Abstract
Short-term exposure to ambient air pollution is linked with adverse cardiovascular effects. While previous research focused primarily on particulate matter-induced responses, gaseous air pollutants also contribute to cause short-term cardiovascular effects. Mechanisms underlying such effects have not been adequately described, however the immediate nature of the response suggests involvement of irritant neural activation and downstream autonomic dysfunction. Thus, this study examines the role of TRPA1, an irritant sensory receptor found in the airways, in the cardiac response of mice to acrolein and ozone. Conscious unrestrained wild-type C57BL/6 (WT) and TRPA1 knockout (KO) mice implanted with radiotelemeters were exposed once to 3 ppm acrolein, 0.3 ppm ozone, or filtered air. Heart rate (HR) and electrocardiogram (ECG) were recorded continuously before, during and after exposure. Analysis of ECG morphology, incidence of arrhythmia and heart rate variability (HRV) were performed. Cardiac mechanical function was assessed using a Langendorff perfusion preparation 24 h post-exposure. Acrolein exposure increased HRV independent of HR, as well as incidence of arrhythmia. Acrolein also increased left ventricular developed pressure in WT mice at 24 h post-exposure. Ozone did not produce any changes in cardiac function. Neither gas produced ECG effects, changes in HRV, arrhythmogenesis, or mechanical function in KO mice. These data demonstrate that a single exposure to acrolein causes cardiac dysfunction through TRPA1 activation and autonomic imbalance characterized by a shift toward parasympathetic modulation. Furthermore, it is clear from the lack of ozone effects that although gaseous irritants are capable of eliciting immediate cardiac changes, gas concentration and properties play important roles.
Keywords: Air pollution, Cardiac, TRPA1, Heart rate variability, Arrhythmia, Acrolein
1. Introduction
Ambient air pollution has been linked to adverse cardiovascular effects by numerous epidemiological, human and animal studies. Although most of the research over the last decade have focused on particulate matter (PM), recent data suggest gaseous pollutants likely contribute significantly to acute decrements in cardiac function and result in increased overall risk for cardiovascular dysfunction (Campen et al., 2014; Hazari et al., 2014; Mills et al., 2015; Shah et al., 2015). In fact, the 2010 American Heart Association document on PM and cardiovascular disease clearly states that “Although PM2.5 mass has rightfully attracted attention as a target for regulation and epidemiological study, more than 98% of the air pollutant mass in the mixture we breathe in urban settings is from gases or vapor-phase compounds…”, not to mention there is still relatively little data describing the effects of gaseous pollutants on the cardiovascular system (Brook et al., 2010). Thus, more studies are needed to determine the effect of individual gases and their causative pathways, particularly given the mechanism underlying the response will undoubtedly differ for the gas alone and when combined with other pollutants such as PM.
In addition to “criteria” pollutant gases like ozone (O3) for which the United States Environmental Protection Agency has set National Ambient Air Quality Standards (NAAQS), non-NAAQS hazardous air pollutants (HAPs) such as acrolein are also frequently present in ambient air pollution, particularly in emissions from combustion processes including exhaust from automobiles, emissions from coal-fired power plants, and emissions from industrial sites (CDC ToxProfile). Acrolein is a gaseous irritant formed during the combustion of petrochemical fuels such as gasoline or diesel and it is considered to pose the greatest relative HAP hazard for non-cancer health effects ((Haussmann, 2012). To that point, we previously showed that a single exposure to either O3 or acrolein causes various cardiovascular effects in rodents; these range from electrocardiogram changes, autonomic imbalance, desensitization of baroreflex, to alterations in hypoxia responsiveness (Farraj et al., 2012; Hazari et al., 2009b; Hazari et al., 2014; Perez et al., 2013). Moreover, many of these effects appear to be latent and are only evident if the body is challenged or encounters a subsequent trigger (Hazari et al., 2012), which would suggest the underlying mechanisms involve the disruption of homeostatic controls which maintain equilibrium of vital bodily systems under changing conditions.
Numerous biological pathways have been described that link air pollution with cardiovascular dysfunction. These include systemic inflammation and oxidative stress, vasoconstriction, enhanced coagulation/thrombosis, autonomic effects due to sensory receptor activation, and the direct effects of translocated particles on the myocardium (Brook et al., 2010). Of these, airway sensory activation may be the most relevant when considering the short-term, reversible, and often latent effects of an acute ambient exposure. An examination of the role of airway sensory nerves in the body indicates that not only do they “sense” or become activated by specific gaseous pollutants but also cause internal homeostatic changes that may predispose to respiratory and cardiovascular dysfunction (Jordt and Ehrlich, 2007; Lee and Pisarri, 2001).
Gaseous air pollutants such as ozone, acrolein, and other reactive aldehydes commonly found in combustion-derived exhaust, stimulate sensory nerves in the airways causing several well-described ventilatory and pulmonary effects (Abbott-Banner et al., 2013; Büch et al., 2013; Jordt, 2011; McDonnell et al., 1983). A subset of these responses is known to be mediated by airway sensors, particularly transient receptor potential cation channels A1 (TRPA1) and V1 (TRPV1), located on pulmonary C-fibers (Nassenstein et al., 2008; Taylor-Clark et al., 2008). It has been suggested that these sensors may also mediate some cardiovascular responses as well (Hazari et al., 2011; Jones et al., 1995; Lee, 2010; Wang et al., 2000) and in fact, our previous studies have demonstrated that pharmacological inhibition of TRPA1 attenuates air pollution-induced cardiac arrhythmia produced by autonomic imbalance in rats (Hazari et al., 2011).
Thus, in the present study we investigated the cardiac effects of exposure to acrolein or ozone inhalation with WT and TRPA1 KO mice. Acrolein was chosen because it is a common gaseous component of urban air pollution and a known TRPA1 activator (Bautista et al., 2006). Only a single study has shown that ozone, albeit in solution and not inhaled, activates TRPA1 (Taylor-Clark and Undem, 2010), yet its irritant properties suggest that such a finding is not unreasonable. Regardless, although both have been traditionally classified as respiratory irritants, data from the last five years have clearly shown that both gases not only also affect the cardiovascular system but other organ systems through pathways which implicate a role for the intrinsic control mechanisms of the body (Bessac and Jordt, 2010; DeJarnett et al., 2014; Miller et al., 2015). The concentration of acrolein used in this study was admittedly high to serve as a proof of concept but certainly there are extreme conditions (e.g. structural fires), occupational settings or cigarette smoke (World Health Organization Concise International Chemical Assessment Document: Acrolein) in which the levels of acrolein exceed those used here. Irrespective of this high concentration, the broader aim was to not only firmly establish a role for TRPA1 in air pollution-induced electrical and mechanical cardiovascular dysfunction but also clarify whether inhalation of acrolein and ozone produce cardiovascular effects through TRPA1.
2. Materials and methods
2.1. Animals
Female C57BL/6 and TRPA1 −/− mice (22 ± 3.8 g) were used in this study (Jackson Laboratory - Bar Harbor, ME). Mice were initially housed five per cage and maintained on a 12-hour light/dark cycle at approximately 22 °C and 50% relative humidity in an AAALAC–approved facility. Food (Prolab RMH 3000; PMI Nutrition International, St. Louis, MO) and water were provided ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee of the U.S. Environmental Protection Agency and are in accordance with the National Institutes of Health Guides for the Care and Use of Laboratory Animals. The animals were treated humanely and with regard for alleviation of suffering. Background controls (B6129PF2/J mouse - Jackson Laboratory - Bar Harbor, ME) were used as appropriate. Only female mice were used so that we could compare the results obtained here to not only our previous findings but also to those of other researchers doing this type of work (Willis et al., 2011); a similar study including a gender comparison is necessary and will be performed in the near future.
