Abstract
We explored the cross-sectional relationships between β-amyloid (Aβ) and inferior temporal tau deposition (IFT Tau) on cognitive performance and whether cognitive reserve (CR) modifies these associations. We studied 156 participants classified into groups of clinically normal (CN=133), mild cognitive impairment (MCI=17) and Alzheimer disease (AD=6) dementia. AMNART IQ served as a proxy of CR and cognitive performance was assessed using the MMSE. In separate linear regression models predicting MMSE, we examined the interactions of CR × global Aβ and CR × IFT tau across all participants and within the CN group alone. In the whole sample, the interaction between CR and IFT tau was significant (p<0.003), such that higher CR participants with elevated IFT tau had better MMSE scores compared with low CR participants with similar levels of IFT tau. The interaction between CR and Aβ status did not reach significance (p=0.093). In CN only, no cross-sectional interactions among CR, Aβ, and IFT tau were observed on MMSE. These findings imply that CR may be protective against early AD processes and enable some individuals to remain cognitively stable despite elevated tau and Aβ burden.
Keywords: Aging, Preclinical Alzheimer’s disease, Amyloid PET imaging, Tau PET imaging, Resilience
Introduction
Advances in positron emission tomography (PET) using radiotracers that bind to β-amyloid (Aβ) has allowed for the in vivo detection of Alzheimer’s disease (AD) pathology in otherwise clinically normal (CN) older adults (Klunk et al. 2004). Based on this evidence, we now understand that AD includes a long preclinical stage whereby CN exhibit biomarker abnormalities approximately 15 years prior to the onset of dementia (Bateman et al. 2012; Rowe et al. 2010; Sperling et al. 2011a). As the field moves toward the identification and treatment of individuals with preclinical AD (Sperling et al. 2011b), the factors that influence the relationships among cognition and pathologic burden have important implications. One important factor is the concept of “cognitive reserve” (CR), which attempts to explain how some individuals are able to maintain normal cognitive performance despite pathological disease burden (Katzman 1993; Stern 2012), thus delaying or reducing the risk of developing symptomatic AD (Bennett et al. 2003; Stern 2009). Several factors that might convey CR include intelligence (IQ), education, engagement in complex occupations, physical exercise and cognitively stimulating activities (Bennett et al. 2003; Rentz et al. 2007; Scarmeas et al. 2009; Scarmeas et al. 2003; Stern 2009; Stern et al. 1992; Stern et al. 1995; R. Wilson et al. 2003; R. S. Wilson et al. 2003; Wilson et al. 2007). Studies using PET and Pittsburgh Compound B (PiB PET) have found that amyloid burden has a weak but persistent effect on cognition and that CR modifies that effect (Kemppainen et al. 2008; Rentz et al. 2010; Roe et al. 2008). With the recent availability of an F18-T807 radiotracer (aka F18-AV-1451) (Chien et al. 2013), we can now image in vivo tau pathology. The availability of these in vivo biomarkers gives us the unique opportunity to explore the impact of both β-amyloid and tau pathology on cognition.
In this study, we explored the relationships of Aβ and tau burden on cognition and whether CR modifies these associations throughout the spectrum of AD, by examining these markers within a cross-sectional sample of clinically normal elderly (CN) and patients with mild cognitive impairment (MCI) and Alzheimer disease (AD) dementia. We also examined these relationships within the CN group alone.
Materials and Methods
Human Subjects
Our sample consisted of 133 CN individuals participating in the Harvard Aging Brain Study and 23 participants diagnosed with either MCI (n=17) or mild AD dementia (n=6) who underwent both C11 -Amyloid PET Imaging using Pittsburgh Compound B (PiB PET) and Tau PET imaging using F18- T807 (T807 PET). They also underwent neuropsychological assessment within 1 year of PET imaging. The study was conducted at the Massachusetts General Hospital (MGH) using protocols and informed consent procedures approved by the Partners Human Research Committee. Informed consent was obtained from all individual participants included in the study.
