Abstract
Background
The high prevalence and severity of methamphetamine (MA) abuse demands greater neurobiological understanding of its etiology.
Methods
Here, we conducted immunoblotting and in vivo microdialysis procedures in Methamphetamine High/Low Drinking (MAH/LDR) mice, as well as in isogenic C57BL/6J mice that varied in their MA-preference/taking, to examine the glutamate underpinnings of MA abuse vulnerability. Neuropharmacological and Homer2 knock-down approaches were also employed in C57BL/6J mice to confirm the role for nucleus accumbens glutamate/Homer2 expression in MA preference/aversion.
Results
We identified a hyper-glutamatergic state within the nucleus accumbens (NAC) as a biochemical trait corresponding with both genetic and idiopathic vulnerability for high MA-preference and -taking. We also confirmed that subchronic, subtoxic MA experience elicits a hyper-glutamatergic state within the NAC during protracted withdrawal, characterized by elevated mGlu1/5 receptor function and Homer2 receptor-scaffolding protein expression. A high MA-preferring phenotype was recapitulated by elevating endogenous glutamate within the NAC shell of mice and we reversed MA-preference/taking by lowering endogenous glutamate and/or Homer2 expression within this subregion.
Conclusions
Our data point to an idiopathic, genetic or drug-induced hyper-glutamatergic state within the NAC as a mediator of MA addiction vulnerability.
Keywords: nucleus accumbens, glutamate, Homer proteins, metabotropic glutamate receptor, NMDA receptor, MAHDR, conditioned place-preference
Introduction
Methamphetamine (MA) is one of the most commonly abused illicit drugs worldwide (1). MA-taking elicits various drug effects, ranging from those with positive to those with negative motivational/affective valence (e.g., euphoria, high energy versus anxiety, dysphoria) (2,3). Individual differences in the perception of drug effects as appetitive or aversive influences risk of continued drug use and addiction (4,5). However, many confounding variables (e.g., prior drug use, trauma or aversive life events) render it difficult to disentangle cause-effect relations in human subjects in any systematic, experimentally-controlled, fashion.
The high rate of MA abuse presumably relates to this drug’s ability to augment forebrain monoaminergic neurotransmission (6,7). However, some preclinical studies question this mechanism for MA abuse/addiction risk (8). In laboratory rodents, acute or subchronic experience with subtoxic MA doses impacts extracellular glutamate within corticostriatal projections (7–11) highly implicated in the addiction neurocircuitry (12,13), arguing that MA-induced perturbations in corticostriatal glutamate may be an antecedent of MA abuse that drives continued use/addiction. Herein, we found that both genetic (induced by selective breeding) and idiopathic individual variation in MA-preference/taking relates to a hyper-glutamatergic state within the nucleus accumbens shell (NACs) and core (NACc) subregions, which can be recapitulated by subchronic MA experience. Importantly, MA-preference can be bi-directionally regulated by manipulating endogenous glutamate within the NACs and reversed by lowering the expression of the glutamate receptor scaffolding protein Homer2. Thus, glutamate hyperactivity within the NAC is both an antecedent and a consequence of MA-taking that provides a viable target for therapeutic intervention in MA abuse/addiction.
Materials and Methods
Experimental Overview
To test the hypothesis that NAC glutamate anomalies accompany genetic predisposition to consume MA, we conducted in vivo microdialysis and immunoblotting procedures on mouse lines selectively bred to exhibit MA High Drinking (MAHDR) and MA Low Drinking (MALDR) (14,15). We next examined whether individual variance in MA-preference/taking might correlate with indices of NAC glutamate transmission in an isogenic model that involved screening a large (N=186) group of inbred C57BL/6J (B6) mice for MA-reward/aversion using place-conditioning procedures, employed to study the genetics and neuropharmacology of MA addiction vulnerability/resiliency and locomotor hyper-activity (7,9,16–18). Subsets of MA-preferring, -neutral or – avoiding mice were then tested either for subsequent MA reinforcement/intake or glutamate-related protein expression within NAC subregions.
As MA-conditioning increased glutamate-related protein expression only in B6 mice expressing a place-preference, we more thoroughly characterized the effects of repeated MA upon biochemical indices of NAC glutamate transmission. To control individual drug experience that can confound data interpretation, we employed a non-contingent, repeated MA injection regimen (10 × 2 mg/kg, IP) that elicits behavioral and dopamine sensitization (7,19,20). The functional relevance of endogenous glutamate/Homer2 scaffolding within the NACs for the manifestation of MA-preference/aversion was determined using neuropharmacological approaches and a small hairpin RNA to knock-down Homer2b expression (shRNA-Homer2b).
The details of the procedures for the experiments are provided in the Supplemental Online Methods section.
Results
Elevated indices of NAC glutamate transmission correspond with high MA-taking in a genetic model of addiction vulnerability/resiliency
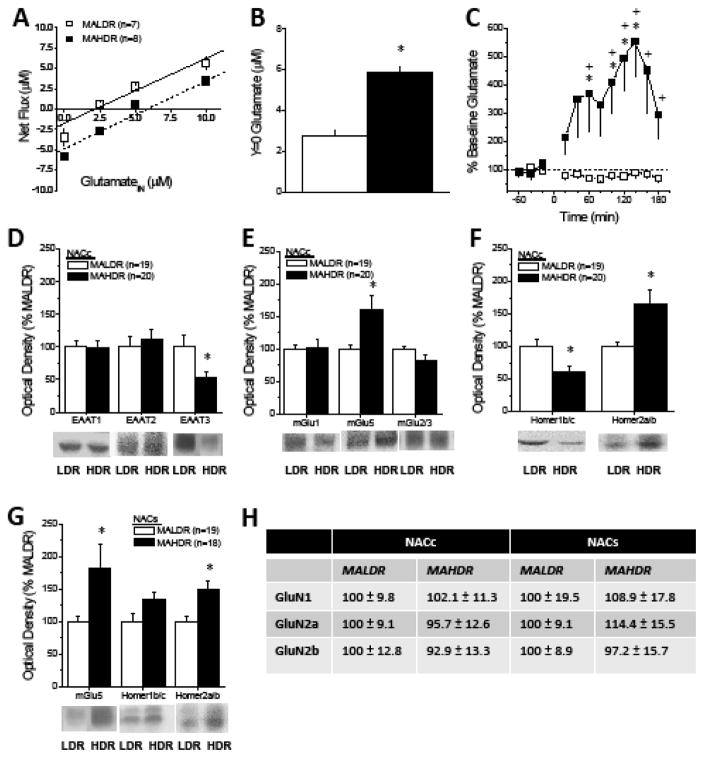
Using no net-flux in vivo microdialysis procedures, we established that the basal extracellular glutamate content within the NAC of MAHDR mice was more than twice that of MALDR mice (Fig. 1a,b); this line difference was replicated using conventional microdialysis procedures [MAHDR=4.17 ± 1.12 pg/sample; MALDR=1.42 ± 0.17 pg/sample; t(13)=2.27, p=0.04]. Despite this, acute MA elicited a robust rise in NAC glutamate only in MAHDR mice (Fig. 1c). These data served as our first indications that a hyper-glutamatergic state within the NAC is a biochemical correlate of high MA-taking.
