Abstract
Glutathione (GSH; γ-glutamyl-cysteinyl-glycine) is a small intracellular thiol molecule which is considered as a strong non-enzymatic antioxidant. Glutathione regulates multiple metabolic functions; for example, it protects membranes by maintaining the reduced state of both α-tocopherol and zeaxanthin, it prevents the oxidative denaturation of proteins under stress conditions by protecting their thiol groups, and it serves as a substrate for both glutathione peroxidase and glutathione S-transferase. By acting as a precursor of phytochelatins, GSH helps in the chelating of toxic metals/metalloids which are then transported and sequestered in the vacuole. The glyoxalase pathway (consisting of glyoxalase I and glyoxalase II enzymes) for detoxification of methylglyoxal, a cytotoxic molecule, also requires GSH in the first reaction step. For these reasons, much attention has recently been directed to elucidation of the role of this molecule in conferring tolerance to abiotic stress. Recently, this molecule has drawn much attention because of its interaction with other signaling molecules and phytohormones. In this review, we have discussed the recent progress in GSH biosynthesis, metabolism and its role in abiotic stress tolerance.
Keywords: Antioxidant, Glyoxalase system, Oxidative stress, Phytochelatin, Thiol, Reactive oxygen species
Introduction
Due to the climate change environmental stresses are frequently associated with plant production significantly. Worldwide, abiotic stresses have been estimated to reduce average crop yields by 50% (Hasanuzzaman et al. 2012a). Understanding plants responses to stress and developing tools for improving stress tolerance are keys to prevent crop loss under abiotic stress conditions.
Abiotic stresses generally result in overproduction of reactive oxygen species (ROS) such as singlet oxygen (1O2), superoxide (O·−2), hydrogen peroxide (H2O2) and hydroxyl radical (OH·). These ROS cause oxidative damage that leads to peroxidation of lipids, oxidation of proteins, inhibition of enzymes, and damage to DNA/RNA. The ROS are also recognized for their signaling action in regulating developmental processes and in the development of stress tolerance (Mittler 2002; Sharma and Dubey 2005). Regulation of ROS is therefore vital for improving the stress resistance of plants and is implemented by an antioxidant defense system composed of a number of antioxidant enzymes and non-enzymatic antioxidants.
Antioxidants are among the key elements those protect plants from the oxidative damages caused by abiotic stresses. Among the non-enzymatic antioxidants, glutathione (GSH; γ-glutamyl-cysteinyl-glycine) is a low molecular weight, water soluble thiol compound that is distributed widely in most plant tissues. Apart from the role in storage and transport of reduced sulfur GSH takes part in the detoxification of ROS, directly or indirectly (Foyer and Noctor 2005a, b). Apart from the ROS detoxification, GSH participates in methylglyoxal (MG) detoxification (Hasanuzzaman et al. 2017). It acts as a co-factor in different biochemical reactions, it interacts with hormones, signaling molecules, and its redox state triggers signal transduction (Foyer and Noctor 2005a, b). Another major function of GSH is the formation of phytochelatins (PCs) that bind heavy metals for safe transport and sequestration in the vacuole (Sharma and Dietz 2006). Thus it plays vital role in detoxifying toxic metals/metalloids and xenobiotics (Srivalli and Khanna-Chopra 2008). The various biochemical properties of GSH give it the potential for involvement in plant growth and development, both under normal growing conditions and under different stress conditions. Glutathione modulates cell proliferation, apoptosis, fibrogenesis, growth, development, the cell cycle, gene expression, protein activity and immune function (Noctor and Foyer 1998; Ogawa 2005; Shao et al. 2008; Nahar et al. 2015a).
Glutathione enhances plant tolerance to different abiotic stresses, including salinity, drought, high temperature (HT), low temperature, and toxic metal stress (Hasanuzzaman and Fujita 2011; Hasanuzzaman et al. 2011, 2012a, b; Luo et al. 2011; Hasanuzzaman and Fujita 2013). Exogenously applied GSH can improve abiotic stress tolerances in plants (Kattab 2007; Salama and Al-Mutawa 2009; Wang et al. 2011; Wu et al. 2011; Chen et al. 2012; Nahar et al. 2015a, b, c). In this review, we concentrate on the biosynthesis and metabolism of GSH and its roles in plants under environmental stress conditions, focusing on recent research findings on GSH and abiotic stress tolerance.
Biochemical nature of glutathione
Glutathione was first identified in yeasts in 1888 but its structure was not described until 1935. Intensive study began in the 1960s due to the discovery of its functions in human body fluids. The clarification of GSH metabolism was credited to Dr. Alton Meister due to his indisputable contribution (Meister and Anderson 1983). Glutathione is often termed as a nonprotein reduced sulfur and is a strong water-soluble antioxidant.
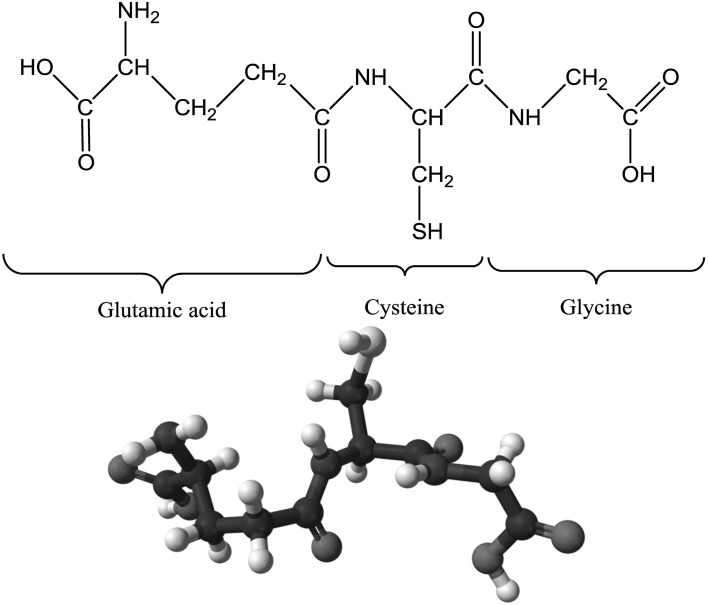
Glutathione is a ubiquitous tripeptide (γ-glutamyl-cysteinyl-glycine; γ-Glu-Cys-Gly) that contains long hydrophilic groups. The structure of GSH includes a bond between the carboxyl group of the glutamate (Glu) side-chain and the amine group of cysteine (Cys) which is attached to a glycine by a peptide bond (Fig. 1). Nevertheless, the thiol group is the main chemically reactive group with respect to its biological and biochemical functions (Zagorchev et al. 2013). The Glu linkage of GSH leads to an increased reactivity with respect to its participation in the γ-glutamyl cycle and protects GSH against attack by aminopeptidases (Wonisch and Schaur 2001). Glutathione is a low molecular weight tripeptide found at high (millimolar) concentration in all aerobic organisms (Hasanuzzaman et al. 2012a; Gill et al. 2013). It is found in the cytosol, endoplasmic reticulum, vacuoles, mitochondria, chloroplasts, peroxisomes, and the apoplast (Noctor and Foyer 1998). For instance, in tobacco mesophyll cells, GSH is distributed as 76, 17, and 7% in chloroplast, vacuole, and cytoplasm, respectively (Berthe-Corti et al. 1992). Some plant species (e.g. soybean, pea, peanut) do not primarily contain GSH; instead, they contain another thiol called homoglutathione (γ-glutamylcysteinyl-β-alanine), which functions in a similar way to GSH and shows the same redox reaction.
Fig. 1.
Chemical structure (above) and ball-and-stick model of the glutathione molecule, C10H17N3O6S
Glutathione biosynthesis and metabolism
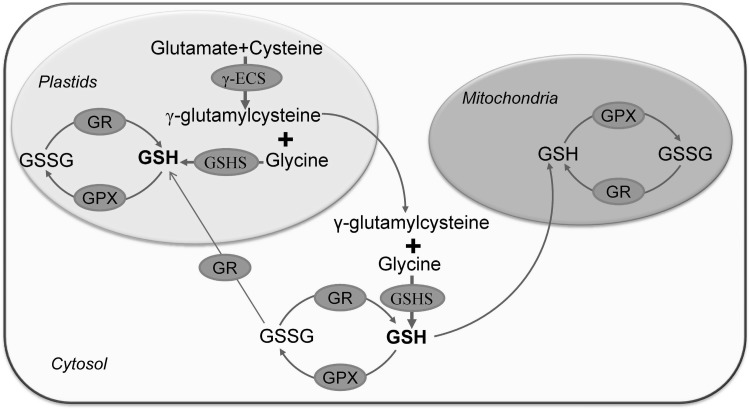
In general, GSH synthesis takes place in the chloroplast, cytosol, and mitochondria (Mahmood et al. 2010; Zechmann and Müller 2010). However, the major processes occur in the chloroplast and consist of a two-step reaction. The reaction starts with three kinds of amino acids Glu, Cys, and glycine. The synthesis of GSH is catalyzed by two enzymes [γ-glutamylcysteine synthetase (γ-ECS) and glutathione synthase (GSHS)]. This synthesis process occurs through a two-step reaction at the expense of two molecules of ATP (Fig. 2). The γ-ECS is found in plastids and GSHS is found both in the cytosol and plastids; for this reason, plastids are considered the site of GSH production in higher plants (Pasternak et al. 2008). The earliest step of GSH biosynthesis consists of a reaction between the γ-carboxyl group of Glu and α-amino group of Cys to create an amide bond to yield γ-ECS; and the γ-ECS enzyme catalyzes this reaction. The next step is GSH formation; which occurs in the presence of the GSHS enzyme by amide bond formation between the α-carboxyl group of the cysteine moiety in γ-glutamylcysteine and the α-amino group of glycine, to form GSH (Hell and Bergmann 1990; Xiang and Oliver 1999). It is also possible to export the γ-ECS from the plastids to the cytosol, where it serves as the precursor to GSH biosynthesis. After the biosynthesis of GSH in the cytosol, it can be efficiently reimported into the plastids or transported into mitochondria (Mahmood et al. 2010).
Fig. 2.
Biosynthesis and metabolism of glutathione. γ-glutamylcysteine synthetase (γ-ECS). First step (in plastid): Glutamate and cysteine together form γ-glutamylcysteine through a reaction catalyzed by the enzyme γ-ECS (γ-glutamylcysteine synthetase). Second step (in cytosol or in plastid): γ-glutamylcysteine and glycine bond together to form GSH via a reaction catalyzed by GSHS: glutathione synthase. The synthesized GSH can be utilized by cellular organelles like the plastids or mitochondria or in the cytosol. GSH participates in ROS scavenging and is converted into GSSG by the enzyme GPX. GSSG can be reconverted/recycled again into GSH by the activity of GR. Nahar et al. (2016b), with Permission from Wiley
Once synthesized in the cytosol, GSH can be imported directly or in other forms, into other organelles to meet metabolic requirements. However, rapid conversion of this reduced GSH into oxidized glutathione or glutathione disulfide (GSSG) can occur through different biochemical reactions within different compartments, and especially during adverse or stress conditions. The activities of glutathione reductase (GR) and glutathione peroxidase (GPX) maintain a balanced state of GSH/GSSG (Mahmood et al. 2010). Different enzyme activities are responsible for the GSH homeostasis and the GSH/GSSG ratio, which are also significantly affected by abiotic stress conditions. The AsA-GSH cycle plays a substantial role in this process. The synthesis of GSH is controlled by several factors; the most vital of these is feedback inhibition of γ-glutamylcysteine synthetase (γ-ECS).
