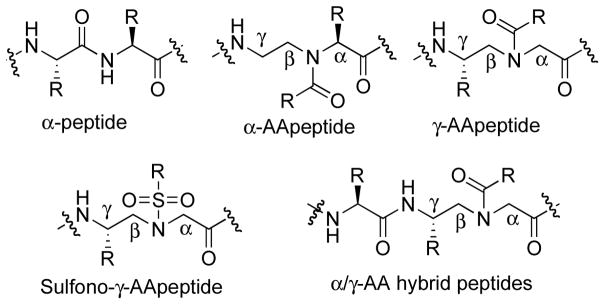
AApeptides have emerged as a new class of peptidomimetics [26–27]. (Fig. 2). They are termed “AApeptides”, [26–27] because they are oligomers of N-Acylated-N-Aminoethyl amino acid (Fig. 2) derived from chiral peptide nucleic acid (PNA) backbones [28–30]. AApeptides can be sub-classified into α-AApeptides and γ-AApeptides based on the different positions of the side chains. In the repeating unit of AApeptides, the chiral side chain is connected to either the α-C or γ-C with respect to the carbonyl group, whereas the other side chain is introduced to the central N through acylation. As each building block in AApeptides is comparable a dipeptide residue in length, AApeptides contain the same number of functional groups compared with conventional peptides of the same lengths. Additionally, as half of the side chains in AApeptides are introduced through acylation by carboxylic acids or acyl chlorides, there is limitless potential for chemodiversity of AApeptides.
Fig. 2.
General structures of canonical α-peptides, α-AApeptides, γ-AApeptides, sulfono-γ-AApeptides, and α/γ-AA hybrid peptides.
AApeptides have already shown great promise in many biological applications. Akin to other classes of peptidomimetics, AApeptides are highly resistant to enzymatic degradation [26–27, 31]. In addition, they also exhibit potential anti-cancer activity by modulating p53/MDM2 interactions [26–27], and have been developed as valuable tracer for targeted imaging in vivo [31]. Meanwhile, they can also mimic the Tat peptide and tightly bind to HIV-1 RNA [32], and act as cargo-carrier to cross mammalian cellular membrane [33–34]. It is important to note that certain AApeptides could also form unique nanostructures including nanoparticles and nanorods, suggesting their further application in the field of biomaterial science [35]. Recently, AApeptides have also been developed to exhibit broad-spectrum antimicrobial activity [20–22, 35–37, 55–60].
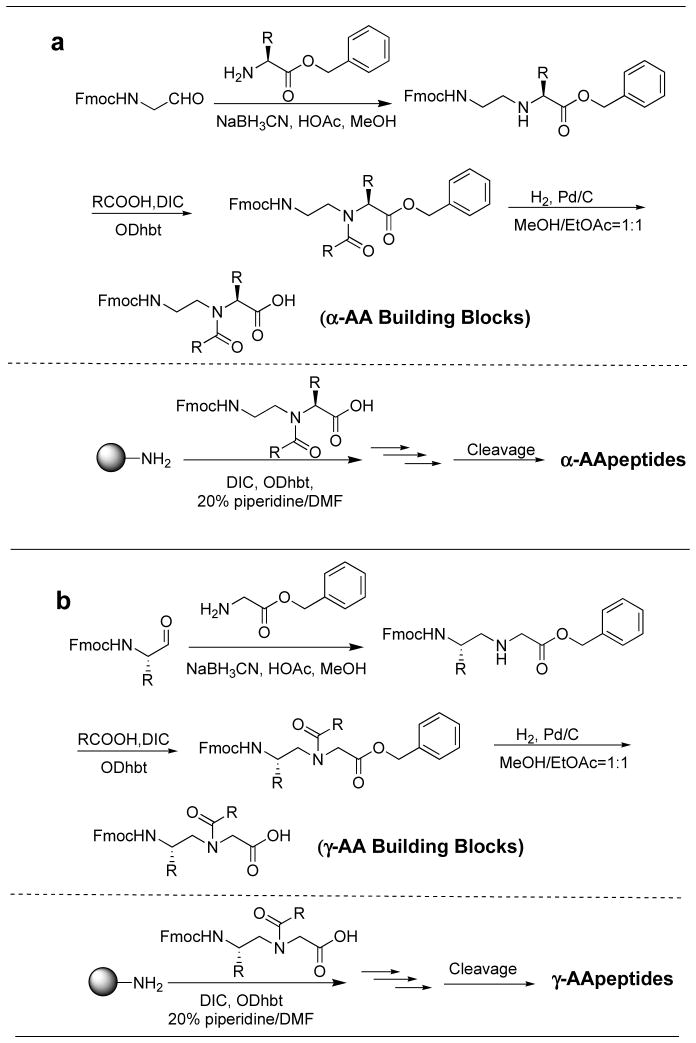
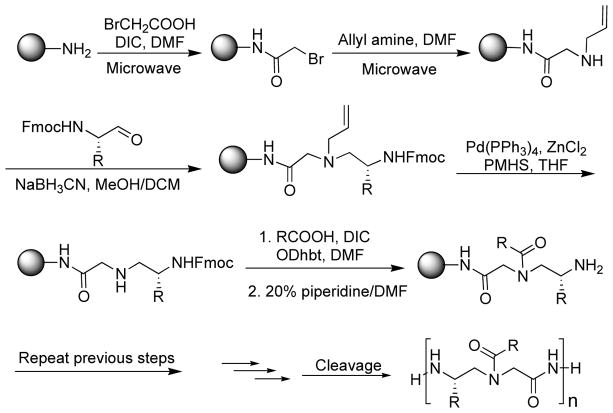
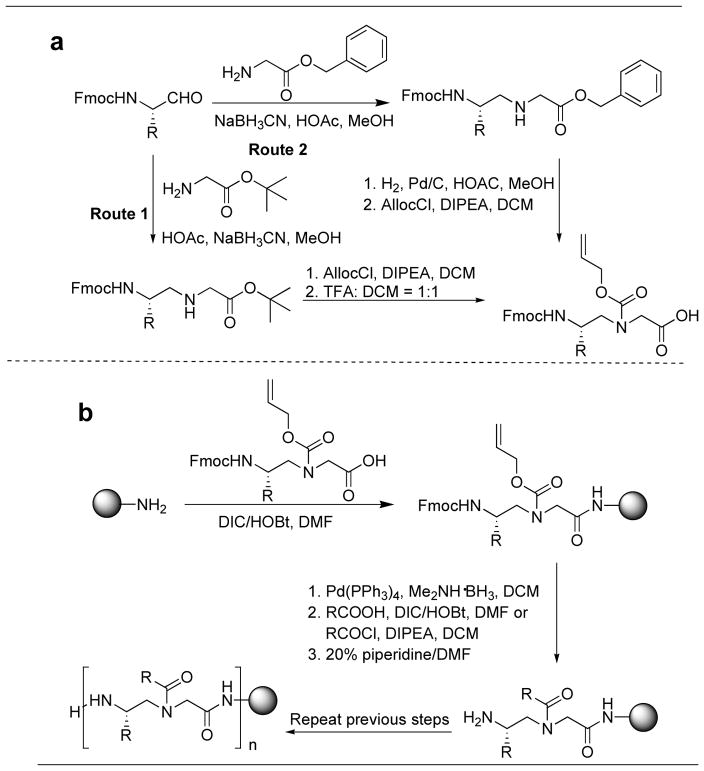
Initially, AApeptides were synthesized by the building block strategy as shown in Fig. (3). Fmoc protected AApeptide building blocks were initially prepared, and then assembled on solid support to afford desired AApeptide sequences [26–27, 31–34]. However, this approach is not straightforward when extensively diversified sequences are needed. Recently, we developed a new method termed submonomeric approach for the preparation of γ-AApeptides (Fig. 4) [36]. This new approach does not require the preparation of building blocks containing two pre-determined side chains, and thus different functional groups can be easily introduced to γ-AApeptide sequences. However, the synthesis on the solid phase is tedious. Our latest strategy for the preparation of γ-AApeptides is the combination of these two types of approaches, in which alloc protected γ-AApeptide building block are prepared and used to synthesize various γ-AApeptides much more conveniently (Fig. 5) [61]. The similar strategy is anticipated to also apply to the synthesis of different α-AApeptides.
