Abstract
Cymbopogon schoenanthus subsp. proximus is a wild plant distributed in subtropical and east Africa extending from the north to the southern parts of Egypt. Widely used in folk medicine, it is the source of the diuretic sesquiterpene proximadiol. Nuclear magnetic resonance metabolomic analysis of polar extracts of shoots from wild, greenhouse, somatic embryos, and direct and indirect organogenic in vitro cultures was carried out. Metabolic profiling yielded 39 compounds, of which common metabolites were 15 (38.4%). Unique metabolites were trehalose (2.5%) in the wild plants, 2-hydroxylisobutyrate, galactarate and tyrosine (7.6%) in indirect organogenic shoots. Tartrate was found only in direct regenerated shoots (2.5%). Metabolites identified in greenhouse and embryogenic shoots showed no unique compounds. Multivariate analysis revealed significant differences between all tested shoots. 4-aminobutyrate, alanine, glutamine, glucose, fructose, and sucrose were the most significantly different metabolites. Proximadiol was identified and quantitatively measured from the non-polar extract of different types of shoots using gas chromatography and mass spectrometry (GC–MS). Concentrations ranged from 3.6 ± 0.03 to 198.6 ± 7.2 µg/100 mg dry weight in regenerated shoots from somatic embryogenesis and in wild plant shoots, respectively. Direct organogenesis yielded the highest in vitro concentration (20.3 ± 0.5 µg/100 mg dry weight). This study reported the metabolic profiling of C. schoenanthus polar extract and identified primary metabolites that are unique to the wild type and shoots regenerated from different in vitro cultures. Proximadiol was quantified and the in vitro culture system yielding the highest concentration relative to the wild plant was identified.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0432-0) contains supplementary material, which is available to authorized users.
Keywords: In vitro tillering, Medicinal plants, Organogenesis, Proximadiol, Somatic embryogenesis, Trigonelline
Introduction
Cymbopogon schoenanthus (L.) Spreng. subsp. proximus (Hochst. ex A. Rich.) Maire and Weiller is a wild aromatic herb distributed in Southern Egypt, subtropical and east Africa (Boulos 1999). In traditional medicine, the plant is used for treatment of a wide range of ailments including influenza, gripes pains, diabetes, cough, hypertension, inflammation, rheumatism, as a bronchodilator and for treatment of renal spasm (Boulos 1983; Batanouny et al. 1999). Other biological activities such as antimicrobial and antioxidant activities were also studied (Selim 2011). Phytochemical studies reported that the less polar fraction (petroleum ether extract) of C. proximus was characterized by the presence of the sesquiterpene bioactive principal compound, proximadiol, along with other sesquiterpenes. Proximol® is the commercial name of the widely used herbal medicine which contains mainly proximadiol and is used for expulsion of renal and ureters calculi through relaxation of the smooth muscle fibers without causing paralysis of the tissues (Radwan 1975; Locksley et al. 1982; El-Askary et al. 2003).
In vitro propagation of medicinal plants is a well-known tool that facilitates a continuous supply of plant tissues, of which can be important in the production of bioactive metabolites. In vitro culture of some Cymbopogon species were carried out through micropropagation and somatic embryogenesis (Baruah and Bordoloi 1989; Bhattacharya et al. 2009; Dey et al. 2010). In our previous studies we reported the regeneration of C. schoenanthus through somatic embryogenesis and direct tillering (El-Bakry and Abelsalam 2012; Abdel-Salam et al. 2015, 2017).
Metabolomic analysis is considered a significant method for studying the metabolome in different biological systems (Fiehn 2002). NMR spectroscopy is a powerful tool as it generates reproducible, rapid and non-destructive measurements. Advances in NMR soft- and hardware facilitate the analysis of the structurally related metabolites (Krishnan et al. 2004; Kim et al. 2011; Sar et al. 2013). It has been used widely in metabolomic analysis of medicinal plants as in Panax ginseng roots, Humulus lupulus and Tussilago farfara (Yanga et al. 2012; Farag et al. 2012; Li et al. 2013).
GC–MS is widely used for the detection of metabolites that possess medicinal, commercial or public value (Rohloff 2015) specifically for non-polar compounds in plants (Halket et al. 2005). GC–MS was used in terpenoid identification from C. martini, C. citratus, C. winterianus and nineteen Indian species from genus Cymbopogon (Raina et al. 2003; Barbosa et al. 2008; Padalia et al. 2011; Kumar and Shukla 2014).
We report in the present work major metabolite differences, using NMR, between shoots grown from in vitro cultures (somatic embryogenesis, direct and indirect organogenesis) and the wild and greenhouse shoots. Also, the quantitative assessment of the bioactive metabolite, proximadiol, in each shoot type using GC–MS was determined. The in vitro culture system yielding the highest concentration of proximadiol was identified.
Materials and methods
Plant material
C. schoenanthus seeds and aerial parts were collected from Aswan, Egypt in March 2014, air dried, and stored in paper bags in the dark at 25 °C.
Chemicals
All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA), except proximadiol, which was purchased from BOC Sciences (NY, USA).
Seed sterilization
Seeds were separated from the inflorescence one day before culturing. Healthy seeds were collected in cheese cloth, washed under tap water for 15 min, in distilled water for 5 min, immersed in 95% ethanol for 1 min, followed by 20% Clorox (5.25% NaOCl) for 20 min with stirring. Under aseptic conditions, seeds were washed 3 times in sterile distilled H2O.
Greenhouse plant growth
Sterile seeds were cultured on hormone-free, half-strength, Murashige and Skoog (Murashige and Skoog 1962) medium with Gamborg B5 vitamins (Gamborg et al. 1968) MSB5 and incubated at 25 °C under 16/8 h light/dark photoperiod for two weeks. Individual seedlings were transferred to garden soil and covered with plastic bags for two days under the same conditions. Humidity was reduced gradually until seedlings adjusted to ambient temperature. Four-week old seedlings were then transferred to the greenhouse under regular irrigation at 25 °C. Shoots were harvested after 10 weeks from seed culture.
In vitro regeneration
-
A.
Somatic embryogenesis (SE) was carried out according to (El-Bakry and Abelsalam 2012) with some modifications. Sterile seeds were cultured on MSB5 containing 3% sucrose supplemented with 1 mg/l 2,4-dichlorophenoxy acetic acid (2, 4-D) and 0.5 mg/l 6-benzyladenine (BA) for 4 weeks followed by subculture onto the same media. Embryogenic calli were transferred to MSB5 media with 1/4th the concentration of growth regulators followed by subculture on media containing 0.2 mg/l BA. Somatic embryo-derived plants were transferred to MSB5 hormone free media for root development.
-
B.
Direct organogenesis (D): Sterile seeds were cultured on MSB5 with 3% sucrose and supplemented with 7.0 mg/l BA and 0.05 mg/l α-Naphthalene acetic acid (NAA) in magenta boxes. After four weeks of culture, 10 ml MSB5 liquid medium with 0.2 mg/l BA was added to each magenta box. Two weeks after the addition of liquid medium, 10 ml MSB5 hormone-free media with 2% sucrose was added to each magenta box (Abdel-Salam et al. 2017).
-
C.
Indirect organogenesis (ID): Sterile seeds were cultured on MSB5 containing 3% sucrose and supplemented with 4 mg/l NAA and 0.5 mg/l BA. The callus produced was sub-cultured on the same medium after 4 weeks in magenta boxes and remained on this media for another 4 weeks. Regenerated shoots were then transferred to MSB5 hormone-free medium with 6% sucrose for rooting (unpublished data).
For all experiments, pH was adjusted to 5.8 before sterilization, and media solidified by adding 2 g/l phytagel. Ten plates or magenta vessels were cultured with 5 explants in each for each of the in vitro culture methods. Incubation was at 25 °C under cool white fluorescent light (3000 lux) for 16/8 h light/dark photoperiod.
Sample collection
Randomly selected replicates (between 6 and 9) were harvested for analysis. For wild and greenhouse plants, one replicate represented a single plant produced from one seed. Shoots were harvested in the vegetative stage. In vitro regenerated shoots were collected from different plants growing in different vessels. Shoots were harvested when they reached the same length. Plants from organogenic cultures (D and ID) were harvested at 10 weeks, while SE shoots were harvested after (20 weeks). It took a longer time for embryo induction and development to attain the same length as organogenic shoots.