2.2. Experimental groups
Wild-type and TRPA1 knockout mice were randomly assigned to one of three exposure groups: (1) Acrolein (Acrl); (2) Ozone (O3); or (3) filtered air (FA). There were 8–12 animals per group.
2.3. Surgical implantation of radiotelemeters
Animals were weighed and then anesthetized using inhaled isoflurane (Isothesia, Butler Animal Health Supply, Dublin OH). Anesthesia was induced by spontaneous breathing of 2.5% isoflurane in pure oxygen at a flow rate of 1 L/min and then maintained by 1.5% isoflurane in pure oxygen at a flow rate of 0.5 L/min; all animals received the analgesic Buprenorphine (0.03 mg/kg, i.p. manufacturer). Using aseptic technique, each animal was implanted subcutaneously with a radiotelemeter (ETA-F10, Data Sciences International, St Paul, MN); the transmitter was placed under the skin to the right of the midline on the dorsal side. The two electrode leads were then tunneled subcutaneously across the lateral dorsal sides; the distal portions were fixed in positions that approximated those of the lead II of a standard electrocardiogram (ECG). Body heat was maintained with a heating blanket both during and immediately after the surgery. Animals were given food and water post-surgery and were housed individually. All animals were allowed 7–10 days to recover from the surgery and reestablish circadian rhythms.
2.4. Radiotelemetry data acquisition
Radiotelemetry methodology (Data Sciences International, Inc., St. Paul, MN) was used to track changes in cardiovascular electrical activity by monitoring heart rate (HR), and ECG waveforms immediately following telemeter implantation, through exposure until 24 h post-exposure. This methodology provided continuous monitoring and collection of physiologic data from individual mice to a remote receiver. Sixty-second ECG segments were recorded every 5 min during the pre- and post-exposure periods. ECG was recorded continuously during exposure (baseline and hours 1–4); HR was automatically obtained from the waveforms (Dataquest ART Software, version 3.01, Data Sciences International, St. Paul, MN, USA). All comparisons of HR, ECG parameters and HRV during exposure were made to the corresponding time of day during pre-/post-exposure; this was always between 8 and 11 am.
2.5. Electrocardiogram (ECG) analysis
ECGAuto software (EMKA Technologies USA, Falls Church VA) was used to visualize individual ECG waveforms, analyze and quantify ECG segment durations and areas, as well as identify cardiac arrhythmias as previously described (Hazari et al., 2009a). Briefly, using ECGAuto, P-wave, QRS complex, and T-wave were identified for individual ECG waveforms and compiled into a library. Analysis of all experimental ECG waveforms was then based on established libraries. The following parameters were determined for each ECG waveform: PR interval (Pstart-R), QRS complex duration (Qstart-S), ST segment interval (S-Tend) and QT interval (Qstart-Tend). QT interval was corrected for HR using the correction formula for mice QTc = QT/(RR/100)1/2 (Mitchell et al., 1998). All ECG streams with <10 s of identifiable cardiac cycles were excluded from ECG parameter calculations. Fig. 2 shows a typical ECG trace as well as examples of the types arrhythmia observed in this study.
Fig. 2.
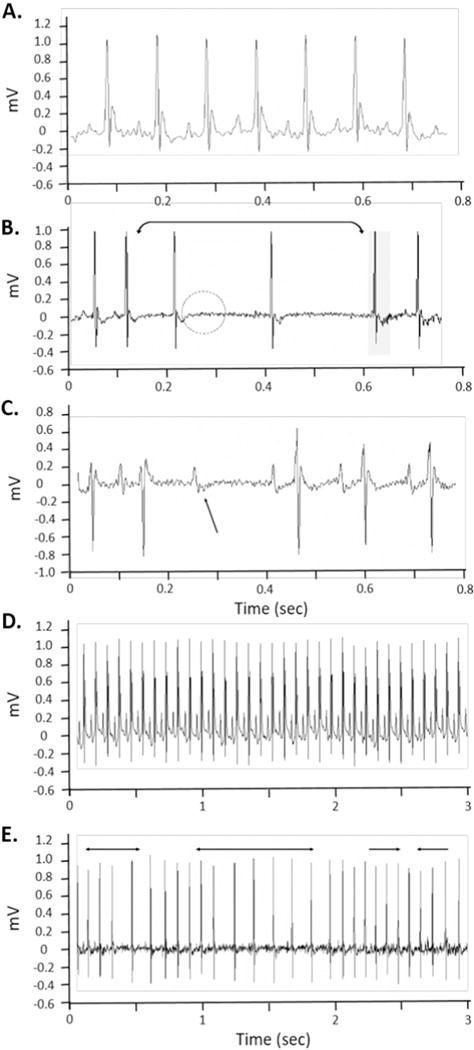
Typical electrocardiogram and cardiac arrhythmia in mice exposed to acrolein and Ozone. A. Typical mouse ECG during normal sinus rhythm. B. Sino-atrial node dysfunction characterized by elongation of the R-R interval and loss of discernable p-waves. C. Non-conducted p-wave, which represents a loss of conduction from the atria to the ventricles. D. Typical mouse ECG during normal sinus rhythm (expanded timescale) E. Dysrhythmia characterized by brief periods of bradycardia dispersed between periods of normal heart rate. All of these arrhythmia are typical of vagal dominance.
2.6. HRV analysis
Heart rate variability (HRV) was calculated as the mean of the differences between sequential RRs for the complete set of ECG waveforms using ECGAuto. For each 1-min stream of ECG waveforms, mean time between successive QRS complex peaks (RR interval), mean HR, and mean HRV-analysis–generated time-domain measures were acquired. The time-domain measures included standard deviation of the time between normal-to-normal beats (SDNN), and root mean squared of successive differences (RMSSD). SDNN represents overall HRV, while RMSSD represents parasympathetic influence over HRV. Analysis of HRV was also conducted in the frequency domain using a fast-Fourier transform. For frequency domain analysis, the signal was analyzed with a Hamming window for segment lengths of 512 samples with 50% overlapping. The spectral power obtained from this transformation represents the total harmonic variability for the frequency range being analyzed. In this study, the spectrum was divided into low-frequency (LF) and high-frequency (HF) regions with frequency bands assigned as 0.15–1.5 and 1.5–5 Hz respectively. LF is generally believed to represent a combination of sympathetic and parasympathetic activity, while HF indicates cardiac parasympathetic (vagal) activity (Ori et al., 1992). All ECG streams with <1 min of identifiable RR intervals were excluded from HRV analysis. Additionally thorough visual inspection was conducted to identify and exclude arrhythmias and artifacts (Rowan et al., 2007).
2.7. Exposure
The study protocol included two days of animal-to-chamber acclimatization prior to exposure. Three or four hour exposures to acrolein or ozone respectively (Exposure) started with 30 min of additional chamber acclimatization. All mice were moved back to their home-cages after the exposure (Post-exposure) and monitored for 24 h (Post-exposure 24 h+) before necropsy. Temperature and humidity of the chambers were maintained at between 70 and 73 °F and 47–52%, respectively, and flow rates were kept constant.