Figure 1. NAC GLU correlates of MA addiction vulnerability/resiliency in a genetic model.
(A) No net-flux in vivo microdialysis procedures were employed to estimate basal GLU content within the shell-core interface of the NAC of MA-naïve mice selectively bred for Methamphetamine High Drinking (MAHDR) and Methamphetamine Low Drinking (MALDR) and (B) an examination of y=0 confirmed higher GLUEC in MAHDR vs. MALDR mice [t(13)=8.93, p<0.0001]. (C) Conventional microdialysis detected marked line differences also in the capacity of acute MA (2 mg/kg, IP) to elevate GLUEC [Genotype X Time interaction [F(11,143)=4.37, p<0.0001], with MAHDR mice exhibiting a large rise in GLUEC [one-way ANOVA, F(11,77)=4.74, p<0.0001], but no change in GLUEC in MALDR animals (one-way ANOVA, p=0.36). Immunoblotting conducted on NACc tissue of MALDR and MAHDR mice revealed line differences in the total protein expression of (D) EAAT3 [t(37)=2.32, p=0.03], (E) mGlu5 monomer [t(37)=2.78, p=0009] and (F) both Homer1b/c [t(37)=2.75, p=0.007] and Homer2a/b [t(37)=3.01, p=0.005]. (G) Although Homer1b/c levels within the NACs did not vary with line (t-test, p=0.27), MAHDR mice also exhibited greater mGlu5 [t(35)=2.28, p=0.03] and Homer2a/b [t(35)=2.08, p=0.04] expression within this subregion. (H) Line differences were not apparent regarding the expression of NMDA receptor subunits within either subregion (t-tests, all p’s>0.20). Samples sizes indicated in parentheses.
Despite no line difference in glutamate clearance/reuptake (Fig. 1a; slopes/Ed: MAHDR= 0.77 ± 0.09; MALDR=0.83 ± 0.07; t-test, p=0.66), MAHDR mice exhibited lower expression of the neuronal-specific EAAT3 glutamate transporter within the NACc (Fig. 1d), which could contribute to their elevated glutamate content. In contrast, MAH/LDR differences were not observed for EAAT3 expression within the adjacent NACs subregion (not shown) or for the expression of the glial-specific EAAT1 and EAAT2 transporters within either subregion (Fig. 1d).
Group1 and Group2 metabotropic glutamate receptor (mGluR) stimulation raises and lowers, respectively, extracellular glutamate levels (21–25). Although no line differences were noted for either total mGlu1 or mGlu2/3 expression, mGlu5 levels were higher within both NACc (Fig. 1e) and NACs (Fig. 1g) of MAHDR mice. mGlu5 is scaffolded by Homer proteins (26) that regulate both basal and stimulated glutamate release within NAC (27). While NAC Homer1b/c levels did not vary by line, Homer2a/b levels were elevated within both NAC subregions (Fig. 1f,g) of MAHDR mice, corresponding with their hyper-glutamatergic state (28,29). Although Homer proteins also scaffold NMDA glutamate receptors (29), line differences were not apparent in NMDA receptor subunit expression within either subregion (Fig. 1h). Although total protein measures cannot inform as to the subcellular localization or function of receptor proteins, these biochemical data point to an mGluR/Homer2-related mechanism as a potential contributor to the hyper-glutamatergic state within NAC subregions accompanying vulnerability to MA intake in our genetic model.
MA-preference predicts subsequent MA-taking and reinforcement in an isogenic model
As illustrated in Suppl. Fig. S1, the majority (57%) of B6 mice tested in our large study (n=186) of MA-induced place-conditioning liked the 2 mg/kg MA dose, as indicated by a conditioned place-preference (CPP). However, approximately 10% of the population tested disliked 2 mg/kg MA, as indicated by a conditioned place-aversion (CPA), while 33% of the animals exhibited neither preference nor aversion and were MA-ambivalent (Neutral).
Although MA-preference/taking can relate to sensitivity to the drug’s psychomotor-activating effects (16,30,31), within the large sample of B6 mice tested, group differences in spontaneous or MA-induced locomotor activity were not detected (Suppl. Table S1). To develop our B6 model further, we employed extinction/reinstatement procedures (18,32) in a subsample of CPP and CPA mice to assay the strength of the memory of MA’s motivational valence during drug withdrawal. Despite exhibiting a MA-conditioned response of similar magnitude at the outset of extinction (Suppl. Fig S2a; see also Fig. 2a,3a), the memory of the MA-induced positive subjective experience was more robust than the negative subjective experience, as indicated by (1) a greater number of days required to extinguish the place-preference vs. –aversion (Suppl. Fig. S2b) and (2) an increased capacity of a MA-priming injection (2 mg/kg, IP) to reinstate the conditioned appetitive response following extinction (Suppl. Fig. S2c). Thus, as for cocaine (33), MA-related memories with a positive valence are more robust and persistent than those with a negative valence. The above results are in line with human studies demonstrating that initial subjective reactivity to amphetamines can predict future drug abuse (34) and perhaps also, addiction severity.
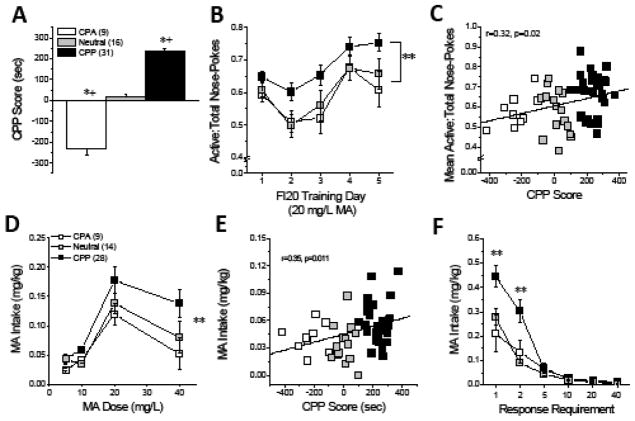
Figure 2. Initial subjective response to MA predicts MA addiction vulnerability in an inbred murine model.
