Abstract
Most gastrointestinal stromal tumors (GISTs) occur in the tubular gastrointestinal tract, but some present apparently outside the GI-tract. In this study, we analyzed 112 GISTs located in the retroperitoneum. These tumors occurred in 55 women and 57 men with a median age of 65 years (range: 21-89 years). Based on clinically or histologically detected connections to GI-tract, 15 tumors were considered likely of gastric, 9 duodenal, and 13 of small intestinal origin. The remaining cases were categorized by location as peripancreatic (n = 25), pelvic (n = 11), mesenteric (n = 4), and of unspecified/miscellaneous sites (n = 35). The tumors varied in size 3-35 cm (median, 15 cm) and by mitotic rate per 5 mm2, 0- >100 (median 10). Histologically the tumors apparently arising outside the GI-tract had features of intestinal (n = 41) and gastric GISTs (n = 25); 9 cases had indeterminate histology. The histologic variants included spindled, epithelioid, vacuolated, nested and myxoid potentially simulating other tumors such as liposarcoma and solitary fibrous tumor. Most GISTs were KIT-positive (106/112 cases), and the remaining 6 tumors were Dog1/Ano1-positive. Five cases showed focal nuclear positivity for MDM2. KIT mutations were detected in 42/59 cases, and PDGFRA mutations in 4/16 KIT wild-type and 3/5 of the KIT-negative tumors analyzed. One pelvic retroperitoneal GIST was SDH-deficient. All 79 patients were dead at last follow-up with a median survival of 14 months, with few survivals > 5 years. Only operable vs. inoperable tumor was a statistically favorable factor in univariate analysis (p<0.01). In multivariate analysis, mitotic rate > 50/5 mm2 was significant for a shorter survival (HR 5.25, 95% CI 1.65-16.8., p<0.01). Histologic and clinicopathologic similarity of extragastrointestinal retroperitoneal GISTs with GISTs of GI-tract suggests their GI tract origin. Potentially overlapping features between GIST and other retroperitoneal tumors necessitate use of multiple diagnostic markers and molecular genetic studies.
Keywords: gastrointestinal stromal tumor, KIT, PDGFRA, SDHB, mutation, prognosis
Introduction
Gastrointestinal stromal tumors (GISTs), usually KIT or PDGFRA mutation-driven tumors, typically occur in the tubular gastrointestinal tract, most commonly in the stomach and small intestine. However, a subset of GISTs appear to be located outside the gastrointestinal tract. These tumors have been designated as extragastrointestinal GISTs or EGISTs.1-5
Only a small number of extragastrointestinal GISTs have been reported in the retroperitoneal location, and clinicopathologic correlation and long-term follow-up data of such tumors are scant. In most cases, the location of the retroperitoneal GISTs was not further specified.3-9
In this study, we analyzed 112 retroperitoneal GISTs in detail with a special attention to precise tumor location, comprehensive immunohistochemical and molecular genetic analysis, including KIT and PDGFRA mutations, succinate dehydrogenase deficiency, and long-term follow-up.
Materials and Methods
Study material
All retroperitoneal tumors coded or diagnosed as leiomyomas, smooth muscle tumors of uncertain malignant potential, or leiomyosarcomas were retrieved from the files of the Armed Forces Institute of Pathology (AFIP) from 1970-1996, a total of approximately 700 cases. During this time period, GISTs were categorized as gastrointestinal smooth muscle tumors, and a substantial number of these cases predated availability of immunohistochemistry and molecular genetics for precise tumor classification.
All tumors were histologically examined and analyzed immunohistochemically at least for KIT expression. By histologic classification done in this study, these tumors included 221 GISTs, 240 true leiomyosarcomas, 34 dedifferentiated liposarcomas, 55 undifferentiated or unclassified sarcomas, and 65 uterine-type leiomyomas in women. There were isolated cases of other entities, such as solitary fibrous tumor, PEComa, and follicular dendritic reticulum cell sarcoma.
Of 112 retroperitoneal GISTs, there was sufficient material and records available the study. The inclusion of cases was based on the following evidence: 106 tumors were KIT-positive and histologically compatible with GIST. Six tumors were compatible with GIST but KIT-negative, yet positive for DOG1/Ano1 and were also classified as GIST.
Clinical data were reviewed from the charts and an effort was made to specify tumor site in the retroperitoneum as accurately as possible. The retroperitoneal location was further divided into the following categories: peripancreatic, pelvic, mesenteric, and undesignated/miscellaneou. Tumors attached to any portion of the GI-tract were not removed from the study but were recognized separately.
Histologic examination included the following parameters: Mitotic rate per 5 mm2, degree of atypia (mild, moderate, severe/pleomorphic), presence of tumor necrosis, spindled vs. epithelioid histology, skeinoid fibers, and possible anatomic connection to any part of the gastrointestinal tract. A histologic assessment was also made whether the tumor had a gastric or intestinal GIST-like histology, observing the histologic features previously described in the spectrum of gastric and intestinal GISTs.10,11 This assessment was blinded from clinical information and showed 87% of accuracy in tumors from known origin. In order to assess the presence of Cajal cells in pancreas, 10 cross sections of pancreas containing ducts of various calibers were examined with KIT immunostaining as described below.