2.8. Ozone exposure
Ozone (O3) exposures took place in whole-body exposure chambers. O3 was generated by passing extra dry oxygen past an arcing transformer in a model V5-0 ozone generator (Ozone Research & Equipment Corp., Phoenix, AZ). Chamber O3 concentrations (0.3 ppm) were regulated and recorded by a PC based automated data system running DASYLab (version 9.0; Measurement Computing Corp., Norton, MA) software. Ozone concentrations were monitored using continuous gas analyzers (model 49i, model 49; Thermo Fisher Scientific, Foxfire, MA) which fed analog signals to the control system. DASYLab then used a feedback loop running a proportional, integral, derivative controller to open or close mass flow controllers to maintain chamber O3 concentration at the desired level. Filtered air control exposures were simultaneously conducted with all chamber conditions maintained and O3 levels < 0.010 ppm.
2.9. Acrolein exposure
Acrolein exposures took place in whole-body plethysmography chambers (Model PLY3213, Buxco Electronics, Inc., Wilmington, NC, USA). Acrolein gas was metered from a 1000 ppm cylinder into a glass mixing chamber where the gas was mixed and diluted with dry filtered air to achieve a final concentration of 3 ppm of acrolein with a total flow of 6 L/min. The actual chamber concentration was measured once per hour using an HP5890 gas chromatograph (GMI Inc., Ramsey, MN, USA) equipped with manual injection, a flame ionization detector and a DB-VRX capillary column.
2.10. Cardiac perfusion
The procedure for cardiac perfusion has been previously described (Tong et al., 2010). Briefly, 24 h after exposure, mice were anesthetized with sodium pentobarbital (80 mg/kg, i.p.). Heparin (100 units) was injected intravenously before removal of heart. The hearts were rapidly removed and placed in ice-cold Krebs-Henseleit buffer, after which the aortas were cannulated. Retrograde perfusion via the aorta was performed under constant pressure (100 cm H2O) above the heart. The non-recirculating perfusate was a Krebs-Henseleit buffer containing (in mmol/L) 120 NaCl, 5.9 KCl, 1.2 MgSO4, 1.75 CaCl2, 25 NaHCO3, and 11 glucose. The buffer was aerated with 95% O2−5% CO2 and maintained at pH 7.4 and a temperature of 37 °C.
For assessment of contractile function, a latex balloon on the tip of a polyethylene catheter was inserted through the left atrium into the left ventricle. The catheter was connected to a pressure transducer (Argon Medical Devices, Athens, TX) at the same height as the heart. The pressure of the left ventricular balloon was inflated to 0–5 cm H2O. A PowerLab system was used to collect and process the heart rate, left ventricular developed pressure (LVDP), and contractility (dP/dt) data (AD Instruments, Milford, MA). All hearts were perfused for 25 min; we then initiated 20 min of global no-flow ischemia by stopping the flow of oxygenated perfusion buffer, followed by 1 h of reperfusion. Onset of ischemic contracture was measured as the time from the start of ischemia until initial contracture (at least 5 cm H2O increase in left ventricular pressure). Recovery of LVDP, expressed as a percentage of the initial pre-ischemic LVDP, was measured at 20, 40 and 60 min of reperfusion after 20 min of ischemia.
2.11. Statistics
All data are expressed as means ± SEM. Statistical analyses of the data were performed with GraphPad Prism 5 (GraphPad software, San Diego CA). A minimum power of 0.8 was used to determine sample size. For HR, ECG intervals and HRV, two-way analysis of variance (ANOVA) for repeated-measures and Bonferroni post hoc tests were used to determine statistical differences. A one-way ANOVA was used to analyze arrhythmia counts. For Langendorff cardiac perfusion data, comparisons between groups were performed by one-way ANOVA followed by Bonferroni post hoc test for multiple comparisons. Comparisons were made across all groups taking into account the multiple endpoints, exposure groups and time points as well as any interactions.
3. Results
3.1. Heart rate
Table 1 lists the average heart rates before, during and after exposure. The Pre-exposure period represents a 4 hour time-matched period one day before exposure. The Post-exposure period represents the 4 h directly following exposure, and the 24 h Post-exposure represents a 4 h time-matched period one day after exposure. All animals experienced an increase in HR when initially placed in the exposure chamber and all progressively decreased over the course of the exposure. There were no significant differences in HR within the strains among any of the exposure groups during any time period, however the WT animals demonstrated a more robust increase in HR in every exposure group compared with the KO animals. Exposure to ozone did produce a decrease in HR in the KO animals compared with the pre-exposure period and compared with ozone-exposed WT animals. In addition, there were no behavioral differences between any of the animal groups either during or after either exposure.
Table 1.
The effect of acrolein or ozone exposure on heart rate.
| Filtered air | Acrolein | Ozone | |
|---|---|---|---|
| WT | |||
| Pre-exposure | 575.9 ± 19.5* | 607.3 ± 34.4 | 563.1 ± 11.3 |
| Exposure | 617.1 ± 9.9 | 638.3 ± 6.6 | 626.6 ± 10.5 |
| Post-exposure | 546.5 ± 13.2 | 573.5 ± 7.1 | 548 ± 9.8 |
| 24 h post-exposure | 525.9 ± 14.2 | 552.4 ± 14.7 | 540.3 ± 8.8 |
| KO | |||
| Pre-exposure | 570.9 ± 19.3 | 575 ± 16.2 | 573.3 ± 9.6 |
| Exposure | 587.9 ± 14.3 | 600.8 ± 12.6 | 562.2 ± 20.7‡ |
| Post-exposure | 584.8 ± 15.8 | 607.5 ± 9.5 | 594.8 ± 13.4 |
| 24 h post-exposure | 552.5 ± 17 | 573.2 ± 15.4 | 571.5 ± 9.4 |
Values are mean (beats/min) ± SEM (n = 8–12).
p < 0.05; significantly different from FA.
p < 0.05; significantly different from WT strain.
3.2. Electrocardiogram (ECG)
Table 2 shows the ECG parameters before, during and after exposure. There were no significant differences in ECG interval durations between animals exposed to acrolein or ozone compared with filtered air during any time period. When compared to the pre-exposure period, WT animals had no significant changes in ECG interval durations during the exposure period. In contrast, KO mice demonstrated slight increases in QRS and QTc interval lengths during acrolein, ozone, and filtered air exposure indicating that these were likely a physiological response of the KOs to placement in the exposure chamber rather than a response to the pollutant.
Table 2.