Subjects were deemed CN at baseline if they met the following criteria: 1) a global Clinical Dementia Rating (CDR) (Morris 1993) score of 0, 2) scores above age and education-adjusted cutoffs on the 30-Minute Delayed Recall of the Logical Memory Story (II) (ADNI based cut-offs; http://www.adni-info.org/), and 3) normal performance on the Mini Mental State Exam (MMSE) (Folstein et al. 1975) using published age and education corrected adjustments (Mungas et al. 1996). Review of medical history and physical and neurological examinations confirmed their status as clinically normal. None of the subjects had a history of alcoholism, drug abuse, head trauma, or current serious medical/psychiatric illness.
MCI and AD patients were recruited from physicians in the Memory Disorders Clinic at MGH. Diagnosis was determined based on a comprehensive neurological exam. Subjects were selected for this analysis if they had a CDR score between 0.5 and 1.0, MMSE >19, underwent PiB-PET and T807 PET imaging within 6 months of each other and neuropsychological testing within 1 year of PET scans.
Neuropsychological Testing
While CN participants underwent yearly neuropsychological testing with a large array of measures, only a limited number of neuropsychological tests were available for MCI/AD subjects. Here we examine performance on the MMSE in order to maximize the sample size. Estimated verbal IQ from the American National Reading Test (AMNART IQ) (Ryan and Paolo 1992) served as a proxy for CR. Given that T807 PET was introduced mid-study, the MMSE that was closest to T807 PET was examined (all of the MMSE scores were within one year of the T807 scan).
PET Imaging
F18 T807 and C11 Pittsburgh Compound B were prepared at MGH using published methods (Gomperts et al. 2008; Johnson et al. 2016). All PET data are acquired using a Siemens/CTI ECAT HR+ scanner (3D mode; 63 image planes; 15.2cm axial field of view; 5.6mm transaxial resolution and 2.4mm slice interval). PiB PET is acquired with an 8.5 to 15 mCi bolus injection followed immediately by a 60-minute dynamic acquisition in 69 frames (12×15 seconds, 57×60 seconds). T807 PET is acquired from 80–100 minutes after a 9.0 to 11.0 mCi bolus injection in 4 × 5-minute frames. PET data are reconstructed and attenuation-corrected. To evaluate the anatomy of cortical T807 binding, each individual PET data set is co-registered to the subject’s MPRAGE data using SPM8. The cortical ribbon and subcortical ROIs defined by MR (described below) are transformed into the PET native space. T807 signal from bilateral inferior temporal ROI was extracted and normalized by cerebellar grey. The inferior temporal region (IFT Tau) was used in all analyses based on autopsy data that suggests that when tau deposition spreads beyond the entorhinal cortex it is more proximal to cognitive impairment in AD (Braak et al. 2006; Johnson et al. 2016; Nelson et al. 2012). PiB PET data are expressed as a DVR with cerebellar grey as reference tissue; regional time-activity curves are used to compute regional DVRs for each ROI using the Logan graphical method. PiB retention is assessed using a large cortical ROI aggregate that includes frontal, lateral temporal and retrosplenial cortices.
MR Imaging
MRI was completed at the MGH Martinos Center on a Siemens TIM Trio 3T System with a 12-channel head coil. Structural T1-weighted volumetric magnetization-prepared, rapid acquisition gradient echo scans (TR/TE/TI=6400/2.8/900ms, flip angle=8°, 1×1×1.2mm resolution) were processed with FreeSurfer v5.1 to define regions of interest in each participant’s native space (Desikan et al. 2006; Fischl et al. 2002).
Statistical Methods
Statistical analyses were completed using R v3 (http://CRAN.R-project.org/doc/). Demographic characteristics were examined using t-tests for continuous variables and χ2 tests for dichotomous variables. Two sets of analyses were completed. First, we examined cross-sectional relationships in the whole sample, controlling for diagnosis (CN versus MCI/AD). Second, analyses were repeated within the CN group alone.
Multiple linear regression analyses were used in the entire sample to predict MMSE performance in relation to PET biomarkers and the interaction between each biomarker and CR (Aβ × CR and IFT Tau × CR). All models included age, level of education, AMNART VIQ and clinical status (CN versus MCI/AD). Model 1 included PiB-PET(Aβ) alone as a predictor. Model 2 included inferior temporal tau aggregation alone (IFT tau) as a predictor. Model 3 included both Aβ and IFT tau as independent predictors. Model 4 included the interaction of CR and Aβ. Model 5 included the interaction of CR and IFT tau. Comparable analyses were completed in the CN only group (Models 6 through 9).
