Abstract
Mung bean having high food value and easily digestible proteins, is one of the socioeconomically important crop of India. Among the varied cultivars, Sona mung is having aroma and hence popularly cultivated in the pockets of Ganga river basin at Bhutnir char village of Malda District in the West Bengal state. In the present study, aroma volatiles with special reference to 2-acetyl-1-pyrroline (2AP) were analyzed using HS–SPME–GCMS from Sona mung bean and compared with non-scented mung bean (PHULE M-9339). 26 volatiles in seeds of Sona mung and 20 in non-scented mung bean were identified, in which 3,7-dimethyl-6-octenal, (2E)-2-decen-1-ol, 2-ethyl-1-dodecanol and 3,5,5-trimethyl-2-cyclohexene-1-one are first time reported. 0.19 ± 0.001 ppm 2AP was recorded in Sona mung seeds whereas it was not detected in non-scented mung bean. PCA analysis indicated that 2AP, octanal, 1 pentanol, decanal, phenylmethanol and 2-nonen-1-ol were the major contributors in the aroma of Sona mung bean. The significantly higher level proline, methylglyoxal and lower level of BADH2 transcript were detected in Sona mung than non-scented mung, suggesting similar 2AP biosynthesis mechanism in Sona mung bean as reported in scented rice, sorghum and soybean.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0414-2) contains supplementary material, which is available to authorized users.
Keywords: Vigna radiata, Sona mung bean, Aroma volatiles, 2-Acetyl-1-pyrroline biosynthesis
Introduction
Mung bean or green gram (Vigna radiata (L.) Wilczek), is one of the important pulse crops of India. It is also cultivated as a pulse in Bangladesh, Thailand, Vietnam, Myanmar, Nepal, Laos Kampuchea, Eastern Malaysia, Southern china, Java, Philippines, East Africa or in the American tropics (Vavilov 1926; Zukovskij 1962; Hanelt 2001). Prehistoric records found that mung bean cultivation is pretty old in India (3500-3000 BP) and more than 40 Indian cultivars were domesticated (Hanelt 2001). It is a socioeconomically important legume crop in Asia, especially in India, Thailand and the Philippines. Many researchers have studied the nutritional and beneficial value of mung bean. Its high food value (1.0–1.5% oil, 3.5–4.5% fiber, 4.5–5.5% ash and 62–65% carbohydrates) and easily digestible protein (25–28%) serves as a major source of dietary protein for part of a nutritionally balance diet in the vast majority of people from Asian countries (Poehlman 1991; Fery 2002; Shanmugasundaram 2007). Hence mung bean is taken as an excellent complement to rice in terms of balanced human nutrition (Sharma et al. 2011). In addition to these excellent dietary protein contents, presence of pleasant aroma adds into the quality of mung bean.
In India, among the several traditionally cultivated mung beans and developed varieties, scented mung bean cultivar locally known as ‘Sona mung bean’ (SMB) is popularly cultivated in the pockets of Ganga river basin at Bhutnir char village of Malda District in the West Bengal state Pal et al. (2010) characterized this cultivar for protein (22.5 ± 0.2% w/w), reducing sugar (29.61 ± 0.1% w/w), total lipid (2.18 ± 0.4% w/w), phospholipids (53.055 ± 0.13% w/w) and sterol (3.58 ± 0.6% w/w) content. Also they investigated fatty acid composition of the triglyceride oil and amino acid composition. Brahmacharya and Ghosh (2002) reported the presence of major basmati aroma volatile 2-acetyl-1-pyrroline (2AP) in this cultivar. Characterization of aroma volatiles contributing in aroma in SMB have not yet been worked out. Due to the presence of unique aroma, SMB is an important genetic resource, hence, Indian Council of Agricultural Research (ICAR), New Delhi has initiated breeding program for SMB improvement with various quality parameters (ICAR 2012). Recently Ghosh (2014), conducted distinctness, uniformity and stability test of SMB. Hence, in the present study, SMB is explored for these characteristics. In addition, the biosynthetic mechanism of major aroma volatile 2AP has been discussed.
Materials and methods
Plant material
Seeds of SMB were collected from the local farmers of Bhutnir char locality belonging to Malda district of West Bengal state, India in air tight zip locked bags (Fig. 1). Authentic seeds of NSMB (PHULE M-9339) were procured from local market, Pune, Maharashtra, India. The collected seeds of SMB and NSMB were stored at −20 °C (Vestfrost, USA) until further use. Seedlings of SMB and NSMB were raised and agro-morphological characters were recorded (Supplementary 1).
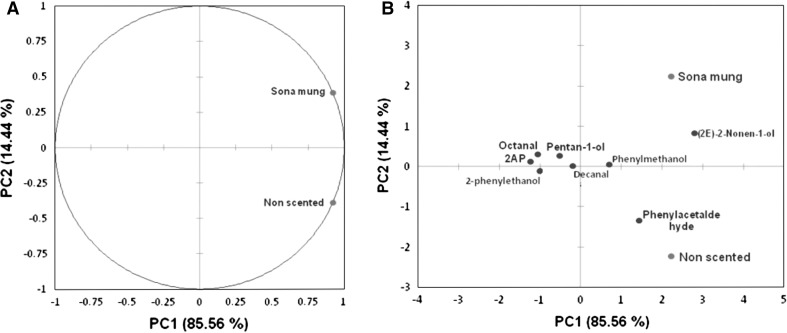
Fig. 1.
