Abstract
We report the first case of thoracic empyema associated with Campylobacter curvus infection. A 65‐year‐old woman with a history of bronchiectasis presented with acute cough and phlegm. The patient reported dyspnoea and left chest pain accompanied by left pleural effusion, despite treatment with sitafloxacin. Curved Gram‐negative rods, eventually identified as C. curvus using 16S ribosomal RNA‐ and atpA‐specific polymerase chain reaction (PCR) and sequencing, were cultured in anaerobic condition of pleural effusion together with Peptostreptococci. The patient recovered after thoracic drainage and treatment with ampicillin/sulbactam and clindamycin. C. curvus, an anaerobe present in human oral cavity, can be associated with extra‐oral infections such as empyema.
Keywords: 16S ribosomal RNA, Campylobacter curvus, lung abscess, pleural effusion
Introduction
Thoracic empyema commonly occurs in patients with poor dental hygiene. Intraoral indigenous bacteria such as Streptococci are most frequently isolated in standard microbiological pleural cultures. However, genetic methods such as amplification of the 16S ribosomal RNA (rRNA) gene using polymerase chain reaction (PCR) combined with clone library analysis suggests a higher incidence of anaerobic bacteria such as Fusobacterium and Prevotella spp. than that estimated previously.
The genus Campylobacter includes 26 species and nine subspecies. Campylobacter jejuni and C. coli are the representative bacteria that cause intestinal and parenteral infection such as sepsis and cholangitis, and are also associated with Guillain–Barre syndrome. On the other hand, C. curvus, C. rectus, C. sputorum, C. concisus, C. gracilis, and C. showae are anaerobes or microaerophiles isolated from the oral cavity of humans, and have been associated with periodontal diseases. Here, we report the first case of empyema associated with C. curvus.
Case Report
A 65‐year‐old woman with a history of bronchiectasis for 12 years presented with acute cough and phlegm. Laboratory tests revealed elevated serum C‐reactive protein level, and a thoracic computed tomography (CT) scan showed a cavitary lesion in the left upper lobe superior lingular segment. The patient was treated with sitafloxacin, considering the diagnosis of lung abscess; however, she developed dyspnoea and left chest pain two weeks later. On examination, body temperature was 37.2°C, pulse rate was 113/min, and peripheral capillary oxygen saturation level was 88% during room air breathing. Leukocytosis (21,400 cells/μL) and high serum C‐reactive protein levels (14.3 mg/dL) persisted, and left pleural effusion appeared on a thoracic CT scan with an increased size of the cavitary lesion in the left lung (Fig. 1A). Thoracentesis was performed; pH of the pleural aspirate was 7.0, with an elevated lactate dehydrogenase level (937 IU/L) and decreased glucose concentration (2 mg/dL). All peripheral blood cultures were negative. An anaerobic culture of pleural effusion showed Peptostreptococcus spp. and curved Gram‐negative rods (GNRs, Fig. 1B), which grew on 5% sheep blood and chocolate agar plates as tiny non‐haemolytic grey colonies after 48 h of incubation at 36°C. The GNRs were catalase‐negative, sensitive to amoxicillin, resistant to clindamycin (E‐test minimum inhibitory concentration >4 µg/mL), but could not be identified using conventional methods.
Figure 1.
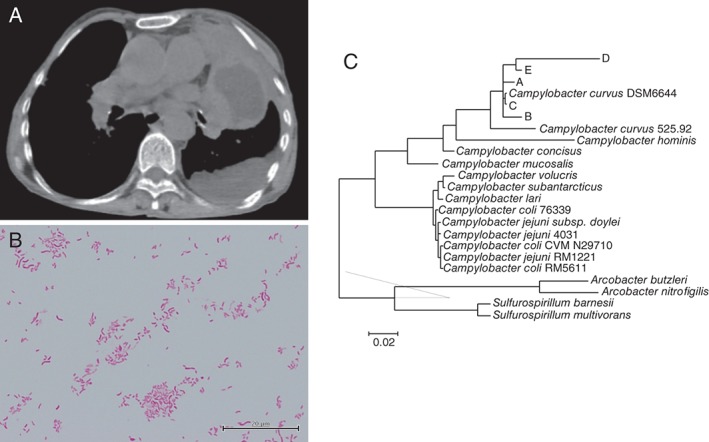
(A) A CT scan on admission. Mass‐like lesion with central low attenuation in the left superior lingular segment suggesting lung abscess together with left pleural effusion was observed. (B) Curve‐shaped Gram‐negative rods observed in anaerobic culture of pleural effusion. (C) A phylogenetic tree for the 16S rRNA. A–E designates the five query sequences derived from the isolated Gram‐negative rods.
Empyema was suspected; accordingly, a chest tube was inserted to drain left pleural effusion, and ampicillin/sulbactam and clindamycin were intravenously administered. The clinical condition of the patient improved gradually, and antibiotic therapy was replaced with oral antibiotics and continued for five weeks.