Results
Demographic Characteristics
Demographic characteristics of the CN and MCI/AD sample are displayed in Table 1. The CN group was older and comprised of more females compared with the MCI/AD group. The CN group was less likely to be Aβ+ and had lower levels of IFT tau. The CN group exhibited higher MMSE scores and less clinical progression on the CDR in comparison with the MCI/AD group. There were no differences between groups in education level or AMNART IQ scores. Although all CN were CDR 0 at baseline, nine CN participants were CDR 0.5 at the neuropsychological testing session closest to the time of T807 scanning. However, none of these individuals met ADNI criteria for Mild Cognitive Impairment and were therefore included in analyses as CN in order to maximize sample size. We did not observe a relationship between CR and amyloid (r=0.034, p=0.673) or between CR and IFT tau (r=−0.065, p=0.422). When examining the CN group alone, we again did not find a relationship between CR and amyloid (r=0.142, p=0.102) or CR and IFT tau (r=0.037, p=0.671).
Table 1.
Descriptive Characteristics
| CN | MCI/AD | Mean Difference | p | |
|---|---|---|---|---|
| Mean (SD) or count (n) |
Mean (SD) or count (n) |
|||
| n | 133 | 17/6 | ||
| Age | 76.17 (6.23) | 69.41 (9.97) | 6.76 | 0.001 |
| Sex (M/F) | 59/74 | 19/4 | 0.001 | |
| Education (years) | 15.91 (2.96) | 16.29 (3.38) | 0.38 | 0.597 |
| Inferior Temporal T807 | 1.20 (0.09) | 1.61 (0.44) | 0.40 | 0.001 |
| PiB | 1.21 (0.21) | 1.50 (0.26) | 0.28 | 0.001 |
| MMSE | 29.18 (1.02) | 26.61(3.06) | 2.57 | 0.001 |
