Abstract
The effect of rutin and gallic acid on growth, phytochemical and defense gene activation of rice (Oryza sativa L.) was investigated. The seeds of rice were primed with different concentrations of rutin and gallic acid (10–60 µg mL−1) to explicate the effect on germination on water agar plates. Further, to study the effect of most effective concentrations of gallic acid (60 µg mL−1) and rutin (50 µg mL−1), greenhouse pot experiment was set up to determine the changes in growth, antioxidant and defense parameters. The results revealed more pronounced effect of gallic acid on total chlorophyll and carotenoids as well as on total flavonoid content and free radical scavenging activities. Gene expression analysis of OsWRKY71, PAL, CHS and LOX genes involved in strengthening the plant defense further validated the results obtained from the biochemical analysis. Microscopic analysis also confirmed reduction in total reactive oxygen species, free radicals like H2O2 and O2 − by exogenous application of gallic acid and rutin. The data obtained thus suggest that both gallic acid and rutin can affect the growth and physiology of rice plants and therefore can be used to develop effective plant growth promoters and as substitute of biofertilizers for maximizing their use in field conditions.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0430-2) contains supplementary material, which is available to authorized users.
Keywords: Antioxidants, Gallic acid, Oryza sativa L., Reactive oxygen species, Rutin
Introduction
Farmer community around the globe often face the issue of low quality yield and loss of soil fertility. Further, ever increasing growing population is adding up to their menace. Therefore, in order to counter the above challenges and keeping in mind the socio-economic aspects in agriculture; development of innovative, environment-friendly and cost effective agronomy techniques to improve the crop yield is the need of hour. One of the emerging fields in this direction is allelopathic science which is not only environmentally friendly but also offers effective solution for the augmentation in crop yield (Wardle et al. 2011; Makoi and Ndakidemi 2012). Allelochemicals, which includes wide range of secondary compounds, are reported to interact with plants thereby positively influencing their growth and development at both cellular as well as molecular level (Hegab et al. 2008; Niakan et al. 2008). Recently, their potential has been successfully harnessed by few of the workers by using them as substitutes for bio-herbicides and biofertilizers (Machado 2007; Makoi and Ndakidemi 2012; Maqbool et al. 2013).
Among the various allelochemicals, phenolic compounds embody a varied collection of plant secondary metabolites like uncomplicated phenylpropanoids, flavonoids, tannins, coumarins, lignin and its precursors and benzoic acid derivatives (Vermerris and Nicholson 2006) etc. These compounds not only act as efficient free radical scavengers but also inhibit the lipid peroxidation process and membrane stabilizers (Arora et al. 2000; Michalak 2006; Verstraeten et al. 2003). They also have marked ameliorative effects towards various abiotic stresses like heavy metals, chilling, osmotic stress and salinity (El-Tayeb et al. 2006; El-Soud et al. 2013; Li et al. 2011; Singh et al. 2013; Ozfidan-Konakci et al. 2015; Chauhan et al. 2017). Among the various class of plant phenolics, coumarin and its derivatives (Razavi 2011; Al-Amiery et al. 2012; Saleh et al. 2015), ferulic acid (Li et al. 2013), ellagic acid (El-Soud et al. 2013) etc. have attracted the interest of researchers for their effect on plant growth and many plant physiological processes. However, still number of plant phenolics with positive effects on various physiological processes linked with seed germination as well as plant growth and development remains unexplored. Amongst them, gallic acid and rutin are two allelochemicals which are extensively dispersed in plant kingdom. They have been mainly reported for their anticarcinogenic, antioxidative, antimutagenic and anti-inflammatory activities (Sharma et al. 2013; Choubey et al. 2015). However, to best of our knowledge the effect of these molecules as bio-priming agents on growth promotion, phytochemical and antioxidant activities remains unexplored. Keeping this in mind two different phenolic acids namely gallic acid and rutin were picked up for assessing their effect on inducing the defensive state and growth parameters of rice (Oryza sativa L.) in a dose dependent manner.
Materials and methods
Plant material and in vitro growth conditions
Surface sterilization of O. sativa seeds (cultivar BLB Pusa Basmati 1, India) was done for 5 min using sodium hypochlorite (2%) and washed with sterile distilled water several times. The sterilized seeds were divided into three groups; first and second group seeds were soaked in different concentration of rutin and gallic acid (10–60 µg mL−1 in water) respectively, for 2 h at 22 °C while the third lot of seeds were immersed in similar set of conditions (unprimed). After treating the seeds, they were left for drying in air cabinet. Primed and unprimed seeds were then plated equidistantly on soft agar plates with moistened blotter discs to record the effect on seed germination.
