Abstract
Background
Given strong environmental influence on both epigenetic marks and allergic asthma in children, the epigenetic alterations in respiratory epithelia may provide insight into allergic asthma.
Objective
To identify DNA methylation and gene expression changes associated with childhood allergic persistent asthma.
Methods
We compared genomic DNA methylation patterns and gene expression in African American children with persistent atopic asthma[N=36] versus healthy controls[N=36]. Results were validated in an independent population of asthmatic children[N=30] using a shared healthy control population[N=36] and in independent population of Caucasian adult atopic asthmatics[N=12] and controls[N=12].
Results
We identified 186 genes with significant methylation changes, differentially methylated regions(DMRs) or differentially methylated probes(DMPs), after adjustment for age, gender, race/ethnicity, batch effects, inflation, and multiple comparisons. Genes differentially methylated included those with established roles in asthma and atopy, genes related to extracellular matrix, immunity, cell adhesion, epigenetic regulation, and airflow obstruction. The methylation changes were substantial (median 9.5%, range:2.6–29.5%). Hypo- and hyper-methylated genes were associated with increased and decreased gene expression respectively (P<2.8x10−6 for DMRs and P<7.8x10−10 for DMPs). Quantitative analysis in 53 differentially expressed genes demonstrated that 32(60%) have significant methylation-expression relationships within 5kb of the gene. 10 loci selected based on the relevance to asthma, magnitude of methylation change, and methylation-expression relationships were validated in an independent cohort of children with atopic asthma. 67/186 genes also have significant asthma-associated methylation changes in nasal epithelia of adult Caucasian asthmatics.
Conclusions
Epigenetic marks in respiratory epithelia are associated with allergic asthma and gene expression changes in inner-city children.
Keywords: DNA methylation, gene expression, microarray, atopic asthma, respiratory epithelia, epigenetic regulation, inner city
INTRODUCTION
The increase in the prevalence, incidence, and severity of asthma over the last 20 years(1) provides strong evidence that exposures play an important role in this disease. While common genetic variants explain only a small portion of asthma heritability(2), epigenetic changes could potentially explain both the non-Mendelian(3) and parent-of-origin(4) patterns of inheritance that are characteristic of asthma. Additionally, epigenetic marks can be influenced by the environment(5), these marks have been shown to affect the expression of transcription factors that alter the maturation of T lymphocytes(6–8), and we have demonstrated a causal relationship between DNA methylation and both Th2 immunity and allergic airway disease in mice(9).
Recently, we have shown that DNA methylation marks in peripheral blood mononuclear cells (PBMCs) are associated with allergic asthma(10) and account for 13.5% of the variation in serum IgE concentrations(11). However, the airway epithelium is the primary interface with the environment, interacts with allergens(12) and other environmental stimuli(13), and represents a potentially important mediator of allergic airway disease. Gene expression profiles of the asthmatic airway epithelium have identified genes associated with exposure to endotoxin and house dust mite allergen(14) as well as cigarette smoke(15), asthma(15), and Th2-high vs –low subphenotypes of disease(16). More recently, it has been demonstrated that gene expression in nasal epithelia is a proxy measure for gene expression of the lower airway epithelium(17). However, no study to date has comprehensively characterized genome-wide DNA methylation patterns and associated changes in gene expression in atopic asthmatic nasal epithelia.
METHODS
Study Populations
Our primary study population consisted of inner-city children aged 10–12 years with both atopy and persistent asthma (cases, N=36) or without atopy or asthma (healthy controls; N=36) (10). Study subjects were recruited by six sites supported by the Inner City Asthma Consortium (ICAC) from census tracts that contain at least 20% of households below the U.S. government poverty level (Supplemental Table S1). We limited our study population to 36 cases and 36 controls with at least 80% ciliated epithelial cells visualized from slides obtained from nasal brushings and by expression of the FOXJ1 gene (Supplemental Table S2). The validation population consisted of 30 African Americans aged 10–12 years with atopic asthma, using the same definition as the derivation cohort, collected by the ICAC independent of the primary study population. The original 36 control samples were used in the validation analysis and will be referred to as ‘shared controls’. The second validation population consisted of 24 Caucasian adults (age range 24–74), 12 with asthma and 12 without asthma, all with at least 80% ciliated epithelial cells visualized from slides obtained from nasal brushings, recruited at National Jewish Health. In this cohort, all asthmatics were also atopic, as assessed by positive skin prick testing for multiple allergens or RAST/Phadiatop tests. Control were required to have IgE level at visit < 100 but did not have additional allergy testing performed.
DNA Methylation and Gene Expression Data Collection
DNA methylation was measured on Illumina’s Infinium Human Methylation 450k BeadChip and validated internally and externally using pyrosequencing with custom designed primers (Supplemental Table S3). Gene expression was assessed on Agilent Human Gene Expression arrays (G3 SurePrint 8x60k). DNA methylation and gene expression array data have been deposited to the Gene Expression Omnibus (GEO) (GSE65205).
Overview of Statistical Analyses
The goal of our analyses was to determine whether DNA methylation and gene expression changes in nasal epithelia are associated with atopic asthma. Overall study design and workflow are presented schematically in Supplemental Figure S1. We identified DNA methylation changes associated with asthma for both single CpG motifs (differentially methylated probes [DMPs]) and differentially methylated regions (DMRs). While identification of DMPs is the most commonly used method for identification of methylation changes in Illumina arrays(18), our rationale for identification of DMRs is three-fold: first, identification of regions is conceptually consistent with what is known about DNA methylation patterns in the human genome(19); second, it increases power to detect associations(20); and third, it has been used in other diseases(21, 22). We also performed two exploratory analyses: (1) to determine if any of the DMRs or DMPs identified in nasal epithelia were associated with nasal corticosteroid use among asthmatics, and (2) to test whether any of the 81 DMRs we previously identified as associated with asthma in PBMCs(10) are also associated with asthma in nasal epithelia.
Statistical Analyses of DNA Methylation Data
Data from the methylation array were normalized using the SWAN method(23) and the normalized M-values were used in all downstream analyses while beta-values, on the scale of 0–100%, are used for tables and figures. Differences between cases and controls are reported as percent methylation changes, using beta-values. We filtered out probes on the 450k Illumina array with known SNPs in European (CEU) and African (YRI) populations within the CpG motif.
We performed the analysis to identify differential methylation in three steps. In brief, these are: infer PEER factors to account for unobserved batch effects(24), fit linear models with limma(25), and use comb-p(26) with a window size of 300 base pairs (bp) to identify regions of sustained low p values or DMRs from the p-values reported by limma. DMPs were identified by calculating q values, corresponding to false discovery rate (FDR), from linear model p-values using the method of Benjamini and Hochberg(27). Following examination of q-q plots, we performed global adjustment for inflation of p values using standard methodology. Details of the analyses are outlined in the diagram in Supplemental Figure S2 and in the methods in the Online Supplement.
Statistical Analyses of Gene Expression Data
The R package limma(25) was used to background-correct, normalize (quantile) and fit linear models for expression data; p-values were based on the moderated t-statistic and q-values were calculated from p-values using the method of Benjamini and Hochberg(27).
Analysis of DNA Methylation and Gene Expression
To understand the relationship of DMRs with gene expression changes, we considered inversely correlated (canonical) vs. positively correlated pairs limiting the analysis to genes within 5kb of a DMR. We calculated the enrichment of inversely correlated pairs in relation to all pairs using the binomial test. To integrate the expression and methylation data, we used a method derived from the comb-p(26) except instead of testing each methylation site for its relation to asthma status, we tested methylation regions and expression probes within 5kb.
Additional Methods
Additional methods are available in the Online Supplement.
RESULTS
There were no significant demographic differences between allergic asthma cases (N=36) and controls (N=36), although collection site approached statistical significance (Table 1). Per study design, all allergic asthma subjects were atopic based on positive skin prick test to at least one indoor allergen (data not shown) and total IgE concentrations, and had airflow limitation as indicated by spirometric measurements (Table 1).