The predicative validity of responses in our MA place-conditioning procedure for addiction vulnerability/resiliency was determined in (A) a cohort of B6 mice expressing divergent subjective responses to the MA-paired compartment [F(2,54)=170.42, p<0.0001; *p<0.05 vs. Neutral, LSD post-hoc tests; one-sample t-tests, for CPA: t(8)=7.30, p<0.0001; for CPP: t(30)=20.46, p<0.0001; for Neutral, p=0.29; +p<0.05 vs. CPP Score = 0]. (B) Early in operant-conditioning (FI20 reinforcement schedule), all mice progressively increased their proportion of responses towards the aperture that delivered 20 mg/L MA; however CPP mice consistently directed a greater proportion of their total responses towards the active hole than the other phenotypes [Phenotype effect: F(2,53)=4.90, p=0.01; FRI20 Day effect: F(4,212)=13.87, p<0.0001; interaction: p=0.79; ** p<0.03 vs. CPA/Neutral, LSD post-hoc tests]. (C) CPP Score correlated positively with the average response allocation during the first 5 days of training (from panel b) [r=0.32, p=0.02; N=56]. (D) For those mice that successfully completed training, a dose-response analysis of MA intake revealed a shift upwards in CPP mice, relative to the other phenotypes [MA dose: F(3,144)=15.85, p<0.0001; Phenotype: F(2,48)=7.02, p=0.002; interaction, p=0.57; ** p<0.05 vs. CPA/Neutral, LSD post-hoc test]. (E) CPP Score predicted the intake of 10 mg/L MA (as well as 40 mg/L, not shown) under an FR5/FI20 reinforcement schedule. (F) A demand-intake analysis of behavior when 10 mg/L MA served as the reinforcer revealed greater MA intake under low demand in CPP mice, relative to the other phenotypes [Phenotype X Schedule: F(5,240)=5.82, p<0.0001; ** p<0.05 vs. CPA/Neutral, LSD post-hoc tests]. Sample sizes are indicated in parentheses and the results of the correlational analyses are presented in their corresponding panels.
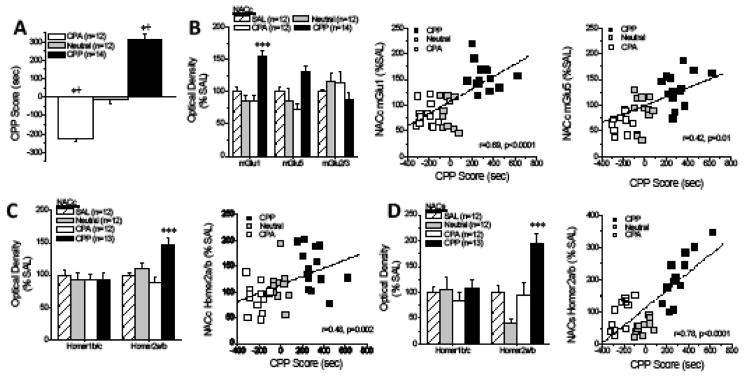
Figure 3. NAC glutamate correlates of MA addiction vulnerability/resiliency in an inbred murine model.
(A) Immunoblotting was employed on MA-induced CPP, CPA and Neutral-B6 mice to assay for indices of protein levels of glutamate transmission within NAC subregions [CPP Score: F(1,37)=121.00, p<0.0001; *p<0.05 vs. Neutral, LSD post-hoc tests; +p<0.05 vs. CPP Score = 0 sec, one-sample t-tests]. (B) Within the NACc, mGlu1 and mGlu5 levels were highest in CPP mice [for mGlu1: [F(3,49)=21.88, p<0.0001, ***p<0.05 vs. all other groups, LSD post-hoc tests; for mGlu5: [F(3,49)=2.70, p=0.06; for mGlu2/3, p=0.34] and the expression of both mGlu1 and mGlu5 was predicted by CPP Score (from panel a). (C) Homer2a/b expression was also elevated within the NACc of CPP mice [for Homer2a/b: F(3,49)=8.92, p<0.0001; ***p<0.05 vs. all other groups, LSD post-hoc tests; for Homer1b/c: p=0.93] with expression also predicted by CPP score. (D) Within the NACs, Homer2a/b levels were also the most highly expressed in CPP mice [F(3,41)=8.10, p<0.0001; *p<0.05 Neutral vs. SAL; ***p<0.05 CPP vs. other groups, LSD post-hoc tests]. Samples sizes are indicated in parentheses.
To further address this issue, we tested the hypothesis that subjective reward/aversion reactivity to MA predicted subsequent MA reinforcement/intake in our B6 model. Even at study outset, CPP-B6 mice directed a greater proportion of their responses towards the nose-poke aperture that delivered MA (a.k.a. active hole; Fig. 2b). Further, CPP Score correlated positively with this early measure of MA reinforcement (Fig. 2c). When we assayed for phenotypic differences in sensitivity to MA reinforcement, the dose-response function for MA intake was shifted upwards in CPP- mice versus Neutral- and CPA-B6 animals (Fig. 2d), with the average intake of both the 10 and 40 mg/L solutions correlated significantly and positively with CPP Score (for 10 mg/L: see Fig. 2e; for 40 mg/L: r=0.37, p=0.04, N=51). Given that CPP Score predicted the intake of 10 mg/L MA (Fig. 2e), we next examined whether CPP Score also predicted motivation for this lower MA dose. In all groups, MA intake dropped precipitously with increasing demand; however, CPP mice consumed significantly more MA under the low-effort FI20 and FR2/FI20 schedules than the other mice tested (Fig. 2f). Not shown, MA intake under these reinforcement schedules was also predicted by CPP Score (for both schedules: r=0.40, p=0.003, N=51). Thus, akin to MAH/LDR mice (15), MA-preference in our B6 model extends across place- and operant-conditioning paradigms.
