Abstract
Background
The most common, persistent concern among breast cancer survivors is the fear that their disease will return, yet few interventions targeting fear of cancer recurrence (FCR) have been developed. This pilot study examined the feasibility, acceptability, and preliminary efficacy of a home-delivered cognitive bias modification (CBM) intervention to reduce FCR. The intervention, Attention and Interpretation Modification for Fear of Breast Cancer Recurrence (AIM-FBCR), targeted two types of cognitive biases (i.e., attention and interpretation biases).
Methods
Breast cancer survivors (n=110) were randomized to receive eight sessions of one of two versions of AIM-FBCR or a control condition program. Computer-based assessments of cognitive biases and a self-report measure of FCR were administered pre-intervention, post-intervention, and 3 months post-intervention.
Results
Improvements in health worries (p=.019) and interpretation biases (rates of threat endorsement, p<.001; and reaction times for threat rejection, p=.007) were found in those who received AIM-FBCR as compared to the control arm. While only 26% of participants who screened into the study agreed to participate, the trial otherwise appeared feasible and acceptable, with 83% of those who began the intervention completing at least 5 of 8 sessions, and 90% reporting satisfaction with the computer-based program used.
Conclusions
This pilot study suggests the promise of AIM-FBCR in reducing FCR in breast cancer survivors. Future research should attempt to replicate these findings in a larger-scale trial using a more sophisticated, user-friendly program and additional measures of improvement in more diverse samples. ClinicalTrials.gov Identifier: NCT01517945.
Keywords: Fear of recurrence, breast cancer, cognitive bias modification, health worries, survivorship
CONDENSED ABSTRACT
Despite the high prevalence of fear of cancer recurrence among survivors, few interventions targeting this issue have been developed and evaluated. This pilot randomized controlled trial demonstrated the feasibility, acceptability, and preliminary efficacy of a home-delivered, computer-based cognitive bias modification intervention targeting fear of cancer recurrence in breast cancer survivors, showing a significant reduction in health worries and interpretation biases among those randomized to receive active treatment.
The most common, persistent concern among breast cancer survivors (BCS) is fear of cancer recurrence (FCR),1 with rates ranging from 47% to as high as 99%.2–5 While some FCR is normative, maladaptive levels of fear can be impairing.3,4,6,7 Increased FCR in BCS is associated with decreased quality of life and increased distress, depression, and maladaptive behaviors, including increased body monitoring, medical visit anxiety, and preoccupation with health.3,4,6 Despite this, few psychosocial interventions have been designed to target FCR.8–10 In fact, BCS report that their greatest unmet need is management of FCR.11
Cognitive models of anxiety posit that cognitive biases, such as selective attention to threat-relevant stimuli (attention bias)12 and interpreting ambiguity in a threatening manner (interpretation bias),13 play a key role in the etiology and maintenance of maladaptive levels of anxiety. Studies focusing on cancer suggest that cognitive biases may contribute to FCR in survivors, implying that such biases may be an important intervention target.14–16,17 A substantial literature has shown that cognitive biases can be altered through brief, computerized interventions referred to as cognitive bias modification (CBM), which involve rapid presentation of stimuli (e.g., 500 ms) and repeated practice (e.g., 360 trials) on cognitive tasks designed to encourage shifts in attention or interpretation.18–20 CBM has demonstrated efficacy in improving a variety of conditions, including social anxiety, panic, and generalized anxiety disorders.19,20
We developed a CBM intervention to target FCR in BCS, adapting a program originally developed for anxiety disorders.21 This intervention, Attention and Interpretation Modification (AIM) for Fear of Breast Cancer Recurrence (AIM-FBCR), attempts to maximize efficacy by targeting both attention and interpretation biases;22 personalizing it with an individual’s greatest worries;23 including a version of AIM-FCBR that included meaningful stimuli, which may have additional psychological benefits;24,25 and allowing completion of the treatment at home. The purpose of this pilot study was to examine the feasibility, acceptability, and preliminary efficacy of AIM-FBCR. We hypothesized that AIM-FBCR would be feasible and would reduce FCR compared to a control condition (CC).
Method
Participants and Procedures
From October 2012 through November 2015, this parallel-group randomized trial recruited a convenience sample of BCS diagnosed with stages 0–III breast cancer (with no history of recurrence or metastases) who completed active treatment ≥3 months prior (could be on hormonal therapy),1 were over age 18, female, English-speaking, and scored ≥3 on the Concerns about Recurrence Scale (CARS) Overall Fear Index, representing at least a low-moderate level of fear (53% of women screened).26 In accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services, following Institutional Review Board (IRB) approval, potential participants were invited to participate through letters, clinic approaches, and advertisements. Informed consent was obtained for each participant. Sample size was determined by resource constraints for this pilot trial, with the expectation of reaching 80% statistical power if the between-group effect size in the primary psychological outcomes of interest (CARS subscales scores) were ≥0.81.