In-vivo experiments
The best concentration of rutin and gallic acid obtained from the seed germination assay was selected for the greenhouse trial. Surface sterilized O. sativa seeds treated with rutin (50 µg mL−1) and gallic acid (60 µg mL−1) were transferred in pots (7.5 cm diameter) containing sterilized soil [pH 7.4, soil: compost (3:1) sieved through 2.5 mm mesh] under natural light conditions in a greenhouse. The sterilized soil was of sandy loam nature with EC 0.35 dS m−1, 150 kg ha−1 available form of nitrogen (alkaline permanganate extractable), 7.5 kg ha−1 available P (0.40 M NaHCO3 extractable), and 76 kg ha−1 potassium (neutral N ammonium acetate extractable). The experiment was designed with following three treatments: (a) control (healthy) plants (b) rutin (50 µg mL−1) and (c) gallic acid (60 µg mL−1) treated seeds. Four weeks after emergence of the seedlings, they were harvested and the data for different growth parameters (shoot and root length) were recorded. Random samples of fresh seedlings were uprooted from every individual group, crushed right away under liquid nitrogen and used for biochemical and gene expression analysis. Seven replications were maintained for each treatment from each set and the data from the replicated pots were then pooled for analysis.
Phytochemical and antioxidant activities after rutin and gallic acid priming
Total chlorophyll (“a” and “b”) and carotenoid contents were measured according to Singh et al. (2016) and Lichtenthaler and Wellburn (1983), respectively. Briefly, 100 mg of powdered leaf sample was extracted with CH3OH:H2O (9:1, v/v) mixture. The reaction mixture was centrifuged at 10,000g for 5 min and the optical density was read at 663, 645, 480 and 510 nm. The total phenolic content (TPC) was calculated using gallic acid (mg per g of dried sample) as standard (Gupta et al. 2017). A sodium carbonate solution (7%; 2.5 mL) and 0.5 mL of Folin Ciocalteu reagent were added to 1 mL methanolic extract of leaf tissue. Further, the reaction was placed in dark for 2 h and the absorbance was recorded at 765 nm. For, total flavonoid content (TFC), methanolic plant tissue (0.5 mL) extract was mixed with sodium nitrite solution (15%; 0.15 mL) and after 6 min the NaOH (4%; 2 mL) solution was added. The volume was made up to 5 mL using sterile water and the absorbance was recorded at 510 nm after 15 min (Gupta et al. 2016). Free radical scavenging activity was quantified by mixing 100 µL of the extracted sample, with 400 µL Tris–HCl (0.1 M, pH 7.4) and 500 µL of methanolic 1, 1-diphenyl-2-picryl-hydrazil solution (0.5 mM). The reaction was kept at room temperature (15 min) and the absorbance was recorded at 517 nm (Brand-Williams et al. 1995). The activity was estimated as: FRSA (%) = [(controlabsorbance − Treatmentabsorbance) (controlasbsorbance)−1] × 100.
The total antioxidant capacity (TAC) was measured by adding 1 mL of reagent solution [sulphuric acid (3.3 mL), sodium phosphate (335 mg) and ammonium molybdate (78.416 mg) in 100 mL] with 100 µL of methanol extracted leaf tissue (Gillespie et al. 2007). The mixture was kept at 100 °C for 1 h and the activity was estimated as: TAC (%) = [(Treatmentabsorbance − controlabsorbance) (controlabsorbance)−1] × 100.
Histochemical analysis of H2O2 and O2−, reactive oxygen species and callose deposition
Localization of H2O2 was conducted using the 3, 3-diaminobenzidine (DAB; Sigma) staining (Fryer et al. 2002). The DAB solution was freshly prepared in sterile water (pH 3.8 with KOH) to avoid auto-oxidation. Segments were kept in 1 mg mL−1 DAB solution for 10 min and kept in the dark for 4 h at 30 °C in the same solution under very slow shaking. After staining for the above mentioned time, stained leaf segments were then bleached in mixture of acetic acid-glycerol-ethanol (1:1:3) (v/v/v) at boiling temperature for 5 min, and then incubated in glycerol-ethanol (1:4) (v/v) solution until final photographs were taken. H2O2 production was localized as a brown color owing to DAB polymerization. Three biological independent repeats were conducted for each experiment. Stained segments were observed under light microscope (Leica, Germany) to obtain images.