Glutathione-induced abiotic stress tolerance
Salinity
Glutathione can aid in improving plant performance under salt stress. Seedling growth in media containing salt is regulated by GSH or the activities of glutathione-dependent and regulating enzymes (Roxas et al. 1997; Hussain et al. 2008). Transgenic tobacco plants overexpressing both GST and GPX biosynthesis genes increased seedling establishment, and seedlings grew faster under salt stress. This effect could be caused by regulation of the glutathione pool (Roxas et al. 1997). The antioxidative function of glutathione reduces oxidative stress, prevents lipid peroxidation, and protects the plasma membrane. Stabilization of the plasma membrane by glutathione helps to reduce passive Na+ influx, which enhances plant salt tolerance. Glutathione also helps to maintain cellular redox balance and performs signaling functions in plants under salt stress (Foyer and Noctor 2005a). Enhanced GSH level was also correlated with enhanced salt tolerance in several studies. For example, wild type canola seedlings showed a threefold increase in GSH and Cys synthesis during exposure to salt stress when compared to transgenic plants that lacked the genes for GSH and Cys synthesis; the wild type also showed greater salinity tolerance, suggesting a protective mechanism for GSH against salt stress (Ruiz and Blumwald 2002).
Glutathione is also associated with the detoxification of methylyglyoxal. It acts as a cofactor in the glyoxalase system and helps to detoxify methylglyoxal in step-by-step reactions involving two enzymes viz. glyoxalase I (Gly I) and glyoxalase II (Gly II). Research findings have demonstrated that GSH acts as a methylglyoxal detoxifier that confers salt stress tolerance (Singla-Pareek et al. 2003). An experiment by El-Shabrawi et al. (2010) conducted with Pokkali (salt tolerant) and IR64 (salt sensitive) rice cultivars, indicated that the GSH level and both Gly I and II activities were significantly higher in Pokkali than in IR64. When both cultivars were exposed to salt stress, Pokkali showed higher GSH/GSSG ratio and ascorbate (AsA)/dehydroascorbate (DHA) ratio, together with higher activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and GPX, when compared to IR64. The higher GSH level and homeostasis (i.e., GSH/GSSH ratio) and the enhanced activity of the antioxidant and glyoxalase systems were associated with the alleviation of salt induced damage in the salt tolerant Pokkali cultivars, as indicated by the reduced Na/K ratio, the status of ROS, and the extent of oxidative DNA damage when compared to the sensitive IR64 cultivar (El-Shabrawi et al. 2010). The protective function against NaCl-induced oxidative stress in tobacco arose by a reduction in protein carbonylation and an improved antioxidant defense and MG detoxification systems triggered by exogenous proline and glycinebetaine application. Increased GSH level, GSH redox state, and activity of GPX, glutathione-S-transferase (GST) and Gly I were associated with salt stress tolerance in tobacco (Hoque et al. 2008). Exogenous GSH (0.5 mM) ameliorated onion bulb epidermis damage, improved membrane permeability, prevented protoplasmic swelling, and maintained cell viability under salt stress (150 mM NaCl).
Salama and Al-Mutawa (2009) described the roles of GSH as ROS scavenger in the improvement of salt stress tolerance. They mentioned a GSH-triggered mitigation in salt-induced alterations in the plasma membrane of onion epidermal cells. Salt stress imposed on two cultivars of canola (Brassica napus L. cv. Serw and cv. Pactol) significantly reduced several plant growth parameters, as well as contents of pigments, total soluble carbohydrates, free amino acids, and total soluble proteins. The amounts of different antioxidant components like GSH, AsA, carotenoids, phenols, proline, and glycine betaine also changed in response to salt stress. Seed priming with exogenous GSH and polyadenylic acid improved the tolerance of Brassica seedlings to salt stress and was associated with increased levels of antioxidants and enhanced activities of SOD and phenol peroxidase antioxidant enzymes (Kattab 2007).
A role of GSH as an antioxidant has been confirmed in several studies on plant growth under salt stress. GSH applied exogenously during salt stress regulated other antioxidant components (both enzymatic and non-enzymatic) and compatible organic solutes or osmoprotectants (Foyer and Noctor 2005a; Kattab 2007; Salama and Al-Mutawa 2009). Glutathione could prevent the loss of photosynthetic pigments under salt stress (Kattab 2007). These responses point to the underlying mechanisms responsible for improved plant growth under salt stress induced by GSH. The GSH redox signaling process enhances salt tolerance, as seen in an Arabidopsis mutant that showed adaptive responses to salt stress, where GSH functioned as a downstream component of signal transduction pathways. This mutant showed hyposensitivity to ABA (abscisic acid) during seed germination which was associated with higher accumulation of GSH. It also reduced stomatal aperture and transpiration rate and performed better root and vegetative development which were attributed due to higher GSH and ABA levels (Shao et al. 2008).
The performances of salt-tolerant (Pusa Vishal) and salt-sensitive (T44) mung bean cultivars were examined following salicylic acid (SA) treatment. Salt tolerant cultivars showed better performance in response to SA application under salt stress when compared to the salt sensitive cultivar; this improved performance was due to a reduction in oxidative stress and improved physiological performance. The tolerant cultivars showed higher GSH content and higher activities of AsA-GSH cycle components, restricted Na+ and Cl− content in the leaves, higher efficiency of PSII, better photosynthetic N-use efficiency, and improved water relations (Nazar et al. 2011). Recently, Nahar et al. (2015c) showed that exogenous GSH could result in coordinated induction of the antioxidant and glyoxalase systems which effectively reduced oxidative damage and MG toxicity, and improved the physiological adaptation of V. radiata seedlings to short-term salt stress (200 mM NaCl, 24 and 48 h).
Hormone induced regulation of GSH and their contribution in abiotic stress tolerance have been proved in several research findings. Increased glutathione level results in salt stress tolerance and global translational changes in Arabidopsis. Both exogenous and endogenous GSH delayed senescence and flowering time. Translatome analysis also exposed that ABA, auxin and jasmonic acid (JA) biosynthesis, and signaling genes were activated during GSH treatment (Cheng et al. 2015). A direct link between cellular redox status and auxin signaling has been reported during characterization of a triple mutant of Arabidopsis (Bashandy et al. 2010). The GST and GPX activities were higher in the auxin autotrophic tobacco callus lines which were suggested to enhance salt stress (100 mmol/L NaCl) tolerance. In the autotrophic tissues, the upregulated antioxidant enzyme activities led to reduced levels of H2O2 and MDA levels under NaCl stress. The auxin autotrophic line also showed higher tolerance to exogenous H2O2 (Csiszár et al. 2004). Cytokinin (in the form of 6-benzyladenine, 6-BA) application showed protective effect against salt stress by improving in photosynthesis and antioxidant capacity. Exogenous 6-BA increased AsA, GSH and proline contents, activities of SOD and POD and decreased O·−2 production rate and MDA content in eggplant under salt stress (Wu et al. 2014). Polyamines (spermidine, spermine and putrescine) application conferred salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. In antioxidant defense system, exogenous polyamines improved GSH level and GSH/(GSSG, glutathione dissulfide) ratio, activities of GR and GPX which defended overproduction of ROS. In glyoxalse system, increased activities of glyoxalse II (which is a GSH dependent enzyme) and enhanced GSH level contributed to reduce toxic methylglyoxal level (Nahar et al. 2016a, b).
Drought
Drought-induced impairment of stomatal conductance, membrane electron transport rate, CO2 diffusion, carboxylation efficiency, and photosynthesis lead to the generation of ROS which causes oxidative damage. Limited growth and developmental processes, reduced growth, and lower final crop yield are some common effects of drought (Pinheiro and Chaves 2011). Glutathione and its metabolic enzymes interact with plant growth regulators, which are regarded as vital in the scheme of plant establishment both under normal and stressful conditions. GST expression in plants is induced by phytohormones such as SA, ethylene, cytokinin, auxin, ABA (Marrs 1996), methyl jasmonate (Moons 2003), and brassinosteroids (Deng et al. 2007). Chen et al. (2012) demonstrated that GST overexpressed in Arabidopsis thaliana plants had a signaling role and it regulated plant development by maintaining GSH pools. The mutant plant atgstu17 synthesized higher levels of GSH and ABA when compared to the wild type. Moreover, supplementation of wild type plants with exogenous GSH resulted in higher ABA content and a similar phenotype to that seen in the mutant, as well as greater drought tolerance.
Glutathione application induced higher levels of ABA, the plant hormone that controls seed germination, regulates stomatal aperture, and reduces transpiration rate (Chen et al. 2012). Drought stress resulted in leaf rolling in Ctenanthe setosa (Rosc.) Eichler (Marantaceae); this leaf rolling was considered as an adaptive nature and was accompanied by an enhanced GSH content and a decreased GSSG content. The level of GSH and the enzymes of the AsA-GSH cycle were associated with ROS detoxification. High GSH content was also correlated with regulation of leaf water content, which may indicate a role in controlling leaf rolling (Saruhan et al. 2009). Glutathione supposedly has growth regulating functions, as elevation of the endogenous GSH level enhances cell division in the root meristematic region (Vernoux et al. 2000); this elongation is an important morphological adaptation to drought.
Numerous studies have confirmed that elevated endogenous GSH levels can confer drought stress tolerance and that GSH ameliorates ROS-induced damages. Drought stress was responsible for severe oxidative stress in B. napus, as indicated by a pronounced rise in H2O2 and lipid peroxidation levels. Drought stress significantly increased GSSG content in the seedlings. Exogenously applied selenium had a beneficial effect because it reduced oxidative stress and improved drought stress tolerance by increasing endogenous GSH levels. The GSH/GSSG ratio and the activities of AsA-GSH cycle enzymes were also increased (Hasanuzzaman and Fujita 2011). Alam et al. (2013) also found similar results for endogenous GSH under drought stress.
Osmotic stress is a primary effect of drought stress. Glutathione and its related antioxidants were observed to reduce osmotic stress in maize and in wheat plants. In wheat, imposition of osmotic stress at the seedling stage increased total GSH and hydroxymethylglutathione. By contrast, in maize, the same level of osmotic stress enhanced GR activity and increased the total amount of hydroxymethylglutathione. Osmotic stress resulted in a great increase in the total GSH content in wheat seedlings and the GR activity in maize seedlings (Kocsy et al. 2002). GSH and glutaredoxins are concerned with detoxification of ROS, but they also transmit redox signals under drought stress (Meyer 2008). The GSH pools in the Arabidopsis atgstu17 mutant were 35% higher compared with wild-type plants (Jiang et al. 2010). Application of exogenous betaine and proline application improved GSH level and maintained high activities of GST and Gly I, thereby protecting lentil plants from oxidative damage and toxic effects of MG (Molla et al. 2014).
Koffler et al. (2014) demonstrated the signaling roles of GSH in Arabidopsis thaliana under drought stress. During drought stress, compartment specific changes in GSH and AsA contents were recorded. Arabidopsis thaliana Col-0, and the GSH and AsA deficient mutants, pad2-1 and vtc2-1, respectively, were exposed to drought stress for 10 days. It was observed that the GSH content strongly decreased in both mutants at the early stages of drought stress, when the stress in leaves was not yet measurable. The authors concluded that GSH was used to indicate drought stress perceived by the roots to the leaves at early time points. The wild type plants also showed better performance under drought stress, compared to mutants. The mutants had reduced GSH and AsA contents, so they had lower activities of antioxidative enzymes, i.e., GR and DHAR (dehydroascorbate reductase) in chloroplasts and peroxisomes. This lower activity of these enzymes occur due to the Calvin cycle malfunctioning and the occurrence of photorespiration under drought stress, which caused greater accumulation of reactive oxygen species compared with the wild type (Koffler et al. 2014).
Glutathione (GSH) was involved in stomatal closure induced by ABA, as its presence in guard cells regulated ABA signaling in the Arabidopsis cad2-1 mutant (Okuma et al. 2011). Akter et al. (2012) showed an involvement of GSH in stomatal closure induced by ABA and methyl jasmonate (MeJA) in Arabidopsis thaliana. They found that exogenously applied GSH decreasing chemicals viz. p-nitrobenzyl chloride (PNBC), iodomethane (IDM), resulted in the depletion of GSH in guard cells of stomata. However, this decrease in GSH could increase the ROS significantly.