Table 1.
Demographic and clinical characteristics of asthma subjects and controls
| Allergic Asthma Subjects (N=36) | Control Subjects (N=36) | p- value † | Replication Allergic Asthma Population (n=30) | |
|---|---|---|---|---|
| Site: Boston | 8 (22.2%) | 15 (41.7%) | 0.071 | -- |
| Dallas | 4 (11.1%) | 1 (2.8%) | 6 (20.0%) | |
| Denver | 7 (19.4%) | 3 (8.3%) | -- | |
| Detroit | 4 (11.1%) | 1 (2.8%) | -- | |
| New York | 6 (16.7%) | 3 (8.3%) | 3 (10.0%) | |
| Washington D.C. | 7 (19.4%) | 13 (36.1%) | -- | |
| Chicago | -- | -- | 7 (23.3%) | |
| Cincinnati | -- | -- | 14 (46.7%) | |
| Age at recruitment (yr) | 11.0 [10.0, 12.0] | 11.0 [10.0, 12.0] | 0.522 | 11.0 [10.0, 12.0] |
| Gender (Male) | 19 (52.8%) | 17 (47.2%) | 0.641 | 19 (63.3%) |
| Participant race: African American (Yes) | 33 (91.7%) | 33 (91.7%) | >0.991 | 30 (100.0%) |
| Participant race: Hispanic or Latino (Yes) | 7 (19.4%) | 3 (8.3%) | 0.171 | 0 (0.0%) |
| How often exposed to smokers: Daily | 10 (27.8%) | 8 (22.2%) | 0.591 | 8 (26.7%) |
| Dog living in the home in the last 6 months (Yes) | 11 (30.6%) | 13 (36.1%) | 0.621 | 9 (30.0%) |
| Cat living in the home in the last 6 months (Yes) | 9 (25.0%) | 12 (33.3%) | 0.441 | 3 (10.0%) |
| Any water/dampness in the last 12 months (Yes) | 9 (25.0%) | 4 (11.1%) | 0.131 | 10 (33.3%) |
| Gas stove, gas range or gas oven (Yes) | 22 (61.1%) | 24 (66.7%) | 0.621 | 12 (40.0%) |
| Allergic to any indoor allergen (Yes) | 36 (100%) | 0 (0%) | ** | 30 (100%) |
| Cockroach IgE (IU/mL) | 0.34 [0.34, 1.53] | 0.34 [0.34, 0.34] | ** | 0.44 [0.34, 25.2] |
| Total serum IgE (kU/l ) | 366.0 [185.0, 785.0] | 29.0 [16.5, 49.5] | ** | 519.5 [181.0, 883.0] |
| Baseline - FEV1(% predicted) ‡ | 88.8 ± 17.5 | 105.4 ± 11.2 | ** | 97.3 ± 19.3 |
| Baseline - FEV1/FVC § | 72.6 ± 10.1 | 84.6 ± 6.4 | ** | 79.6 ± 8.1 |
| Asthma Medications: Albuterol | 34 (94.4%) | 0 (0%) | **¶ | 30 (100.0%) |
| Inhaled Steroids | 29 (80.6%) | 0 (0%) | 22 (73.3%) | |
| Nasal Steroids | 9 (25%) | 0 (0%) | 25 (83.3%) | |
| Montelukast | 15 (41.7%) | 0 (0%) | 11 (36.7%) |
Data are presented as mean ± standard deviations, medians [interquartile range] or numbers (%).
P-values were calculated with the use of the Chi-Square1 test for categorical variables and the Mann-Whitney2 test for continuous variables.
FEV1 denotes forced expiratory volume in one second
FVC denotes forced vital capacity
Medication data missing on 14 controls
By protocol design
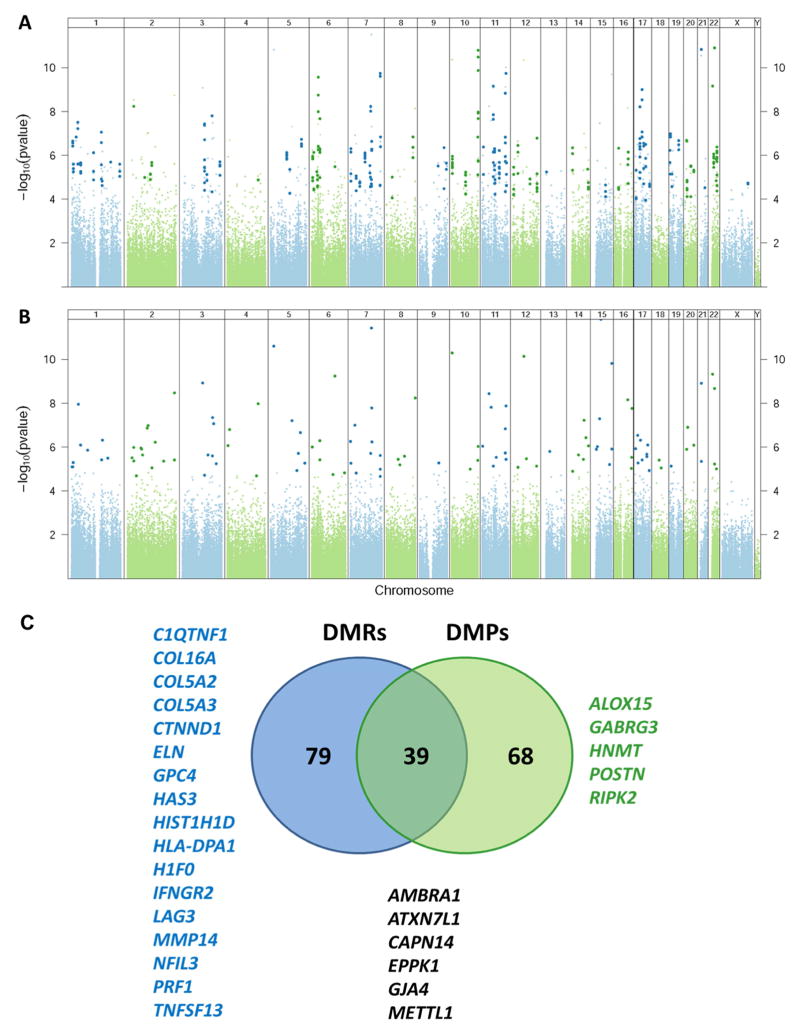
After adjusting for age, gender, race/ethnicity, unknown batch effects, removing probes with known European and African SNPs at the CpG motif, and performing global adjustment for inflation, we identified 119 genome-wide significant DMRs associated with 118 unique genes (Figure 1A; Table 2 and Supplemental Table S4) and 118 single CpG motifs (DMPs) associated with 107 unique genes (Figure 1B; Table 2 and Supplemental Table S5). The median percent methylation change between allergic asthmatics and controls was 6.8% (range 2.6–28.8%) for DMRs and 13.6% (range 5.2–29.5%) for DMPs. 39 genes were identified in both the DMR and DMP analysis but a number of genes only have DMPs or DMRs as these two analytical approaches are complementary. Some genes/loci that have a highly significant CpG (DMP) will not be represented in the DMR analysis because there are no neighboring CpGs with low p values as Illumina array does not interrogate all CpGs and sequence data would be needed to examine this further. On the other hand, DMR analysis identifies regions of sustained low p values in multiple CpG sites where coverage on the Illumina array is available but not all of these loci have one highly significant CpG (DMP). Representative histograms of percent methylation estimates (beta values) in allergic asthmatic and controls are shown in Supplemental Figure S3. Among the 186 (DMR and/or DMP) allergic asthma-associated differentially methylated genes in the nasal epithelia are genes with established roles in asthma and atopy - arachidonate 15-lipoxygenase (ALOX15), calpain 14 (CAPN14), histamine N-methyltransferase (HNMT), and periostin (POSTN) (Figure 1C). Moreover, components of the extracellular matrix (COL16A1, COL5A2, COL5A3, ELN, HAS3, MMP14), genes related to immunity (IFNGR2, HLKA-DPA1, LAG3, NFIL3, PRF1, TNFSF13), cell adhesion (CTNND1, EPPK1, GJA4), epigenetic regulation (ATXN7L1, H1F0, HIST1H1D, METTL1), airway obstruction (GABRG3), obesity (C1QTNF1, GPC4), and autophagy (AMBRA1) are also differentially methylated in nasal epithelia of atopic asthmatics compared to controls (Figure 1C). None of the allergic asthma-associated DMRs/DMPs were significantly associated with nasal corticosteroid use among asthmatics after adjustment for multiple comparisons. Moreover, the small degree of overlap in allergic asthma-associated epigenetic changes in PBMC and nasal epithelia (3 of the 81 PBMC DMRs are significant in nasal epithelia at q<0.05; Supplemental Table S6) is not surprising given that PBMCs are a surrogate for an asthma-associated immune response, while nasal epithelia are directly exposed to the environment that may influence DNA marks and the course of asthma. Moreover, nasal epithelia are a more pure cell population than PBMCs and some of the methylation changes present in specific mononuclear cell subsets such as Th2 T-cell subsets for example may be diluted by the presence of other cell types. The 3 DMRs that are present in both cell types may be associated with genetic variants (methylation quantitative trait loci or mQTLs)(28). We also performed an enrichment analysis of methylation marks within areas of open chromatin, as assessed by DNAse I hypersensitivity site (DHS) analysis, in cell lines profiled by ENCODE(29). This analysis identified strongest enrichment of allergic asthma-associated DNA methylation changes in DHSs in epithelial cells (q<6.57x10−11), suggesting that our analysis identified DNA methylation changes in open areas of chromatin in the cell type of interest (Supplemental Figure S4). To begin to explore the influence of environmental exposures on the nasal epithelial epigenome, we identified 48 DMRs that are significantly associated with environmental tobacco smoke (ETS) after adjusting for age, gender, race/ethnicity, unknown batch effects, and allergic asthma. 48 DMRs are in 46 unique genes (Supplemental Table S7), one of which (SFRP2) overlaps with the analysis of direct cigarette smoke exposure on methylation of small airway epithelia that identified three genes after adjustment for genome-wide comparisons(30).
