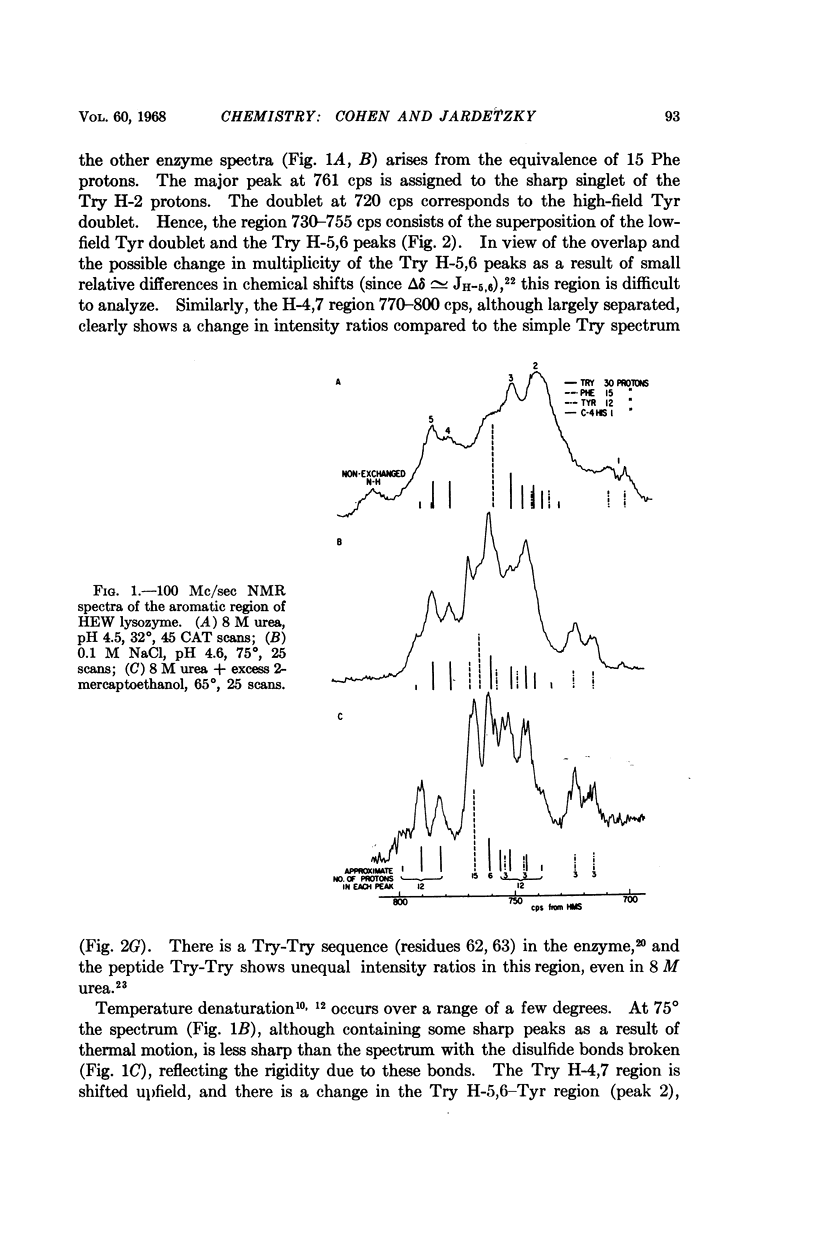
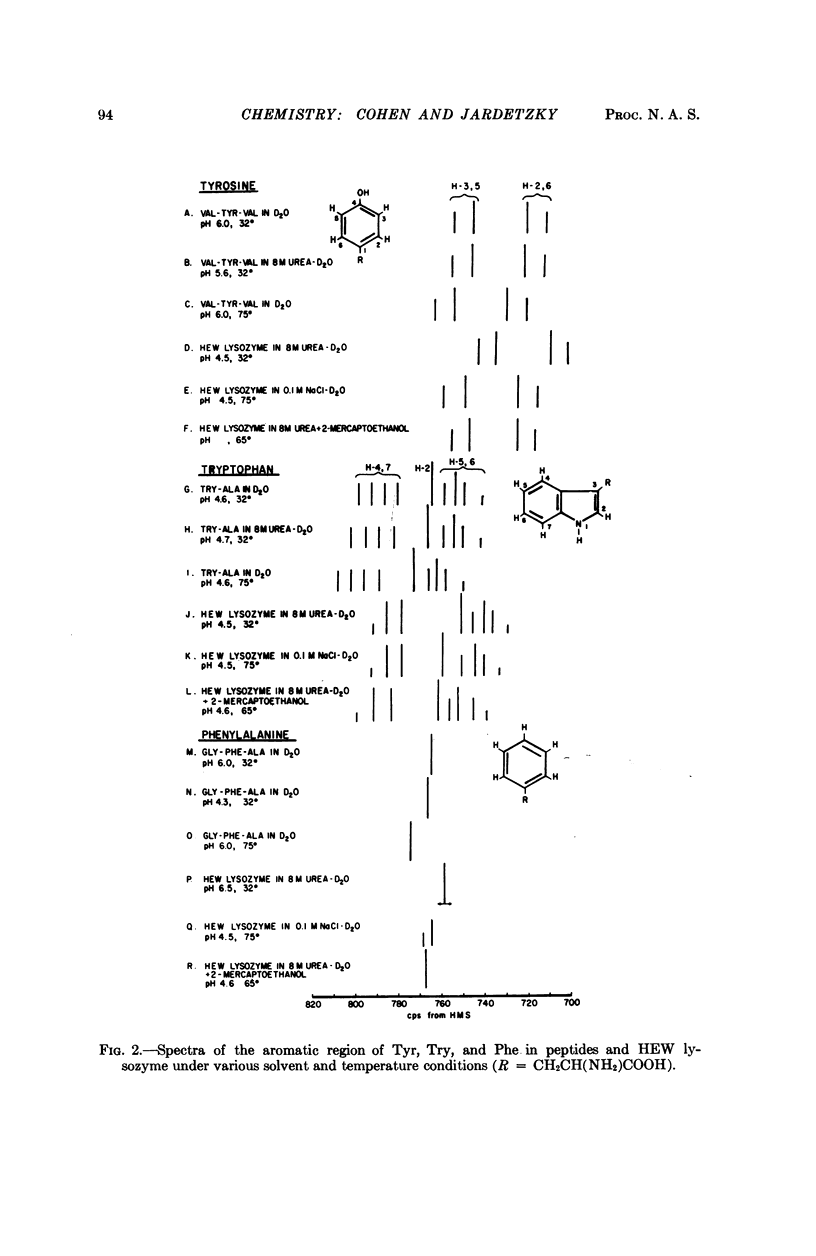
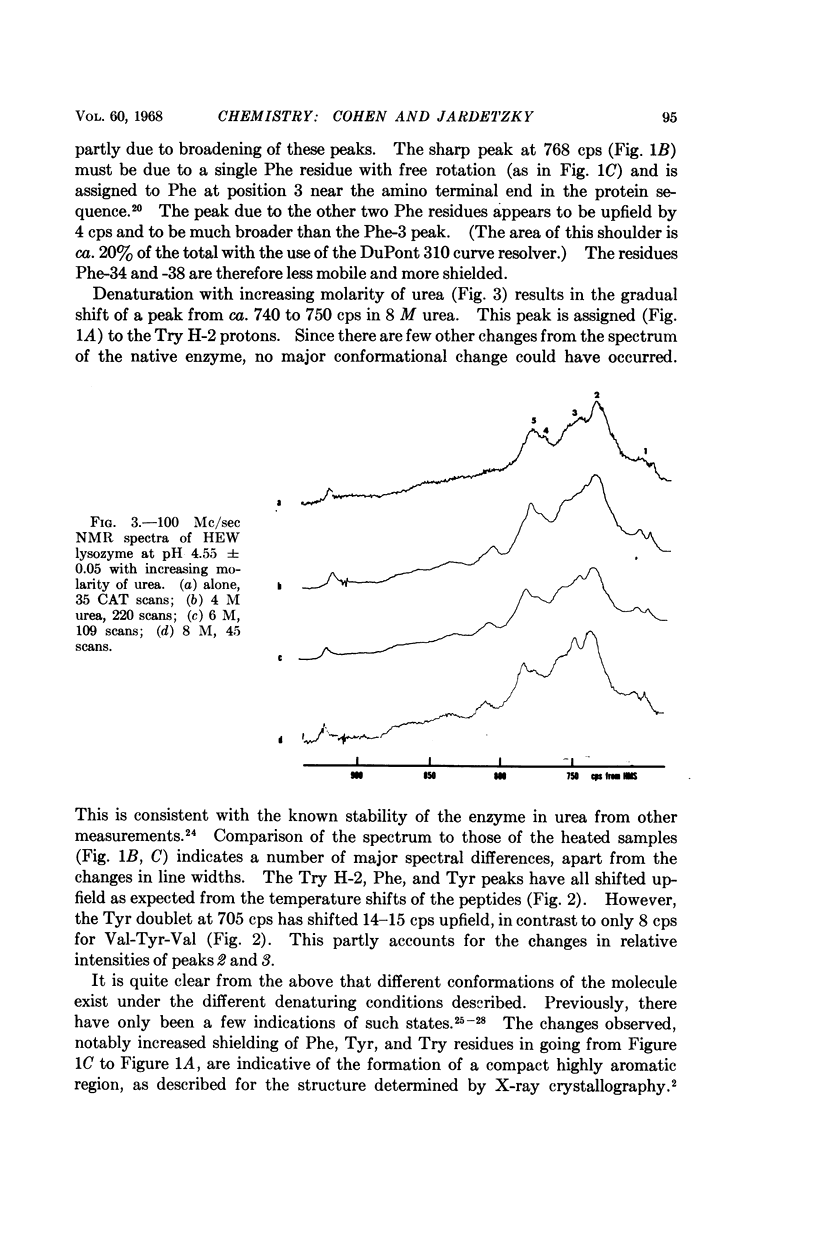
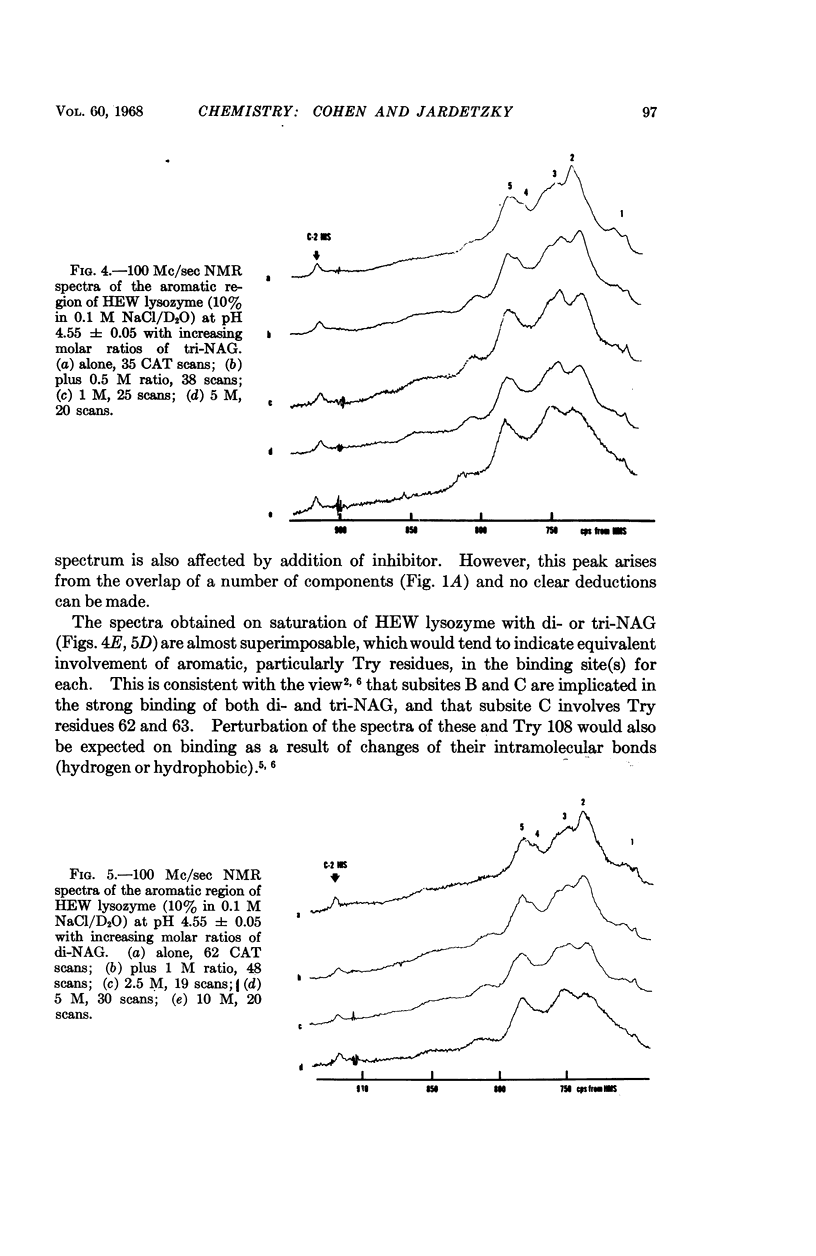
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E. Studies on the reduction and re-formation of protein disulfide bonds. J Biol Chem. 1961 May;236:1361–1363. [PubMed] [Google Scholar]
- Bak B., Pedersen E. J., Sundby F. Proton magnetic resonance spectra of porcine and bovine insulin and of the A and B chain of bovine insulin. J Biol Chem. 1967 Jun 10;242(11):2637–2645. [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Bradbury J. H., Wilairat P. Proton magnetic resonance spectroscopy of histidine residues in proteins. Biochem Biophys Res Commun. 1967 Oct 11;29(1):84–89. doi: 10.1016/0006-291x(67)90545-1. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- Chipman D. M., Grisaro V., Sharon N. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J Biol Chem. 1967 Oct 10;242(19):4388–4394. [PubMed] [Google Scholar]