Immunohistochemical studies were mainly performed using multitumor blocks containing 30-60 cases, with the sample sizes being 5-15 times larger than those in tumor arrays from 0.6 mm core samples. In 30 cases with no blocks available, KIT or DOG1/Ano1-immunostain was performed on unstained slides, or occasionally on a restained negative control slide. Additional markers studied were beta-catenin, CD34, h-caldesmon, desmin, MDM2, MyoD1, myogenin, S100 protein, SDHA, SDHB, SMA, and STAT6. Immunostaining was performed using Leica Bond Max automation. The staining protocols are tabulated in Supplementary data 1.
DNA was extracted from formalin-fixed and paraffin-embedded tissue following previously published procedure. 12 The selected mutation hot-spots in KIT: exons 9, 11, 13, and 17, PDGFRA: exons 12, 14, and 18, BRAF exon 15, KRAS exon 2, and PIK3CA exon 20 were PCR amplified. Sanger sequencing of PCR amplification products were completed by Macrogen USA (Rockville, MD). The sequences were analyzed following alignment with following NCBI Reference Sequences: KIT NM_000222.2 and NP_000213.1, PDGFRA NM_006206.4 and NP_006197.1, and PIK3CA NM_006218.2 and NP_006209.2 (www.ncbi.nml.nih.gov).
Follow-up was performed based on social security death index, other public records, and follow-up notes available in the files. Statistical analyses were performed with EZR version 1.32. software.13 The prognostic value of the categorical data for overall survival was determined through univariate Kaplan-Meier survival estimates with log-rank test. Multivariate Cox's proportional hazard models were performed to analyze the association of survival and independent categorical variables including age (<65 vs. ≧65 years old), sex (male vs. female), tumor size (<10 vs. 10≦ <20 vs. ≧20), tumor histology (epithelial vs. spindle vs. mixed subtypes), tumor atypia (mild vs. moderate), tumor necrosis (present vs. absent), mitotic count (≦10 vs. 10< ≦50 vs. <50), liver metastasis (present vs. absent), operability (operable vs. inoperable), and gene mutation (wild type vs. KIT Ex11del vs. KIT Ex11dup vs. KIT Ex11pm vs. KIT Ex9dup vs.PDGFR mutant). A backward selection technique with a threshold of p=0.05 was used to select variables in the final model, whereas factors not significant were removed stepwise from the model. Cases with missing information were eliminated from the statistical analysis of that parameter.
Results
Clinical features
There were 112 patients: 55 women (median age: 69 years, range 31-89 years) and 57 men (median age 61 years, range, 21-85 years). The patients presented variably with abdominal pain, increased abdominal girth, gastrointestinal bleeding, and in some cases, with a palpable abdominal mass or septic episodes prompting the detection of an abdominal mass.
Although all tumors were surgically designated as retroperitoneal tumors, analysis of the records, and in some cases, histologic examination, indicated involvement and likely origin from the stomach in 15 cases, small intestine in 13 cases, and duodenum in 9 cases. In the remaining cases, the tumor location was further specified as peripancreatic (n = 25), pelvic (n = 11), and small intestinal mesenteric (n = 4). Three tumors were adherent to inferior vena cava and one was subhepatic in the right upper quadrant. The retroperitoneal location of the remaining 31 cases was not further specified. Eight patients had multiple synchronous retroperitoneal tumors.
The surgical procedures were characterized as excisions in 49 cases, debulking or partial excision in 11 cases, and incisional biopsy in 28 cases; 35 cases were considered inoperable. Liver metastases developed in 15 cases. All cases predated availability of imatinib treatment.
Outcomes
At the last follow-up, 79 patients were dead at 0-39 years with a median survival of 14 months. No patient was found to be alive, but 32 patients were lost to follow-up due to incomplete demographics. Of the 79 patients with follow up, cumulative number of deceased patients was 37 in 1 year, 50 in 2 years, and 61 in 3 years. Of the remaining 18 patients,7 patients survived 3-5 years, 6 patients 5-10 years, 3 patients 10-20 years, and 2 patients > 20 years: one 27.4 years, and another 39 years. The cause of death, was known to be tumor-related in all 12 cases when known. None of the patients had received imatinib or other tyrosine kinase inhibitors, as this study cohort predated availability of these therapies.
The only prognostically favorable factor in univariate analysis was an operable tumor (p<0.01), whereas patient age < 65 years, tumor size < 10 cm, mitotic rate <10/5 mm2, lack of necrosis, KIT/PDGFRA mutation type or wild-type, or absent liver metastases, were not identified as statistically favorable factors (Supplementary Figs. 1 and 2). In multivariate analysis, mitotic rate > 50/5 mm2 was significant for a shorter survival (HR 5.25, 95% CI 1.65-16.8., p<0.01). Kaplan-Meier plots comparing histological variables and mutation types are shown in Supplementary Figures 1 and 2.
Pathology
In the 81 cases with specified tumor size, most tumors were large with a median size of 15 cm (range, 3-35 cm). While only 14 tumors were < 10 cm, many tumors with unspecified size were characterized as large to extremely large. Multiple synchronous retroperitoneal tumors were present in 8 cases. Grossly the tumors were often described as cystic, tan, hemorrhagic, and lobulated. Other common descriptors included pink, fleshy, and rubbery.