| AMNART | 121.59 (8.75) | 121.22 (8.01) | 0.37 | 0.850 |
| Global CDR (1/0.5/0) | 0.03 (0.13) | 0.41 (0.05) | 0.38 | 0.001 |
Notes.
Abbreviations: MMSE=Mini Mental Status Exam, CDR=Clinical Dementia Rating, MCI= Mild Cognitive Impairment, AD= Alzheimer’s disease, AMNART- American National Adult Reading Test
Cross-Sectional Results of Multiple Linear Regression Analyses Predicting MMSE in the Whole Sample (CN + MCI/AD)
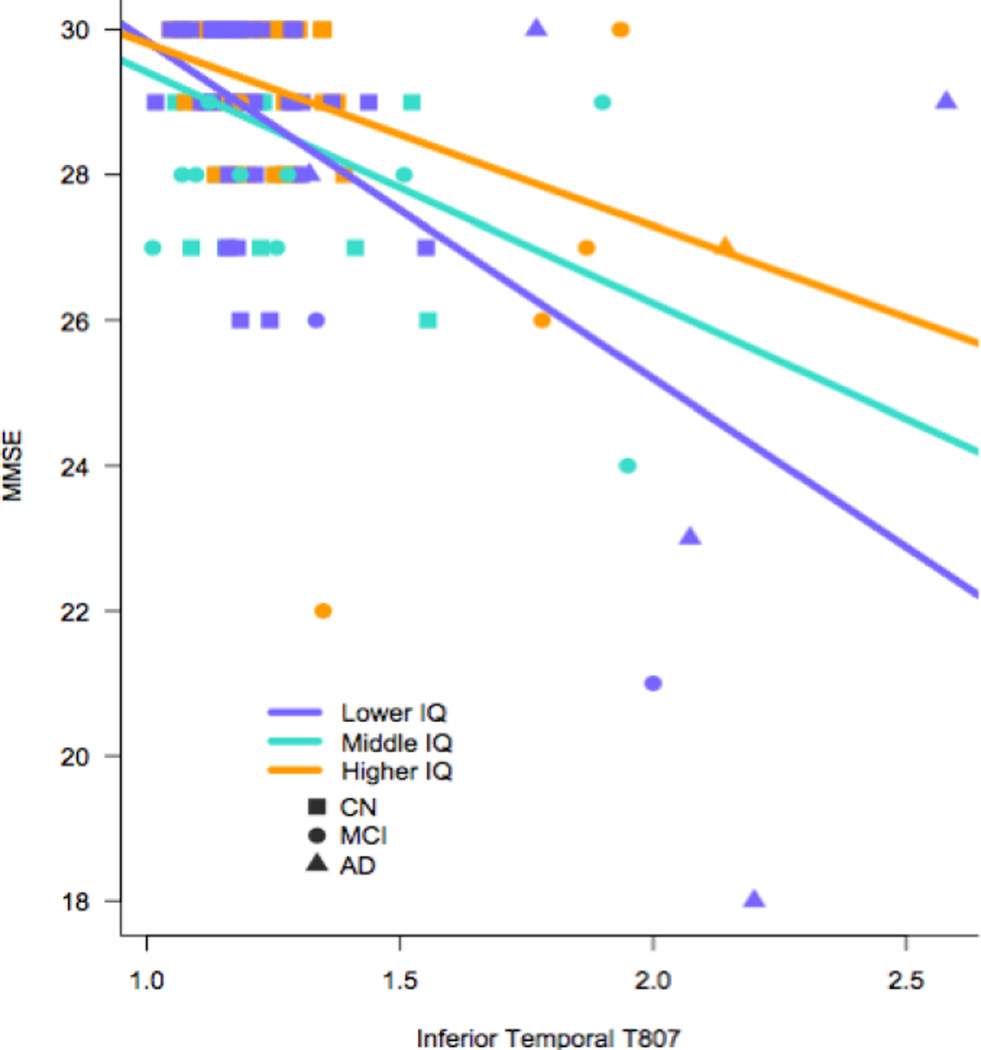
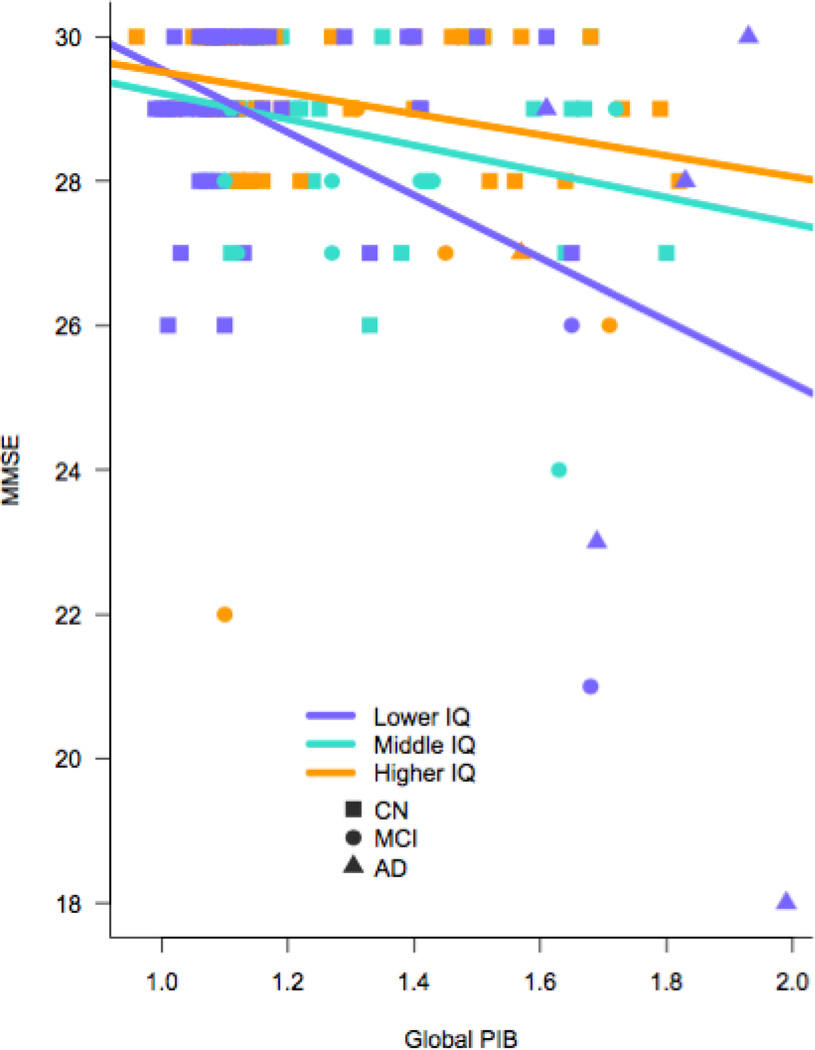
As expected, performance on the MMSE was related to clinical status in all models (see Table 2). Aβ deposition (Model 1) and IFT tau (Model 2) were significant contributors to MMSE score, wherein lower performance was related to higher biomarker values. When both Aβ and IFT tau were included together in the model, only IFT tau was a predictor of MMSE performance (Model 3). Furthermore, there was an interaction between CR and IFT tau such that higher IFT tau showed a greater association with lower MMSE score among low CR participants than high CR participants (Model 5). Figure 1 provides a visual representation of this model showing the relationship between slope in MMSE and biomarker abnormality in tertiles of low (VIQ<118), medium, (119<VIQ<125), and high (VIQ>126–132) AMNART scores. This same interaction with CR did not reach statistical significance for Aβ, though was at trend level (Model 4, see Figure 2).
Table 2.
Cross-Sectional Results of Multiple Linear Regression Analysis Predicting MMSE in Whole Sample
| t | B(SE) | p | F | df | p | Adj. R2 | |
|---|---|---|---|---|---|---|---|