Samples of each type of shoot were immersed directly in liquid nitrogen to quench all metabolic processes. The frozen plant samples were stored at −80 °C for 4 h, and then lyophilized for 24 h. The dried samples were ground to a powder. The wild plants were air dried, which is the common method used in drug production, and homogenized in liquid nitrogen.
Metabolic profiling using NMR spectroscopy
-
A.
Metabolite extraction
From each sample, 20 mg of dried and homogenized material was used. Metabolites were extracted using a constant ratio of 2:2:1 methanol:chloroform:water (Kim et al. 2010) and by using the dry mass to water loss ratio (Bligh and Dyer 1959; Wu et al. 2008). The hydrophilic upper layer was removed from the extract and dried under vacuum for 24 h.
-
B.
Sample preparation and data collection
Each dry sample was re-suspended in 620 μl of NMR buffer (1 mM TMSP (internal standard(, [3-(trimethylsilyl)-2,2′,3,3′-tetradeuteropropionic acid, 100 mM sodium phosphate buffer at pH 7.3 and 0.1% sodium azide, in 99.9 atom % D2O].
The data was collected at 700 MHz with a Bruker Avance™ III spectrometer using a spectral width of 16.0 ppm and 64 K points resulting in an acquisition time of 2.9 s. On-resonance pre-saturation was used for solvent suppression during a 3 s recycle delay. The first increment of the presat-noesy spectra was collected with 120 scans, 4 dummy scans, 3 s relaxation delay, and pre-saturation at the residual water frequency. The 90º pulse widths were measured for each sample using the automatic pulse calculation experiment (pulscal) in TopSpin 2.1.1 (BrukerBioSpin, Billerica, MA). Two dimensional 1H–13C HSQC data were collected for one sample from each shoot type (in vitro regenerated, greenhouse and wild shoots) at 700 MHz using a Bruker hsqcedetgpsisp2.2 pulse sequence. The 1H was observed in the F2 channel with a spectral width of 11 ppm while the 13C was observed in the F1 channel with a spectral width of 180 ppm.
-
C.
Metabolic profiling and statistical analysis of NMR data
Metabolomic analysis from somatic embryogenesis, direct organogenesis, indirect organogenesis, greenhouse, and wild plant shoots was carried out by comparing 1H NMR data with Chenomx NMR Suite library of compounds (Chenomx Inc., Edmonton, Alberta, Canada). For verification of Chenomx assignments, specifically in the case of overlapping peaks, 1H–13C HSQC data was analysed and compared with 1H–13C HSQC data available online in MMCD (Madison Metabolomics Consortium Database) (http://mmcd.nmrfam.wisc.edu/).
To label the spectra, Mnova software was used. Principal components analysis (PCA) and hierarchical cluster analysis (HCA) was performed on the bucket tables generated from AMIX using MetaboAnalyst 2.0 (MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis) with 95% confidence intervals (Xia et al. 2012). The PCA analysis was done with 0.5–10.0 ppm spectral region (excluding the water region of 4.733–4.833 ppm) using 0.01 ppm bucket widths and the advanced bucketing option in AMIX, which is based on individual peaks within the spectra that do not shift significantly (constant temperature and pH). The spectral bins were normalized to total intensity. For each scores plot generated during the analysis, two-sample Hotelling’s T2 statistic (T2), F-value (Ft), and critical F-values (Fc) were calculated using MatLab R2010b (Goodpaster and Kennedy 2011)_ENREF_3. The chemical shifts of significantly changing metabolites were determined with pair-wise fold change analysis using both MetaboAnalyst 2.0 and AMIX software, and then identified by using Chenomx. Metabolic pathway network analysis was carried out using KEGG database and MetaboAnalyst 2.0 (pathway analysis) according to significant metabolites based on fold change analysis.
Quantitative analysis of proximadiol using GC–MS
-
A.
Extraction
Shoots were extracted according to the reference method (Jueun et al. 2014) with some modifications. Shoots derived from wild, greenhouse, and in vitro regenerated tissues were collected and dipped into liquid nitrogen. The collected shoots were then dried by lyophilization for about 24–48 h. From the original number of replicates, 3 were randomly chosen for GC–MS analysis.
To 100 mg of each sample, 2 ml of methanol:water (1:1 v/v) was added and thoroughly mixed using a vortex mixer for 30 s followed by sonication at 40 °C for 20 min. Next, 2 ml of chloroform were added to each extraction mixture and vortex mixed for an additional 30 s. The resulting two phases were separated by centrifugation at 2000×g for 10 min and the lower non-polar (chloroform) layer was transferred to a new tube. An additional 2 ml of chloroform was added and the mixtures were kept closed for 24 h at room temperature. The two layers were then separated as described before and the chloroform layer of each sample was pooled with the corresponding, previously obtained one. Each chloroform extract was filtered with a 0.22 µl syringe filter and then evaporated under vacuum until complete dryness. The resulting residues were individually dissolved in 1 ml chloroform from which aliquots for GC–MS analysis were taken.
-
B.
Identification and quantification
GC–MS measurements were performed on an HP Agilent 5890 GC supplied with an RtX-5 column [length: 30 m, inner diameter (ID): 0.25 mm, film thickness (df): 0.25 µm] connected with VG 70 S mass unit. Injection temperature was set at 250 °C and oven temperature was programmed as follows: Initially at 70 °C for 0 min, (70–150 °C) at 10 °C/min, (150–210 °C) at 5 °C/min and finally (210–300 °C) at 10 °C/min for 10 min. All experiments were done using splitless mode at a flow rate of 1 ml/min and scanning range 50–450 m/z. A sample volume of 1 µl was used for injection, and each sample was studied in triplicate.
A proximadiol standard was used as a reference for analysis of the tested samples. Proximadiol was detected at Rt = 17:31 min with a base ion peak at 149 m/z. Based on the retention time and mass fragmentation pattern, qualitative analysis of proximadiol in all shoots samples was carried out. Quantitative determination of proximadiol in the examined shoots was carried out using the external standard calibration curve. At first, the calibration curve was constructed by preparing 1 mg/ml of standard proximadiol in chloroform from which four different dilutions were made to give final concentrations of 0.5, 1, 10, and 50 µg/ml. One microliter of each solution was injected into the GC–MS. The base peak at 149 m/z was found to be unique to proximadiol, and the curve was generated by plotting the area under this peak (AUP) for each dilution versus the corresponding concentration. The linear regression equation and regression coefficient (r2) were also determined.
Data were analyzed using Minitab 17 software by one way ANOVA. Proximadiol concentrations in different shoots were compared by Fisher least significant difference (LSD) method with 95% confidence level.
Results and discussion
In vitro plant regeneration
In vitro propagation can be used to obtain a rapid and constant production of plant materials. Some of these plants contain metabolites of pharmaceutical importance. Hence, developing a regeneration protocol for these plants is considered a reliable source for medicinal compounds production (Nalawades and Tsay 2004; Debnath et al. 2006).
In this study, seeds from wild plants (W) (Fig. 1a) were used for both the generation of the greenhouse plants (G) (Fig. 1b) and as a source of explants for in vitro regeneration. In vitro regeneration was carried out through somatic embryogenesis, direct organogenesis and indirect organogenesis.
Fig. 1.
a Wild plant; b greenhouse plants; c shoots from somatic embryogenesis; d direct regenerated shoots; e indirect regenerated shoots. Figures from c–e represent in vitro regenerated shoots prior to rooting process
For somatic embryogenesis, embryogenic calli and somatic embryos were induced on MSB5 medium supplemented with 1 mg/l 2, 4-D and 0.5 mg/l BA. Somatic embryos maturation and regeneration of plants (Fig. 1e) took place after transferring somatic embryos to a medium lacking 2, 4-D. The effect of 2, 4-D in combination of BA on somatic embryogenesis of genus Cymbopogon has been reported (Mathur et al. 1988; Baruah and Bordoloi 1989; Dey et al. 2010).
Direct organogenesis (Fig. 1c) was carried out on MSB5 medium supplemented with high concentration of BA (7 mg/l) in combination with 0.05 mg/l NAA. Similarly, a relatively higher concentration of BA was found to give the best results in shoot multiplication experiments in different plant species such as wheat and banana (Mokhtari et al. 2013; Bhosale et al. 2011).