Histologically 61 tumors showed intestinal GIST histology being composed of spindle cells often showing Verocay body-like arrangements, or hemangiopericytoma-like patterns (Fig. 1). Only 2 cases had skeinoid fibers. Gastric GIST histology was seen in 35 cases including tumors with palisaded and vacuolated histology, sclerosing spindle cell appearance, or epithelioid morphology (Fig. 2). Indeterminate histology, often with high mitotic rates, was seen in 15 cases. This included tumors with diffuse sheets of cells (Fig. 3A), nested patterns with myxoid matrix remotely resembling a chordoma (Fig. 3B), and perivascular sparing patterns (Fig. 3C, D). A majority of tumors had spindle cell histology (n = 81), with 12 cases showing epithelioid and 19 cases a mixed spindled-epithelioid pattern. Nuclear atypia was mild in 82 cases and moderate in 30 cases with focal pleomorphism occurring in 6 cases (4 with epithelioid and 2 with spindle cell overall histology). The mitotic rate varied 0 - >100/5mm2 (median 10), and tumor necrosis was present in 57 cases. Only 7 tumors fell into low-risk prognostic groups (Group 2: 1 case, group 3a: 6 cases).
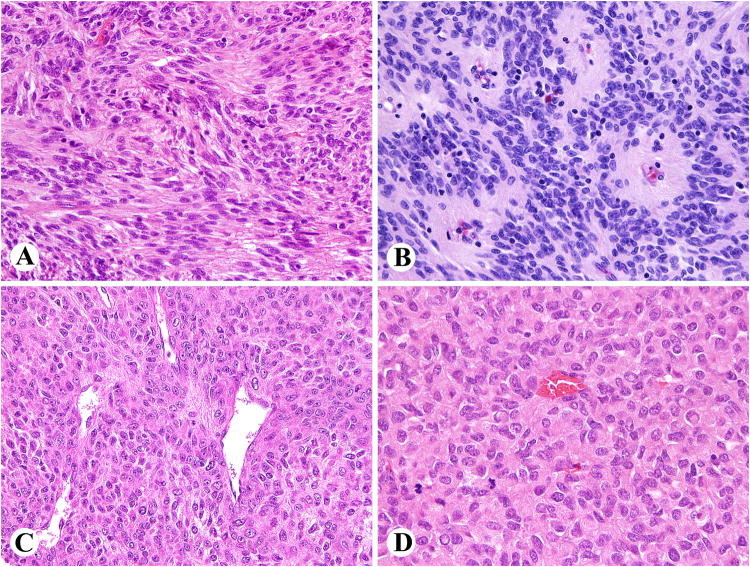
Fig. 1.
Examples of small intestinal-like GIST histology in retroperitoneal GISTs. A. Verocay body-like spaces and vague palisading. B. Perivascular pseudorosettes. C. Hemangiopericytoma-like pattern. D. Irregular Verocay body-like formations in a highly cellular tumor.
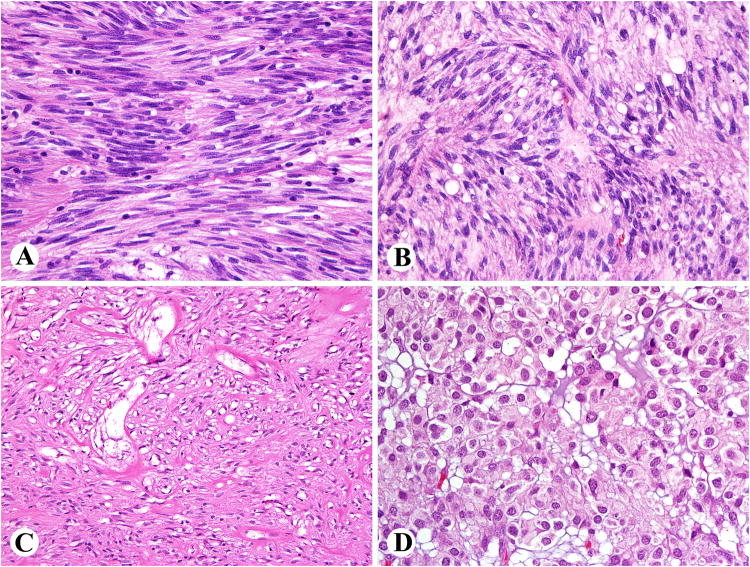
Fig. 2.
Examples of gastric GIST-like histology in retroperitoneal GISTs. A, B. Tumors with palisaded and vacuolated histology. C. Sclerosing spindle cell pattern. D. Distinctly epithelioid histology seen in a subset of gastric GISTs.
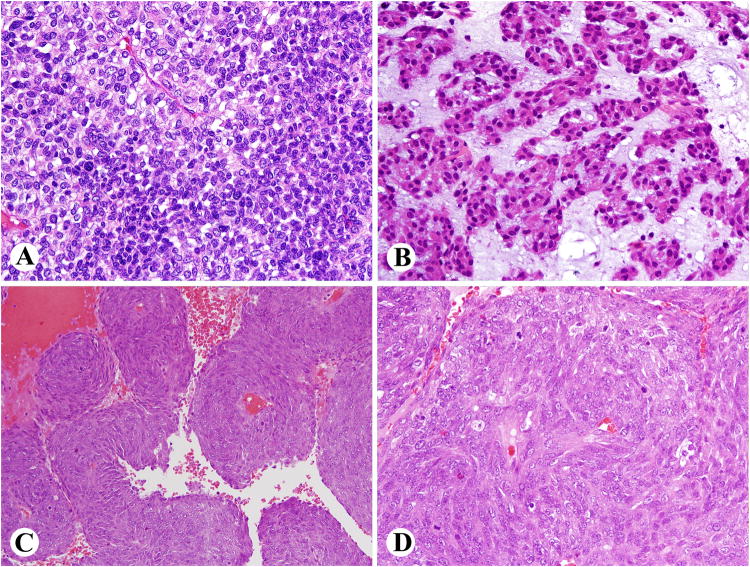
Fig. 3.