Elevated NAC Homer2 expression corresponds with traits that predict MA addiction vulnerability in an isogenic model
Relative to the other groups tested, MA-preferring CPP-B6 mice exhibited elevated NACc expression of mGlu1, mGlu5 and Homer2a/b, and CPP Score correlated with protein expression for all 3 proteins (Fig. 3b,c). Although NACs mGluR expression did not vary with MA-preference (univariate ANOVAs, all p’s>0.10; data not shown), CPP-B6 mice exhibited higher Homer2a/b expression within NACs, with expression strongly correlated with CPP Score (Fig. 3d). In contrast, no obvious relation existed between MA-preference and the expression of Homer1b/c (Fig. 3c,d; one-way ANOVAs, p’s>0.90), mGlu2/3 (e.g., Fig. 3b), EAAT2 and EAAT3 or NMDA receptor subunits (Suppl. Table S2).
Elevated indices of NAC glutamate transmission are pharmacodynamic consequences of MA exposure
Repeated MA injections elicited a time-dependent increase in basal extracellular glutamate content within the NAC of B6 mice (Fig. 4a; x-intercept); however, the elevated extracellular glutamate exhibited by MA-injected mice did not relate in any obvious manner to a change in the Ed/clearance (Drug X Withdrawal ANOVA, all p’s>0.25; Fig. 4a). Repeated MA also elicited a time-dependent sensitization of the capacity of low-dose MA (1 mg/kg) to increase extracellular glutamate in the NAC (Fig. 4b) – a finding in line with prior reports for both non-contingent and behavior-contingent delivery of intravenous MA (8,9).
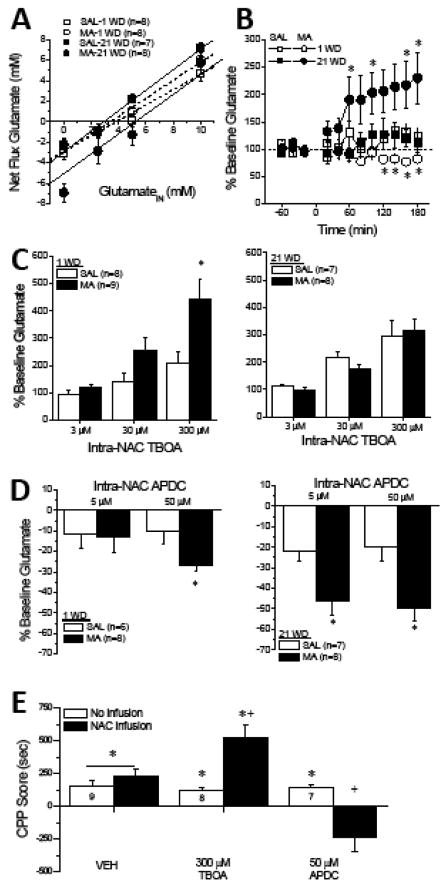
Figure 4. Endogenous glutamate within the NAC bi-directionally regulates MA-preference.
(A) No net-flux in vivo microdialysis procedures revealed that repeated MA (10 daily injections of 2 mg/kg, IP) increased basal extracellular glutamate content (y=0), but only at the 21-day withdrawal time-point (21 WD) [Treatment X Withdrawal: F(1,30)=7.80, p=0.009]. No group difference in the Ed was apparent when the slopes of the linear regressions for each plot were compared (Treatment X Withdrawal ANOVA, all p’s>0.08). *p<0.05 vs. SAL; +p<0.05 vs. 1 WD (t-tests). (B) A significant Treatment X Withdrawal X Time interaction was observed for the change in extracellular glutamate elicited by a challenge injection of 1 mg/kg MA, administered at either 1 or 21 WD [F(11,319)=3.12, p=0.001]. This interaction reflected the fact that the MA challenge injection lowered glutamate below baseline in MA-treated animals at 1 WD, but these same mice exhibited a robust glutamate response to the same challenge at 21 WD [Withdrawal X Time interaction: F(11,176)=2.93, p=0.001]. In contrast, the MA challenge elicited a modest glutamate rise in SAL animals that was comparable between microdialysis sessions [Withdrawal X Time ANOVA, all p’s>0.05]. *p<0.05 vs. SAL (tests for simple effects). (C) Repeated MA treatment sensitized mice to the capacity of the EAAT inhibitor TBOA to increase NAC glutamate, but this effect was apparent only in early withdrawal [for 1 WD, Dose X Treatment: F(2,30)=6.62, p=0.004; for 21 WD, Dose X Treatment: p=0.39; *p<0.05 for MA vs. SAL, t-tests]. (D) Repeated MA treatment also sensitized mice to the capacity of the mGlu2/3 autoreceptor agonist APDC to reduce NAC glutamate, irrespective of withdrawal [Dose X Treatment: F(2,48)=4.64, p=0.004; 3-way interaction: p=0.94], with SAL-MA differences in the magnitude of the glutamate reduction observed at both 1 WD [50 μM: t(12)=2.59, p=0.02] and 21 WD [5μM: t(14)=2.95, p=0.01; 50μM: t(13)=3.38, p=0.005; *p<0.05 for MA vs. SAL]. (E) In a separate cohort of B6 mice, the repeated pairing of MA (4 × 2 mg/kg) elicited a place-preference when mice were tested in a drug-free state (no infusion; open bars; Drug X Side, p>0.75]. However, raising and lowering endogenous glutamate via infusion of 300 μM TBOA or 50 μM APDC into the NACs, respectively, potentiated and reversed the place-preference, relative to vehicle (VEH)-infused animals [Drug X Side: F(1,21)=17.84, p<0.0001; *p<0.05 Paired vs. Unpaired or conditioning; +p<0.05 vs. VEH, t-tests]. Samples sizes are indicated in the data bars.
In vivo microdialysis and immunoblotting were then employed to understand the molecular basis of the hyper-glutamatergic state observed in MA-injected mice. Consistent with their elevated basal glutamate, MA-treated mice exhibited greater glutamate sensitivity to the EAAT inhibitor TBOA; however, this effect was apparent in early, but not later, withdrawal (Fig. 4c), and did not coincide with changes in EAAT2/3 expression within the NACs (Suppl. Fig. S3a) or NACc (not shown; Treatment X Withdrawal ANOVA, all p’s>0.10; n=9–12). In contrast, intra-NAC infusion of the system Xc- substrate cystine did not influence extracellular glutamate, irrespective of the animals’ MA history (Suppl. Fig. S3b–d). Combined, these results argued that a history of repeated MA elicits only transient changes in EAAT function in vivo, without influencing sodium-independent glutamate transport within this region.