For detection of O2 −, nitroblue tetrazolium (NBT; Sigma) was used. Leaves segments were kept in 0.1% NBT (w/v) prepared in NaN3 (10 mM) in potassium phosphate buffer (10 mM; pH 7.8) for 20 min (Rao and Davis 1999). Thereafter, stained segments were bleached to remove chlorophyll in acetic acid–glycerol–ethanol (1:1:3) (v/v/v) solution at boiling temperature for 5–8 min. Further, leaf segments were then incubated in a glycerol–ethanol (1:4) (v/v) solution until photographs were taken.
The reactive oxygen species (ROS) generated in leaves was visualized using the fluorescent probe 5-(and 6)-carboxy-2′,7′-dichloro dihydrofluorescein diacetate (DCF-DA) (Sigma). The primed and unprimed leaves were kept in for 3 min in DCF-DA (10 μM) in 2-morpholinoethanesulfonic acid; 10 mM; pH 6.1). Leaves were observed in epifluorescence microscope (excitation 480/40 nm, emission 527/30 nm) using a GFP filter set (Beneloujaephajri et al. 2013). Callose deposition was also analysed using aniline blue (0.01%). For callose visualization, leaf segments were further bleached as aforementioned and examined for the presence of cell wall deposits using a UV epifluorescence microscope (Ellinger et al. 2013).
Transcription analysis by real-time RT-PCR
Total RNA was isolated by trizol (Invitrogen) method as per the manual protocol. RNA was reverse-transcribed with random primers using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Actin gene was taken as the internal control. All the primers involved in the experiment were reported by Liu et al. (2007) and Hao et al. (2011) (Table S1). Real time RT-PCR was done as described by Hao et al. (2011). The PCR reactions were performed in triplicate. To analyze the real-time PCR data the 2−ΔΔCT method was used as previously reported by Livak and Schmittgen (2001).
Data analysis
The result was expressed as the mean of triplicates. Significant means were analyzed using Tukey’s multiple comparison test at P < 0.05 by SPSS package (SPSS V16.0, SPSS Inc., Chicago, IL) and principal component analysis (PCA) was applied to select appropriate components in current investigation.
Results and discussion
The seed germination assay was set up to measure shoot and root length of rice primed with different concentration of rutin and gallic acid. Rutin and gallic acid showed the most significant results at 50 and 60 μg mL−1 concentrations respectively (Fig. S1). Rutin at the concentration of 50 μg mL−1 significantly (P ≤ 0.05) stimulated plumule and radical length which showed an increase of 2.98 and 2.91 fold compared to the control seedlings, respectively (Table S2). However, gallic acid at a concentration of 60 μg mL−1 significantly (P ≤ 0.05) enhanced plumule (1.69 fold) and radical (2.65 fold) lengths as compared to the control seedlings, respectively (Table S2).
In the previous reports, a stimulatory or inhibitory effect of phenolic compounds on germination and early seedling growth have been observed depending on the compound taken, its concentration, and the plant selected (Reigosa et al. 1999). The results obtained here demonstrated that the effect of rutin and gallic acid is concentration-dependent which was more prominent on growth rather than germination of rice seedlings (Mata et al. 1998). Differential responses were obtained by Saleh et al. (2015) who showed the effect of these compounds on germination of faba bean seeds while Mata et al. (1998) observed the effect on growth parameters.
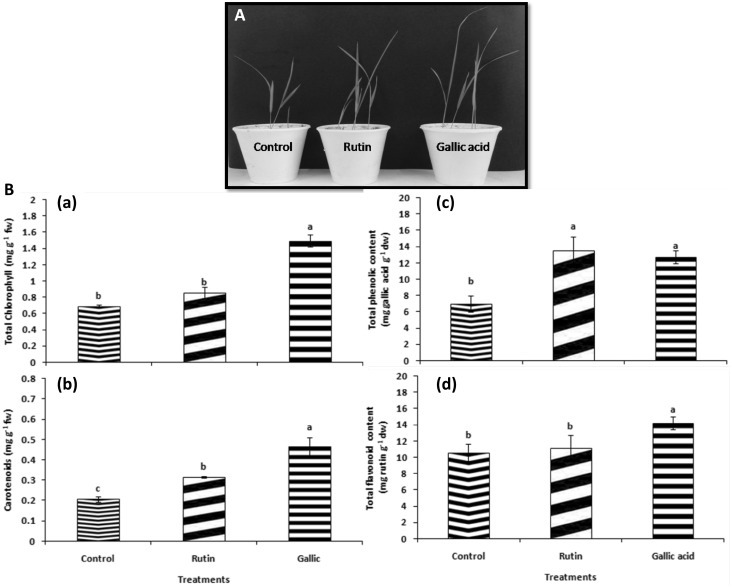