Fig. 3.
Synthesis of AApeptides. a, Synthesis of α-AApeptide building blocks and α-AApeptides; b, Synthesis of γ-AApeptide building blocks and γ-AApeptides. ODhbt = 3-Hydroxy-4-oxo-3,4-dihydro-1,2,3-benzotriazine.
Fig. 4.
The submonomeric approach for the synthesis of γ-AApeptides.
Fig. 5.
Synthesis of γ-AA peptides. a, synthesis of N-alloc γ-AApeptide building block. b, synthesis of γ-AApeptide sequence; Alloc = allyloxycarbonyl.
Design of Antimicrobial AApeptides
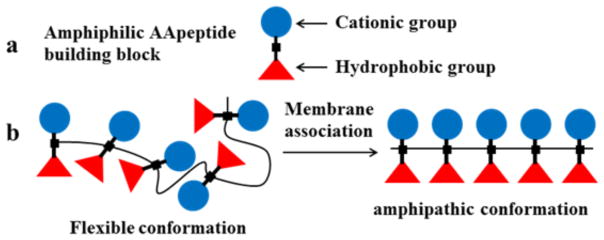
Due to the limited conformational backbone flexibility [20, 22], high stability [26–27, 31] and unlimited derivatizing potential [26–27], we envisioned that AApeptides may be the privileged molecular framework for designing novel antimicrobial agents that mimic structure, function and mechanism of HDPs. Compared to canonical peptides, AApeptides have more dihedral angles, leading to increased rotational freedom. As such, we initially hypothesized that antimicrobial AApeptides could be designed as homo-oligomers of amphipathic AApeptide building blocks. AApeptides containing amphiphilic building blocks are expected to adopt globally amphipathic structures upon association with bacterial membranes (Fig. 6). Fine tuning of these antimicrobial activity and selectivity is readily achieved by varying the ratio and nature of the hydrophobic and cationic groups. Based on this hypothesis, a series of antimicrobial α-AApeptides and γ-AApeptides were designed to mimic HDPs.
Fig. 6.
Schematic representation of the design of antimicrobial AApeptides. a, the basic components of amphiphilic AApeptide building blocks; b, the formation of amphipathic AApeptides upon association with bacterial membranes. Reproduced with permission from ref 58. Copyright 2016 Royal Society of Chemistry.
ANTIMICROBIAL α-AAPEPTIDES
Linear Antimicrobial α-AApeptides
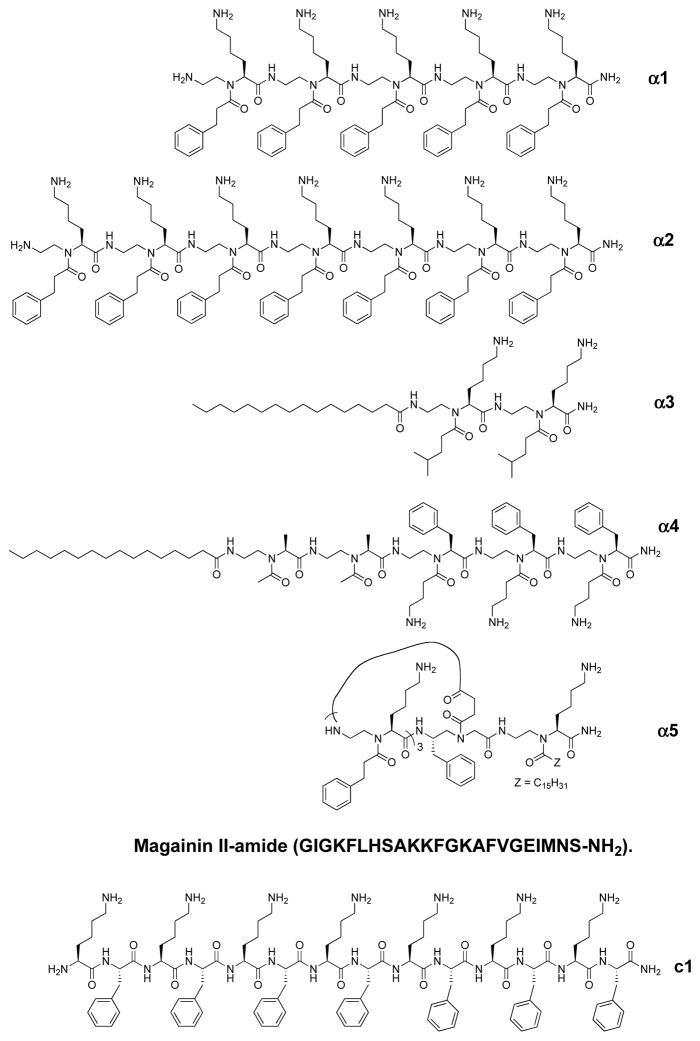
We initially started with the design of linear α-AApeptide sequences. A focused library including α1 and α2 was made (Fig. 7) [22]. These linear sequences were believed to mimic natural linear HDPs, and they were designed with the conception that, although globally amphipathic structures are critical for activity, well-defined secondary structures should not impact the potency of antimicrobial agents [19, 24–25]. As expected, although sequences consisting of 1–4 building blocks were not active [22], the sequence α1, containing five amphiphilic building blocks, was active against Bacillus subtilis and Staphylococcus epidermidis (Fig. 7 and Table 1), consistent to our hypothesis shown in Fig. (6). Inspired by these results, we next studied the activity of the longer sequence containing seven amphiphilic building blocks. To our delight, the more potent antimicrobial AApeptide α2 was identified to show good activity toward both Gram-positive and Gram-negative strains [22]. Mechanistic studies were conducted for α2 subsequently. Fluorescence microscopy and SEM micrographs analysis suggested α2 probably eradicate bacteria by disrupting bacterial membranes [22]. In addition, both α1 and α2 exhibited excellent activity toward bacteria because none of them has any hemolytic activity up to 250 μg/mL. Intriguingly, the regular peptide c1, which is composed of alternating Phe and Lys residues, only showed very weak antibacterial activity at the tested concentrations, echoing the necessity for the presence of global amphipathic structure. It suggests that the unique linear AApeptide backbone leads to a stronger capability to disrupt bacterial membranes than conventional peptides. In addition, the results indicate that the number of cationic and hydrophobic groups greatly affects antimicrobial activity, even though the global amphipathicity can be achieved in sequences. In summary, these early results proved that the straightforward design and modular programmability for the development of antimicrobial α-AApeptides.