Figure 1.
Differentially methylated regions (DMRs) (A) and differentially methylated single-CpG probes (DMPs) (B) in nasal epithelia are associated with asthma after controlling for age, gender, race/ethnicity, technical variables, and batch effects. (A) Manhattan plot of the adjusted p-values for disease status (asthma/control) from the linear model. Each dot represents a p value for a probe on the Illumina 450k array that has been adjusted by the significance of neighboring probes within 300 bases according to their correlation. Probes within statistically significant DMRs after adjustment for genome-wide comparisons are identified by darker larger symbols. (B) Manhattan plot of the false discovery rate (FDR) adjusted p-values (q-values) for disease status (asthma/control) from the linear model. Probes with q<0.05 are highlighted by darker larger symbols. (C) Venn diagram depicting gene-based overlap of DMRs and DMPs. Representative genes of clinical/biological relevance to asthma that are discussed in the text are highlighted
Table 2.
10 differentially methylated regions (DMRs) and 10 differentially methylated probes (DMPs) with the most pronounced DNA methylation changes in allergic asthmatics compared to controls.
| Type | Chr | Start | End | Adjusted p value | % Methylation Difference | Nearest Gene | Gene Distance | CpG Island Distance |
|---|---|---|---|---|---|---|---|---|
| DMR | chr11 | 36030085 | 36030086 | 0.002895 | −28.8% | LDLRAD3 | 0 | 63631 |
| DMR | chr7 | 1.06E+08 | 1.06E+08 | 1.32E-06 | −27.3% | ATXN7L1 | −4084 | 3152 |
| DMR | chr12 | 58162286 | 58162287 | 2.12E-05 | −27.0% | METTL1 | 63 | 2286 |
| DMR | chr10 | 4386801 | 4386802 | 2.28E-05 | −26.1% | LINC00703 | −39635 | −481323 |
| DMR | chr15 | 1.02E+08 | 1.02E+08 | 9.70E-05 | −25.1% | PCSK6 | 0 | 51207 |
| DMR | chr22 | 19471092 | 19471093 | 4.13E-04 | −24.4% | CDC45 | 0 | 3281 |
| DMR | chr3 | 1.02E+08 | 1.02E+08 | 3.78E-04 | −23.2% | LOC152225 | 177435 | 325159 |
| DMR | chr15 | 39544143 | 39544144 | 0.01364 | −22.9% | C15orf54 | 0 | −328383 |
| DMR | chr15 | 45449436 | 45449437 | 6.83E-07 | −22.7% | DUOX1 | 0 | 4731 |
| DMR | chr8 | 1.45E+08 | 1.45E+08 | 0.003634 | −21.5% | EPPK1 | −17562 | 3314 |
| DMP | chr16 | 88558222 | 88558223 | 1.43E-05 | −29.5% | ZFPM1 | 0 | 0 |
| DMP | chr11 | 36030085 | 36030086 | 6.23E-06 | −28.8% | LDLRAD3 | 0 | 63631 |
| DMP | chr7 | 1.06E+08 | 1.06E+08 | 4.09E-09 | −27.3% | ATXN7L1 | −4084 | 3152 |
| DMP | chr12 | 58162286 | 58162287 | 5.03E-08 | −27.0% | METTL1 | 63 | 2286 |
| DMP | chr10 | 4386801 | 4386802 | 5.03E-08 | −26.1% | LINC00703 | −39635 | −481323 |
| DMP | chr15 | 1.02E+08 | 1.02E+08 | 2.39E-07 | −25.1% | PCSK6 | 0 | 51207 |
| DMP | chr22 | 19471092 | 19471093 | 8.17E-07 | −24.4% | CDC45 | 0 | 3281 |
| DMP | chr6 | 2977292 | 2977293 | 4.99E-04 | −24.1% | SERPINB6 | −4893 | 5330 |
| DMP | chr3 | 1.02E+08 | 1.02E+08 | 8.15E-07 | −23.2% | LOC152225 | 177435 | 325159 |
| DMP | chr15 | 39544143 | 39544144 | 2.62E-05 | −22.9% | C15orf54 | 0 | -328383 |
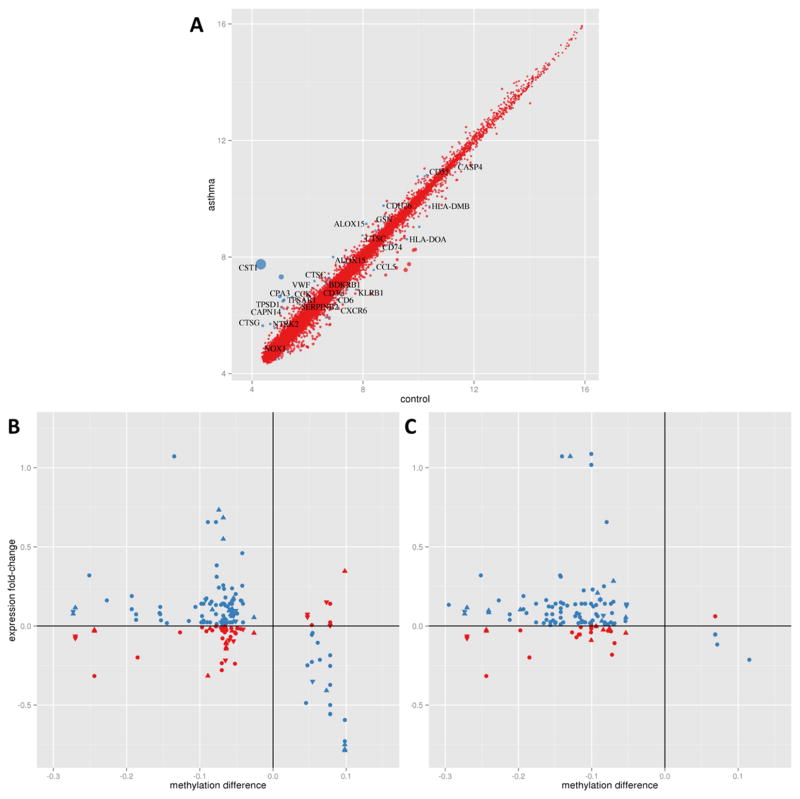
Gene expression analysis of nasal epithelia identified 53 differentially expressed genes (Figure 2A; Supplemental Table S8A). Among differentially expressed genes are genes associated with asthma and allergy - ALOX15, bradykinin B1 receptor (BDKRB1), cadherin 26 (CDH26), calpain 14 (CAPN14), cathepsins C and G (CTSC, CTSG), cystatin SN (CST1), gelsolin (GSN), mast cell carbohypeptidase CPA3 and tryptases (TPSAB1 and TPSD1), NADPH oxidase 1 (NOX1), neurotrophic tyrosine kinase receptor (NTRK2), plasminogen activator inhibitor 2 (SERPINB2), and von Willebrand factor (VWF). Moreover, a number of immune related genes with high relevance to asthma (CASP4, CCK, CCL5, CCL26, CD3G, CD55, CD6, CD74, CXCR6, HLA-DMB, HLA-DOA, KLRB1, TCRA) are also differentially expressed in atopic asthmatic compared to control samples.