Indirect organogenesis was performed on MSB5 medium fortified with 4 mg/l NAA and 0.5 mg/l BA, which produced organogenic calli that eventually yielded green shoots (Fig. 1d). Auxins were found to play a role in the control of tiller angle in rice, which enhanced rice growth and density (Wang et al. 2007).
Metabolomic analysis using NMR spectroscopy
NMR based metabolomics is a credible technique used widely to identify and quantify a variety of metabolites in different plant species, tissues and organs (Krishnan et al. 2004). In the present study, the polar fraction from different shoot types (W, G, SE, D, and ID) from C. schoenanthus subsp. proximus was analyzed using NMR (700 MHz Bruker Avance™III spectrometer).
From the different types of shoots a total of 39 metabolites were identified. 1H NMR spectral analysis indicated that all shoot types shared the presence of some metabolites. In the aliphatic region (δ 0.5–3.0 ppm) where amino acids are mostly located, all shoots were found to contain alanine, asparagine, isoleucine, leucine, proline and threonine (Appendix 1a, 1b in ESM). Also from the sugar region (δ 3.0–5.5 ppm), d-glucose, fructose and sucrose were found in all shoots (Appendix 1c in ESM). Appendix 1d in ESM presents 1D and 2D spectra of the aromatic region (δ 5.5–10 ppm).
On the other hand some metabolites were determined to exist in only certain types of shoots, for example, trigonelline was identified in the down field region δ 8.0–9.5 ppm (Appendix 2 in ESM) of all types of shoot except the greenhouse shoots (Table 1). 1H NMR revealed the presence of a singlet proton resonating at δ 9.13 ppm which is ascribed to the H-2 of the aromatic ring of trigonelline. Signals at δH 8.08 ppm (1H, t, J = 7.37, H–5) and δH 8.84 ppm (2H, t, J = 8.8, H-4 and H-6) were detected. Additionally 1H–13C connectivities were determined based on HSQC assignments (Table 1) and values were consistent with those reported in the literature (Campo et al. 2010; Machado et al. 2013; Öman et al. 2014). This is the first report of trigonelline’s presence in the genus Cymbopogon. Trigonelline biosynthesis and accumulation were found to be related to stress conditions (Cho et al. 1999; Tramontano and Jouve 1997). Plants in in vitro culture system are known to be exposed to stress as a result of gelling agents, large tissue mass and warm temperatures which limits diffusion for gases such as O2, CO2 and ethylene (Jackson 2005). Also, wild plants growing in arid habitats are under water stress conditions (Boulos 1999). Greenhouse plants have been grown under favorable environmental conditions which may explain the absence of the alkaloid trigonelline in such shoots. Trigonelline has important biological properties as anticancer, neuro-protectant, and anti-diabetic (Hirakawa et al. 2005; Zhou et al. 2012; Hamden et al. 2013). This may be related to the traditional use of this plant as an anti-diabetic (Boulos 1983). Trehalose was found to be present only in the wild plant shoots. The wild plant grows in deserts, in high temperatures (harsh environment) and, since trehalose is a well-known osmoprotectant that protects the proteins and membrane structure of cells (Paul et al. 2008), it can serve a vital function in protecting the plant in its natural habitat. The protective role of trehalose in the family Poaceae has also been reported by Garcia et al. (1997), Ghasempour et al. (1998) and Henry et al. (2014).
Table 1.
List of identified metabolites in the polar extract of C. schoenanthus shoots (W = wild, G = greenhouse, D = direct organogenic, ID = indirect organogenic, SE = somatic embryogenic) with their chemical formula, chemical shift and coupling constant
| Compound name | Chemical formula | 13C (ppm) (functional group or specific C) | 1H (ppm) (functional group or specific H, multiplicity) | J (Hz) | W | G | SE | D | ID | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2-Hydroxy isobutyrate | C4H8O3 | 29.3 (CH3) | 1.30 (CH3, s) | – | _ | _ | _ | _ | √ |
| 2 | 4-Aminobutyrate | C4H9NO2 | 26.4 (CH2) 37.0 | 1.90 (CH2, m) | – | √ | √ | √ | √ | _ |
| (CH2) 42.0 (CH2) | 2.3 (CH2, t) | 7.35 | ||||||||
| 3.0 (CH2, t) | 7.40 | |||||||||
| 3 | 4-Hydroxy benzoate | C7H6O3 | 117.6 (CH) | 6.90 (CH, d) | 8.59 | √ | √ | √ | √ | √ |
| 7.80 (CH, d) | 8.60 | |||||||||
| 4 | Acetate | C2H4O2 | 26.1 (CH3) | 1.92 (CH3, s) | – | √ | √ | _ | _ | √ |
| 5 | Alanine | C3H7NO2 | 53.5 (Cα) | 3.78 (Hα, q) | 7.2 | √ | √ | √ | √ | √ |
| 18.9 (Cβ) | 1.47 (Hβ, d) | 7.26 | ||||||||
| 6 | Asparagine | C4H8N2O3 | 54.2 (Cα) | 4.01 (Hα, q) | 4.29 | √ | √ | √ | √ | √ |
| 37.4 (Cβ) | 2.95 (Hβ, dd) | 4.15, 16.9 | ||||||||
| 2.88 (Hβ, dd) | 7.75, 16.9 | |||||||||
| 7 | Betaine | C5H11NO2 | 56.2 (CH3) | 3.26 (CH3, s) | – | √ | √ | √ | √ | √ |
| 69.2 (CH2) | 3.90 (CH2, s) | – | ||||||||
| 8 | Choline | C5H14NO | 58.6 (Cα) | 4.06 (Hα,m) | – | √ | √ | √ | √ | √ |
| 56.8 (Cγ) | 3.21 (Hγ, s) | – | ||||||||
| 70.4 (Cβ) | 3.52 (Hβ, m) | – | ||||||||
| 9 | cis-Aconitate | C6H6O6 | 127.0 (CH) | 5.71 (CH, s) | – | √ | _ | √ | √ | _ |
| 46.4 (CH2) | 3.12 (CH2, s) | |||||||||
| 10 | Citrate | C6H8O7 | 47.5 (CH2) | 2.71 (CH2, d) | 15.65 | √ | √ | √ | _ | _ |
| 47.5 (CH2) | 2.57 (CH2, d) | 15.65 | ||||||||
| 11 | Dimethylamine | C2H7N | 37.0 (CH3) | 2.73 (CH3, s) | – | _ | √ | √ | √ | √ |
| 12 | Ethanolamine | C2H7NO | 60.5 (CH2) | 3.8 (CH2, overlapped) | 5.21 | _ | _ | √ | _ | √ |
| 3.13 (CH2, t) | ||||||||||
| 13 | Ferulate | C10H10O4 | 117.6 (5CH) | 7.17 (5CH, dd) | 1.87, | √ | √ | √ | _ | _ |
| 143.7 (3CH) | 7.27 (3CH, d) | 9.9, 1.78 | ||||||||
| 124.8 (2CH) | 7.34 (3CH, d) | 15.98 | ||||||||
| 124.5 (9CH) | 6.34 (2CH, d) | 16.0 | ||||||||
| 6.92 (9CH, d) | 8.6 | |||||||||
| 14 | Formate | CH2O2 | – | 8.44 (CH, s) | – | √ | √ | √ | √ | √ |