- Dahlquist F. W., Jao L., Raftery M. On the binding of chitin oligosaccharides to lysozyme. Proc Natl Acad Sci U S A. 1966 Jul;56(1):26–30. doi: 10.1073/pnas.56.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMAGUCHI K., KURONO A. STRUCTURE OF MURAMIDASE (LYSOZYME). I. THE EFFECT OF GUANIDINE HYDROCHLORIDE ON MURAMIDASE. J Biochem. 1963 Aug;54:111–122. [PubMed] [Google Scholar]
- Hammes G. G., Swann J. C. Influence of denaturing agents on solvent structure. Biochemistry. 1967 Jun;6(6):1591–1596. doi: 10.1021/bi00858a004. [DOI] [PubMed] [Google Scholar]
- JIRGENSONS B. Optical rotation and viscosity of native and denatured proteins. II. Influence of temperature and concentration. Arch Biochem Biophys. 1952 Dec;41(2):333–344. doi: 10.1016/0003-9861(52)90462-1. [DOI] [PubMed] [Google Scholar]
- JOLLES P., JAUREGUI ADELL J., JOLLES J. LE LYSOZYME DE BLANC D'OEUF DE POULE: DISPOSITION DES PONTS DISULFURES. C R Hebd Seances Acad Sci. 1964 Apr 13;258:3926–3928. [PubMed] [Google Scholar]
- Johnson L. N., Phillips D. C. Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Angstrom resolution. Nature. 1965 May 22;206(4986):761–763. doi: 10.1038/206761a0. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Fasman G. D. Fluorescence of lysozyme and lysozyme substrate complexes. Separation of tryptophan contributions by fluorescence difference methods. J Biol Chem. 1967 Oct 25;242(20):4644–4651. [PubMed] [Google Scholar]
