Abstract
Introduction
Ibrutinib, a first-in-class covalent inhibitor of Bruton’s tyrosine kinase (BTK), is approved in many countries for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL) and for previously untreated disease with a 17p deletion and, most recently, as a frontline therapy for CLL. In controlled trials in CLL, ibrutinib produced high response rates and improved survival in both the frontline and relapsed settings. While ibrutinib controls CLL with impressive efficacy, it only infrequently induces complete remissions, particularly of relapsed CLL, and does not eradicate minimal residual disease. Finally, ibrutinib is extremely expensive, has off-target toxicities, and requires indefinite therapy.
Areas covered
In this article, we provide an overview of the CLL therapeutic landscape and discuss the pharmacokinetic and pharmacodynamic aspects of ibrutinib. Major clinical trials of ibrutinib in CLL are summarized, and its safety profile explored.
Expert Opinion
Ibrutinib represents a transformative advance in CLL management and has validated BTK as a therapeutic target in this disease, but has some limitations, leading to the emergence of other BTK inhibitors and mechanism-based combination strategies. Given complete BTK occupancy at lower doses of ibrutinib and declining levels of BTK on ibrutinib therapy, lower doses of ibrutinib in CLL are being explored.
Keywords: Bruton tyrosine kinase, B-cell receptor, chronic lymphocytic leukemia, dosing, ibrutinib, pharmacokinetic, pharmacodynamics
1. Introduction
The last several years have seen an unprecedented increase in the availability of targeted therapeutics for chronic lymphocytic leukemia (CLL), the most common leukemia in the Western hemisphere, with 18,960 new cases estimated in the United States this year, with 4,660 deaths.[1] Indeed, gains in the treatment of CLL were hailed as the “advance of the year” in 2015 by the American Society of Clinical Oncology. This reflects the translation of key concepts underlying the biology of the disease, chiefly the critical importance of signaling via the B-cell receptor (BCR) pathway (reviewed in ref. [2]) and the exquisite dependence of CLL cells on the B-cell lymphoma 2 (BCL2) anti-apoptotic protein for survival.[3] Ibrutinib, an irreversible inhibitor of Bruton tyrosine kinase (BTK), a key signaling molecule in the BCR pathway, has undoubtedly revolutionized the landscape of CLL therapy and heralded a paradigm shift in the management of relapsed CLL.[4] Chemoimmunotherapy (CIT), using a number of different combinations based on age and overall fitness, remains the standard of care for initial therapy of most patients with CLL, except the minority of treatment-naïve patients with deletion 17p, who should receive ibrutinib.[5] The recent Food and Drug Administration (FDA) approval of ibrutinib in the frontline setting for all patients with CLL based on findings from the RESONATE-2 trial[6] is poised to greatly expand the use of this expensive drug, at least in the United States, which calls for a critical appraisal of this agent in CLL. In this review, we evaluate the currently available data with ibrutinib in CLL, emphasizing the pharmacokinetic and pharmacodynamic aspects.
2. Overview of the market
The therapeutic armamentarium for CLL has expanded considerably in recent years, and the landscape continues to evolve. Ibrutinib was initially granted accelerated approval by the US FDA in February 2014 for patients with CLL after at least one prior therapy.[7] High response rates (91% overall response rate (ORR), including 20% partial response with lymphocytosis (PRL)) were seen at the FDA-approved 420 mg once daily dose in a phase I/II study in 85 patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL).[8] This was subsequently converted to full approval upon the demonstration of statistically significant improvements in progression-free survival (PFS) and overall survival (OS) in a head-to-head trial versus ofatumumab (RESONATE) in patients with relapsed CLL.[9] Ibrutinib achieves high ORRs in patients with CLL and deletion 17p/TP53 aberrations,[10] and was separately approved for this highly refractory group of patients in any line of therapy. In the phase II RESONATE-17 trial, among 144 patients with previously treated CLL/SLL and deletion 17p, the ORR was 82.6%, including 17.4% PRL, and 79.3% of patients were alive and progression-free at 12 months.[11] Most recently, the findings of the RESONATE-2 trial,[6] which compared ibrutinib to chlorambucil in previously untreated patients with CLL led to the approval of ibrutinib in the frontline setting. “Real-world” data on 95 patients receiving ibrutinib through a compassionate use program in Sweden were recently published.[12] Nearly two-thirds of patients had del17p/TP53 mutation and Rai stage III/IV disease, and 28% had bulky lymphadenopathy. At median follow-up of 10.2 months, the ORR was 84% and PFS 77%, mirroring the clinical trial results in general. PFS and OS were significantly shorter among patients with del17p/TP53 mutation, and ibrutinib was overall well-tolerated.[12] Acalabrutinib is a second-generation, more selective, irreversible inhibitor of BTK currently in phase III trials in CLL. In a phase I/II study in 61 patients with relapsed CLL, an ORR of 95% was reported, including 85% with partial remission (PR) and 10% with PRL; the remaining 5% of patients had stable disease.[13] Other selective inhibitors of BTK include ONO/GS-4059 and BGB-3111. In a phase I trial of the former in patients with relapsed/refractory B-cell malignancies, 24 of 25 (96%) CLL patients responded, and no maximal tolerated dose (MTD) was reached.[14] No dose-limiting toxicities (DLTs) or MTD were identified in a phase I trial of BGB-3111 either, and 6 of 8 (75%) CLL patients had an objective response.[15]
The first-in-class phosphatidylinositol-3-kinase (PI3K) delta isoform inhibitor idelalisib was approved by the FDA in combination with rituximab in 2014 for patients with relapsed CLL and co-morbidities for whom rituximab alone would be considered appropriate therapy.[16] Obinutuzumab, a type II, glycoengineered monoclonal antibody against CD20, was FDA-approved in 2013 in combination with chlorambucil for upfront therapy in patients with CLL and co-morbidities based on the superiority of this combination over that of rituximab and chlorambucil (in terms of PFS) and chlorambucil alone (in terms of PFS and OS) in a phase III trial (CLL 11) in previously untreated patients with CLL and a Cumulative Illness Rating Scale score higher than 6 or compromised renal function.[17] Finally, venetoclax, a selective, small-molecule “BH3-mimetic” inhibitor of BCL2,[18] received accelerated approval from the FDA in 2016 for CLL patients with deletion 17p after at least one prior therapy. In a phase I study in relapsed or refractory CLL/SLL, the drug produced an ORR of 79% and notably, a CR rate of 20%, including 5% who had no minimal residual disease (MRD) on flow cytometry.[19] A nearly identical ORR (79.4%) was reported in a phase II study confined to relapsed or refractory CLL patients with deletion 17p.[20]
2.1 Limitations of current CLL therapeutics
Despite the remarkable pace of drug development and approval for CLL in recent years, there remain several areas of unmet need. Durable remissions and functional “cures” have been reported with fludarabine, cyclophosphamide and rituximab (FCR) CIT in patients with somatic hypermutation of the variable region of the immunoglobulin heavy chain gene (IGHV) and favorable cytogenetic characteristics (e.g., deletion 13q, normal, trisomy 12),[21] and it appears that abbreviated courses of FCR (e.g., 3 cycles) may be sufficient for those patients who achieve MRD-negative status.[22] However, long-term outcomes with CIT are far less gratifying in the setting of unmutated IGHV, deletion 11q and in particular, deletion 17p, and therapy-related myeloid neoplasms remain a problem after FCR.[23] IGHV-unmutated CLL may be particularly sensitive to ibrutinib due to high levels of phosphorylated BTK, correlating with higher proliferative capacity.[24]
2.2 Limitations of ibrutinib
While ibrutinib certainly provides benefits in previously difficult to treat CLL cases, such as disease with unmutated IGHV and patients with deletion 17p [10, 20], cures remain elusive with ibrutinib monotherapy, the drug induces very few CRs, particularly in previously treated patients,[25] and resistance-conferring mutations (e.g., in BTK or in the downstream molecule phospholipase C gamma 2 (PLCγ2)) emerge in some patients on ibrutinib therapy,[26] as do other resistant subclones, e.g., those bearing deletion 8p with additional driver mutations.[27] Finally, complex karyotype has been shown to be a powerful predictor of inferior outcomes among patients with relapsed or refractory CLL treated with ibrutinib-based regimens.[28] Combination strategies are therefore needed, and combinations that appear promising preclinically, e.g., that of ibrutinib with venetoclax,[29] are being pursued in clinical trials, with or without the addition of obinutuzumab (NCT02756897 NCT02758665 NCT02427451).
