Abstract
Here we report the use of the hexadehydro-Diels–Alder (HDDA) reaction for the de novo construction of a benzenoid ring in fused polycyclic heteroaromatic carbazole (i.e., [2,3]-benzoindole) skeletons. The strategy allows creation of highly substituted benzenoids. We also describe the HDDA-enabled chemical synthesis of the natural product alkaloids mahanimbine and koenidine. Trapping of the intermediate carbazolyne with a conjugated enal–proceeding through formal [2+2] cycloaddition, 4π-electrocyclic ring-opening, and 6π-electrocyclic ring-closing events—constitutes a robust method for producing pyranocarbazoles.
Graphical Abstract

Carbazoles have a long1 and rich history in the fields of heterocyclic and medicinal chemistries and recently have garnered considerable attention as a chromophoric platform for the development of organic electroluminescent2 materials. Hundreds of carbazole-containing alkaloid secondary metabolites are known.3 Collectively these exhibit manifold categories of bioactivity.4
Numerous approaches for carbazole synthesis use construction of one of the benzenoid rings as a strategic tactic.3,5 Methods using condensation, elimination, or oxidative aromatization reactions are common. Cyclizations or cycloadditions involving alkynes or allenes have also been used. These often give rise to the new six-membered carbocycle at precisely the oxidation state of benzene. The examples shown in Figures 1a–c include electrocyclic ring closure,6 [2+2+2] cyclization of three isolated alkyne units,7 and the tetradehydro-Diels–Alder8,9 reaction.
Figure 1.
a–c. Three known types of alkyne-containing substrates that produce a benzenoid moiety of carbazoles. d) The hexadehydro-Diels–Alder (HDDA) cascade of the diynamide substrate 1 produces substituted carbazoles 3 via the carbazolyne 2.
Here we describe a new strategy for carbazole assembly that capitalizes on the hexadehydro-Diels–Alder (HDDA) cascade.10 The process involves a sequential net 4+2 cycloisomerization reaction between a 1,3-diyne and a diynophile to produce a benzyne intermediate,11 followed by one of several different modes of trapping reactions.10,12,13,14 This cascade constitutes a powerful and versatile strategy for synthesis of benzenoid derivatives in which the benzene ring itself has been assembled in de novo fashion from the six reacting alkyne carbon atoms. The purely thermal nature of the reaction conditions lends itself to the discovery of new types of aryne trapping reactions that can be complementary to those possible with arynes generated by conventional15 means.
In the studies we present here, the relevant and enabling intermediate is a carbazolyne (2, Figure 1d), a rare member16 of the family of arynes. We have used a number of different trapping agents Nu–El to capture the intermediate carbazolynes. Collectively, these demonstrate the versatility of this approach for preparing carbazoles bearing a wide variety of substituents and other structural variations. Finally, we further demonstrate the strategic power of this approach17 through efficient chemical syntheses of the mahanine alkaloids mahanimbine (12) 18 and koenidine (19)19 in which three of the four rings in these pyranocarbazoles are constructed in a single operation.
In Table 1 we have shown examples of the types of carbazoles that can be prepared using this HDDA approach. Many different types of trapping agents, both internal (tethered) and external, are effective, and often highly so. Various substituents R1 and R2 are accommodated on the alkyne termini [e.g., TMS, H, CO2Me, and alkynyl (on the diynophile) and alkyls (on the diyne)] and the benzene ring in the triyne 4 can carry an additional substituent (e.g., 6h). Taken together, these types of modifications can be envisioned to allow for considerable flexibility in the substitution pattern that can be accessed in the carbazole products 6.
Table 1.
HDDA constructiona of carbazoles 6 from triyne substrates 4 via trapping of the carbazolynes 5
Reactions were carried out in 1,2-dichloroethane (DCE), chloroform, or THF at temperatures of 90–100 °C (see SI) with an initial concentration of 4 = 0.02 M; the following amounts of external trapping agents were used:
2 equiv,
5 equiv,
10 equiv.