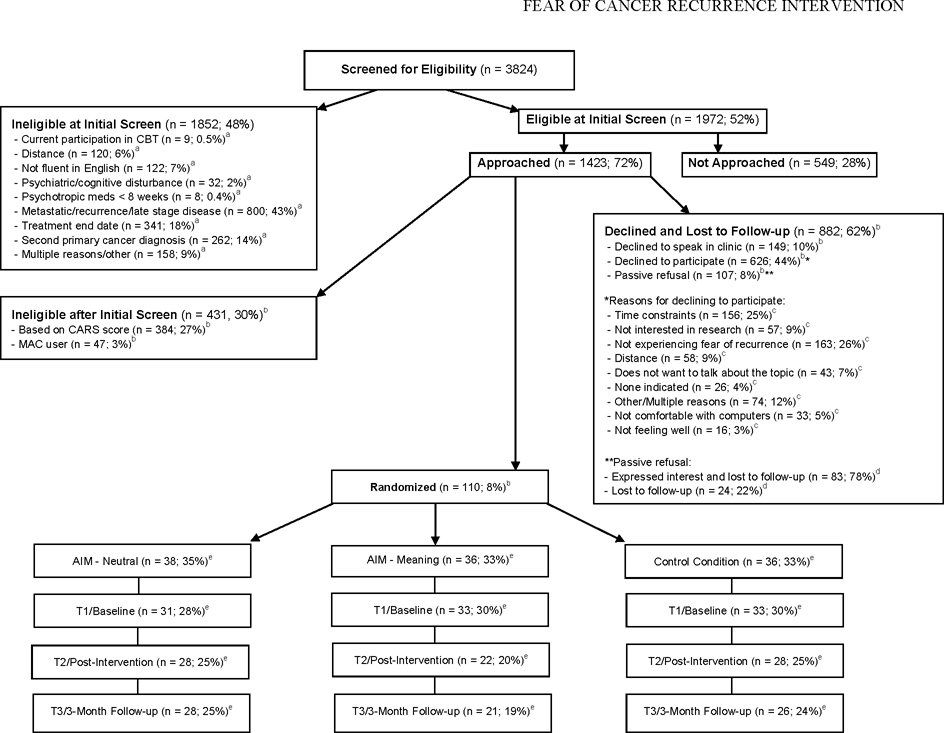
With a goal of enrolling 25 completers per arm for this pilot study, accounting for ~30% attrition, 110 participants were randomized (1:1:1) to receive one of two versions of AIM-FBCR (n=38 for the neutral version; n=36 for the meaning version) or a control condition (CC) program (n=36). Ninety-seven participants provided T1 data (12% attrition), and 78 completed the intervention and provided T2 data (20% attrition from T1). Of these 78, 75 provided T3 data (23% attrition from T1). See Figure 1. Participants were blinded to assignment. The allocation sequence was generated by an institutional program that uses randomly permuted blocks. Randomization was stratified by disease stage (stages 0–I versus stages II–III). Cognitive bias and psychosocial outcomes assessments were administered pre-intervention (T1), post-intervention (T2), and 3 months post-intervention (T3).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the current study. CBT=Cognitive-behavioral therapy. CARS=Concerns About Recurrence Scale. AIM-Neutral=Attention and Interpretation Modification for Fear of Breast Cancer Recurrence Neutral Version. AIM-Meaning=Attention and Interpretation Modification for Fear of Breast Cancer Recurrence Meaning Version. aDenominator is total ineligible at screen. bDenominator is total approached. cDenominator is total declined. dDenominator is total passive refusal. eDenominator total randomized.
Cognitive Bias Assessments
Stimuli
The stimulus pool for the assessments and interventions was developed by compiling stimuli from previous studies of cognitive biases16–18 and piloting them with 10 women endorsing FCR (CARS ≥3). The study team removed the least emotionally-charged stimuli, yielding a final set of 83 neutral-threat word pairs, 107 meaning-threat word pairs, and 78 threat-neutral word/ambiguous sentence pairs, all matched for length and frequency of use.
Bias assessments
Attention bias was assessed via a modified dot probe task.27 In this task, individuals with an attention bias toward threat information should theoretically respond more quickly (lower reaction time, fewer milliseconds) to congruent trials (in which a probe replaces a threatening stimulus) because their attention will be captured by the threatening stimulus. In contrast, reaction times should be slower for incongruent trials (in which the probe shows up in the opposite location of the threatening stimulus) because they will have to disengage their attention from the location of the threatening stimulus in order to identify the probe (more milliseconds). The task used in this study had 144 trials comprised of combinations of 2 (probe type: E/F) by 2 (probe position: top/bottom) by 2 (neutral word position: top/bottom) by 9 (words) by 2 repetitions. Following presentation of a fixation cue, the computer presented neutral-threat word pairs (e.g., “sculpture-malignant”) for 500 ms followed by a probe (the letter E or F) in the previous location of one of the words. Participants were instructed to indicate whether the letter was an E or an F with a click of the corresponding button on the mouse. Attention bias “bottom bias” scores were calculated according to recent guidelines28 by subtracting reaction times for theoretically fast trial types (probe congruent with threat word) from slow trial types (probe incongruent with threat word), using trials in which the probe appeared in the bottom location.
Interpretation bias was assessed via a word-sentence association paradigm (WSAP)29 with 118 trials of word-sentence pairings,2 measuring endorsement rates and reaction times for threat and benign interpretations of ambiguous sentences. Each WSAP trial began with a fixation cross for 500 ms, followed by a word representing either the threat or benign interpretation of an ambiguous sentence appearing on the screen for 500 ms, followed by the ambiguous sentence (e.g., “Suspicious mass” or “Thorough” appeared before the sentence, “The technician takes additional scans.”). Participants were asked to indicate whether the word and sentence were related or not by pressing keyboard keys. We calculated the percentage of threat (i.e., “Rate of Threat Endorsement”) and benign interpretations (i.e., “Rate of Benign Endorsement”) endorsed and the mean reaction time to make each response (e.g., “Reaction Time for Threat Rejection”).