Involvement of hormone and GSH in improving drought stress tolerance was mentioned in several previous studies. Higher glutathione content improved drought stress tolerance and global translational changes in Arabidopsis. Abscisic acid, auxin and JA biosynthesis, and signaling genes were also activated during GSH treatment as revealed from transcriptomic study (Cheng et al. 2015). Drought-induced glutathione transferase, GST8 was identified in Arabidopsis. GST8 RNA levels were strongly induced by 2,4-D and NAA (two synthetic auxins) and by IAA (Bianchi et al. 2002). The iaaM-OX transgenic lines with higher endogenous indole-3-acetic acid (IAA) level and IAA pre-treated wild type Arabidopsis plants exhibited enhanced drought stress resistance. Moreover, endogenous and exogenous auxin positively modulated the expression levels of multiple abiotic stress-related genes and ROS metabolism, contents of GSH and GSSG and activity of GR. Some carbon metabolites including amino acids, organic acids, sugars, sugar alcohols and aromatic amines were also significantly affected by auxin under drought stress. Endogenous and exogenous auxin positively modulated root architecture especially the lateral root number (Shi et al. 2014).
Extreme temperature
Extreme temperatures at either high or low ranges are major environmental adversities that limit plant production (Hasanuzzaman et al. 2013). A beneficial role of GSH under HT stress has been investigated in few studies. The GSH pools, with their temporal and spatial changes, together with the GSH redox state and its regulation and roles in redox signaling and defense processes, are important components for the thermotolerance (Szalai et al. 2009). The glutathione pool is associated with the HT stress response of maize at the seedling stage. Total GSH levels and the GSH/GSSG ratio were increased by HT stress (40 °C, 1 day) treatment in chilling-sensitive maize genotypes, whereas GR activity and total hydroxyl methylglutathione content increased in both chilling sensitive and tolerant maize genotypes (Kocsy et al. 2002). Coleus blumei and Fagus sylvatica L. plants were exposed to heat stress (35 °C), which resulted in oxidative stress. However, after a short-term acclimation to low temperature, the seedlings of both species showed modulations in the GSH pool and activity of GR, although in different ways, which was correlated with the HT stress tolerance of these species. The GSH level and GR activity were higher in F. sylvatica than in Coleus blumei, so F. sylvatica also showed better tolerance to HT. The HT susceptibility of C. blumei was associated with a differential loss of GR and GSH at low temperature (Peltzer et al. 2002).
A higher content of total GSH was suggested to confer HT tolerance in T. aestivum, Z. mays and V. radiata (Nieto-Sotelo and Ho 1986; Dash and Mohanty 2002; Nahar et al. 2015a). Efficient elimination of H2O2 was possible due to the elevated GSH level in heat stressed mustard seedlings, which was facilitated by improved GR activity (Dat et al. 1998). Higher GSH levels at the reproductive stage in apple plants enhanced their capacity for HT acclimation. Apple peels exposed to HT and excessive solar radiation showed increased endogenous GSH levels, which improved the acclimation capacity under HT stress. Exogenous application of AsA + benzoic acid and SA increased the endogenous GSH level by 33.97 and 31.81%, respectively, compared to the control, and acclimation was again enhanced (Zhang et al. 2008). The AsA–GSH cycle was up-regulated in response to elevated temperatures in two-year-old Malus domestica Borkh. plants exposed to 40 °C for 0, 2, 4, 6, and 8 h. Highest amount of total GSH, and total and reduced AsA contents were recorded under HT exposure for 2 h. Activity and expression of DHAR, ascorbate peroxidase (APX), and GR increased following up to 4 h of HT. Thus, upregulation of the AsA-GSH cycle showed protective effects HT-induced oxidative damages at the vegetative stage of M. domestica (Ma et al. 2008).
Higher total GSH contents increased antioxidative protection and electron consumption in Soldanella alpina and Ranunculus glacialis, which in turn enhanced thermotolerance (Laureaua et al. 2011). Hasanuzzaman et al. (2012b) investigated the regulatory role of exogenous nitric oxide (NO) in alleviating HT-induced damage of T. aestivum seedlings. High temperature (38 °C) induced oxidative damage to the wheat seedlings, whereas application of exogenous NO protected the seedlings from oxidative damage due to increases in GSH levels, GSH/GSSG ratio, and other components of the AsA-GSH cycle (e.g., APX and GR activities), AsA content, and activity of GSH metabolizing enzymes, including GPX and GST. Increased chlorophyll content and better phenotypic appearance were also documented in NO supplemented plants exposed to HT, when compared to plants exposed to HT stress alone (Hasanuzzaman et al. 2012b). Nahar et al. (2015b) showed the pretreatment of V. radiata seedlings with GSH (1.0 mM) resulted in better physiological performance along with improved antioxidant and glyoxalase systems. They also observed the reduced MG and oxidative stress under short term heat stress (42 °C, 24 and 48 h). Heat stressed seedlings those were supplemented with GSH improved chl content and leaf RWC and also increased APX (only after 24 h), MDHAR, DHAR, GR, glutathione peroxidase (GPX), GST (increased only after 24 h), catalase (CAT). Increased Gly I and Gly II activities and improved endogenous GSH content and the GSH/GSSG ratio, while a reduction of GSSG content were also observed in those seedlings. The supplementation of GSH with drought stress resulted in significant decrease in MDA, H2O2 and MG content, O·−2 generation rate and LOX activity (Nahar et al. 2015b).
The relationship between protection from chilling stress (5 °C, 7 days) and total GSH or GR activity was studied by Kocsy et al. (2001) in Zea mays. When compared to an untreated control, a combination of two herbicide safeners improved the GSH pool and its precursors, Cys and γ-ECS. An increase in the total GSH level was correlated with enhanced GR activity. These alterations in GSH precursors or pools resulted in a 50–75% increase in protection of maize plants against chilling. Cold treatment of maize at different low temperatures (2, 5, 10, or 15 °C) also induced a greater increase in total GSH content and GR activity in tolerant genotypes than in a sensitive genotype. The GSH/GSSG ratio and the ratio of reduced to oxidized hydroxymethylglutathione (hmGSH/hmGSSG) were increased by the same low temperature treatments in a frost-tolerant wheat variety (Kocsy et al. 2002).
When compared to the normal (25/18 °C) growth condition, a chilling (15/8 °C) temperature resulted in withering symptoms, with significantly increased H2O2, O·−2, and malondialdehyde (MDA) levels in Cucumis sativus cv. Jinchun 4 seedlings. Supplementation of low-temperature-treated seedlings with exogenous silicon (Si) resulted in an improved phenotype and reduced oxidative damage, which were correlated with an increase in GSH and AsA levels, and enhanced activities of enzymes involved in AsA-GSH cycle (i.e., GPX and SOD) (Liu et al. 2009). Higher GSH levels and higher activities of enzymes of the AsA-GSH cycle also enhanced the tolerance to low temperature stress (0 °C for 2, 4, 6, 8, 12, 24, 48, and 72 h) in strawberry cultivars (Luo et al. 2011). Low temperature stress (13 °C) in Dunaliella salina increased the total GSH pool by 20–100% and the total ascorbate pool by 10–50%, as well as increasing SOD; these were considered vital responses to suit that environment (Haghjoua et al. 2009). Over-expression of GST in transgenic rice enhanced the germination and growth rate at low temperature (Takesawa et al. 2002). Studies on GSH under extreme temperature stress confirmed that GSH enhanced tolerance in different growth stages of plants including the seedling, vegetative and reproductive stages. However, most of the research findings regarding responses to extreme temperatures showed that the antioxidant activity of GSH enhanced temperature stress tolerance. Studies also showed GSH to regulate other antioxidant components that also induced temperature stress tolerance. As with other stresses, GSH is thought to act as a signaling molecule or as a growth regulator to improve extreme temperature stress tolerance in plants (Hasanuzzaman et al. 2012b). This hypothesis deserves further study.
Toxic metals/metalloids
Several transition metals/metalloids are essential for plant growth (this is also the case for most other organisms); however, many of these compounds are toxic when present in excess amounts (Zitka et al. 2013). Unfortunately, due to rapid industrialization, emissions of metals to the environment have increased and now exceed those from natural sources. Metal/metalloid toxicity often disrupts the normal growth and physiology of plants, thereby substantially hampering productivity. One major consequence of metal/metalloid toxicity is the excess generation of ROS and subsequent oxidative stress (Hasanuzzaman and Fujita 2013). However, as already described, plants can reduce this damage by activating defense strategies to enhance antioxidative defenses and restore physiological processes (Hall 2002).
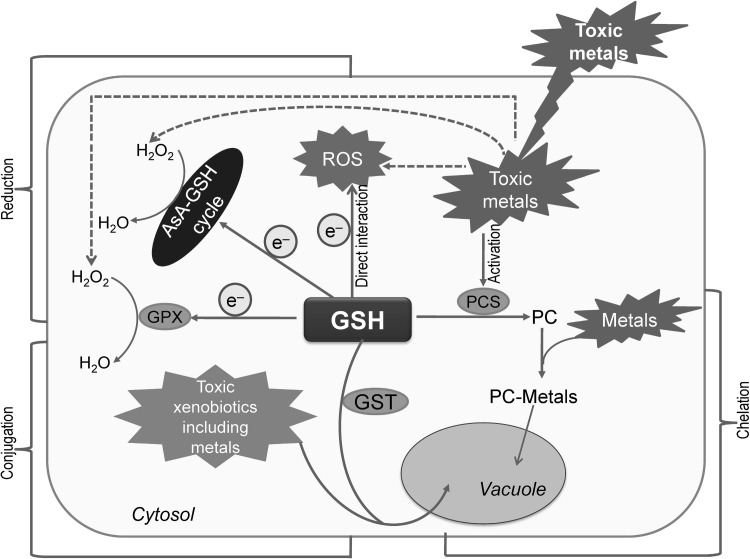
Glutathione and related thiols are potential components that reduce metal/metalloid toxicity in plants (Fig. 3). In line with its important functions in the maintenance of cellular redox homeostasis, GSH also participates in the detoxification of toxic metals/metalloids. Glutathione can protect the plant cells from metal/metalloid toxicity in three possible ways: (1) direct quenching of ROS; (2) conjugation of toxic metals and other xenobiotics to GST; and (3) acting as a precursor for the synthesis of phytochelatins (PCs).
Fig. 3.
GSH mediated toxic metal and xenobiotic detoxification in a plant cell; PC: phytochelatin, PCS: phytochelatin synthase. Dotted arrows indicate the induction of ROS production. Protection of plant cells from metal/metalloid toxicity by GSH occurs in three possible ways: (1) reduction: direct quenching of ROS which is occurred by AsA-GSH cycle together with other components of antioxidant system, (2) conjugation: toxic metals/xenobiotics conjugation by the activity of glutathione S-transferase (GST) and transportation of conjugates into vacuole, (3) chelation: GSH acts as a precursor for the synthesis of phytochelatins (PC). Metals are bound to the thiol (–SH) group of GSH and forms PC by the activity of enzyme phytochelatin synthase (PCS, γ-Glu-Cys transpeptidase); the PC-Metal complex is also transported into the vacuole. Nahar et al. (2016b), with Permission from Wiley
Several reports have showed GSH-induced protective effects in mitigating metal/metalloid toxicity. A few reports showed that GSH content is increased in response to metal exposure due to the enhanced activities of proteins responsible for GSH biosynthesis (Xiang and Oliver 1999). In an in vitro study, Ruegsegger et al. (1990) found 3-to 4-fold increase in GSHS activity in P. sativum when exposed to Cd. Bergmann and Rennenberg (1993) also demonstrated a time-dependent increase in GSHS activity in same species upon exposure to Cd. Wang et al. (2011) reported some injury symptoms, such as leaf chlorosis, necrosis and stunting in barley seedlings when subjected to Cd (5 μM). However, the addition of GSH (20 mg L−1) in the Cd culture medium can alleviate the Cd-induced growth inhibition and leaf injury symptoms significantly. Exogenous GSH resulted in the increase of plant dry weight in the following two barley varieties: Weisuobuzhi (by 42.3%) and Dong 17 (by 70.6%). The reduced shoot/root Cd concentrations of 33.8/42.2% in Weisuobuzhi and 59.4/66.3% in Dong 17 relative to the Cd alone treatment reflects that GSH can suppress plant Cd uptake (Wang et al. 2011).