We then probed for MA’s effects upon mGlu2/3 autoreceptor function to account for the MA-induced rise in basal glutamate content. APDC reduced NAC extracellular glutamate levels; however, MA-injected mice were more, rather than less, sensitive to this APDC effect (Fig. 4d). While unexpected, this outcome likely does not reflect non-selective effects of APDC upon mGlu1/5 receptors, based on the doses selected for study (35) and evidence that mGlu1/5 stimulation elevates extracellular glutamate in forebrain (21–24; Suppl. Fig S4c). As increased autoreceptor sensitivity could not explain the hyper-glutamatergic state of MA-injected animals, we examined for changes in glutamate release elicited by either NMDA or mGlu1/5 receptor stimulation. Irrespective of withdrawal, there was no significant MA effect on NMDA-stimulated glutamate release within the NAC, although immunoblotting revealed moderate, time-independent, reductions in NMDA receptor subunits within the NACc of MA-injected animals (Suppl. Fig. S4a,b). However, consistent with their hyper-glutamatergic profile, MA-treated animals exhibited greater glutamate release in response to infusions of the mGlu1/5 agonist DHPG – an effect only apparent at 21 days withdrawal (Suppl. Fig. S4c). An attempt to relate the MA-sensitization of mGlu2/3 and mGlu1/5 function to changes in total protein expression failed to detect group differences in mGlu2/3 or mGlu5 levels within either NAC subregion (Suppl. Fig. S4d), although MA-injected animals exhibited lower mGlu1 expression within the NACs (Suppl. Fig. S4d′). While total protein expression cannot inform as to the plasma membrane localization of the receptors/transporters studied herein, this latter result is interesting as DHPG-elicited glutamate release within the NAC is mGlu1-dependent (21).
Endogenous glutamate within the NACs gates the motivational valence of MA
Consistent with the hypothesis that a hyper-glutamatergic state drives MA-preference, TBOA infusion increased place-preference magnitude, while APDC infusion elicited a robust place-aversion (Fig. 4e). Neither TBOA nor APDC significantly influenced the locomotor activity of the animals during the test session (not shown; one-way ANOVA, p=0.12). These data indicate that the hyper-glutamatergic state induced by MA exposure is causal to the motivational valence of MA.
Elevated Homer2 within the NACs enhances MA-seeking and –taking
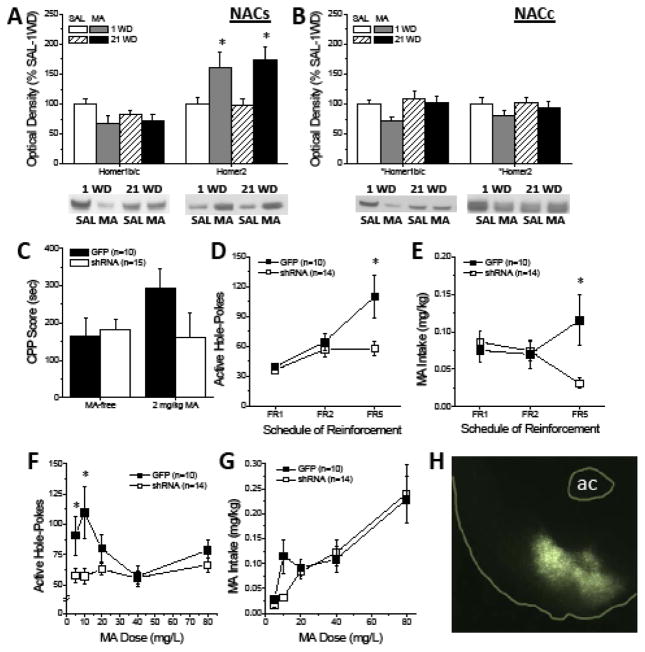
Homer proteins regulate NAC extracellular glutamate levels in vivo (27). Thus, we determined how MA history impacted NAC Homer protein expression. Interestingly, repeated MA history lowered and raised, respectively, Homer1b/c and Homer2a/b levels within the NACs (Fig. 5a). In contrast, repeated MA did not alter Homer expression within the NACc, although there was a trend for reduced protein levels at 1 day withdrawal (Fig. 5b). We next employed a small hairpin RNA (shRNA)-Homer2b strategy (36) to knock-down protein expression within the NACs (Fig. 5h), prior to behaviorally testing B6 mice. Relative to mice infused with green fluorescent protein (GFP) control, shRNA-Homer2b did not impact the expression of a MA-conditioned place-preference when mice were tested in a MA-free state (Fig. 5c, left), nor did it influence the locomotor activity of the animals during conditioning (Suppl. Table S3). However, the magnitude of the place-preference trended upwards in GPF-infused animals when mice were tested immediately following a 2 mg/kg MA priming injection, while that exhibited by shRNA-infused animals was unaffected by the interoceptive MA cue (Fig. 5c). This result argues that reducing NACs Homer2b expression does not alter the MA-conditioned reward but blunts the positive motivational valence of MA’s interoceptive effects.
Figure 5. Repeated MA experience elevates NACs Homer2 expression to promote MA-seeking and – taking.
(A) Immunoblotting conducted on the NACs of B6 mice treated repeatedly with MA (10 IP injections of 2 mg/kg) revealed a moderate, albeit significant, reduction in Homer1b/c expression relative to SAL controls, irrespective of withdrawal [Treatment effect: F(1,36)=4.61, p=0.04; n’s=11–12]. Interestingly, these same animals exhibited a polar opposite increase in Homer2a/b expression [Treatment effect: F(1,31)=5.47, p=0.03; n’s=11–12]. (B) No changes in Homer protein expression were observed within the adjacent NACc (Treatment X Withdrawal ANOVAs, all p’s>0.20; n’s=10–12). For both panels a & b, representative immunoblots are provided, corresponding with their respective datasets above and *p<0.05 denotes a main Treatment effect (p<0.05). (C) When tested in a MA-free state, an intra-NACs infusion of AAV-shRNA-Homer2b did not impact the magnitude of a MA-conditioned place-preference induced by 4 pairings of 2 mg/kg MA. However, only GFP animals exhibited a relative increase in place-preference magnitude when tested in the presence of a 2 mg/kg MA interoceptive cue, however the group difference in this regard was shy of statistical significance [AAV X Side X Test: F(1,23)=3.04, p=0.09]. (D) During operant conditioning for reinforcement by 10 mg/L MA, shRNA-infused mice emitted fewer nose-pokes than green fluorescent protein (GFP) controls with increasing response requirement [AAV X Schedule: F(2,44)=5.92, p=0.005; *p<0.05 vs. GFP, t-tests]. (E) In parallel, shRNA-infused mice consumed less MA with increasing response requirement during this training phase [AAV X Schedule: F(2,44)=7.84, p=0.001; *p<0.05 vs. GFP, t-tests]. (F) shRNA-Homer2b infusion also flattened the MA dose-nose-poke response function under an FR1/FI20 schedule of reinforcement [AAV X Dose: F(4,88)=4.75, p=0.002; *p<0.05 vs. GFP, t-tests]. (G) Although it appeared that shRNA-Homer2b infusion reduced the intake of 10 mg/L MA, there was no systematic effect of knock-down on the MA dose-intake function [AAV X Dose, p>0.3]. (H) Visualization of the GFP reporter by fluorescent microscopy indicating neuronal transduction within the NACs at 10X magnification. For panels c–g, sample sizes are indicated in parentheses.