PCA plot of SMB and NSMB based on nine major odor active compounds (a), PCA plot showing separation of odor active compounds (b)
Characterization of 2AP by HS-SPME coupled with GC-FID
HS-SPME parameters were optimized with respect to the sample form (intact and powdered seeds), seed weight (750–1750 mg with an interval of 250 mg), water quantity (100–400 µl with an interval of 100 µl), extraction temperature (60–90 °C with an interval of 10 °C), pre-incubation time (20–35 min with an interval of 5 min) and adsorption time (15–30 min with an interval of 5 min) for maximum adsorption of 2AP. Area count of 2AP was considered for optimization of extraction conditions. For the analysis, vigorous and even sized seeds were crushed into fine powder and transferred into 4 ml screw cap vials (15 × 45 mm) with PTFE silicon septa (Chromatography research supplies, Louiseville, KY, USA) and subjected to Head Space—Solid Phase Micro Extraction (HS-SPME) analysis. The carboxen/DVB/PDMS SPME fiber of 1 cm long attached to the manual holder (Supleco, Bellefonte, PA, USA) was selected for analysis based on previous report (Grimm et al. 2001). The SPME fiber was desorbed for 5 min in GC injector (GC 17A, Shimadzu, Japan) having 250 °C temperature with BP-20 capillary column (SGE, Ringwood, Australia) (30 m × 0.32 µm) and flame ionization detector (FID). The GC oven Program was used as follows: oven temp was kept at 50 °C for 1 min, and ramped at the rate 10 °C/min up to 100 °C and 1 min hold, further ramped to 240 °C at the rate 20 °C/min with final hold of 1 min. Analyses was repeated for three times with both accessions. The 2AP peak was identified by matching 2AP standard as a reference material. Quantification of 2AP from the seeds, germinating and vegetative stages of SMB was done following the method of Mathure et al. (2011) and Wakte et al. (2010) respectively from our laboratory.
Characterization of aroma volatiles by HS-SPME coupled with GC–MS
Following the optimized HS-SPME conditions and GC-oven program, aroma volatiles characterization was done in SMB and NSMB. Separation of volatiles was done using GC–MS (Varian 430-GC and 210-MS, Japan) with a Factor Four capillary column VF5-MS, 30 m × 0.25 mm × 0.25 μm of 5% diphenyl—95% dimethyl polysilosane (Varian, Inc., Palo Alto, CA) with research grade helium (99.999%) as the carrier gas under a constant flow of 28.6 cm/s (1 ml/min), in the split less mode coupled with mass spectrometry. The injector temperature was 260 °C and the transfer line was held at 230 °C. The detection was performed by a Saturn III mass spectrometer in the EI mode (ionization energy, 70 eV; source temperature, 180 °C). The MS was operated in the scan mode from m/z 20 to 400. Identification of volatiles was done by the presence of selected ions and their ratio and comparing the MS spectra to the reference spectra in the National Institute of Standards and Technology (NIST, ver. 2.0f, 2008) mass spectral database. Every identified compound was confirmed by matching the retention index (RI) values. Series of n-alkane (C8–C20) was used for calculation of retention indices (Van Den Dool and Kratz 1963). The PCA analysis was performed using mean peak area of 8 major odor active compounds to distinguish SMB and NSMB.
Quantitative analysis of proline and methylglyoxal (MG)
Free proline and MG from leaves of SMB and NSMB at germinating and vegetative stage was determined following the methods (Bates et al. 1973; Yadav et al. 2005). Quantification of MG was done by following the method described by Wild et al. (2012). Quantification of proline and MG was performed in triplicate.
BADH2 gene expression analysis
The total RNA was isolated using Plant RNA isolation kit (Sigma, USA) as per manufacturer’s instructions. mRNA purification was carried out using Oligotex mRNA Mini Kit (Qiagen) as per manufacturer’s instructions and resulting mRNA was further used for first strand cDNA synthesis using ImProm-II™ Reverse Transcription System (Promega, USA) following manufacturer’s instructions. Based on BADH2 gene sequences of rice deposited in NCBI database (XM_015795403), gene specific primers were designed from the conserved sequence of gene. Using these primers, gene specific fragment was amplified and sequenced. Based on these sequences, mung bean specific primers were designed (F: 5′-CTCTTCCCATGGACACATTC-3′ and R: 5′-AAACATGTCACAGATGCCAA-3′) and used for real time expression analysis. SYBR Green Brilliant® II QPCR Master Mix (Stratagene, USA) was used, 20 µl reaction performed in Real time PCR, Real Plex (Eppendorf, Germany). qPCR conditions were kept as follows: initial denaturation at 95 °C for 5 min followed by 35 cycles having denaturation at 95 °C for 1 min, annealing at 57 °C for 30 s and extension for 1 min at 72 °C. qRT-PCR reactions were performed in triplicates and repeated twice. The gene specific amplification and primer specificity of EF1α and BADH2 primers were checked using melt curve analysis and stability and efficiency of primers were analyzed using standard curve (Supplementary 2). The expression of BADH2 was normalized with expression of EF1α gene and ∆ct value was calculated. Based on the ∆ct value fold change in relative gene expression was calculated using 2−∆∆ct method (Livak and Schmittgen 2001).
Results and discussion
Characterization of 2AP by HS-SPME coupled with GC-FID
Among the two different plant material forms, weight and water quantity, powdered seeds of 1.5 g with 300 µl water was found to be the most effective combination for maximum release of 2AP in the headspace. Among the different extraction temperatures and time, 80 °C with 30 min pre-incubation and 20 min adsorption showed maximum release of 2AP (Supplementary 3). Mathure et al. (2011) reported 1 g rice containing 300 μl of water, incubation at 80 °C for 30 min pre-incubation and 20 min adsorption were optimum conditions for 2AP quantification.