For trapping reactions that involve inequivalent trapping atoms or groups, the nucleophilic component could add to either Ca or Cb in the electrophilic carbazolynes 5. However, we never observed an isomeric product arising from attack of the nucleophile at Ca (cf. 6a, 6e, 6f, 6g, and 6h).20 This essentially perfect level of regioselectivity is deserving of comment. Aryne 5 (R1 = TMS and R2 = Me; i.e., 2 in Figure 1) is computed [DFT: SMD(ClCH2CH2Cl)/M06-2X/6-31G(d)] to be significantly distorted, having a very large difference of 24° (see Table 2) between the intra-annular angles at its nominally sp-hybridized atoms Ca vs. Cb. It is now well established21 that this ring-distortion allows one to account for (or predict in advance) the sense of the regioselectivity shown by unsymmetrical trapping agents of the Nu–El class. Consistent with this, the nucleophilic portion of the trapping agent (Nu) shows a high preference for adding to Cb, the atom having higher in-plane p-character and, accordingly, greater electrophilicity (δ+) in the reactive, strained alkyne. This is portrayed in 5 at the top of Table 1.
Table 2.
Computed (DFT) geometric distortion of 2 vs. analogous benzynes lacking the TMS and/or having the electronegative NTs moiety replaced by a methylene group

| ||||
|---|---|---|---|---|
| Angle (°) | 2 | 2(-TMS) | 2(CH2) | 2(CH2-TMS) |
| ∠a | 114.8° | 117.7° | 120.8° | 125.3° |
| ∠.b | 138.8° | 136.0° | 134.1° | 129.9° |
| ∠b–∠a | 24.0° | 18.3° | 13.3° | 4.6° |
To gain a better sense of the factors that contribute to the highly biased geometry of these carbazolynes, we also calculated the extent of distortion in several analogs of 2. These are shown in Table 2. The remote TMS substituent is more than an innocent bystander. Its removal results in a reduction of the ring distortion (see 2(–TMS), where b – a = 18.3°), presumably a response to the change in the buttressing force the TMS imposes on its adjacent substituents. Nonetheless, the trapping of 5 (R1 = H and R2 = Me) with, for example, acetic acid also proceeded with high regioselectivity, giving 6e as the only observed product. To understand the impact of the electronegative NTs substituent adjacent to carbon a, the geometries of the methylene replacement analogs 2(CH2) and 2(CH2-TMS) were also computed. These show, in turn, a substantial reduction in the extent of the internal distortion (b –a = 13.3° and 4.6°, respectively), which shows that the inductive effect of the NTs is a major contributor to the polarization of the alkyne in carbazolyne 2. A similar effect has been observed for the computed geometric distortions of 6,7-indolyne vs. 4,5-indanyne.21b
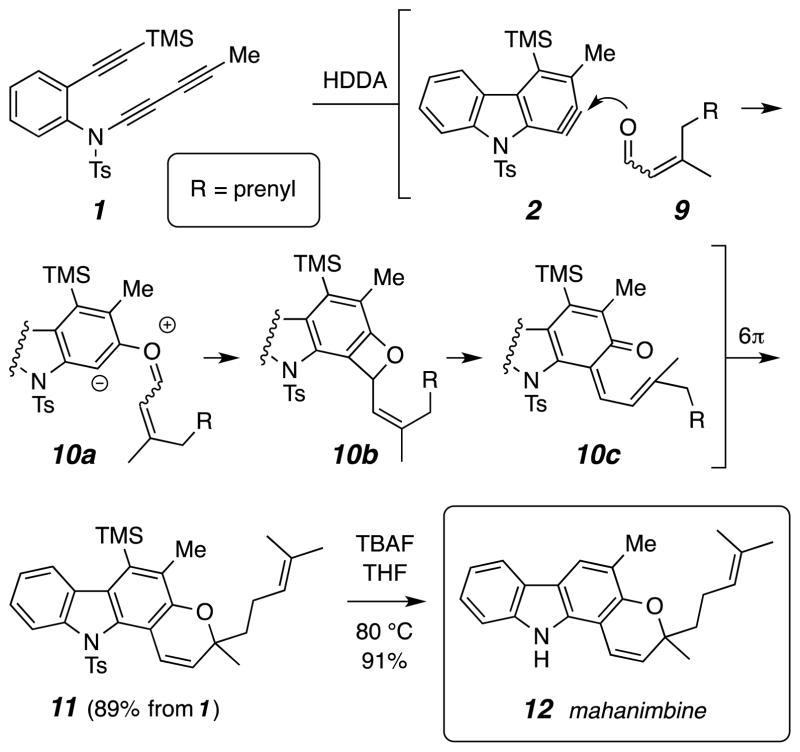
We then explored whether this type of reaction could serve as an enabling strategy for the synthesis of mahanine alkaloids like mahanimbine (12, Scheme 1) and koenidine (19, Scheme 2). These natural substances are found in Murraya koenigii (the curry tree) and have been ingested by humans, including for medicinal purposes, for eons. Koenidine was recently reported to show insulin sensitizing and blood glucose lowering properties in mice. 22 Each of the carbazoles 12 and 19 bears a fused pyran ring, a hallmark of many of the carbazoles found in Murraya koenigii, indigenous to Asia.