Figure 2.
DNA methylation changes are associated with changes in gene expression in nasal epithelia. (A) Asthma-associated gene expression changes after adjusting for age, gender, and race/ethnicity. Blue dots indicate 27 genes with q<0.05 for the asthma vs control comparison with high functional relevance to asthma and atopy. The size of the dot is proportional to asthma vs control fold change. Expression changes in genes within 5 kilobases of the nearest DMR (B) and DMP (C). In both panels, x-axis methylation difference is represented by the mean % methylation difference in asthma subjects compared to controls; y-axis expression difference is represented by the mean fold change in asthma subjects compared to controls (on the log2 scale). The blue symbols represent hypomethylated genes that were associated with increased gene expression as well as some hypermethylated genes associated with decreased gene expression. The red symbols represent methylation changes that were not associated with expected gene expression differences. Upward triangles indicate DMR/DMP location upstream of the gene, circles represent DMRs in the gene body, and downward triangles refer to DMR/DMPs downstream of the gene.
Given the substantial number of immune–related differentially expressed genes, we examined the function, general tissue/cell expression patterns(31), and immune cell specific expression patterns(32) of all 53 differentially expressed genes to discern which genes were likely contributed by immune cells in the respiratory epithelium (Supplemental Table S8B). This analysis revealed 11 of the 53 of the genes as highly likely to be contributed by immune cells – B-cells (HLA-DMB and HLA-DOA), mast cells (CPA3, CTSG, TPSAB1, and TPSD1), NK cells (KLRB1), T cells (CD3G, CD6, TCRA) and multiple immune cells (CST1). This is consistent with published findings in the lung epithelium; for example, differential expression of mast cell genes (CPA3, TPSAB1, and TPSD1) in the airway epithelium of asthmatics with different immune subtypes of asthma (Th2 high and Th2 low) is due to accumulation of intraepithelial mast cells with a unique protease phenotype in Th2-high asthma(33). The remaining 42 genes are specifically expressed by epithelial cells, contributed both by epithelial and immune cells, or have unknown expression patterns.
The relationship between DNA methylation and gene expression revealed enrichment of hypomethylated genes associated with increased gene expression, and hypermethylated genes associated with decreased gene expression (binomial P<2.8x10−6 for enrichment in inverse relationships in asthma-related DMRs [Figure 2B] and P<7.8x10−10 for allergic asthma-associated DMPs [Figure 2C]) within 5 kilobase (kb) distance of the transcription start site (TSS). However, a number of DNA methylation changes were not associated with canonical gene expression differences (Figure 2B and 2C). While these results are similar when we use 2kb or 3kb distance, beyond 5kb of the TSS we begin to lose the enrichment in anti-correlated methylation-expression pairs. We did not observe an enrichment in negatively correlated methylation-expression relationships in gene promoters compared to gene bodies, as has been previously reported(34); however, this analysis was underpowered. We implemented QTL mapping to identify methylation marks that control gene expression (methyl-eQTR or quantitative trait region).
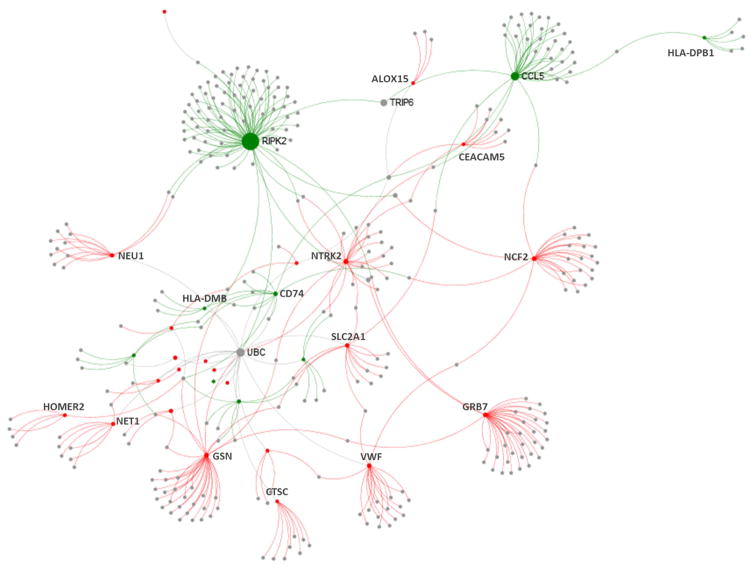
We performed two analyses – one centered on differentially methylated genes (union of DMRs and DMPs from Figure 1; 186 unique genes) and the other focused on 53 differentially expressed genes (Figure 2A). Of the 186 allergic asthma-associated differentially methylated genes, 158 have methylation and expression data within 5kb distance and, of these, 16 (10%) have significant relationships of DNA methylation and gene expression within 5kb (Supplemental Table S9A). Of the 53 allergic asthma-associated differentially expressed genes, 32 (60%) have significant relationships of DNA methylation and gene expression within 5kb (Supplemental Table S9B). We observed a strong enrichment in significant methylation-expression pairs with 5kb of differentially expressed genes compared to all methylation-expression pairs within 5kb distance of each other in the genome (enrichment p=3.2x10−6). Both analyses identified predominantly canonical inverse correlations of methylation and expression (15/ o f the 16 genes in the methylation-centered analysis and 32 of the 53 in the expression-centered analysis). Importantly, both analyses demonstrated that ALOX15 has significant relationship of DNA methylation and gene expression. Upstream regulator analysis of the 47 allergic asthma-associated genes that demonstrate canonical inverse relationships between methylation and expression, using Ingenuity Pathway Analysis (IPA), revealed a significant enrichment (p<1x10−4) in cytokines (IL-13, IL-4, IL-6, IFN-γ , and others) as well as transcription factors (CIITA) and growth factors (TGF-β) known to regulate gene expression profiles in asthma (Supplemental Table S10). Protein-protein interaction (PPI) analysis of the 47 allergic asthma-associated genes with inverse relationships of methylation and expression revealed a network of proteins with the largest hub being RIPK2 (Figure 3). RIPK2 or CARD3 is a component of signaling complexes in both the innate and adaptive immune pathways, is critical for NOD-mediated NF-κB activation and cytokine production(35), and silencing of its expression attenuates allergic airway inflammation in mice(36). Smaller hubs in the network include other proteins important in immunity and identified in our analysis. Taken together these analyses support biological and disease relevance of our findings.
Figure 3.