We next trained the same cohort of mice to self-administer oral MA. Unlike GFP-infused mice, shRNA-infused animals failed to augment their nose-poking behavior under the higher reinforcement schedules during self-administration training (Fig. 5d). In parallel, shRNA-infused animals consumed less MA under higher demand schedules than did GFP controls (Fig. 5e). Thus, shRNA-Homer2b infusion reduced the reinforcing efficacy and associated intake of MA. Further, the MA dose-response function in GFP controls was inverted U-shaped, with mice responding most robustly for 5 and 10 mg/L MA under a simple fixed interval 1 (FI1) reinforcement schedule. In contrast, the dose-response function in shRNA-infused animals was completely flat (Fig. 5f). Despite the rather obvious effect of shRNA infusion upon responding for MA, the MA dose-intake functions for the two virus-infused groups were very similar, with the exception of the lower intake of 10 mg/L MA exhibited by shRNA-Homer2b mice (Fig. 5g). Thus, Homer2b expression within the NACs is important for MA reinforcement and, to a lesser extent intake, particularly under scenarios of high response requirement and/or relatively low MA concentrations.
Discussion
Through study of both genetic (selective breeding) and inbred murine models of MA abuse and reward vulnerability, we discovered that a hyper-glutamatergic state within NAC is a biochemical trait corresponding with a high MA-preferring/taking/seeking phenotype, largely resembling that produced by subchronic MA experience. Causal relations between high MA-preference and glutamate hyper-activity within the NACs were established via bi-directional neuropharmacological manipulations of endogenous glutamate and transgenic knock-down of Homer2 expression within this subregion. Together, the present results argue that a hyper-glutamatergic state within the NAC is both an antecedent and consequence of MA abuse of relevance to the etiology and treatment of MA addiction.
Inter-relations between MA-preference and MA-taking in murine models of MA abuse
Individual differences in sensitivity to MA’s rewarding/aversive effects influence risk of continued MA use/addiction in humans (2,3). Consistent with this, the divergent MA-taking phenotypes of MAH/LDR mice (a well-validated genetic model of vulnerability/resiliency to MA addiction-related traits) reflect marked line differences in the motivational valence of MA’s effects, with the high MA-taking MAHDR line exhibiting high sensitivity to the rewarding properties of low-dose MA, but insensitivity to high-dose MA-aversion (15). The B6 progenitor of the MA drinking lines has been reported to consume less MA than the DBA/2J progenitor (14); however, herein, B6 mice voluntarily consumed unadulterated MA solutions and exhibited oral MA reinforcement. Consistent with a relation between initial MA-preference/avoidance and subsequent drug-taking, the rate of operant-response acquisition was faster and the total daily MA intake was greater in the subset of B6 mice exhibiting MA reward (i.e., CPP-B6 mice), relative to B6 mice exhibiting MA-ambivalence (Neutral-B6) or -aversion (CPA-B6). Further, the MA-conditioned response expressed by B6 mice following subchronic drug exposure predicted both subsequent MA reinforcement and voluntary intake. Notwithstanding a full dose-response analysis of place-conditioned behavior, our study outcomes related to individual variance in MA reward/aversion in isogenic B6 mice are very much in line with average differences between the MAH/LDR lines (15) and argue that individual differences in the initial perception of MA’s interoceptive effects as positive or negative (i.e., MA-preference or avoidance) predict risk of subsequent MA-taking also in an isogenic model of MA abuse. Further, examination of published (15) and recent place-conditioning data from MALDR mice at a late stage in selection also indicate individual differences, such that about one-third (35%) exhibit CPP, while the rest exhibit a neutral response (23%) or CPA (42%; Suppl. Fig. S5). By extension then, psychobiological factors influencing initial MA-preference/avoidance in both models, be they genetic or epigenetic, are those with high relevance MA addiction risk/resiliency.
Although MA was a more efficient reinforcer in CPP- vs. Neutral/CPA-B6 mice, the majority of Neutral/CPA B6 mice acquired the operant-response for MA reinforcement and consumed some unadulterated MA across a range of doses. This outcome may seem counterintuitive as one would have predicted little to no MA reinforcement/intake in CPA or neutral animals. However, consistent with prior results for cocaine-induced place-conditioning in rats (33), the results of our extinction/reinstatement study indicate that the memory of MA’s aversive effects is short-lived in B6 mice (~4 days). Further, when re-exposed to MA following extinction of the place-aversion, CPA-B6 mice expressed ambivalence, not aversion, to the drug’s interoceptive effects. Thus, the weak memory of MA’s aversive properties, coupled with the fact that mice can titrate their MA dosing under operant-conditioning procedures to a level that is reinforcing, likely explains the observation of intact, albeit less efficient, MA reinforcement in Neutral/CPA mice.
A hyper-glutamatergic state within the NAC predicts MA abuse vulnerability
In animal models the separation of cause-effect relationships pertaining to potential biomarkers of disease risk/resiliency is tractable to a greater degree than in human subjects. The MAH/LDR selected lines have proven very useful in the discovery of biochemical and genetic correlates of high/low MA-taking (15,37,38). Extending this prior work, the present study identified marked line differences in biochemical indices of NAC glutamate function, with MAHDR mice exhibiting what is most succinctly described as a hyper-glutamatergic state, characterized by elevated basal and MA-stimulated increases in extracellular glutamate, lowered EAAT3 levels, and elevated mGlu5 and Homer2a/b expression. Most strikingly, the NAC hyper-glutamatergic profile of MA-naïve MAHDR mice resembles that observed within the NAC of rats (8) or mice withdrawn from subchronic MA exposure (see Table 1). As discussed below, the MA-injection regimen selected for this study produces both behavioral and neurochemical (7,19,20) sensitization in rodents. Thus, genetic vulnerability to high MA-taking is associated with a “pre-sensitized” glutamate state within the NAC. Most remarkably, the NAC hyper-glutamatergic state observed in MAHDR mice bears strong resemblance to that observed in our presumably isogenic B6 model of MA vulnerability/resiliency (Table 1). Although this B6 model precludes any post-mortem investigation of potential protein correlates of MA vulnerability/resiliency in the drug-naïve state, the strong positive correlations between MA-preference and NAC mGlu1, mGlu5 and Homer2a/b expression in B6 mice, coupled with the generalization of a “MA-preferring” phenotype across place- and operant-conditioning paradigms, provides independent correlative evidence for a link between MA abuse vulnerability and NAC glutamate hyper-activity that complements our results for MAH/LDR animals.