| 15 | Fructose | C6H12O6 | 78.17 (3CH) | 4.12 (3CH, m) | 12.72 | √ | √ | √ | √ | √ |
| 66.11 (6CH2) | 4.02 (6CH2, dd) | 1.04 | ||||||||
| 71.95 (5CH) | 4.00 (5CH, m) | |||||||||
| 66.56 (1CH) | 3.56 (1CH, m) | |||||||||
| 16 | Galactarate | C6H10O8 | 74.5 (CH) | 3.90 (CH, s) | – | _ | _ | _ | _ | √ |
| 17 | Glucose | C6H12O6 | 98.9 (1αCH) | 4.66 (1αCH, d) | 7.88 | √ | √ | √ | √ | √ |
| 94.8 (1βCH) | 5.24 (1βCH, d) | 3.68 | ||||||||
| 74.4 (2αCH) | 3.53 (2αCH, m) | |||||||||
| 72.6 (4CH) | 3.41 (4CH, m) | |||||||||
| 63.7 (6CH) | 3.74 (5βCH, m) | |||||||||
| 18 | Glutamine | C5H10N2O3 | 33.7 (Cγ) | 2.46 (Hγ, m) | √ | √ | √ | √ | √ | |
| 29.1 (Cβ) | 2.14 (Hβ, m) | 6.3 | ||||||||
| 57.0 (Cα) | 3.78 (Hα, t) | |||||||||
| 19 | Glycine | C2H5NO2 | 44.3 (Cα) | 3.56 (Hα, s) | – | √ | √ | √ | √ | √ |
| 20 | Glycolate | C2H4O3 | 64.0 (CH2) | 3.90(CH2, s) | – | _ | √ | _ | √ | √ |
| 21 | Homoserine | C4H9NO3 | 35.1 (Cβ) | 2.10(Hβ, m) | – | _ | √ | _ | √ | √ |
| 22 | Isobutyrate | C4H8O2 | 22.0 (CH3) | 1.10 (CH3, d) | 7.02 - | _ | _ | √ | √ | √ |
| 2.3 (CH, m) | – | |||||||||
| 23 | Isoleucine | C6H13NO2 | 26.8 (Cγ) | 1.29 (Hγ, m) | – | √ | √ | √ | √ | _ |
| 17.5 (Cγ) | 1.00 (Hγ, d) | 7.0 | ||||||||
| 0.91 (H, t) | 7.1 | |||||||||
| 24 | Lactate | C3H6O3 | 22.17 (CH3) | 1.33 (CH3, d) | 6.88 | √ | √ | √ | √ | _ |
| 25 | Leucine | C6H13NO2 | 24.7 (Cδ) | 0.98 (Hδ, t) | 6.1 | √ | √ | √ | √ | √ |
| 26 | Malonate | C3H4O4 | 50.2 (Cβ) | 3.1 (Hβ, S) | – | √ | _ | √ | _ | _ |
| 27 | Phenylalanine | C9H11NO2 | 39.2 (CH2) | 3.10 (CH2, m) | 7.0 | _ | _ | √ | _ | √ |
| 7.31 (CH, d) | ||||||||||
| 28 | Proline | C5H9NO2 | 64.2 (Cα) | 4.13 (Hα, dd) | 8.6, 6.4 | √ | _ | √ | √ | √ |
| 49.0 (Cδ) | 3.35, 3.4 (Hδ, dt) | 11.65 | ||||||||
| 31.9 (Cβ) | 2.36 (Hβ, m) | 7.1 | ||||||||
| 26.7 (Cγ) | ||||||||||
| 29 | Pyruvate | C3H4O3 | 29.3 (CH3) | 2.35 (CH3, s) | – | _ | √ | √ | _ | _ |
| 30 | Succinate | C4H6O4 | 37.1 (CH2) | 2.41 (CH2, s) | – | √ | √ | √ | √ | √ |
| 31 | Sucrose | C12H22O11 | 95.1 (1CH) | 5.42 (1CH, d) | 3.89 | √ | √ | √ | √ | √ |
| 79.8 (3′CH) | 4.22 (3′CH, d) | 8.838.6 | ||||||||
| 76.8 (4′CH) | 4.06 (4′CH, t) | |||||||||
| 73.9 (2CH) | 3.56 (2CH, m) | |||||||||
| 72.1 (3CH) | 3.48 (3CH, m) | |||||||||
| 65.3 (6CH2) | 3.83 (6CH2, m) | |||||||||
| 64.2 (1′CH2) | 3.69 (1′CH2, s) | |||||||||
| 72.1 (3CH) | 3.48 (3CH, m) | |||||||||
| 32 | Tartrate | C4H6O6 | 76.5 (CH) | 4.33 (CH, s) | – | _ | _ | _ | √ | _ |
| 33 | Threonine | C4H9NO3 | 63.4 (Cα) | 3.55 (Hα, d) | 5.20 6.49 | √ | _ | √ | _ | √ |
| 23.0 (Cγ) | 1.33 (Hγ, d) | |||||||||
| 4.2 (CH, m) | ||||||||||
| 34 | trans-Aconitate | C6H6O6 | 134.0 (CH) | 6.59 (CH, s) | √ | √ | √ | √ | √ | |
| 40.4 (CH2) | 3.45 (CH2, s) | |||||||||
| 35 | Trehalose | C12H22O11 | 96.1 (1CH) | 5.20 (1CH, d) | 3.73 | √ | _ | _ | _ | _ |
| 74.0 (2CH) | 3.67 (2CH, dd) | 10, 3.97 | ||||||||
| 3.46 (CH, t) | 9.54 | |||||||||
| 36 | Trigonelline | C7H7NO2 | 148.2 (2CH) | 9.10 (CH, s) | 8.80 7.37 | √ | _ | √ | √ | √ |
| 147.4 (4,6CH) | 8.84 (CH, t) | |||||||||
| 130.5 (5CH) | 8.08 (CH, t) | |||||||||
| 51.05 (1CH3) | 4.4 (CH3, s) | |||||||||
| 37 | Tyrosine | C9H11NO3 | 38.2 (Cβ) | 3.00 (CH2, q) | 7.30 | _ | _ | _ | _ | √ |
| 14.30 | ||||||||||
| 38 | Valine | C5H11NO2 | 63.0 (Cα) | 3.6 (H, d) | 4.54 7.00 | √ | √ | √ | √ | √ |
| 20.8 (Cγ) | 0.98 (Hγ, d) 1.03 | 7.00 | ||||||||
| 19.5 (Cγ) | (Hγ, d) | |||||||||
| 39 | Xanthine | C5H4N4O2 | 140.3 (CH) | 7.90 (CH, s) | – | _ | _ | √ | _ | √ |
Metabolites were identified using both 1H and 1H–13C HSQC NMR spectra, Chenomx, and online databases. The check mark indicates the presence of the compound in the corresponding type of shoot
Similarly unique to the indirect shoots were 2-hydroxylisobutyrate, galactarate, and tyrosine. Tyrosine is an aromatic amino acid and is considered a precursor for the synthesis of many metabolites including tocochromanols (vitamin E), plastoquinones, isoquinoline alkaloids and betacyanin (Sarin 2003; Tzin and Galili 2010). Exogenous addition of benzyl adenine was found to decrease tyrosine production in suspension cultures of Phytolacca americana (Hirano et al. 1992). In our study, we used high concentration from benzyl adenine in the case of direct regenerated plants (7 mg/l) which may have contributed to the inhibition of tyrosine production in those plants or decreased its biosynthesis to an undetectable level. Ubalua and Mbanaso (2014), Apio et al. (2015) reported that tyrosine has an important role in embryogenic callus production from different plants. This may explain that this compound has been metabolized during somatic embryogenesis stages (induction, maturation and germination) in the case of SE shoots.
In the present work, tartrate was found only in the direct regenerated shoots. Biosynthesis of tartrate in higher plants is carried out through catabolism of ascorbic acid onto oxalate and threonine, the latter is oxidized to tartrate (Loewus and Loewus 1987; DeBolt et al. 2006). Our data shows that, threonine is present in wild shoots, embryogenic shoots and indirect regenerated shoots and absent in direct regenerated shoot. This may suggest that threonine in direct regenerated shoots was metabolized into tartrate. Appendix 3 in ESM shows the identified compounds in different shoot types using 1D and 2D NMR spectra.
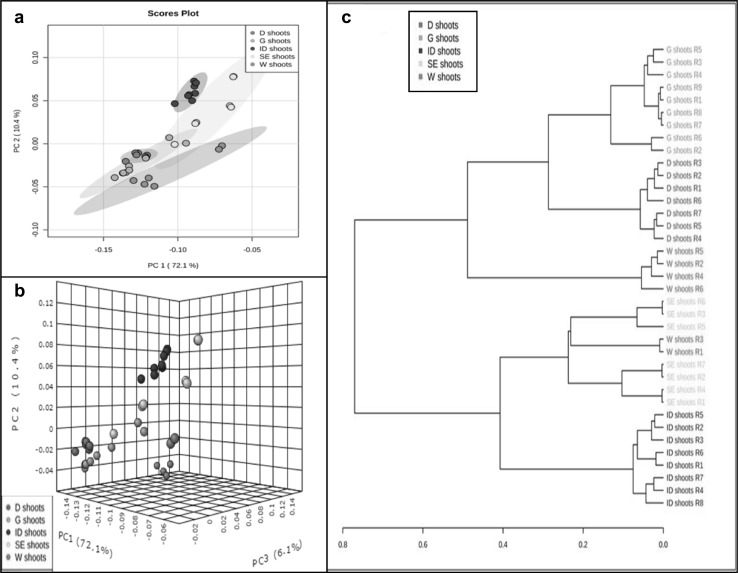
Principal component analysis was conducted to distinguish between the metabolic profiles of different types of shoots (Fig. 2a, b show all plant samples grouped in 2D and 3D scores plots with ovals corresponding to 95% confidence intervals). Overall, 82.5% of the total variance can be explained in PC1 and PC2, while 86.6% of the total variance can be explained when PC3 is included.