The effect of acrolein or ozone exposure on electrocardiogram parameters.
| PR (msec)
| ||||
|---|---|---|---|---|
| Filtered air | Acrolein | Ozone | ||
| WT | ||||
| Pre-exposure | 35.16 ± 0.59* | 32.65 ± 0.51 | 34.14 ± 0.74 | |
| Exposure | 37.02 ± 0.4 | 34.33 ± 0.73 | 35.26 ± 0.58 | |
| Post-exposure | 35.34 ± 0.54 | 32.82 ± 0.52 | 34.25 ± 0.59 | |
| KO | ||||
| Pre-exposure | 30.34 ± 0.65 | 29.35 ± 0.35 | 30.17 ± 0.69‡ | |
| Exposure | 30.95 ± 0.77 | 31.36 ± 0.75‡ | 31.97 ± 0.75‡ | |
| Post-exposure | 32.33 ± 0.98 | 31.84 ± 0.75 | 32.75 ± 0.74 | |
| QRS (msec)
| ||||
| Filtered air | Acrolein | Ozone | ||
|
| ||||
| WT | ||||
| Pre-exposure | 13.41 ± 0.52 | 11.9 ± 0.08 | 12.45 ± 0.3 | |
| Exposure | 12.4 ± 0.35 | 11.14 ± 0.28 | 12.23 ± 0.18 | |
| Post-exposure | 13.28 ± 0.48 | 11.74 ± 0.02 | 12.34 ± 0.28 | |
| KO | ||||
| Pre-exposure | 11.96 ± 0.34 | 12.43 ± 0.31 | 11.66 ± 0.25 | |
| Exposure | 13.64 ± 0.56† | 14.21 ± 0.58†,‡ | 13.45 ± 0.5† | |
| Post-exposure | 11.78 ± 0.35 | 12.39 ± 0.33 | 11.71 ± 0.36 | |
| QTc (msec)
| ||||
| Filtered air | Acrolein | Ozone | ||
|
| ||||
| WT | ||||
| Pre-exposure | 74.58 ± 3.07 | 65.94 ± 2.08 | 71.58 ± 2.69 | |
| Exposure | 67.98 ± 2.14 | 68.1 ± 1.73 | 69.39 ± 1.34 | |
| Post-exposure | 73.93 ± 3.93 | 63.36 ± 2.15 | 69.95 ± 2.95 | |
| KO | ||||
| Pre-exposure | 72.38 ± 1.18 | 71.81 ± 1.34 | 72.58 ± 3.11 | |
| Exposure | 82.91 ± 3.39†,‡ | 84.74 ± 4.07†,‡ | 81.93 ± 4.02†,‡ | |
| Post-exposure | 67.68 ± 1.76 | 70.18 ± 1.21 | 67.29 ± 2.38 | |
Values are mean ± SEM (n = 8–12).
p < 0.05; significantly different from FA.
p < 0.05; significantly different from pre-exposure period.
p < 0.05; significantly different from WT strain.
3.3. Heart rate variability (HRV)
Exposure to acrolein caused significant increases in all measures of HRV in WT animals, including the time domain measurements SDNN and RMSSD (Fig. 1A and B), as well as the high and low frequency domain measurements (Fig. 1C and D) when compared with filtered air and KO mice. There was no effect of acrolein on KO mice nor was there any effect of ozone exposure on either WT or KO mice.
Fig. 1.
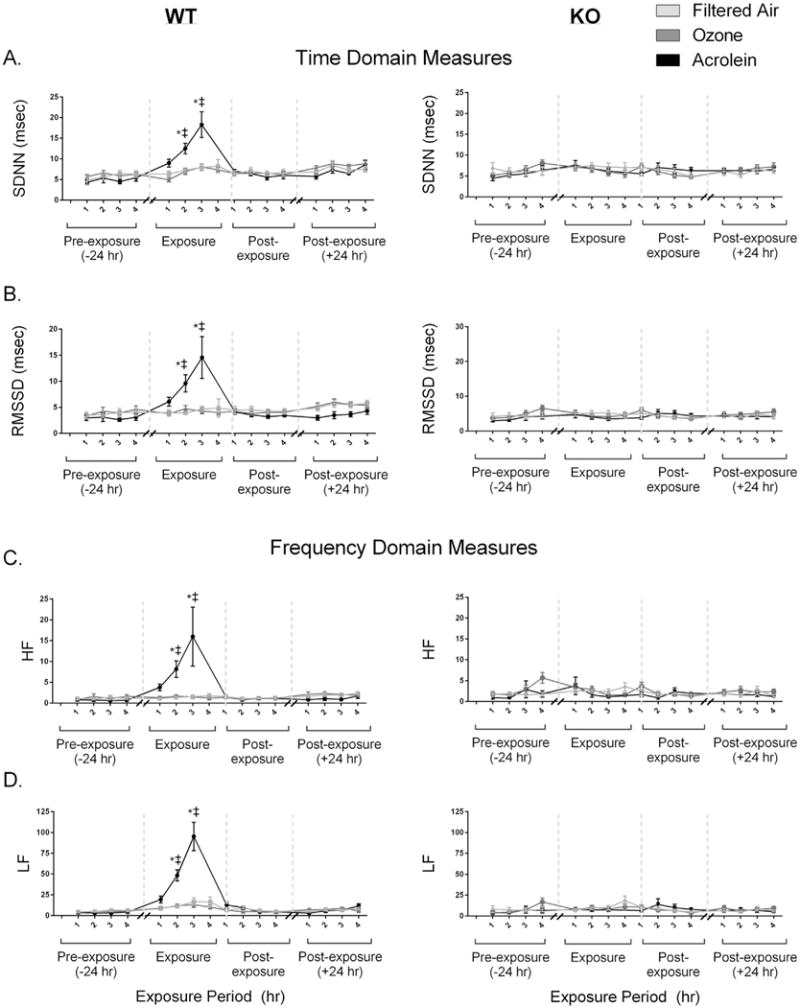
Exposure to acrolein but not ozone increases all measures of heart rate variability (HRV). Wildtype (WT) mice experienced an increase in SDNN, RMSSD, LF/HF, LF and HF during exposure to acrolein. These increases in HRV were not observed in TRPA1 KO animals. There was no effect of ozone exposure on either the WT or KO animals. Values are mean ± SEM. *p < 0.05; significantly different from FA. ‡p < 0.05; significantly different from KO strain (n = 8–12).
3.4. Cardiac arrhythmias
Typical ECG and cardiac arrhythmias observed in mice exposed to acrolein and ozone are shown in Fig. 2. There was a significant increase in the number of arrhythmia during the 3 hour exposure period to acrolein (27.8 ± 10.8) when compared with filtered air (2.2 ± 0.9) (Fig. 3). No other significant differences in arrhythmias were observed between any exposure groups at any time period.
Fig. 3.
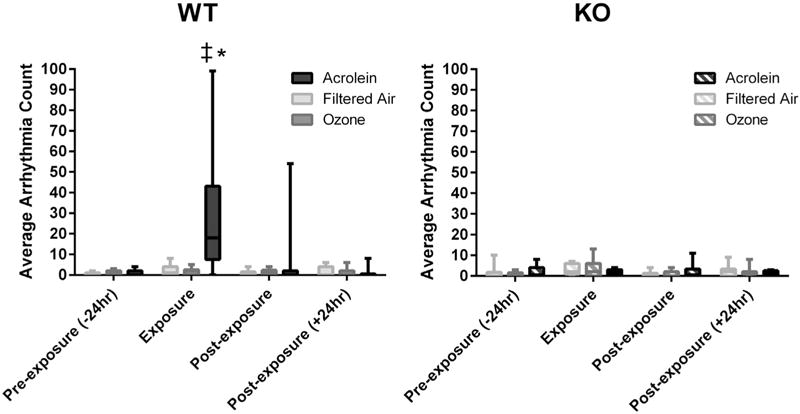
Exposure to acrolein increases cardiac arrhythmias in wildtype mice. WT mice experienced an increase in arrhythmia during exposure to acrolein. Values are total number of arrhythmia occurrences during the pre-exposure, exposure and post-exposure periods. There were no significant differences in arrhythmia counts between any of the groups during any other period. *p < 0.0001; significantly different from FA. ‡p < 0.0001; significantly different from KO strain (n = 8–12).
