Abstract
Propofol (2,6-diisopropylphenol) is one of the safest and most commonly used anaesthetic agents for intravenous general anaesthesia. However, in clinical practice, a large inter-individual variability in response to propofol is observed. To limit the risk of adverse effects, pharmacogenetic investigations are recommended. The aim of our study was to verify the impact of genetic changes c.516G>T in the CYP2B6, c.98T>C in the UGT1A9 and c.1075A>C in the CYP2C9 genes on the individual propofol pharmacokinetic profile in the Polish patients undergoing general anaesthesia. Eighty-five patients from the Department of Anaesthesiology and Intensive Therapy, Regional Hospital in Poznan, Poland, anaesthetised with propofol for surgery, were enrolled in the study. We have genotyped CYP2B6, UGT1A9 and CYP2C9 polymorphisms with the use of pyrosequencing. HPLC measurements of propofol plasma concentration were applied for a pharmacokinetic analysis of the anaesthetic. We identified poor (20), intermediate (42) and rapid (23) metabolisers of propofol, which constituted 24%, 49% and 27% of the group, respectively. Homozygotes c.516 T/T in the CYP2B6 gene were statistically more often found in the rapid metabolisers group (p < 0.05). However, polymorphisms c.98T>C in the UGT1A9 and c.1075A>C in the CYP2C9 genes did not affect the pharmacokinetic profile of propofol. The mean propofol retention time (MRT) correlated with the patient’s body mass index (BMI) (p < 0.05). From all the analysed changes, only polymorphism c.516G>T in the CYP2B6 gene and BMI affect the metabolism rate of propofol and may play an important role in the optimisation of propofol anaesthesia.
Keywords: Propofol, Individual response, Pharmacokinetics, General anaesthesia, Genotyping
Introduction
Propofol is one of the safest and most commonly used anaesthetic agents for intravenous general anaesthesia. However, in clinical practice, a large inter-individual variability, including adverse reactions, is observed in response to this anaesthetic (Pasin et al. 2015). Changes between individuals in the pharmacokinetics of propofol result in differences in the required dose of anaesthetic needed for efficient general anaesthesia (Karwacki et al. 2014). This variability is mostly assigned to the genetic polymorphism of genes coding for enzymes participating in the biotransformation pathway of propofol (Kübler 2005; Mikstacki et al. 2013). The need for gene profiling in anaesthesia has been suggested many times recently (Landau et al. 2012).
Propofol is metabolised mainly in the liver by cytochrome P450 2B6 (CYP2B6) and cytochrome P450 2C9 (CYP2C9) or by UDP-glucuronosyltransferase 1A9 (UGT1A9) (Restrepo et al. 2009).
UGT1A9, playing a key role in the biotransformation of propofol, is responsible for conjugation with glucuronic acid of around 70% of the metabolised anaesthetic. Because the enzyme present in the liver, kidney, colon, ovary and testis is involved in the elimination process of important drugs, such as irinotecan and flavopiridol, the polymorphism of the UGT1A9 gene is a subject of pharmacogenetic studies. Among the most essential variants of the UGT1A9 gene, leading to decreased enzyme activity, are three known amino acid changes: p.M33T, p.D256N and p.Y242X. Sequence variation in codon 33 (c.98T>C, rs72551330, UGT1A9*3) was identified previously in the Polish population with an allele C frequency of 0.016 (Zakerska et al. 2013). This substitution is defined as affecting the pharmacokinetic profile and catalytic efficiency of binding propofol to UGT1A9 (Korprasertthaworn et al. 2012). An association of this variant with a reduced glucuronidation level and liver failure in patients treated with entacapone and irinotecan was observed (Villeneuve et al. 2003; Martignoni et al. 2005).
CYP2B6 and CYP2C9, catalysing hydroxylation of propofol in humans, participate in the biotransformation of a wide range of drugs. A variable expression level of these enzymes due to a highly polymorphic nature of genes CYP2B6 and CYP2C9 makes them relevant pharmacogenes. In the context of propofol response, the most common single nucleotide polymorphism (SNP) c.516G>T (p.Q172H, rs3745274) in exon 4 of the CYP2B6 gene was analysed in several investigations. The effect of this SNP was proved to be substrate-specific, and usually led to a disturbed gene expression.