Treatment Conditions
AIM-FBCR Intervention
AIM-FBCR consisted of eight, 30-minute personalized treatment sessions administered twice a week for four weeks, as done in prior studies.19–21,27,30 Session 1 was completed in the clinic; sessions 2–8 were completed at home. Sessions involved completion of an attention modification task (CBM-A) followed by an interpretation modification task (CBM-I). Two versions of AIM-FCBR were developed. AIM-Neutral modeled prior studies that paired neutral and threatening words in CBM-A.27 AIM-Meaning was developed to determine if there was any added value of redirecting attention toward a positive stimulus in CBM-A, as research, including our own, has suggested that increasing a sense of meaning in life plays a protective role in adjustment among cancer patients.24,25,31 We thus piloted a version of AIM-FBCR that was slightly modified, redirecting attention away from threat and toward positive and meaningful stimuli.
CBM-A consisted of 160 trials of a modified dot probe task27 involving either neutral-threat pairs (e.g., “sculpture-malignant”) in the neutral version (AIM-Neutral) or meaning-threat pairs (e.g., “grandchild-malignancy”) in the meaning version (AIM-Meaning). CBM-A was identical to the attention bias assessment, except that in order to direct attention away from threat, the probes always replaced the neutral words (e.g., “magazine”) for AIM-Neutral or meaning words (e.g., “grandchild”) for AIM-Meaning. CBM-I consisted of 100 trials3 of a modified WSAP30 that were identical to the interpretation bias assessment, except that participants received feedback about their responses. The program provided positive feedback (“You are correct!”) when participants endorsed benign interpretations or rejected threat interpretations. Negative feedback (“You are incorrect.”) was provided when participants endorsed threat interpretations or rejected benign interpretations. The two versions of AIM-FBCR were identical for CBM-I. See online figure.
Control Condition (CC)
The CC followed identical procedures as AIM-FBCR; however, in the CBM-A task, trials included 10 neutral-threat pairs and 10 meaning-threat pairs, and the probe replaced neutral/meaning and threat words with equal frequencies. In the CBM-I task, participants were reinforced 50% of the time for making either benign or threat interpretations.
Personalization and Ideographic Selection
Participants rated (−3 to +3) a pool of words and sentences for how threatening or meaningful they were to them personally prior to their first session. Study staff selected the 20 words rated as most threatening (if receiving AIM-Neutral or CC) or 20 pairs of the most threatening and meaningful words (if receiving AIM-Meaning) to personalize CBM-A. Staff selected the 50 most threatening ambiguous sentences to personalize CBM-I.
Additional Measures
Demographic, medical, and mental health information was assessed pre-intervention, with physical and mental health status updates obtained post-intervention and at follow-up.
Feasibility and Acceptability
We assessed feasibility by measuring the proportions of patients who agreed to be screened, consented, declined participation, or dropped out. Reasons for refusal were assessed. Completers were defined as completing at least 5 of the 8 sessions. Acceptability was evaluated during a post-treatment qualitative exit interview in which participants were asked how satisfied they were with the program overall. Our acceptability target was mostly feedback indicating satisfaction and minimal dissatisfaction.
Primary Outcome (Efficacy of AIM-FBCR vs. CC)
The primary FCR outcome was the CARS,26 a widely-used, reliable 30-item self-report measure that assesses the extent and nature of women’s FCR. Subscales include: Overall Fear (4 items about the frequency/intensity of FCR), Health Worries (11 items about potential medical treatment or declines in physical health), Womanhood Worries (7 items about womanhood, sexuality, romantic relationships, and identity), Role Worries (6 items about responsibilities at work, at home, and with friends and family), and Death Worries (2 items about the possibility of death).26 Items were rated on a scale 1 (no fear) to 6 (intense/frequent fear) for Overall Fear, and from 0 ("Not at all") to 4 ("Extremely”) on all other scales. Mean scores were calculated for each subscale.
Data Analysis
Descriptive statistics were computed to characterize feasibility and acceptability. Differences between subsets of participants (e.g., between treatment conditions) were evaluated using Pearson χ2 tests, Fisher’s exact tests, and t-tests. To evaluate preliminary efficacy, repeated outcomes (e.g., T1, T2, and T3 CARS scores) were entered into Generalized Estimating Equation (GEE)32 models, clustering by participant and including a time main effect (dummy-coded for T2 and T3 with T1 as the reference group), a randomization assignment main effect, and a time by randomization interaction effect. Statistical significance of model terms was evaluated using Wald’s χ2 statistic. Covariates were also included. Standardized Hedges’ g33 was used as a measure of effect size. Data were analyzed according to the intention-to-treat principle.
To evaluate the impact of missing data, analyses were run again with datasets created using multilevel multiple imputation (MI) conducted with the SAS MMI_IMPUTE macro.34,35 Data were imputed at the item level. Results from imputation models were pooled to derive parameter estimates. A reliable change index (RCI) was calculated for FCR.36,37 The proportions of participants demonstrating reliable change in the AIM-FBCR and CC arms were compared using a Pearson χ2 test.4 Associations between changes in cognitive biases and changes in FCR were examined using Pearson’s correlation coefficient.
Post-treatment exit interviews were audio-taped, transcribed, coded, and analyzed by two blinded, independent coders (E.S. and K.R.) using thematic content analysis38 and established techniques39 to achieve consensus about responses related to program satisfaction. Analyses were performed using IBM SPSS version 21 (IBM Corp., Armonk, NY, 2013) and SAS version 9.4 (SAS Inst. Inc., Cary, NC, 2013).
Results
Participant Characteristics
See Table 1 for participant characteristics. Compared to AIM-FBCR participants, CC participants were significantly further out from treatment (p=.036) and had significantly different hormonal treatment histories (p=.028).
Table 1.