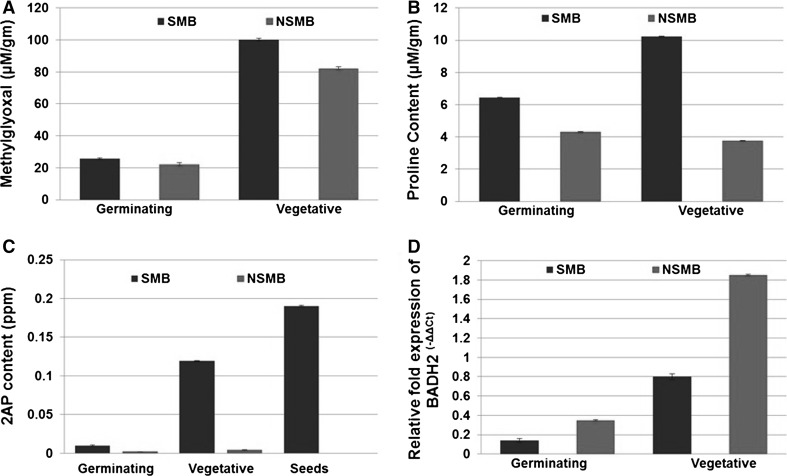
0.19 ± 0.001 ppm 2AP was recorded in SMB seeds whereas 2AP was not detected in NSMB seeds. Also, 2AP content at germinating and vegetative stages in SMB was 0.01 ± 0.001 and 0.119 ± 0.001 ppm respectively, whereas NSMB recorded negligible amount (Fig. 2a). The 2AP content increased with the growth stages and maximum amount was recorded in seeds. Brahmachary and Ghosh (2002) qualitatively detected 2AP in aromatic mung bean seeds but not in ordinary mung bean. 2AP has also been recorded as the key flavor compound in aromatic vegetable soybean (Fushimi and Masuda 2001). Wu et al. (2009) and Arikit et al. (2011a) recorded 0.28–1.16 ppm 2AP in seeds. In scented rice seeds, 2AP content was reported to vary from 0.032 to 0.552 ppm (Mathure et al. 2011).
Fig. 2.
2AP, free proline, MG contents and BADH2 transcript level in SMB and NSMB
Characterization of aroma volatiles by HS-SPME coupled with GC–MS
Total 28 volatiles were recorded in seeds of SMB and NSMB cultivars (Table 1). Amongst them, 26 were detected in SMB and 20 were in NSMB. 8 compounds along with 2AP were specifically detected in SMB. 19 compounds were commonly detected in both the accessions. The volatile compounds were further classified on the basis of their chemical nature (Table 1).
Table 1.
Comparative account of volatiles in SMB and NSMB seeds
| Sr. no. | Compound | Odor description | RI | Mean peak area ± SE | ||
|---|---|---|---|---|---|---|
| Expt. | Ref. | SMB | NSMB | |||
| 1. Aliphatic Aldehyde | ||||||
| 1 | Hexanala | Green, grassy, grass, apple, rancid, fatty | 798 | 796 | 1419.7 ± 134.7 | 897.3 ± 34.7 |
| 2 | Heptanala | Fresh, floral, rancid, fatty, citrus | 904 | 905 | 1132.7 ± 52.9 | ND |
| 3 | Octanala | Citrus, fruity, floral, fatty, soapy, green, oily, fresh | 1004 | 1005 | 1365 ± 60.2 | ND |
| 4 | Nonanala | Strong fruity, floral | 1061 | 1050 | ND | 2357.3 ± 124.6 |
| 5 | Decanala | Sweet waxy, floral | 1198 | 1204 | 2967 ± 148.7 | 875.7 ± 67.5 |
| 6 | (6Z)-6-Nonenal | Muskmelon, fruity | 1152 | 1132 | 542.3 ± 57.4 | 357 ± 39 |
| 7 | 3,7-Dimethyl-6-octenalb | Floral, rosy | 1168 | 1165 | 260.3 ± 31.3 | 142.7 ± 43.7 |
| 2. Aromatic Aldehyde | ||||||
| 1 | Phenylacetaldehyde | Intense, green, floral, rosy | 1040 | 1044 | 3764.7 ± 164.9 | 3120 ± 143.2 |
| 3. Aliphatic Alcohol | ||||||
| 1 | 1-Pentanola | Banana, apricot like | 835 | 800 | 2787.3 ± 103.6 | 433 ± 34 |
| 2 | 1-Hexanola | Vegetal, sticky, fatty | 887 | 888 | 1024 ± 42.7 | 913.7 ± 37.9 |
| 3 | Oct-1-en-3-ola | Fatty, woody, cucumber | 966 | 969 | 1211.7 ± 80.1 | ND |
| 4 | (2E)-2-Nonen-1-ola | Green | 1108 | 1105 | 13,864 ± 977.6 | 2504 ± 193.5 |
| 5 | (2E)-2-Decen-1-olb | Waxy | 1204 | 1197 | 397 ± 28.5 | 239.3 ± 22.4 |
| 6 | 2-Ethyl-1-dodecanolb | Citrus | 1589 | 1591 | 168 ± 12.5 | 124 ± 14 |
| 4. Aromatic Alcohol | ||||||
| 1 | Phenylmethanola | Floral | 1031 | 1036 | 5667.3 ± 263.8 | 1514.3 ± 50.6 |
| 2 | 2-phenylethanol | Soft, like roses | 1125 | 1121 | 310 ± 12.9 | 366.7 ± 50.4 |
| 3 | p-Cymene-8-ol | Herbaceous celery-like | 1173 | 1172 | 772.3 ± 75.6 | 307.3 ± 49.9 |
| 5. Ketone | ||||||
| 1 | 6-Methyl-5-hepten-2-one | Banana like | 984 | 986 | 770 ± 34.3 | ND |
| 2 | 3,5,5-Trimethyl-2-cyclohexene-1-oneb | Peppermint-like | 1130 | 1135 | 460 ± 65.6 | 748 ± 35.6 |
| 3 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | Musty, woody, sweet | 1138 | 1141 | 545.3 ± 33 | 331 ± 38.8 |
| 6. Pyrroline ketone | ||||||
| 1 | 2-Acetyl-1-pyrrolinea | Popcorn, cooked Jasmine, Basmati rice | 924 | 926 | 310.3 ± 26.3 | ND |
| 7. Furanone | ||||||
| 1 | 4-Methyldihydro-2(3H)-furanone | Coconut toasted, nutty, celery | 916 | 909 | 701.3 ± 86.8 | ND |
| 8. N-containing aromatic | ||||||
| 1 | Tetramethylpyrazine | Pungent, walnut, green, nutty, roasty, toasty | 1088 | 1086 | 514.3 ± 53.7 | 336.3 ± 39.1 |