3.5. Cardiac mechanical effects
Post-exposure (pre-ischemic) and post-ischemic left ventricular pressure measurements are summarized in Table 3. Exposure to acrolein significantly increased LVDP 24 h post-exposure (129.9 ± 10.3 cm H2O) compared with filtered air-exposed controls (86.4 ± 7.8 cm H2O) (Fig. 4). There were no other significant differences in cardiac mechanical function between any other groups at any other time points.
Table 3.
Cardiac mechanical effects of exposure to acrolein or ozone.
| LVDP post-ischemia recovery (% LVDPbaseline) |
||||||
|---|---|---|---|---|---|---|
| Strain/exposure | LVDP baseline (cm H2O) |
dP/dT (+) (cm H2O/s) |
dP/dT (−) (cm H2O/s) |
20 min | 40 min | 60 min |
| WT/FA | 86.4 ± 7.86 | 3527 ± 243 | −2794 ± 296 | 32.0 ± 9.3 | 38.1 ± 10.7 | 44.0 ± 13.7 |
| WT/acrolein | 129.9 ± 10.33* | 4501 ± 415 | −3411 ± 363 | 34.1 ± 5.6 | 37.3 ± 6.3 | 39.9 ± 9.6 |
| WT/ozone | 74.2 ± 9.60 | 3209 ± 379 | −2363 ± 351 | 50.5 ± 12.7 | 51.9 ± 15.4 | 50.6 ± 15.6 |
| KO/FA | 101.9 ± 8.72 | 5297 ± 721 | −3267 ± 268 | 14.4 ± 3.8 | 23.4 ± 7.1 | 24.4 ± 8.4 |
| KO/acrolein | 83.6 ± 7.32 | 3980 ± 500 | −2416 ± 209 | 35.1 ± 13.3 | 58.6 ± 34.9 | 34.7 ± 12.8 |
| KO/ozone | 80.8 ± 16.96 | 3830 ± 601 | −3560 ± 1214 | 30.4 ± 6.9 | 41.9 ± 12.0 | 49.0 ± 17.7 |
dP/dtmax = maximum 1st derivative of the change in left ventricular pressure/time; dP/dtmin = minimum 1st derivative of the change in left ventricular pressure/time. Values are mean ± SEM (n = 8–12).
p < 0.05; significantly different from FA.
Fig. 4.
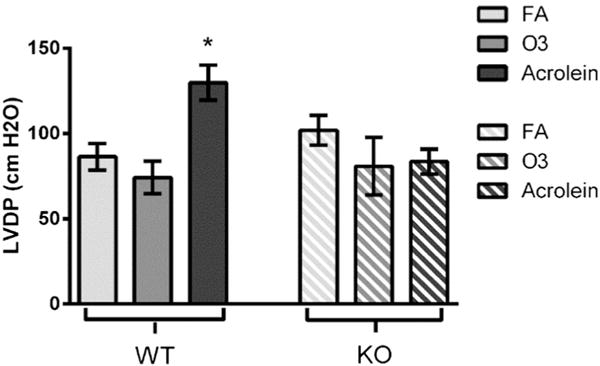
Exposure to acrolein increases left ventricular developed pressure 24 h post-exposure. WT animals exposed to acrolein experienced an increase in LVDP 24 h post-exposure when compared to FA controls. Exposure to ozone did not produce any changes in LVDP. *p < 0.05; significantly different from FA (n = 8–12).
4. Discussion
The results of this study demonstrate that TRPA1 channels found on airway sensory nerves, which can be described as sensors for a broad array of environmental irritants, play a role in the cardiovascular response of mice to acrolein. The data presented here also suggests that a single exposure to gaseous irritant pollutants causes not only cardiac electrical disturbances, which are commonly observed in humans (Peters et al., 2000), but acute, nearly imperceptible changes in cardiac mechanical function as well; these findings corroborate not only our previous work (Kurhanewicz et al., 2014) but the work of others (Tong et al., 2010). More importantly, the current study confirms that gaseous air pollutants have effects beyond the respiratory system affecting not only cardiac function but also the homeostatic control of the cardiovascular system as indicated by changes in HRV. Although blood pressure was not measured in this study, our previous results (Perez et al., 2013) confirm that the effects of acrolein exposure likely involve subtle disruptions to intrinsic cardiovascular controls. In order to elucidate how gaseous air pollutants may cause this cardiac dysfunction, we investigated the role of TRPA1 by using high concentrations of acrolein as a putative positive control and lower concentrations of ozone as a more relevant and real-world exposure. Acrolein produced abnormal modulation of autonomic balance and an increased incidence of arrhythmia through TRPA1, which also mediated an increase in LVDP. In contrast, exposure to ozone did not appear to cause any significant effects despite the fact that mild cardiovascular effects of ozone at similar levels have been documented by our lab in the past and by others (Barath et al., 2013; Farraj et al., 2012; Farraj et al., 2015; Kurhanewicz et al., 2014; Wang et al., 2013; Wu et al., 2010).
Sensory receptors in the respiratory system, from the nose down to the lower lungs, respond to gaseous irritants and initiate visceral (i.e. autonomic) reflexive changes which impact the function of the cardiovascular system. While it is unlikely that inhalation of these gases affects the heart or vasculature by causing direct toxicity or damage, there is certainly enough data that suggests signals initiated in the airways can lead to altered cardiovascular regulation, compensatory deficits, and increased sensitivity to subsequent exposures (Widdicombe and Lee, 2001). For example, the prevalence of arrhythmias, although not life-threatening in and of themselves, are increased in people exposed to smoke (Carey and Thevenin, 2009). Thus, these irritant gases may not be overtly deleterious but rather may increase the risk of an adverse response if another stressor is encountered and the body is unable to maintain homeostasis. We previously showed that acrolein exposure increases risk by desensitizing the baroreflex (Hazari et al., 2014), which maintains blood pressure, and therefore systemic perfusion, by altering heart rate. As such, the baroreflex, as an internal sensor of blood pressure, operates and exerts its effects through the sympathetic and parasympathetic branches of the autonomic nervous system. Therefore any exposure-related changes in autonomic balance, which can be measured through HRV, might result in altered, and possibly impaired, regulation of the cardiovascular system. Although yet unsubstantiated, TRPA1 activation by acrolein may represent the initiating event for this baroreflex desensitization given its association with autonomic imbalance as we have shown here.
While the results from this study do not provide direct evidence of serious cardiac morbidity or premature mortality as a result of acute exposure to airway irritants, they may reflect a transient instability that can worsen if exposure continues over a longer period. Moreover, such dysregulation in individuals with pre-existing regulatory issues or disease resulting in poor compensatory capacity could be more seriously impacted. Performing studies in at-risk humans is problematic, so as a result these fundamental relationships in autonomic cardiac dysfunction may only be dissected in rodent models. As data examining autonomic function in rodents in the past had to be interpreted cautiously due to the use of anesthesia, the current results were produced from conscious unrestrained mice that could be monitored continuously during exposure, thus improving discernibility of toxicological effects relevant to human scenarios. Still, HRV data in rodents is not easily interpretable given that several dynamic factors contribute to it (Rowan et al., 2007).