Retroperitoneal GISTs with histological patterns nonspecific for tissue origin or differentiation. A. A sheet like pattern with smaller and larger cells. B. Nests of cells surrounded by myxoid matrix remotely resembling chordoma. C, D. An example showing perivascular preservation pattern with pseudoangiomatoid spaces.
Immunohistochemical profiles
KIT was detected in 106/112 tumors (95%), usually with strong positivity in all or most tumor cells. DOG1/Ano1-positive cases (81/87, 93%) included the 6 KIT-negative cases, of which 3/5 cases analyzed has a PDGFRA mutation. CD34 was present in 53/86 cases (62%), in >25% of tumor cells in all but 4 cases. Smooth muscle actin was detected in in 26/84 cases (31%), usually in >50% of tumor cells. Desmin was focally expressed in a PDGFRA mutant, KIT-negative GIST. Heavy-caldesmon was present in 27/73 cases (37%). One tumor (1/79) had an SDHB-loss, with retained SDHA. Nuclear MDM2 was focally present in 7/72 cases (in 2-60%of tumor cells, median 7%). None of the 61-72 cases tested showed nuclear positivity for beta-catenin, myogenin, MyoD1, STAT6, or definitive tumor cell positivity for S100 protein.
Mutation analysis
Molecular genetic studies were performed on 95 cases including 59 confirmed GISTs and 36 diagnostically challenging tumors. In the latter cases, detection of KIT/PDGFRA activating mutations could potentially support GIST diagnosis in the absence of KIT or DOG1/Ano1-expression. However, no such cases were identified. One KIT- and DOG1-negative, KIT mutant (p.Gly565Val (c.1781G>T substitution in KIT exon 11) and BRAF V600E mutant, S100 protein-positive tumor, was considered most likely melanoma and was excluded from the study.
The detected mutations are presented in detail in supplementary data Table 2. KIT mutations predicted to be activating were detected in 42 (71%) of the 59 analyzed GISTs. There were 28 in-frame deletions typically involving 5′ part of exon 11 (KIT juxtamembrane domain) with p.Trp557_Lys558del being the most common (n=6). In eight tumors, 9 single nucleotide substitutions affecting codons 551, 553, 557, 559 (n=4), 560 and 576 were identified. Four GISTs carried 2 to 7 codon internal tandem duplications. In one case, duplication of the p.Ala502_Tyr503 was detected in KIT exon 9. Four (25%) of 16 KIT wild-type GISTs carried PDGFRA mutations: one p.Val561Asp substitution in exon 12, two identical p.Ile843_Asp846 deletions, and one p.Asp842Val substitution in exon 18. Moreover, 4 PIK3CA exon 20 mutations were detected in 3 (5.5%) of 55 analyzed tumors including 2 KIT-mutant GISTs. KIT mutation assay failed in one of those cases. All 53 GISTs studied were wild type for BRAF exon 15 and KRAS exon 2.
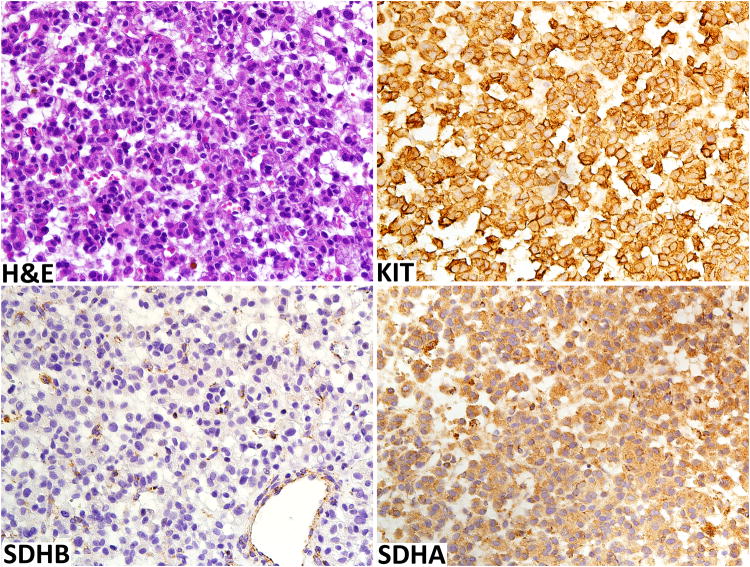
One of the KIT/PDGFRA wild-type GIST was SDH-deficient. This 15 cm retroperitoneal tumor occurred in a 45-year-old man in a pelvic basin between rectum and bladder extending anteriorly to abut the rectus abdominis muscle. The tumor was composed of rather uniform epithelioid cells, similar to those typically seen gastric SDH-deficient GISTs (Fig. 4). This tumor was positive for KIT, DOG1/Ano1, and showed loss of SDHB with labeling in capillaries and tumor-infiltrating mononuclear cells only while retaining SDHA expression (Fig. 4). This patient has also a history of desmoid fibromatosis, but there was no previous history of GIST. The patient was lost to follow-up.
Fig. 4.