As illustrated in Fig. 2a, the rice seeds inoculated with rutin (50 μg mL−1) and gallic acid (60 μg mL−1) treatments, resulted in increase (P ≤ 0.05) in plant growth than the control plants in greenhouse experiment. The growth parameters (shoot and root length) were significantly augmented when seeds were treated with rutin and gallic acid (Table 1). The highest increment in shoot and root length was found to be 1.28 and 2.05 fold, respectively higher in 60 μg mL−1gallic acid treatment followed by 50 μg ml−1 rutin treatment (1.14 and 1.38 fold respectively) over the control plants as showed in Table 1. The biochemical parameters data additionally supported the growth parameters results as pronounced increase was observed in rutin and gallic acid primed seedlings than the control plants. Total content of chlorophyll in leaves was increased (P < 0.05) in treatments primed with rutin and gallic acid (Fig. 1Ba). The augmentation in total chlorophyll and carotenoids by 2.20 and 2.26 fold, respectively was found in gallic acid treatment proceeded by rutin (1.26 and 1.53 fold, respectively) as compared to control (Fig. 1Ba, Bb). The accumulation of chlorophyll in plants is well known to be linked with the incorporation of pigment into highly organized lipoprotein arrangement in the chloroplast. In a study conducted by Singh and Chaturvedi (2014), ameliorative effect of cinnamic acid (CA) was observed in maize plants as contents of chlorophyll a, b and carotenoids were significantly higher in the presence of 0.05 mM CA under salinity stress in comparison to only salinity stressed plants. In the present investigation too it can be speculated that the increase in total chlorophyll and carotenoid contents in pretreated gallic and rutin rice seedlings probably might have resulted in higher photosynthetic rates due to higher photosynthetic pigments thereby improving the overall plant growth.
Fig. 2.
Effect of rutin (Ru) and gallic acid (Ga) inoculation on a free radical scavenging activity and b total antioxidant capacity of rice. Bars indicate the standard errors of the means from three replicates. Columns with different letters are statistically different according to the Tukey’s test (P < 0.05) among treatment
Table 1.
Effect of rutin (Ru) and gallic acid (Ga) on growth parameters of rice in greenhouse experiment
| Treatments | Shoot length (cm) | Root length (cm) |
|---|---|---|
| Control | 18.00 ± 0.58b | 1.95 ± 0.03c |
| Rutin (50 µg/mL) | 20.50 ± 0.87ab | 2.70 ± 0.17b |
| Gallic acid (60 µg/mL) | 23.00 ± 1.15a | 4.00 ± 0.11a |
Results are means of three replicates ± SD and means within a column followed by the same letter are not significantly different (P ≤ 0.05)
Fig. 1.
Comparison of growth and biochemical parameters between different treatments (a) effect of different treatments on plant growth promoting traits of rice and (b) (a) total chlorophyll (mg g−1 fw), (b) carotenoid content (mg g−1 fw), (c) total phenolic (mg g−1 dw) and (d) total flavonoid content (mg g−1 dw) of rice after treatment with rutin, gallic acid and control. Bars indicate the standard errors of the means from three replicates. Columns with different letters are statistically different according to the Tukey’s test (P < 0.05) among treatment
However, higher TPC was recorded in seedlings primed with rutin (13.52 mg gallic g−1) followed by gallic acid (12.71 mg gallic g−1) treatment, which were about 1.40 and 1.31 folds respectively higher than the control seedlings (Fig. 1Bc). The maximum TFC content was found in gallic acid (14.22 mg rutin g−1) followed by rutin (11.10 mg rutin g−1), which were 1.51 and 1.18 folds, respectively in elevated amount than the control plants (Fig. 1Bd). Priming by exogenous application of compounds or microbes is well known phenomenon for making the plants respond faster and stronger to abiotic and biotic challenge. Among the various pathways triggered, induction of bioactive secondary metabolites like flavonoids and phenolic acids play a noteworthy role in plant defense. Recently, El-Soud et al. (2013) established that pre-treatment of chickpea seedlings with ellagic acid resulted in higher phenylalanine ammonia lyase activity, a key enzyme acting as a connector between primary and secondary metabolism under osmotic stress as compared to the untreated seedlings. Likewise, Saleh et al. (2015) reported induction of salicylic, syringic, gallic and ferulic acids in coumarin primed wheat seedlings. The enhanced TPC and TFC in the present investigation thus, provide an evidence of direct or indirect role played by rutin and gallic acid in regulation of shikimic acid biosynthetic pathway.