Table 1.
Comparison of the major findings regarding the NAC glutamate phenotype of: (1) MA-injected B6 mice (10 × 2 mg/kg, IP) vs. saline controls (SAL-injected); 2) MAHDR vs. MALDR selected lines; and 3) MA-conditioned (4 × 2 mg/kg, IP) B6 mice exhibiting a conditioned place-preference (CPP) vs. a conditioned place-aversion (CPA).
| Biochemical index of NAC glutamate function | MA- vs SAL- injected | MAHDR vs MALDR | CPP vs CPA |
|---|---|---|---|
| Basal extracellular glutamate content | ↑ | ↑ | n.d. |
| MA-elicited glutamate release | ↑ ( 21 WD) | ↑ | n.d. |
| EAAT3 levels or EAAT function | ↓ (21 WD) | ↓ | -- |
| GluN1 levels or NMDA function | ↓ (protein) --(function) |
– | – |
| mGlu1 levels or function | ↑ (function) ↓ (receptor) |
-- | ↑ (core) *correlated trait |
| mGlu5 levels | – | ↑ | -- *correlated trait |
| Homer1b/c | -- | ↓ (core) | -- |
| Homer2a/b | ↑ (shell) | ↑ | ↑ *correlated trait |
↑denotes relative increase, ↓ denotes relative decrease, –denotes no change, n.d. denotes not determined.
”correlated trait” denotes that the variable of interest was positively correlated with CPP Score.
Interestingly, the similar hyper-glutamatergic profile of the NAC of MAHDR, CPP-B6 and MA-sensitized B6 mice is not observed within the mPFC - a major glutamatergic afferent to the NAC (39). Whereas MAHDR mice also exhibit increased mPFC basal extracellular glutamate content, MA-stimulated glutamate release and Homer2a/b expression in the mPFC are lower in MAHDR vs. MALDR animals and none of the line differences in our biochemical indices of mPFC glutamate function are recapitulated in MA-sensitized mice (7). Further, there is no overlap between the glutamate profiles of the NAC (Fig. 3) versus mPFC of MA-preferring CPP-B6 mice (7). In fact, akin to MAHDR animals, CPP-B6 mice exhibit reduced mPFC expression of mGlu5 and Homer2a/b (7). Importantly, in all three models, the measurements of glutamate-related protein expression within NAC subregions (present study) and mPFC (7) were derived from the same animals, arguing that the NAC hyper-glutamate profile observed across MAHDR, CPP-B6 and MA-sensitized animals is neuroanatomically selective. The functional relevance of the hyper-glutamatergic state for MA-preference also appears to be neuroanatomically selective. Although elevating endogenous glutamate with TBOA within either the NACs or mPFC (7) augments a MA place-preference, an intra-mPFC infusion of APDC to lower endogenous glutamate does not impact place-conditioning in B6 mice (7). Such findings argue that basal glutamate hyperactivity within prefrontal corticoaccumbens pathways is sufficient to augment MA reward; however, endogenous glutamate within the NACs terminal region is critical for MA reward, with NAC glutamate hypofunction driving a strong MA aversion, even in individuals originally exhibiting signs of initial MA-preference. As both MAHDR (15) and CPP-B6 mice exhibit greater MA intake than their low MA-preferring counterparts, these neuropharmacological results provide direct cause-effect evidence in support of NACs glutamate hyper-activity in driving the positive motivational valence of MA that contributes subsequently to high levels of drug-taking behavior.
A hyper-glutamatergic state is a consequence of subchronic MA experience
Popular, current, theories regarding the glutamatergic basis of psychomotor stimulant addiction are derived almost exclusively from cocaine research and posit that drug-taking, -seeking, and behavioral sensitization result from drug-elicited anomalies in glutamate signaling primarily within NAC subregions (40–42). Herein, we extend to mice the handful of published studies describing the effects of subtoxic (herein, ≤2 mg/kg) MA treatment or MA self-administration upon glutamate-related biochemistry within the NAC of rats (8,9,11,19,43). Replicating earlier results (8,9,11), acute MA has little impact on extracellular glutamate within mouse NAC; however, its capacity to elevate NAC glutamate levels sensitizes with the passage of time in withdrawal for mice with repeated MA experience. Intriguingly, only in this latter regard are MA-induced changes in NAC glutamate similar to those reported in comparable studies of cocaine (e.g., 40–44). In fact, the basal hyper-glutamatergic state observed within the NAC of MA-withdrawn mice is akin to that observed in alcohol-experienced animals (eg., 25–27,45), but polar-opposite that produced by cocaine (21–23,25,40–42,44). Also akin to alcohol (25–27,45) but opposite to cocaine (44), MA-experienced animals exhibited increased NAC Homer2 expression during withdrawal and MA-sensitized glutamate release was temporally coincident with increased sensitivity to DHPG-stimulated glutamate release, indicating increased mGlu1 function (21,22).
Other glutamate-related biochemical changes also distinguish MA from cocaine. First, the basal hypo-glutamate state of cocaine-experienced animals is associated with blunted function/expression of mGlu2/3 autoreceptors, EAATs, system Xc- (a.k.a. cystine-glutamate exchanger), and Homer1 levels (21,25,27,41,42,44). In contrast, MA did not affect system Xc- function or Homer1 expression and the glutamate response to infusions of the EEAT inhibitor TBOA or the mGlu2/3 agonist APDC during early withdrawal indicated increased, rather than decreased, protein function. These findings, coupled with earlier results from MA-abstinent rats (8,9,43), argue that certain NAC glutamatergic consequences of repeated MA exposure are very distinct from those produced by repeated cocaine, lending further credence to the hypothesis that the hypo-glutamatergic state reported in a rat model of MA relapse (9) reflects neuroadaptations induced by extinction learning, rather than a pharmacodynamic response to the drug itself.