Pelvic retroperitoneal SDH-deficient GIST is highly cellular composed of uniform epithelioid cells. Like KIT-mutant GIST, this tumor is KIT-positive. SDHB-expression is lost but preserved in endothelia and macrophages. SDHA-expression is retained in tumor cells.
Subgroups of tumors by location
Peripancreatic tumors were the largest group of GISTs with a specified location, a total of 25 cases. These tumors were often surgically considered pancreatic tumors or (pseudo)cysts. They were further described to be retrogastric/lesser sac masses (n = 7), localized at the mesentery (n = 2), and one each at the hepatic veins, head of pancreas, and junction of the pancreatic body and tail. Gastric GIST-like histology was seen in 9 cases and intestinal GIST-like histology in 10 cases, with 6 cases being histologically indeterminate. Median survival of the 15 patients with follow-up was 12 months, and only 2 patients survived > 5 years. One of those survivors had a hyalinized GIST with gastric GIST-like morphology and mitotic count of 0/5mm2.
The ten pancreatic specimens containing ducts of various sizes did not show KIT-positive elements other than mast cells and occasional vascular endothelial cells. No elongated mesenchymal cell showing Cajal cell morphology were identified. Representative images are shown in Supplementary Figure 3.
Pelvic location was specified for 11 tumors. One of these tumors was an SDH-deficient GIST and described above in detail. The 7 patients with follow-up survived 1-71 months (median, 17 months). Six of these tumors had intestinal GIST-like histology, 3 were gastric GIST-like, and 2 indeterminate.
Discussion
In this clinicopathologic study, we analyzed 112 retroperitoneal GISTs, sometimes designated as extragastrointestinal GISTs (EGISTs). A small number of retroperitoneal GISTs have been reported in two series and isolated case reports.3-9 It is clear that these tumors are true GIST by their histology and immunophenotypes, and GIST-type KIT mutations, as reported in one series. 4 However, more precise location was not given in any of those cases. 3-9
Whether GISTs truly arise from the extragastrointestinal sites such as retroperitoneum is debatable. Most of the retroperitoneal GISTs reported here and in the literature have been large, > 10 cm. It can be reasoned that any large abdominal extragastrointestinal GIST is located at some proximity of segments of the tubular gastrointestinal tract. GISTs forming external masses around the stomach or intestines with only tenuous connections to those organs can create a clinical appearance of an extragastrointestinal GIST. This was especially true before the nature of GIST became widely understood. Furthermore, if such “extragastrointestinal” tumors are excised without a gastrointestinal resection, there is a pathologic impression of an extragastrointestinal GIST. Common extension of GISTs outward from the GI-tract can make them look extragastrointestinal. 10,11,14,15
Critical analysis of the origin of surgically defined retroperitoneal GISTs in this study showed that origin from the gastrointestinal tract could be confirmed or strongly suspected in 36 cases. Furthermore, histologic analysis showed gastric GIST histology suggestive of gastric origin in 15 cases and intestinal GIST histology suggesting intestinal origin in 35 cases. This evidence indicates that a significant portion, perhaps all, of the retroperitoneal GISTs, originate in the gastrointestinal tract proper and belong to the clinicopathologic spectrum of advanced gastric and intestinal GISTs rather than being an entity of their own.
The possibility for GIST originating outside the gastrointestinal tract may rarely exist, for example from developmental anomalies such as enteric duplications cysts (intestinal duplications) that could contain Cajal cells or related stem cells known for ancestry of GIST. Some authors have detected Cajal cells outside of gastrointestinal tract such as omentum2, although to our knowledge, this has not been widely confirmed and has not been specifically observed in retroperitoneal soft tissues. Also, Cajal cells have been reported in feline pancreas, based on KIT immunohistochemistry and electron microscopy.16 In this study, we failed to identify Cajal cells comparable with intestinal Cajal cells in human pancreas.
The most common specified location for retroperitoneal GIST in this study was peripancreatic (25 cases). These GISTs included cases with both intestinal and gastric GIST-like histologic features. The former likely originate from the duodenum, whereas gastric-like GISTs include tumors that arise from the posterior wall of stomach extending into the peripancreatic area. There was no evidence that these tumors arose from the pancreas itself.
There are approximately 25 reports of “pancreatic extragastrointestinal GISTs” in the literature (Supplementary data Table 3). In 14 of those cases, there is evidence to suggest gastric or duodenal origin. In 4 cases, the tumor involved duodenal wall, which was illustrated in the lastcited report.5,17-19 In 5 cases the tumor involved the uncinate process of the pancreas located immediately adjacent to the duodenal wall. 20-24 Furthermore, 5 of the tumors reported as pancreatic GISTs also involved stomach, and gastric resection was also performed as part of surgery.25-29 In most of the remaining cases, the tumors were large well exceeding the dimensions of pancreas. None of the reports convincingly documented purely intrapancreatic location for the tumor.30-40
In general, the prognosis of retroperitoneal GISTs in this pre-imatinib study cohort was poor with the median survival of the 79 patients with follow-up being only 14 months. Furthermore, only 17 of those patients (22%) survived more than 3 years, 10 patients (13%) > 5 years, and 4 patients >10 years. The only favorable factor in univariate analysis was an operable tumor. These data indicate that most retroperitoneal GISTs are advanced malignant tumors. The patient survival has been longer in some more recent patient cohorts, probably because of availability of imatinib treatment.4,5 Although we did not have cause of death data in the majority of patients, the facts that all patients with known cause of death died of tumor and that survivals were short with a few exceptions, suggest that most deaths were tumor-related.