Fig. 2.
PCA score plot for types of shoots based on 1H NMR data and buckets created using AMIX software. a, b 2D and 3D scores plot comparing polar extracts of the bucket tables created with 0.5–10.0 ppm spectral regions; 0.05 ppm bucket widths and advance bucketing. PC1, PC2, and PC3 represent ~90% of the total variance. The ovals in the 2D scores plot indicate 95% Hotellings confidence intervals. c Hierarchical cluster analysis (HCA) dendrogram showing metabolite relationship between different types of shoots (W wild, G greenhouse, D direct organogenic, ID indirect organogenic, SE somatic embryogenic)
The metabolome similarities between different shoot sources were detected using HCA and shown in Fig. 2c. The resulting dendogram showed that the direct regenerated shoots were closely similar to both the greenhouse and the wild shoots. Regeneration through direct organogenesis has been documented as a safe method for obtaining true-to-type plants (Salvi et al. 2001; Gunathilake et al. 2008). Also, our results showed that the indirect regenerated shoots were metabolically different from the wild and greenhouse shoots. Plants regenerated through callus-mediated organogenesis were known to have a higher percentage of somaclonal variations (Ramírez-Mosqueda and Iglesias-Andreu 2015). The high probability of variation incidence may give an explanation to why our indirect regenerates displayed noticeable metabolic differences when compared to the wild shoots.
Pair-wise, score plot comparisons show complete separation between different shoot types (Appendix 4 in ESM). Although score plot data of greenhouse shoots overlapped with that of shoots from somatic embryogenesis, the separation was statistically significant. Matlab analysis showed significant differences between all pair-wise analyses in different shoots (Table 2). Similar significant differences were also reported in PCA analysis of cucumber green house plants and different types of in vitro regenerated plants (Filipecki et al. 2005).
Table 2.
Two-sample Hotellings T2 test, and F-test values
| G versus D | G versus ID | W versus D | W versus G | W versus ID | D versus ID | W versus SE | G versus SE | D versus SE | ID versus SE | |
|---|---|---|---|---|---|---|---|---|---|---|
| T2 | 329.98 | 129.73 | 548.54 | 237.88 | 501.57 | 264.95 | 295.81 | 126.98 | 372.87 | 782.94 |
| F true | 153.99 | 60.23 | 253.17 | 110.44 | 231.49 | 123.01 | 135.58 | 58.20 | 169.48 | 358.84 |
| F critical | 4.54 | 4.54 | 4.67 | 4.60 | 4.67 | 4.60 | 4.75 | 4.60 | 4.75 | 4.60 |
| Significant status | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
F true > F critical indicates a statistically significant separation between the groups (W wild, G greenhouse, D direct organogenic, ID indirect organogenic, SE somatic embryogenic)
Fold change analysis in pair-wise groups (Fig. 3) based on 1H NMR data was carried out to identify the metabolite variation between different shoot sources and the degree of variation for each metabolite. The data showed a significant decrease in the concentrations of the amino acids alanine, asparagine and glutamine in the wild plant shoots compared to other types of shoots (Fig. 3a–d). Wild plants grow in sandy soil that is water deficient, and glutamine and valine concentrations were reported to decrease in response to water deficiency (Barnett and Naylor 1966). On the other hand, higher concentrations of betaine, proline, and trans-aconitate were recorded in the wild plant in comparison with the greenhouse, indirect, and embryogenic shoots, respectively (Fig. 3a, c, d). Proline is known as an osmolyte and a reactive oxygen species scavenger, while betaine accumulation in tissue is known to increase under stress conditions (Verbruggen and Hermans 2008; Szabados and Savoure 2009; Burg and Ferraris 2009). Such metabolites would positively enhance the plant’s survival in its natural, harsh habitat. Alanine, glutamine and asparagine biosynthesis from the alanine, aspartate and glutamate metabolism pathway have an important role in development since these compounds are considered to be the precursors for amino acyl tRNA biosynthesis (Appendix 5 in ESM). Our results indicate that the indirect regenerated shoots have higher accumulation of the amino acids alanine, asparagine, glutamine and valine when compared with embryogenic, direct, greenhouse, and wild shoots. Higher accumulation of the amino acids valine, asparagine and glutamine in shoot-differentiated callus was observed in Vanilla planifolia (Palama et al. 2010). In contrast, the amino acids arginine, asparagine, and serine were abundant in shoots produced from Silybum marianum somatic embryogenesis when compared to non-embryogenic calli (Khan et al. 2015).
Fig. 3.
Significant metabolite fold changes (FC) based on 1H NMR (fold change threshold = 1). Differences in metabolite concentrations between pair-wise groups of shoots (W wild, G greenhouse, D direct organogenic, ID indirect organogenic, SE somatic embryogenic). The unique metabolites (a qualitative difference) do not appear in this analysis
Greenhouse shoots contained higher concentrations of sucrose and fructose in comparison with direct and indirect regenerated shoots (Fig. 3e, f). Shoots regenerated through somatic embryogenesis showed significantly lower concentrations of the monosaccharides glucose and fructose, and higher concentration of sucrose when compared with other types of shoots. Glucose and fructose concentrations have been shown to vary greatly during the developmental stages and their concentrations considerably declined by the later stages of embryo maturation in black and white spruce Iraqi and Tremblay (2001). Also, sucrose concentration was significantly higher in embryogenic callus than in non-embryogenic callus of sugarcane plants (Mahmud et al. 2014).
γ-Aminobutyric acid (4-aminobutyrate) increased 23 fold in D shoots compared to SE shoots (Fig. 3i), increased 6 and 5.7 folds when compared with G shoots and ID shoots (Fig. 3e, i), respectively, and increased 3.4 fold compared to W shoots (Fig. 3b). This compound accumulation was found to be induced by anoxia in rice seedling, coleoptiles and in tea leaves (Aurisano et al. 1995; Kato-Noguchi and Ohashi 2006; Mei et al. 2016) and to be involved in stress tolerance in wheat (Reggiani et al. 1993). In direct regenerated cultures, plants may suffer from deficiency of oxygen due to the higher number of regenerated shoots (15–27 shoot/explant) in a limited space (Abdel-Salam et al. 2017) when compared to plants regenerated via somatic embryogenesis and indirect organogenesis (El-Bakry and Abelsalam 2012), which produce fewer shoots (Fig. 1). γ-Aminobutyric acid is biosynthesized through different pathways including the metabolism of arginine and proline, alanine, aspartate and glutamate metabolism pathway and butanoate metabolism pathway (Bouche and Fromm 2004, and Appendix 5 in ESM).
Fold change analysis showed significant differences among some other compounds that we were unable to identify using the Chenomx database. These unknowns are listed in Table 3. The metabolite with 1H chemical shifts of 7.51 and 7.53 ppm increased 86.6 and 12.99 fold in ID shoots compared with SE and G shoots. Other unknowns with 1H chemical shifts 2.64, 2.63, and 2.62 ppm increased 27.9, 27.6 and 18.6 folds respectively in SE shoots compared with G shoots.
Table 3.