| 2 | 1H-indolea | Floral, sweet, burnt | 1307 | 1304 | 277.7 ± 19.8 | 143 ± 24.6 |
| 9. Ether | ||||||
| 1 | 1,2-dimethoxy-4-methylbenzene | Musty | 1208 | 1209 | 396.3 ± 27.1 | 175.3 ± 2 |
| 10. Alkane | ||||||
| 1 | Tridecane | Gasoline-like | 1313 | 1320 | 248.3 ± 29.8 | ND |
| 2 | 2,4,6-trimethyldecane | – | 1313 | 1320 | ND | 89 ± 6.4 |
| 11. Sesquiterpene hydrocarbon | ||||||
| 1 | δ-Elemene | Fruity, dry | 1364 | 1347 | 125 ± 16.6 | ND |
aCompounds confirmed by authentic standards
bCompounds first time detected in mung bean
RT Retention Time, RI Retention Index, Expt. Experimental value, Ref. Reference value, ND Not detected
2AP, a pyrroline ketone with popcorn like aroma was specifically detected in the SMB seeds (Table 1). Several studies reported that 2AP is biosynthesized naturally as a major aroma compound in rice (Buttery et al. 1983a; Widjaja et al. 1996; Mahatheeranont et al. 2001; Yoshihashi 2002), Pandanus amaryllifolius Roxb. (Buttery et al. 1983b), Bassia latifolia Roxb. (Midya and Brahmachary 1996), Vallaris glabra Ktze. (Wongpornchai et al. 2003), soybean (Fushimi and Masuda 2001; Wu et al. 2009), mung bean (Brahmachary and Ghosh 2002), sorghum (Ayyanger 1938; Yundaeng et al. 2013) and cucumber (Pramnoi et al. 2013). Presence of 2AP in SMB, confirmed as the major aroma compound.
Among the 7 aliphatic aldehydes, heptanal and octanal were specifically detected in SMB. Hexanal, decanal, (6Z)-6-nonenal and 3,7-dimethyl-6-octenal were identified in both SMB and NSMB but elevated in SMB. Nonanal was detected only in NSMB (Table 1). Phenylacetaldehyde is an aromatic aldehyde, was observed to be increased content in SMB than the NSMB (Table 1). 3,7-Dimethyl-6-octenal or Citronellal was reported first time in mung bean. It is monoterpenoid compound, naturally occurs in essential oils (Singh et al. 2008). Remaining aliphatic aldehydes were reported earlier in other mung bean cultivars (Lovergren et al. 1979; Lee and Shibamoto 2000). Aldehydes are the lipid oxidation products and can produce a wide range of flavors and odors (Sun et al. 2010). Among these aldehyde compounds, hexanal, heptanal, octanal, nonanal, decanal and phenylacetaldehyde were identified as odor-active compounds, responsible for aroma in scented rice varieties (Yang et al. 2008; Mathure et al. 2014; Hinge et al. 2015). In SMB, heptanal and octanal were specifically detected, whereas hexanal, decanal, (6Z)-6-nonenal, 3,7-dimethyl-6-octenal and phenylacetaldehyde were present in elevated amount than NSMB. This indicates that aliphatic aldehydes compounds contributed in the pleasant aroma of SMB.
Among all aliphatic and aromatic alcohols, oct-1-en-3-ol detected only in SMB and remaining alcohols were commonly detected in both the cultivars. 1-pentanol, 1-hexanol, (2E)-2-nonen-1-ol, phenylmethanol and p-cymene-8-ol showed significantly increased peak area in SMB than NSMB. Therefore these alcohols might be contributing into the aroma of SMB. (2E)-2-Decen-1-ol and 2-ethyl-1-dodecanol are first time reported in mung bean seeds, in which (2E)-2-decen-1-ol with Waxy type odor and 2-ethyl-1-dodecanol with citrus type odor were reported earlier in natural plant systems (Eyres et al. 2005). Most of alcohol compounds were previously reported in rice (Buttery et al. 1988, 1999; Yang et al. 2008; Mathure et al. 2014).
Ketone containing compound, 6-methyl-5-hepten-2-one was detected only in SMB. It was reported earlier in rice and other fruit crops (Bryant and McClung 2011; Oomah et al. 2014; Hinge et al. 2015). 2,6,6-Trimethyl-2-cyclohexene-1,4-dione was commonly detected in both mung beans and previously reported in aromatic rice (Bryant and McClung 2011). Similarly, 3,5,5-trimethyl-2-cyclohexene-1-one was first time commonly detected in both mung beans. 4-Methyldihydro-2(3H)-furanone was detected only in SMB and may be associated with its aroma.