Long term survival > 10 years was observed in 3 patients and survival >5≤10 years in 6 patients. These tumors behaved comparable to primary GISTs with favorable or low-grade clinical course. Some tumors had previously known favorable features, such as low mitotic rate, gastric histology, and internal tandem duplications in exon 11 of KIT, as shown for gastric GISTs in an earlier study. 41 However, these features were not statistically significant due to the small number of such cases.
GISTs in the retroperitoneal location may be challenging to diagnose due to their considerable morphologic variation. They can histologically simulate dedifferentiated liposarcoma, leiomyosarcoma, solitary fibrous tumor, and PEComa. Occasional MDM2 expression in GIST is a pitfall in the differential diagnosis between GIST and dedifferentiated liposarcoma. Previous studies have reported MDM2 gene amplification in GISTs with a frequency of 3-5%42,43, but among highly malignant GISTs, 9% frequency of MDM2 amplification was reported.43 Although some retroperitoneal GISTs have perivascular hemangiopericytoma-like patterns, KIT and DOG1/Ano1 expression and lack of nuclear STAT6 expression help to distinguish them from solitary fibrous tumors that similar to GISTs, can develop undifferentiated morphologies. Immunohistochemical detection of melanocytic markers for PEComa help to rule out this diagnostic possibility. Distinction of GIST from leiomyosarcoma is usually straightforward, as the latter tumors are almost never KIT-positive and rarely DOG1/Ano-1-positive, with desmin expression very common, while rare in GIST.
The potential of GIST to “dedifferentiate”, i.e. to lose histological features or expression of KIT or DOG1/Ano-1 typical of GISTs or evolve into tumors with aberrant phenotypes, may also complicate the differential diagnosis.44,45 Dedifferentiated GIST with loss of KIT and DOG1/Ano1-expression is diagnostically challenging if differentiated tumor components are not detected. Dedifferentiated GISTs have been reported to arise spontaneously, or in some cases, following imatinib therapy. These tumors may also have heterologous rhabdomyosarcomatous and angiosarcomatous differentiation.44,45 which were not detected in out series.
In conclusion, GISTs presenting in the retroperitoneal space are a heterogeneous group of tumors most of which resemble gastric or intestinal GISTs and are likely of GI tract origin. The most common retroperitoneal locations are peripancreatic area and pelvis. These tumors have usually poor outcomes, with rare exceptions. Their differential diagnosis of non-GIST sarcomas can be challenging and is generally solved by KIT, DOG1/Ano1 and other immunohistochemical studies and KIT/PDGFRA mutation analysis.
Supplementary Material
Supplementary Figure 1. Kaplan Meier plots illustrating differences in outcome of operable vs. inoperable retroperitoneal GISTs (A) and of retroperitoneal GISTs with various mitotic rates (B).
Supplementary Figure. 2. Kaplan Meier-Plots illustrating differences in outcome for retroperitoneal GISTs with various KIT or PDGFRA mutation types.
Supplementary Figure 3. A-C. Kit immunostain shows a number of positive cells with features of mast cells. D. In addition, occasional capillary endothelia are also KIT-positive. No distinct Cajal cells are seen.
Table 1.
Tumor sizes, mitotic rates, and median overall survivals of patients with retroperitoneal GISTs by apparent tumor origin or more defined anatomic site, KIT mutation type, and histologic likeness to gastric vs. intestinal GISTs, and mitotic rate
| Non-overlapping categories by likely tumor origin or location (number of cases) | Maximum diameter of the main tumor in cm Range (median) | Mitoses/5 mm2 Range (median) | Gastric like (G) - Small intestinal-like (S) – or indeterminate (I) histology G – S - I | Overall survival in months Range (median) |
|---|---|---|---|---|
| Gastric (n = 15) | 4-32 (21) | 0-82 (14) | 8 - 2 - 5 | 0-87 (16) |
| Duodenal (n = 9) | 7-18 (14) | 4-53 (13) | 2 - 7 - 0 | 1-39 (12) |
| Small intestinal (n = 13) | 10-29 (17) | 0-75 (17) | 0 -12-1 | 0-149 (17) |
| Mesenteric (n = 4) | 10-20 (11) | 0-22 (2) | 0 - 4 - 0 | 1-19 (11) |
| Peripancreatic (n = 25) | 6-30 (12) | 0-70 (13) | 9 - 10- 6 | 2-189 (12) |
| Pelvic (n = 11) | 7-20 (14) | 3- >100 | 3 - 6 - 2 | 1-329 (21) |
| Other and unspecified (n = 35) | 3-35 (20) | 0- 65 (5) | 13 - 20 - 2 | 0-468 (13) |
| Groups by KIT mutation type, histological likeness to gastric vs. intestinal GIST, and mitotic rate | ||||
| KIT exon 11 deletion (n = 27) | 4.5-30 (19) | 0-65 (14) | 0-143 (14)* | |
| KIT exon 11 substitution (n = 9) | 9-30 (12) | 0-22 (4) | 3-12 (10) * | |
| KIT exon 11 internal tandem duplication (n = 4) | 15-26 (19) | 2-11 (7) | 8-85 (74)* | |
| Gastric GIST-like histology (n = 35) | 3-35 (17) | 0-65 (5) | 0-329 (25)* | |
| Intestinal GIST-like histology (n = 61) | 4.5-35 (14) | 0-73 (10) | 0-468 (14)* | |
| Mitotic rate ≤ 5/5 mm2 (n = 45) | 3-35 (18) | 0-5 (1) | 0-448 (22)* | |
| Mitotic rate > 5/5 mm2 (n = 65) | 4.5-32 (15) | 6 - >100 (17) | 0-149 (12)* |
Not statistically significant.