| Overall Model 1 | 21.66 | 5,148 | 0.000 | 0.40 | |||
| Age | −0.81 | −0.01 (0.02) | 0.422 | ||||
| Education | 2.30 | 0.09 (0.04) | 0.023 | ||||
| VIQ | 2.23 | 0.03 (0.01) | 0.027 | ||||
| Clinical Status | −7.05 | −2.55 (0.36) | 0.000 | ||||
| PiB | −3.27 | −1.70 (0.52) | 0.001 | ||||
| Overall Model 2 | 28.82 | 5,148 | 0.000 | 0.48 | |||
| Age | −1.72 | −0.03 (0.02) | 0.088 | ||||
| Education | 2.02 | 0.08 (0.04) | 0.045 | ||||
| VIQ | 2.24 | 0.03 (0.01) | 0.027 | ||||
| Clinical Status | −4.97 | −1.85 (0.37) | 0.000 | ||||
| T807 | −5.73 | −3.47 (0.61) | 0.000 | ||||
| Overall Model 3 | 24.09 | 6,147 | 0.000 | 0.48 | |||
| Age | −1.61 | −0.03 (0.02) | 0.109 | ||||
| Education | 2.04 | 0.08 (0.04) | 0.044 | ||||
| VIQ | 2.29 | 0.03 (0.01) | 0.024 | ||||
| Clinical Status | −4.86 | −1.82 (0.38) | 0.000 | ||||
| PiB | −0.84 | −0.47 (0.56) | 0.401 | ||||
| T807 | −4.62 | −3.20 (0.69) | 0.000 | ||||
| Overall Model 4 | 18.76 | 6,147 | 0.000 | 0.41 | |||
| Age | −0.71 | −0.01(0.02) | 0.477 | ||||
| Education | 2473 | 0.10 (0.04) | 0.015 | ||||
| VIQ | −1.18 | −0.08 (0.07) | 0.239 | ||||
| Clinical Status | −6.81 | −2.47 (0.36) | 0.000 | ||||
| PiB | −1.95 | −12.55 (6.44) | 0.053 | ||||
| PiB×VIQ | 1.69 | 0.09 (0.05) | 0.093 | ||||
| Overall Model 5 | 26.77 | 6,147 | 0.000 | 0.50 | |||
| Age | −1.64 | −0.03(0.02) | 0.103 | ||||
| Education | 2.14 | 0.08 (0.04) | 0.034 | ||||
| VIQ | −2.43 | −0.15 (0.06) | 0.016 | ||||
| Clinical Status | −5.21 | −1.89 (0.36) | 0.000 | ||||
| T807 | −3.58 | −20.07 (5.61) | 0.000 | ||||
| T807×VIQ | 2.97 | 0.14 (0.05) | 0.003 |
Figure 1.