Another limitation of ibrutinib is its significant off-target toxicity, e.g., atrial fibrillation (6–9%) and bleeding (grade 3/4 in up to 6%). Several studies have implicated inhibition of other kinases in ibrutinib-induced bleeding and atrial fibrillation. First, ibrutinib’s effect on cardiac signaling through the phosphatidylinositol-3-kinase (PI3K)/Akt cassette has been suggested as the mechanism underlying atrial fibrillation.[30] Second, several studies have implicated ibrutinib in causing platelet dysfunction.[31] Ibrutinib impairs both collagen and von Willebrand factor-dependent platelet functions,[32] as well as collagen-mediated platelet aggregation.[33] Other targets of ibrutinib include the epidermal growth factor receptor (EGFR),[34] interleukin-2-inducible kinase (ITK),[35] T-cell receptor axis signaling and TEC kinases.[13] While the Cys481 residue of BTK is the primary target of ibrutinib, several other kinases that contain cysteine residues homologous to Cys481 of BTK are also inhibited at low nanomolar concentrations.[36] These off-target actions of ibrutinib and their effects on the immune system and hematopoietic cells are not yet well-defined. Table 1 lists kinases inhibited by ibrutinib, along with the corresponding IC50 values. Some off-target effects of ibrutinib have been recognized for their potential therapeutic benefit in other diseases, e.g., inhibition of ITK and resultant Th1 “skewing” in Th2-mediated diseases like asthma, inhibition of BTK and TEC in overactive osteoclasts in osteoporosis and rheumatoid arthritis, and inhibition of BTK, ITK and TEC in mast cells in allergic conditions and pancreatic tumors.[37]
Table 1.
IC50 values and fold selectivity for inhibition of enzymatic activity by ibrutinib.
| Kinase | IC50, nM | BTK selectivity, fold |
|---|---|---|
| BTK | 0.5 | -- |
| BLK* | 0.5 | 1 |
| BMX* | 0.8 | 1.6 |
| CSK | 2.3 | 4.6 |
| FGR | 2.3 | 4.6 |
| BRK | 3.3 | 6.6 |
| HCK | 3.7 | 7.4 |
| EGFR* | 5.6 | 11.2 |
| YES | 6.5 | 13 |
| ErbB2* | 9.4 | 18.8 |
| ITK* | 10.7 | 21.4 |
| JAK3* | 16.1 | 32.2 |
| FRK | 29.2 | 58.4 |
| LCK | 33.2 | 66.4 |
| RET | 36.5 | 73 |
| FLT3 | 73 | 146 |
| TEC* | 78 | 156 |
| ABL | 86 | 172 |
| FYN | 96 | 192 |
| RIPK2 | 152 | 304 |
| c-SRC | 171 | 342 |
| LYN | 200 | 400 |
| PDGFRα | 718 | 1436 |
| FMS | 5545 | >10,000 |
| FER | 8070 | >10,000 |
| JAK1 | >10,000 | >10,000 |
| JAK2 | >10,000 | >10,000 |
| NEK2 | >10,000 | >10,000 |
| p38 | >10,000 | >10,000 |
| PI3K | >10,000 | >10,000 |
| PLK1 | >10,000 | >10,000 |
| RSK1 | >10,000 | >10,000 |
| SYK | >10,000 | >10,000 |
Kinases that contain a cysteine residue aligning with Cys-481 in BTK. Reproduced, with permission, from reference [36]: Honigberg LA et al. Proc Natl Acad Sci U S A 2010;107:13075–80.
Yet another limitation of ibrutinib is its prohibitive cost. In a recent analysis, the average lifetime cost of treatment of CLL per patient in the US was estimated to be over $700,000 for patients beginning therapy in the 2017–2025 period.[38] In this analysis, the incremental cost per life-year gained was $204,000 and that per quality-adjusted life-year gained was $262,000.[38] Another recent analysis published before the approval of the drug for frontline use in the US compared the costs of CLL treatment before the approval of ibrutinib to the “current” cost using ibrutinib as salvage therapy and to the potential “future” cost, assuming approval of ibrutinib for first-line use.[39] Estimated 10-year pharmaceutical costs per newly diagnosed patient and per treated patient, respectively, were: $45,659 and $157,446 for the historical scenario, $77,948 and $268,788 for the “current” scenario for ibrutinib, and $164,141 and $566,002 for the potential “future” scenario.[39] Total out-of-pocket cost per treated patient with newly diagnosed CLL under Medicare Part D increased from $325 under the historical scenario to $8,800 under the “current” scenario and to $35,564 under the “future” scenario.[39] In the United Kingdom, the upper limit for approval of a drug for National Health Service (NHS) use is £30,000 per year of good-quality life or £50,000 for a drug given towards the end of life. Ibrutinib was initially included in the UK Cancer Drugs Fund (CDF) for patients with CLL who have received at least one prior therapy and for whom treatment or re-treatment with purine analog-based therapy would not be appropriate.[40] Earlier this year, the National Institute for Health and Care Excellence (NICE) provisionally recommended against making ibrutinib, priced at £5151 for a 4-week course, available for the treatment of CLL patients in the UK through the National Health Service.[41] More recently, however, NICE has asked the manufacturer of ibrutinib to put forward a case for its inclusion in the CDF, particularly for treatment-naïve patients with deletion 17p or a TP53 mutation.[42]
3. Introduction to the compound
Ibrutinib (formerly PCI-32765) is an orally administered, potent (IC50, 0.5 nM), irreversible inhibitor of BTK, forming a covalent bond with the cysteine 481 residue in the adenosine triphosphate (ATP)-binding domain of the kinase.[36] Besides its current FDA approval status for all patients with CLL as discussed above, the drug is also indicated for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy[43] and those with Waldenstrom’s macroglobulinemia.[44]
4. Chemistry
The chemical name for ibrutinib is 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1Hpyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one (Box 1). It’s molecular formula is C25H24N6O2, and its molecular weight is 440.5 g/mol.[45]
5. Therapeutic target and mechanism of action
BCR signaling plays a key role in CLL pathogenesis, and BTK has a pivotal role in BCR signaling in both normal and malignant B-cells.[2, 46] BTK is important for antigen-induced BCR activation in both normal, mature B-cells and in CLL lymphocytes and mediates survival and proliferation via amplification of downstream effector pathways, e.g., PI3K/Akt, mitogen activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and nuclear factor kappa B (NF-κB).[2, 46] Additionally, autonomous BCR signaling has also been described in CLL, which is not, however, associated with activating mutations in any of the BCR components.[46]
Ibrutinib inactivates BTK through irreversible, covalent binding to the Cys481 residue in the ATP-binding domain of BTK.[36] BTK has been shown to be critical for the development and expansion of CLL and an important target of ibrutinib.[47] Indeed, BTK point mutations (C481S) that convert ibrutinib to a reversible inhibitor, as well as activating mutations in PLCγ2, located immediately downstream of BTK, have been implicated in the pathogenesis of acquired resistance to ibrutinib in CLL.[26] The success of the more selective BTK inhibitors, acalabrutinib and ONO/GS-4059, in heavily pretreated patients with CLL, further testifies to the relevance of BTK as a major therapeutic target in this disease.[13, 14]
6. Pharmacodynamics