Exposure to acrolein produced significant increases in SDNN, RMSSD, LF power and HF power in WT mice; this response was not present in acrolein-exposed TRPA1 KO animals. While decreased HRV is commonly accepted as an indicator of heightened cardiovascular risk in humans (Gold et al., 2000), increased HRV has also been linked to adverse cardiovascular outcomes (Stein et al., 2005). Indeed, other reports note increased HRV as an indicator of heightened risk. For example, increased RMSSD has been shown to be associated with elevated risk of air pollution-induced arrhythmia (Davoodi et al., 2010) and increases in HRV have been demonstrated preceding post-operative atrial fibrillation (Amar et al., 2003). Additionally, increases in vagal tone have been associated with adverse cardiovascular events in type II diabetics (Eguchi et al., 2010), and linked with increased mortality in heart failure patients and the elderly (de Bruyne et al., 1999; Stein et al., 2005). With regard to the animal models, we have consistently shown that exposure to different types of air pollutants such as residual oil fly ash (Carll et al., 2015; Farraj et al., 2011), diesel exhaust (Carll et al., 2013; Hazari et al., 2011), ozone (Farraj et al., 2012) and acrolein (Hazari et al., 2014) all lead to an increase in HRV in rodent models. This is not entirely surprising given increased HRV, and in particular RMSSD and HF, indicate parasympathetic modulation which is a well-characterized reflex response to airway sensory activation (Lee and Pisarri, 2001). Thus, it is clear the relationship between HRV and cardiac dysfunction cannot be overly simplified to say increased HRV is good whereas decreased HRV is bad. Instead, it is the imbalance of this homeostatic mechanism, regardless of direction of change, which likely produces the increase in risk. In fact, observation of simultaneous electrical disturbances in the ECG, like AV node block, along with an increase in HRV may confirm the underlying shift from normal function.
In our studies, increased HRV, and therefore parasympathetic modulation, was observed with an increased incidence of sinus node dysfunction and dysrhythmia (Fig. 2) during acrolein exposure in normal animals. Increased vagal tone can cause such changes in the P-wave to P-wave interval independent of normal breathing. This condition is known as non-respiratory sinus arrhythmia and it differs from respiratory sinus arrhythmia (RSA) in that it is not associated with respiration but rather occurs when the outflow of parasympathetic tone predominates over sympathetic tone (Deboor et al., 2005; McMullen et al., 2012) and unlike RSA is grossly irregular. Furthermore, non-respiratory sinus arrhythmia or dysrhythmia when observed with sinus node dysfunction can indicate sick sinus syndrome (Keller and Lemberg, 2006), which is a group of abnormal heart rhythms that appear not only as a blocked sinus signal but also as random intervals of bradycardia and tachycardia. Although these parameters fit what we observed with acrolein, it still remains to be determined whether it represents a toxicological effect that leads to adverse systemic outcomes; yet it would not be unreasonable to suspect that such a change might predispose an individual to subsequent triggered responses. Increases in HRV associated with increased randomness of the heart rate, that is, a high degree of non-respiratory sinus arrhythmia have been found to be strongly associated with risk of mortality (McMullen et al., 2012; Stein, 2004).
Interpreting the frequency domain of the HRV data is a bit more challenging. The cardiac cycle and R-R interval variability are affected by multiple control mechanisms including autonomic modulation at the SA node, the dynamic regulation of the vasculature, as well as endocrine/paracrine, endothelial and mechanical factors. Additionally, complex control mechanisms including baroreflex and respiratory sinus arrhythmia can also drive changes in these parameters. Numerous studies have shown that the HF power component of HRV is strongly associated with cardio vagal activity (Billman, 2013; Chess et al., 1975; Heathers, 2014; Piccirillo et al., 2009). In contrast, there is a growing body of evidence directly countering the claim that LF power is proportional to cardiac sympathetic nerve activity (Billman, 2013; Goldstein et al., 2011; Reyes del Paso et al., 2013). In fact, a number of studies suggest that LF power may better reflect other mechanisms exerting regulatory control over the cardiac cycle such as baroreflex activity in response to vasomotor tone (Goldstein et al., 2011; Moak et al., 2007; Rahman et al., 2011). Investigators have also used LF/HF, which was popularized in the 1980s, as a measure of sympatho-vagal balance (Pagani et al., 1984), however more recent analysis of this metric has cast doubt on its interpretation (Billman, 2013; Heathers, 2014; Reyes del Paso et al., 2013). Although the two branches of the autonomic nervous system may act reciprocally, they can also be co-activated or completely uncoupled and function independently of one-another (Amar et al., 2003; Reyes del Paso et al., 2013). Thus, theoretically the HF and LF power measurements may provide information relating to different physiological control mechanisms. Namely that HF power may be more strongly related to vagal influence while LF may provide information about not only parasympathetic factors but also a mix of sympathetic and other factors such as blood pressure control mechanisms including modulation of vasomotor tone (Billman, 2013; Reyes del Paso et al., 2013).
Notably, in this study exposure to ozone produced no discernable adverse cardiac effects in either WT or KO mice. While these results corroborate both our previous findings and those of others (Barath et al., 2013; Farraj et al., 2012; Farraj et al., 2015; Wang et al., 2013; Wu et al., 2010) they also highlight the fact that the cardiovascular response to low level or near-ambient ozone is quite variable and requires further investigation. We assumed a role for TRPA1 given the findings of Taylor-Clark and Undem (Taylor-Clark and Undem, 2006) who showed activation of TRPA1 on airway C-fibers by high concentrations of ozone (approximately 7.2 ppm) in an ex-vivo system. Therefore, the lack of discernable effects in this study may be due to the lower concentrations of ozone applied in our study as well as differences intrinsic to the in-vivo system which would certainly have changed the exposure profile (i.e. presence of epithelium, lining fluid, etc.). To that point, the activity level of the subjects also contributes to the dose of ozone received as exercise has been shown to increase the dose of ozone delivered to the lungs (Hatch et al., 1994). In this study, where the mice were primarily at rest, the effective dose of ozone delivered to the lower airways would be relatively low. Additionally, phenotypic differences between the subtypes of C-fibers being targeted by acrolein and ozone may also account for some of the differences observed in our responses. Acrolein is known to primarily impact the upper airways and not penetrate much past the larynx while ozone can reach deep into the lung. As such, C-fibers demonstrate phenotypic differences in their sensitivities and responses to chemical and mechanical stimuli based on location (Lee and Pisarri, 2001) (Coleridge and Coleridge, 1984). Hence, differences in activation of C-fiber subtypes may account for the disparate responses observed.