Significantly different metabolites chemical shifts based on fold change analysis among pair-wise shoots (W wild, G greenhouse, D direct organogenic, ID indirect organogenic, SE somatic embryogenic)
| Comparison | 1H Chemical shift (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| Unknown 1 | Unknown 2 | Unknown 3 | Unknown 4 | Unknown 5 | Unknown 6 | Unknown 7 | |
| D versus ID | 7.13 | 3.07 | 1.65 | 0.23 | 0.25 | 0.19 | 0.18 |
| W versus D | 5.24 | 3.65 | 1.25 | – | – | – | – |
| 3.63 | |||||||
| W versus ID | 0.27 | 0.18 | 0.19 | – | – | – | – |
| G versus D | 3.75 | 3.66 | 3.36 | 2.67 | 0.84 | – | – |
| 2.66 | 0.83 | ||||||
| 2.65 | |||||||
| 2.63 | |||||||
| G versus ID | 7.53 | 6.99 | 2.65 | 0.84 | – | – | – |
| 7.51 | 6.98 | 2.64 | |||||
| 2.63 | |||||||
| 2.62 | |||||||
| G versus W | 3.87 | 3.71 | 3.553 | 3.367 | – | – | – |
| W versus SE | 3.65 | 3.35 | 3.21 | 1.22 | 1.18 | – | – |
| 3.64 | |||||||
| G versus SE | 2.64 | 2.63 | 2.62 | 1.22 | 1.20 | 1.19 | 1.18 |
| 1.17 | |||||||
| D versus SE | 5.41 | 4.07 | 3.63 | 3.36 | 2.37 | 1.18 | 1.17 |
| 5.42 | |||||||
| ID versus SE | 7.51 | 1.19 | 1.18 | 0.92 | – | – | – |
| 7.53 | 1.17 | ||||||
Proximadiol identification and quantification using GC–MS
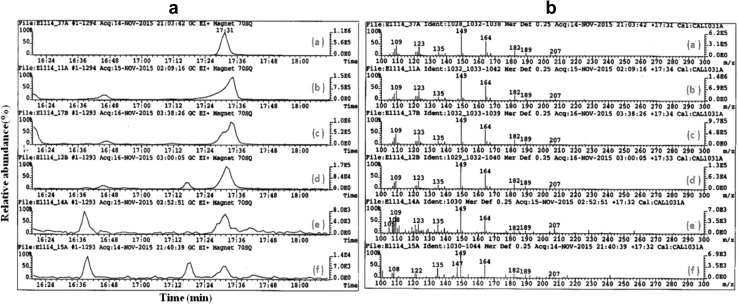
Non-polar extracts from different types of shoots (3 replicates each) were used for proximadiol quantification using GC–MS. All samples were tested for the presence of proximadiol by comparing the retention time and mass spectra with that of the reference standard. Figure 4a represents the TIC (total ion chromatogram) of the standard proximadiol along with the other tested shoots in the expansion range of Rt = 16–19 min. Proximadiol was found to be detected at Rt = 17:31 min under the GC–MS conditions defined in the methodology section. The proximadiol peak was detected in all types of shoots and further confirmed by investigating the mass spectra corresponding to this peak (Fig. 4b). All mass spectra showed identical fragmentation patterns as that recorded for the standard; some ions were found to be characteristic to proximadiol, including m/z 207, 189, 182, 164, and 149.
Fig. 4.
a The TIC expansion range 16:00–19:00 min for the 149 Da ion, b mass fragmentation patterns. Samples shown: a proximadiol standard, b wild, c greenhouse, d direct regenerated, e indirect regenerated, f somatic embryo shoots. Proximadiol standard peak at Rt ~ 17:31
Proximadiol quantification in each shoot type was assigned using the linear regression equation. An external calibration curve was generated using four different concentrations of the standard, and linear regression analysis yielded the regression equation with regression coefficient r2 = 0.995, where X is the concentration of proximadiol in µg/ml and Y is the area under the peak of the ion m/z 149. Wild shoots have the highest recorded significant proximadiol concentration (198.6 ± 7.2 µg/100 mg dry weight) followed by the greenhouse, direct organogenic, indirect organogenic, and the lowest concentration were in SE shoots (Table 4). Several reports have demonstrated that many factors are determinants in the production of secondary metabolites by plants. Developmental stages and stress factors such as temperature variation and drought are among these factors (Figueiredo et al. 2008; Radušienė et al. 2012). C. schoenanthus naturally grows in an arid climate with a scarce supply of water and usually harvested for medicinal use when it becomes fully grown; these, along with other, factors may contribute to the trigger of proximadiol biosynthesis and also could explain the relatively higher proximadiol concentration in the wild shoots compared to other laboratory-raised shoots. Accordingly, future optimization of growth conditions like temperature or culture age and elicitors additions would help attain the best in vitro conditions for metabolite production.
Table 4.
Proximadiol concentrations (µg/100 mg dry weight) in different types of shoots
| Shoot type | Concentration |
|---|---|
| Wild | 198.6 ± 7.2 a |
| Greenhouse | 121.5 ± 7.7 b |
| Direct regenerated | 20.3 ± 0.5 c |
| Indirect regenerated | 3.8 ± 0.01 d |
| Somatic embryogenesis | 3.6 ± 0.03 d |
Numbers followed by different letter are significantly different at P < 0.05 according to Fisher test
According to our results, we observed that the shoot systems with higher levels of proximadiol concentrations such as wild and greenhouse shoots also showed a significant decrease in the concentration of asparagine and an increase in the concentrations of the monosaccharides glucose and fructose. Glucose and fructose are produced from starch and sucrose metabolism and then converted to glyceraldhyde-3-phosphate in the glycolysis pathway, which is also involved in isoperene biosynthesis (a terpenoid precursor) through the non-mevalonate pathway. Biosynthesis of terpenoids from glucose has been reported in many studies (Eisenreich et al. 1996; Arigoni et al. 1997; Adam et al. 1998). Also, glucose and alanine were found to be incorporated in linalyl acetate biosynthesis in Mentha citrate (Fowler et al. 1999).
Conclusion
This study reports the metabolic profiling of the polar extracts, as well as, primary metabolites characteristic of the wild plant and of shoots regenerated in vitro through organogenesis (direct and indirect) and through somatic embryogenesis. Also, two important secondary metabolites (proximadiol and trigonelline) have been identified and quantified using GC–MS and NMR spectroscopy in the polar extracts of the wild plant. The presence of proximadiol (source of the commercial drug Proximol®) has been quantified in all in vitro systems investigated. Direct organogenesis was identified as the culture producing the highest concentrations.
To our knowledge, this work represents the first metabolic profiling study for the genus Cymbopogon. C. schoenanthus, confined in its geographical distribution to subtropical Africa extending from the north to the southern part of Egypt, and used extensively in folk medicine, previously has been poorly studied. Genetic assessment, physiological, biochemical and biotechnological studies have been very limited in this species.
Future research on the genetic and physiological manipulation of in vitro cultures and the use of greenhouse plants, have the potential to be good sources of bioactive compounds in particular when coupled to horticultural practices. Also it may provide propagules for both ex situ conservation and habitat restoration. This may alleviate collection pressure of the plant from the wild and help in genetic conservation of the species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the Culture Affairs and Missions Sector, Ministry of Higher Education, Egypt. Authors are also grateful for SC-INBRE (2 P20 GM103499) and NSF HBCU-UP (HRD-1332516) for providing NMR facility support.