Fig. 7.
The structures of antimicrobial α-AApeptides, magainin and the regular peptide c1.
Table 1.
The antimicrobial and hemolytic activity of α-AApeptides. The microbial organisms used were Escherichia Coli (JM 109), Bacillus subtilis (BR151), methicillin-resistant Staphylococcus epidermidis (RP62A), vancomycin-resistant Enterococcus faecalis (ATCC 700802), methicillin-resistant Staphylococcus aureus (ATCC 33592), Klebsiella pneumoniae (ATCC 13383), and Pseudomonas aeruginosa (ATCC 27853). Magainin, and an α-peptide c1, bearing alternative Phe and Lys residues, were used for comparison in the experiment. “-” indicates no test was conducted.
| Organism | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| α1 | α2 | α3 | α4 | α5 | Magainin | α-peptide c1 | |
| E. coli | 13 | 5 | >50 | 30 | - | 40 | >100 |
| K. pneumoniae | >50 | >50 | >50 | 8 | 5 | - | >100 |
| P. aeruginosa | >50 | >50 | >50 | 8 | 10 | - | >100 |
| B. subtilis | 8 | 2 | 2 | 2 | - | 40 | >100 |
| S. epidermidis (MRSE) | 20 | 10 | 10 | 4 | 1 | >50 | >100 |
| E. faecalis (VRE) | >50 | >50 | 1 | 4 | 1 | - | >100 |
| S. aureus (MRSA) | >50 | >50 | 5 | 4 | 4 | - | >100 |
| Hemolysis (HC50) | >250 | >250 | >250 | >250 | 150 | >250 | >100 |
LIPO ANTIMICROBIAL α-AAPEPTIDES
We next made our efforts in the development of lipo α-AApeptides, in order to study the impact of lipid tails on the activity of α-AApeptides [37]. It is widely known that the lipopeptide antibiotics are heavily dependent on their lipid tails for antibacterial activity, those lipid tails are responsible for penetration and disruption of bacterial membranes. [38] Hence, even with smaller number of cationic and hydrophobic groups, lipidated sequences might be even more active in bacteria killing than non-lipidated linear sequences [39]. Notably, although naturally occurring [40–42] lipopeptides are frequently used as antibiotic agents, their antimicrobial mechanisms are not same as HDPs. This is because natural lipopeptides are generated during metabolic processes in microbes [38, 43]. Their charges could cationic [44], neutral [45], or even anionic. It is also why many natural lipo-antibiotics do not exhibit broad-spectrum activity.
As overall structures of lipo α-AApeptides are still cationic and globally amphipathic, we hypothesized that they should also mimic the antimicrobial mechanism of HDPs. Therefore, they are expected to exert bactericidal activity by disrupting membranes of both Gram-positive and Gram-negative bacteria. Indeed, alkylation improved the hydrophobicity of positively charged sequences and reinforced the interaction with cytoplasmic membranes, and thereby greatly enhanced the antibacterial activity of α-AApeptides. For instance, α3 [37], containing just two amphiphilic building blocks, has excellent activity toward Gram-positive bacteria (Table 1 and Fig. 7). Fine tuning by including one amphiphilic building block and two hydrophobic building blocks led to the identification of lipo-α-AApeptide α4 that was active against all tested strains [37]. Fluorescence microscopy indicates that similar to HDPs, lipo-α-AApeptides could also kill bacteria by acting on bacterial membranes [37].
CYCLIC ANTIMICROBIAL α-AAPEPTIDES
We conceived to further modify α-AApeptides by introducing both cyclization and lipidation. These newly developed lipocyclic α-AApeptides demonstrated important improvements in antimicrobial activity against both Gram-positive and Gram-negative bacteria [46]. The design was straightforward. Amphipathic α-AApeptide building blocks were used to form cyclic α-AApeptides of different sizes. Lipid tails were attached to cyclic rings [46]. Interestingly, Lipocyclic α-AApeptides contain three amphiphilic building blocks and a C6 lipid tail did not inhibit bacterial growth. However, the sequence comprised of the same ring structure, a C12 lipid tail displayed activity against Gram-positive bacteria. Inclusion of the lipid tail C16 led to the identification of α5 (Table 1 and Fig. 7), which showed excellent activity toward a range of bacteria. The reason why sequences with shorter tails show less potent activity may be due to incapability of short lipid tails to penetrate bacterial membranes. Since Gram-negative bacteria have both outer and inner membranes, these sequences were even less active. It should be noted that the large ring size does not always ensure more potent antimicrobial activity. In fact, α5 has a relatively smaller ring size yet exhibited the most potent and broad-spectrum activity, particularly toward Gram-positive bacteria. We anticipated that α5 may have the optimal ring size. Indeed, it is known that many natural cyclic antimicrobial peptides have rings of six to eight amino acid residues [44]. Again, the subsequent fluorescence microscopic studies suggest lipocyclic α-AApeptides could compromise the membranes of bacteria, akin to HDPs. Compared to the linear α-AApeptide and pexiganan, the HDP derivatives, this new class of lipocyclic α-AApeptides eradicates bacteria with enhanced potency and broader-spectrum activity. Additionally, it should be mentioned that the lead lipocyclic α-AApeptide has been shown to mimic HDPs to modulate immune response. This was observed by their ability to antagonize TLR4-induced nuclear factor kappalight-chain-enhancer of activated B cells (NF-κB), and to inhibit production of TNF-α [47–48]. It is interesting that linear analogues did not show effective anti-inflammatory activity, which suggests that both lipidation and cyclization are of importance for antimicrobial and immunomodulatory activity [46].
ANTIMICROBIAL γ-AAPEPTIDES
Linear Antimicrobial γ-AApeptides
The exploration of antimicrobial α-AApeptides shed light on the design of antimicrobial γ-AApeptides, since they have similar molecular frameworks. In our early effort, we designed and synthesized a series of linear γ-AApeptide sequences based on the design principle for antimicrobial α-AApeptides, and tested their activity toward a panel of Gram-negative and Gram-positive bacteria (Table 2 and Fig. 8) [20].
Table 2.
The antimicrobial and hemolytic activities of γ-AApeptides. Bacterial strains are same as strains shown in Table 1. “-” indicates no test was carried out.