Visual Representation of Model 5: Interaction Among MMSE Score, Inferior Temporal Tau, and Levels of Cognitive Reserve in Whole Sample (CN, MCI, AD)
Notes: purple= Low IQ (VIQ<118), turquoise=Middle IQ (119<VIQ<125), orange=High IQ (VIQ>126–132), unadjusted data shown.
Figure 2.
Visual Representation of Model 4: Interaction Among MMSE Score, Global Amyloid Burden, and Levels of Cognitive Reserve in Whole Sample (CN, MCI, AD)
Notes: purple= Low IQ (VIQ<118), turquoise=Middle IQ (119<VIQ<125), orange=High IQ (VIQ>126–132), unadjusted data shown.
Cross-Sectional Results of Multiple Linear Regression Analysis Predicting MMSE in CN Only Sample
As shown in Model 6 (see Table 3), less Aβ aggregation was associated with better MMSE. Similarly, in Model 7, less IFT tau was associated with better MMSE. Model 8 shows that there is no interaction between Aβ and CR on MMSE performance. Similarly, Model 9 shows no interaction between IFT tau deposition and CR on MMSE performance.
Table 3.
Cross-Sectional Results of Multiple Linear Regression Analysis Predicting MMSE in Clinically Normal Older Adults Only
| t | Β(SE) | p | F | df | p | Adj. R2 | |
|---|---|---|---|---|---|---|---|
| Overall Model 6 | 5.16 | 3,124 | 0.001 | 0.11 | |||
| Age | −1.48 | −0.02 (0.01) | 0.141 | ||||
| Education | 1.26 | 0.04 (0.03) | 0.209 | ||||
| VIQ | 2.74 | 0.03 (0.01) | 0.007 | ||||
| PiB | −2.21 | −0.93 (0.42) | 0.029 | ||||
| Overall Model 7 | 6.66 | 3,124 | 0.000 | 0.15 | |||
| Age | −0.76 | −0.01(0.01) | 0.451 | ||||
| Education | 1.74 | 0.06(0.03) | 0.084 | ||||
| VIQ | 2.35 | 0.02(0.01) | 0.020 | ||||
| T807 | −3.01 | −2.88(0.96) | 0.003 | ||||
| Overall Model 8 | 4.34 | 4,124 | 0.001 | 0.11 | |||
| Age | −1.50 | −0.02 (0.01) | 0.135 | ||||
| Education | 1.09 | 0.04 (0.03) | 0.277 | ||||
| VIQ | 1.51 | 0.09 (0.06) | 0.134 | ||||
| PiB | 0.85 | 4.93 (5.79) | 0.396 | ||||
| PiB×VIQ | −1.01 | −0.05 (0.05) | 0.312 | ||||
| Overall Model 9 | 5.53 | 4.124 | 0.000 | 0.15 | |||
| Age | −0.95 | −0.01(0.01) | 0.343 | ||||
| Education | 1.74 | 0.06 (0.03) | 0.085 | ||||
| VIQ | −0.09 | −0.09 (0.16) | 0.457 | ||||
| T807 | −2.64 | −2.62 (0.99) | 0.009 | ||||
| T807×VIQ | 0.97 | 0.09 (0.09) | 0.335 |
Discussion
We report that higher Aβ and IFT tau are associated with lower cognitive performance across the AD trajectory suggesting that these biomarkers are not benign but have a deleterious effect on cognitive performance even in cognitively normal older adults. Across the whole sample, the interaction between CR and Aβ in predicting MMSE did not reach statistical significance (p=0.093) whereas the interaction between CR and tau was significant (p<0.003). The reduced strength of the interaction between Aβ and CR with cognition compared to tau may be related to the more proximal association of tau to cognitive impairment (Delacourte et al. 2002; Nelson et al. 2012), indicating that tau takes precedent over Aβ deposition along the AD trajectory. Among CN individuals, we found a relation between AD biomarkers and MMSE performance at baseline, but no modifying effect of CR on MMSE in relation to either AD biomarker. Overall, these findings offer support that CR may afford a protective effect on cognition throughout the AD spectrum.