Pre-Intervention Participant Characteristics & Group Differences.
| AIM-FBCR n=64 n(%) |
CC n=33 n(%) |
p | |
|---|---|---|---|
| Age (Years; M, SD)a | 55.8(7.4) | 53.9(10.3) | .347 |
| Stage | .380 | ||
| 0 | 6(9.4) | 4(11.5) | |
| I | 29(45.3) | 10(26.9) | |
| II | 27(42.2) | 16(48.5) | |
| III | 2(3.1) | 3(9.1) | |
| Type of Treatment | .765 | ||
| Surgery Only | 6(9.4) | 4(12.1) | |
| Surgery + Radiation | 19(29.7) | 7(21.2) | |
| Surgery + Chemo | 11(17.2) | 6(18.2) | |
| Surgery + Radiation + Chemo | 28(43.8) | 16(48.5) | |
| Hormonal Treatmentc | .028 | ||
| None | 12(18.8) | 7(23.3) | |
| Prior | 5(7.8) | 8(26.7) | |
| Current | 47(73.4) | 15(50.0) | |
| Type of Surgeryc | .884 | ||
| Lumpectomy | 39(60.9) | 19(61.3) | |
| Mastectomy | 15(23.4) | 8(25.8) | |
| Prophylactic Mastectomy | 10(15.6) | 4(12.9) | |
| Time Since Treatment (Years; M, SD) | 3.8(3.0) | 5.4(4.3) | .036 |
| Education | .069 | ||
| High School | 7(10.9) | 9(27.3) | |
| College | 21(32.8) | 12(36.4) | |
| Graduate Degree | 36(56.3) | 12(36.4) | |
| Race/Ethnicityc | .665 | ||
| White | 46(73.0) | 24(75.0) | |
| Black | 7(11.1) | 3(9.4) | |
| Hispanic | 3(4.8) | 4(12.5) | |
| Asian/Pacific Islander | 7(11.1) | 1(3.1) | |
| Relationship Statusa | .060 | ||
| Married | 43(67.2) | 15(46.9) | |
| Divorced | 4(6.3) | 3(9.4) | |
| Single | 10(15.6) | 8(25.0) | |
| Widowed | 1(1.6) | 4(12.5) | |
| Separated | 2(3.1) | 2(6.3) | |
| Cohabitating | 4(6.3) | 0(0.0) | |
| At Least One Childa | 45(70.3) | 24(72.7) | .810 |
Missing 1 response
Missing 2 responses
Missing 3 responses
Study Feasibility and Acceptability
The study appeared feasible, as 57% of individuals approached (n=809/1423) agreed to be screened with the CARS, 26% of individuals screened with the CARS who were eligible (n=110/426) agreed to participate, and 83% of those who began the intervention (n=78/94) completed at least 5 of 8 sessions (M=6.9, SD=2.5). Twelve percent of those enrolled dropped out of the study following consent, and 10% dropped out after their first session. See Figure 1 for reasons for declining. There were no significant demographic differences between completers and non-completers. However, completers scored significantly lower on the CARS Role Worries subscale (p=.019) at T1.
Thematic analysis of the available exit interviews (n=77) revealed that 90% (n=69/77) reported being at least somewhat satisfied with the computer program. Despite this general satisfaction, 52% (n=40/77) suggested a more user-friendly, visually appealing interface such as a mobile phone application.
Efficacy
Patterns of findings in the two versions of AIM-FBCR were similar (ps>.10); thus we collapsed across versions. See online tables for results by treatment arm. Controlling for age, cancer stage, time since treatment, education level, and hormonal treatment history did not impact the significance level of findings below, so unadjusted results are presented.
FCR
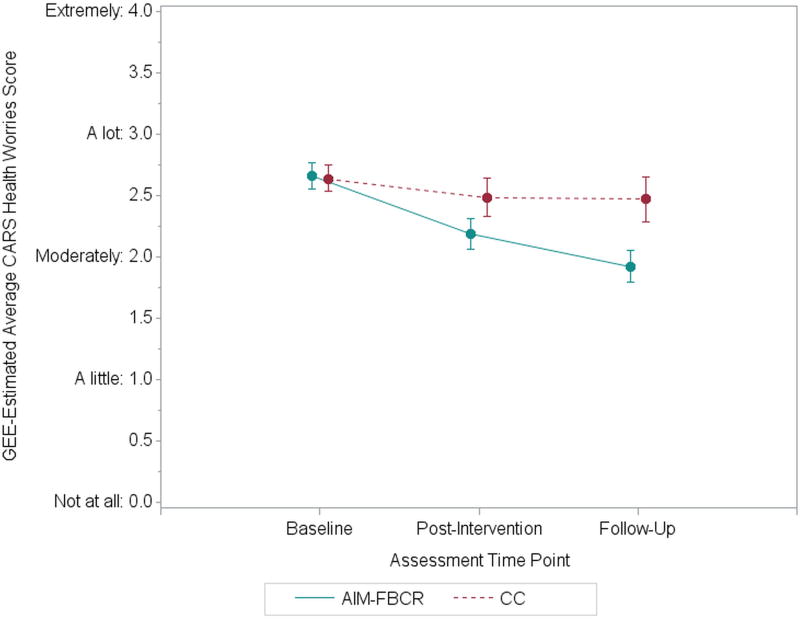
There was a significant Time × Condition interaction for CARS Health Worries scores (p=.019). Specifically, compared with their health worries at T1 (M=2.60, SD=0.89, for AIM-FBCR; M=2.56, SD=0.72, for CC), AIM-FBCR participants experienced somewhat greater reductions at T2 than the CC group (M=2.07, SD=0.91, versus M=2.39, SD=0.99; between-groups g=0.35; p=.095) and significantly greater reductions with a moderate-sized effect at T3 (M=1.83, SD=0.98, versus M=2.36, SD=0.99; between-groups g=0.54; p=.005). See Figure 1 and Table 2. Examining the RCI, 45% (22 of 49) of AIM-FBCR participants demonstrated reliable improvement in health worries from T1 to T3 as compared to 23% (6 of 26) of CC participants (p=.063). When analyses were conducted with MI, improvements in CARS Health Worries for AIM-FBCR relative to CC remained significant at T3.