Acknowledgments
Supported as a part of NIH intramural research program.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai S, Hishima T, Takazawa Y, et al. Gastrointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol Int. 2001;51:524–531. doi: 10.1046/j.1440-1827.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 3.Reith JD, Goldblum JR, Lyles RH, et al. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Oda Y, Kawaguchi K, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–488. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Goh BK, Chow PK, Kesavan SM, et al. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg. 2009;13:1094–1098. doi: 10.1007/s11605-009-0828-4. [DOI] [PubMed] [Google Scholar]
- 6.Takao H, Yamahira K, Doi I, et al. Gastrointestinal stromal tumor of the retroperitoneum: CT and MR findings. Eur Radiol. 2004;14:1926–1929. doi: 10.1007/s00330-004-2404-3. [DOI] [PubMed] [Google Scholar]
- 7.Takizawa I, Morishita H, Matsuki S, et al. Primary gastrointestinal stromal tumor in the retroperitoneum. Int J Urol. 2006 Sep;13(9):1245–8. doi: 10.1111/j.1442-2042.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Kojima A, Nagata S, et al. KIT-negative gastrointestinal stromal tumor of the abdominal soft tissue: a clinicopathologic and genetic study of 10 cases. Am J Surg Pathol. 2011;35:1287–1295. doi: 10.1097/PAS.0b013e3182206f15. [DOI] [PubMed] [Google Scholar]
- 9.Kim KH, Nelson SD, Kim DH, et al. Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases. Anticancer Res. 2012;32:923–937. [PubMed] [Google Scholar]
- 10.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lasota J, Felisiak-Golabek A, Wasag B, et al. Frequency and clinicopathologic profile of PIK3CA mutant GISTs: molecular genetic study of 529 cases. Mod Pathol. 2016;29:275–282. doi: 10.1038/modpathol.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 15.Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006;391:322–329. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Diamant NE, Huizinga JD. Interstitial cells of Cajal: pacemaker cells of the pancreatic duct? Pancreas. 2011;40:137–143. doi: 10.1097/MPA.0b013e3181f690ff. [DOI] [PubMed] [Google Scholar]
- 17.Daum O, Klecka J, Ferda J, et al. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch. 2005;446:470–472. doi: 10.1007/s00428-004-1200-4. [DOI] [PubMed] [Google Scholar]
- 18.Saif MW, Hotchkiss S, Kaley K. Gastrointestinal stromal tumors of the pancreas. JOP. 2010;11:405–406. [PubMed] [Google Scholar]
- 19.Aziret M, Cetinkunar S, Aktas E, et al. Pancreatic gastrointestinal stromal tumor after upper gastrointestinal hemorrhage and performance of Whipple procedure: A case report and literature review. Am J Case Rep. 2015;16:509–513. doi: 10.12659/AJCR.893803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan BM, Pai RK, Van Dam J. Diagnosis of pancreatic gastrointestinal stromal tumor by EUS guided FNA. JOP. 2008;9:192–196. [PubMed] [Google Scholar]
- 21.Beltrame V, Gruppo M, Pastorelli D, Pizzi S, Merigliano S, Sperti C., 1 Extra-gastrointestinal stromal tumor of the pancreas: case report and review of the literature. World J Surg Oncol. 2014;12:105. doi: 10.1186/1477-7819-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian YT, Liu H, Shi SS, Xie YB, Xu Q, Zhang JW, Zhao DB, Wang CF, Chen YT. Malignant extra-gastrointestinal stromal tumor of the pancreas: report of two cases and review of the literature. World J Gastroenterol. 2014;20:863–868. doi: 10.3748/wjg.v20.i3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanek M, Pędziwiatr M, Matłok M, et al. Laparoscopic removal of gastrointestinal stromal tumors of uncinate process of pancreas. Wideochir Inne TechMaloinwazyjne. 2015;10:311–315. doi: 10.5114/wiitm.2015.52141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan M, Jones S, Jenkins J, et al. Pancreatic GIST in a Patient with Limited Stage Small Cell Lung Cancer: A Case Report and Review of Published Cases. Case Rep Oncol Med. 2016;2016:9604982. doi: 10.1155/2016/9604982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaura K, Kato K, Miyazawa M, et al. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol. 2004;19:467–470. doi: 10.1111/j.1440-1746.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 26.Trabelsi A, Yacoub-Abid LB, Mtimet A, et al. Gastrointestinal stromal tumor of the pancreas: A case report and review of the literature. N Am J Med Sci. 2009;1:324–326. [PMC free article] [PubMed] [Google Scholar]
- 27.Čečka F, Jon B, Ferko A, et al. Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas. Hepatobiliary Pancreat Dis Int. 2011;10:330–332. doi: 10.1016/s1499-3872(11)60056-8. [DOI] [PubMed] [Google Scholar]