Further, the gallic acid treated seedlings (P < 0.05) increased FRSA (1.08 fold) followed by treatment having rutin as compared to the control plants (Fig. 2a). However, insignificant value of TAC was found in rutin treatment, which was 1.13 fold higher than the control (Fig. 2b). The obtained results can be very well corroborated with the increased accumulation of total phenolic content as they are well known antioxidants and improve the nonenzymatic antioxidant capacity of plants by their FRSA (Karamac et al. 2005; Michalak 2006).
Reduction in hydrogen peroxide content was observed in leaf tissues of treatments having rutin and gallic acid (Fig. 3A). This visible reduction was observed by DAB staining (Fig. 3A). The gallic acid (60 μg mL−1) treatment showed least brown stain deposit in comparison with the control and rutin (50 µg mL−1) treated plants (Fig. 3A). In the control plants, superoxide content was highest on the leaf surface (Fig. 3B1). Visual observations of superoxide radical in leaves can be correlated with the H2O2 results as strong blue coloration was found in the leaves of control treatment plants. However, in comparison to the control plants, the plants treated with rutin and gallic acid showed less blue pigmentation mostly in veins and sparsely in the interveinal regions (Fig. 3B2, B3). Likewise, histochemical analysis of ROS in rice leaves using DCF-DA also revealed maximal expanse of ROS localization in the midrib region of healthy plants than the treated ones (Fig. 3C1). In contrast to the control, lesser ROS was found in gallic acid treatment followed by rutin (Fig. 3C2, C3). ROS such as H2O2 and O2 − are usually produced in plants as toxic by products of aerobic metabolism whose concentration increases when plants are exposed to a variety of both biotic and abiotic stresses (Bailey-Serres and Mittler 2006). Similarly, Chauhan et al. (2017) reported increased production of H2O2 and O2 − in both root and shoot during arsenic stress. The excess production and accumulation of ROS is linked with lipid peroxidation (Foyer et al. 1994) which can damage ultra structure, disturb the normal plant metabolism, and can even lead to cell death. Application of phenolic acids for alleviation of different kinds of stress is being taken up by the research community globally as in some of the recent investigations priming of cucumber seedlings with phenolic acids like ferulic and cinnamic acid attenuated dehydration-induced oxidative stress via expression of antioxidant enzymes, lessening of H2O2, MDA contents and by increasing total antioxidant capacity (Sun et al. 2012; Li et al. 2013). Likewise, application of ferulic acid, ellagic acid and proline mitigated various stresses by reducing ROS levels in plants (Ozden et al. 2009; El-Soud et al. 2013). The reduced levels of ROS and free radicals like H2O2 and O2 − could be possibly by the triggering of the antioxidant enzymes, total phenolics and flavonoids directly due to exogenic treatment of gallic acid and rutin.
Fig. 3.
Effect of rutin (Ru) and gallic acid (Ga) inoculation on a hydrogen peroxide detected by DAB staining (×10, scale bar 100 µm), b superoxide radical detected by NBT staining (×10, scale bar 100 µm), c ROS production (×10, scale bar 100 µm) and d callose deposition (×20, scale bar 200 µm) in leaves of rice. Black arrows denote H2O2 production, superoxide generation and callose deposition in different treatments
Plants have been bestowed with pre-invasive structural defenses and local modifications of cell walls that protect them from the attacking pest and pathogens (Van Kan 2006). However, regardless of the innate defense plant must rapidly regulate gene expression to adapt its physiology to changing environmental conditions. Interestingly, callose, a structural barrier localized by an intense blue-green fluorescence after aniline blue staining, was also found to be majorly distributed in the interveinal regions cells of rice leaves (Fig. 3D). However, in comparison to the control plants, the plants treated with gallic acid showed high callose deposition mostly in veins and sparsely in the interveinal regions (Fig. 3D2, D3), indicating the role of gallic acid in strengthening the defense response in plants.
