Abstract
Somatic embryos were induced from internodal segment derived callus of Oldenlandia umbellata L., in MS medium supplemented with different concentrations of 2,4-Dichlorophenoxy acetic acid (2,4-D). Initially calli were developed from internodes of microshoots inoculated in 2.5 µM NAA supplemented medium. Then calli were transferred to 2,4-D added medium for somatic embryogenesis. Nutritional stress coupled with higher concentration of 2,4-D triggered somatic embryogenesis. Nutritional stress was induced by culturing callus in a fixed amount of medium for a period up to 20 weeks without any external supply of nutrients. Addition of 2.5 µM 2,4-D gave 100% embryogenesis within 16 weeks of incubation. Callus mass bearing somatic embryos were transferred to germination medium facilitated production of in vitro plantlets. MS medium supplemented with 2.5 µM benzyl adenine and 0.5 µM α-naphthalene acetic acid produced 15.33 plants per culture within 4 weeks of culture. Somatic embryo germinated plants were then hardened and transferred to green house.
Keywords: 2,4-D; Nutritional stress; Oldenlandia umbellata; Plant regeneration; Somatic embryos
Introduction
Generally plants show flowering and seed set during unfavorable conditions as a mechanism of survival (Potters et al. 2007). Likewise, studies on somatic embryogenesis revealed that stress has a major role in the induction of somatic embryos (Potters et al. 2007; Jin et al. 2014). Somatic embryogenesis in plants occurs as a result of a series of physiological, biochemical and genetic changes in asexual cells in response to a number of reasons, of which stress is an important one. The stressful conditions induces somatic embryogenesis by chromatin reorganization that lead to ‘accidental’ release of the embryogenic program (Feher 2005). A number of studies also support such views (Steward et al. 2002; Williams et al. 2003; Law and Suttle 2005; Zavattieri et al. 2010). Early stages of somatic embryogenesis are characterized by the induction of many stress-related genes (Davletova et al. 2001) leading to the hypothesis that somatic embryogenesis is an extreme stress response of cultured plant cells (Dudits et al. 1995; Talapatra et al. 2014). In the present study, the influence of nutritional stress in the development of somatic embryos from Oldenlandia umbellata L. callus.
Oldenlandia umbellata L. (Family Rubiaceae) is a small profusely branched biennial herbaceous plant native to Indian subcontinent. The red coloured dye from the roots of the plant makes the plant both pharmacologically and industrially important (Siva 2007; Siva et al. 2009a, 2012; Mahibalan et al. 2016). Though the plant is well known for its medicinal and industrial applications, there were very few reports of the in vitro production of high yielding plants. Somatic embryogenesis could be applicable for the high frequency multiplication of O. umbellata plants in vitro.
Materials and methods
Induction of embryogenic calli
Internodal segments (1–1.5 cm) derived from microshoots (Krishnan and Siril 2015) were inoculated in MS medium supplemented with 2.5 μM NAA for the induction of callus. Agar gelled (0.75%, extra pure, bactograde suitable for plant tissue culture, SISCO Research Laboratories, Mumbai, India) MS medium fortified with different concentrations of 2,4-D (1.0, 2.5, 5.0 or 10.0 μM) (Sigma-Aldrich, St. Louis, US) was used for the development of somatic embryos from callus. Each culture tube was filled with 15 mL of MS media. The pH of the medium was adjusted to 5.8 before autoclaving at 121 °C and 108 kPa pressure for 15 min. Cultures were incubated at 25 ± 2 °C in a culture room with 40 µmol m−2 s−1 irradiance provided by cool white fluorescent tubes (40 W; Philips, India) and were exposed to a photoperiod of 16 h and 55 ± 5% of relative humidity.
Effect of duration of incubation on somatic embryogenesis
To determine the influence of duration of incubation, embryogenic calli developed on 2,4-D (1.0, 2.5, 5.0 & 10 µM) containing media were incubated for different duration (4, 8, 12, 16, 20 weeks) without any external supply of nutrients and percentage of embryogenesis was recorded.