Scheme 1.
Key step in the enal trapping of benzyne 2, establishing the tetracyclic pyranocarbazole core structure of mahanimbine (12)
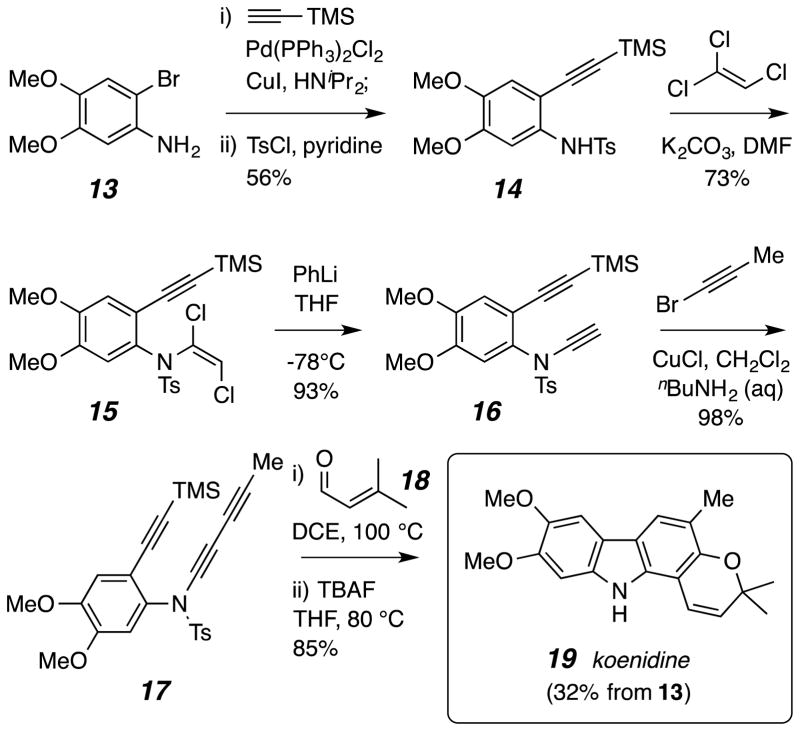
Scheme 2.
Synthesis of koenidine (19)
Enals were recently reported to produce benzopyrans (or chromenes) by trapping o-benzyne. 23 If the engagement of the benzyne by the nucleophilic carbonyl oxygen atom in the enal initiates the capture event, then the distortion present in a benzyne like 2 should dictate the proper outcome required for assembly of the requisite skeleton for mahanimbine (12) and koenidine (19). In the event (Scheme 1), when triyne 1 was heated in the presence of citral (9, 2.0 equiv), a very clean transformation to 11 allowed its isolation in 89% yield after chromatographic purification. This reaction proceeds, presumably, through the intermediate 1,3-zwitterion 10a, benzoxetene 10b, and alkenyl quinonemethide 10c, prior to the ultimate electrocyclic ring-closure to the pyran. This transformation proceeds with 100% atom efficiency and is mediated solely by thermal energy—no reagents or catalysts are used. Removal of the TMS and Ts groups in 11 proved to be trivial under the action of tetrabutylammonium fluoride (TBAF) in THF at 80 °C, which smoothly provided 12.
To prepare koenidine (19), the triyne 17, the dimethoxy analog of 1, was required. The preparation of this substrate, starting from the commercially available bromide 13, is shown in Scheme 2. This is representative of the strategy used to prepare each of the HDDA substrates used in this study (see the Supporting Information for those details). Particularly noteworthy is the construction of the ynamide bond24 in 16 via the 1,2-dichlorovinylsulfonamide 15.25 In our hands this strategy proved to be more reproducible and serviceable for the sulfonamide 14 than one using the ethynyl iodonium salt HC≡C(Ph)I+TfO−.26 Heating 17 with β,β-dimethylacrolein followed by TBAF treatment to strip away the TMS and Ts groups completed this efficient synthesis of koenidine.