Understanding the relation of CR to cognitive performance in the context of preclinical AD has important implications for early diagnosis and enrollment into AD prevention trials. At baseline, we did not find an association between CR, Aβ and tau burden when analyses were restricted to CN adults. These findings are consistent with our previous report where CR failed to modify cognitive performance in CN with a canonical composite of less challenging tasks but did emerge with the inclusion of more challenging tests of memory (Rentz et al. 2010). It is not surprising that our use of the MMSE, a simple screening measure of global cognition, failed to detect a protective effect of CR at baseline in CN adults, given that most CN score at ceiling. Future work will examine whether CR modifies the association between AD markers of pathology and cognition among the CN sample using more challenging tests as well as longitudinal cognitive decline. Nevertheless, this initial study examining the full spectrum of AD provide compelling evidence that the protection inferred by CR may act through mechanisms that mask the detrimental effect of tau aggregation.
A recent neuropathology study by Bennett et al. (2012) found that both Aβ and IFT tau were related to cognition in persons without cognitive impairment. However, a recent meta-analysis of PiB PET imaging and cognition found the effect of Aβ on cognition in CN appears small (Hedden et al. 2013). In contrast, IFT tau, in our sample, had a much stronger relation to cognition than Aβ, consistent with the hypothetical model that Aβ deposition may occur at an earlier stage than inferior temporal tau (Hardy, 2009; Jack et al. 2013) and with other studies that suggest neocortical tau may be more proximal to AD-related cognitive impairment or decline (Braak et al. 2006; Johnson et al. 2016; Nelson et al. 2012). Furthermore, degree of tangle pathology has been related to the severity and duration of AD dementia and not Aβ burden alone (Arriagada et al. 1992a; Arriagada et al. 1992b; Price and Morris 1999). The fact that we observed a stronger interaction between tau and CR in predicting MMSE across the clinical spectrum than between Aβ and CR is also consistent with tau being more closely linked with cognition. This tau, CR interaction further suggests that CR may exert a protective effect on cognition even after tau pathology has started to spread from the medial temporal lobe to the neocortex. Given that higher CR has been hypothesized to be associated with a faster progression of cognitive symptoms after disease onset (Stern 2009), it will be important for future work to understand if this acceleration is related to a corresponding biomarker signature.
Tangle pathology imaged by T807 is specific for paired helical filament tangles in AD (Marquie et al. 2015) and not to other tangle pathology seen in frontotemporal and other atypical AD dementias (Wolk et al. 2012) or chronic traumatic encephalopathy (McKee et al. 2009). Our understanding of IFT tau in CN requires further exploration, as we understand the role tau plays in aging and other neurodegenerative diseases (Delacourte et al. 2002). Even the concept of cognitive reserve can be applied to many different types of pathologies and is not solely related to AD (Bieliauskas et al. 2007; Sumowski et al. 2009).
There are several limitations to this study. In order to maximize sample size across the whole sample of CN, MCI and AD, we used the MMSE as our measure of cognition. While this was optimal across all diagnostic groups, examination of more challenging or specific tests of cognition will be preferable when exploring the relations of cognition, CR and AD biomarkers in CN populations. Furthermore, we chose to use AMNART as our proxy of CR to be consistent with our previous report (Rentz et al. 2010) but recognize that there are other factors that also contribute to CR including level of education, occupational status and even amount of cognitive activities performed during life. In the future, it will be important to explore whether a composite of reserve factors would be preferable to exploring the multiple dimensions of CR on cognition and whether there are differential modifying effects of CR on Aβ and tau-related performance in a group of individuals with subjective cognitive decline. While our sample tended to be well educated with high IQ, despite this skewed distribution, we were still able to identify these interactions. Unlike a recent article that described a modifying effect of cognitive activity/reserve on amyloid burden in ApoE-4 carriers (Wirth et al. 2014), we were unable to examine this relationship as APOE genotyping was not available for our entire sample. In future work, we will attempt to tease out the relationship between E4 status and CR on both Aβ and IFT tau accumulation. Finally, we plan on exploring the longitudinal effects of CR on cognition, particularly as we collect more longitudinal data.