Germination of somatic embryos
Somatic embryos persist on callus mass with maturity were transferred along with embryogenic calli to the regeneration medium. MS medium fortified with a combination of BA and NAA was used for germination of somatic embryos. After 4 weeks of incubation, the plants that were easily detached from callus mass were hardened and transferred to green house.
Experimental design and statistical analysis
All experiments were conducted using a completely randomized block design. Every treatment was composed of three replications and each replication block was represented by seven culture tubes. Data on various parameters were subjected to analysis of variance to determine levels of significance and mean separation was done using Duncan’s Multiple Range Test (DMRT, p < 0.05, Duncan 1955). Data scored in percentages were subjected to arcsine transformation before subjected to statistical analysis and converted back to percentages for the presentation in tables (Snedecor and Cochran 1962).
Result and discussion
Induction of embryogenic callus
Internodal segments inoculated in 2.5 μM NAA media produced callus. The calli were further subcultured in different concentrations of 2,4-D added media for somatic embryogenesis. Initially, calli showed a compact, friable or sticky appearance within 4 weeks. Upon extending duration of incubation period, the nature of callus and percentage of embryogenesis were increased. Callus raised in different concentrations of 2,4-D added media showed difference in growth and morphology. As the period of incubation increased, the percentage of embryogenesis also increased. Following 8-weeks of incubation in MS medium containing 2.5 μM 2,4-D, produced 25% of embryogenesis and 16 weeks incubation in this medium produced 100% embryogenic callus (Fig. 1a–d). An incubation period up to 20 weeks leads to the production of 100% embryogenic callus in medium containing 2.5, 5.0 and 10 μM 2,4-D (Table 1). Previous reports on somatic embryogenesis suggest that a number of stress factors have a positive influence on somatic embryogenesis. For example, certain plant hormones especially auxins induce somatic embryogenesis (Su et al. 2009; Yang et al. 2012). 2,4-D is the most frequently used auxin for the induction of somatic embryogenesis in plants. It is well established that the in vitro development of somatic embryos were strongly influenced by the exogenously supplied auxin. Presence of auxin in the medium possibly influences the gene expression of differentiating cells by increased demethylation of DNA (Lo Schiavo et al. 1989; Steward et al. 2002; Yang and Zhang 2010). Thus, the pro embryonic cells will produce all gene products for globular stage of somatic embryogenesis. After the induction, further development of somatic embryos can be achieved by removing auxin/reducing auxin concentration from the initiation media. This is because the developing embryonic cells also produce some mRNAs and proteins that inhibit further development of embryogenesis in auxin supplemented media. That is why the removal/reduction of auxin from the medium or transfer of developing somatic embryos to new medium is necessary during somatic embryogenesis (Zimmerman 1993; Yang and Zhang 2010). Similarly, in the present study the prolonged nutritional stress along with higher levels of exogenous auxin might be the reason for the increased percentage of induction of somatic embryos from callus.
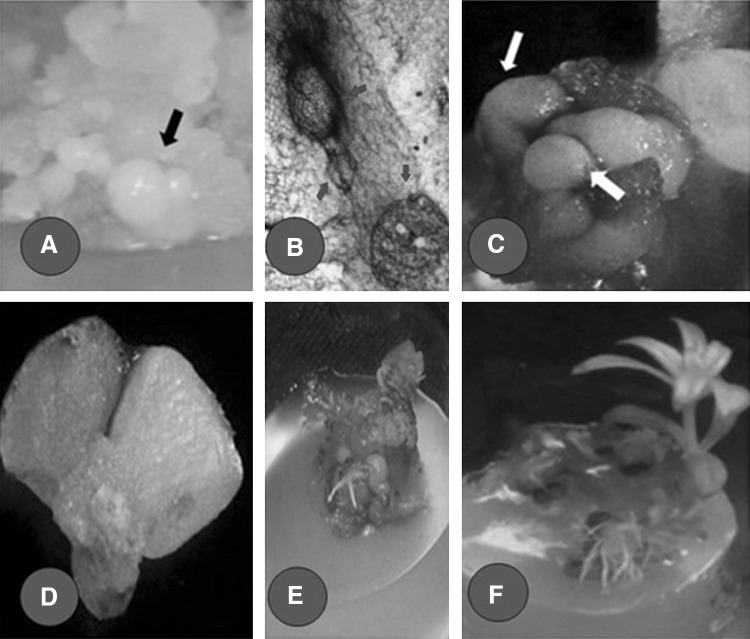
Fig. 1.