Because ibrutinib binds irreversibly and covalently to BTK, the enzyme is permanently inhibited. Furthermore, because there is only one site (Cys481) for drug binding, a one to one stoichiometric concentration of ibrutinib is needed. Hence, a concentration of the drug that occupies >95% of the BTK molecules in CLL lymphocytes is considered ideal. Such measurements are achieved using BTK occupancy assays and were performed in the context of early phase I and II studies.[8, 48] BTK occupancy is measured using a highly specific fluorescent affinity probe, whose binding to the BTK active site had previously been shown to tightly correlate with blockade of BCR signaling and in vivo efficacy.[36] ≥95% BTK occupancy was achieved 4 hours post-dose at a dose of 2.5 mg/kg/day of ibrutinib in the phase I study in relapsed/refractory B-cell malignancies.[48] Dose escalation proceeded up to three dose levels above the level at which full BTK occupancy was observed (the 5 dose levels were 1.25, 2.5, 5, 8.3 and 12.5 mg/kg/day), and all dosing cohorts from 2.5 through 12.5 mg/kg/day had ≥95% BTK occupancy and similar clinical response rates.[48]
At a signaling level, ibrutinib binding inhibits activity of BTK, autophosphorylation of the enzyme, and abrogates activation of downstream survival pathways (PI3K, ERK, NF-κB), inducing modest apoptosis and inhibiting activation-induced proliferation of CLL cells in vitro. It effectively blocks survival signals provided externally to CLL cells from the microenvironment.[49] These actions were observed in primary, patient-derived CLL cells (from blood, lymph nodes and bone marrow) as well as in mouse models.[50–52] Additionally, the drug blocks the interaction of CLL cells with tissue homing chemokines (CXCL12, CXCL13, CCL19), possibly through blockade of BCR-induced activation of lymphocyte cytosolic protein 1 (LCP1)[53] and restoration of the balance between expression levels of the homing receptors CCR7 and CXCR4 and the S1P receptor 1 (S1P1).[54] Figure 1 depicts the major pathways and consequences of BTK activation in CLL cells. Additionally, ibrutinib down-regulates secretion of BCR-dependent chemokines (CCL3, CCL4) by CLL cells, both in vitro and in vivo, and causes a transient early lymphocytosis in mouse models.[52, 55] This characteristic “redistribution lymphocytosis” is routinely and uniquely observed in patients receiving ibrutinib or other BCR pathway inhibitors and is believed to be a result of mobilization of CLL cells from their protective tissue microenvironmental niches into the peripheral blood, where they eventually undergo apoptosis from the lack of prosurvival signals.[56] The observations that ibrutinib abolishes the adhesion of CLL cells to fibronectin and VCAM-1 and reduces CLL cell surface expression of key adhesion molecules such as CD49d, CD29 and CD44, mechanistically explain this treatment-induced lymphocytosis.[55, 57] In a mouse model of aggressive TCL1 CLL-like disease, BTK inhibition by ibrutinib reduced surface membrane levels of CXCR4, CD49d and other adhesion/homing receptors, resulting in rapid redistribution of CLL cells from spleens and lymph nodes into the circulation, and failure of these cells to home to spleens.[58]
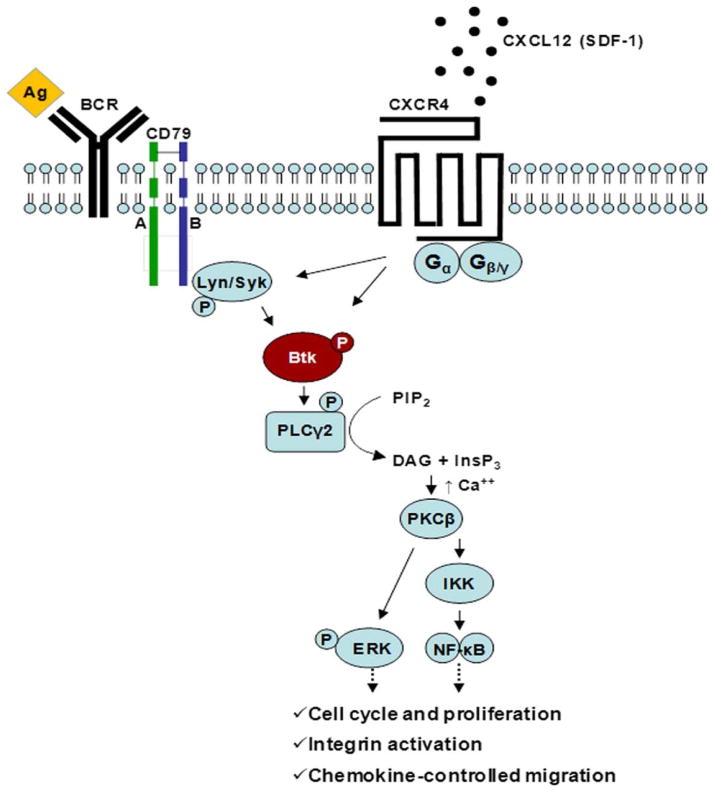
Figure 1. BTK signaling pathways.
BTK in involved in the signaling of multiple receptors that together control cell migration, adhesion, survival and proliferation. Activation of BTK is triggered upon BCR stimulation, for example, after antigen (Ag) binding, as shown on the left hand side. The BCR signaling pathway is thought to play a major role in mediating B-cell survival and proliferation in normal and neoplastic B-cells. In addition, BTK mediates signals derived from chemokine receptors, such as the CXCR4 receptor, which binds to the chemokine CXCL12 (SDF-1) to mediate homing and migration. Both CXCR4 and the BCR also regulate adhesion molecules such as integrins, which are essential for tissue homing and retention. Consequently, inhibition of BTK results in redistribution of tissue-resident CLL cells into the blood and inhibition of re-homing. Reproduced, with permission, from [102].
The redistribution lymphocytosis characteristic of treatment with ibrutinib has been extensively investigated. While an efflux of tumor cells from the tissue compartments into the blood has been conclusively shown,[59] mathematical models have estimated the fraction of the tissue CLL cells redistributed into the blood during ibrutinib therapy to be 23.3% ± 17% of the total tissue disease burden, arguing that the reduction of tissue disease burden is due more to CLL cell death and less to egress from nodal compartments.[60] While the lymphocytosis resolves within 8 months in the majority of patients, it may last for over a year in some patients.[61] Importantly, this persistent lymphocytosis does not represent disease progression or clonal evolution, and these patients do not have inferior PFS.[61]
BTK belongs to the TEC family kinases (TFKs), and other members of this family (TEC, ITK, BMX, RLK/TXK) are also targeted by ibrutinib, albeit at higher concentrations (e.g., 78 nM for TEC).[37] Other kinases targeted by ibrutinib include the HER family kinases EGFR, HER2/ErbB2 and HER4/ErbB4, and JAK3 (Table 1).[37, 46] Ibrutinib inhibits yet other kinases at low nanomolar concentrations, e.g., BRK, CSK, FRG, HCK, BLK; indeed, the IC50 for BLK is the same as for BTK, i.e., 0.5 nM.[36, 37] Much attention has been directed towards ibrutinib’s irreversible inhibition of ITK in T-cells, resulting in subversion of Th2 immunity and potentiation of Th1-based immune responses.[35] This action of ibrutinib may underlie the synergism between the drug and immune checkpoint inhibitors (anti-PD1/PDL1 monoclonal antibodies) in mouse models of a number of tumor types.[62] Conversely, ibrutinib has been reported to antagonize rituximab-dependent NK-cell mediated cytotoxicity[63, 64] and impair the phagocytosis of rituximab-coated patient-derived CLL cells,[65] but these effects have not been demonstrated in murine xenograft models[64] or observed in clinical trials,[66, 67] possibly due to the promotion by ibrutinib of both positive and negative interactions with anti-CD20 monoclonal antibodies.[68]
Recently, correlative studies from a clinical trial examining the effects of ibrutinib on the CLL microenvironment were published.[69] Serum levels of key chemokines and inflammatory cytokines decreased significantly in patients on ibrutinib. Ibrutinib decreased overall T-cell numbers and particularly the Th17 subset of CD4+ T-cells, along with reduced expression of activation markers and PD1 on T-cells, inhibited secretion of CXCL13, decreased the chemo-attraction of CLL cells, and interfered with the interactions of macrophages and CLL cells in the bone marrow microenvironment.[69]
7. Pharmacokinetics and metabolism
Ibrutinib is absorbed after oral administration with a median time to maximal plasma concentration (Tmax) of 1 to 2 hours.[48] Ibrutinib exposure increases with dosage up to 840 mg daily. The steady-state area under the curve (AUC, mean ± standard deviation) observed in patients on 420 mg daily is 680 ± 517 ng·h/mL.[70] No food restrictions are warranted.[71]
Like other kinase inhibitors, ibrutinib binds to plasma proteins. Reversible binding of ibrutinib to human plasma protein in vitro was 97.3% with no concentration dependence in the range of 50 to 1000 ng/mL (Table 2). The apparent volume of distribution at steady state (Vd,ss/F, the sum of the volumes of the central and peripheral compartments) was approximately 10,000 L.[72]
Table 2.