Finally and as evidence of the potential that this benzyne plus enal trapping reaction has for the construction of additional types of polycyclic skeletons, we show the reaction between the triyne 20 (which happens to bear a trifluoromethyl substituent27 on the diynophile) and the designer exocyclic enal 21.28 When heated at 120 °C for 15 h,29 the spirocyclic pyran 22 was smoothly produced.
In summary, we have shown that an HDDA cascade is a general strategy for the preparation of substituted carbazoles. The intermediate, unsymmetrical carbazolynes can be captured by a variety of nucleophilic trapping agents with perfect regioselectivity. Factors that contribute to the distortion of these intermediate arynes have been probed through a simple DFT study. Efficient reactions of carbazolynes with 3,3-disustituted enals comprise a key strategy for construction of the tetracyclic pyranocarbozole rings of the alkaloids mahanimbine (12) and koenidine (19). The de novo construction of a highly substituted, central benzenoid ring constitutes a significant strategic advance.12i,30
Supplementary Material
Scheme 3.
Example of use of an exocyclic enal—21—to introduce the spirocyclic pyran subunit in the product—22
Acknowledgments
This research was supported by the National Institutes of Health (GM65597). NMR spectral data were collected with instrumentation acquired through the NIH Shared Instrumentation Grant program (S10OD011952). T. W. received support from a Wayland E. Noland Fellowship and a University of Minnesota Graduate School Doctoral Dissertation Fellowship.
Footnotes
The authors declare to have no competing financial interests.
The Supporting Information is available free of charge on the ACS Publications website.
New compound preparation, spectroscopic characterization data, and copies of 1H and 13C NMR spectra (PDF).
References
- 1.Campbell N, Barclay BM. Chem Rev. 1947:359–380. doi: 10.1021/cr60127a001. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Sun J, Zhang J. A review on synthesis of carbazole-based chromophores as organic light-emitting materials. Curr Org Chem. 2012;16:2014–2025. [Google Scholar]
- 3.(a) Knölker H-J, Reddy KR. Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev. 2002;102:4303–4427. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]; (b) Knölker H-J, Reddy KR. In: In The Alkaloids: Chemistry and biology of carbazole alkaloids. Cordell GA, editor. Vol. 65. Academic Press; New York: 2008. pp. 1–430. [DOI] [PubMed] [Google Scholar]; (c) Schmidt AW, Reddy KR, Knölker H-J. Chem Rev. 2011;112:3193–3328. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- 4.Głuszyńska A. Eur J Med Chem. 2015;94:405–426. doi: 10.1016/j.ejmech.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 5.For recent reviews of carbazole synthesis see: Roy J, Jana AK, Mal D. Tetrahedron. 2012;68:6099–6121.Bauer I, Knölker HJ. Top Curr Chem. 2012;309:203–253. doi: 10.1007/128_2011_192.
- 6.Tohyama S, Choshi T, Azuma S, Fujioka H, Hibino S. Heterocycles. 2009;79:955–965. [Google Scholar]
- 7.Witulski B, Alayrac C. Angew Chem Int Ed. 2002;41:3281–3284. doi: 10.1002/1521-3773(20020902)41:17<3281::AID-ANIE3281>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Esperón MF, Rodríguez D, Castedo L, Saá C. Org Lett. 2005;7:2213–2216. doi: 10.1021/ol050609a. [DOI] [PubMed] [Google Scholar]
- 9.For a description of this nomenclature see: Baire B, Niu D, Willoughby PH, Woods BP, Hoye TR. Nature Protocols. 2013;8:501–508. doi: 10.1038/nprot.2013.017.