- 28.Meng L, Fang SH, Jin M. An unusual case of pancreatic and gastric neoplasms (2010: 12b). Malignant GISTs originating from the pancreas and stomach. Eur Radiol. 2011;21:663–665. doi: 10.1007/s00330-010-1893-5. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosio MR, Rocca BJ, Mastrogiulio MG, et al. Cystic gastrointestinal stromal tumors of the pancreas simulating cystoadenocarcinoma. Report of three cases and short review of the literature. Histol Histopathol. 2014;29:1583–1591. doi: 10.14670/HH-29.1583. [DOI] [PubMed] [Google Scholar]
- 30.Neto MR, Machuca TN, Pinho RV, et al. Gastrointestinal stromal tumor: report of two unusual cases. Virchows Arch. 2004;444:594–596. doi: 10.1007/s00428-004-1009-1. [DOI] [PubMed] [Google Scholar]
- 31.Showalter SL, Lloyd JM, Glassman DT, et al. Extra-gastrointestinal stromal tumor of the pancreas: case report and a review of the literature. Arch Surg. 2008;143:305–308. doi: 10.1001/archsurg.2007.68. [DOI] [PubMed] [Google Scholar]
- 32.Harindhanavudhi T, Tanawuttiwat T, Pyle J, et al. Extra-gastrointestinal stromal tumor presenting as hemorrhagic pancreatic cyst diagnosed by EUS-FNA. JOP. 2009;10:189–91. [PubMed] [Google Scholar]
- 33.Padhi S, Sarangi R, Mallick S. Pancreatic extragastrointestinal stromal tumors, interstitial Cajal like cells, and telocytes. JOP. 2013;14:1–14. doi: 10.6092/1590-8577/1293. [DOI] [PubMed] [Google Scholar]
- 34.Rao RN, Vij M, Singla N, Kumar A. Malignant pancreatic extra-gastrointestinal stromal tumor diagnosed by ultrasound guided fine needle aspiration cytology. A case report with a review of the literature. JOP. 2011;12:283–6. [PubMed] [Google Scholar]
- 35.Vij M, Agrawal V, Kumar A, et al. Gastrointestinal stromal tumors: a clinicopathological and immunohistochemical study of 121 cases. Indian J Gastroenterol. 2010;29:231–236. doi: 10.1007/s12664-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 36.Kim HH, Koh YS, Park EK, et al. Primary extragastrointestinal stromal tumor arising in the pancreas: report of a case. Surg Today. 2012;42:386–390. doi: 10.1007/s00595-011-0080-x. [DOI] [PubMed] [Google Scholar]
- 37.Wegge JI, Bartholomew DM, Burke LH, et al. Pancreatic extra-gastrointestinal stromal tumour masquerading as a bleeding duodenal mass. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serin KR, Keskin M, Güllüoğlu M, Emre A. An atypically localized gastrointestinal stromal tumor: a case report of pancreas gastrointestinal stromal tumor. Ulus Cerrahi Derg. 2013;29:42–44. doi: 10.5152/UCD.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbulut S, Yavuz R, Otan E, et al. Pancreatic extragastrointestinal stromal tumor: A case report and comprehensive literature review. World J Gastrointest Surg. 2014;6:175–82. doi: 10.4240/wjgs.v6.i9.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph P, Goyal R, Bansal P, et al. Pancreatic extra-gastrointestinal stromal tumour with documentation of C-kit mutation: a case report. J Clin Diagn Res. 2015;9(4):ED17–8. doi: 10.7860/JCDR/2015/13018.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasota J, Dansonka-Mieszkowska A, Stachura T, et al. Gastrointestinal stromal tumors with internal tandem duplications in 3′ end of KIT juxtamembrane domain occur predominantly in stomach and generally seem to have a favorable course. Mod Pathol. 2003;16:1257–1264. doi: 10.1097/01.MP.0000097365.72526.3E. [DOI] [PubMed] [Google Scholar]
- 42.Wallander ML, Layfield LJ, Tripp SR, Schmidt RL. Gastrointestinal stromal tumors: clinical significance of p53 expression, MDM2 amplification, and KIT mutation status. Appl Immunohistochem Mol Morphol. 2013;21:308–312. doi: 10.1097/PAI.0b013e31826ea7c0. [DOI] [PubMed] [Google Scholar]
- 43.Tornillo L, Duchini G, Carafa V, et al. Patterns of gene amplification in gastrointestinal stromal tumors (GIST) Lab Invest. 2005;85:921–931. doi: 10.1038/labinvest.3700284. [DOI] [PubMed] [Google Scholar]
- 44.Antonescu CR, Romeo S, Zhang L, et al. Dedifferentiation in gastrointestinal stromal tumor to an anaplastic KIT-negative phenotype: a diagnostic pitfall: morphologic and molecular characterization of 8 cases occurring either de novo or after imatinib therapy. Am J Surg Pathol. 2013;37:385–392. doi: 10.1097/PAS.0b013e31826c1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu P, Fei Y, Wang Y, et al. Recurrent retroperitoneal extra-GIST with rhabdomyosarcomatous and chondrosarcomatous differentiations: a rare case and literature review. Int J Clin Exp Pathol. 2015;8:9655–9661. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan Meier plots illustrating differences in outcome of operable vs. inoperable retroperitoneal GISTs (A) and of retroperitoneal GISTs with various mitotic rates (B).
Supplementary Figure. 2. Kaplan Meier-Plots illustrating differences in outcome for retroperitoneal GISTs with various KIT or PDGFRA mutation types.
Supplementary Figure 3. A-C. Kit immunostain shows a number of positive cells with features of mast cells. D. In addition, occasional capillary endothelia are also KIT-positive. No distinct Cajal cells are seen.