Table 2.
Effects of AIM-FBCR.
| Attention and Interpretation Modification for Fear of Breast Cancer Recurrence (AIM-FBCR) |
Control Condition (CC) | AIM-FBCR vs. CC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| T1 n=64 M (SD) |
T2 n=50 M (SD) |
T3 n=49 M (SD) |
T1 vs. T2 g |
T1 vs. T3 g |
T1 n=33 M (SD) |
T2 n=28 M (SD) |
T3 n=26 M (SD) |
T1 vs. T2 g |
T1 vs. T3 g |
GEEa T1 vs. T2 β (95% CI) |
T2b g |
GEEa T1 vs. T3 β (95% CI) |
T3b g |
GEEa Time × Treatment χ2 |
|
| Fear of Recurrence (CARS) | |||||||||||||||
| Overall Fearc | 4.29 (1.00) | 3.63 (1.21) | 3.25 (1.00) | 0.61 | 1.04 | 4.40 (1.12) | 3.93 (1.28) | 3.44 (1.22) | 0.39 | 0.82 | 0.13 (−0.28,0.53) | 0.25 | 0.11 (−0.30,0.53) | 0.18 | 0.44 |
| Health Worriesd | 2.60 (0.89) | 2.07 (0.91) | 1.83 (0.98) | 0.59 | 0.83 | 2.56 (0.72) | 2.39 (0.87) | 2.36 (0.99) | 0.23 | 0.24 | 0.32 (−0.04,0.68)† | 0.35 | 0.57 (0.17,0.98)** | 0.54 | 7.95* |
| Womanhood Worriesd | 1.15 (1.01) | 0.79 (0.84) | 0.70 (0.97) | 0.39 | 0.45 | 1.06 (0.91) | 0.95 (0.89) | 0.82 (0.81) | 0.12 | 0.28 | 0.25 (−0.02,0.52)† | 0.20 | 0.21 (−0.12,0.55) | 0.13 | 3.30 |
| Role Worriesd | 1.89 (1.01) | 1.46 (1.03) | 1.33 (1.02) | 0.42 | 0.56 | 1.96 (0.84) | 1.75 (0.89) | 1.69 (0.92) | 0.25 | 0.30 | 0.22 (−0.17,0.60) | 0.29 | 0.36 (−0.02,0.74)† | 0.37 | 3.54 |
| Death Worriesd | 3.02 (1.12) | 2.56 (1.27) | 2.40 (1.30) | 0.39 | 0.52 | 3.26 (0.92) | 3.07 (1.18) | 2.85 (1.24) | 0.18 | 0.38 | 0.21 (−0.25,0.66) | 0.41 | 0.20 (−0.31,0.71) | 0.35 | 0.79 |
| Attention Biase | |||||||||||||||
| Bottom Bias | −6.8 (38.7) | 4.1 (26.7) | 1.3 (23.0) | −0.32 | −0.25 | 2.3 (42.6) | −2.8 (24.7) | 5.9 (29.3) | 0.14 | −0.10 | −16.4 (−38.2,5.3) | −0.27 | −4.8 (−26.0,6.4) | 0.18 | 2.87 |
| Interpretation Biasf | |||||||||||||||
| Benign Endorsement (%) | 73.5 (14.3) | 89.1 (15.0) | 82.4 (19.7) | 1.07 | 0.53 | 75.0 (14.3) | 95.7 (3.2) | 91.0 (9.2) | 1.92 | 1.30 | −5.4 (−12.2,1.5) | −0.55 | −8.1 (−15.7,0.5)* | −0.52 | 4.38 |
| Threat Endorsement (%) | 53.6 (19.4) | 13.7 (16.8) | 17.4 (17.3) | 2.18 | 1.95 | 53.8 (18.7) | 46.3 (24.0) | 41.0 (25.6) | 0.36 | 0.59 | 32.8 (21.2,44.4)** | 1.65 | 23.4 (11.2,35.6)** | 1.14 | 30.96** |
| Benign Endorsement (RT, ms) |
1388 (542) | 529 (422) | 1464 (849) | 1.74 | 0.14 | 1403 (461) | 542 (281) | 1356 (613) | 2.21 | 0.22 | −26 (−179,127) | 0.03 | 34 (−161,228) | −0.14 | 0.81 |
| Threat Rejection (RT, ms) | 1542 (579) | 553 (437) | 814 (479) | 1.89 | 1.35 | 1596 (579) | 934 (356) | 1165 (514) | 1.35 | 0.78 | 320 (120,520)** | 0.93 | 293 (39,547)* | 0.71 | 9.84** |
Abbreviations: T1=Pre-intervention, T2=Post-intervention, T3=3-month follow-up, CARS=Concerns About Recurrence Scale, M=Mean, SD=Standard Deviation, g=Hedges’ g effect size estimate, GEE=Generalized estimating equations, β=Unstandardized regression coefficient for the Time × Treatment interaction at a given time point, CI=Confidence interval, RT=Reaction time.
Note: Effect sizes and β values from GEE results have been transformed such that positive values represent improvement or improvement in AIM-FBCR relative to CC (i.e., increases in benign endorsement and decreases in FCR, attention bias, threat endorsement, benign endorsement reaction time, and threat rejection reaction time).