References
- Abdel-Salam AM, Chowdhury K, El-Bakry AA. Effect of sugar types, culture age, concentrations of 2, 4-D and sucrose on somatic embryogenesis of Cymbopogon schoenanthus subsp. proximus. Plant Tissue Cult Biotechnol. 2015;25:51–62. doi: 10.3329/ptcb.v25i1.24125. [DOI] [Google Scholar]
- Abdel-Salam AM, Chowdhury K, El-Bakry AA. Micropropagation through in vitro tillering from seed culture of the medicinal plant Cymbopogon schoenanthus subsp. proximus. Asian J Appl Sci. 2017;5:31–40. [Google Scholar]
- Adam KP, Thiel R, Zapp J, Becker H. Involvement of the mevalonic acid pathway and the glyceraldehydes–pyruvate pathway in terpenoid biosynthesis of the liverworts Ricciocarpos natans and Conocephalum conicum. Arch Biochem Biophys. 1998;354:181–187. doi: 10.1006/abbi.1998.0666. [DOI] [PubMed] [Google Scholar]
- Apio HB, Alicai T, Baguma Y, Mukasa SB, Bua A, Taylor N. Production of friable embryogenic callus and regeneration of Ugandan farmer-preferred cassava genotypes. Afr J Biotechnol. 2015;22:1854–1864. [Google Scholar]
- Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH. Terpenoid biosynthesis from 1-deoxy-d-xylulose in higher plants by intra-molecular skeletal rearrangement. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurisano N, Bertani A, Reggiani R. Anaerobic accumulation of 4-aminobutyrate in rice seedlings; causes and significance. Phytochemistry. 1995;38:1147–1150. doi: 10.1016/0031-9422(94)00774-N. [DOI] [Google Scholar]
- Barbosa LCA, Pereira UA, Martinazzo AP, Maltha CRA, Teixeira RR, Melo EC. Evaluation of the chemical composition of Brazilian commercial Cymbopogon citratus (D.C.) Stapf samples. Molecules. 2008;13:1864–1874. doi: 10.3390/molecules13081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NM, Naylor AW. Amino acid and protein metabolism in Bermuda grass during water stress. Plant Physiol. 1966;44:1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah A, Bordoloi DN. High frequency plant regeneration of Cymbopogon martini (Roxb.)Wats. by somatic embryogenesis and organogenesis. Plant Cell Rep. 1989;8:483–485. doi: 10.1007/BF00269054. [DOI] [PubMed] [Google Scholar]
- Batanouny KH, Abou-Tabl S, Shabana M, Soliman F. Wild medicinal plants in Egypt. An inventory to support conservation and sustainable use. Cairo: Academy of Scientific Research and Technology; 1999. [Google Scholar]
- Bhattacharya S, Bandopadhtay TK, Ghosh PD. Somatic embryogenesis in Cymbopogon pendulus and evaluation of clonal fidelity of regenerates using ISSR marker. Sci Hortic. 2009;123:505–513. doi: 10.1016/j.scienta.2009.10.011. [DOI] [Google Scholar]
- Bhosale UP, Dubhashi SV, Rathod HP. In vitro shoot multiplication in different species of banana. Asian J Plant Sci Res. 2011;1:23–27. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bouche N, Fromm H. GABA in plants: just a metabolite? Trends Plant Sci. 2004;9:110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Boulos L. Medicinal plants of North Africa. Michigan: Reference Publication Inc.; 1983. [Google Scholar]
- Boulos L. Flora of Egypt. Cairo: Al Hadara publishing; 1999. [Google Scholar]
- Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem. 2009;283:7309–7313. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo G, Berregi L, Caracena R, Zuriarrain J. Quantitative determination of caffeine, formic acid, trigonelline and 5-(hydroxymethyl) furfural in soluble coffees by 1H NMR spectrometry. Talanta. 2010;81:367–371. doi: 10.1016/j.talanta.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Cho Y, Lightfoot DA, Wood AJ. Trigonelline concentrations in salt stressed leaves of cultivated Glycine max. Phytochemistry. 1999;52:1235–1238. doi: 10.1016/S0031-9422(99)00410-0. [DOI] [Google Scholar]
- Debnath M, Malik CP, Bisen PS. Micropropagation: a tool for the production of high quality plant-based medicines. Curr Pharm Biotechnol. 2006;7:33–49. doi: 10.2174/138920106775789638. [DOI] [PubMed] [Google Scholar]
- DeBolt S, Cook DR, Ford CM. l-Tartaric acid synthesis from vitamin C in higher plants. Proc Nat Acad Sci. 2006;6:5608–5613. doi: 10.1073/pnas.0510864103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey T, Bhattacharya S, Ghosh PD. Somatic embryogenesis from rhizome explants of Cymbopogon winterianus. Biol Plant. 2010;54:325–328. doi: 10.1007/s10535-010-0056-5. [DOI] [Google Scholar]
- Eisenreich W, Menhard B, Hyland PJ, Zenk MH, Bacher A. Studies on the biosynthesis of taxol: the taxane carbon skeleton is not of mevalonoid origin. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Askary HI, Meselhy MR, Galal AM. Sesquiterpenes from Cymbopogon proximus. Molecules. 2003;8:670–677. doi: 10.3390/80900670. [DOI] [Google Scholar]
- El-Bakry AA, Abelsalam AM. Regeneration from embryogenic callus and suspension cultures of the wild medicinal plant Cymbopogon schoenanthus. Afr J Biotechnol. 2012;11:10098–10107. [Google Scholar]
- Farag MA, Porzel A, Schmidt J, Wessjohann LA. Metabolite profiling and fingerprinting of commercial cultivars of Humulus lupulus L. (hop): a comparison of MS and NMR methods in metabolomics. Metabolomics. 2012;8:492–507. doi: 10.1007/s11306-011-0335-y. [DOI] [Google Scholar]
- Fiehn O. Metabolomics-the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting volatile and essential oil production in plants: volatile components and essential oils. Flavour Fragr J. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- Filipecki M, Wiśniewski A, Yin Z, Malepszy S. The heritable changes in metabolic profiles of plants regenerated in different types of in vitro culture. Plant Cell Tissue Organ Cult. 2005;82:349–356. doi: 10.1007/s11240-005-2585-8. [DOI] [Google Scholar]
- Fowler DJ, Hamilton JTG, Humphrey AJ, O’Hagan D. Plant terpene biosynthesis. The biosynthesis of linalyl acetate in Mentha citrata. Tetrahedron Lett. 1999;40:3803–3806. doi: 10.1016/S0040-4039(99)00532-8. [DOI] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Garcia AB, Engler JA, Lyer S, Gerats T, Montagu MV, Gaplan AB. Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol. 1997;11:159–169. doi: 10.1104/pp.115.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasempour HR, Gaff DF, Williams RPW, Gianello RD. Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses. Plant Growth Regul. 1998;24:185–191. doi: 10.1023/A:1005927629018. [DOI] [Google Scholar]
- Goodpaster AM, Kennedy MA. Quantification and statistical significance analysis of group separation in NMR-based metabolomics studies. Chemometr Intell Lab Syst. 2011;109:162–170. doi: 10.1016/j.chemolab.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunathilake PMPC, Abayagunawardhana N, Wilamasiri S, Sangakkara UR, Eeswara JP. Direct shoot organogenesis from nodal and leaf explants of Munronia pinnata (Wall.)Theob: a valuable medicinal plant. Trop Agric Res. 2008;20:213–225. [Google Scholar]
- Halket JM, Waterman D, Przyborowska AM, Patel RKP, Fraser PD, Bramley PM. Chemical derivatization and mass spectral libraries in metabolic profiling by GC–MS and LC/MS. J Exp Bot. 2005;56:219–243. doi: 10.1093/jxb/eri069. [DOI] [PubMed] [Google Scholar]
- Hamden K, Bengara A, Amri Z, Elfeki A. Experimental diabetes treated with trigonelline: effect of key enzymes related to diabetes and hypertension, β-cell and live function. Mol Cell Biochem. 2013;381:85–94. doi: 10.1007/s11010-013-1690-y. [DOI] [PubMed] [Google Scholar]
- Henry C, Bledsoe SW, Siekman A, Kollman A, Waters BM, Feil R, Stitt M, Lagriminin LM. The trehalose pathway in maize: conservation and gene regulation in response to the diurnal cycle and extended darkness. J Exp Bot. 2014;65:5959–5973. doi: 10.1093/jxb/eru335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa N, Okauchi R, Miura Y, Yagasaki K. Anti-invasive activity of niacin and trigonelline against cancer cells. Biosci Biotechnol Biochem. 2005;69:653–658. doi: 10.1271/bbb.69.653. [DOI] [PubMed] [Google Scholar]
- Hirano H, Sakuta M, Komamine A. Inhibition by cytokinin of the accumulation of betacyanin in suspension cultures of Phytolacca americana. Z Naturforsch C. 1992;47:705–710. [Google Scholar]