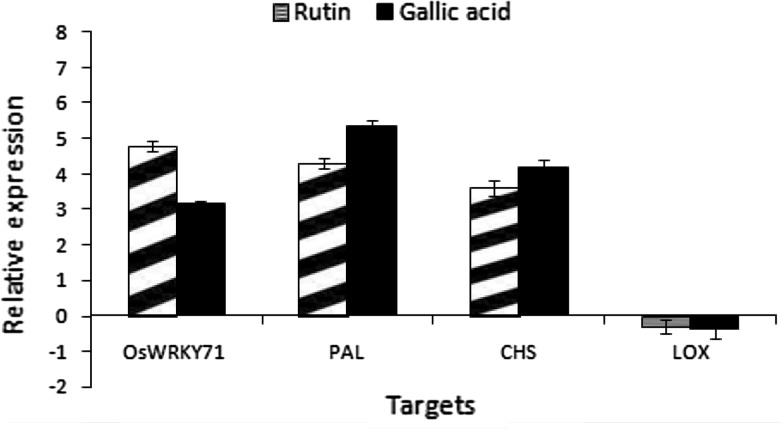
RT-qPCR was used to determine the expression of defense gene in rice plants primed with rutin, gallic acid and control (Fig. 4). Results indicated that rice plants primed with rutin and gallic acid treatments showed the highest level of OsWRKY71, PAL and CHS transcription compared to the control plants (Fig. 4). OsWRKY71 expression was up-regulated (4.8 and 3.15 fold) in rutin and gallic acid treatments, respectively with respect to control. Gallic acid treatment showed the highest level of PAL (5.33 fold) and CHS (4.19 fold) transcription compared to the control plants, suggesting its role in promoting the defense response by activation of these two genes (Fig. 4). However, there was not much significant difference in the LOX transcript levels treated with rutin and gallic acid as compared to control. Among the different defense response factors generated in plants, WRKY factors function in synchronizing a wide array of stress responses under biotic and abiotic stresses in several plants (Kim et al. 2000; Asai et al. 2002). OsWRKY71 has been reported to be induced by defense signalling molecules like methyl jasmonate (MeJA), SA, and 1-aminocyclo-propane-1-carboxylic acid (ACC). Thus, its induction in rutin and gallic acid treatment thus suggests the plausible role played by OsWRKY71 in strengthening the defense response.
Fig. 4.
The expression of target genes in the rice plants inoculated with rutin, gallic acid and control analyzed by quantitative RT-PCR. Transcript levels of target genes were calculated relatively to the actin gene. Error bars represent the standard error of the mean from three independent experiments
Likewise, PAL and CHS are the key enzymes of phenylpropanoid pathway producing phenolic compounds and flavonoid/isoflavonoid biosynthesis pathway respectively (Dixon and Paiva 1995; Dao et al. 2011). In the present investigation, higher transcription of PAL and CHS genes in gallic acid and rutin pretreated seedlings are in correlation with higher induction of total phenolic and flavonoid content. Present findings are well supported by observations recorded by El-Soud et al. (2013) who observed that ellagic acid primed seedlings showed maximum CHS activity and flavonoid content under _0.4 MPa. Similarly, when salicylic acid was applied to common bean plantlets increase in CHS and PAL activities was observed thereby enhancing the defensive state of bean plants (Campos et al. 2003). Further, decrease in LOX transcript levels in comparison to the control plants can be related with the higher accumulation of phenolic compounds which are reported to inhibit lipid peroxidation and help in maintaining the membrane integrity by trapping the lipid alkoxyl radical (Milic et al. 1998; Verstraeten et al. 2003).
Moreover, the data was validated by PCA as grouping of treatments revealed formation of three different clusters: first having only control, second having rutin and third having gallic acid treatment. A separate group formed by rutin and gallic acid treatments where all plant growth parameters were significantly (P < 0.05) enhanced. The PC1 contributed 78.60%, and PC2 contributed 21.40% of the total variance (Fig. S2).
Conclusions
The result of this study suggest that rutin and gallic acid not only affected the growth of rice seedlings but also ameliorated the plants defensive state via enhancement in TPC, TFC, callose and reduction in total ROS. Since, modern agriculture is constantly challenged with the need to lessen the health risks and environmental damage caused by synthetic chemicals and growth promoters, the present study highlights the prospective use of natural molecules in survival and triumphant establishment in competitive environments. Also the findings obtained may be translated into endeavours aimed to develop effective plant growth promoters and substitute of biofertilizers, thus maximizing their use in field conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are gratified to the Director, CSIR-CIMAP, Lucknow, India, for providing required facilities and encouragement during the investigation.
Compliance with ethical standards
Conflict of interest
There exists no potential conflict of interest among the authors.
Footnotes
Akanksha Singh and Rupali Gupta have contributed equally to this work.