Protein-protein interactome analysis of 47 allergic asthma-associated genes with inverse relationship of methylation and expression. The interactome was created using NetworkAnalyst(70) and the InnateDB PPI dataset. The nodes are colored based on their methylation and expression (green are downregulated and hypermethylated while red are upregulated and hypomethylated). The sizes of nodes are proportional to their betweenness centrality values.
To determine the validity of these findings, we selected 10 loci for pyrosequencing and included an independent population of children with allergic asthma from the inner city (Table 1). 10 loci were chosen to represent genes with known relevance to asthma, loci with largest methylation changes, and methylation marks that affect gene expression. Four allergic asthma-associated DMRs or DMPs were selected based on the relevance to asthma (ALOX15, HLA-DPA1, GJA4, and POSTN), three were selected based on the extent of methylation differences between asthmatics and controls (LDLRAD3, ATXN7L1, and METTL1), and three additional loci were selected based on the allergic asthma specific methylation-expression relationships (CCL5, CTSC, and CXCR6). Nine of these 10 loci validated internally in the original study population (one-tailed t-test P<0.05), and the CTSC locus approached statistical significance (P=0.12) (Table 3 and Supplemental Table S11) if using nominal p values and 8/10 if stringent Bonferroni correction is applied. More importantly, all 10 loci validated in an independent population of children with allergic asthma residing in the inner city compared to controls from the original population (shared control design; one-tailed t-test P<0.05; Table 2) and methylation levels in two sets of allergic asthmatic cases are comparable (Supplemental Table S11). Significance levels were very similar if adjustment for age, gender, and race/ethnicity was included, demonstrating the robustness of these associations with allergic asthma. We also examined expression of these ten genes by qPCR and showed that half of them have significant changes in allergic asthma compared to controls and are also significantly inversely correlated with methylation in the original study population as well as independent allergic asthmatics (Table 3).
Table 3.
Pyrosequencing and qPCR validation of selected DNA methylation marks in the same asthma cases and controls and an independent population of asthmatics. Green denotes hypomethylation and decreased expression while red indicated hypermethylation and increased expression in asthma cases compared to controls.
| (A) Selected DMRs/DMPs from the Illumina analysis | |||
|---|---|---|---|
| DMR/DMP Coordinates | Gene | Illumina Adjusted p Value | Illumina Methylation Change |
| chr17:4541333-4541334 | ALOX15 | 7.82x10−4 | −10.1 |
| chr6:33041220- 33041697 | HLA- DPA1 | 1.04x10−6 | 9.8 |
| chr1:35258778- 35258933 | GJA4 | 6.00x10−3 | −9.8 |
| chr13:38172802-38172803 | POSTN | 7.25 x10−4 | −11.6 |
| chr11:36030085-36030086 | LDLRAD3 | 6.23x10−6 | −28.8 |
| chr7:105521115-105521116 | ATXN7L1 | 4.09x10−9 | −27.3 |
| chr12:58162286-58162287 | METTL1 | 5.03x10−8 | −27 |
| chr3:45984742-45985168 | CXCR6 | 7.30x10−4 | 5.9 |
| chr17:34202460-34202461 | CCL5 | 0.017 | 1.9 |
| chr11:88059526-88059527 | CTSC | 1.51x10−3 | −1.1 |
| (B) Internal validation | |||||||
|---|---|---|---|---|---|---|---|
| Coordinates of Measured Cs | Gene | Methylation Change | One-tailed t-test | Expression Fold Change | One-tailed t-test | Methylation-Expression Correlation | Correlation p value |
| chr17:4541333 | ALOX15 | −8.7 | 1.55x10−7 | 2.0 | 0.0258 | −0.39 | 5.53x10−4 |
| chr6:33041528 | HLA- DPA1 | 9 | 2.10x10−4 | 0.6 | 0.0361 | −0.46 | 2.90x10−5 |
| chr1:35258850, 35258855 | GJA4 | −3.4, −7.8 | 6.0x10−5, 1.74x10−3 | 1.7 | 0.0913 | −0.0061, −0.028 | 0.9595; 0.810 |
| chr13:38172802 | POSTN | −8.9 | 1.70x10−4 | 5.8 | 1.84x10−4 | −0.35 | 1.71x10−3 |
| chr11:36030086 | LDLRAD3 | −20.9 | 2.18x10−7 | 1.2 | 0.263 | −0.093 | 0.424 |
| chr7:105521115 | ATXN7L1 | −24.8 | 2.60x10−14 | 0.9 | 0.375 | 0.048 | 0.682 |
| chr12:58162286 | METTL1 | −22.8 | 8.25x10−11 | 0.8 | 0.214 | 0.054 | 0.643 |
| chr3:45984839, 45984841 | CXCR6 | 5.7, 6.0 | 9.89x10−4, 1.48x10−3 | 0.5 | 0.0197 | −0.32; −0.27 | 5.42x10−3; 0.0193 |
| chr17:34202460 | CCL5 | 4.4 | 0.048 | 0.8 | 0.152 | −0.29 | 9.94x10−3 |
| chr11:88059526 | CTSC | −4.7 | 0.12 | 1.6 | 9.30x10−5 | −0.36 | 1.40x10−3 |
| (C) External validation | |||||||
|---|---|---|---|---|---|---|---|
| Coordinates of Measured Cs | Gene | Methylation Change | One-tailed t-test | Expression Fold Change | One-tailed t-test | Methylation-Expression Correlation | Correlation p value |
| chr17:4541333 | ALOX15 | −10.6 | 7.50x10−10 | 1.5 | 0.0697 | −0.092 | 0.449 |
| chr6:33041528 | HLA- DPA1 | 8.1 | 1.27x10−3 | 0.5 | 0.00109 | −0.47 | 4.20x10−5 |
| chr1:35258850, 35258855 | GJA4 | −4.7, −13.7 | 2.03x10−8, 2.26x10−8 | 7.1 | 1.27x10−5 | −0.16; −0.13 | 0.172; 0.303 |
| chr13:38172802 | POSTN | −13.6 | 6.5x10−9 | 7.3 | 9.73x10−5 | −0.57 | <1x10−5 |
| chr11:36030086 | LDLRAD3 | −23.8 | 8.80x10−9 | 1.5 | 0.0282 | −0.13 | 0.298 |
| chr7:105521115 | ATXN7L1 | −31.1 | 4.91x10−20 | 0.9 | 0.346 | 0.11 | 0.347 |
| chr12:58162286 | METTL1 | −27.1 | 6.02x10−14 | 1.3 | 0.149 | −0.076 | 0.537 |
| chr3:45984839, 45984841 | CXCR6 | 4.2, 4.7 | 0.0227, 0.0188 | 0.7 | 0.0632 | −0.30; −0.31 | 0.0125; 9.49x10−3 |
| chr17:34202460 | CCL5 | 7.8 | 1.6x10−3 | 1.0 | 0.419 | −0.25 | 0.0393 |
| chr11:88059526 | CTSC | −14.5 | 8.2x10−5 | 1.5 | 0.00232 | −0.46 | 7.30x10−5 |
To further examine validity and generalizability of our findings, we profiled nasal epithelia from adult Caucasian subjects, 12 atopic asthmatic and 12 controls (cohort characteristics in Supplemental Table S12), on Illumina methylation arrays. Using the same methods applied to our primary cohort, we identified 1766 DMPs and 370 DMRs, representing 1108 unique genes, significantly associated with asthma (genome-wide adjusted p<0.05) after adjustment for age, gender and batch effects. Of the 186 genes with significant methylation changes in the primary cohort of atopic asthmatic African American children from the inner city, 67 of them (36%) have at least one genome-wide significant DMP and/or DMR in adult Caucasian asthmatics (Supplemental Table S13). Similarly, 56 genes (30%) also overlap with genes that have IL-13 responsive CpGs in cultured nasal epithelial cells from 57 unrelated adult lung donors(37). These results demonstrate external validity and generalizability of some of our findings to asthma regardless of age, race/ethnicity, specific exposures, and conditions (cultured vs fresh cells, nasal vs lung epithelia).