GEE Wald chi-squared results for the Time × Treatment interactions predicting outcome
Between-group effect sizes comparing AIM-FBCR to CC at post-intervention and 3-month follow-up
Possible scores range from 1 to 6
Possible scores range from 0 to 4
Missing data: 9 T1 (7 AIM-FBCR & 2 CC), 6 T2 (4 AIM-FBCR & 2 CC), 7 T3 (AIM-FBCR)
Missing data: 3 T1 (AIM-FBCR), 3 T2 (AIM-FBCR), 5 T3 (4 AIM-FBCR & 1 CC)
=p<.01,
=p<.05,
=p<.10
AIM-FBCR participants exhibited reductions in the other CARS subscales, as well, though these were not significantly different than reductions for CC participants (ps>.10; between-group gs ranging from 0.20 to 0.41 at post-intervention and 0.13 to 0.37 at follow-up).
Cognitive biases
For the dot probe, inaccurate trials were excluded, and reaction times for both bias assessments were Winsorized.40 There was no significant Time × Condition effect for attention bias. However, there were significant Time × Condition effects for Rate of Threat Endorsement (p<.001) and Reaction Time for Threat Rejection (p=.007). Specifically, compared with their T1 Rate of Threat Endorsement (M=53.6, SD=19.4, for AIM-FBCR; M=53.8, SD=18.7, for CC) and Reaction Time for Threat Rejection (M=1542, SD=579, for AIM-FBCR; M=1596, SD=579, for CC), AIM-FBCR participants experienced greater reductions than the CC group at T2, with large-sized effects in both Rate of Threat Endorsement (M=13.7, SD=16.8, versus M=46.3, SD=24.0; between-groups g=1.65; p<.001) and Reaction Time for Threat Rejection (M=553, SD=437, versus M=934, SD=356; g=0.93; p=.002). Improvements were similar at the T3 assessment (ps<.05). Also of note, improvements in Reaction Time for Threat Rejection at T2 were significantly correlated with improvements in CARS Health Worries (r(73)=.24, p=.042). When analyses were conducted with MI, improvements in Rate of Threat Endorsement for AIM-FBCR relative to CC remained significant at T2 and T3, and improvements in Reaction Time for Threat Rejection were still marginally significant at T2 and no longer significant at T3.
Discussion
The current study evaluated the feasibility, acceptability, and preliminary efficacy of an intervention targeting cognitive biases associated with FCR. In a sample of BCS with at least moderate levels of FCR, AIM-FBCR significantly reduced health worries. Levels of worry in women who received the intervention decreased from high-moderate to less than moderate three months post-intervention, whereas worry levels in women who received the CC remained relatively stable (Figure 1). Although quantitatively small, this improvement is potentially clinically meaningful, especially given the minimal time and financial cost of AIM-FBCR. Additionally, the rate of AIM-FBCR participants reliably improving was approximately two times the rate among CC participants.
Our finding that Health Worries was the only CARS subscale that improved is likely due to the stimuli used to populate the AIM-FBCR intervention, which were threatening health- and treatment-related words and sentences. There may have also been placebo improvement in the CC arm; this seems particularly likely for the Overall Fear subscale, on which the CC group showed substantially more improvement over time than the other subscales. This may be due to its use as a screening measure, which primed responses indicating benefit. Similar patterns were observed in a recent study testing a gratitude intervention, which resulted in improvements in only one of the CARS subscales, Death Worries,10 and not the Overall Fear subscale, suggesting that a strength of the CARS is its ability to truly tap into different aspects of multidimensional FCR.
The hypothesized mechanism of change in CBM interventions is reduction in cognitive biases. Indeed, AIM-FBCR participants demonstrated decreased rates of negative interpretations compared to CC participants. Consistent with previous CBM trials,41,42 however, no significant group differences emerged on the attention bias assessment. Thus, it is possible that observed changes in FCR are mostly due to changes in interpretation biases. Alternatively, it is possible that the program did affect attention, but the dot probe task, which some consider unreliable,43 was not sensitive to this.
Although not as large in magnitude, CC participants also experienced improvements. In addition to general placebo effects, improvements in the CC group may be due to essentially receiving a “diluted” treatment. In effect, they received feedback that they were correct in accepting a benign/rejecting a threatening interpretation in CBM-I or when they were redirected away from threatening stimuli in CBM-A, both of which happened 50% of the time with the CC intervention. Finally, there may be effects resulting from exposure to anxiety-provoking stimuli.
While some metrics of feasibility were strong, such as the majority of participants who started the intervention completing it, only 26% of women who screened in with the CARS agreed to participate. Feasibility was negatively affected by the need to complete one session in the office and the reliance on Windows-based computers. One of the most common participant complaints was technical difficulties with the AIM-FBCR computer program. This was a recognized limitation of this pilot study, and future studies should refine the technology. Nonetheless, findings suggest the potential of a CBM approach in reducing FCR, either alone or as a complement to psychotherapy.44
The results of the current study suggest the promise of AIM-FBCR as a low-cost, easily disseminated, home-delivered intervention with the potential to reduce FCR. Study strengths include the randomized controlled and mixed methods design. Limitations include a small, homogenous sample; potential selection bias due to study refusal, with only approximately one-quarter of women who screened into the study enrolling, and attrition; and reliance on a self-report measure of FCR. In addition, differences among participants who dropped out may have played a role in some of the observed results. Importantly, however, improvement in health worries was robust at follow-up, accounting for missing data.