- 10.Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP. Nature. 2012;490:208–212. doi: 10.1038/nature11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Bradley AZ, Johnson RP. J Am Chem Soc. 1997;119:9917–9918. [Google Scholar]; (b) Miyawaki K, Suzuki R, Kawano T, Ueda I. Tetrahedron Lett. 1997;38:3943–3946. [Google Scholar]
- 12.(a) Yun SY, Wang K, Lee N, Mamidipalli P, Lee D. J Am Chem Soc. 2013;135:4668–4671. doi: 10.1021/ja400477r. [DOI] [PubMed] [Google Scholar]; (b) Karmakar R, Mamidipalli P, Yun SY, Lee D. Org Lett. 2013;15:1938–1941. doi: 10.1021/ol4005905. [DOI] [PubMed] [Google Scholar]; (c) Wang K, Yun SY, Mamidipalli P, Lee D. Chem Sci. 2013;4:3205–3211. [Google Scholar]; (d) Mamidipalli P, Yun SY, Wang K, Zhou T, Xia Y, Lee D. Chem Sci. 2014;5:2362–2367. [Google Scholar]; (e) Lee N, Yun SY, Mamidipalli P, Salzman RM, Lee D, Zhou T, Xia Y. J Am Chem Soc. 2014;136:4363–4368. doi: 10.1021/ja500292x. [DOI] [PubMed] [Google Scholar]; (f) Karmakar R, Yun SY, Wang K, Lee D. Org Lett. 2014;16:6–9. doi: 10.1021/ol403237z. [DOI] [PubMed] [Google Scholar]; (g) Karmakar R, Yun SY, Chen J, Xia Y, Lee D. Angew Chem Int Ed. 2015;54:6582–6586. doi: 10.1002/anie.201412468. [DOI] [PubMed] [Google Scholar]; (h) Karmakar K, Ghorai S, Xia Y, Lee D. Molecules. 2015;20:15862–15880. doi: 10.3390/molecules200915862. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Karmakar R, Wang KP, Yun SY, Mamidipalli P, Lee D. Org Biomol Chem. 2016;14:4782–4788. doi: 10.1039/c6ob00524a. [DOI] [PubMed] [Google Scholar]
- 13.(a) Vandavasi JK, Hu W, Hsiao C, Senadia GC, Wang JJ. RSC Adv. 2014;4:57547–57552. [Google Scholar]; (b) Liang Y, Hong X, Yu P, Houk KN. Org Lett. 2014;16:5702–5705. doi: 10.1021/ol502780w. [DOI] [PubMed] [Google Scholar]; (c) Nobusue S, Yamane H, Miyoshi H, Tobe Y. Org Lett. 2014;16:1940–1943. doi: 10.1021/ol5004934. [DOI] [PubMed] [Google Scholar]; (d) Kerisit N, Toupet L, Larini P, Perrin L, Guillemin J, Trolez Y. Chem - Eur J. 2015;21:6042–6047. doi: 10.1002/chem.201500633. [DOI] [PubMed] [Google Scholar]; (e) Watanabe T, Curran DP, Taniguchi T. Org Lett. 2015;17:3450–3453. doi: 10.1021/acs.orglett.5b01480. [DOI] [PubMed] [Google Scholar]; (f) Chen Z, Shan W, Yin J, Yu G, Liu S. Acta Chimica Sinica. 2015;73:1007–1012. [Google Scholar]; (g) Zhang MX, Shan W, Chen Z, Yin J, Yu GA, Liu SH. Tetrahedron Lett. 2015;56:6833–6838. [Google Scholar]
- 14.(a) Niu D, Hoye TR. Nat Chem. 2014;6:34–40. doi: 10.1038/nchem.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen J, Baire B, Hoye TR. Heterocycles. 2014;88:1191–1200. doi: 10.3987/COM-13-S(S)83. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Woods BP, Baire B, Hoye TR. Org Lett. 2014;16:4578–4581. doi: 10.1021/ol502131r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Woods BP, Hoye TR. Org Lett. 2014;16:6370–6373. doi: 10.1021/ol503162k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Pogula VD, Wang T, Hoye TR. Org Lett. 2015;17:856–859. doi: 10.1021/ol5037024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Luu Nguyen Q, Baire B, Hoye TR. Tetrahedron Lett. 2015;56:3265–3267. doi: 10.1016/j.tetlet.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen J, Palani V, Hoye TR. J Am Chem Soc. 2016;138:4318–4321. doi: 10.1021/jacs.6b01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.For a summary of these methods see, e.g.: Tadross PM, Stoltz BM. Chem Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. and references therein.
- 16.(a) Brown RFC, Choi N, Coulston KJ, Eastwood FW, Ercole F, Horvath JM, Mattinson M, Mulder RJ, Ooi HC. Liebigs Ann/Recueil. 1997:1931–1940. [Google Scholar]; (b) Goetz AE, Silberstein AL, Corsello MA, Garg NK. J Am Chem Soc. 2014;136:3036–3039. doi: 10.1021/ja501142e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The first application of a HDDA reaction to the synthesis of a natural product (herbindole B) was recently reported (ref 12i).