For the CYP2C9 gene, over 65 haplotypes have been described, including insertions, deletions and substitutions (http://www.cypalleles.ki.se/cyp2c9.htm). In global studies, two non-synonymous changes, p.R144C (c.430C>T, rs1799853, CYP2C9*2) and p.I359L (c.1075A>C, rs1057910, CYP2C9*3), determining a poor metabolising phenotype, are intensively analysed. A substrate-dependent decrease in the activity of this enzyme may occur. Rare alleles, CYP2C9*6 (c.818delA, rs933213) resulting in a lack of enzyme activity and allele CYP2C9*4 (p.I359T), have been identified in patients suffering from side effects after phenytoin application (Restrepo et al. 2009).
Awareness of the consequences of important changes in the UGT1A9, CYP2B6 and CYP2C9 genes in response to propofol would make it possible to increase the safety of patients undergoing general intravenous anaesthesia. The aim of this study was to verify the impact of genetic changes c.516G>T in the CYP2B6, c.98T>C in the UGT1A9 and c.1075A>C in the CYP2C9 genes on the individual propofol pharmacokinetic profile in the Polish patients under general anaesthesia.
Materials and methods
Patients
Eighty-five Polish patients (32 women and 53 men) undergoing propofol general anaesthesia (10 mg/mL propofol injectable emulsion; Diprivan, AstraZeneca, Macclesfield, UK) for laryngological surgery in the Department of Anaesthesiology and Intensive Therapy, Regional Hospital in Poznan, Poland, were enrolled in this study. All participants gave their informed consent. No history of addiction to alcohol or nicotine of patients was reported. Patients involved in the study represented classes I and II of the American Society of Anesthesiologists (ASA) scale. The study was approved by the Ethical Committee of the Poznan University of Medical Sciences, Poznan, Poland (resolution no. 653/09).
Anaesthesia was induced with propofol (2 mg/kg), followed by a continuous infusion at the rate of 8 mg/kg/h plus boluses (20–30 mg). Additionally, fentanyl was used to maintain anaesthesia. The infusion time, total dose of propofol, sex, age and body mass index (BMI) were monitored. The characteristics of the patient group are shown in Table 1.
Table 1.
Characteristics of the patient group, with clinical parameters
| Parameter | ||
|---|---|---|
| Sex | Women | 32 |
| Men | 53 | |
| Age | Mean | 44.3 |
| Range | 31–56 | |
| BMI | Mean | 27 |
| Range | 20.1–44.8 | |
| Total dose of propofol (mg) | Mean | 691.4 |
| Range | 130–2200 | |
| Infusion time (min) | Mean | 47 |
| Range | 10–145 |
All subjects were screened for plasma propofol concentration in five time points as follows: at the end of anaesthesia and 5, 10, 20 and 30 min later. The whole study group was also genotyped for UGT1A9, CYP2B6 and CYP2C9.
Molecular analysis
Genomic DNA was isolated from the peripheral blood of all participants using the method with guanidine isothiocyanate (GTC). Three polymorphic changes, p.Q172H (c.516G>T) in the CYP2B6, p.M33T (c.98T>C) in the UGT1A9 and p.I359L (c.1075A>C) in the CYP2C9 genes, were analysed using pyrosequencing. The amplification and genotyping conditions of a UGT1A9 gene fragment have been published previously (Zakerska et al. 2013). The PCR procedure of fragments containing codons 172 of the CYP2B6 and 359 of the CYP2C9 genes was carried out in a total volume of 30 μL using 0.75 U of FIREPol® DNA Polymerase, 2.5 μL 10× buffer, 2.0 μL dNTP mix (2.5 mM each dNTP), 1.5 mM MgCl2 solution, 80 ng DNA and 0.2 μM of each primer (Table 2). Amplification involved 50 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 60 s. All reagents were obtained from Solis BioDyne (Tartu, Estonia). The PCR products were analysed in 1.5 % agarose gels electrophoresis. Pyrosequencing was performed by the PSQ™ 96MA system (Qiagen) using PyroMark™ Gold Q96 Reagents (Qiagen GmbH, Hilden, Germany), as described by the manufacturer.
Table 2.