Future research should attempt to replicate these findings in a larger-scale RCT using a more sophisticated, user-friendly program that can be used on portable devices in diverse samples. It may also be valuable to test a more diverse pool of recurrence-related stimuli to target other domains of FCR. Trials should include clinician-rated and neurobiological measures of improvement (e.g., EEG)45 and may screen for cognitive biases at baseline. Treatment response moderators and dose modifications should also be examined. Finally, given that FCR is one of the greatest concerns for survivors of a variety of cancers,46 if it proves efficacious, this approach could also be adapted for other survivor populations.
Figure 2.
Change in CARS Health Worries over Time. Values represent estimated marginal means from GEE models with a Time × Condition interaction predicting CARS Health Worries. Response scale anchors for CARS Health Worries items are presented on the y-axis. Error bars represent the standard errors of estimated marginal means for each condition at each time point. CARS=Concerns About Recurrence Scale. AIM-FBCR=Attention and Interpretation Modification for Fear of Breast Cancer Recurrence. CC=Control Condition. GEE=Generalized Estimating Equations.
Acknowledgments
FUNDING
Wendy G. Lichtenthal reports grants from the National Institutes of Health Center for Translational Science Center (grant UL1 TR00457 [pilot funding]), the T.J. Martell Foundation, and the National Cancer Institute (grants K07 CA172216 and P30 CA008748) for work performed as part of the current study.
Collaborators on the CTSC grant were Vivian Zayas, Ph.D. and Erik Helzer, Ph.D. Special thanks to Caraline Craig, Maria Farberov, Greta Jankauskaite, Polly Korbel, Corinne Catarozoli, Emine Tanoglu and the invaluable support of Kristen Cognetti, NP, Elizabeth Comen, MD, Maura Dickler, MD, Megan Dunne, ANP, Teresa Gilewski, MD, Shari Goldfarb, MD, Clifford Hudis, MD, Kathleen Keenan, ANP, Shanu Modi, MD, Monica Morrow, MD, FACS, Martha Rodriguez, ANP, Rebecca Steed, NP, Steven Sugarman, MD, and Tiffany Traina, MD.
Footnotes
DISCLOSURES
There are no conflict of interest disclosures from any authors.
AUTHOR CONTRIBUTIONS
Wendy G. Lichtenthal: Conceptualization, methodology, software, formal analysis, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration, and funding acquisition. Geoffrey W. Corner: Conceptualization, formal analysis, data curation, writing–original draft, writing–review and editing, visualization, project administration, and supervision. Elizabeth Slivjak: Software, formal analysis, investigation, data curation, writing–original draft, writing– review and editing, visualization, and project administration. Kailey Roberts: Formal analysis, data curation, writing–original draft, writing–review and editing, and visualization. Yuelin Li: Conceptualization, methodology, formal analysis, writing–review and editing, visualization, supervision, and funding acquisition. William Breitbart: Conceptualization, methodology, writing–review and editing, and funding acquisition. Stephanie Lacey: Data curation, writing–original draft, writing–review and editing, visualization, supervision, and project administration. Malwina Tuman: Software, formal analysis, investigation, data curation, writing–original draft, writing– review and editing, visualization, and project administration. Katherine DuHamel: Conceptualization, methodology, and writing–review and editing. Victoria Blinder: Methodology, formal analysis, data curation, and writing–review and editing. Courtney Beard: Conceptualization, methodology, software, formal analysis, investigation, resources, data curation, writing–review and editing, visualization, supervision, and project administration.
We changed the eligibility criterion from ≥3 months post-treatment to ≥1 year post-treatment due to concerns about treatment-related cognitive side effects impacting study performance. We subsequently changed the criterion back to ≥3 months because experts in neurocognitive functioning in breast cancer survivors indicated performance would be adversely affected by any residual side effects that may be present.
At the beginning of the study, participants completed 144 trials. In August 2013, 13 ambiguous situations were removed from the assessment stimuli in order improve the intervention.
Four participants received 96 or 98 trials due to a system error.
In calculating an individual’s RCI, 2-month test-retest reliability from a study using a Dutch version of the CARS was used as an estimate of each subscale’s reliability.34
References
- 1.Ashing-Giwa KT, Lim JW. Examining emotional outcomes among a multiethnic cohort of breast cancer survivors. Oncology nursing forum. 2011;38(3):279–288. doi: 10.1188/11.ONF.279-288. [DOI] [PubMed] [Google Scholar]
- 2.Johnson Vickberg SM. Fears about breast cancer recurrence: Interviews with a diverse sample. Cancer Practice. 2001;9(5):237–243. doi: 10.1046/j.1523-5394.2001.009005237.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch L, Bertram H, Eberle A, et al. Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psycho-oncology. 2014;23(5):547–554. doi: 10.1002/pon.3452. [DOI] [PubMed] [Google Scholar]
- 4.Tewari A, Chagpar AB. Worry about breast cancer recurrence: a population-based analysis. The American surgeon. 2014;80(7):640–645. [PubMed] [Google Scholar]
- 5.Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. Journal of women's health. 2011;20(9):1307–1313. doi: 10.1089/jwh.2010.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer Journal. 2006;12(5):432–443. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Mast ME. Survivors of breast cancer: illness uncertainty, positive reappraisal, and emotional distress. Oncology nursing forum. 1998;25(3):555–562. [PubMed] [Google Scholar]