| Organism | MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| γ1 | γ2 | γ3 | γ4 | γ5 | γ6 | γ7 | γ8 | |
| E. coli | 5 | 5 | 5 | 5 | - | 2 | 4 | 4 |
| K. pneumoniae | >50 | 5 | 5 | 3 | 8 | 3 | - | - |
| P. aeruginosa | >50 | >50 | 5 | 3 | 8 | 5 | 6 | 4 |
| B. subtilis | 3 | 2 | 3 | 3 | 1 | - | - | - |
| S. epidermidis (MRSE) | 8 | 5 | 4 | 3 | 2 | 1 | 2 | 2 |
| E. faecalis (VRE) | 15 | 5 | 5 | 4 | 5 | 2 | 2 | 2 |
| S. aureus (MRSA) | 15 | 5 | 4 | 3 | 1 | 1 | 4 | 2 |
| Hemolysis (HC50) | >500 | 300 | >500 | >500 | 100 | 100 | 75 | 100 |
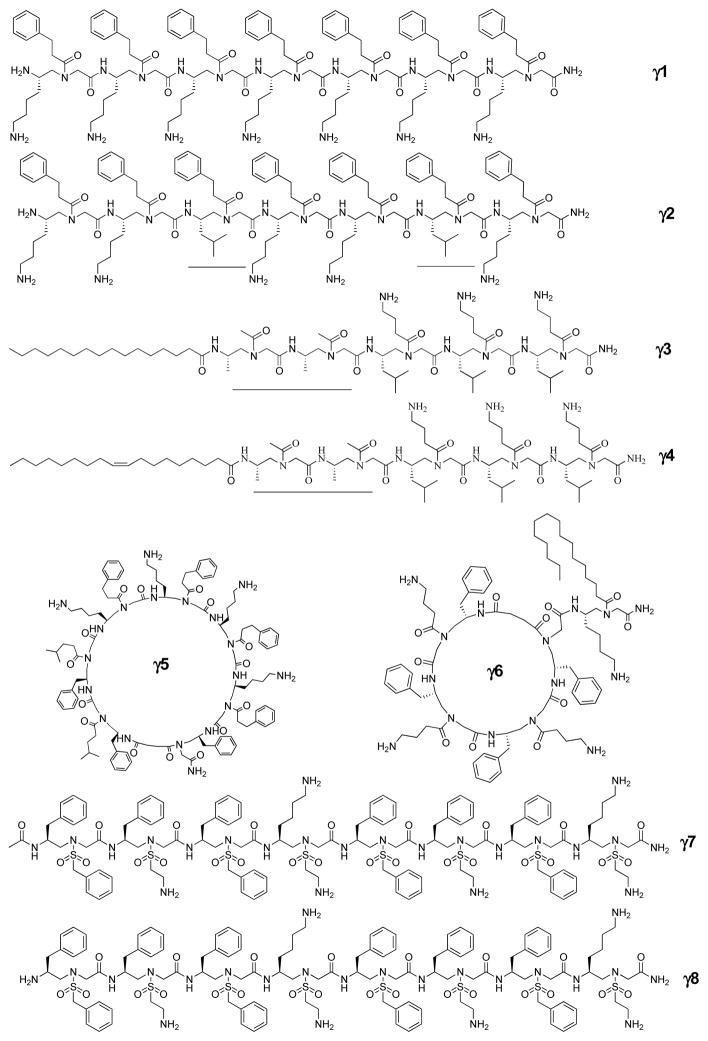
Fig. 8.
The representative antimicrobial γ-AApeptides. The hydrophobic building blocks are marked by lines.
As predicted, the γ-AApeptide γ1 (Table 2 and Fig. 8) which is comprised of seven amphiphilic building blocks exhibited good activity against Gram-positive bacteria. Similar to α-AApeptides, γ-AApeptides containing less amphiphilic building blocks showed much weaker activity [20], suggesting that the numbers of cationic and hydrophobic groups are all important for effective disruption of bacterial membranes [20]. It was also noted that when the overall hydrophobicity increases, the antimicrobial activity of γ-AApeptides could also be improved; however, it may also increase hemolytic activity and cytotoxicity. Intriguingly, the hemolytic activity and cytotoxicity could be mitigated if more cationic charges are introduced. Therefore, it is envisioned that more potent and selective antimicrobial γ-AApeptides could be identified through fine tuning. Indeed, substitution of two amphiphilic building blocks in γ1 with two hydrophobic building blocks led to discovery of more potent antimicrobial γ-AApeptide γ2 [21]. Although γ2 was still not active toward P. aeruginosa, it effectively killed both Gram-positive and other Gram-negative strains, including multidrug resistant pathogens. It should be mentioned that it even inhibited the growth of the USA100 lineage MRSA strain. It is significant as this strain has acquired resistance to most conventional antibiotics. Moreover, γ2 could also arrest the growth of B. anthracis, which is the notorious strain for causing lethal condition, anthrax. These results suggest that antimicrobial γ-AApeptides could be developed for bio-defense in the future. Furthermore, γ1 and γ2 only exhibit limited toxicity, as their HC50’s are all more than 300 μg/mL. It is reasonable as mammalian cell membranes are zwitterionic, whereas bacterial membrane surface is embedded with negative charges that favor for electrostatic interactions with γ-AApeptides. Mechanistic studies such as fluorescence microscopy and drug resistance studies all suggested that γ2 may mimic mechanism of action of HDPs and kill bacterial pathogens by disrupting bacterial membranes [20].
Lipo Antimicrobial γ-AApeptides
Similarly to that of lipo α-AApeptides, we hypothesized that lipo γ-AApeptides could also be employed for antimicrobial development. As they were designed to mimic HDPs, they were expected to display broad-spectrum activity. Indeed, the best leads, γ3 and γ4, were identified (Table 2 and Fig. 8) [21]. Interestingly, γ4, the sequence with more potent and broader-spectrum activity than γ3 toward all bacterial strains, was less hemolytic. We reasoned such a favorable selection for bacteria may be due to the higher sensitivity of bacterial membranes to unsaturated alkyl tails in comparison to mammalian cells. The finding is significant as it may inspire the future development of antibiotic agents with lipid tails in the future. One in particular is that these lipo γ-AApeptides were more potent antimicrobial agents than the linear sequence γ2 [20], particularly towards the Gram-negative bacterium P. aeruginosa, and fungus C. albicans, even though they contain shorter lengths of γ-AApeptide fragments, further demonstrating the importance of lipidation. Subsequent fluorescence microscopy, membrane depolarization, as well as drug resistance studies are all in consistency with our hypothesis that lipo γ-AApeptides could compromise bacterial membranes, and mimic the mechanism of action of HDPs, and do not induce resistance in bacteria readily [21].
Cyclic Antimicrobial γ-AApeptides
We further extended our exploration to cyclic γ-AApeptides that mimic the structure and function of HDPs [49]. Cyclic peptide antibiotics such as gramicidin S, pro-tegrin I, tyrocidine, and polymyxin B are widely found in nature. The backbones of these cyclic peptides are more rigid than linear peptides, allowing substituents to be positioned with reduced rotational freedom [18]. It is envisioned that amphipathic cyclic peptides may possess more potent antimicrobial activity than linear peptides, as their structures are conformationally constrained. We therefore expected that cyclic antimicrobial γ-AApeptides could be designed based on the rational for the design of linear γ-AApeptides. We anticipated that the cyclization of amphiphilic building blocks would lead to the formation of globally amphipathic conformations which favors the interaction with bacterial membranes. Inclusion of extra hydrophobic building blocks was expected to enhance the antimicrobial activity. [20] This is seen for γ5 in Fig. 8 and Table 2 [49].
Lipo-Cyclic Antimicrobial γ-AApeptides
Based on our previous findings, cyclization could reduce conformational freedom and enhance interaction with bacterial membranes [49]. Lipidation could confer antimicrobial agent with ability to penetrate bacterial membranes. Indeed, natural lipocyclic peptides daptomycin and colistin are currently used as the “last-resort” antibiotics to treat bacterial infections. However, the effort devoted to the development of unnatural antimicrobial lipocyclic peptidomimetics is rare. Thus, we envisioned that γ-AApeptides with both cyclization and lipidation could have synergistic effect on killing bacterial pathogens. To test our hypothesis, a focused library of lipocyclic γ-AApeptides were designed. As expected, compared with cyclic γ-AApeptides without lipid tails, these lipocyclic γ-AApeptides (such as γ6) (Table 2 and Fig. 8) exhibited particularly more potent activity toward Gram-negative bacteria. Intriguingly, they blocked lipopolysaccharide (LPS) activated Toll-like receptor 4 (TLR4) signaling, which suggests that lipocyclic γ-AApeptides could modulate immune response and also directly kill bacterial pathogens. Moreover, our recent findings suggest that these lipocyclic γ-AApeptides could inhibit the formation of biofilm more effectively than conventional antibiotics, possibly because their lipid tails could penetrate and inhibit the growth of biofilms. Fluorescent microscopic studies again suggested that γ6 could arrest the growth of bacteria through the disruption of membranes of bacteria [49].