Summary of pharmacokinetic parameters of ibrutinib.
| Route of administration | Oral, without regard to food intake. |
| Absorption | Median Tmax 1–2 hrs. Steady state AUC at 420 mg/d dose = 680 ± 517 ng.h/ml. |
| Distribution | 97.3% plasma protein bound in vitro (reversible). Vd at steady state 683 L. |
| Metabolism | Primarily through CYP3A. Active dihydrodiol metabolite. DDIs with moderate to strong CYP3A inducers and inhibitors. |
| Elimination | Mainly as metabolites via feces; high first-pass effect. Half-life 4 to 6 hours. |
Abbreviations: Tmax, time to maximal plasma concentration; AUC, area under the curve; Vd, volume of distribution; CYP, cytochrome P450; DDIs, drug-drug interactions.
Ibrutinib is metabolized to several metabolites in the liver, primarily by cytochrome P450 CYP3A,[73] and to a minor extent by CYP2D6; this is considered the main route of elimination (Table 2). The active metabolite, PCI-45227, is a dihydrodiol derivative with inhibitory activity towards BTK approximately 15 times lower than that of ibrutinib.[70] The range of the mean metabolite to parent ratio for PCI-45227 at steady-state is 1 to 2.8.[70]
In line with a high first-pass effect, the apparent oral clearance of ibrutinib is approximately 2000 and 1000 L/h in fasted and fed conditions, respectively.[72] The half-life of ibrutinib is 4 to 6 hours and the drug does not accumulate after repeated oral dosing.[48]
Ibrutinib is not significantly cleared renally; urinary excretion of metabolites is <10% of the dose.[74] In concert, creatinine clearance (CrCL) >25 mL/min has no influence on the exposure to ibrutinib. There are no data in patients with severe renal impairment (CrCL < 25 mL/min) or in patients on dialysis.[70] Ibrutinib, mainly in the form of metabolites, is eliminated primarily via feces.[74] As mentioned above, these metabolites are generated in the liver. As a result, ibrutinib AUC and maximal plasma concentration (Cmax) do increase progressively in the presence of mild, moderate and severe liver impairment.[70] For patients with mild liver impairment (Child-Pugh class A), the recommended dose is 140 mg daily (one capsule).[70] Ibrutinib is not recommended in the setting of moderate or severe liver impairment (Child-Pugh classes B and C).[70] The systemic clearance of ibrutinib is not altered by gender, and dose adjustment by age is not warranted.[70]
8. Pharmacogenetics
It is too early to comment on pharmacogenetic variability as there are limited data. In a phase I study conducted in 15 Japanese patients with relapsed/refractory B-cell malignancies (6 with CLL/SLL), the ORR was 73.3% and the most common (occurring in ≥20% of patients) adverse events (AEs) were neutropenia, anemia, nasopharyngitis, increased bilirubin, and rash.[75] One patient in the CLL/SLL cohort experienced grade 3 pneumonia and sepsis.[75] Overall, these data are similar to observations in Caucasian populations.
9. Clinical efficacy
In a phase I study in 56 patients with relapsed or refractory B-cell lymphoma or CLL, dose escalation of ibrutinib continued through 12.5 mg/kg/day without reaching a MTD.[48] Notably, full occupancy of the BTK active site occurred at 2.5 mg/kg/day.[48] The ORR in 50 evaluable patients was 60%, including a CR rate of 16%. Median PFS in all patients was 13.6 months. Table 3 summarizes the major trials of ibrutinib in CLL, either as single agent, in combination with a CD20 monoclonal antibody, or with CIT. The results of 3-year (median) follow-up of 132 patients receiving ibrutinib as a single agent[25] demonstrated that longer treatment was associated with improvement in response quality and durability of remissions.[25] Progression was uncommon, occurring primarily in some patients with relapsed deletion 17p and/or deletion 11q disease.[25] Outcomes after ibrutinib discontinuation on clinical trials have been reported to be poor, but this likely reflects the unavailability of other effective therapies at the time.[76, 77] In the MD Anderson Cancer Center experience, 33 of 127 patients discontinued ibrutinib, 14 due to disease progression and 14 due to AEs or sudden death.[76] In the Ohio State University experience, 76 of 308 patients discontinued ibrutinib, 31 because of disease progression and 37 because of AEs or sudden cardiac death.[77] In general, Richter’s transformations tended to occur early, while most CLL progressions were late events.[77] Seven Richter’s transformations occurred in the Swedish “real-world” cohort of 95 poor-prognosis CLL patients who received ibrutinib through a compassionate use program.[12]
Table 3.
Major clinical trials of ibrutinib in CLL/SLL.