Among N-containing aromatic compounds, tetramethylpyrazine and 1H-indole were detected in both with higher in SMB (Table 1). Tetramethylpyrazine is naturally exists in different food and fermented foods products (Buttery and Ling 1995; Buttery et al. 1999; Fan et al. 2012). It was firstly isolated from cultures of a strain of Bacillus subtilis, having characteristic smell of fermented soybean and it is the favourite food as ‘natto’ among Japanese people (Kosuge and Kamiya 1962). The Flavor and Extract Manufacturers Association (FEMA) recognized it as a safe compound for its use in the food industry to enhance the flavour of the products (Xiao et al. 2014). Hence, along with 2AP (pyrroline ketone) N-containing aromatic compounds could be taken as major contributors in the aroma of SMB seeds. 1,2-dimethoxy-4-methylbenzene belonging to ether class was detected in both the cultivars with no significant content difference.
Among the 2 alkane compounds, tridecane was observed only in SMB and 2,4,6-trimethyldecane in NSMB (Table 1). These compounds were previously reported in raw or cooked aromatic rices. Alkane compounds were unable to add any contribution towards aroma in rice grains (Bryant and McClung 2011). Uneven distribution of alkane confirmed their inability towards aroma. Sesquiterpene hydrocarbon, δ-Elemene was only observed in SMB which was reported previously in aromatic rice but its contribution in aroma is not yet confirmed (Liu et al. 2008).
The aroma profile is one of the most important characteristics of various food products (Careri et al. 1993; Virgili et al. 1994). Among the volatile compounds contributing in aroma, specific compounds have potential role to distinguishing cultivars. In SMB, along with 2AP, heptanal, octanal, oct-1-en-3-ol, 6-methyl-5-hepten-2-one, 4-methyldihydro-2(3H)-furanone, tridecane and δ-elemene were specifically detected hence these could be considered as the aroma volatiles distinguishing SMB from NSMB. Decanal, 1-pentanol, 1-hexanol, p-cymene-8-ol, tetramethylpyrazine and 1,2-dimethoxy-4-methylbenzene were found synthesized at significantly higher levels in SMB and thus contribute towards aroma in SMB.
Differentiation of SMB from NSMB based on PCA analysis
Based on reported odor active values, 8 major compounds including 2AP were considered for the PCA analysis (Fig. 1). The PCA analysis revealed that 8 major volatiles could distinguish SMB from NSMB with 85.56 and 14.44% variance (Fig. 1a). SMB was separated into positive side of PC1 and PC2 whereas NSMB was placed into positive quadrant in PC1 and negative side of PC2. At individual compound level, 2AP, octanal, 1-pentanol, decanal, phenylmethanol and (2E)-2-nonen-1-ol contributed in the separation of SMB and placed it at positive side of the PC2 (Fig. 1b). 2-Phenylethanol and phenylacetaldehyde were loaded into PC1 and were the contributors for separation of NSMB from SMB. Thus, the analysis highlighted that 2AP, octanal, 1-pentanol, decanal, phenylmethanol and (2E)-2-nonen-1-ol were the major contributors for aroma in SMB.
Biosynthetic mechanism of 2AP
Quantitative analysis of proline and MG
The free proline was found to be higher in SMB at germination and vegetative stages (6.457 ± 0.004 and 10.224 ± 0.001 µM/g respectively) than in NSMB (4.305 ± 0.004 and 3.767 ± 0.004 µM/g respectively) (Fig. 2b). In these two stages, 2AP contents were also higher. It is now widely accepted that proline is the precursor of 2AP (Yoshihashi et al. 2002; Kaikavoosi et al. 2015). The higher content of proline in vegetative stage in SMB suggests its role as precursor for the synthesis of 2AP in SMB.
MG content was also followed the same trend as that of proline where its higher contents were recorded in SMB (25.8 ± 0.004 and 100.2 ± 0.024 µM/g) than NSMB (22.2 ± 0.016 and 82.2 ± 0.003 µM/g) at germination and vegetative stages respectively (Fig. 2c). Wu et al. (2009) reported significantly higher MG level in scented soybean than that of non-scented. Similarly, Huang et al. (2008) reported higher level of MG in scented rice calli than that of non-scented rice calli. Huang et al. (2007) demonstrated that MG reacts with P5C, to form 2AP. In the present study, higher levels of MG might be contributing towards higher levels of 2AP in SMB.
BADH2 gene expression analysis
In NSMB, 0.35 and 1.85 fold increase in BADH2 transcript levels was recorded at germination and vegetative stage respectively than SMB (0.14 and 0.80) (Fig. 2d). In these stages, inverse correlation between BADH2 transcript abundance and 2AP was recorded. 2AP biosynthesis studies in fragrant rice, Soybean and Sorghum have very well demonstrated that non-functionality of BADH2 leads in the synthesis of 2AP (Bradbury et al. 2005; Wu et al. 2009; Yundaeng et al. 2013). Fitzgerald et al. (2008) reported that the BADH2 expression was higher in non-fragrant rice varieties than fragrant genotypes. Arikit et al. (2011b) demonstrated that the expression levels of GmAMADH2 in soybean were much lower in aromatic varieties than non-aromatic ones. These reports clearly establish recessive nature of BADH2 fragrant allele in all 2AP synthesized species and can also be applicable to SMB.