HELICAL ANTIMICROBIAL γ-AAPEPTIDES
As we recently discovered that sulfono-γ-AApeptides could form helical structure in solution, we next explored the ability of sulfono-γ-AApeptides to mimic the helical HDP magainin 2, in order to identify new antibiotics with novel mechanisms, so as to combat antibiotic resistance [50]. To date only a few classes of peptidomimetics have been investigated to mimic the helical folding and antibacterial activity of magainin 2, such as β-peptides [10, 51–52], peptoids [53], and oligoureas [54]. As a new class of unnatural helical foldamer and a subclass of γ-AApeptides, (Fig. 2) [55], sulfono-γ-AApeptides have been shown to adopt well-defined helical conformation in solution. Interestingly, they resemble α-helix well because they have similar helical pitches and diameters (Fig. 3) [55]. Since they are resistant to enzymatic degradation, sulfono-γ-AApeptides may overcome obstacles faced by magainin 2 for antibiotic development. Moreover, as half of their side chains could be introduced through the reaction of the backbone with a variety of sulfonyl chlorides, sulfono-γ-AApeptides are envisioned to exhibit enormous chemical diversity. [26, 31, 55–56]. Our early reported antimicrobial AApeptides [20–12, 49, 57–59] were designed based on the formation of extended structures based on amphiphilic building blocks, which interact with the membranes of bacteria. As such, it was intriguing to investigate if helical sulfono-γ-AApeptides could also be designed to mimic amphipathic structure and antibacterial activity of magainin 2, which may enable us to gain insight into the design of novel antibiotic agents. It should be noted that CD studies suggested that sulfono-γ-AApeptides possess high helical folding propensity, because pronounced helicity could be observed in the sequence comprised of as less as five building blocks [26, 55]. Interestingly, 2D-NMR studies indicate that unlike α-helix in which there are 3.6 residues per helical turn, sulfono-γ-AApeptides have a helical pattern of four side chains per turn, which makes the design of amphipathic helical structure straightforward. [55].
According to the above-mentioned structural model, a series of sulfono-γ-AApeptides of different lengths, cationic charges, and hydrophobicity were designed, in order to establish the principle that governs the structure–function relationship existing in sulfono-γ-AApeptides for potential application in antibiotic development. As expected, γ7 and γ8 possessed much more potent antimicrobial activity than pexiganan (Fig. 8, Table 2). Indeed, they are among the best antibacterial helical peptidomimetics reported to date [10, 19, 51–54]. The sequence γ8 lacks an acetyl group at the N-terminus and is less helical suggested by small angle X-ray scattering (SAXS), however, showed better antimicrobial activity as well as less hemolytic activity and cytotoxicity toward mammalian cells compared with γ7. This is consistent to the findings that defined secondary structures are not necessary for potent antimicrobial activity [19, 24–25].
Similarly to previous findings [20, 22], antimicrobial activities rely on the lengths of the sequences. The activity of sequence generally increases as the length of the sequence elongates. There are two factors which may account for the results. First, only when sulfono-γ-AApeptides contain five or more sulfono-γ-AApeptide building blocks, they could form discernible helical structures. Shorter sequences may be incapable of forming helical conformations in solution, thus no amphipathic structure is present for membrane interaction. In addition, antibacterial activity of HDPs are largely dependent on the number of cationic charges and hydrophobic groups. Sequences normally fail to selectively bind and interact with bacterial membranes with insufficient hydrophilicity and hydrophobicity. In order to possess good activity, HDPs usually need > +5 charges [3].
Notably, in addition to amphipathicity, the nature of hydrophobic groups also contributes enormously to the activity. Sequences containing isopropyl groups instead of phenyl groups found in γ7 and γ8 show significantly impaired activity. We reasoned that due to the less hydrophobicity of the isopropyl group than the aromatic phenyl moiety, the sequences may not arise strong interactions with membranes of bacteria.
The subsequent mechanistic studies including time-killing and fluorescence microscopic experiments are all in agreement with the hypothesis that sulfono-γ-AApeptides could compromise membranes of both Gram-positive and Gram-negative bacteria, analogues to the bactericidal mechanism of HDPs. Moreover, this class of antibacterial foldamer is resistant to proteolytic hydrolysis. The findings could direct the design of a new class of foldameric agents combating antibiotic resistance [50].
α/γ-AA Hybrid Peptides as Antimicrobial Agents
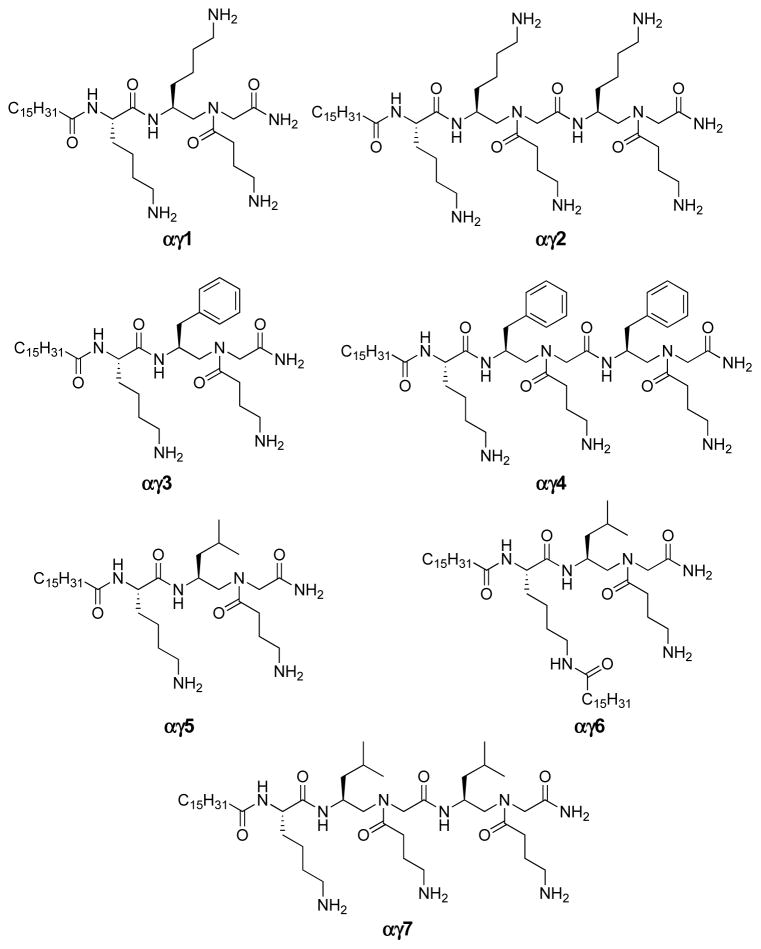
We also utilized a hybrid backbone containing both canonical α-peptide units and γ-AApeptide building blocks to generate short hybrid antimicrobial peptides so as to further develop antimicrobial agents [60]. Previous findings suggest short peptides or peptoids with lipid tails could lead to improved antibiotic agents [38, 62], as the addition of a hydrophobic tail increases lipophilicity of the sequences and thereby enhancing their interaction with bacterial membranes. Based on the above perception, short sequences might be active with the inclusion of a lipid tail. In addition, the recent studies of antibacterial hybrid peptidic oligomers revealed good potency and selectivity [13, 63]. Although their antimicrobial mechanism is elusive, the findings suggested that short lipidated hybrid peptidic oligomers may exhibit favorable activing and selectivity toward bacteria. To confirm our hypothesis, we designed a series of α/γ-AA hybrid oligomers (Table 3 and Fig. 9). These peptides are comprised of γ-AApeptide building blocks and one lysine amino acid residue. Each γ-AA peptide building block contains either two cationic charges, or one cationic group and one hydrophobic group. Therefore, their global amphipathic structures are expected to mimic HDPs. Lipidation was achieved by attaching one or two C16 lipid tails to the α- or at both the α- and ε-NH2 groups in the lysine residue.