- Iraqi D, Tremblay FM. Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Biol. 2001;52:2301–2311. doi: 10.1093/jexbot/52.365.2301. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Aeration stress in plant tissue cultures. In: Hvoslef-Eide AK, Preil W, editors. Liquid culture systems for in vitro plant propagation. Dordrecht: Springer; 2005. pp. 459–473. [Google Scholar]
- Jueun L, Youngae J, Jeoung-Hwa S, Ho KK, Byeong CM, Do HR, Geum-Sook H. Secondary metabolite profiling of Curcuma species grown at different locations using GC-TOF and UPLC/Q-TOF MS. Molecules. 2014;19:9535–9551. doi: 10.3390/molecules19079535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H, Ohashi C. Effects of anoxia on amino acid levels in rice coleoptiles. Plant Prod Sci. 2006;9:383–387. doi: 10.1626/pps.9.383. [DOI] [Google Scholar]
- Khan AM, Abbasi BH, Ali H, Ali M, Adil M, Hussain I. Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organ Cult. 2015;120:127–139. doi: 10.1007/s11240-014-0587-0. [DOI] [Google Scholar]
- Kim HK, Choi YH, Verpoorte R. NMR-based metabolomics analysis of plants. Nat Protoc. 2010;5:536–549. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]
- Kim HK, Choi YH, Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol. 2011;29:269–275. doi: 10.1016/j.tibtech.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Kruger NJ, Ratcliffe RG. Metabolite fingerprinting and profiling in plants using NMR. J Exp Bot. 2004;56:255–265. doi: 10.1093/jxb/eri010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Shukla A. GC/MS analysis of essential oil isolated from the roots of Cymbopogon Winterianus Jowitt. J Chem Chem Sci. 2014;4:35–41. [Google Scholar]
- Li Z, Zhi H, Zhang F, Sun H, Zhang L, Jia J, Xing J, Qin X. Metabolomic profiling of the antitussive and expectorant plant Tussilago farfara L. by nuclear magnetic resonance spectroscopy and multivariate data analysis. J Pharm Biomed Anal. 2013;75:158–164. doi: 10.1016/j.jpba.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Locksley HD, Fayez MBE, Radwan AS, Chari VM, Cordell GA, Wagner H. Constituents of local plants XXV, constitution of the antispasmodic principle of Cymbopogon proximus. Planta Med. 1982;45:20–22. doi: 10.1055/s-2007-971233. [DOI] [PubMed] [Google Scholar]
- Loewus FA, Loewus MW. Biosynthesis and metabolism of ascorbic-acid in plants. CRC Crit Rev Plant Sci. 1987;5:101–119. doi: 10.1080/07352688709382235. [DOI] [Google Scholar]
- Machado ART, Lage GA, Medeiros FS, Filho JDS, Pimenta LPS. Quantitative analysis of trigonelline in some Annona species by proton NMR spectroscopy. Nat Prod Bioprospect. 2013;3:158–160. doi: 10.1007/s13659-013-0051-6. [DOI] [Google Scholar]
- Mahmud I, Thapaliya M, Boroujerdi A, Chowdhury K. NMR-based metabolomics study of the biochemical relationship between sugarcane callus tissues and their respective nutrient culture media. Anal Bioanal Chem. 2014;406:5997–6005. doi: 10.1007/s00216-014-8002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AK, Ahuja PS, Pandey B, Kukreja AK, Mandal S. Screening and evaluation of somaclonal variations for quantitative and qualitative traits in an aromatic grass Cymbopogon winterianus Jowitt. Plant Breed. 1988;101:321–334. doi: 10.1111/j.1439-0523.1988.tb00305.x. [DOI] [Google Scholar]
- Mei X, Chen Y, Zhang L, Fu X, Weil Q, Grierson D, Zhou Y, Huang Y, Dong F, Yang Z. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari A, Alizadeh H, Samadi BY, Omidi M, Otroshy M, Moeini Z. Effect of plant growth regulators on direct shoot regeneration of wheat immature embryonic Explants. J Agric Eng Biotechnol. 2013;1:74–80. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiol. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nalawades M, Tsay H. In vitro propagation of some important Chinese medicinal plants and their sustainable usage. In Vitro Cell Dev Biol Plant. 2004;40:143–154. doi: 10.1079/IVP2003504. [DOI] [Google Scholar]
- Öman T, Tessem M, Bathen T, Bertilsson H, Angelsen A, Hedenström M, Andreassen T. Identification of metabolites from 2D 1H–13C HSQC NMR using peak correlation plots. BMC Bioinform. 2014;15:413–420. doi: 10.1186/s12859-014-0413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalia RC, Verma RS, Chanotiya CS, Anju Y. Chemical fingerprinting of the fragrant volatiles of nineteen Indian cultivars of Cymbopogon Spreng. (Poaceae) Rec Nat Prod. 2011;5:290–299. [Google Scholar]
- Palama TL, Menard P, Fock I, Choi YH, Bourdon E, Govinden-Soulange J, Bahut M, Payet B, Verpoorte R, Kodja H. Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): proteomic and metabolic responses at early stage. BMC Plant Biol. 2010;10:82–90. doi: 10.1186/1471-2229-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y. Trehalose metabolism and signaling. Annu Rev Plant Biol. 2008;59:417–441. doi: 10.1146/annurev.arplant.59.032607.092945. [DOI] [PubMed] [Google Scholar]
- Radušienė J, Karpavičienė B, Stanius Z. Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Bot Lith. 2012;18:101–108. [Google Scholar]
- Radwan AS. An analytical method for proximadiol, the active principle of Cymbopogon proximus. Planta Med. 1975;27:93–97. doi: 10.1055/s-0028-1097767. [DOI] [PubMed] [Google Scholar]
- Raina VK, Srivastava SK, Aggarwal KK, Syamasundar KV, Khanuja SPS. Essential oil composition of Cymbopogon martinii from different places in India. Flavour Fragr J. 2003;18:312–315. doi: 10.1002/ffj.1222. [DOI] [Google Scholar]
- Ramírez-Mosqueda MA, Iglesias-Andreu LG. Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tissue Organ Cult. 2015;123:657–664. doi: 10.1007/s11240-015-0868-2. [DOI] [Google Scholar]
- Reggiani R, Aurisano N, Mattana M, Bertani A. ABA induce 4-aminobutyrate accumulation in wheat seedlings. Phytochemistry. 1993;3:605–609. doi: 10.1016/0031-9422(93)85324-K. [DOI] [Google Scholar]
- Rohloff J. Analysis of phenolic and cyclic compounds in plants using derivatization techniques in combination with GC–MS-based metabolite Profiling. Molecules. 2015;20:3431–3462. doi: 10.3390/molecules20023431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi ND, George L, Eapen S. Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tissue Organ Cult. 2001;66:113–119. doi: 10.1023/A:1010638209377. [DOI] [Google Scholar]
- Sar SV, Kim HK, Meissner A, Verpoorte R, Choi YH. Nuclear magnetic resonance spectroscopy for plant metabolite profiling. In: Weckwerth W, Kahl G, editors. The handbook of plant metabolomics. Weinheim: Wiley-VCH Verlag GmbH & Co.; 2013. pp. 57–76. [Google Scholar]
- Sarin R. Enhancement of optimum alkaloid production in callus culture of Papaver rhoeas Linn. Indian J Biotechnol. 2003;2:271–272. [Google Scholar]
- Selim SA. Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas Aceites. 2011;62:55–61. doi: 10.3989/gya.033810. [DOI] [Google Scholar]
- Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2009;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Tramontano WA, Jouve D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle regulators. Phytochemistry. 1997;44:1037–1040. doi: 10.1016/S0031-9422(96)00715-7. [DOI] [Google Scholar]
- Tzin V, Galili G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol Plant. 2010;3:956–972. doi: 10.1093/mp/ssq048. [DOI] [PubMed] [Google Scholar]
- Ubalua AO, Mbanaso E. Somatic embryogenesis n two Nigerian cassava cultivars (Sandpaper and TMS 60444) J Evol Biol Res. 2014;6:9–12. doi: 10.5897/JEBR2013.0054. [DOI] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Wang DK, Pei KM, Fu YP, Sun ZX, Li SJ, Liu HQ, Tang K, Han B, Tao YZ. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa) Gene. 2007;394:13–24. doi: 10.1016/j.gene.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wu H, Southam AD, Hines A, Viant MR. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal Biochem. 2008;372:204–212. doi: 10.1016/j.ab.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0-a comprehensive server for metabolomics data analysis. Nucleic Acids Res. 2012;40:W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanga S, ShinbY Hyuna S, Choa S, Bangb K, Leec D, Choid SP, Cho H. NMR-based metabolic profiling and differentiation of ginseng roots according to cultivation ages. J Pharm Biomed Anal. 2012;58:19–26. doi: 10.1016/j.jpba.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem. 2012;19:3523–3531. doi: 10.2174/092986712801323171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.