DISCUSSION
Our findings demonstrate that methylation marks in the nasal epithelia of children with allergic asthma are associated with changes in gene expression. Moreover, the magnitude of these allergic asthma-associated epigenetic signals is substantial when compared with other diseases(38, 39). These findings suggest that genes that have been previously reported to be differentially expressed in the airway epithelium of allergic asthmatics(14–16) may be regulated by epigenetic mechanisms.
The effect of aeroallergens(12) and other environmental stimuli(13) on the respiratory epithelium is a key mediator of asthma. Gene expression profiles of the allergic asthmatic airway(14–16) and nasal(17) epithelia demonstrate that these cells are involved in an active biological process. Our findings demonstrate a clear relationship between DNA methylation and gene expression in the nasal epithelia of young allergic asthmatics using a stringent analytical pipeline to ensure that methylation changes we identified are not false positives due to influence of demographic factors or technical variables. More than half of the differentially expressed genes in our analysis have significant associations with methylation marks; this includes asthma and allergy genes ALOX15(40), CAPN14(41), CTSC(42), CST1(43), GSN(44), NTRK2(45), and VWF(46) as well as a number of immune and extracellular matrix genes. Moreover, genes have previously identified as differentially expressed in the respiratory epithelia but did not reach statistical significance in our expression analysis, such as periostin(15, 17), are also differentially methylated. Finally, genes with the largest absolute changes in methylation observed in allergic asthma (LDLRAD3, ATXN7L1, and METTL1) have not previously been implicated in allergic asthma and represent biological candidates for future investigation. There is recent evidence that expression of ALOX15 is regulated by demethylation of H3K27me3 at the ALOX15 promoter following IL-4 treatment in epithelial cells, providing support for the specific genes identified in our analysis(47).
Importantly, the magnitude of DNA methylation changes at some of the loci is large, consistent with that observed in other diseased tissue(38, 39) and these loci may become important therapeutic targets in the future, provided additional evidence from cohorts with larger sample sizes. DNA methylation changes have been shown to drive tumor formation and malignant progression(48), appear to critical to disease pathogenesis, and represent novel therapeutic targets in cancer. DNA methyltransferase (DNMT) inhibitors, such as 5-azacitidine and decitabine, have been approved for the treatment of myelodysplastic syndrome(49, 50), and are being tested in solid tumors(51, 52). Methylation changes in nasal epithelia of allergic asthmatics are on average ~10 times larger than DNA methylation changes in peripheral blood that are associated with childhood allergic asthma(10) or other diseases of the airway such as COPD(18). The strength of the DNA methylation signal in the nasal epithelia of children with asthma was detectable even in the limited sample size of the study population. Moreover, many of the methylation marks validated in an even smaller cohort of adult Caucasian asthmatics, demonstrating generalizability of our findings to asthma. Given similarities in the nasal and airway/bronchial epithelial transcriptomes(17), it is logical to speculate that DNA methylation in nasal epithelia could be used as a biomarker of exposure or disease; however, further work in larger cohorts will be needed to explore this biomarker potential..
The majority of allergic asthma-associated methylation marks we identified in the nasal epithelia are hypomethylated and within gene bodies. Our results are in concordance with previous reports that have shown hypomethylation of specific genes in asthma(10, 53–55) and other airway diseases such as COPD(18). Similarly, cigarette smoke exposure is more often associated with hypomethylated regions in airway epithelia(30) and large blocks of hypomethylation have been identified in cancer(56, 57). While early studies focused specifically on promoter methylation as a mechanism of gene regulation(51), more recent research has demonstrated the significance of gene body methylation(58, 59) in transcriptional regulation in malignant(34) and non-malignant diseases(22). Our study is the first to show an association of gene body methylation and gene expression in allergic asthma.
There are several potential causes for these methylation changes. First, nasal epithelia are subject to a number of environmental exposures that are relevant to allergic asthma and are also known to shape the epigenome; these include cigarette smoke(30, 60), air pollution(61), and farming aerosols(62). While we were unable to evaluate the impact of exposures on the epigenome due to lack of exposure assessment in our study, this is an important future direction for the field. Second, epigenetic marks may influence the severity of allergic asthma. Previous work has shown association of DNA methylation in specific gene loci (IL-6 and iNOS) in the nasal epithelia(53) and buccal cells (IFN-3)(63). Finally, microbial species are also known to influence the epigenome. Differential effect of rhinovirus infection in asthmatics (N=6) compared to non-asthmatic (N=3) nasal epithelia on methylation of a small noncoding RNA locus (SNORA12) has been recently demonstrated(64).
There are several limitations to our study. First, our study design precludes us from differentiating epigenetic marks associated with allergy versus those associated with asthma. However, since most children with asthma also are atopic, our findings among children allergic asthma are generalizable to a larger population of children with asthma. Secondly, we were able to evaluate the impact of exposures on the epigenome in limited fashion using questionnaires and not exposure assessments. We did not collect longitudinal samples and were unable to examine longitudinal variation in DNA methylation due to changes in exposure. The current study is an observational study with small sample size showing an association between methylation biomarkers and childhood persistent asthma, and further work will be required to address the stability of the biomarker over time. The relationship between the environment and epigenome, especially in relation to conditions like allergic asthma, remains a particularly important area of future research. Thirdly, our small sample size precluded us from assessing methylation marks in relation to disease heterogeneity and asthma endotypes and future work in larger cohorts will be required to address this question. Fourthly, while all our subjects are self-reported African American or of Dominican/Haitian descent, there is substantial genetic admixture of African ancestry in these populations(65, 66) and we did not examine the effect of genetics on DNA methylation(67, 68). The fourth limitation is the inability to detect differences in methyl- vs. hydroxyl-methyl cytosine, which is thought to be a mark for de-methylation(69). Finally, our association analysis is unable to distinguish between DNA methylation marks being the cause or consequence of disease.
Despite these limitations, our study identified substantial and consistent DNA methylation changes in the nasal epithelia of children with allergic asthma residing in the inner city compared to non-diseased controls, and these methylation marks were internally and externally validated in both children and adults. These methylation changes are associated with gene expressionand need to be further evaluated in larger cohorts for their potential as biomarkers and therapeutic targets . The peculiar spatial and biological relation of epigenetic changes in the nasal epithelia of asthmatics suggests that these cells and these mechanisms may provide insight into the etiology and pathogenesis of this persistent public health concern(1).
Supplementary Material
KEY MESSAGES.
Expression of genes related to extracellular matrix, immunity, cell adhesion, epigenetic regulation, and airflow obstruction are epigenetically regulated in nasal epithelia and associated with asthma.
The methylation changes in nasal epithelia are substantial (median 9.5%, range: 2.6–29.5%) and similar in magnitude to those observed in other diseases.
More than one third of methylation changes identified by our analysis appear to be generalizable to atopic asthma regardless of age, regardless of age, race/ethnicity, and specific exposures.
Acknowledgments
This research was supported by the National Institute of Allergy and Infectious Diseases (N01-AI90052), National Heart, Lung and Blood Institute (R01-HL101251), the National Institute for Environmental Health Sciences (P01-ES18181), and the National Center for Advancing Translational Sciences (UL1TR000075).