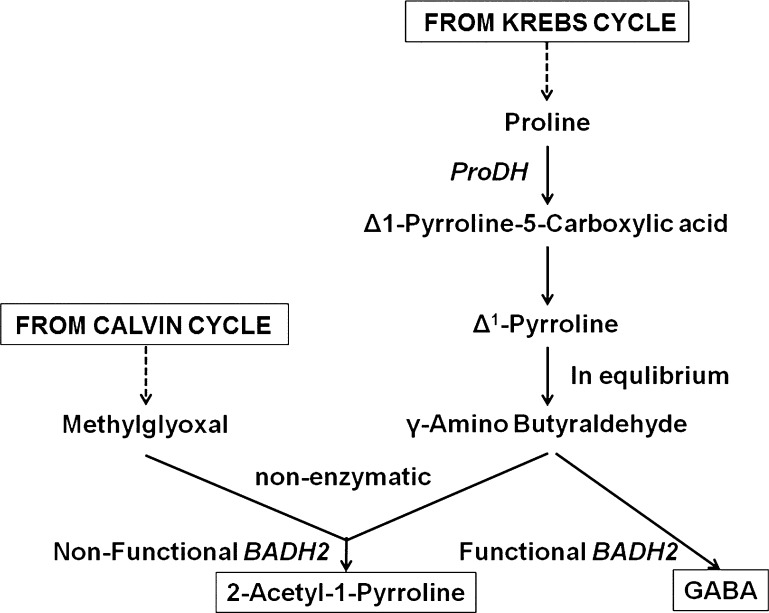
Recently 2AP biosynthetic mechanism has been proposed by several researchers in scented rice, soybean and sorghum (Bradbury et al. 2005; Fitzgerald et al. 2008, Wu et al. 2009; Zanan et al. 2016). As per their reports, non-functionality of BADH2 leads in the accumulation of GABald/Δ1-pyrroline which non-enzymatically reacts with MG to form 2AP (Bradbury et al. 2008; Chen et al. 2008; Kaikavoosi et al. 2015). MG is derived from dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P) in the glycolytic pathway (Phillips and Thornalley 1993). Higher levels of proline, methylgloxyl and lower expression of BADH2 in SMB suggest similar mechanism of biosynthesis in SMB (Fig. 3). According to Pal et al. (2010) in SMB, 2AP is produced through Maillard reaction than enzymatic pathway. It is well known fact that the Maillard reaction proceeds rapidly from around 140 to 165 °C. Our present studies rules out this possibility as 2AP quantification was done at much below temperature. In addition, sensory test done at room temperature using 1.7% KOH (Sood and Siddiq 1978), recorded 2AP in SMB seeds against standard 2AP (data not shown).
Fig. 3.
Proposed biosynthetic mechanism of 2AP in SMB
SMB is an important genetic resource, cultivated in small pockets, hence demands urgent need of preservation. For this the farmers need to be promoted for large scale cultivation. It is the only cultivar that biosynthesized 2AP, and having similar mechanism like rice, sorghum and soybean hence, characterization of BADH2 gene responsible for 2AP biosynthesis needs to be done.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to Dr. P. Srinivas (Central Food and Technology Research Institute, Mysore, India) for generous gift of authentic 2AP.
References
- Arikit S, Yoshihashi T, Wanchana S, Tanya P, Juwattanasomran R, Srinives P, Vanavichit A. A PCR-based marker for a locus conferring aroma in vegetable soybean (Glycine max L.) Theor Appl Genet. 2011;122:311–316. doi: 10.1007/s00122-010-1446-y. [DOI] [PubMed] [Google Scholar]
- Arikit S, Yoshihashi T, Wanchana S, Uyen TT, Huong NTT, Wongpornchai S, Vanavichit A. Deficiency in the amino aldehyde dehydrogenase encoded by GmAMADH2, the homologue of rice Os2AP, enhances 2-acetyl-1-pyrroline biosynthesis in soybeans (Glycine max L.) Plant Biotechnol J. 2011;9(1):75–87. doi: 10.1111/j.1467-7652.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- Ayyanger GRR. Studies in Sorghum. J Madras Univ. 1938;11:131–143. [Google Scholar]
- Bates L, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradbury LMT, Henry RJ, Jin Q, Russell FRF, Waters DLE. A perfect marker for fragrance genotyping in rice. Mol Breed. 2005;16:279–283. doi: 10.1007/s11032-005-0776-y. [DOI] [Google Scholar]
- Bradbury LMT, Gillies SA, Brushett DJ, Waters DLE, Henry RJ. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Biol. 2008;68:439–449. doi: 10.1007/s11103-008-9381-x. [DOI] [PubMed] [Google Scholar]
- Brahmachary RL, Ghosh M. Vaginal pheromone and other unusual compounds in mung bean aroma. J Sci Ind Res. 2002;61:625–629. [Google Scholar]
- Bryant RJ, McClung AM. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011;124:501–513. doi: 10.1016/j.foodchem.2010.06.061. [DOI] [Google Scholar]
- Buttery RG, Ling LC. Volatile flavor components of corn tortillas and related products. J Agric Food Chem. 1995;43:1878–1882. doi: 10.1021/jf00055a023. [DOI] [Google Scholar]
- Buttery RG, Juliano BO, Ling LC. Identification of rice aroma compound 2-acetyl-1-pyrroline in Pandan leaves. Chem Ind. 1983;23:478. [Google Scholar]
- Buttery RG, Ling LC, Juliano BO, Turnbaugh JG. Cooked rice aroma and 2-Acetyl- 1-pyrroline. J Agric Food Chem. 1983;31:823–826. doi: 10.1021/jf00118a036. [DOI] [Google Scholar]
- Buttery RG, Turnbaugh JG, Ling LC. Contributions of volatiles to rice aroma. J Agric Food Chem. 1988;36:1006–1009. doi: 10.1021/jf00083a025. [DOI] [Google Scholar]
- Buttery RG, Orts WJ, Takeoka GR, Nam Y. Volatile flavor components of rice cakes. J Agric Food Chem. 1999;47:4353–4356. doi: 10.1021/jf990140w. [DOI] [PubMed] [Google Scholar]
- Careri M, Mangia A, Barbieri G, Bolzoni L, Virgili R, Parolari G. Sensory property relationships to chemical data of Italian-type dry-cured ham. J Food Sci. 1993;58:968–972. doi: 10.1111/j.1365-2621.1993.tb06090.x. [DOI] [Google Scholar]
- Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, Cheng Z, Liu X, Xu M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20(7):1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyres G, Dufour JP, Hallifax G, Sotheeswaran S, Marriott PJ. Identification of character-impact odorants in coriander and wild coriander leaves using gas chromatography-olfactometry (GCO) and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC x GC-TOFMS) J Sep Sci. 2005;28(9–10):1061–1074. doi: 10.1002/jssc.200500012. [DOI] [PubMed] [Google Scholar]