| Trial (reference) | Population studied (n) | Phase and design | Response rates | Survival |
|---|---|---|---|---|
| [8] | Relapsed or refractory (n=85) with a median of 4 prior therapies | Ib/II, two doses: 420 mg (n=51) and 840 mg (n=34) | ORR 71% with both doses; another 20% and 15% had PRL, respectively; 2 CRs at 420 mg/d | At 26 months, estimated PFS 75% and OS 83%, median PFS not reached |
| [100] | Frontline, elderly (≥65, n=31) | Ib/II, initially two doses, later 840 mg dose discontinued | ORR 71% after median f/u of 22.1 months (13% CRs, 3% nPRs, 55% PRs) | At 24 months, estimated PFS 96.3% and OS 96.6%, median PFS not reached |
| RESONATE [9] | Relapsed or refractory (n=391); median 3 prior therapies in ibrutinib group and 2 in ofatumumab group; crossover allowed | III, RCT (1:1) of ibrutinib 420 mg/d vs. ofatumumab (300 mg in week 1, followed by 2000 mg weekly x 7, then every 4 weeks x 16) | ORR 42.6% vs. 4.1% favoring ibrutinib; another 20% in ibrutinib group had PRL | At median f/u of 9.4 months, median PFS not reached vs. 8.1 months; PFS at 6 months 88% vs. 65%; OS 90% vs. 81% at 12 months |
| RESONATE-17 [11] | Relapsed or refractory with deletion 17p (n=144); median 2 prior therapies | II; single arm (420 mg/d) | ORR 82.6% (including 17.4% PRL, 3 patients with CR/CRi) | At median f/u of 13 months, median PFS not reached; 79.3% PFS at 12 months |
| [10] | Treatment-naïve (n=35) or relapsed or refractory (n=16) with TP53 aberrations (47 had deletion 17p) | II, single arm (420 mg/d) | ORR 97% (55% PR + 42% PRL) among previously untreated; 80% (40% PR + 40% PRL) among R/R | At 24 months, estimated PFS for all patients 82% and OS 80% (ITT); 24-month OS 84% in previously untreated and 74% in R/R pts |
| RESONATE-2 [6] | Previously untreated, elderly (≥65, n=269); crossover allowed | III, RCT (1:1) of ibrutinib 420 mg/d vs. chlorambucil (up to 12 28-day cycles; 0.5 mg/kg (could be ↑ to 0.8 mg/kg) on days 1 and 15 | ORR 86% with ibrutinib vs. 35% with chlorambucil (CRs 4% vs. 2%); 4% in ibrutinib group had PRL | At median f/u of 18.4 months, estimated PFS not reached vs. 18.9 months; estimated OS at 24 months 98% vs. 85%; 84% reduction in risk of progression or death with ibrutinib |
| [66] | High risk (del17p, TP53 mutation, del11q, PFS < 36 months after CIT, n=40); median 2 prior therapies | II, single arm, ibrutinib 420 mg/d plus rituximab (375 mg/m2, weekly in cycle 1, then once per cycle till cycle 6) | ORR 95% (87% PR + 8% CR); 1 patient achieved MRD-negative CR; 2 CRs occurred in patients with deletion 17p | At 18 months, PFS in all patients 78%; 72.4% in patients with deletion 17p or TP53 mutation (n=20) |
| [67] | Patients who had failed ≥2 prior therapies; PLL (n=2) and RT also included (n=3); median 3 prior therapies | Ib/II, ibrutinib 420 mg/d plus ofatumumab x 12 (ibrutinib lead-in, n=27; concurrent start, n=20; ofatumumab lead-in, n=24) | ORRs in CLL/SLL pts (n=66) 100%, 79% and 71% in the 3 groups, respectively (overall 83.3%); 1 CR and 54 PRs | Estimated 12-month PFSs for all patients 89%, 85% and 75% in the 3 groups, respectively |
| HELIOS [101] | Relapsed or refractory (n=578) without deletion 17p; crossover allowed | III, RCT (1:1) of bendamustine (70 mg/m2/dose x 2) plus rituximab x 6 cycles plus ibrutinib 420 mg/d or placebo | ORR 83% in IBR group vs. 68% in BR group; CR/CRi rate 10% vs. 3%; MRD-negativity rates 13% vs. 5% | At median f/u of 17 months, estimated PFS not reached in IBR group vs. 13.3 months in BR group; at 18 months, PFS 79% vs. 24% |
Abbreviations: CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; ORR, overall response rate; CR, complete remission; CRi, complete response with incomplete count recovery; PR, partial response; PRL, partial response with lymphocytosis; nPR, nodular partial response; MRD, minimal residual disease; RCT, randomized controlled trial; PFS, progression-free survival; OS, overall survival; R/R, relapsed/refractory; IBR, ibrutinib, bendamustine and rituximab; BR, bendamustine and rituximab; f/u, follow-up.
10. Safety, tolerability, toxicity, and potential off-target effects related to safety liabilities
In the phase I study of ibrutinib mentioned above, most AEs were grade 1 or 2 in severity and self-limited, and DLTs did not occur.[48] Ibrutinib was well-tolerated in the two trials that led to accelerated and full approval, respectively, for relapsed CLL, with predominantly grade 1/2 adverse events, e.g., nausea, diarrhea, fatigue, pyrexia and upper respiratory infection.[8, 9] In the registration trial in the frontline setting, AEs occurring in ≥20% of patients on ibrutinib included diarrhea, fatigue, cough and nausea; additionally, peripheral edema, dry eye, arthralgia, neutropenia (including grade 3) and vomiting occurred in ≥10% of patients.[6] Infections in CLL patients have been shown to decline over time during ibrutinib therapy.[78]
Atrial fibrillation (AF) and flutter have occurred in 6–9% of patients treated with ibrutinib.[70] This effect has been attributed to the binding of ibrutinib to BTK and TEC in the heart and subsequent inhibition of cardioprotective PI3K/Akt signaling.[30] Other targets of ibrutinib, viz., HER2/ErbB2, HER4/ErbB4 and BMX play important roles in cardiac physiology, and their inhibition could represent additional mechanisms through which ibrutinib could cause AF and cardiac dysfunction.[37]
Bleeding is a relatively common AE of ibrutinib and grade ≥3 bleeding events have occurred in up to 6% of patients.[70] Bleeding events of any grade, including bruising and petechiae, have occurred in approximately half of patients.[70] In the Swedish “real-world” experience, 46 of 95 patients developed hematomas, without any major bleeding.[12] The low frequency of subarachnoid hemorrhages in several patients receiving concomitant warfarin in the phase 1b-2 trial of ibrutinib prompted the exclusion of concurrent therapy with oral vitamin K antagonists in all subsequent studies of ibrutinib.[4] Additionally, ibrutinib is typically held for 3 to 7 days before and after surgical procedures.[4] Both BTK and TEC play important roles in collagen-induced platelet adhesion mediated through glycoprotein (GP) VI, and TEC is able to compensate for loss of function of BTK in this setting.[79, 80] However, BTK is essential for von Willebrand factor (vWF)-induced (GPIb-dependent) platelet aggregation and thrombus formation.[81] The risk of bleeding in CLL patients treated with ibrutinib does appear to decrease with continued therapy.[82]
Mice lacking BTK and TEC show severe osteopetrosis caused by a defect in bone resorption.[83] Receptor activator of NF-κB (RANK) and ITAM-harboring adaptors signal through BTK, TEC, B-cell linker (BLNK) and PLCγ to activate an essential calcium signal in osteoclasts, and inhibition of TEC reduces osteoclastic bone resorption in models of osteoporosis and inflammation-induced bone destruction.[83] While this could argue for a therapeutic role of ibrutinib in diseases of enhanced osteoclastic bone resorption such as osteoporosis or rheumatoid arthritis, these findings raise concern over the long-term effects of ibrutinib treatment on bone homeostasis, particularly since systematic studies of bone density have not been performed in patients receiving ibrutinib.[37]
Inhibition of ITK and resultant modulation of cellular immunity have been invoked as potentially contributing to the development of panniculitis, an unusual, recently recognized AE in ibrutinib-treated patients.[84] Even more recently, the development of painful, purpuric skin nodules with a Th1-rich lymphocytic infiltrate (as opposed to the expected Th2 milieu) on ibrutinib therapy in a patient with CLL that resolved with cessation of the drug has been reported,[85] consistent with ITK inhibition by ibrutinib.[35] Brittle nails and textural hair changes have been reported during long-term therapy of CLL with ibrutinib, but the underlying mechanisms remain unknown.[86]
11. Drug-drug interactions
As mentioned in the Pharmacokinetics section, ibrutinib is primarily metabolized by cytochrome P450 enzyme 3A.[73] Consistent with this observation, co-administration of ketoconazole, a strong CYP3A inhibitor, increased Cmax and AUC of ibrutinib by 29- and 24-fold, respectively, in healthy volunteers,[73] indicating that concomitant administration of ibrutinib with strong or moderate inhibitors of CYP3A should be avoided.[70] For strong CYP3A inhibitors used short-term (e.g., antifungals and antibiotics for ≤7 days, e.g., ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin), interruption of ibrutinib therapy should be considered[70] and chronic use of strong CYP3A inhibitors should be avoided. If a moderate CYP3A inhibitor must be used, the dose of ibrutinib should be reduced to 140 mg daily (from the approved dose of 420 mg daily).[70] Patients taking concomitant strong or moderate CYP3A4 inhibitors should be monitored more closely for signs of toxicity. Grapefruit and Seville oranges should be avoided during ibrutinib treatment, as these contain moderate inhibitors of CYP3A.[70] Not only strong CYP3A inhibitors, but strong CYP3A activators (e.g., carbamazepine, rifampin, phenytoin and St. John’s Wort) also should be avoided; indeed, administration of ibrutinib with rifampin, a strong CYP3A inducer, decreased ibrutinib Cmax and AUC by approximately 13- and 10-fold, respectively.[73]
12. Dosing routes
Ibrutinib is dispensed as 140 mg capsules. It is dosed orally. Since the recommended daily dose in CLL is 420 mg, patients take 3 capsules orally per day.