- 18.Isolation: Joshi BS, Kamat VN, Gawd DH. Tetrahedron. 1970;26:1475–1482. doi: 10.1016/s0040-4020(01)92976-x.Recent syntheses: Hesse R, Gruner KK, Kataeva O, Schmidt AW, Knölker HJ. Chem Eur J. 2013;19:14098–14111. doi: 10.1002/chem.201301792.Li X, Song W, Tang W. J Am Chem Soc. 2013;135:16797–16800. doi: 10.1021/ja408829y.
- 19.Isolation: Narasimhan NS, Paradkar MV, Chitguppi VP, Kelkar SL. Indian J Chem. 1975;13:993–999.Recent synthesis: Schuster C, Rönnefahrt M, Julich-Gruner KK, Jäger A, Schmidt AW, Knölker H–J. Synthesis. 2016;48:150–160.
- 20.Our analytical methods should have allowed detection of as little as 1% of such a byproduct.
- 21.(a) Hamura T, Ibusuki Y, Sato K, Matsumoto T, Osamura Y, Suzuki K. Org Lett. 2003;5:3551–3554. doi: 10.1021/ol034877p. [DOI] [PubMed] [Google Scholar]; (b) Cheong PHY, Paton RS, Bronner SM, Im GYJ, Garg NK, Houk KN. J Am Chem Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org Lett. 2010;12:96–99. doi: 10.1021/ol902415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel OPS, Mishra A, Maurya R, Saini D, Pandey J, Taneja I, Raju KSR, Kanojiya S, Shukla SK, Srivastava MN, Wahajuddin M, Tamrakar AK, Srivastava AK, Yadav PP. J Nat Prod. 2016;79:1276–1284. doi: 10.1021/acs.jnatprod.5b00883. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Huang C, Wu L. Eur J Org Chem. 2012:3507–3519. [Google Scholar]
- 24.DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem Rev. 2010;110:5064–5106. doi: 10.1021/cr100003s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansfield SJ, Campbell CD, Jones MW, Anderson EA. Chem Commun. 2015;51:3316–3319. doi: 10.1039/c4cc07876d. [DOI] [PubMed] [Google Scholar]
- 26.(a) Witulski B, Stengel B. Angew Chem, Int Ed. 1999;38:2426–2430. [PubMed] [Google Scholar]; (b) Alayrac C, Schollmeyer D, Witulski B. Chem Commun. 2009:1464–1466. doi: 10.1039/b820291e. [DOI] [PubMed] [Google Scholar]
- 27.Tresse C, Guissart C, Schweizer S, Bouhoute Y, Chany AC, Goddard ML, Blanchard N, Evano G. Adv Synth Catal. 2014;356:2051–2060. [Google Scholar]
- 28.Burkhard JA, Guérot C, Knust H, Rogers-Evans M, Carreira EM. Org Lett. 2010;12:1944–1947. doi: 10.1021/ol1003302. [DOI] [PubMed] [Google Scholar]
- 29.(a) This HDDA cycloisomerization proceeds more slowly than the other examples reported here, consistent with the presence of the CF3-group, one of the rare substituents that destabilizes adjacent radical character relative to a hydrogen atom. In turn, this outcome is entirely in line with previous mechanistic studies supporting the stepwise (diradical) nature of the HDDA cyclization reaction.b–dLiang Y, Hong X, Yu P, Houk KN. Org Lett. 2014;16:5702–5705. doi: 10.1021/ol502780w.Marell DJ, Furan LR, Woods BP, Lei X, Bendelsmith AJ, Cramer CJ, Hoye TR, Kuwata KT. J Org Chem. 2015;80:11744–11754. doi: 10.1021/acs.joc.5b01356.Wang T, Niu D, Hoye TR. J Am Chem Soc. 2016;138:7832–7835. doi: 10.1021/jacs.6b03786.
- 30.It was once deemed that “bonds within aromatic rings are not considered to have potential strategic character.” Corey EJ, Howe WJ, Orf HW, Pensak DA, Petersson G. J Am Chem Soc. 1975;97:6116–6124.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.