a Embryogenic calli with different stages of somatic embryos developed after 16 weeks of continuous incubation on MS medium containing 2.5 µM 2,4-D. b C.S of embryogenic callus showing the initial stage of development of globular embryo. c, d Stereo microscopic images of globular and heart shaped embryos. Globular and heart shaped stages are indicated with arrow mark. e Callus with germinating somatic embryos on MS medium supplemented with 2.5 µM BA and 0.5 µM NAA. f Plantlets developed from somatic embryo on MS plus 2.5 µM BA and 0.5 µM NAA
Table 1.
Effect of duration of callus incubation on MS medium supplemented with different concentrations of 2,4 -D on somatic embryogenesis
| Conc. of 2,4-D | Duration of incubation in weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 12 Weeks | 16 Weeks | 20 Weeks | ||||||
| Type of callus | % of embryo genesis | Type of callus | % of embryo genesis | Type of callus | % of embryo genesis | Type of callus | % of embryo genesis | Type of callus | % of embryo genesis | |
| 1.0 | Friable | 0.00 ± 0.00 | Sticky | 0.00 ± 0.00b | Compact | 4.17 ± 0.17c | Embryogenic | 12.50 ± 1.22b | Embryogenic | 66.67 ± 8.33b |
| 2.5 | Compact sticky | 0.00 ± 0.00 | Compact Embryogenic |
25.00 ± 1.22a | Embryogenic | 87.50 ± 2.22a | Embryogenic | 100.00 ± 0.00a | Embryogenic | 100.00 ± 0.00a |
| 5.0 | Sticky | 0.00 ± 0.00 | Compact | 0.00 ± 0.00b | Embryogenic | 37.50 ± 1.22b | Embryogenic | 75.00 ± 1.43a | Embryogenic | 100.00 ± 0.00a |
| 10.0 | Sticky | 0.00 ± 0.00 | Compact Embryogenic |
8.33 ± 0.17b | Embryogenic | 70.83 ± 1.17a | Embryogenic | 91.67 ± 2.33a | Embryogenic | 100.00 ± 0.00a |
| Treatment Df (n − 1) = 3 | – | – | 8.00** | – | 39.33*** | – | 19.00*** | – | 16.00*** | |
Means within a column followed by same letters are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test
NS non significant
*** F value is highly significant at p < 0.001 level; **significant at p < 0.01 level; *significant at p < 0.05
Germination of somatic embryos
Callus mass bearing somatic embryos were transferred to germination medium resulted in the production of plantlets (Fig. 1e, f). MS medium supplemented with different concentrations of BA and NAA served as germination medium. A combination of 2.5 μM BA and 0.5 μM NAA produced significantly (p < 0.05) higher number of plants than other BA and NAA combinations. On an average 15 plants per culture were emerged from 2.5 μM BA and 0.5 μM NAA combinations (Table 2). The overall development of plantlet from somatic embryos were duly documented (Fig. 2). The plants derived through somatic embryos were further hardened and transferred to a greenhouse.
Table 2.