In order to identify GNRs, total DNA was extracted from bacterial colonies and PCR was performed to amplify 16S rRNA gene using commercially available primers (Thermo Fisher Scientific, Waltham, Massachusetts, USA) or atpA gene by using primers previously reported 1. The amplicons were sequenced, and analysed with SILVA rRNA database (https://www.arb‐silva.de/), GenomeSync database (http://genomesync.org/), or GenBank using Basic Local Alignment Search Tool. Phylogenetic relationships were determined using the neighbour‐joining method. Five query sequences (364–874 bp) derived from hypervariable regions of 16S rRNA gene and one sequence (700 bp) from atpA gene were compared with all available bacterial genomes. All five query sequences showed the highest match alignment (98–100%) with the genome of DSM 6644 strain of C. curvus (GenBank accession ID: GCA_000376325.1) and atpA sequence matched with 525.92 strain of C. curvus (GenBank accession ID: CP000767.2) (98%), whereas they only partially aligned with the genome of other Campylobacter species.
Discussion
In reviewing the first case of empyema associated with C. curvus, we discovered two clinically important issues: (1) oral C. curvus can be associated with abscess in the respiratory system, and (2) genetic analysis of hypervariable regions of 16S rRNA is necessary to identify this microorganism.
The oral Campylobacter spp. such as C. curvus and C. rectus are associated with extra‐oral abscesses. In addition to the present case, only three cases of C. curvus‐associated abscess in the liver or the lungs have been reported 2, 3. Campylobacter rectus has also been identified in 10 cases of intracranial, vertebral, breast, or chest wall abscesses, and in one case of thoracic empyema 4. Moreover, C. rectus was the most prevalent Campylobacter spp. in the subgingival plaque samples from the patients with periodontitis (68/90 cases; 76%), followed by C. gracilis (67%) and C. concisus (24%). In contrast, C. curvus was identified only in two of 90 (2%) patients with periodontitis 5. However, other oral microorganisms such as Streptococci and Peptostreptococci are co‐isolated from cultures of C. curvus‐associated abscess and empyema, supporting that C. curvus originated from the oral cavity during these infections. Importantly, it is difficult to determine whether Campylobacter spp. isolated from abscesses or empyemas are true pathogens or by‐standers, because other microorganisms such as Streptococci and other anaerobes are co‐isolated in most of the cases with C. curvus‐ or C. rectus‐associated infection.
Diagnoses for all cases of extra‐oral abscess due to C. curvus and C. rectus required 16S rRNA gene sequencing. Identification of specific pathogens causing empyema using standard culture methods is difficult because of the following reasons: (1) antibiotics are usually administered prior to pleural effusion sampling, (2) some pathogenic bacteria cannot be cultured or are difficult to culture, and (3) anaerobes are often difficult to identify with conventional methods. Campylobacter curvus could be cultured; however, it could not be identified without genome sequencing that targets hypervariable 16S rRNA regions, suggesting that a substantial number of Campylobacter‐mediated empyema cases are undiagnosed.
In conclusion, oral Campylobacter spp. such as C. curvus should be considered as a possible pathogen for abscesses in the respiratory system and other organs.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Funding Statement
This study is partially supported by a Research Grant from Japan Agency for Medical Research and Development.
Acknowledgments
We would like to thank Miki Miyazawa for bacterial cultures, and Chinatsu Konno, Hiroshi Kamiguchi, and Hideyuki Matsuzawa for technical support for PCR and sequencing. We would also like to thank Kirill Kryukov for his excellent assistance and suggestions pertaining to bioinformatics analysis.
Horio, Y. , Shiraishi, Y. , Watanabe, N. , Inoue, S. , Imanishi, T. and Asano, K. (2017) Empyema associated with Campylobacter curvus infection. Respirology Case Reports, 5 (4), e00234. doi: 10.1002/rcr2.234.
Associate Editor: Wei Shen Lim
References
- 1. Miller WG, Yee E, Jolley KA, et al. 2014. Use of an improved atpA amplification and sequencing method to identify members of the Campylobacteraceae and Helicobacteraceae. Lett. Appl. Microbiol. 58:582–590. [DOI] [PubMed] [Google Scholar]
- 2. Han XY, Tarrand JJ, and Rice DC. 2005. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J. Clin. Microbiol. 43:2513–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wetsch NM, Somani K, Tyrrell GJ, et al. 2006. Campylobacter curvus‐associated hepatic abscesses: a case report. J. Clin. Microbiol. 44:1909–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogata T, Urata T, Nemoto D, et al. 2017. Thoracic empyema caused by Campylobacter rectus . J. Infect. Chemother. 23:185–188. [DOI] [PubMed] [Google Scholar]
- 5. Henne K, Fuchs F, Kruth S, et al. 2014. Shifts in Campylobacter species abundance may reflect general microbial community shifts in periodontitis progression. J. Oral Microbiol. 6:25874. [DOI] [PMC free article] [PubMed] [Google Scholar]