13. Regulatory affairs
The remarkable activity of ibrutinib is reflected in the fact that this drug was selected for all four expedited programs of the FDA, i.e., fast-track and breakthrough therapy designation, priority review, and accelerated approval.[7] As discussed above, ibrutinib was initially approved in the United States for patients with CLL/SLL who had received at least one prior therapy. It was subsequently approved for patients with CLL/SLL with deletion 17p, and most recently for previously untreated patients with CLL/SLL. In the European Union, it is also now approved for CLL patients across all lines of therapy, i.e., in the frontline or relapsed settings, irrespective of the presence or absence of del17p/TP53 mutation. The same is true of ibrutinib’s Health Canada approval status. In Australia, ibrutinib is indicated for the treatment of patients with CLL/SLL who have received at least one prior therapy or as first line in patients with CLL with 17p deletion, while in the United Kingdom, the availability of ibrutinib through the NHS is currently restricted to patients with relapsed CLL for whom purine analog-based therapy is considered inappropriate.
14. Conclusion
Ibrutinib is a highly effective and generally well-tolerated oral drug that has ushered in a new era in CLL treatment. Important limitations include the rarity of CRs in the relapsed setting and achievement of MRD-negative status with ibrutinib monotherapy.[87] The need for indefinite therapy poses unique challenges given the high cost of ibrutinib. Finally, the significant potential for off-target toxicities as well as the development of resistance are additional short-comings.
15. Expert Opinion
Ibrutinib undoubtedly represents a great advance in the treatment of CLL and is a one of few examples in oncology of successful therapeutic targeting of a kinase that is not mutated.[88] However, while highly effective at controlling disease, best monotherapy responses with ibrutinib are typically PRs, necessitating indefinite use of the drug.[87] Achieving MRD-negative CR in the bone marrow is associated with superior PFS and OS, and MRD status is the single best post-treatment predictor of long-term outcomes after CIT.[87] The inability to achieve MRD eradication, or even CR in the majority of patients, is an important shortcoming of ibrutinib, invoking the need for laboratory-based rational combination strategies, such as with venetoclax,[29] proteasome inhibitors,[89] heat shock protein 90 inhibitors[90], selective inhibitors of nuclear export[91] and caspase activators.[92]
As discussed above, the high cost of ibrutinib, like that of other targeted, oral anti-cancer agents, especially given the need for indefinite therapy and its recent approval in the frontline setting in the US, creates a considerable societal economic burden.[38, 39] The cost of lifelong tyrosine kinase inhibitor therapy has already been noted to be unsustainable by experts in chronic myeloid leukemia,[93] and one could easily draw a parallel with the situation with the oral kinase inhibitors such as ibrutinib and idelalisib in CLL.[38] Finally, while generally well-tolerated, ibrutinib does have some significant off-target toxicities, notably atrial fibrillation[30] and bleeding,[32, 33, 79–81] and it is conceivable that the more selective and specific irreversible BTK inhibitor, acalabrutinib, which does not inhibit other kinases such as EGFR, TEC and ITK at pharmacologically active concentrations, will possess an advantage over ibrutinib in this regard.[13] Similar considerations apply to other selective BTK inhibitors in earlier stages of clinical development.[14, 15]
Drug cost, toxicities, and off-target effects of ibrutinib may be mitigated by lowering the total daily dose. The optimal dose of ibrutinib should inhibit or bind to >95% of cellular BTK protein. Prior investigations in murine models demonstrated that total BTK protein levels increased after B-cell receptor stimulation in normal B-cells[94] or malignant CLL B-cells, tested in an adoptive transfer CLL murine model.[58] As a corollary, inhibition of BTK by ibrutinib in this murine model resulted in a decline in total BTK along with internalization of CXCR4 receptors.[58] Consistent with these data, total BTK protein was decreased in primary circulating CLL cells obtained from patients undergoing ibrutinib therapy (Figure 2).[95] Furthermore, in CLL patient-derived lymphocytes, the decline was also observed for BTK transcript levels. Ibrutinib attenuates BTK-dependent NF-κB activation, resulting from both BCR[96, 97] and toll-like receptor (TLR)[98] activation. NF-κB regulates the level of BTK protein through transcriptional control,[99] providing another mechanism by which ibrutinib therapy may lead to reduced levels of BTK protein. Collectively, these observations suggest that for stochiometric inhibition of the enzyme, a lower level of BTK protein would mean a lower concentration of ibrutinib required to effectively inhibit it. In fact, the 420 mg dose of ibrutinib may result in higher levels of circulating ibrutinib than are required to establish full BTK occupancy, thus increasing the likelihood of off-target binding of the drug, potentially leading to AEs.
Figure 2. Ibrutinib therapy decreases total protein and mRNA transcript levels of BTK.
Primary CLL cells from patients were isolated fresh from peripheral blood samples prior to treatment (baseline), and at 2, 4, and 12 weeks of 420 mg daily oral dosing of ibrutinib. Blood samples were collected from six patients in green-top tubes and CLL cells were isolated by Ficoll-Hypaque gradient. Cell pellets were used for total BTK protein levels (A) and BTK mRNA levels (B) from six patients with CLL. For the former, cell lysates were prepared and after protein quantitation, immunoblots were run to analyze total BTK protein levels. Immunoblots were quantitated and plotted as a percentage of the control level (baseline sample). For mRNA transcript levels, RT-PCR assay was performed in triplicate from each sample as described previously.[29] Student’s t-tests (two-tailed) were performed using the GraphPad Prism6 software (GraphPad Software, Inc. San Diego, CA) to compare values in untreated samples compared to all treated samples and the p values for the comparisons in figure A and figure B are 0.0001 and 0.0003, respectively. Reproduced, with permission, from [95].
In light of the above considerations, we have designed a pilot clinical protocol (NCT02801578, full protocol available as supplemental file) to identify the optimal dose of ibrutinib that can inhibit BTK protein after a cycle at the full dose (420 mg/d). In this study design, each patient will receive the full dose (three capsules daily) during the first cycle and then progressively lower doses, i.e., 280 mg/d (two capsules daily) during the second cycle and 140 mg/d (one capsule daily) during the third cycle. Extensive sampling for correlative studies will be performed to cover plasma pharmacokinetics and a number of pharmacodynamic endpoints, including but not limited to BTK occupancy, levels of total and phospho-BTK, BTK mRNA and protein levels, and NF-κB activity, at several different time points. The findings from this pilot study could justify a larger and more definitive trial of lower doses of ibrutinib in CLL with clinical endpoints in the future.
Drug summary box.
| Drug name: Ibrutinib (formerly PCI-32765) |
| Phase: IV (approved worldwide) |
| Indication: Chronic lymphocytic leukemia (all patients in the United States, relapsed/refractory or with deletion 17p in other countries) |
| Pharmacology description/mechanism of action: Irreversible (covalent) inhibitor of Bruton’s tyrosine kinase, an important mediator of B-cell receptor signaling |
| Route of administration: Oral; 3 capsules (140 mg each) per day |
Chemical structure:

|
| Pivotal trials: RESONATE [9] RESONATE-17 [11] RESONATE-2 [6] |
Acknowledgments
Funding
This work was supported by the U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute (P30 CA016672).
Footnotes
Declaration of interest
VV Gandhi has received research support from Pharmacyclics LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.SEER stat fact sheets: Chronic lymphocytic leukemia (CLL)[Internet]; 2016 - [cited2016 May/17].