Germination of somatic embryos of O. umbellata L. in different BA containing medium
| Hormone | Number somatic embryos germinated/culture | Height of SE raised plants | |
|---|---|---|---|
| BAP (µM) | NAA (µM) | ||
| 2.5 | 0.25 | 7.67 ± 1.20b | 5.03 ± 0.41a |
| 5 | 0.25 | 8.00 ± 2.31b | 5.10 ± 0.12a |
| 2.5 | 0.5 | 15.33 ± 1.20a | 5.23 ± 0.48a |
| 5 | 0.5 | 9.00 ± 2.08b | 4.53 ± 0.20a |
| 2.5 | 1.0 | 6.00 ± 2.08b | 5.00 ± 0.15a |
| 5 | 1.0 | 5.00 ± 2.08b | 5.47 ± 0.12a |
| Treatment Df (n − 1) = 5 | 3.754* | 1.178NS | |
Means within a column followed by same letters are not significantly (p < 0.05) different as determined by Duncan’s multiple range test
*** F value is highly significant at p < 0.001 level; **significant at p < 0.01 level; *significant at p < 0.05
NS non-significant
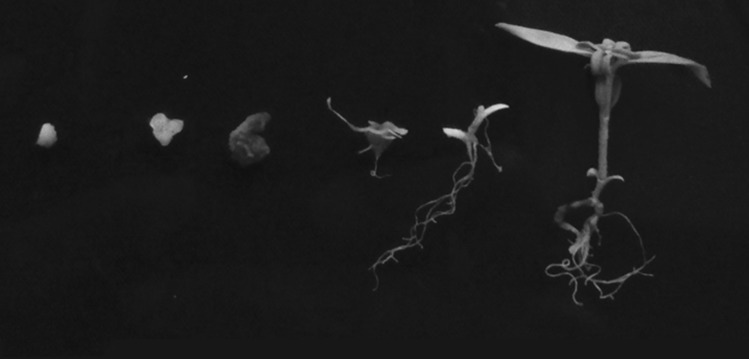
Fig. 2.
Different stages of somatic embryogenesis from globular stage to fully germinated stage
The protocol discussed above is an effective way for in vitro multiplication of O. umbellata. In an early report (Rao and Bahadur 1990) on O. umbellata, somatic embryogenesis was achieved by the addition of BA at 0.2 mg L−1, ascorbic acid at 0.1% and sucrose at 1.5%, under diffused light (300 lx). Similarly Siva et al. (2009b) has reported somatic embryogenesis and organogenesis from callus using a combination of 1.5 mg L−1 BA, 0.3 mg L−1 NAA and 1% coconut milk. Optimized media combinations in the previous report use complex organic additives along with BA and NAA for organogenesis that diminishes reproducibility of such techniques. The present study is mainly focused on the impact of nutritional stress in somatic embryogenesis from O. umbellata, and the study reveals that prolonged nutritional stress can induce 100% somatic embryogenesis (without any complex additives in the medium) from callus which can result in high turnout in tissue culture of O. umbellata. Further, the molecular mechanism behind the spontaneous triggering of somatic embryogenesis during nutritional stress needs to be studied.
Acknowledgements
The authors are thankful to Dr. Suharabeevy, Professor and Head, Department of Botany University of Kerala for facilities provided. SKSR thank University of Kerala, Thiruvananthapuram, India for granting University JRF (Ac E1B1/43700/2011 dt. 26/12/11).
References
- Davletova S, Mészáros T, Miskolczi P, Oberschall A, Török K, Magyar Z, Dudits D, Deák M. Auxin and heat shock activation of a novel member of the calmodulin like domain protein kinase gene family in cultured alfalfa cells. J Exp Bot. 2001;52:215–221. doi: 10.1093/jexbot/52.355.215. [DOI] [PubMed] [Google Scholar]
- Dudits D, Györgyey J, Bögre L, Bako L. Molecular biology of somatic embryogenesis. In: Thorpe TA, editor. In vitro embryogenesis in plants. Dordrecht: Kluwer Academic Publishers; 1995. pp. 267–308. [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Feher A. Why somatic plant cells start to form embryos? In: Mujid A, Samaj J, editors. Somatic embryogenesis. Plant cell monographs. Berlin: Springer; 2005. pp. 85–101. [Google Scholar]
- Jin F, Hu L, Yuan D, Xu J, Gao W, He L, Yang X, Zhang X. Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J. 2014;12:161–173. doi: 10.1111/pbi.12123. [DOI] [PubMed] [Google Scholar]
- Krishnan SRS, Siril EA. Enhanced in vitro shootregeneration in Oldenlandia umbellata L. by using quercetin: anaturally occurring auxin-transport Inhibitor. P Natl Acad Sci India Sect B Biol Sci. 2015 [Google Scholar]