Our study demonstrates that autonomic nervous system function can be modulated by TRPA1, and appears to also play a role in the acrolein-induced cardiac arrhythmia and mechanical changes (Table 4). Stimulation of the nasal or laryngeal mucosa with an irritant such as acrolein has been shown to cause a slowing of the HR in combination with an irregular rhythm (Kratschmer, 2001), however there are very little data demonstrating a link between airway irritation and cardiac dysrhythmia. TRPA1 activation increases the release of glutamate, a centrally-acting neurotransmitter that facilitates transmission in the nucleus tractus solitarius (Sun et al., 2009). Although yet unproven, it is conceivable that this modulation of central neurotransmission can result in autonomic modulation and altered cardiovascular function. Similar mechanisms have already been demonstrated in models of cigarette smoke irritation (Mutoh et al., 2000) as well as others (Paton and Nolan, 2000; Simms et al., 2006), while TRPA1’s role in modulating other “autonomic” activity (e.g. breathing) in the brainstem is also known (Tani et al., 2015). Another possible contributing mechanism is neuroinflammation from the release of neuropeptides; which not only affect the airways locally but can also have central neural as well as systemic effects. However, we do not have any data to substantiate this in our model. TRPA1 also appears to play a role in the increase in LVDP 24 h after exposure. We have also shown myocardial dyssynchrony and increased left ventricular stroke volume in C57BL/6 mice 24 h after acrolein (Thompson et al., 2016). Similarly, Tong et al. (Tong et al., 2010) showed a trend toward increasing LVDP after particulate matter exposure. It is possible that the increase in LVDP observed here is a compensatory response to increased stroke volume to maintain cardiac output.
Table 4.
Summary of exposure effects.
| Exposure group | HR | SDNN | RMSSD | LF | HF | PR | QRS | QTc | Arrhythmia | LVDP baseline | LVDP post-ischemia recovery | dP/dT (+) | dP/dT (−) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acrolein | NE | ↑ | ↑ | ↑ | ↑ | NE | NE | NE | ↑ | ↑ | NE | NE | NE |
| Ozone | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE |
| Filtered air | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE |
In conclusion, we showed here that a single acute exposure to acrolein disrupts normal autonomic balance and produces arrhythmia in healthy, young mice. We previously showed that TRPA1 mediates increased arrhythmia through sympathetic modulation in rats exposed to diesel exhaust. Although this is seemingly an opposite effect to what is described here, it is not entirely surprising given the previous results were demonstrated in a model of hypertension, which can cause decreased HRV. Regardless, disruption of autonomic homeostasis can increase the risk of subsequent adverse cardiovascular events as was seen here with the increased incidence of arrhythmia. Acute exposure to airway irritants may not cause overt functional effects, but rather may produce latent or subclinical effects that are only manifested when the host is challenged by a subsequent stressor or disease state. However, the likelihood of this occurring clearly depends on not only the level (i.e. concentration, duration, etc.) of exposure, but the characteristics of the pollutant as well. The underlying health of the host must also be considered because the greatest risk may be in subjects with eroded or impaired compensatory capacity as is seen with chronic respiratory and cardiovascular disease. Despite a somewhat better understanding of this mechanism, all of these factors must be accounted for if a proper assessment of risk is to be made.
Acknowledgments
We are grateful to Najwa Haykal Coates, Keith Chesnutt, and Kimberly Stratford for their technical assistance in carrying out these experiments. We thank Dr. Ian Gilmour for his careful review of this manuscript. This work was funded by the joint UNC Curriculum in Toxicology-EPA Training Agreement, CR83515201 and EPA Cooperative Agreement, CR83578501.
Footnotes
Disclaimer: This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA, nor does mention of trade names.
Conflict of interest statement
The authors state that there is no conflict of interest.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- Abbott-Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr Top Med Chem. 2013;13:310–321. doi: 10.2174/1568026611313030008. [DOI] [PubMed] [Google Scholar]
- Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–1268. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- Barath S, Langrish JP, Lundbäck M, Bosson JA, Goudie C, Newby DE, Sandström T, Mills NL, Blomberg A. Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol Sci. 2013;135:292–299. doi: 10.1093/toxsci/kft157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Büch T, Schäfer E, Steinritz D, Dietrich A, Gudermann T. Chemosensory TRP channels in the respiratory tract: role in toxic lung injury and potential as “sweet spots” for targeted therapies. Rev Physiol Biochem Pharmacol. 2013;165:31–65. doi: 10.1007/112_2012_10. [DOI] [PubMed] [Google Scholar]
- Campen M, Robertson S, Lund A, Lucero J, McDonald J. Engine exhaust particulate and gas phase contributions to vascular toxicity. Inhal Toxicol. 2014;26:353–360. doi: 10.3109/08958378.2014.897776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MG, Thevenin BJ. High-resolution 12-lead electrocardiograms of on-duty professional firefighters: a pilot feasibility study. J Cardiovasc Nurs. 2009;24(Suppl 4):261–267. doi: 10.1097/JCN.0b013e3181a4b250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Lust RM, Hazari MS, Perez CM, Krantz QT, King CJ, Winsett DW, Cascio WE, Costa DL, Farraj AK. Diesel exhaust inhalation increases cardiac output, bradyarrhythmias, and parasympathetic tone in aged heart failure-prone rats. Toxicol Sci. 2013;131:583–595. doi: 10.1093/toxsci/kfs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Haykal-Coates N, Winsett DW, Hazari MS, Ledbetter AD, Richards JH, Cascio WE, Costa DL, Farraj AK. Cardiomyopathy confers susceptibility to particulate matter-induced oxidative stress, vagal dominance, arrhythmia and pulmonary inflammation in heart failure-prone rats. Inhal Toxicol. 2015;27:100–112. doi: 10.3109/08958378.2014.995387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess GF, Tam RM, Calaresu FR. Influence of cardiac neural inputs on rhythmic variations of heart period in the cat. Am J Phys. 1975;228:775–780. doi: 10.1152/ajplegacy.1975.228.3.775. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Davoodi G, Sharif AY, Kazemisaeid A, Sadeghian S, Farahani AV, Sheikhvatan M, Pashang M. Comparison of heart rate variability and cardiac arrhythmias in polluted and clean air episodes in healthy individuals. Environ Health Prev Med. 2010;15:217–221. doi: 10.1007/s12199-009-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150:1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- Deboor SS, Pelter MM, Adams MG. Nonrespiratory sinus arrhythmia. Am J Crit Care. 2005;14:161–162. [PubMed] [Google Scholar]