ABBREVIATIONS
- DMP
Differentially methylated probe
- DMR
Differentially methylated region
- FDR
false discovery rate
- ICAC
Inner City Asthma Consortium
- PBMC
peripheral blood mononuclear cells
- PEER
Probabilistic estimation of expression residuals
- QTL
Quantitative trait locus
CAPSULE SUMMARY
DNA methylation changes substantial in magnitude are associated with atopic asthma and gene expression in African American inner city children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Lockett GA, Holloway JW. Genome-wide association studies in asthma; perhaps, the end of the beginning. Current opinion in allergy and clinical immunology. 2013;13:463–469. doi: 10.1097/ACI.0b013e328364ea5f. [DOI] [PubMed] [Google Scholar]
- 3.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nature reviews Immunology. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 4.Moffatt MF, Cookson WO. The genetics of asthma. Maternal effects in atopic disease. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1998;28(Suppl 1):56–61. doi: 10.1046/j.1365-2222.1998.0280s1056.x. discussion 65–56. [DOI] [PubMed] [Google Scholar]
- 5.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proc Natl Acad Sci U S A. 2007;104:17052–17057. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. The Journal of biological chemistry. 2007;282:700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 9.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA. In utero supplementation with methyl donors enhances allergic airway disease in mice. The Journal of clinical investigation. 2008:118. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Yang IV, Pedersen BS, Liu AH, O’Connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SF, Gill MA, Calatroni A, David G, Hennessy CE, Davidson EJ, Zhang W, Gergen P, Togias A, Busse WW, DAS DNA Methylation Changes and Childhood Asthma in the Inner City. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.01.025. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, Willis-Owen SA, Laprise C, Wong KC, Davies GA, Hudson TJ, Binia A, Hopkin JM, Yang IV, Grundberg E, Busche S, Hudson M, Ronnblom L, Pastinen TM, Schwartz DA, Lathrop GM, Moffatt MF, Cookson WO. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature. 2015 doi: 10.1038/nature14125. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi VD, Davidson C, Asaduzzaman M, Nahirney D, Vliagoftis H. House dust mite interactions with airway epithelium: role in allergic airway inflammation. Current allergy and asthma reports. 2013;13:262–270. doi: 10.1007/s11882-013-0349-9. [DOI] [PubMed] [Google Scholar]
- 13.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nature medicine. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 14.Yang IV, Tomfohr J, Singh J, Foss CM, Marshall HE, Que LG, McElvania-Tekippe E, Florence S, Sundy JS, Schwartz DA. The clinical and environmental determinants of airway transcriptional profiles in allergic asthma. American journal of respiratory and critical care medicine. 2012;185:620–627. doi: 10.1164/rccm.201108-1503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. American journal of respiratory and critical care medicine. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole A, Urbanek C, Eng C, Schageman J, Jacobson S, O'Connor BP, Galanter JM, Gignoux CR, Roth LA, Kumar R, Lutz S, Liu AH, Fingerlin TE, Setterquist RA, Burchard EG, Rodriguez-Santana J, Seibold MA. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. The Journal of allergy and clinical immunology. 2014;133:670–678. e612. doi: 10.1016/j.jaci.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu W, Baccarelli A, Carey VJ, Boutaoui N, Bacherman H, Klanderman B, Rennard S, Agusti A, Anderson W, Lomas DA, DeMeo DL. Variable DNA methylation is associated with chronic obstructive pulmonary disease and lung function. American journal of respiratory and critical care medicine. 2012;185:373–381. doi: 10.1164/rccm.201108-1382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolzhenko E, Smith AD. Using beta-binomial regression for high-precision differential methylation analysis in multifactor whole-genome bisulfite sequencing experiments. BMC bioinformatics. 2014;15:215. doi: 10.1186/1471-2105-15-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcucci G, Yan P, Maharry K, Frankhouser D, Nicolet D, Metzeler KH, Kohlschmidt J, Mrozek K, Wu YZ, Bucci D, Curfman JP, Whitman SP, Eisfeld AK, Mendler JH, Schwind S, Becker H, Bar C, Carroll AJ, Baer MR, Wetzler M, Carter TH, Powell BL, Kolitz JE, Byrd JC, Plass C, Garzon R, Caligiuri MA, Stone RM, Volinia S, Bundschuh R, Bloomfield CD. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, Guardela BJ, Tedrow JR, Zhang Y, Singh MK, Correll M, Schwarz MI, Geraci M, Sciurba FC, Quackenbush J, Spira A, Kaminski N, Schwartz DA. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2014;190:1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile Within Array Normalization for Illumina Infinium HumanMethylation450 BeadChips. Genome biology. 2012;13:R44. doi: 10.1186/gb-2012-13-6-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stegle O, Parts L, Durbin R, Winn J. A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS computational biology. 2010;6:e1000770. doi: 10.1371/journal.pcbi.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical applications in genetics and molecular biology. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen BS, Schwartz DA, Yang IV, Kechris KJ. Comb-p: software for combining, analyzing, grouping and correcting spatially correlated P-values. Bioinformatics. 2012;28:2986–2988. doi: 10.1093/bioinformatics/bts545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 28.Zhang D, Cheng L, Badner JA, Chen C, Chen Q, Luo W, Craig DW, Redman M, Gershon ES, Liu C. Genetic control of individual differences in gene-specific methylation in human brain. American journal of human genetics. 2010;86:411–419. doi: 10.1016/j.ajhg.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstein M. Genomics: ENCODE leads the way on big data. Nature. 2012;489:208. doi: 10.1038/489208b. [DOI] [PubMed] [Google Scholar]
- 30.Buro-Auriemma LJ, Salit J, Hackett NR, Walters MS, Strulovici-Barel Y, Staudt MR, Fuller J, Mahmoud M, Stevenson CS, Hilton H, Ho MW, Crystal RG. Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Human molecular genetics. 2013;22:4726–4738. doi: 10.1093/hmg/ddt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabbott NA, Baillie JK, Brown H, Freeman TC, Hume DA. An expression atlas of human primary cells: inference of gene function from coexpression networks. BMC genomics. 2013;14:632. doi: 10.1186/1471-2164-14-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benoist C, Lanier L, Merad M, Mathis D. Consortium biology in immunology: the perspective from the Immunological Genome Project. Nature reviews Immunology. 2012;12:734–740. doi: 10.1038/nri3300. [DOI] [PubMed] [Google Scholar]
- 33.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. The Journal of allergy and clinical immunology. 2010;125:1046–1053. e1048. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 35.Nachbur U, Stafford CA, Bankovacki A, Zhan Y, Lindqvist LM, Fiil BK, Khakham Y, Ko HJ, Sandow JJ, Falk H, Holien JK, Chau D, Hildebrand J, Vince JE, Sharp PP, Webb AI, Jackman KA, Muhlen S, Kennedy CL, Lowes KN, Murphy JM, Gyrd-Hansen M, Parker MW, Hartland EL, Lew AM, Huang DC, Lessene G, Silke J. A RIPK2 inhibitor delays NOD signalling events yet prevents inflammatory cytokine production. Nature communications. 2015;6:6442. doi: 10.1038/ncomms7442. [DOI] [PubMed] [Google Scholar]
- 36.Goh FY, Cook KL, Upton N, Tao L, Lah LC, Leung BP, Wong WS. Receptor-interacting protein 2 gene silencing attenuates allergic airway inflammation. J Immunol. 2013;191:2691–2699. doi: 10.4049/jimmunol.1202416. [DOI] [PubMed] [Google Scholar]