References
- Al-Amiery AA, Al-Bayati RI, Saour KY, Radi MF. Cytotoxicity, antioxidant, and antimicrobial activities of novel 2-quinolone derivatives derived from coumarin. Res Chem Intermed. 2012;38:559–569. doi: 10.1007/s11164-011-0371-2. [DOI] [Google Scholar]
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–109. doi: 10.1006/abbi.1999.1525. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Mittler R. The roles of reactive oxygen species in plant cells. Plant Physiol. 2006;141:311. doi: 10.1104/pp.104.900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneloujaephajri E, Costa A, L’Haridon F, Métraux JP, Binda M. Production of reactive oxygen species and wound-induced resistance in Arabidopsis thaliana against Botrytis cinerea are preceded and depend on a burst of calcium. BMC Plant Biol. 2013;13:160. doi: 10.1186/1471-2229-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Campos ÂD, Ferreira AG, Hampe MMV, Antunes IF, Brancão N, Silveira EP, Silva JBD, Osório VA. Induction of chalcone synthase and phenylalanine ammonia-lyase by salicylic acid and Colletotrichum lindemuthianum in common bean. Braz J Plant Physiol. 2003;15:129–134. doi: 10.1590/S1677-04202003000300001. [DOI] [Google Scholar]
- Chauhan R, Awasthi S, Tripathi P, Mishra S, Dwivedi S, Niranjan A, Mallick S, Tripathi P, Pande V, Tripathi RD. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.) Ecotoxicol Environ Saf. 2017;138:47–55. doi: 10.1016/j.ecoenv.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Choubey S, Varughese LR, Kumar V, Beniwal V. Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharma Patent Anal. 2015;4:305–315. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- Dao TTH, Linthorst HJM, Verpoorte R. Chalcone synthase and its functions in plant resistance. Phytochem Rev. 2011;10:397–412. doi: 10.1007/s11101-011-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, Somerville SC, Voigt CA. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013;161:1433–1444. doi: 10.1104/pp.112.211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Soud WA, Hegab MM, AbdElgawad H, Zinta G, Asard H. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol Biochem. 2013;71:173–183. doi: 10.1016/j.plaphy.2013.07.007. [DOI] [PubMed] [Google Scholar]
- El-Tayeb MA, El-Enany AE, Ahmed NL. Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.) Plant Growth Regul. 2006;50:191–199. doi: 10.1007/s10725-006-9118-2. [DOI] [Google Scholar]
- Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. doi: 10.1111/j.1365-3040.1994.tb00146.x. [DOI] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Gillespie KM, Chae JM, Ainsworth EA. Rapid measurement of total antioxidant capacity in plants. Nat Prot. 2007;2:867–870. doi: 10.1038/nprot.2007.100. [DOI] [PubMed] [Google Scholar]
- Gupta R, Singh A, Gupta MM, Pandey R. Cumulative role of bioinoculants on growth, antioxidant potential and artemisinin content in Artemisia annua L. under organic field conditions. World J Microbiol Biotechnol. 2016;32:167–177. doi: 10.1007/s11274-016-2130-4. [DOI] [PubMed] [Google Scholar]
- Gupta R, Singh A, Srivastava M, Singh V, Gupta MM, Pandey R. Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopa monnieri under Meloidogyne incognita stress. Sci Rep. 2017;7:41867. doi: 10.1038/srep41867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Wang L, He Y, Liang J, Tao R. Expression of defense genes and activities of antioxidant enzymes in rice resistance to rice stripe virus and small brown planthopper. Plant Physiol Biochem. 2011;49:744–751. doi: 10.1016/j.plaphy.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Hegab MM, Khodary SEA, Hammouda O, Ghareib HR. Autotoxicity of chard and its allelopathic potentiality on germination and some metabolic activities associated with growth of wheat seedlings. Afr J Biotechnol. 2008;7:884–892. [Google Scholar]
- Karamac M, Kosiñska A, Pegg RB. Comparison of radical-scavenging activities for selected phenolic acids. Pol J Food Nut Sci. 2005;14:165–170. [Google Scholar]
- Kim CY, Lee SH, Park HC, Bae CG, Cheong YH, Choi YJ, Han CD, Lee SY, Lim CO, Cho MJ. Identification of rice blast fungal elicitor-responsive genes by differential display analysis. Mol Plant Microbe Interact. 2000;13:470–474. doi: 10.1094/MPMI.2000.13.4.470. [DOI] [PubMed] [Google Scholar]