Primers used for the amplification and pyrosequencing of the CYP2B6 and CYP2C9 genes
| Direction | Primer name | Sequence | Product length | |
|---|---|---|---|---|
| Amplification | Forward (*) | CYP2B6_Q172Hf | 5′-CCTGCTGCTTCTTCCTAGGG-3′ | 83 bp |
| Reverse | CYP2B6_Q172Hr | 5′-GACGATGGAGCAGATGATGTTG-3′ | ||
| Forward (*) | CYP2C9_I359Lf | 5′-ATGCAAGACAGGAGCCACATG-3′ | 181 bp | |
| Reverse | CYP2C9_I359Lr | 5′-GGGACTTCGAAAACATGGAGTTG-3′ | ||
| Pyrosequencing | Reverse | CYP2B6_Q172Hseq | 5′-TGATGTTGGCGGTAAT-3′ | |
| Reverse | CYP2C9_I359Lseq | 5′-TGGGGAGAAGGTCAA-3′ |
(*) = primers labelled with biotin
Pharmacokinetic analysis
Propofol concentration in plasma samples was measured using the HPLC/UV system (P580A; Dionex, Germany) coupled to a fluorescence detector (RF2000; Dionex, Germany) detector. As an analytical standard, propofol obtained from Toronto Research Chemicals (Toronto, Canada) was used. Plasma samples (150 μL) were mixed with 150 μL of 2 M trichloroacetic acid (TCA) and certifugated at 10,000 × g for 10 min. An aliquot of the supernatant was injected onto an analytical C18 reversed-phase column (Hypersil GOLD, 250 mm × 4.6 mm × 5 μm, Germany) maintained at 30 °C. The mobile phase constituted 0.6 % (v/v) orthophosphoric (V) acid and acetonitrile (50:50) at a flow rate of 1.0 ml/min. The elution profiles of propofol were monitored fluorometrically at an excitation wavelength of 270 nm and an emission wavelength of 310 nm. Plasma concentrations of propofol were determined by Chromeleon software version 6.80 (Dionex, Germany). For each analysis, the RSD (percentage of relative standard deviation) was calculated and for the HPLC/UV and fluorescence method, it was below 2.5 %. All samples were analysed in duplicate.
As the pharmacokinetic parameter, the mean retention time (MRT) was calculated for each patient using PKSolver software (Zhang et al. 2010). Observed MRT values were assigned to a percentile rank for a score of 25 and 75.
Statistical analysis
All correlation analyses were performed using Student’s t-test for Pearson’s linear correlation coefficient, whereas correlation between metabolic profiles and genetic variants was proved using the Chi-squared and Fisher’s tests. The value indicating statistical significance was set at p ≤ 0.05. All calculations were performed using STATISTICA 12.0 software (StatSoft).
Results
A total of 85 individuals were successfully screened for genetic variants p.Q172H (c.516G>T) in the CYP2B6, p.M33T (c.98T>C) in the UGT1A9 and p.I359L (c.1075A>C) in the CYP2C9 genes, using pyrosequencing.
The results showed that allele CYP2B6*9 (c.516T) was present in the study group with a frequency of 18 %, while the frequencies of alleles UGT1A9*3 (c.98C) and CYP2C9*3 (c.1075C) were 2% and 4.7%, respectively.
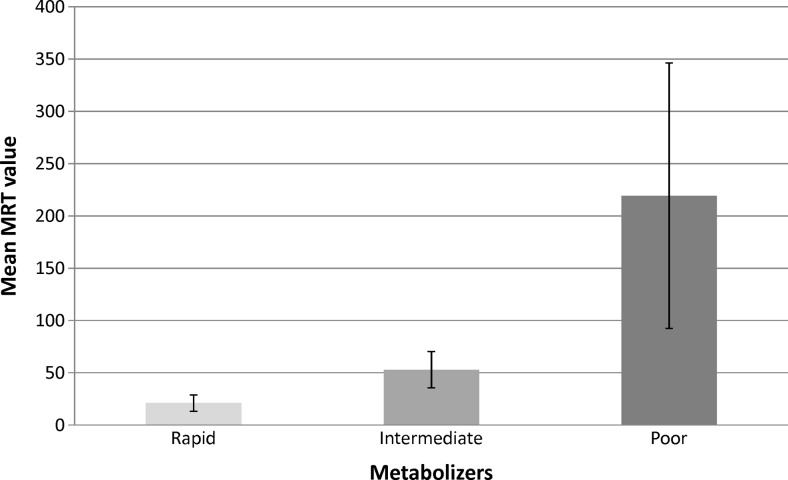
Based on the plasma propofol concentration in five time points within 30 min after stopping anaesthetic infusion and on clinical data, the MRT was calculated for each patient (Table 3). We decided to use this independent pharmacokinetic parameter due to the proved high correlation and lack of statistically significant difference between MRT and t1/2. In our studied patients group, the MRT of propofol was in the range of 8–504 min. Using percentile rank, we identified poor (20), intermediate (42) and rapid (23) metabolisers of propofol, which constituted 24 %, 49 % and 27 % of the group, respectively (Fig. 1).