- 2.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–84. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. Preclinical paper showing that CLL is exquisitely dependent on BCL2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Jones JJ, Woyach JA, Johnson AJ, Flynn JM. Entering the era of targeted therapy for chronic lymphocytic leukemia: Impact on the practicing clinician. J Clin Oncol. 2014;32:3039–47. doi: 10.1200/JCO.2014.55.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain N, O’Brien S. Initial treatment of CLL: Integrating biology and functional status. Blood. 2015;126:463–70. doi: 10.1182/blood-2015-04-585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37. doi: 10.1056/NEJMoa1509388. Pivotal trial that led to approval of ibrutinib for previously untreated patients with CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Claro RA, McGinn KM, Verdun N, et al. FDA approval: Ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3586–90. doi: 10.1158/1078-0432.CCR-14-2225. [DOI] [PubMed] [Google Scholar]
- 8••.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. Phase 1/2 study of ibrutinib in relapsed CLL showing unprecedented efficacy and good tolerability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. Pivotal trial that led to approval of ibrutinib for the treatment of relapsed CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. Lancet Oncol. 2015;16:169–76. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.O’Brien S, Jones JA, Coutre S, et al. Efficacy and safety of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic leukemia with 17p deletion: Results from the phase II RESONATE™-17 trial. Blood. 2014;124:327. Pivotal trial that led to approval of ibrutinib for the treatment of CLL with del17p in any line of therapy. [Google Scholar]
- 12.Winqvist M, Asklid A, Andersson PO, et al. Real--world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: Data from 95 consecutive patients treated in a compassionate use program. Haematologica. 2016 doi: 10.3324/haematol.2016.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–32. doi: 10.1056/NEJMoa1509981. Phase 1/2 trial of selective BTK inhibitor acalabrutinib in relapsed CLL showing high efficacy with minimal toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–9. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam C, Grigg AP, Opat S, et al. The BTK inhibitor, bgb-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: Initial report of a phase 1 first-in-human trial. Blood. 2015;126:832. [Google Scholar]
- 16••.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. Pivotal trial that led to approval of idelalisib and rituximab for relapsed CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. doi: 10.1056/NEJMoa1313984. Pivotal trial that led to approval of obinutuzumab plus chlorabucil for frontline therapy of CLL. [DOI] [PubMed] [Google Scholar]
- 18••.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 doi: 10.1038/nm.3048. Preclinical paper describing discovery of a selective BCL2 antagonist, venetoclax. [DOI] [PubMed] [Google Scholar]
- 19••.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22. doi: 10.1056/NEJMoa1513257. Phase 1/2 study of venetoclax in relapsed CLL showing high efficacy, including CRs and MRD eradication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30019-5. Pivotal trial that led to approval of venetoclax for relapsed/refractory CLL with del17p. [DOI] [PubMed] [Google Scholar]
- 21•.Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide and rituximab achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2015 doi: 10.1182/blood-2015-09-667675. Long-term follow-up of FCR-treated CLL patients suggesting functional “cures” in a subset of patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Strati P, Keating MJ, O’Brien SM, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123:3727–32. doi: 10.1182/blood-2013-11-538116. This paper showed that abbreviated courses of FCR may suffice for patients who achieve MRD eradication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: Distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–50. doi: 10.3109/10428194.2014.957203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo A, Lu P, Galanina N, et al. Heightened BTK-dependent cell proliferation in unmutated chronic lymphocytic leukemia confers increased sensitivity to ibrutinib. Oncotarget. 2016;7:4598–610. doi: 10.18632/oncotarget.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. First desription of BTK and PLCγ2 mutations conferring resistance to ibrutinib in CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Burger JA, Landau DA, Taylor-Weiner A, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat Commun. 2016;7:11589. doi: 10.1038/ncomms11589. Demonstration that ibrutinib resistance may develop through other mechanisms as well. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson PA, O’Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer. 2015;121:3612–21. doi: 10.1002/cncr.29566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3705–15. doi: 10.1158/1078-0432.CCR-14-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-akt signaling. Blood. 2014;124:3829–30. doi: 10.1182/blood-2014-10-604272. This paper first provided a mechanistic explanation of ibrutinib-induced atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 31.Alberelli MA, Innocenti I, Sica S, Laurenti L, De Candia E. PO-54 - clinical and laboratory characterization of platelet dysfunction caused by ibrutinib treatment in patients with chronic lymphocytic leukemia. Thromb Res. 2016;140(Suppl 1):S196. doi: 10.1016/S0049-3848(16)30187-6. [DOI] [PubMed] [Google Scholar]; 3848(16):30187–6. Epub 2016 Apr 8. [Google Scholar]
- 32.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von willebrand factor-dependent platelet functions. Blood. 2014;124:3991–5. doi: 10.1182/blood-2014-06-583294. [DOI] [PubMed] [Google Scholar]
- 33.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–7. doi: 10.1038/leu.2014.247. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Wang M, Wang L, et al. Selective antitumor activity of ibrutinib in EGFR-mutant non-small cell lung cancer cells. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju204. Print 2014 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. Demonstration of ITK inhibition in T cells by ibrutinib and resultant “Th1 skewing”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Honigberg LA, Smith AM, Sirisawad M, et al. The bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. Preclinical paper reporting discovery of ibrutinib and description of its kinome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berglof A, Hamasy A, Meinke S, et al. Targets for ibrutinib beyond B cell malignancies. Scand J Immunol. 2015;82:208–17. doi: 10.1111/sji.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain N, Chen Q, Ayer T, et al. Prevalence and economic burden of CLL in the era of oral targeted therapies. Blood. 2015;126:871. [Google Scholar]
- 39.Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract. 2015;11:252–8. doi: 10.1200/JOP.2014.002469. [DOI] [PubMed] [Google Scholar]
- 40.Cancer drugs fund decision summary: Ibrutinib for the treatment of relapsed or refractory chronic lymphatic leukaemia[Internet] 2015 Jan;2015 Available from: https://www.england.nhs.uk/wp-content/uploads/2015/01/ncdf-summ-ibrutnb-relps-rfract-cll.pdf. [Google Scholar]
- 41.Ibrutinib for treating chronic lymphocytic leukaemia [Internet] Vol. 2016. NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE; 2016. May, Available from: https://www.nice.org.uk/guidance/GID-TAG492/documents/appraisal-consultation-document. [Google Scholar]
- 42.NICE asks company to put leukaemia drug forward for new cancer drugs fund[Internet] Vol. 2016. National Institute for Health and Care Excellence; 2016. Jun 1st, Available from: https://www.nice.org.uk/news/press-and-media/nice-asks-company-to-put-leukaemia-drug-forward-for-new-cancer-drugs-fund. [Google Scholar]
- 43••.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. Report of the trial that led to ibrutinib’s initial approval in relapsed/refractory mantle cell lymphoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372:1430–40. doi: 10.1056/NEJMoa1501548. Report of the trial that led to approval of ibrutinib for Waldenstrom’s macroglobulinemia. [DOI] [PubMed] [Google Scholar]
- 45.Davids MS, Brown JR. Ibrutinib: A first in class covalent inhibitor of bruton’s tyrosine kinase. Future Oncol. 2014;10:957–67. doi: 10.2217/fon.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponader S, Burger JA. Bruton’s tyrosine kinase: From X-linked agammaglobulinemia toward targeted therapy for B-cell malignancies. J Clin Oncol. 2014;32:1830–9. doi: 10.1200/JCO.2013.53.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL) Blood. 2014;123:1207–13. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. Report on the phase 1 trial of ibrutinib in relapsed/refractory B-cell malignancies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. Important preclinical paper describing mechanisms of action of ibrutinib in CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–95. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman SE, Sun X, McAuley EM, et al. Modeling tumor-host interactions of chronic lymphocytic leukemia in xenografted mice to study tumor biology and evaluate targeted therapy. Leukemia. 2013;27:2311–21. doi: 10.1038/leu.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Ponader S, Chen SS, Buggy JJ, et al. The bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. Important preclinical paper describing mechanisms of action of ibrutinib in CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubovsky JA, Chappell DL, Harrington BK, et al. Lymphocyte cytosolic protein 1 is a chronic lymphocytic leukemia membrane-associated antigen critical to niche homing. Blood. 2013;122:3308–16. doi: 10.1182/blood-2013-05-504597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrussi L, Capitani N, Martini V, et al. Enhanced chemokine receptor recycling and impaired S1P1 expression promote leukemic cell infiltration of lymph nodes in chronic lymphocytic leukemia. Cancer Res. 2015;75:4153–63. doi: 10.1158/0008-5472.CAN-15-0986. [DOI] [PubMed] [Google Scholar]