Exogenous GSH not only improved growth and nutrient uptake but also increased the endogenous GSH content, while decreasing the Cys content of leaf tissue of rice seedlings under chromium (Cr, 100 µM) stress. It also markedly increased the contents of acetic, lactic, citric, tartaric, and malic acid in the root, while decreasing the Cr concentration of root and shoot. The reduction in Cr concentration by exogenous GSH supports the metal chelation and sequestration activity of GSH. The growth-regulating functions of GSH were confirmed by the regulation of biomolecule contents and nutrient uptake, as well as plant growth (Qiu et al. 2013). Adding GSH in different concentrations (1, 5, 10 or 25 mg L−1) to media containing different concentrations (1, 5, 50, 100 or 200 mg L−1) of Pb(NO)3 can ameliorate the adverse effects of heavy metals on morphogenesis of Spilanthes calva L. by enhancing survival percentage, shoot number and shoot length (Shankar et al. 2012).
Glutathione improves both the growth parameters and physiological activities in barley. A combined supplementation of Dong 17 barley seedlings with Cd (5 μM) together with GSH (20 mg L−1) increased photosynthesis by 130.2%, transpiration by 68.9% and stomatal conductance by 52.4%, when compared to seedlings exposed to Cd alone. Glutathione treatment also promoted the maintenance of a better number and ultrastructure of plastids and mitochondria. In plastids, well-developed thylakoid membranes properties, parallel patterns of lamellae, and more unfolded starch grains have been demonstrated. Glutathione also resulted in well-structured mitochondrial cristae and uniform chromatin distribution in nuclei was observed. The improved membrane and anatomical structures were due to the antioxidant functions of GSH. The mechanisms underlying the improvement in physiological processes under stress indicates various others roles for GSH which deserve further research (Wang et al. 2011).
Chen et al. (2010) studied the antioxidant functions of GSH that enhanced Cd stress tolerance in rice. Oxidative stress is reduced due to the regulation of antioxidant enzymes by GSH which renders better survival of the rice plants. It increased POX activity and decreased CAT activity, while maintaining a better structure of the leaf and root, which aided in conferring tolerance (Cai et al. 2011). Dramatic depressions in O·−2, H2O2, and MDA accumulation were seen after the supplementation of GSH in rice seedlings subjected to Cd and the antioxidant system modulation is considered to be the main reason. When GSH application is done exogenously, it can increase the endogenous GSH levels and can also decrease the AsA and nonprotein thiol levels, while maintaining activities of monodehydroascorbate reductase (MDHAR), DHAR and GPX similar to those of the non-treated seedlings. The activities of CAT and APX were significantly increased compared to Cd-stressed plants (Chen et al. 2010). The oxidative stress induced by Cd in Ceratophyllum demersum was alleviated by exogenous Zn application, which effectively restored thiols. The application of Zn also inhibited oxidation of AsA and GSH, and maintained the redox state balance. Zn addition also restored and enhanced the activities of AsA-GSH enzymes (i.e., APX, MDHAR, DHAR, and GR), GST, and GPX, which all together conferred tolerance to Cd stress (Aravind and Prasad 2005). Upregulation of GSH and GSH dependent enzymes, modulation of AsA-GSH cycle components by exogenous polyamines application improved toxic metal stress tolerance. An increased GSH content and activities of antioxidant enzymes such as SOD, CAT, GST, MDHAR, DHAR and GR which reduced oxidative stress and enhanced cadmium tolerance of exogenous spermine treated mung bean plant (Nahar et al. 2016a). In other study, exogenous spermidine increased AsA and GSH contents, activities of APX, DHAR, GR and CAT which reduced ROS production and oxidative stress under aluminium stress (Nahar et al. 2017).
Beside playing a key role in the antioxidant defense system and improving the physiological processes under heavy metal toxicity conditions, GSH also binds the toxic metals. Transportation of toxic metals to safe sequestration sites within the plant cell not only ensures a sound cellular environment but also presents an ideal perspective to abolish the toxic metals from environment by the process of bioremediation and phytoremediation (Peuke and Rennenberg 2005). The reduction of the free metal content within the plant cell can be achieved by two ways: the synthesis of specific chelators and the sequestration of metal complexes (Smeets et al. 2009; Cuypers et al. 2010). The high affinity for metals imparted by the GSH thiol (–SH) group, coupled with the fact that GSH is a precursor of PCs. It is the reason why GSH is considered to be a very important biomolecule in the process of scavenging metals (Jozefczak et al. 2012). Enzymatic oligomerization of GSH results in phytochelatins formation to give a generalized structure of (γ-Glu-Cys)n-Gly (n = 2–11). Phytochelatins can bind metal and then can be transported into the vacuole. The formation of PCs largely depends on whether heavy metals are present or not. It also varies with the activity of PC synthase (PCS, γ-Glu-Cys transpeptidase), a PC synthesizing enzyme (Fig. 3). The activation of the PCS enzyme by the metal itself mainly regulates the PC formation. Phytochelatins are reported to chelate Cd most effectively, followed by As (Jozefczak et al. 2012); the other preference order for chelation of metals are Ag > Bi > Pb > Zn > Cu > Hg > Au (Pasternak et al. 2008). The vacuole contains the sequestered PC-ion complexes, thereby reducing toxic metal effects.
In plants, PCs can confer tolerance to heavy metal, as indicated by numerous recent studies, including Cd tolerance in B. juncea (Mohamed et al. 2012), Populus nigra (Iori et al. 2012), Oenothera odorata (Son et al. 2012), T. aestivum (Hentz et al. 2012), and Linum usitatissimum (Najmanova et al. 2012); As tolerance in Nicotiana tabacum (Shukla et al. 2012) and Arabidopsis thaliana (Huang et al. 2012); Zn and Cu tolerance in Arabidopsis thaliana (Huang et al. 2012); and Mn tolerance in Vitis vinifera (Yao et al. 2012). Glutathione conjugates metals by forming PCs, which can be translocated. In between roots and shoots of Arabidopsis plant, PCs go through a long distance transport and are also identified in the xylem and phloem saps of B. napus. The concentrations of PCs and Cd in the phloem sap is higher than xylem sap; which indicates that translocation of PCs occurs largely via the phloem. Again, in the phloem sap the high ratios of [PC]/[Cd] and [GSH]/[Cd] designate the PCs and GSH as the long-distance carriers for Cd (Mendoza-Cozatl et al. 2008). Arabidopsis thaliana overexpressing AtPCS1 and GSH1 showed greater PC formation and enhanced tolerance for Cd and As accumulation than single-gene transgenic lines (Guo et al. 2008). The two glyoxalase pathway genes (Gly I and Gly II), individually and together, in Nicotiana tabacum plants enhanced the levels of both GSH and PCs and imparted tolerance against Zn stress (Singla-Pareek et al. 2006). Overexpression of the AtPCS1 gene encoding PCS in Brassica juncea resulted in tolerance to Cd, As, and Zn stresses (Gasic and Korban 2007a, b). High intracellular GSH content and enhanced activities of PCS were observed in Lotus japonicus, where the biosynthesis of PCs and homophytochelatins are involved in regulation of heavy metal sequestration (Asada 1994). In the absence of PC formation, conjugation of GSH with both the exogenous and endogenous organic toxicants (hydrophobic) can be formed, which are then transported and sequestered in the vacuole (Fig. 3; Peuke and Rennenberg 2005; Hasanuzzaman et al. 2012a). Paulose et al. (2013) found that the Arabidopsis plants overexpressing γ-glutamyl cyclotransferase (GGCT) genes showed better tolerance to Cd and As which is due to higher turnover of GSH because GGCT is responsible for Glu recycling.
Glutathione-induced oxidative stress tolerance
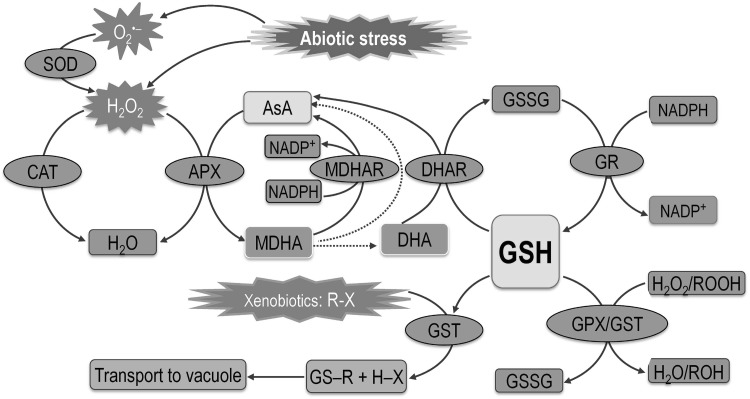
Glutathione is a strong antioxidant; hence, it has a vital role in antioxidant defense. Under adverse environmental conditions, excessive amounts of ROS are formed, which leads to oxidative stress. However, plants have developed well-equipped antioxidant defense systems, and GSH is one of the major molecules that forms an integral part of the AsA-GSH cycle that scavenges H2O2. Glutathione reacts with O·−2, OH· and H2O2 form adducts with reactive electrophiles (glutathiolation). Glutathione is also an efficient electron donor during the ROS detoxification process and oxidation to GSSG (Asada 1994). Further, GSH can be regenerated by GR using nicotinamide adenine dinucleotide phosphate (NADPH) as the substrate (Fig. 4; Noctor and Foyer 1998; Hasanuzzaman et al. 2012a). Because of its abundance in almost all of the cellular organelles GSH participates actively in various metabolic processes under stress conditions (Fig. 4). Glutathione not only detoxifies ROS, but also modulates antioxidant enzymes.
Fig. 4.
Mechanisms of ROS detoxification by different antioxidant enzymes. AsA-GSH cycle consist of AsA (ascorbate) and GSH (glutathione), and antioxidant enzymes APX (ascorbate peroxidase), MDHAR (monodehydroascorbate reductase), DHAR (dehydroascorbate reductase), GR (glutathione reductase). MDHA-monodehydroascorbate, DHA-dehydroascorbate. Dotted lines denote non-enzymatic conversions. R may be an aliphatic, aromatic, or heterocyclic group; X may be a sulfate, nitrite, or halide group. Abiotic stresses generate reactive oxygen species/ROS. The SOD (superoxide dismutase) is considered the first line of defense in the ROS detoxification process and converts O·−2 to H2O2. This H2O2 can be converted to H2O by CAT (catalase) activity or H2O2 enters the AsA-GSH cycle, where APX, using AsA, converts H2O2 to H2O. While participating in the ROS detoxification process, AsA is oxidized to DHA (dehydroascorbate reductase). AsA is regenerated through the AsA-GSH cycle enzymes MDHAR and DHAR in a step-by-step reaction. In the AsA-GSH cycle, GSH takes part in detoxification of ROS (by the activity of enzyme GPX/glutathione peroxidase or GST/glutathione-S-transferase) or xenobiotics (by the activity of GST). The GSH is converted into GSSG (glutathione disulfide) during ROS detoxification and this GSSG is recycled again to GSH by the activity of GR. Adapted from Hasanuzzaman et al. (2012a)
Glutathione helps to maintain the reduced state of constituents of the AsA-GSH pathway and thus detoxify ROS. The DHAR enzyme uses GSH to convert oxidized ascorbic acid (DHA) to AsA (Foyer and Noctor 2011); GSH also reduces DHA by a nonenzymatic process in the chloroplast at alkaline pH (Foyer and Halliwell 1976). Glutathione is a substrate for GPX when converting the lipid hydroperoxide or H2O2 into a non-toxic form or water (Noctor et al. 2002). Besides, GST uses GSH to convert toxic xenobiotics to non/less toxic complexes that are transported to the vacuole (Srivalli and Khanna-Chopra 2008). Apart from its direct role in ROS scavenging, GSH also maintains the reduced state of other antioxidants like tocopherol and zeaxanthin and indirectly protects cell membranes. Glutathione also protects proteins from denaturation under stressful conditions. It also transmits signals for maintaining cellular homeostasis in plants, thereby protecting the plant from stress-induced damage (Noctor 2006). A histochemical study of Sedum alfredii revealed that inhibition of GSH biosynthesis during Cd stress overproduced H2O2 and O·−2 (Jin et al. 2008a). It is also reported that Zn toxicity may involve inhibition of NADPH oxidase, and GSH synthesis, which may lead to increased H2O2 and O·−2 accumulation (Jin et al. 2008b). In the last couple of decades several researchers reported that exogenous application of GSH provided better protection against oxidative stress and improved physiology under environmental stress (Table 1).