Table 3.
The antimicrobial and hemolytic activities of α/γ-AA hybrid peptides. The bacterial strains are same as those in Table 1.
| Organism | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| αγ1 | αγ2 | αγ3 | αγ4 | αγ5 | αγ6 | αγ7 | |
| E. coli | 5 | 3 | 4 | 4 | 2 | 25–50 | 4 |
| P. aeruginosa | 2 | 2 | 2 | 2 | 2 | 3 | 2 |
| S. epidermidis (MRSE) | 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| E. faecalis (VRE) | 5 | 4 | 2 | 2 | 2 | 2 | 2 |
| S. aureus (MRSA) | 4 | 4 | 2 | 2 | 2 | 2 | 2 |
| Hemolysis (H50) | >500 | >500 | 150 | 250 | 100 | 400 | 350 |
Fig. 9.
The representative antimicrobial α/γ-AA hybrid peptides.
To our delight, despite the short sequence, αγ1 showed excellent activity toward an array of pathogens (Table 3 and Fig. 9). Critically, αγ1 was highly selective for various bacteria. Interesting, αγ1 was superior to pexiganan in terms of both activity and hemolytic activity. Moreover, the selectivity is also improved over previously developed lead lipo-γ-AApeptide [21]. Both antibacterial activity and hemolytic activity of these hybrid oligomers could also be adjusted by varying charges and hydrophobicity of the sequences. For example, bearing one more hydrophilic γ-AApeptide building block, α/γ-AA hybrid peptide αγ2 displayed enhanced selectivity compared with αγ1 (Table 3). With a decreased ratio of hydrophobicity to hydrophilicity, αγ2 still retained antibacterial activity without inducing hemolysis. The results also confirmed again that the increase in hydrophobicity enhances antimicrobial activity, as seen for αγ3, αγ4, αγ5, and αγ7. Compared to αγ1 and αγ2, these compounds with increased hydrophobicity show better antimicrobial activity. This is in consistency with the previous conception that increased hydrophobicity correlates to increased antimicrobial activity, although hydrophobicity is accompanied with toxicity, which compromises the selectivity. It is also interesting that most hybrid peptidic oligomers bearing two C16 alkyl tails failed to display good activity against any bacteria. Although αγ6 exhibited activity against a few bacterial strains, its activity was not as broad-spectrum as that of hybrid peptides containing one tail. We reasoned that they may form stable micelle structures which do not dissociate easily on bacterial membranes.
The following mechanistic studies also suggest that by permeating and damaging bacterial membranes the lipo-α/γ-AA hybrid peptidic oligomers could resemble HDPs and rapidly eradicate bacteria. We envision that lipo-α/γ-AA hybrid peptides could hold promise to combat drug resistance due to their simplicity and high selectivity.
CONCLUSION
Last two decades have witnessed the emerging development of antimicrobial peptidomimetics. One of the most successful examples is the antimicrobial arylamides developed by DeGrado et al., which are currently in Phase III clinical trials [1]. As a new class of peptidomimetics, AApeptides have demonstrated their potential as antimicrobial agents that mimic structure and function of HDPs. Akin to other classes of peptidomimetics, AApeptides have enhanced stability, enormous potential for derivatization, and straightforward design for optimization. The future development of AApeptides for antimicrobial application could be taken on the following aspects. First of all, in order to obtain more potent and selective AApeptides, different functional groups will have to be investigated. In the meantime, since generally it is more challenging to develop antimicrobial agents to kill Gram-negative bacteria that are believed to be more critical at the moment, more research focus should be given to development of antimicrobial AApeptides that have more potent activity against Gram-negative bacteria. Furthermore, in vivo efficacy of these peptidomimetics on the mouse model should be carried out in the near future, so as to evaluate the potential of AApeptides as a new generation of antibiotic agents combating drug-resistance.
Acknowledgments
This work was supported by NSF CAREER award (1351265) and NIH 1R01GM112652-01A1.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.America IDSO. Combating Antimicrobial Resistance: Policy Recommendations to Save Lives. Clin Infect Dis. 2011;52(suppl 5):S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marr AK, Gooderham WJ, Hancock REW. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6(5):468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 4.Choi S, Isaacs A, Clements D, Liu D, Kim H, Scott RW, Winkler JD, DeGrado WF. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc Natl Acad Sci U S A. 2009;106(17):6968–6973. doi: 10.1073/pnas.0811818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tew GN, Scott RW, Klein ML, DeGrado WF. De Novo Design of Antimicrobial Polymers, Foldamers, and Small Molecules: From Discovery to Practical Applications. Acc Chem Res. 2010;43(1):30–39. doi: 10.1021/ar900036b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RW, DeGrado WF, Tew GN. De novo designed synthetic mimics of antimicrobial peptides. Curr Opin Biotechnol. 2008;19(6):620–627. doi: 10.1016/j.copbio.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu M, Maier E, Benz R, Hancock REW. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli. Biochemistry. 1999;38(22):7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer RI, Lu W. α-Defensins in human innate immunity. Immunol Rev. 2012;245(1):84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 9.Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevitz D, Zuckermann RN, Barron AE. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc Natl Acad Sci U S A. 2008;105(8):2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson AJ, Pomerantz WC, Weisblum B, Gellman SH, Palecek SP. Antifungal Activity from 14-Helical β-Peptides. J Am Chem Soc. 2006;128(39):12630–12631. doi: 10.1021/ja064630y. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson AJ, Pomerantz WC, Neilsen KJ, Gellman SH, Palecek SP. Effect of Sequence and Structural Properties on 14-Helical β-Peptide Activity against Candida albicans Planktonic Cells and Biofilms. ACS Chem Biol. 2009;4(7):567–579. doi: 10.1021/cb900093r. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson AJ, Flessner RM, Gellman SH, Lynn DM, Palecek SP. Polyelectrolyte Multilayers Fabricated from Anti-fungal β-Peptides: Design of Surfaces that Exhibit Antifungal Activity Against Candida albicans. Biomacromolecules. 2010;11(9):2321–2328. doi: 10.1021/bm100424s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen CA, Ziegler HL, Nielsen HM, Frimodt-Møller N, Jaroszewski JW, Franzyk H. Antimicrobial, Hemolytic, and Cytotoxic Activities of β-Peptoid–Peptide Hybrid Oligomers: Improved Properties Compared to Natural AMPs. ChemBioChem. 2010;11(10):1356–1360. doi: 10.1002/cbic.201000232. [DOI] [PubMed] [Google Scholar]