- Law RD, Suttle JC. Chromatin remodeling in plant cell culture: patterns of DNA methylation and histone H3w and H4 acetylation vary during growth of asynchronous potato cell suspensions. Plant Physiol Biochem. 2005;43:527–534. doi: 10.1016/j.plaphy.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Lo Schiavo F, Pitto L, Giuliano G, Torti G, Nutironchi V, Marazziti D, Vergara R, Selli S, Terzi M. DNA methylation of embryogenic carrot cell cultures and its variations ascaused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet. 1989;77:325–331. doi: 10.1007/BF00305823. [DOI] [PubMed] [Google Scholar]
- Mahibalan S, Rao PC, Khan R, Basha A, Siddareddy R, Masubuti H, Fujimoto Y, Begum AS. Cytotoxic constituents of Oldenlandia umbellata and isolation of a new symmetrical coumarin dimer. Med Chem Res. 2016 [Google Scholar]
- Potters G, Pasternak T, Guisez Y, Palme KJ, Jansen M. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rao GP, Bahadur B. Somatic embryogenesis and plant regeneration in self-incompatible Oldenlandia umbellata L. (Rubiaceae) Phytomorphology. 1990;40:95–101. [Google Scholar]
- Siva R. Status of natural dyes and dye yielding plants in India. Curr Sci. 2007;92:916–925. [Google Scholar]
- Siva R, Mudgal G, Rajesh D, Khan NF, Vijayakumar V, Rajasekaran C. Characterization of novel pH indicator of natural dye Oldenlandia umbellata L. Nat Prod Res. 2009;23:1210–1217. doi: 10.1080/14786410802696635. [DOI] [PubMed] [Google Scholar]
- Siva R, Rajasekaran C, Mudgal G. Induction of somatic embryogenesis and organogenesis in Oldenlandia umbellata L., a dye-yielding medicinal plant. Plant Cell Tiss Org. 2009;98:205–211. doi: 10.1007/s11240-009-9553-7. [DOI] [Google Scholar]
- Siva R, Mayes S, Behera SK, Rajasekaran C. Anthraquinones dye production using root cultures of Oldenlandia umbellata L. Ind Crop Prod. 2012;37:415–419. doi: 10.1016/j.indcrop.2011.12.027. [DOI] [Google Scholar]
- SnedecorGW Cochran WG. Statistical methods. Iowa: The Iowa State University Press; 1962. [Google Scholar]
- Steward N, Ito M, Yamaguchi Y, Koizumu N, Sano H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J BiolChem. 2002;277:37741–37746. doi: 10.1074/jbc.M204050200. [DOI] [PubMed] [Google Scholar]
- Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009;59:448–460. doi: 10.1111/j.1365-313X.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapatra S, Ghoshal N, Raychaudhuri SS. Molecular characterization, modeling and expression analysis of a somatic embryogenesis receptor kinase (SERK) gene in Momordica charantia L., during somatic embryogenesis. Plant Cell Tiss Org. 2014;116:271–283. doi: 10.1007/s11240-013-0401-4. [DOI] [Google Scholar]
- Terzi M. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. TheorAppl Genet. 1989;77:325–331. doi: 10.1007/BF00305823. [DOI] [PubMed] [Google Scholar]
- Williams L, Zhao J, Morozava N, Li Y, Avivi Y, Grafi G. Chromatin reorganization accompanying cellular differentiation is associated with modifications of histone H3, redistribution of HP1, and activation of E2F-target genes. Dev Dyn. 2003;228:113–120. doi: 10.1002/dvdy.10348. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang X. Regulation of somatic embryogenesis in higher plants. CrcCr Rev Plant Sci. 2010;29:36–57. doi: 10.1080/07352680903436291. [DOI] [Google Scholar]
- Yang X, Zhang X, Yuan D, Jin F, Zhang Y, Xu J. Transcript profiling reveals complex auxin signaling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biol. 2012;12:110–129. doi: 10.1186/1471-2229-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol. 2010;13:12–13. doi: 10.2225/vol13-issue1-fulltext-4. [DOI] [Google Scholar]
- Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. Plant Cell. 1993;5:1411–1423. doi: 10.1105/tpc.5.10.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]