Table 1.
Protective effect of glutathione under abiotic stress conditions
| Plant species | Stress level | Glutathione supplementation | Protective effects | References |
|---|---|---|---|---|
| Allium cepa cv. Giza 6 | Salinity (150 mM NaCl, soaked epidermal cells for 3 h) | 0.5 mM GSH, soaked epidermal cells for 3 h | Reduced cell membrane permeability coefficient (Ks) Decreased Number of dead and swollen cells |
Salama and Al-Mutawa (2009) |
| B. napus vs. Serw and Pactol | Salinity (100 and 200 mM NaCl) | 100 mg L−1 GSH, seed soaking for 24 h | Improved shoot and root growth Increased SOD, CAT, POX, GPX and APX activities Increased the contents of total soluble carbohydrates, soluble proteins, phenols and free amino acid Increased total photosynthetic pigment contents |
Kattab (2007) |
| V. radiata cv. Binamoog-1 | Salinity (200 mM NaCl, 24 and 48 h) | 1 mM GSH, 24 and 48 h | Reduced MDA and H2O2 content, O·−2 generation rate, and LOX activity Reduced Pro content Improved Chl b and total Chl contents Lessened the reduction rate of RWC Increased SOD, APX, GR, GST and GPX activities |
Nahar et al. (2015c) |
| O. sativa | Salinity (200 mM NaCl) | 2 mM GSH, foliar spray | Increased the number of filled grains per panicle Increased spikelet fertility Increased yield per panicle |
Hussain et al. (2016) |
| V. radiata cv. Binamoog-1 | Drought (25% PEG, −0.7 MPa) | 1 mM GSH, 24 and 48 h | Reduced MDA content, O·−2 generation rate and LOX activity Restored the decreased Chla and total Chl contents Increased SOD, CAT, APX, MDHAR, DHAR, GR, GST and GPX activities Reduced MG content and increased Gly I and Gly II activities |
Nahar et al. (2015a) |
| Cicer arietinum and C. reticulatum Ladiz. | Drought (withheld irrigation, 10 days) | 10 and 100 mM, foliar spray | Reduced CAT, APX and GR activities Increased AsA content |
Çevik and Ünyayar (2015) |
| V. radiata cv. Binamoog-1 | High temperature (42 °C, 24 h and 48 h) | 0.5 mM GSH, 24 h seedling pretreatment | Restored leaf RWC Increased Chl b and total Chl contents Reduced MDA and H2O2 contents along with LOX activity Increased CAT, GPX, DHAR, MDHAR and GR activities Reduced MG content and increased Gly I and Gly II activities |
Nahar et al. (2015b) |
| Hordeum vulgare cvs. Dong 17 and Weisuobuzhi | Metal toxicity (5.0 μM CdCl2) | 20 mg L−1 GSH | Alleviated growth inhibition Reduced Cd concentrations in leaves and roots Increased net photosynthetic rate P(n), stomatal conductance G(s) and transpiration rate T(r) |
Wang et al. (2011) |
| O. sativa lines 117 and 41 | Metal toxicity (100 µM Cr6+) | 300 µM GSH | Increased K, Mg, Fe and Mg contents in both shoot and root Reduced Cr contents in both shoot and root Increased GSH contents in leaf, stem and root |
Qiu et al. (2013) |
| Spilanthes calva | Metal toxicity (1, 5, 50, 100, or 200 mg L−1 Pb, 28 days) | 10 mg L−1 GSH | Minimized the toxic effects of Pb Produced multiple shoots |
Shankar et al. (2012) |
|
Gossypium hirsutum cvs. Coker 312 and TM-1 |
Metal toxicity (50 μM Cd, 7 days) | 50 μM GSH | Minimized the contents of various Stress markers (TTC reduction, TSP, MDA, H2O2) Reduced leaf Cd content Increased SOD, POD, APX and GR activities |
Daud et al. (2016) |
| Gossypium hirsutum cv. TM-1 | Metal toxicity (500 μM Pb) | 50 μM GSH | Increased net photosynthetic rate, stomatal conductance, intercellular CO2 concentration and transpiration rate Reduced MDA, H2O2, total soluble proteins contents and Pb uptake in leaves Increased CAT, SOD, POD, GR and APX activities |
Khan et al. (2016) |
Methylglyoxal detoxification: a vital role of glutathione
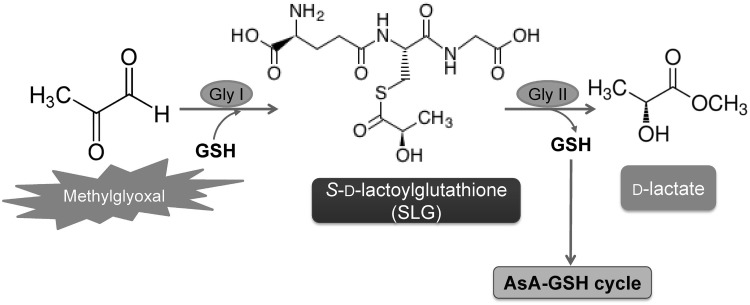
One of the most important functions of GSH is its involvement in the MG detoxification system. MG is an organic compound that is developed under different abiotic stress conditions and cause toxicity to plants. It is a cytotoxic α-oxoaldehyde compound that is highly reactive and produced through different enzymatic and non-enzymatic reactions. Methylglyoxal can create oxidative stress and thus cause considerable damage to plant cells. The enzymatic functions of biochemical reactions including those of antioxidant enzymes can also be disrupted by MG (Wang et al. 2009; Desai et al. 2010). So, in case of conferring tolerance against the abiotic stress, MG detoxification should be considered as an important strategy (Hoque et al. 2008). Gly I (lactoylglutathione lyase; EC 4.4.1.5) and Gly II (hydroxyacylglutathione hydrolase; EC 3.1.2.6) these two enzymes, using GSH as a cofactor, make up the glyoxalase system that detoxifies MG in plants (Fig. 5). These two enzymes use GSH to convert toxic MG and other 2-oxoaldehydes to their 2-hydroxyacids. The detoxification process is a two-step reaction. Gly I first converts MG to S-d-lactoylglutathione (SLG) using GSH, and Gly II then converts SLG to d-lactic acid, simultaneously regenerating GSH (Yadav et al. 2008). This MG detoxifying action of GSH has been confirmed and supported by a number of studies.
Fig. 5.
Roles of glutathione in methylglyoxal (MG) detoxification. First step: The enzyme GlyI (glyoxalase-I) utilizes GSH to convert MG to S-d-lactoylglutathione (SLG). Second step: S-d-lactoylglutathione (SLG) is converted to d-lactate by the activity of GlyII (glyoxalase-II) and GSH is regenerated in this step
Signaling cross talk of glutathione
Glutathione is considered to be a signaling molecule in several studies. Several schemes have been suggested for GSH-induced signal transmission pathways by different researchers, but no complete pathways or mechanisms for GSH-induced signal transmission have been discovered yet. The following is a discussion of some feasible proposed pathways:
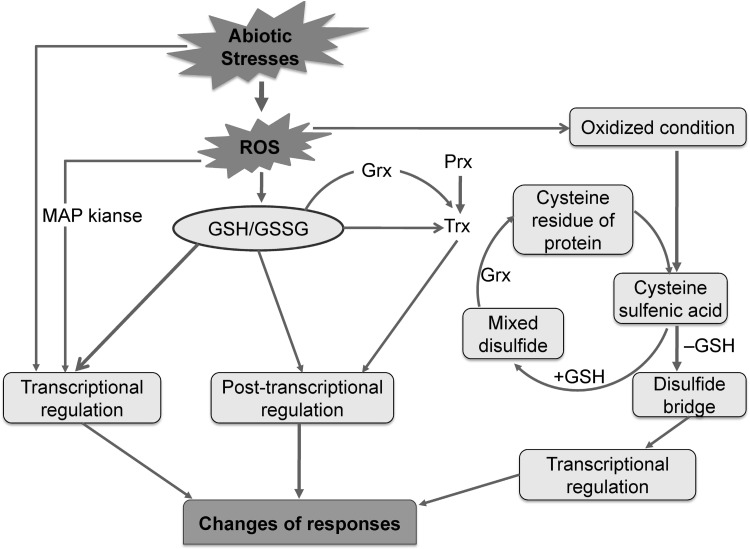
Under different abiotic stress conditions, GSH (either alone or with H2O2 or some other ROS) triggers adaptive or cell death processes through an intercellular signaling system (Foyer and Noctor 2005a, b). Glutathionylation is the posttranslational modification of protein cysteine residues by the addition of glutathione. An involvement of glutathione is suggested in redox signaling processes, where glutathionylation of protein affects the processes of transcription. Glutathione can execute protection against abiotic stress conditions. Stress conditions cause disturbance to the cellular redox homeostasis, thereby influencing transcription factors. It also influences the translation, post-translational modification of proteins, regulation of metabolic processes, and the expression of genes. GSH is actively involved in this signaling process (Szalai et al. 2009; Kumar et al. 2010) but the details of signal transduction pathway is still unknown. A generalized model is presented in Fig. 6 (Szalai et al. 2009).
Fig. 6.
Schematic representation of GSH induced signal transmission under abiotic stress condition. Several pathways are proposed for signal transmission by GSH. Stress induced ROS and the subsequent stress signal may be transmitted through a mitogen activated protein (MAP) kinase cascade to modulate transcriptional regulation. The GSH/GSSG redox system can control redox signaling and can directly participate in transcriptional regulation. Oxidative stressand GSH/GSSG-modulated glutathionylation has been confirmed for a number of proteins including Grx (glutaredoxin) and several GSTs. Redox changes in Trxs (thioredoxin) are important because they target the intercellular disulfide bonds of proteins. Trxs are inactivated by glutathionylation. The reduction of Trxs may be regulated by GPXs (glutathione peroxidases) and Prxs (peroxiredoxins). Trxs can also be activated by deglutathionylation, which is activated by Grxs (glutaredoxins, members of the Trx superfamily). Trx and the GSH/Grx redox systems have important roles in signaling. Interactions between GSH, GSH/GSSG, Trx, Grx, and Prx in stress signaling pathways still require intensive study. Glutathione directly or indirectly regulates the transcriptional or post-translational processes by interacting with other redox systems. Abiotic stress generates ROS/oxidative conditions in the cell. The oxidative environment helps to convert cysteine residues of proteins into cysteine sulfenic acid, which is regulated by GSH and Grx (glutaredoxin). Oxidation of protein then occurs by conversion of cysteine sulfenic acid into cysteine sulfinic acid and cysteine sulfonic acid. Deglutathionization of the cysteine sulfenic acid then occurs to form the disulfide bridge, which transmits the oxidative stress signal to regulate transcriptional factors. Nahar et al. (2016a), with Permission from Wiley
Glutathione modulates the transcriptional or post-translational levels by maintaining interaction with other redox systems directly or indirectly. Stress generates the ROS and creates an oxidative environment. The family of proteins, peroxiredoxins (Prxs) can catalyze the reduction of H2O2 in the presence of GSH and/or other thiols. Prx, with Cys, in its thiolate (S−) form in their active sites (as below).