- DeJarnett N, Conklin DJ, Riggs DW, Myers JA, O’Toole TE, Hamzeh I, Wagner S, Chugh A, Ramos KS, Srivastava S, Higdon D, Tollerud DJ, DeFilippis A, Becher C, Wyatt B, McCracken J, Abplanalp W, Rai SN, Ciszewski T, Xie Z, Yeager R, Prabhu SD, Bhatnagar A. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K, Schwartz JE, Pickering TG, Hoshide S, Ishikawa J, Shimada K, Kario K. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens Res. 2010;33:737–742. doi: 10.1038/hr.2010.61. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Hazari MS, Haykal-Coates N, Lamb C, Winsett DW, Ge Y, Ledbetter AD, Carll AP, Bruno M, Ghio A, Costa DL. ST depression, arrhythmia, vagal dominance, and reduced cardiac micro-RNA in particulate-exposed rats. Am J Respir Cell Mol Biol. 2011;44:185–196. doi: 10.1165/rcmb.2009-0456OC. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Hazari MS, Winsett DW, Kulukulualani A, Carll AP, Haykal-Coates N, Lamb CM, Lappi E, Terrell D, Cascio WE, Costa DL. Overt and latent cardiac effects of ozone inhalation in rats: evidence for autonomic modulation and increased myocardial vulnerability. Environ Health Perspect. 2012;120:348–354. doi: 10.1289/ehp.1104244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farraj AK, Walsh L, Haykal-Coates N, Malik F, McGee J, Winsett D, Duvall R, Kovalcik K, Cascio WE, Higuchi M, Hazari MS. Cardiac effects of seasonal ambient particulate matter and ozone co-exposure in rats. Part Fibre Toxicol. 2015;12:12. doi: 10.1186/s12989-015-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–1261. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25:794–810. doi: 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicol Sci. 2009a;110:224–234. doi: 10.1093/toxsci/kfp092. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. A single exposure to particulate or gaseous air pollution increases the risk of aconitine-induced cardiac arrhythmia in hypertensive rats. Toxicol Sci. 2009b;112:532–542. doi: 10.1093/toxsci/kfp214. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Callaway J, Winsett DW, Lamb C, Haykal-coates N, Krantz QT, King C, Costa DL, Farraj AK. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Griggs J, Winsett DW, Haykal-Coates N, Ledbetter A, Costa DL, Farraj AK. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovasc Toxicol. 2014;14:52–63. doi: 10.1007/s12012-013-9228-9. [DOI] [PubMed] [Google Scholar]
- Heathers JA. Everything Hertz: methodological issues in short-term frequency-domain HRV. Front Physiol. 2014;5:177. doi: 10.3389/fphys.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JF, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats and rabbits. J Physiol. 1995;489(Pt 1):203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE. Trigeminal TRPs and the scent of pain. Pain. 2011;152:4–5. doi: 10.1016/j.pain.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Ehrlich BE. TRP channels in disease. Subcell Biochem. 2007;45:253–271. doi: 10.1007/978-1-4020-6191-2_9. [DOI] [PubMed] [Google Scholar]
- Keller KB, Lemberg L. The sick sinus syndrome. Am J Crit Care. 2006;15:226–229. [PubMed] [Google Scholar]
- Kratschmer F. On reflexes from the nasal mucous membrane on respiration and circulation. Respir Physiol. 2001;127:93–104. doi: 10.1016/s0034-5687(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Walsh L, Farraj A, Hazari MS. Ozone co-exposure modifies cardiac responses to fine and ultrafine ambient particulate matter in mice: concordance of electrocardiogram and mechanical responses. Part Fibre Toxicol. 2014;11:54. doi: 10.1186/s12989-014-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY. TRPA1 ion channels: a gateway to airway irritation and reflex responses induced by inhaled oxidants. J Physiol. 2010;588:747–748. doi: 10.1113/jphysiol.2010.187286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Horstman DH, Hazucha MJ, Seal E, Jr, Haak ED, Salaam SA, House DE. Pulmonary effects of ozone exposure during exercise: dose-response characteristics. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:1345–1352. doi: 10.1152/jappl.1983.54.5.1345. [DOI] [PubMed] [Google Scholar]
- McMullen MK, Whitehouse JM, Shine G, Towell A. Respiratory and non-respiratory sinus arrhythmia: implications for heart rate variability. J Clin Monit Comput. 2012;26:21–28. doi: 10.1007/s10877-011-9327-8. [DOI] [PubMed] [Google Scholar]
- Miller DB, Karoly ED, Jones JC, Ward WO, Vallanat BD, Andrews DL, Schladweiler MC, Snow SJ, Bass VL, Richards JE, Ghio AJ, Cascio WE, Ledbetter AD, Kodavanti UP. Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol Appl Pharmacol. 2015;286:65–79. doi: 10.1016/j.taap.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 2015;5:e006946. doi: 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Phys. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–1529. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Joad JP, Bonham AC. Chronic passive cigarette smoke exposure augments bronchopulmonary C-fibre inputs to nucleus tractus solitarii neurones and re-flex output in young guinea-pigs. J Physiol. 2000;523(Pt 1):223–233. doi: 10.1111/j.1469-7793.2000.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis. Cardiol Clin. 1992;10:499–537. [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, Cerutti S, Malliani A. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl. 1984;2:S383–S385. [PubMed] [Google Scholar]
- Paton JF, Nolan PJ. Similarities in reflex control of laryngeal and cardiac vagal motor neurones. Respir Physiol. 2000;119:101–111. doi: 10.1016/s0034-5687(99)00105-x. [DOI] [PubMed] [Google Scholar]
- Perez CM, Ledbetter AD, Hazari MS, Haykal-Coates N, Carll AP, Winsett DW, Costa DL, Farraj AK. Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Toxicol Sci. 2013;132:467–477. doi: 10.1093/toxsci/kft008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Piccirillo G, Ogawa M, Song J, Chong VJ, Joung B, Han S, Magri D, Chen LS, Lin SF, Chen PS. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm. 2009;6:546–552. doi: 10.1016/j.hrthm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman F, Pechnik S, Gross D, Sewell L, Goldstein DS. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res. 2011;21:133–141. doi: 10.1007/s10286-010-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Rowan WH, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc Toxicol. 2007;7:28–51. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- Shah ASV, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE. Disinhibition of the cardiac limb of the arterial baroreflex in rat: a role for metabotropic glutamate receptors in the nucleus tractus solitarii. J Physiol. 2006;575:727–738. doi: 10.1113/jphysiol.2006.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK. Increased randomness of heart rate could explain increased heart rate variability preceding onset of atrial fibrillation. J Am Coll Cardiol. 2004;44:668–669. doi: 10.1016/j.jacc.2004.05.009. author reply 669. [DOI] [PubMed] [Google Scholar]
- Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol. 2005;16:954–959. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- Sun B, Bang SI, Jin YH. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport. 2009;20:1002–1006. doi: 10.1097/WNR.0b013e32832d2219. [DOI] [PubMed] [Google Scholar]
- Tani M, Yazawa I, Ikeda K, Kawakami K, Onimaru H. Long-lasting facilitation of respiratory rhythm by treatment with TRPA1 agonist, cinnamaldehyde. J Neurophysiol. 2015;114:989–998. doi: 10.1152/jn.00282.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol. 2006;101:950–959. doi: 10.1152/japplphysiol.00222.2006. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LC, Ledbetter AD, Haykal-Coates N, Cascio WE, Hazari MS, Farraj AK. Acrolein inhalation alters myocardial synchrony and performance at and below exposure concentrations that cause ventilatory responses. Cardiovasc Toxicol. 2016 doi: 10.1007/s12012-016-9360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Cheng WY, Samet JM, Gilmour MI, Devlin RB. Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovasc Toxicol. 2010;10:259–267. doi: 10.1007/s12012-010-9082-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones JF, Jeggo RD, de Burgh Daly M, Jordan D, Ramage AG. Effect of pulmonary C-fibre afferent stimulation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats. J Physiol. 2000;526(Pt 1):157–165. doi: 10.1111/j.1469-7793.2000.t01-1-00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Jiang R, Zhao Z, Song W. Effects of ozone and fine particulate matter (PM(2.5)) on rat system inflammation and cardiac function. Toxicol Lett. 2013;217:23–33. doi: 10.1016/j.toxlet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109(Suppl 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. J Fed Am Soc Exp Biol. 2011;25(Suppl 12):4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-F, Kuo IC, Su TC, Li YR, Lin LY, Chan CC, Hsu SC. Effects of personal exposure to particulate matter and ozone on arterial stiffness and heart rate variability in healthy adults. Am J Epidemiol. 2010;171:1299–1309. doi: 10.1093/aje/kwq060. [DOI] [PubMed] [Google Scholar]