Our neuropharmacological results demonstrating that endogenous glutamate within the NACs regulates MA reward during withdrawal confirm the functional relevance of a MA-induced hyper-glutamatergic state for addiction-related behavior. This outcome has direct clinical implications for treatment intervention in MA abuse, arguing in favor of “anti-glutamate” therapeutic strategies for attenuating MA’s rewarding/reinforcing effects. Combined, our data from MAH/LDR and B6 mice indicate that any therapeutic approach to raise basal extracellular glutamate transmission would either be ineffective (e.g., cystine) or deleterious (e.g., TBOA) for MA abuse prevention/intervention. Indeed, Homer2 maintains and bidirectionally regulates NAC extracellular glutamate levels in vivo (c.f., 27), possibly accounting for the blunted MA reinforcement/reward exhibited by shRNA-Homer2b animals. The mechanism(s) through which Homer2 maintains NAC glutamate are still unknown (27). Recently, however, mGlu5-Homer1 interactions were identified as important in exocytotic glutamate release from astrocytes (46). This raises the intriguing possibility that Homer2 regulation of calcium dynamics in astrocytes may be involved in the capacity of this isoform to regulate extracellular glutamate levels in forebrain. However, the concordant results between the phenotypes of MAHDR, CCP-B6 and MA-sensitized mice argues that idiopathic or MA-induced up-regulation of Homer2-dependent changes in NAC extracellular glutamate is the “bad” that leads to the “ugly” state of increased MA abuse vulnerability and addiction severity.
Supplementary Material
Acknowledgments
The authors would like to thank the following individuals for their excellent technical assistance (in alphabetical order): Hannah M. Barrett, John J. Holloway, Dan Maliniak, Courtney L. McKenna, Ganesh Rajasekar, Katherine O. Travis, Lawrence E. Urman, Melissa G. Wroten. This work was supported by grants from the National Institute on Drug Abuse to K.K.S. (DA024038, DA039168), T.J.P. (P50 DA018165) and T.E.K. (DA027525), the Department of Veterans Affairs (T.J.P.), the W.M. Keck Foundation (T.E.K.) as well as by funds from the Australian Research Council (M.K.).
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Contributions
K.K.S., K.D.L, R.R.C, M.C, C.N.B., T.E.K and T.J.P. designed the studies. K.K.S., K.D.L., R.R.C., M.C., E.F., C.N.B., B.W.M., S.G.Q., D.M., and A.B.T. performed the experiments. T.J.P. generated and provided the MAH/LDR mice. G.v.J. and M.K. provided the viral vectors. K.K.S. and C.N.B. analyzed data. K.K.S., K.D.L. and R.R.C. wrote the paper. K.K.S., K.D.L., R.R.C., C.N.B., M.K., T.J.P., and T.E.K. edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report 2015. United Nations publication, Sales No. E.15.XI.6; 2015. [Google Scholar]
- 2.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan J, Butler R, Wheeler A. Initiation into methamphetamine use: qualitative findings from an exploration of first time use among a group of New Zealand users. J Psychoactive Drugs. 2009;41:11–17. doi: 10.1080/02791072.2009.10400670. [DOI] [PubMed] [Google Scholar]
- 4.Chait LD. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav Pharmacol. 1993;4:191–199. [PubMed] [Google Scholar]
- 5.de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–60. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- 6.Chiu VM, Schenk JO. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr Drug Abuse Rev. 2012;5:227–242. doi: 10.2174/1874473711205030227. [DOI] [PubMed] [Google Scholar]
- 7.Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, et al. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur J Neurosci. 2016;43:689–702. doi: 10.1111/ejn.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–22. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, et al. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol. 2012;17:758–69. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- 11.Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015;1628(Pt A):174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips TJ, Shabani S. An animal model of differential genetic risk for methamphetamine intake. Front Neurosci. 2015;9:327. doi: 10.3389/fnins.2015.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdani N, Parker CC, Shen Y, Reed ER, Guido MA, Kole LA, et al. Hnrnph1 Is A Quantitative Trait Gene for Methamphetamine Sensitivity. PLoS Genet. 2015;11:e1005713. doi: 10.1371/journal.pgen.1005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant CD, Kole LA, Guido MA, Cheng R, Palmer AA. Methamphetamine-induced conditioned place preference in LG/J and SM/J mouse strains and an F45/F46 advanced intercross line. Front Genet. 2012;3:126. doi: 10.3389/fgene.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han WY, Du P, Fu SY, Wang F, Song M, Wu CF, et al. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: Involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharmacol Biochem Behav. 2014;119:80–87. doi: 10.1016/j.pbb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW, et al. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front Systems Neurosci. 2014;8:70. doi: 10.3389/fnsys.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szumlinski KK, Balogun MY, Maisonneuve IM, Glick SD. Interactions between iboga agents and methamphetamine-sensitization: Studies of locomotion and stereotypy in rats. Psychopharmacology. 2000;151:234–241. doi: 10.1007/s002130000478. [DOI] [PubMed] [Google Scholar]
- 21.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate vulnerability to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- 24.Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- 25.Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 26.Shirashi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, et al. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology. 2011;214:791–804. doi: 10.1007/s00213-010-2086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 32.Szumlinski KK, Price KL, Frys KA, Middaugh LD. Unconditioned and conditioned factors contribute to the “reinstatement” of cocaine place conditioning following extinction in C57BL/6 mice. Behav Brain Res. 2002;136:151–160. doi: 10.1016/s0166-4328(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 33.Su ZI, Santoostaroam A, Wenzel J, Ettenberg A. On the persistence of cocaine-induced place preferences and aversions in rats. Psychopharmacology. 2013;229:115–123. doi: 10.1007/s00213-013-3086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- 35.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 36.Klugmann M, Szumlinski KK. Targeting Homer genes using AAV: Lessons learned from behavioural and neurochemical studies. Behav Pharmacol. 2008;19:485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eastwood EC, Phillips TJ. Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol. 2014;19:370–379. doi: 10.1111/adb.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips TJ, Mootz JR, Reed C. Identification of Treatment Targets in a Genetic Mouse Model of Voluntary Methamphetamine Drinking. Int Rev Neurobiol. 2016;126:39–85. doi: 10.1016/bs.irn.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Berendse HK, Galis DE, Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–367. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 40.Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.04.003. ePub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: A two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge drinking up-regulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buscemi L, Ginet V, Lopatar J, Montana V, Pucci L, Spagnuolo P, et al. Homer1 Scaffold Proteins Govern Ca2+ Dynamics in Normal and Reactive Astrocytes. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw078. pii: bhw078 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.