Prx-SOH characterizes the sulfenic acid intermediate that perpetuate to react with GSH to detoxify OH− and to form Prx-SSG. Later on Prx-S− is formed. The sulfenic acid intermediate or combined thiolate (Prx-S−) are linked with signaling process. Thiol chemistry, GPX reaction or nonenzymatic metal catalyzed oxidations are described for signal transmission. H2O2 does not react with thiols. In case of H2O2 or peroxynitrite reactions with a thiol, which are catalyzed by metal, produce either a sulfinic (–SO2H) or sulfonic (–SO3H) acid. These cannot be reduced easily. H2O2 in low concentrations, react spontaneously with the thiolate anion (S−), which results in the formation of sulfenic acid that has been described to participate in signal transmission (Foyer and Noctor 2005a, b; Szalai et al. 2009; Kumar et al. 2010). Cysteine sulfenic acid and some other fates of this compound might be controlled by GSH (Kumar et al. 2010). The gradual oxidation of this Cys sulfenic acid to Cys sulfinic acid and Cys sulfonic acid can occur and results in oxidation of the protein. The formation of Cys sulfenic acid can be reversed by GSH. Deglutathionylation of Cys sulfenic acid forms a disulfide bridge that can transmit the signal of oxidative stress and influence the transcriptional factors (Fig. 6). Szalai et al. (2009) suggested that the GSH/GSSG redox pair mainly controls redox signaling. Glutathione may be involved in the transcriptional regulation. The stress-induced ROS can have effects on gene expression which may be imparted through a mitogen activated protein kinase (MAP) cascade for transcriptional regulation.
GSSG (oxidized form of GSH) can exchange with protein sulfhydryls and proceed to produce protein–glutathione mixed disulfides (protein–SSG) (protein–SSG can be reconverted into protein-SH reacting with GSH. GS indicates Glu-Cys ligase catalytic subunit) (Szalai et al. 2009).
Mixed disulfides, i.e., protein–SSG, possess a longer half-life than GSSG (because of protein folding). For the action of GSH in cell signaling this exchange reaction renders a vital mechanism. Significant numbers of protein containing critical thiols are involved in the signaling. The functions of the receptors/proteins involved in ubiquitinylation, several protein kinases and transcription factors can be altered upon formation of mixed disulfides. However, once GSSG is produced upon reaction between GSH and ROS, it can be reduced to GSH (by NADPH) by GR.
The function of GSH as an antioxidant that confers abiotic stress tolerance is well established, but the mechanism underlying this defense response is unknown. Cross-communication of GSH with other signaling molecules helps in adaptation to abiotic stress (Ghanta and Chattopadhyay 2011), as suggested by several research findings on GSH cross-talk with signaling molecules. Glutathione and its redox state have major functions in the regulation of plant growth, development, and stress responses (Noctor and Foyer 1998; May et al. 1998). The antioxidative property of GSH is involved in the reduction of H2O2, which in turn alters the redox state of the GSH/GSSG couple. The GSH/GSSG ratio and H2O2 perform a signaling role by affecting proteins during transcription, translation, post-translational processes, and the metabolic processes conducted by the proteins (Dietz 2008; Quan et al. 2008). H2O2 regulates the GSH pool in maize under various abiotic stresses and imposes posttranslational regulation of γ-ECS and GR transcript levels, thereby controlling GSH synthesis, the GSH/GSSG ratio, and GR activity (Kellős et al. 2008). This interaction between H2O2 and GSH served as a stress signal that enhanced chilling tolerance in mungbean (Yu et al. 2003). Noctor et al. (2002) established the involvement of GSH in redox signaling because inter and intracellular GSH were linked by transport across the membranes. Oxidative stress-induced glutathionylation has been recognized as a mechanism for induction and regulation of several proteins, including Grx (glutaredoxin), Trxs (thioredoxin) and GSTs, which are involved in environmental stress responses (Dixon et al. 2005; Reichheld et al. 2007). For example, Grx and GST proteins were induced in Arabidopsis under oxidative stress (Dixon et al. 2005).
Different hormones (e.g. ethylene, JA, and NO) can affect expression of GST at the transcript level. However, this response depends on the growth stage and the tolerance level of the plant (Moons 2005). Abscisic acid may regulate redox signaling by affecting the GSH/GSSG ratio (Pastori and Foyer 2002). Intracellular GSH regulates stomatal movement by acting together with signal molecules. Jasmonic acid regulates Arabidopsis GSH concentration and genes for GSH metabolism (Sasaki-Sekimoto et al. 2005). Glutathione functions as a signal molecule during MeJA signaling in guard cells. In Arabidopsis, intracellular GSH regulates MeJA-induced stomatal movement (Jahan et al. 2008). Signaling roles for GSH were confirmed in carrot vacuoles, where GSH modulates a slow vacuolar channel activity that is also dependent on cytosolic pH (Scholz-Starke et al. 2004). Exogenous SA caused differential changes in the γ-ECS transcript and GSH levels, GR transcript levels, and GR activity in maize genotypes that varied in stress tolerance (Kellős et al. 2008). Salicylic acid altered GSH level and GR activity in soybean cell suspension (Knörzer et al. 1999). Overexpression of the SA metabolism gene resulted in changes in both SA and GSH concentrations in rice that were correlated with oxidative stress tolerance (Kusumi et al. 2006). Nitric oxide can regulate GSH synthesis by regulating genes related to GSH biosynthesis or metabolism (Innocenti et al. 2007). Interaction of GSH with NO forms S-nitrosoglutathione (GSNO) which is involved in nitrogen-based signaling pathways (Neill et al. 2002). Cadmium stress activated a GSNO signal that effectively conferred cadmium stress tolerance in pea plants (Barroso et al. 2006; Hasanuzzaman et al. 2010). Expression of the γ-ECS and GSH synthase genes have been upregulated by NO application in Medicago trunculata roots which led to a significant increase in root GSH level (Kusumi et al. 2006). An involvement of glutathione is suggested in the Ca2+-dependent protein kinase activation which is associated with stress signal pathways. In tobacco GSH and GSSG treatment modulated Ca2+ level, calcium signaling and gene expression (Gómez et al. 2004). Treatment with H2O2 increased the Ca2+ concentration in low temperature stress affected tobacco plant (Price et al. 1994).
Concluding remarks and future perspectives
Glutathione occupies a central position in antioxidant defense; thus, it represents a potent molecule for plant protection against various abiotic stresses. The function of GSH as an antioxidant that confers abiotic stress tolerance is being well established, with evidence from many plant studies. Increased levels of endogenous GSH allow efficient scavenging of ROS, thereby providing the plant cell with relief from oxidative stress experienced during abiotic stresses. Exogenous application of GSH also confers abiotic stress tolerance by maintaining normal physiological functions and improving growth and development. Increases in endogenous GSH levels and GSH/GSSG ratios or application of exogenous GSH are correlated with sound plant health due to maintenance of better plant physiology and biochemistry from many points of view. Higher GSH levels maintain osmotic balance by induction of osmoprotectants and they prevent damage to lipids, amino acids, and polysaccharides, thereby preventing damage to membranes, photosynthetic pigments, mitochondria, and other organelles which ultimately confer tolerance to abiotic stresses (Nahar et al. 2015a, b, c). GSH is indispensable in the detoxification of xenobiotics and heavy metals and for enhancing toxic metal tolerance, as demonstrated by many research findings.
Considerable attempt has been made in underlying GSH biosynthesis, metabolism, and the role played by GSH in plant stress tolerance. Nevertheless, the results with respect to overall stress responses are still extremely variable. The mechanism underlying the GSH defense response is still not established. Thus, future studies need to identify the genes responsible for GSH biosynthesis and the cellular mechanisms underlying the regulation of enzymes related to GSH metabolism or degradation. How the amount of GSH is regulated by biosynthesis/degradation and the required amount of GSH and its regulation in different components of cell are not known. Cross-talk between GSH and other signaling molecules would be anticipated during adaptation to abiotic stress, but this aspect is still poorly studied. The involvement of GSH in signaling pathways, as well as the molecular basis of interactions between GSH and other molecules (e.g., phytohormones, NO, H2O2, and Ca2+) during signaling, still need to be resolved. Some plant species show limitations in GSH synthesis under adverse condition, so the potential for using exogenous GSH to increase GSH levels in stressed plants is now being investigated by many researchers. However, the exact doses and the optimal methods of application are still matters for further research. Various posttranslational modifications such as nitrosylation, hydroxylation, and glutathionylation are involved in stress signaling. The involvement of GSH in these processes is not clearly elucidated. Transcriptomics, proteomics, metabolomics, ionomics and micromics together have been a bloom in revealing plant stress responses and the mechanisms that underlie these responses in different stress response studies. These fields can be incorporated with GSH, its interaction with other hormonal/signaling molecules and cellular redox induced stress signal response and abiotic stress tolerance in plants.
Acknowledgements
The authors wish to thank Mr. Md. Mahabub Alam and Mr. Jubayer-Al-Mahmud, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan for providing several supporting articles and critical reading of the manuscript draft. Kamrun Nahar is grateful to the Japan Government Ministry of Education, Culture, Sports, Science, and Technology (MEXT) for financial support.
Author's contribution
MH planned and designed the work, drawn the figures and wrote some parts. KN wrote the paper and constructed the figures. MF contributed critically to the improvement and editing the manuscript. All authors contributed to improving the paper and approved the final manuscript.
Abbreviations
- γ-ECS
γ-Glutamylcysteine synthetase
- ABA
Abscisic acid
- APX
Ascorbate peroxidase
- AsA
Ascorbate
- CAT
Catalase
- Cys
Cysteine
- DHAR
Dehydroxyascorbate reductase
- Glu
Glutamine
- Gly I
Glyoxalase I (Lactoylglutathione lyase)
- Gly II
Glyoxalase II (Hydroxyacylglutathione hydrolase)
- GPX
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione
- GSHS
Glutathione synthase
- GSNO
S-Nitrosoglutathione
- GSSG
Glutathione disulfide
- GST
Glutathione S-transferase
- JA
Jasmonic acid
- MAP
Mitogen activated protein
- MDA
Malondialdehyde
- MG
Methylglyoxal
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- PC
Phytochelatin
- PCS
Phytochelatin synthase
- POD
Peroxidase
- ROS
Reactive oxygen species
- SA
Salicylic acid
- SLG
S-d-Lactoylglutathione
- SOD
Superoxide dismutase
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Mirza Hasanuzzaman and Kamrun Nahar have contributed equally to this work.
Contributor Information
Mirza Hasanuzzaman, Email: mhzsauag@yahoo.com.
Masayuki Fujita, Phone: +8187-891-3033, Email: fujita@ag.kagawa-u.ac.jp.