- 8.Butow PN, Bell ML, Smith AB, et al. Conquer fear: protocol of a randomised controlled trial of a psychological intervention to reduce fear of cancer recurrence. BMC cancer. 2013;13:201. doi: 10.1186/1471-2407-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheu C, Lebel S, Courbasson C, et al. Protocol of a randomized controlled trial of the fear of recurrence therapy (FORT) intervention for women with breast or gynecological cancer. BMC cancer. 2016;16(1):291. doi: 10.1186/s12885-016-2326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto AK, Szczesny EC, Soriano EC, Laurenceau JP, Siegel SD. Effects of a Randomized Gratitude Intervention on Death-Related Fear of Recurrence in Breast Cancer Survivors. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2016 doi: 10.1037/hea0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors' supportive care needs 2–10 years after diagnosis. Supportive Care in Cancer. 2007;15(5):515–523. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Haim Y, Dominique L, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Ouimet AJ, Gawronski B, Dozois DJA. Cognitive vulnerability to anxiety: A review and integrative model. Clinical Psychology Review. 2009;29:459–470. doi: 10.1016/j.cpr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.DiBonaventura MD, Erblich J, Sloan RP, Bovbjerg DH. A computerized Stroop task to assess cancer-related cognitive biases. Behavioral medicine. 2010;36(2):37–43. doi: 10.1080/08964280903521321. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod C, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behaviour research and therapy. 1992;30(2):151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- 16.Glinder JG, Beckjord E, Kaiser CR, Compas BE. Psychological adjustment to breast cancer: Automatic and controlled responses to stress. Psychology & Health. 2007;22(3):337–359. [Google Scholar]
- 17.Miles A, Voorwinden S, Mathews A, Hoppitt LC, Wardle J. Cancer fear and the interpretation of ambiguous information related to cancer. Cognition and Emotion. 2009;.23(4) [Google Scholar]
- 18.Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: a meta-analytic review. Behavior therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- 20.Beard C. Cognitive bias modification for anxiety: current evidence and future directions. Expert review of neurotherapeutics. 2011;11(2):299–311. doi: 10.1586/ern.10.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beard C, Weisberg RB, Amir N. Combined cognitive bias modification treatment for social anxiety disorder: a pilot trial. Depression and anxiety. 2011;28(11):981–988. doi: 10.1002/da.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch CR, Clark DM, Mathews A. Imagery and interpretations in social phobia: support for the combined cognitive biases hypothesis. Behavior Therarpy. 2006;37(3):223–236. doi: 10.1016/j.beth.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Dear BF, Sharpe L, Nicholas MK, Refshauge K. Pain-related attentional biases: the importance of the personal relevance and ecological validity of stimuli. The Journal of Pain. 2011;12(6):625–632. doi: 10.1016/j.jpain.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Menne-Lothmann C, Viechtbauer W, Hohn P, et al. How to boost positive interpretations? A meta-analysis of the effectiveness of cognitive bias modification for interpretation. PloS. one. 2014;9(6):e100925. doi: 10.1371/journal.pone.0100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickberg SM, Bovbjerg DH, DuHamel KN, Currie V, Redd WH. Intrusive thoughts and psychological distress among breast cancer survivors: global meaning as a possible protective factor. Behav. Med. 2000;25(4):152–160. doi: 10.1080/08964280009595744. [DOI] [PubMed] [Google Scholar]
- 26.Vickberg SM. The Concerns About Recurrence Scale (CARS): a systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav. Med. 2003;25(1):16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 27.Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of abnormal psychology. 2009;118(1):28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price RB, Kuckertz JM, Siegle GJ, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological assessment. 2015;27(2):365–376. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beard C, Amir N. Interpretation in Social Anxiety: When Meaning Precedes Ambiguity. Cognitive therapy and research. 2009;33(4):406–415. doi: 10.1007/s10608-009-9235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard C, Amir N. A multi-session interpretation modification program: changes in interpretation and social anxiety symptoms. Behaviour research and therapy. 2008;46(10):1135–1141. doi: 10.1016/j.brat.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitbart W, Rosenfeld B, Pessin H, Applebaum A, Kulikowski J, Lichtenthal WG. Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(7):749–754. doi: 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. American journal of epidemiology. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 33.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 34.Enders CK. Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- 35.Mistler SA. SAS Global Forum. Vol. 2013. San Francisco, CA: 2013. A SAS® Macro for Applying Multiple Imputation to Multilevel Data. [Google Scholar]
- 36.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 37.van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psycho-oncology. 2008;17(11):1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- 38.Morse JM. Designing funded qualitative research. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks, CA: Sage Publications, Inc; 1994. pp. 220–235. [Google Scholar]
- 39.Hill CE, Knox S, Thompson BJ, Williams EN, Hess SA, Ladany N. Consensual Qualitative Research: An Update. Journal of Counseling Psychology. 2005;52(2):196–205. [Google Scholar]
- 40.Price RB, Kuckertz JM, Siegle GJ, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological assessment. 2014 doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boettcher J, Leek L, Matson L, et al. Internet-based attention bias modification for social anxiety: a randomised controlled comparison of training towards negative and training towards positive cues. PloS one. 2013;8(9):e71760. doi: 10.1371/journal.pone.0071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlbring P, Apelstrand M, Sehlin H, et al. Internet-delivered attention bias modification training in individuals with social anxiety disorder--a double blind randomized controlled trial. BMC psychiatry. 2012;12:66. doi: 10.1186/1471-244X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Frontiers in psychology. 2014;5:1368. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuckertz JM, Amir N, Boffa JW, et al. The effectiveness of an attention bias modification program as an adjunctive treatment for Post-Traumatic Stress Disorder. Behaviour research and therapy. 2014;63:25–35. doi: 10.1016/j.brat.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Toole L, Dennis TA. Attention training and the threat bias: an ERP study. Brain and cognition. 2012;78(1):63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. Journal of Cancer Survivorship. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]