- Markley J. L., Meadows D. H., Jardetzky O. Nuclear magnetic resonance studies of helix-coil transitions in polyamino acids. J Mol Biol. 1967 Jul 14;27(1):25–40. doi: 10.1016/0022-2836(67)90349-x. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Bhatnagar G. M. Unfolding reactions of proteins. II. Spectral and optical rotatory dispersion studies in urea, guanidinium chloride, and 2-chloroethanol. Biochemistry. 1967 Jun;6(6):1638–1650. doi: 10.1021/bi00858a010. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Manifestations of the tertiary structures of proteins in high-frequency nuclear magnetic resonance. J Am Chem Soc. 1967 Nov 22;89(24):6332–6341. doi: 10.1021/ja01000a061. [DOI] [PubMed] [Google Scholar]
- Meadows D. H., Markley J. L., Cohen J. S., Jardetzky O. Nuclear magnetic resonance studies of the structure and binding sites of enzymes. I. Histidine residues. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1307–1313. doi: 10.1073/pnas.58.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlino V. J., Martin R. B. Chemical shift nonequivalence in proton magnetic resonance spectra of glycyl peptides. J Am Chem Soc. 1967 Jun 21;89(13):3107–3111. doi: 10.1021/ja00989a004. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Jardetzky O. Systematic analysis of chemical shifts in the nuclea magnetic resonance spectra of Peptide chains, I. Glycine-containing dipeptides. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2212–2219. doi: 10.1073/pnas.58.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Butler L., Gerring M., Hartdegen F. J., Pecoraro R. Studies on the enzymic activity of lysozyme, 3. The binding of saccharides. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1088–1095. doi: 10.1073/pnas.57.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VANHOLDE K. E. PHYSICAL STUDIES OF MURAMIDASE (LYSOZYME). II. PH-DEPENDENT DIMERIZATION. J Biol Chem. 1964 Aug;239:2516–2524. [PubMed] [Google Scholar]
- Sternlicht H., Wilson D. Magnetic resonance studies of macromolecules. I. Aromatic-methyl interactions and helical structure effects in lysozyme. Biochemistry. 1967 Sep;6(9):2881–2892. doi: 10.1021/bi00861a032. [DOI] [PubMed] [Google Scholar]