References
- Akter N, Sobahan MA, Uraji M, Ye W, Hossain MA, Mori IC, Nakamura Y, Murata Y. Effects of depletion of glutathione on abscisic acid- and methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2012;76:2032–2037. doi: 10.1271/bbb.120384. [DOI] [PubMed] [Google Scholar]
- Alam MM, Hasanuzzaman M, Nahar K, Fujita M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2013;7:1053–1063. [Google Scholar]
- Aravind P, Prasad MNV. Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physiol Biochem. 2005;43:107–116. doi: 10.1016/j.plaphy.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of photooxidative stress and amelioration of defense systems in plants. Boca Raton: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Barroso JB, Corpa FJ, Carreras A, Rodriguez-Serrano M, Esteban FJ, Fernandez-Ocana A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Rio LA. Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot. 2006;57:1785–1793. doi: 10.1093/jxb/erj175. [DOI] [PubMed] [Google Scholar]
- Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell. 2010;22:376–391. doi: 10.1105/tpc.109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann L, Rennenberg H. Glutathione metabolism in plants. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser W, editors. Sulfur nutrition and assimilation in higher plants. The Hague: SPB Academic; 1993. pp. 109–123. [Google Scholar]
- Berthe-Corti L, Hulsch R, Nevries U, Eckardt-Schupp F. Use of batch and fed-batch fermentation for studies on the variation of glutathione content and its influence on the genotoxicity of methyl-nitro-nitrosoguanidine in yeast. Mutagenesis. 1992;7:25–30. doi: 10.1093/mutage/7.1.25. [DOI] [PubMed] [Google Scholar]
- Bianchi MW, Roux C, Vartanian N. Drought regulation of GST8, encoding the Arabidopsis homologue of ParC/Nt107 glutathione transferase/peroxidase. Physiol Plant. 2002;116:96–105. doi: 10.1034/j.1399-3054.2002.1160112.x. [DOI] [PubMed] [Google Scholar]
- Cai Y, Cao F, Wei K, Zhang G, Wu F. Genotypic dependent effect of exogenous glutathione on Cd-induced changes in proteins, ultrastructure and antioxidant defense enzymes in rice seedlings. J Hazard Mater. 2011;192:1056–1066. doi: 10.1016/j.jhazmat.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Çevik S, Ünyayar S. The effects of exogenous application of ascorbate and glutathione on antioxidant system in cultivated Cicer arietinum and wild type C. reticulatum under drought stress. J Nat Appl Sci. 2015;19(1):91–97. [Google Scholar]
- Chen F, Wang F, Wu F, Mao W, Zhang G, Zhou M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem. 2010;48:663–672. doi: 10.1016/j.plaphy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012;158:340–351. doi: 10.1104/pp.111.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MC, Ko K, Chang WL, Kuo WC, Chen GH, Lin TP. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015;83:926–939. doi: 10.1111/tpj.12940. [DOI] [PubMed] [Google Scholar]
- Csiszár J, Szabó M, Erdei L, Márton L, Horváth F, Tari I. Auxin autotrophic tobacco callus tissues resist oxidative stress: the importance of glutathione S-transferase and glutathione peroxidase activities in auxin heterotrophic and autotrophic calli. J Plant Physiol. 2004;161:691–699. doi: 10.1078/0176-1617-01071. [DOI] [PubMed] [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- Dash S, Mohanty N. Response of seedlings to heat-stress in cultivars of wheat: growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. J Plant Physiol. 2002;159:49–59. doi: 10.1078/0176-1617-00594. [DOI] [Google Scholar]
- Dat JF, Foyer CH, Scott IM. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998;118:1455–1461. doi: 10.1104/pp.118.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud MK, Mei L, Azizullah A, Dawood M, Ali I, Mahmood Q, Ullah W, Jamil M, Zhu SJ. Leaf-based physiological, metabolic, and ultrastructural changes in cultivated cotton cultivars under cadmium stress mediated by glutathione. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-016-6739-5. [DOI] [PubMed] [Google Scholar]
- Deng Z, Zhang X, Tang W, Oses-Prieto JA, Suzuki N, Gendron JM, Chen H, Guan S, Chalkley RJ, Peterman TK, Burlingame AL, Wang ZY. A proteomics study of brassinosteroid response in Arabidopsis. Mol Cell Proteom. 2007;6:2058–2071. doi: 10.1074/mcp.M700123-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can J Physiol Pharmacol. 2010;88:273–284. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- Dietz KJ. Redox signal integration: from stimulus to networks and genes. Physiol Plant. 2008;133:459–468. doi: 10.1111/j.1399-3054.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Grundy NM, Edwards R. Stress-induced protein S-lutathionylation in Arabidopsis. Plant Physiol. 2005;138:2233–2244. doi: 10.1104/pp.104.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245:85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts. A proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell Environ. 2005;28:1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x. [DOI] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic K, Korban SS. Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta. 2007;225:1277–1285. doi: 10.1007/s00425-006-0421-y. [DOI] [PubMed] [Google Scholar]
- Gasic K, Korban SS. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol. 2007;64:361–369. doi: 10.1007/s11103-007-9158-7. [DOI] [PubMed] [Google Scholar]
- Ghanta S, Chattopadhyay S. Glutathione as a signaling molecule: another challenge to pathogens. Plant Signal Behav. 2011;6:783–788. doi: 10.4161/psb.6.6.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trived DK, Ahmad I, Pereira E, Tuteja N. Glutathione reductase and glutathione: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem. 2013;70:204–212. doi: 10.1016/j.plaphy.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Gómez LD, Noctor G, Knight MR, Foyer CH. Regulation of calcium signalling and gene expression by glutathione. J Exp Bot. 2004;55:1851–1859. doi: 10.1093/jxb/erh202. [DOI] [PubMed] [Google Scholar]
- Guo J, Dai X, Xu W, Ma M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere. 2008;72:1020–1026. doi: 10.1016/j.chemosphere.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Haghjoua MM, Shariatia M, Smirnoff N. The effect of acute high light and low temperature stresses on the ascorbate–glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol Plant. 2009;135:272–280. doi: 10.1111/j.1399-3054.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. doi: 10.1093/jxb/53.366.1. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Fujita M. Selenium pretreatment up-regulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res. 2011;143:1758–1776. doi: 10.1007/s12011-011-8998-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Fujita M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology. 2013;22:584–596. doi: 10.1007/s10646-013-1050-4. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am J Plant Physiol. 2010;5:295–324. doi: 10.3923/ajpp.2010.295.324. [DOI] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353–365. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Teixeira da Silva JA, Fujita M. Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateswarlu B, Shanker AK, Shanker C, Maheswari M, editors. Crop stress and its management: perspectives and strategies. New York: Springer; 2012. pp. 261–315. [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2012;6:1314–1323. [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar N, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci. 2017;18:200. doi: 10.3390/ijms18010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Bergmann L. γ-glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Hentz S, McComb J, Miller G, Begonia M, Begonia G. Cadmium uptake, growth and phytochelatin contents of Triticum aestivum in response to various concentrations of cadmium. World Environ. 2012;2:44–50. doi: 10.5923/j.env.20120203.05. [DOI] [Google Scholar]
- Hoque MA, Banu MN, Nakamura Y, Shimoishi Y, Murata Y. Proline and glycinebetaine enhance antioxidant and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. Plant Physiol. 2008;165:813–882. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang Y, Peng JS, Zhong C, Yi HY, Ow DW, Gong JW. Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis. Plant Physiol. 2012;158:1779–1788. doi: 10.1104/pp.111.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain TM, Hazara M, Sultan Z, Saleh BK, Gopal GR. Recent advances in salt stress biology: a review. Biotechnol Mol Biol Rev. 2008;3:8–13. [Google Scholar]
- Hussain BMN, Akram S, Sharif-Ar-Raffi Burritt DJ, Hossain MA. Exogenous glutathione improves salinity stress tolerance in rice (Oryza sativa L.) Plant Gene Trait. 2016;7(11):1–17. [Google Scholar]
- Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, Stefano M, Delledonne M, Puppo A, Baudouin E, Frendo P. Glutathione synthesis is regulated by nitric oxide in Medicagotruncatula roots. Planta. 2007;225:1597–1600. doi: 10.1007/s00425-006-0461-3. [DOI] [PubMed] [Google Scholar]
- Iori V, Pietrini F, Massacci A, Zacchini A. Induction of metal binding compounds and antioxidative defence in callus cultures of two black poplar (P. nigra) clones with different tolerance to cadmium. Plant Cell Tissue Org Cult. 2012;108:17–26. doi: 10.1007/s11240-011-0006-8. [DOI] [Google Scholar]
- Jahan MS, Ogawa K, Nakamura Y, Shimoishi Y, Mori IC, Murata Y. Deficient glutathione in guard cells facilitates abscisicacid-induced stomatal closure but does not affect light-induced stomatal opening. Biosci Biotechnol Biochem. 2008;72:2795–2798. doi: 10.1271/bbb.80407. [DOI] [PubMed] [Google Scholar]
- Jiang HW, Liu MJ, Chen IC, Huang CH, Chao LY, Hsieh HL. A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010;154:1646–1658. doi: 10.1104/pp.110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Yang X, Islam E, Liu D, Mahmood Q. Effects of cadmium on ultrastructure and antioxidative defense system in hyperaccumulator and non-hyperaccumulator ecotypes of Sedum alfredii Hance. J Hazard Mater. 2008;156:387–397. doi: 10.1016/j.jhazmat.2007.12.064. [DOI] [PubMed] [Google Scholar]
- Jin X, Yang X, Islam E, Liu D, Mahmood Q, Li H, Li J. Ultrastructural changes, zinc hyperaccumulation and its relation with antioxidants in two ecotypes of Sedum alfredii Hance. Plant Physiol Biochem. 2008;46:997–1006. doi: 10.1016/j.plaphy.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Jozefczak M, Remans T, Vangronsveld J, Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012;13:3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattab H. Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline condition. Aust J Basic Appl Sci. 2007;1:323–332. [Google Scholar]
- Kellős T, Tímár I, Szilágyi V, Szalai G, Galiba G, Kocsy G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008;10:563–572. doi: 10.1111/j.1438-8677.2008.00071.x. [DOI] [PubMed] [Google Scholar]
- Khan M, Daud MK, Basharat A, Khan MJ, Azizullah A, Muhammad N, Muhammad N, Rehman Z, Zhu SJ. Alleviation of lead-induced physiological, metabolic, and ultramorphological changes in leaves of upland cotton through glutathione. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-015-5959-4. [DOI] [PubMed] [Google Scholar]
- Knörzer OC, Lederer B, Durner J, Böger P. Antioxidative defense activation in soybean cells. Physiol Plant. 1999;107:294–302. doi: 10.1034/j.1399-3054.1999.100306.x. [DOI] [Google Scholar]
- Kocsy G, von Ballmoos P, Rüegsegger A, Szalai G, Galiba G, Brunold C. Increasing the glutathione content in a chilling-sensitive maize genotype using safeners increased protection against chilling-induced injury. Plant Physiol. 2001;127:1147–1156. doi: 10.1104/pp.010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsy G, Szalai G, Galiba G. Induction of glutathione synthesis and glutathione reductase activity by abiotic stresses in maize and wheat. Sci World J. 2002;2:1726–1732. doi: 10.1100/tsw.2002.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler BE, Luschin-Ebengreuth N, Stabentheiner E, Müller M, Zechmann B. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Sci. 2014;227:133–144. doi: 10.1016/j.plantsci.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Singla-Pareek SL, Sopory SK. Glutathione homeostasis: crucial for abiotic stress tolerance in plants. In: Pareek A, Sopory SK, Bohnert JH, Govindjee, editors. Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. New York: Springer; 2010. pp. 263–282. [Google Scholar]
- Kusumi K, Yaeno T, Kojo K, Hirayama M, Hirokawa D, Yara A, Iba K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol Plant. 2006;128:651–666. doi: 10.1111/j.1399-3054.2006.00786.x. [DOI] [Google Scholar]