- 37.Nicodemus-Johnson J, Naughton KA, Sudi J, Hogarth K, Naurekas ET, Nicolae DL, Sperling AI, Solway J, White SR, Ober C. Genome-Wide Methylation Study Identifies an IL-13-induced Epigenetic Signature in Asthmatic Airways. American journal of respiratory and critical care medicine. 2016;193:376–385. doi: 10.1164/rccm.201506-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Aryee MJ, Padyukov L, Fallin MD, Hesselberg E, Runarsson A, Reinius L, Acevedo N, Taub M, Ronninger M, Shchetynsky K, Scheynius A, Kere J, Alfredsson L, Klareskog L, Ekstrom TJ, Feinberg AP. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nature biotechnology. 2013;31:142–147. doi: 10.1038/nbt.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, Ji H, Potash JB, Sabunciyan S, Feinberg AP. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature genetics. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindley AR, Crapster-Pregont M, Liu Y, Kuperman DA. 12/15-lipoxygenase is an interleukin-13 and interferon-gamma counterregulated-mediator of allergic airway inflammation. Mediators of inflammation. 2010 doi: 10.1155/2010/727305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lampe WR, Park J, Fang S, Crews AL, Adler KB. Calpain and MARCKS protein regulation of airway mucin secretion. Pulmonary pharmacology & therapeutics. 2012;25:427–431. doi: 10.1016/j.pupt.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imoto Y, Tokunaga T, Matsumoto Y, Hamada Y, Ono M, Yamada T, Ito Y, Arinami T, Okano M, Noguchi E, Fujieda S. Cystatin SN upregulation in patients with seasonal allergic rhinitis. PloS one. 2013;8:e67057. doi: 10.1371/journal.pone.0067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Candiano G, Bruschi M, Pedemonte N, Caci E, Liberatori S, Bini L, Pellegrini C, Vigano M, O'Connor BJ, Lee TH, Galietta LJ, Zegarra-Moran O. Gelsolin secretion in interleukin-4-treated bronchial epithelia and in asthmatic airways. American journal of respiratory and critical care medicine. 2005;172:1090–1096. doi: 10.1164/rccm.200409-1185OC. [DOI] [PubMed] [Google Scholar]
- 45.Nassenstein C, Braun A, Erpenbeck VJ, Lommatzsch M, Schmidt S, Krug N, Luttmann W, Renz H, Virchow JC., Jr The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med. 2003;198:455–467. doi: 10.1084/jem.20010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imaoka H, Punia N, Irshad A, Ying S, Corrigan CJ, Howie K, O'Byrne PM, Gauvreau GM, Sehmi R. Lung homing of endothelial progenitor cells in humans with asthma after allergen challenge. American journal of respiratory and critical care medicine. 2011;184:771–778. doi: 10.1164/rccm.201102-0272OC. [DOI] [PubMed] [Google Scholar]
- 47.Han H, Xu D, Liu C, Claesson HE, Bjorkholm M, Sjoberg J. Interleukin-4-mediated 15-lipoxygenase-1 trans-activation requires UTX recruitment and H3K27me3 demethylation at the promoter in A549 cells. PloS one. 2014;9:e85085. doi: 10.1371/journal.pone.0085085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu DH, Waterland RA, Zhang P, Schady D, Chen MH, Guan Y, Gadkari M, Shen L. Targeted p16Ink4a epimutation causes tumorigenesis and reduces survival in mice. The Journal of clinical investigation. 2014;124:3708–3712. doi: 10.1172/JCI76507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, Leighton JK, Patel H, Rahman A, Sridhara R, Wang YC, Pazdur R. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- 50.Saba HI. Decitabine in the treatment of myelodysplastic syndromes. Therapeutics and clinical risk management. 2007;3:807–817. [PMC free article] [PubMed] [Google Scholar]
- 51.Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glockner S, Piantadosi S, Gabrielson E, Pridham G, Pelosky K, Belinsky SA, Yang SC, Baylin SB, Herman JG. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 52.Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, Sebree R, Rodgers K, Hooker CM, Franco N, Lee B, Tsai S, Delgado IE, Rudek MA, Belinsky SA, Herman JG, Baylin SB, Brock MV, Rudin CM. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer discovery. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baccarelli A, Rusconi F, Bollati V, Catelan D, Accetta G, Hou L, Barbone F, Bertazzi PA, Biggeri A. Nasal cell DNA methylation, inflammation, lung function and wheezing in children with asthma. Epigenomics. 2012;4:91–100. doi: 10.2217/epi.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales E, Bustamante M, Vilahur N, Escaramis G, Montfort M, de Cid R, Garcia-Esteban R, Torrent M, Estivill X, Grimalt JO, Sunyer J. DNA Hypomethylation at ALOX12 Is Associated with Persistent Wheezing in Childhood. American journal of respiratory and critical care medicine. 2012;185:937–943. doi: 10.1164/rccm.201105-0870OC. [DOI] [PubMed] [Google Scholar]
- 55.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Scientific reports. 2013;3:2164. doi: 10.1038/srep02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, Briem E, Zhang K, Irizarry RA, Feinberg AP. Increased methylation variation in epigenetic domains across cancer types. Nature genetics. 2011;43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timp W, Bravo HC, McDonald OG, Goggins M, Umbricht C, Zeiger M, Feinberg AP, Irizarry RA. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. Genome medicine. 2014;6:61. doi: 10.1186/s13073-014-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, Xie B, Daley GQ, Church GM. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nature biotechnology. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulis M, Heath S, Bibikova M, Queiros AC, Navarro A, Clot G, Martinez-Trillos A, Castellano G, Brun-Heath I, Pinyol M, Barberan-Soler S, Papasaikas P, Jares P, Bea S, Rico D, Ecker S, Rubio M, Royo R, Ho V, Klotzle B, Hernandez L, Conde L, Lopez-Guerra M, Colomer D, Villamor N, Aymerich M, Rozman M, Bayes M, Gut M, Gelpi JL, Orozco M, Fan JB, Quesada V, Puente XS, Pisano DG, Valencia A, Lopez- Guillermo A, Gut I, Lopez-Otin C, Campo E, Martin-Subero JI. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nature genetics. 2012;44:1236–1242. doi: 10.1038/ng.2443. [DOI] [PubMed] [Google Scholar]
- 60.Wan ES, Qiu W, Baccarelli A, Carey VJ, Bacherman H, Rennard SI, Agusti A, Anderson W, Lomas DA, Demeo DL. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Human molecular genetics. 2012 doi: 10.1093/hmg/dds135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA methylation changes after exposure to traffic particles. American journal of respiratory and critical care medicine. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin JC, Riedler J, Mazaleyrat N, Weber J, Karvonen AM, Hirvonen MR, Braun-Fahrlander C, Lauener R, von Mutius E, Kabesch M, Tost J. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68:355–364. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 63.Lovinsky-Desir S, Ridder R, Torrone D, Maher C, Narula S, Scheuerman M, Merle D, Kattan M, DiMango E, Miller RL. DNA methylation of the allergy regulatory gene interferon gamma varies by age, sex, and tissue type in asthmatics. Clinical epigenetics. 2014;6:9. doi: 10.1186/1868-7083-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McErlean P, Favoreto S, Jr, Costa FF, Shen J, Quraishi J, Biyasheva A, Cooper JJ, Scholtens DM, Vanin EF, de Bonaldo MF, Xie H, Soares MB, Avila PC. Human rhinovirus infection causes different DNA methylation changes in nasal epithelial cells from healthy and asthmatic subjects. BMC medical genomics. 2014;7:37. doi: 10.1186/1755-8794-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vergara C, Murray T, Rafaels N, Lewis R, Campbell M, Foster C, Gao L, Faruque M, Oliveira RR, Carvalho E, Araujo MI, Cruz AA, Watson H, Mercado D, Knight-Madden J, Ruczinski I, Dunston G, Ford J, Caraballo L, Beaty TH, Mathias RA, Barnes KC. African ancestry is a risk factor for asthma and high total IgE levels in African admixed populations. Genetic epidemiology. 2013;37:393–401. doi: 10.1002/gepi.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galanter JM, Gignoux CR, Torgerson DG, Roth LA, Eng C, Oh SS, Nguyen EA, Drake KA, Huntsman S, Hu D, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, LeNoir MA, Meade K, Serebrisky D, Borrell LN, Rodriguez-Cintron W, Estrada AM, Mendoza KS, Winkler CA, Klitz W, Romieu I, London SJ, Gilliland F, Martinez F, Bustamante C, Williams LK, Kumar R, Rodriguez-Santana JR, Burchard EG. Genome-wide association study and admixture mapping identify different asthma-associated loci in Latinos: the Genes-environments & Admixture in Latino Americans study. The Journal of allergy and clinical immunology. 2014;134:295–305. doi: 10.1016/j.jaci.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, Gilad Y, Pritchard JK. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome biology. 2011;12:R10. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fraser HB, Lam LL, Neumann SM, Kobor MS. Population-specificity of human DNA methylation. Genome biology. 2012;13:R8. doi: 10.1186/gb-2012-13-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature reviews Genetics. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 70.Xia J, Benner MJ, Hancock RE. NetworkAnalyst--integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic acids research. 2014;42:W167–174. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.