- 14.Huang ML, Shin SBY, Benson MA, Torres VJ, Kirshenbaum K. A Comparison of Linear and Cyclic Peptoid Oligomers as Potent Antimicrobial Agents. ChemMedChem. 2012;7(1):114–122. doi: 10.1002/cmdc.201100358. [DOI] [PubMed] [Google Scholar]
- 15.Hua J, Scott RW, Diamond G. Activity of antimicrobial peptide mimetics in the oral cavity: II. Activity against periopathogenic biofilms and anti-inflammatory activity. Mol Oral Microbiol. 2010;25(6):426–432. doi: 10.1111/j.2041-1014.2010.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua J, Yamarthy R, Felsenstein S, Scott RW, Markowitz K, Diamond G. Activity of antimicrobial peptide mimetics in the oral cavity: I. Activity against biofilms of Candida albicans. Mol Oral Microbiol. 2010;25(6):418–425. doi: 10.1111/j.2041-1014.2010.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RLA, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Käch A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. Peptidomimetic Antibiotics Target Outer-Membrane Biogenesis in Pseudomonas aeruginosa. Science. 2010;327(5968):1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 18.Obrecht D, Robinson JA, Bernardini F, Bisang C, DeMarco SJ, Moehle K, Gombert FO. Recent Progress in the Discovery of Macrocyclic Compounds as Potential Anti-Infective Therapeutics. Curr Med Chem. 2009;16(1):42–65. doi: 10.2174/092986709787002844. [DOI] [PubMed] [Google Scholar]
- 19.Claudon P, Violette A, Lamour K, Decossas M, Fournel S, Heurtault B, Godet J, Mély Y, Jamart-Grégoire B, Averlant-Petit MC, Briand JP, Duportail G, Monteil H, Guichard G. Consequences of Isostructural Main-Chain Modifications for the Design of Antimicrobial Foldamers: Helical Mimics of Host-Defense Peptides Based on a Heterogeneous Amide/Urea Backbone. Angew Chem, Int Ed. 2010;49(2):333–336. doi: 10.1002/anie.200905591. [DOI] [PubMed] [Google Scholar]
- 20.Niu Y, Padhee S, Wu H, Bai G, Harrington L, Burda WN, Shaw LN, Cao C, Cai J. Identification of γ-AApeptides with potent and broad-spectrum antimicrobial activity. Chem Commun. 2011;47(44):12197–12199. doi: 10.1039/c1cc14476f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu Y, Padhee S, Wu H, Bai G, Qiao Q, Hu Y, Harrington L, Burda WN, Shaw LN, Cao C, Cai J. Lipo-γ-AApeptides as a New Class of Potent and Broad-Spectrum Antimicrobial Agents. J Med Chem. 2012;55(8):4003–4009. doi: 10.1021/jm300274p. [DOI] [PubMed] [Google Scholar]
- 22.Padhee S, Hu Y, Niu Y, Bai G, Wu H, Costanza F, West L, Harrington L, Shaw LN, Cao C, Cai J. Non-hemolytic α-AApeptides as antimicrobial peptidomimetics. Chem Commun. 2011;47(34):9729–9731. doi: 10.1039/c1cc13684d. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt MA, Weisblum B, Gellman SH. Interplay among Folding, Sequence, and Lipophilicity in the Antibacterial and Hemolytic Activities of α/β-Peptides. J Am Chem Soc. 2007;129(2):417–428. doi: 10.1021/ja0666553. [DOI] [PubMed] [Google Scholar]
- 24.Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. Mimicry of Antimicrobial Host-Defense Peptides by Random Copolymers. J Am Chem Soc. 2007;129(50):15474–15476. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 25.Ivankin A, Livne L, Mor A, Caputo GA, DeGrado WF, Meron M, Lin B, Gidalevitz D. Role of the Conformational Rigidity in the Design of Biomimetic Antimicrobial Compounds. Angew Chem, Int Ed. 2010;49(45):8462–8465. doi: 10.1002/anie.201003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Y, Hu Y, Li X, Chen J, Cai J. γ-AApeptides: design, synthesis and evaluation. New J Chem. 2011;35(3):542–545. [Google Scholar]
- 27.Hu Y, Li X, Sebti SM, Chen J, Cai J. Design and synthesis of AApeptides: A new class of peptide mimics. Bioorg Med Chem Lett. 2011;21(5):1469–1471. doi: 10.1016/j.bmcl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Dragulescu-Andrasi A, Rapireddy S, Frezza BM, Gayathri C, Gil RR, Ly DH. A Simple γ-Backbone Modification Pre-organizes Peptide Nucleic Acid into a Helical Structure. J Am Chem Soc. 2006;128(31):10258–10267. doi: 10.1021/ja0625576. [DOI] [PubMed] [Google Scholar]
- 29.Tedeschi T, Sforza S, Corradini R, Marchelli R. Synthesis of new chiral PNAs bearing a dipeptide-mimic monomer with two lysine-derived stereogenic centres. Tetrahedron Lett. 2005;46(48):8395–8399. [Google Scholar]
- 30.Falkiewicz B, Kowalska K, Kolodziejczyk AS, Wiśniewski K, łankiewicz L. Synthesis of New Chiral Peptide Nucleic Acid (PNA) Monomers by a Simplified Reductive Animation Method. Nucleosides Nucleotides. 1999;18(3):353–361. [Google Scholar]
- 31.Yang Y, Niu Y, Hong H, Wu H, Zhang Y, Engle JW, Barnhart TE, Cai J, Cai W. Radiolabeled γ-AApeptides: a new class of tracers for positron emission tomography. Chem Commun. 2012;48(63):7850–7852. doi: 10.1039/c2cc33620k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu Y, Jones AJ, Wu H, Varani G, Cai J. γ-AApeptides bind to RNA by mimicking RNA-binding proteins. Org Biomol Chem. 2011;9(19):6604–6609. doi: 10.1039/c1ob05738c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu Y, Bai G, Wu H, Wang RE, Qiao Q, Padhee S, Buzzeo R, Cao C, Cai J. Cellular Translocation of a γ-AApeptide Mimetic of Tat Peptide. Mol Pharm. 2012;9(5):1529–1534. doi: 10.1021/mp300070w. [DOI] [PubMed] [Google Scholar]
- 34.Bai G, Padhee S, Niu Y, Wang RE, Qiao Q, Buzzeo R, Cao C, Cai J. Cellular uptake of an α-AApeptide. Org Biomol Chem. 2012;10(6):1149–1153. doi: 10.1039/c2ob06679c. [DOI] [PubMed] [Google Scholar]
- 35.Niu Y, Wu H, Huang R, Qiao Q, Costanza F, Wang XS, Hu Y, Amin MN, Nguyen AM, Zhang J, Haller E, Ma S, Li X, Cai J. Nanorods Formed from a New Class of Peptidomimetics. Macromolecules. 2012;45(18):7350–7355. [Google Scholar]