- Li Q, Yu B, Gao Y, Dai AH, Bai JG. Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. J Plant Physiol. 2011;168:927–934. doi: 10.1016/j.jplph.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Li DM, Nie YX, Zhang J, Yin JS, Li Q, Wang XJ, Bai JG. Ferulic acid pretreatment enhances dehydration-stress tolerance of cucumber seedlings. Biol Planta. 2013;5:711–717. doi: 10.1007/s10535-013-0326-0. [DOI] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Liu X, Bai X, Wang X, Chu C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol. 2007;164:969–979. doi: 10.1016/j.jplph.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machado S. Allelopathic potential of various plant species on downy brome. Agron J. 2007;99:127–132. doi: 10.2134/agronj2006.0122. [DOI] [Google Scholar]
- Makoi JH, Ndakidemi PA. Allelopathy as protectant, defence and growth stimulants in legume cereal mixed culture systems. N Z J Crop Hortic Sci. 2012;40:161–186. doi: 10.1080/01140671.2011.630737. [DOI] [Google Scholar]
- Maqbool N, Wahid A, Farooq M, Cheema ZA, Siddique KHM. Allelopathy and abiotic stress interaction in crop plants. In: Cheema ZA, Farooq M, Wahid A, editors. In Allelopathy. Berlin, Heidelberg: Springer; 2013. pp. 451–468. [Google Scholar]
- Mata R, Macías ML, Rojas IS, Lotina-Hennsen B, Toscano RA, Anaya AL. Phytotoxic compounds from Esenbeckia yaxhoob. Phytochem. 1998;49:441–449. doi: 10.1016/S0031-9422(98)00110-1. [DOI] [Google Scholar]
- Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15:523. [Google Scholar]
- Milić BL, Djilas SM, Čanadanović-Brunet JM. Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem. 1998;61:443–447. doi: 10.1016/S0308-8146(97)00126-X. [DOI] [Google Scholar]
- Niakan M, Tajari M, Ghorbanli M. Effects of salinity on allelopathic potential of canola (Brassica napus L.) Allelopath J. 2008;21:329–337. [Google Scholar]
- Ozden M, Demirel U, Kahraman A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci Hortic. 2009;119:163–168. doi: 10.1016/j.scienta.2008.07.031. [DOI] [Google Scholar]
- Ozfidan-Konakci C, Yildiztugay E, Kucukoduk M. Upregulation of antioxidant enzymes by exogenous gallic acid contributes to the amelioration in Oryza sativa roots exposed to salt and osmotic stress. Environ Sci Poll Res. 2015;22:1487–1498. doi: 10.1007/s11356-014-3472-9. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313X.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Razavi SM. Plant coumarins as allelopathic agents. Int J Biol Chem. 2011;5:86–90. doi: 10.3923/ijbc.2011.86.90. [DOI] [Google Scholar]
- Reigosa MJ, Souto XC, Gonz L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999;28:83–88. doi: 10.1023/A:1006269716762. [DOI] [Google Scholar]
- Saleh AM, Madany MM, González L. The effect of coumarin application on early growth and some physiological parameters in Faba Bean (Vicia faba L.) J Plant Growth Regul. 2015;34:233–241. doi: 10.1007/s00344-014-9459-4. [DOI] [Google Scholar]
- Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Exp Opin Investig Drugs. 2013;22:1063–1079. doi: 10.1517/13543784.2013.805744. [DOI] [PubMed] [Google Scholar]
- Singh PK, Chaturvedi VK. Impact of cinnamic acid on physiological and anatomical changes in maize plants (Zea mays L.) grown under salinity stress. J Stress Physiol Biochem. 2014;10:44–54. [Google Scholar]
- Singh PK, Singh R, Singh S. Cinnamic acid induced changes in reactive oxygen species scavenging enzymes and protein profile in maize (Zea mays L.) plants grown under salt stress. Physiol Mol Biol Plants. 2013;19:53–59. doi: 10.1007/s12298-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Gupta R, Pandey R. Rice seed priming with picomolar rutin enhances rhizospheric Bacillus subtilis CIM colonization and plant growth. PLoS One. 2016 doi: 10.1371/journal.pone.0146013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WJ, Nie YX, Gao Y, Dai AH, Bai JG. Exogenous cinnamic acid regulates antioxidant enzyme activity and reduces lipid peroxidation in drought-stressed cucumber leaves. Acta Physiol Planta. 2012;34:641–655. doi: 10.1007/s11738-011-0865-y. [DOI] [Google Scholar]
- Van Kan JA. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006;11:247–253. doi: 10.1016/j.tplants.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Vermerris W, Nicholson R. Families of phenolic compounds and means of classification. In: Vermerris W, Nicholson R, editors. Phenolic Compound Biochemistry. Netherland: Springer; 2006. pp. 3–25. [Google Scholar]
- Verstraeten SV, Keen CL, Schmitz HH, Fraga CG, Oteiza PI. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Rad Biol Med. 2003;34:84–92. doi: 10.1016/S0891-5849(02)01185-1. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Karban R, Callaway RM. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol. 2011;26:655–662. doi: 10.1016/j.tree.2011.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.