In summary, we found that AD biomarkers of Aβ and IFT tau deposition have a negative association on cognitive performance but CR modifies that relationship. Higher IFT Tau had a stronger and more direct association with poorer cognition than Aβ burden. Our analyses provide further evidence of the protective effect of CR in the course of Alzheimer’s disease, and suggest that CR may exert its beneficial effect by reducing the detrimental cognitive consequences of tau aggregation. Understanding the factors that mediate the relationships among cognition and pathologic burden have important implications for early diagnosis and identifying individuals for AD prevention trials.
Acknowledgments
Funding:
The National Institute on Aging (P01 AG036694, RO1 AG037497, RO1EB014894) and the Alzheimer’s Association IIRG-08-90934 funded this study.
Conflict of Interest:
D. Rentz received research support from the NIH grants P01 AG036694, R01 MH090291, U01 AG024904, R01 AG027435, R01 AG037497 and P50 AG005134, Alzheimer Association grant IIRG-08-90934 and Fidelity Biosciences. She has also served as a paid consultant for Eli Lilly, Lundbeck Pharmaceuticals, Janssen Alzheimer Immunotherapy, Biogen, Idec and Neurotrack. These relationships are not related to the content in the manuscript.
E. Mormino received funding from NIH grant F32AG044054 and P01 AG036694 and has served as a paid consultant for Janssen Pharmaceuticals and Biogen Idec. These relationships are not related to the content in the manuscript.
K. Papp was supported by NIA grant T32AG023480-08, the Charles King Trust, and NIH grant P01 AG036694 and has served as a paid consultant for Biogen Idec. These relationships are not related to the content in the manuscript.
R. Betensky received funding from NIH grants R01 CA075971, R03 CA165070, UL 1RR025758, P50 NS051343, P50 NS051343, P30 CA006516, P50 AG005134, P01 AG036694, R01 NS070834, R01 NS070834 and R01 AG026484.
R. Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen. R. Sperling receives research support from Janssen Pharmaceuticals, and Eli Lilly and Co. These relationships are not related to the content in the manuscript. She also receives research support from the following grants: P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, Fidelity Biosciences, Harvard NeuroDiscovery Center, and the Alzheimer’s Association.
K. Johnson has served as paid consultant for Bayer, Bristol-Myers Squibb, GE Healthcare, Janssen Alzheimer’s Immunotherapy, Siemens Medical Solutions, and Genzyme. He is a site coinvestigator for Lilly/Avid, Bristol-Myers Squibb, Pfizer, Janssen Immunotherapy, and Navidea. He has spoken at symposia sponsored by Janssen Alzheimer’s Immunotherapy and Pfizer. T. Benzinger has served on an advisory board for Eli Lilly and has received research funding from Avid Radiopharmaceuticals. These relationships are not related to the content in the manuscript. K. Johnson receives funding from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497 and the Alzheimer’s Association grant ZEN-10-174210.
Footnotes
Ethical Approval:
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Arriagada PV, Growdon JH, Hedley-White ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992a;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992b;42(9):1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72(4):599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Wright EC, Kronfol Z, Lok AS, et al. Cognitive reserve and neuropsychological functioning in patients infected with hepatitis C. Journal of the International Neuropsychological Society. 2007;13(4):687–692. doi: 10.1017/S1355617707070877. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Sergeant N, Wattez A, Maurage CA, Lebert F, Pasquier F, et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol. 2002;37(10–11):1291–1296. doi: 10.1016/s0531-5565(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Annals of Neurology. 2008;63(1):112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating Scale (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46(3):700–706. doi: 10.1212/wnl.46.3.700. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6:53–62. [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Archives of Neurology. 2003;60(3):359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011a;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011b;3(111):111cm133. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Annals of Neurology. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, et al. Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45:55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol. 2009;31(8):913–926. doi: 10.1080/13803390902740643. [DOI] [PubMed] [Google Scholar]
- Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene–environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. The Journal of Neuroscience. 2014;34(25):8612–8617. doi: 10.1523/JNEUROSCI.4612-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Madeira C, Saxton JA, Snitz BE, Lopez OL, et al. Amyloid imaging in dementias with atypical presentation. Alzheimers Dement. 2012;8(5):389–398. doi: 10.1016/j.jalz.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]