- Laureaua C, Blignyb R, Streba P. The significance of glutathione for photoprotection at contrasting temperatures in the alpine plant species Soldanella alpina and Ranunculus glacialis. Physiol Plant. 2011;143:246–260. doi: 10.1111/j.1399-3054.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Lin SH, Xu PL, Wang XJ, Bai JG. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric Sci China. 2009;8:1075–1086. doi: 10.1016/S1671-2927(08)60315-6. [DOI] [Google Scholar]
- Luo Y, Tang H, Zhang Y. Production of reactive oxygen species and antioxidant metabolism about strawberry leaves to low temperatures. J Agric Sci. 2011;3:89–96. [Google Scholar]
- Ma YH, Ma FW, Zhang JK, Li MJ, Wang YH, Liang D. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci. 2008;175:761–766. doi: 10.1016/j.plantsci.2008.07.010. [DOI] [Google Scholar]
- Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA. Ascorbate and glutathione: protectors of plants in oxidative stress. In: Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA, editors. Ascorbate–glutathione pathway and stress tolerance in plants. Berlin: Springer; 2010. pp. 209–229. [Google Scholar]
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol–peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ. The integration of glutathione homeostasis and redox signaling. J Plant Physiol. 2008;165:1390–1403. doi: 10.1016/j.jplph.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Castagna A, Ranieri A, di Toppi LS. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem. 2012;57:15–22. doi: 10.1016/j.plaphy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Molla MR, Ali MR, Hasanuzzaman M, Al-Mamun MH, Ahmed A, Nazim-Ud-Dowla MAN, Rohman MM. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not Bot Horti Agrob. 2014;42:73–80. [Google Scholar]
- Moons A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal- and hypoxic stress-induced and differentially salt stressresponsive in rice roots. FEBS Lett. 2003;553:427–432. doi: 10.1016/S0014-5793(03)01077-9. [DOI] [PubMed] [Google Scholar]
- Moons A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs) Vitam Horm. 2005;72:155–202. doi: 10.1016/S0083-6729(05)72005-7. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants. 2015 doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot. 2015;112:44–54. doi: 10.1016/j.envexpbot.2014.12.001. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol Plant. 2015;59:745–756. doi: 10.1007/s10535-015-0542-x. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Fujita M. Physiological roles of glutathione in conferring abiotic stress tolerance to plants. In: Gill SS, Tuteja N, editors. Abiotic Stress Response in Plants. Weinheim: Wiley; 2016. pp. 151–179. [Google Scholar]
- Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ Sci Pollut Res. 2016;23:21206–21218. doi: 10.1007/s11356-016-7295-8. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Suzuki T, Fujita M. Polyamines-induced aluminum tolerance in mung bean: a study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology. 2017;26:58–73. doi: 10.1007/s10646-016-1740-9. [DOI] [PubMed] [Google Scholar]
- Najmanova J, Neumannova E, Leonhardt T, Zitka O, Kizek R, Macek T, Mackova M, Kotrba P. Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind Crop Prod. 2012;36:536–542. doi: 10.1016/j.indcrop.2011.11.008. [DOI] [Google Scholar]
- Nazar R, Iqbal N, Syeed S, Khan NA. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol. 2011;168:807–815. doi: 10.1016/j.jplph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurat RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot. 2002;53:1237–1247. doi: 10.1093/jxb/53.372.1237. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Ho T-HD. Effect of heat shock on the metabolism of glutathione in maize roots. Plant Physiol. 1986;82:1031–1035. doi: 10.1104/pp.82.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- Ogawa K. Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal. 2005;7:973–981. doi: 10.1089/ars.2005.7.973. [DOI] [PubMed] [Google Scholar]
- Okuma E, Jahan MS, Munemasa S, Hossain MA, Muroyama D, Islam MM, Ogawa K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Mori IC, Murata Y. Negative regulation of abscisic acid-induced stomatal closure by glutathione in Arabidopsis. J Plant Physiol. 2011;168:2048–2055. doi: 10.1016/j.jplph.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Pasternak M, Lim B, Wirtz M, Hell R, Cobbett CS, Meyer AJ. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J Cell Mol Biol. 2008;53:999–1012. doi: 10.1111/j.1365-313X.2007.03389.x. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH. Common components, networks, and pathways of cross-tolerance to stress. The central role of ‘‘redox’’ and abscisic acid-mediated controls. Plant Physiol. 2002;129:460–468. doi: 10.1104/pp.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose B, Chhikara S, Coomey J, H-I Jung, Vatamaniuk O, Dhankher OP. A γ-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell. 2013;25:4580–4595. doi: 10.1105/tpc.113.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer D, Dreyer E, Polle A. Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol Biochem. 2002;40:141–150. doi: 10.1016/S0981-9428(01)01352-3. [DOI] [Google Scholar]
- Peuke AD, Rennenberg H. Phytoremediation. EMBO Rep. 2005;6:497–501. doi: 10.1038/sj.embor.7400445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot. 2011;62:869–882. doi: 10.1093/jxb/erq340. [DOI] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B, Zeng F, Cai S, Wu X, Haider SI, Wu F, Zhang G. Alleviation of chromium toxicity in rice seedlings by applying exogenous glutathione. J Plant Physiol. 2013;170:772–779. doi: 10.1016/j.jplph.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol. 2008;50:2–16. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell. 2007;19:1851–1865. doi: 10.1105/tpc.107.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxas VP, Smith RK, Allen ER, Allen RD. Over-expressionof glutathione S-transferase/glutathione peroxidase enhances thegrowth of transgenic tobacco seedlings during stress. Nat Biotechnol. 1997;15:988–991. doi: 10.1038/nbt1097-988. [DOI] [PubMed] [Google Scholar]
- Ruegsegger A, Schmutz D, Brunold C. Regulation of glutathione synthesis by cadmium in Pisum sativum. Plant Physiol. 1990;93:1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JM, Blumwald E. Salinity-induced glutathione synthesis in Brassica napus. Planta. 2002;214:965–969. doi: 10.1007/s00425-002-0748-y. [DOI] [PubMed] [Google Scholar]
- Salama KHA, Al-Mutawa MM. Glutathione-triggered mitigation in salt-induced alterations in plasmalemma of onion epidermal cells. Int J Agric Biol. 2009;11:639–642. [Google Scholar]
- Saruhan N, Terzi R, Saglam A, Kadioglu A. The relationship between leaf rolling and ascorbate-glutathione cycle enzymes in apoplastic and symplastic areas of Ctenanthe setosa subjected to drought Stress. Biol Res. 2009;42:315–326. doi: 10.4067/S0716-97602009000300006. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005;44:653–668. doi: 10.1111/j.1365-313X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- Scholz-Starke J, Angeli AD, Ferraretto C, Paluzzi S, Gambale F, Carpaneto A. Redox-dependent modulation of the carrot SV channel by cytosolic pH. FEBS Lett. 2004;576:449–454. doi: 10.1016/j.febslet.2004.09.052. [DOI] [PubMed] [Google Scholar]
- Shankar V, Thekkeettil V, Sharma G, Agrawal V. Alleviation of heavy metal stress in Spilanthes calva L. (antimalarial herb) by exogenous application of glutathione. In Vitro Cell Dev Biol Plant. 2012;48:113–119. doi: 10.1007/s11627-011-9409-9. [DOI] [Google Scholar]
- Shao HB, Chu LY, Lu ZH, Kang CM. Primary antioxidant free radical scavenging and redox signalling pathways in higher plant cells. Int J Biol Sci. 2008;4:8–14. doi: 10.7150/ijbs.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005;46:209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- Shi H, Chen L, Ye T, Liu X, Ding K, Chan Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol Biochem. 2014;82:209–217. doi: 10.1016/j.plaphy.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Shukla D, Kesari R, Mishra S, Dwivedi S, Tripathi RD, Nath P, Trivedi PK. Expression of phytochelatin synthase from aquatic macrophyte Ceratophyllum demersum L. enhances cadmium and arsenic accumulation in tobacco. Plant Cell Rep. 2012;31:1687–1699. doi: 10.1007/s00299-012-1283-3. [DOI] [PubMed] [Google Scholar]
- Singla-Pareek SL, Reddy MK, Sopory SK. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci. 2003;100:14672–14677. doi: 10.1073/pnas.2034667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 2006;140:613–623. doi: 10.1104/pp.105.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets K, Opdenakker K, Remans T, van Sanden S, van Belleghem F, Semane B, Horemans N, Guisez Y, Vangronsveld J, Cuypers A. Oxidative stress-related responses at transcriptional and enzymatic levels after exposure to Cd or Cu in a multipollution context. J Plant Physiol. 2009;166:1982–1992. doi: 10.1016/j.jplph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Son KH, Kim DY, Koo N, Kim KR, Kim JG, Owens G. Detoxification through phytochelatin synthesis in Oenothera odorata exposed to Cd solutions. Environ Exp Bot. 2012;75:9–15. doi: 10.1016/j.envexpbot.2011.08.011. [DOI] [Google Scholar]
- Srivalli S, Khanna-Chopra R. Role of glutathione in abiotic stress tolerance. In: Khan NA, Singh S, Umar S, editors. Sulfur assimilation and abiotic stress in plants. Berlin: Springer; 2008. pp. 207–225. [Google Scholar]
- Szalai G, Kellős T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plantsunder abiotic stress conditions. Plant Growth Regul. 2009;28:66–80. doi: 10.1007/s00344-008-9075-2. [DOI] [Google Scholar]
- Takesawa T, Ito M, Kanzaki H, Kameya N, Nakamura I. Over-expression of glutathione S-transferase in transgenic rice enhances germination and growth at low temperature. Mol Breed. 2002;9:93–101. doi: 10.1023/A:1026718308155. [DOI] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenace of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu J, Wu L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem Pharmacol. 2009;77:1709–1716. doi: 10.1016/j.bcp.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen F, Cai Y, Zhang G, Wu F. Modulation of exogenous glutathione in ultrastructure and photosynthetic performance against Cd stress in the two barley genotypes differing in Cd tolerance. Biol Trace Elem Res. 2011;144:1275–1288. doi: 10.1007/s12011-011-9121-y. [DOI] [PubMed] [Google Scholar]
- Wonisch W, Schaur R. Chemistry of glutathione. In: Grill D, Tausz M, De Kok LJ, editors. Significance of glutathione to plant adaptation to the environment. Dordrecht: Kluwer; 2001. pp. 13–26. [Google Scholar]
- Wu JC, Sun SH, Ke YT, Xie CP, Chen FX. Effects of glutathione on chloroplast membrane fluidity and the glutathione circulation system in young loquat fruits under low temperature stress. Acta Hortic. 2011;887:221–225. doi: 10.17660/ActaHortic.2011.887.36. [DOI] [Google Scholar]
- Wu X, He J, Chen J, Yang S, Zha D. Alleviation of exogenous 6-benzyladenine on two genotypes of eggplant (Solanum melongena Mill.) growth under salt stress. Protoplasma. 2014;251:169–176. doi: 10.1007/s00709-013-0535-6. [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. Glutathione and its central role in mitigating plant stress. In: Pessarakli M, editor. Handbook of plant and crop stress. Basel: Marcel Dekker; 1999. pp. 697–708. [Google Scholar]
- Yadav SK, Singla-Pareek SL, Sopory SK. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact. 2008;23:51–68. doi: 10.1515/DMDI.2008.23.1-2.51. [DOI] [PubMed] [Google Scholar]
- Yao Y, Xu G, Mou D, Wang J, Ma J. Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemosphere. 2012;89:150–157. doi: 10.1016/j.chemosphere.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Yu CW, Murphy TM, Lin CH. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol. 2003;30:955–963. doi: 10.1071/FP03091. [DOI] [PubMed] [Google Scholar]
- Zagorchev L, Seal CE, Kranner I, Odjakova MA. Central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci. 2013;14:7405–7432. doi: 10.3390/ijms14047405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B, Müller M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma. 2010;246:15–24. doi: 10.1007/s00709-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen S, Li Y, Di B, Zhang J, Liu Y. Effect of high temperature and excessive light on glutathione content in apple peel. Front Agric China. 2008;2:97–102. doi: 10.1007/s11703-008-0002-x. [DOI] [Google Scholar]
- Zitka O, Krystofova O, Hynek D, Sobrova P, Kaiser J, Sochor J, Zehnalek J, Babula P, Ferrol N, Kizek R, Adam V. Metal transporters in plants. In: Gupta DK, Corpas FJ, Palma JM, editors. Metal transporters in plants. New York: Springer; 2013. pp. 19–41. [Google Scholar]