- 55•.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. Important preclinical paper describing mechanisms of action of ibrutinib in CLL. [DOI] [PubMed] [Google Scholar]
- 56.ten Hacken E, Burger JA. Molecular pathways: Targeting the microenvironment in chronic lymphocytic leukemia--focus on the B-cell receptor. Clin Cancer Res. 2014;20:548–56. doi: 10.1158/1078-0432.CCR-13-0226. [DOI] [PubMed] [Google Scholar]
- 57.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res. 2015;21:4642–51. doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SS, Chang BY, Chang S, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2015 doi: 10.1038/leu.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herman SE, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: Correlative analyses from a phase II study. Leukemia. 2014;28:2188–96. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wodarz D, Garg N, Komarova NL, et al. Kinetics of CLL cells in tissues and blood during therapy with the BTK inhibitor ibrutinib. Blood. 2014;123:4132–5. doi: 10.1182/blood-2014-02-554220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–7. doi: 10.1182/blood-2013-09-527853. Showed that prolonged lymphocytosis on ibrutinib is not associated with inferior outcomes in CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015;112:E966–72. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123:1957–60. doi: 10.1182/blood-2014-01-547869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duong MN, Matera EL, Mathe D, et al. Effect of kinase inhibitors on the therapeutic properties of monoclonal antibodies. MAbs. 2015;7:192–8. doi: 10.4161/19420862.2015.989020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borge M, Belen Almejun M, Podaza E, et al. Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages. Haematologica. 2015;100:e140–2. doi: 10.3324/haematol.2014.119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: A single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–9. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: A phase 1b/2 study. Blood. 2015;126:842–50. doi: 10.1182/blood-2014-12-617522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skarzynski M, Niemann CU, Lee YS, et al. Interactions between ibrutinib and anti-CD20 antibodies: Competing effects on the outcome of combination therapy. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niemann CU, Herman SE, Maric I, et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib - findings from an investigator-initiated phase II study. Clin Cancer Res. 2016;22:1572–82. doi: 10.1158/1078-0432.CCR-15-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.IMBRUVICA prescribing information.[Internet] Vol. 2016. U.S. Food and Drug Administration; 2016. Available from: http://www.imbruvica.com/docs/librariesprovider3/default-document-library/prescribing_information.pdf?sfvrsn=14. [Google Scholar]
- 71.de Jong J, Sukbuntherng J, Skee D, et al. The effect of food on the pharmacokinetics of oral ibrutinib in healthy participants and patients with chronic lymphocytic leukemia. Cancer Chemother Pharmacol. 2015;75:907–16. doi: 10.1007/s00280-015-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marostica E, Sukbuntherng J, Loury D, et al. Population pharmacokinetic model of ibrutinib, a bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol. 2015;75:111–21. doi: 10.1007/s00280-014-2617-3. [DOI] [PubMed] [Google Scholar]
- 73.de Jong J, Skee D, Murphy J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect. 2015;3:e00156. doi: 10.1002/prp2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheers E, Leclercq L, de Jong J, et al. Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: An open-label, phase I, single-dose study in healthy men. Drug Metab Dispos. 2015;43:289–97. doi: 10.1124/dmd.114.060061. [DOI] [PubMed] [Google Scholar]
- 75.Tobinai K, Ogura M, Ishizawa K, et al. Safety and tolerability of ibrutinib monotherapy in japanese patients with relapsed/refractory B cell malignancies. Int J Hematol. 2016;103:86–94. doi: 10.1007/s12185-015-1900-3. [DOI] [PubMed] [Google Scholar]
- 76.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–7. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–7. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015 doi: 10.1182/blood-2015-04-639203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oda A, Ikeda Y, Ochs HD, et al. Rapid tyrosine phosphorylation and activation of bruton’s tyrosine/Tec kinases in platelets induced by collagen binding or CD32 cross-linking. Blood. 2000;95:1663–70. [PubMed] [Google Scholar]
- 80.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of btk. Blood. 2003;102:3592–9. doi: 10.1182/blood-2003-04-1142. [DOI] [PubMed] [Google Scholar]
- 81.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108:2596–603. doi: 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015 doi: 10.3324/haematol.2015.126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinohara M, Koga T, Okamoto K, et al. Tyrosine kinases btk and tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 84.Fabbro SK, Smith SM, Dubovsky JA, Gru AA, Jones JA. Panniculitis in patients undergoing treatment with the bruton tyrosine kinase inhibitor ibrutinib for lymphoid leukemias. JAMA Oncol. 2015;1:684–6. doi: 10.1001/jamaoncol.2015.0457. [DOI] [PubMed] [Google Scholar]
- 85.Mulvey JJ, Nuovo GJ, Magro CM. Cutaneous, purpuric painful nodules upon addition of ibrutinib to RCVP therapy in a CLL patient: A distinctive reaction pattern reflecting iatrogenic Th2 to Th1 milieu reversal. Am J Dermatopathol. 2016 doi: 10.1097/DAD.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 86.Bitar C, Farooqui MZ, Valdez J, et al. Hair and nail changes during long-term therapy with ibrutinib for chronic lymphocytic leukemia. JAMA Dermatol. 2016 doi: 10.1001/jamadermatol.2016.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thompson PA, Wierda WG. Eliminating minimal residual disease as a therapeutic end point: Working toward cure for patients with CLL. Blood. 2016;127:279–86. doi: 10.1182/blood-2015-08-634816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davids MS. Boldly targeting kinases without mutations. Blood. 2014;123:1119–21. doi: 10.1182/blood-2013-12-543322. [DOI] [PubMed] [Google Scholar]
- 89.Lamothe B, Cervantes-Gomez F, Sivina M, Wierda WG, Keating MJ, Gandhi V. Proteasome inhibitor carfilzomib complements ibrutinib’s action in chronic lymphocytic leukemia. Blood. 2015;125:407–10. doi: 10.1182/blood-2014-07-585364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woyach JA, Johnson AJ. Targeted therapies in CLL: Mechanisms of resistance and strategies for management. Blood. 2015;126:471–7. doi: 10.1182/blood-2015-03-585075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hing ZA, Mantel R, Beckwith KA, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015;125:3128–32. doi: 10.1182/blood-2015-01-621391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel V, Keating MJ, Wierda WG, Gandhi V. Preclinical combination of TP-0903, an AXL inhibitor and B-PAC-1, a procaspase-activating compound with ibrutinib in chronic lymphocytic leukemia. Leuk Lymphoma. 2016;57:1494–7. doi: 10.3109/10428194.2015.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Experts in chronic myeloid leukemia. Price of drugs for chronic myeloid leukemia (CML), reflection of the unsustainable cancer drug prices: Perspective of CML experts. Blood. 2013;121:4439–42. doi: 10.1182/blood-2013-03-490003. Using CML as an example, this paper draws attention to the astronomical prices of cancer drugs in the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nisitani S, Satterthwaite AB, Akashi K, Weissman IL, Witte ON, Wahl MI. Posttranscriptional regulation of bruton’s tyrosine kinase expression in antigen receptor-stimulated splenic B cells. Proc Natl Acad Sci U S A. 2000;97:2737–42. doi: 10.1073/pnas.050583597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cervantes-Gomez F, Kumar Patel V, Bose P, Keating MJ, Gandhi V. Decrease in total protein level of bruton’s tyrosine kinase during ibrutinib therapy in chronic lymphocytic leukemia lymphocytes. Leukemia. 2016;30:1803–4. doi: 10.1038/leu.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton’s tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;191:1735–44. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–54. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton’s tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B cells. J Biol Chem. 2008;283:11189–98. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 99.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 100.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: An open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 102.Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) Leuk Lymphoma. 2013;54:2385–91. doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]