- Fan W, Xu Y, Qian MC (2012) Identification of aroma compounds in Chinese “Moutai” and “Langjiu” liquors by normal phase liquid chromatography fractionation followed by gas chromatography/olfactometry. In: Qian MC, Shellhammer TH (eds) Flavor chemistry of wine and other alcoholic beverages, American Chemical Society publication, Washington DC, 1104, pp 303–338
- Fery RL. New opportunities in Vigna. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria: ASHS press; 2002. pp. 424–428. [Google Scholar]
- Fitzgerald TL, Waters DLE, Henry RJ. The effect of salt on betaine aldehyde dehydrogenase transcript levels and 2-acetyl-1-pyrroline concentration in fragrant and non-fragrant rice (Oryza sativa) Plant Sci. 2008;175:539–546. doi: 10.1016/j.plantsci.2008.06.005. [DOI] [Google Scholar]
- Fushimi T, Masuda R (2001) 2-acetyl-1-pyrroline concentration of the aromatic vegetable soybean “Dadacha-Mame”. In: Lumpkin T, Shanmugasundaram S (eds) Proceeding of the 2nd international vegetable soybean conference. Washington State University, Pullman, p 39
- Ghosh M. Preserving an indigenous cultivar. Curr Sci. 2014;107(12):1945. [Google Scholar]
- Grimm CC, Bergman C, Delgado JT, Bryant R. Screening for 2-Acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. J Agric Food Chem. 2001;49:245–249. doi: 10.1021/jf0008902. [DOI] [PubMed] [Google Scholar]
- Hanelt P. Institute of plant genetics and crop plant research, mansfeld’s encyclopedia of agricultural and horticultural crops, 1-5. Berlin: Springer; 2001. [Google Scholar]
- Hinge V, Patil H, Nadaf A. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl Biochem Biotechnol. 2015;178(4):619–639. doi: 10.1007/s12010-015-1898-2. [DOI] [PubMed] [Google Scholar]
- Huang TC, Huang YW, Hung HJ, Ho CT, Wu ML. Δ1-Pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J Agric Food Chem. 2007;55:5097–5102. doi: 10.1021/jf0700576. [DOI] [PubMed] [Google Scholar]
- Huang TC, Teng CS, Chang JL, Chuang HS, Ho CT, Wu ML. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J Agric Food Chem. 2008;56:7399–7404. doi: 10.1021/jf8011739. [DOI] [PubMed] [Google Scholar]
- ICAR (2012). All India coordinated research project on MULLaRP. In: Proceedings and recommendations, annual group meet on mungbean and urdbean, Indian council of agricultural research. http://www.aicrpmullarp.res.in/pdf/kharif_12_13.pdf. Accessed 25 Mar 2016
- Kaikavoosi K, Kad TD, Zanan RL, Nadaf AB. 2-Acetyl-1-Pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) gene transformation. Appl Biochem Biotech. 2015;177(7):1466–1479. doi: 10.1007/s12010-015-1827-4. [DOI] [PubMed] [Google Scholar]
- Kosuge T, Kamiya H. Discovery of a pyrazine in a natural product: tetramethylpyrazine from cultures of a strain of Bacillus subtilis. Nature. 1962;193:776. doi: 10.1038/193776a0. [DOI] [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J Agric Food Chem. 2000;48:4290–4293. doi: 10.1021/jf000442u. [DOI] [PubMed] [Google Scholar]
- Liu T, Fan W, Xu Y. Characterization of volatile and semi-volatile compounds in Chinese rice wines by headspace solid phase microextraction followed by gas chromatography—mass spectrometry. J Inst Brew. 2008;114(2):172–179. doi: 10.1002/j.2050-0416.2008.tb00323.x. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovergren NV, Fisher GS, Legendre MG, Schuller WH. Volatile constituents of dried legumes. J Agric Food Chem. 1979;27(4):851. doi: 10.1021/jf60224a055. [DOI] [Google Scholar]
- Mahatheeranont S, Keawsaard S, Dumri K. Quantification of the rice aroma compound, 2-Acetyl-1-pyrroline, in uncooked Khao Dawk Mali 105 brown rice. J Agric Food Chem. 2001;49(2):773–779. doi: 10.1021/jf000885y. [DOI] [PubMed] [Google Scholar]
- Mathure SV, Wakte KV, Jawali N, Nadaf AB. Quantification of 2-Acetyl-1-pyrroline and other rice aroma volatiles among Indian scented rice cultivars by HS-SPME/GC-FID. Food Anal Method. 2011;4(3):326–333. doi: 10.1007/s12161-010-9171-3. [DOI] [Google Scholar]
- Mathure SV, Jawali N, Thengane RJ, Nadaf AB. Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food Chem. 2014;142:383–391. doi: 10.1016/j.foodchem.2013.07.066. [DOI] [PubMed] [Google Scholar]
- Midya S, Brahmachary R. The aroma of Bassia flower. Curr Sci. 1996;71:430. [Google Scholar]
- Oomah BD, Razafindrainibe M, Drover JC. Headspace volatile components of Canadian grown low-tannin faba bean (Vicia faba L.) genotypes. J Sci Food Agric. 2014;94(3):473–481. doi: 10.1002/jsfa.6272. [DOI] [PubMed] [Google Scholar]
- Pal M, Brahmachary RL, Ghosh M. Comparative studies on physicochemical and biochemical characteristics of scented and non-scented strains of mung Beans (Vigna radiata) of Indian origin. Legum Res. 2010;33(1):1–9. [Google Scholar]
- Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem. 1993;212:101–105. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
- Poehlman JM. The mungbean. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.; 1991. [Google Scholar]
- Pramnoi P, Somta P, Chankaew S, Juwattanasomran R, Srinives P. A single recessive gene controls fragrance in cucumber (Cucumis sativus L) J Genet. 2013;92:147–149. doi: 10.1007/s12041-013-0228-0. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram S. Exploit mungbean with value added products. Acta Hortic. 2007;752:99–102. doi: 10.17660/ActaHortic.2007.752.12. [DOI] [Google Scholar]
- Sharma OP, Bambawale OM, Gopali JB, Bhagat S, Yelshetty S, Singh SK, Anand R, Singh OP (2011) Field guide mungbean and urdbean, National Centre for Integrated Pest Management 2011. http://www.ncipm.org.in/A3P/UI/Home/publish/Field%20Guide.pdf. Assessed 20 Dec 2015
- Singh N, Kaul VK, Megeji NW, Singh V, Ahuja PS. Essential oil composition of three accessions of Dracocephalum heterophyllum Benth. Cultivated at Palampur, India. Nat Prod Res. 2008;22(11):927–936. doi: 10.1080/14786410701642847. [DOI] [PubMed] [Google Scholar]
- Sood BC, Siddiq EA. A rapid technique for scent determination in rice. Indian J Genet Plant Breed. 1978;38(2):268–275. [Google Scholar]
- Sun W, Zhao Q, Zhao H, Zhao M, Yang B. Volatile compounds of Cantonese sausage released at different stages of processing and storage. Food Chem. 2010;121(2):319–325. doi: 10.1016/j.foodchem.2009.12.031. [DOI] [Google Scholar]
- Van Den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- Vavilov NI. Studies on the origin of cultivated plants. Leningrad: Institute of Applied Botany and Plant Breeding; 1926. [Google Scholar]
- Virgili R, Parolari G, Bolzoni L, Barbieri G, Mangia A, Careri M, Spagnoli S, Panari G, Zannoni M. Sensory-chemical relationships in Parmigiano-Reggiano cheese. LWT Food Sci Technol. 1994;27:491–495. doi: 10.1006/fstl.1994.1098. [DOI] [Google Scholar]
- Wakte KV, Thengane RJ, Jawali N, Nadaf AB. Optimization of HS-SPME conditions for quantification of 2-acetyl-1-pyrroline and study of other volatiles in Pandanus amaryllifolius Roxb. Food Chem. 2010;121:595–600. doi: 10.1016/j.foodchem.2009.12.056. [DOI] [Google Scholar]
- Widjaja R, Craske JD, Wootton M. Comparative studies on volatile components of non-fragrant and fragrant rices. J Sci Food Agric. 1996;70(2):151–161. doi: 10.1002/(SICI)1097-0010(199602)70:2<151::AID-JSFA478>3.0.CO;2-U. [DOI] [Google Scholar]
- Wild R, Ooi L, Srikanth V, Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-l-cysteine assay. Anal Bioanal Chem. 2012;403:2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- Wongpornchai S, Sriseadka T, Choonvisase S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze) J Agric Food Chem. 2003;51:457–462. doi: 10.1021/jf025856x. [DOI] [PubMed] [Google Scholar]
- Wu ML, Chou KL, Wu CR, Chen JK, Huang TC. Characterization and the possible formation mechanism of 2-Acetyl-1-Pyrroline in aromatic vegetable soybean (Glycine max L.) J Food Sci. 2009;74(5):192–197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Hou X, Lyu X, Xi L, Zhao J. Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate. Biotech Biofuels. 2014;7:106. doi: 10.1186/1754-6834-7-106. [DOI] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun. 2005;337:61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Yang DS, Shewfelt RL, Lee KS, Kays SJ. Comparison of odor-active compounds from six distinctly different rice flavor types. J Agric Food Chem. 2008;56(8):2780–2787. doi: 10.1021/jf072685t. [DOI] [PubMed] [Google Scholar]
- Yoshihashi T. Quantitative analysis on 2-acetyl-1-pyrroline of aromatic rice by stable isotope dilution method and model studies on its formation during cooking. J Food Sci. 2002;67(2):619–622. doi: 10.1111/j.1365-2621.2002.tb10648.x. [DOI] [Google Scholar]
- Yoshihashi T, Huong NTT, Inatomi H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J Agric Food Chem. 2002;50:2001–2004. doi: 10.1021/jf011268s. [DOI] [PubMed] [Google Scholar]
- Yundaeng C, Somta P, Tangphatsornruang S, Wongpornchai S, Srinives P. Gene discovery and functional marker development for fragrance in sorghum (Sorghum bicolor (L.) Moench) Theor Appl Genet. 2013;26(11):2897–2906. doi: 10.1007/s00122-013-2180-z. [DOI] [PubMed] [Google Scholar]
- Zanan R, Khandagale K, Hinge V, Elangovan M, Henry FJ, Nadaf A. Characterization of fragrance in sorghum (Sorghum bicolor (L.) Moench) grain and development of a gene-based marker for selection in breeding. Mol Breed. 2016;36:146. doi: 10.1007/s11032-016-0582-8. [DOI] [Google Scholar]
- Zukovskij PM. Cultivated plants and their wild relatives. London: Commonwealth Agriculture Bureau; 1962. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.