- 36.Wu H, Amin MN, Niu Y, Qiao Q, Harfouch N, Nimer A, Cai J. Solid-Phase Synthesis of γ-AApeptides Using a Submonomeric Approach. Org Lett. 2012;14(13):3446–3449. doi: 10.1021/ol301406a. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Amin MN, Padhee S, Wang RE, Qiao Q, Bai G, Li Y, Mathew A, Cao C, Cai J. Lipidated Peptidomimetics with Improved Antimicrobial Activity. ACS Med Chem Lett. 2012;3(8):683–686. doi: 10.1021/ml3001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makovitzki A, Avrahami D, Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci U S A. 2006;103(43):15997–16002. doi: 10.1073/pnas.0606129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chongsiriwatana NP, Miller TM, Wetzler M, Vakulenko S, Karlsson AJ, Palecek SP, Mobashery S, Barron AE. Short alkylated peptoid mimics of antimicrobial lipopeptides. Antimicrob Agents Chemother. 2011;55(1):417–420. doi: 10.1128/AAC.01080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu G, Abraham T, Rapp J, Vastey F, Saad N, Balmir E. Daptomycin: evaluation of a high-dose treatment strategy. Int J Antimicrob Agents. 2011;38(3):192–196. doi: 10.1016/j.ijantimicag.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Beiras-Fernandez A, Vogt F, Sodian R, Weis F. Daptomycin: a novel lipopeptide antibiotic against Gram-positive pathogens. Infect Drug Resist. 2010;3:95–101. doi: 10.2147/IDR.S6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kvitko CH, Rigatto MH, Moro AL, Zavascki AP. Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother. 2011;66(1):175–179. doi: 10.1093/jac/dkq390. [DOI] [PubMed] [Google Scholar]
- 43.Makovitzki A, Baram J, Shai Y. Antimicrobial Lipopolypeptides Composed of Palmitoyl Di- and Tricationic Peptides: In Vitro and in Vivo Activities, Self-Assembly to Nanostructures, and a Plausible Mode of Action. Biochemistry. 2008;47(40):10630–10636. doi: 10.1021/bi8011675. [DOI] [PubMed] [Google Scholar]
- 44.Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60(6):1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 45.Morris MI, Villmann M. Echinocandins in the management of invasive fungal infections, part 1. Am J Health-Syst Pharm. 2006;63(18):1693–1703. doi: 10.2146/ajhp050464.p1. [DOI] [PubMed] [Google Scholar]
- 46.Padhee S, Smith C, Wu H, Li Y, Manoj N, Qiao Q, Khan Z, Cao C, Yin H, Cai J. The Development of Antimicrobial α-AApeptides that Suppress Proinflammatory Immune Responses. ChemBioChem. 2014;15(5):688–694. doi: 10.1002/cbic.201300709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowdish DME, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock REW. Impact of LL-37 on anti-infective immunity. J Leukocyte Biol. 2005;77(4):451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 48.Scott MG, Gold MR, Hancock REW. Interaction of Cationic Peptides with Lipoteichoic Acid and Gram-Positive Bacteria. Infect Immun. 1999;67(12):6445–6453. doi: 10.1128/iai.67.12.6445-6453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Niu Y, Padhee S, Wang RE, Li Y, Qiao Q, Bai G, Cao C, Cai J. Design and synthesis of unprecedented cyclic γ-AApeptides for antimicrobial development. Chem Sci. 2012;3(8):2570–2575. [Google Scholar]
- 50.Li Y, Wu H, Teng P, Bai G, Lin X, Zuo X, Cao C, Cai J. Helical Antimicrobial Sulfono-γ-AApeptides. J Med Chem. 2015;58(11):4802–4811. doi: 10.1021/acs.jmedchem.5b00537. [DOI] [PubMed] [Google Scholar]
- 51.Porter EA, Weisblum B, Gellman SH. Mimicry of Host-Defense Peptides by Unnatural Oligomers: Antimicrobial β-Peptides. J Am Chem Soc. 2002;124(25):7324–7330. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- 52.Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. Antibiotics: Non-haemolytic β-amino-acid oligomers. Nature. 2000;404(6778):565–565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 53.Patch JA, Barron AE. Helical Peptoid Mimics of Magainin-2 Amide. J Am Chem Soc. 2003;125(40):12092–12093. doi: 10.1021/ja037320d. [DOI] [PubMed] [Google Scholar]
- 54.Violette A, Fournel S, Lamour K, Chaloin O, Frisch B, Bri-and JP, Monteil H, Guichard G. Mimicking Helical Antibacterial Peptides with Nonpeptidic Folding Oligomers. Chem Biol. 2006;13(5):531–538. doi: 10.1016/j.chembiol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Wu H, Qiao Q, Hu Y, Teng P, Gao W, Zuo X, Wojtas L, Larsen RW, Ma S, Cai J. Sulfono-γ-AApeptides as a New Class of Nonnatural Helical Foldamer. Chem -Eur J. 2015;21(6):2501–2507. doi: 10.1002/chem.201406112. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Li Y, Bai G, Niu Y, Qiao Q, Tipton JD, Cao C, Cai J. γ-AApeptide-based small-molecule ligands that inhibit Aβ aggregation. Chem Commun. 2014;50(40):5206–5208. doi: 10.1039/c3cc46685j. [DOI] [PubMed] [Google Scholar]
- 57.Niu Y, Wang RE, Wu H, Cai J. Recent development of small antimicrobial peptidomimetics. Future Med Chem. 2012;4(14):1853–1862. doi: 10.4155/fmc.12.111. [DOI] [PubMed] [Google Scholar]
- 58.Niu Y, Wu H, Li Y, Hu Y, Padhee S, Li Q, Cao C, Cai J. AApeptides as a new class of antimicrobial agents. Org Biomol Chem. 2013;11(26):4283–4290. doi: 10.1039/c3ob40444g. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Smith C, Wu H, Padhee S, Manoj N, Cardiello J, Qiao Q, Cao C, Yin H, Cai J. Lipidated Cyclic γ-AApeptides Display Both Antimicrobial and Anti-inflammatory Activity. ACS Chem Biol. 2014;9(1):211–217. doi: 10.1021/cb4006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Smith C, Wu H, Teng P, Shi Y, Padhee S, Jones T, Nguyen AM, Cao C, Yin H, Cai J. Short Antimicrobial Lipo-α/γ-AA Hybrid Peptides. ChemBioChem. 2014;15(15):2275–2280. doi: 10.1002/cbic.201402264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H, Teng P, Cai J. Rapid Access to Multiple Classes of Peptidomimetics from Common γ-AApeptide Building Blocks. Eur J Org Chem. 2014;2014(8):1760–1765. [Google Scholar]
- 62.Ghosh C, Manjunath GB, Akkapeddi P, Yarlagadda V, Hoque J, Uppu DSSM, Konai MM, Haldar J. Small Molecular Antibacterial Peptoid Mimics: The Simpler the Better! J Med Chem. 2014;57(4):1428–1436. doi: 10.1021/jm401680a. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt MA, Weisblum B, Gellman SH. Unexpected Relationships between Structure and Function in α,β-Peptides: Antimicrobial Foldamers with Heterogeneous Backbones. J Am Chem Soc. 2004;126(22):6848–6849. doi: 10.1021/ja048546z. [DOI] [PubMed] [Google Scholar]