Table 3.
Summary of the pharmacokinetic and genetic data
| Patient number | Sex | Age | BMI | Total dose of propofol (mg) | Infusion time (min) | MRT (min) | CYP2C9 c.1075A>C | CYP2B6 c.516G>T | UGT1A9 c.98T>C |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | 29.8 | 300 | 65 | 49.8 | AA | GT | TT |
| 2 | F | 53 | 24.1 | 200 | 35 | 21.7 | AA | GG | TT |
| 3 | M | 52 | 30.9 | 500 | 10 | 178.7 | AA | GG | TT |
| 4 | M | 50 | 24.5 | 500 | 89 | 65.2 | AC | GG | TT |
| 5 | M | 37 | 24.7 | 700 | 59 | 35.8 | AA | GG | TT |
| 6 | F | 39 | 20.1 | 240 | 23 | 44 | AA | GT | TT |
| 7 | F | 56 | 37.2 | 130 | 14 | 10.5 | AA | GG | TT |
| 8 | F | 30 | 29.8 | 500 | 35 | 27.6 | AA | GG | TT |
| 9 | M | 30 | 25.8 | 600 | 33 | 37.9 | AA | GG | TT |
| 10 | M | 52 | 27.8 | 450 | 36 | 158.4 | AA | GG | TT |
| 11 | M | 31 | 23.6 | 360 | 22 | 47.5 | AA | GG | TT |
| 12 | M | 47 | 31.6 | 400 | 25 | 34 | AA | GG | TT |
| 13 | M | 51 | 44.8 | 430 | 23 | 37.3 | AA | GG | TT |
| 14 | M | 52 | 26.9 | 290 | 14 | 37.2 | AA | GT | TT |
| 15 | F | 53 | 31.3 | 570 | 45 | 98.4 | AA | GG | TT |
| 16 | M | 37 | 27.8 | 300 | 15 | 70.8 | AA | GT | TT |
| 17 | M | 51 | 26.3 | 550 | 49 | 48.3 | AA | GG | TT |
| 18 | M | 53 | 31.2 | 560 | 65 | 40.3 | AC | GT | TT |
| 19 | F | 49 | 37.0 | 400 | 24 | 467 | AA | GG | TT |
| 20 | M | 49 | 27.8 | 650 | 43 | 55.3 | AA | GT | CT |
| 21 | F | 48 | 23.5 | 350 | 18 | 25.9 | AA | GG | TT |
| 22 | M | 48 | 22.2 | 540 | 55 | 74 | AA | GG | TT |
| 23 | M | 52 | 20.9 | 300 | 20 | 33.8 | AA | GG | TT |
| 24 | M | 31 | 26.6 | 340 | 18 | 41.9 | AA | GG | TT |
| 25 | F | 32 | 21.3 | 340 | 22 | 54.7 | AA | GT | TT |
| 26 | F | 53 | 24.7 | 370 | 16 | 62.1 | AA | GG | TT |
| 27 | F | 52 | 23.1 | 330 | 13 | 59.5 | AA | GG | TT |
| 28 | M | 53 | 29.1 | 260 | 40 | 48.2 | AA | GG | TT |
| 29 | M | 49 | 30.7 | 500 | 25 | 28 | AA | GT | TT |
| 30 | M | 48 | 26.8 | 350 | 35 | 58 | AA | GT | TT |
| 31 | M | 52 | 22.9 | 430 | 15 | 15.8 | AA | GT | TT |
| 32 | F | 31 | 19.2 | 810 | 87 | 151 | AA | GG | TT |
| 33 | M | 51 | 30.0 | 780 | 45 | 29.2 | AA | GG | TT |
| 34 | F | 53 | 22.7 | 410 | 30 | 16.8 | AA | GG | TT |
| 35 | M | 35 | 35.2 | 840 | 53 | 198.9 | AA | GG | TT |
| 36 | M | 51 | 29.4 | 430 | 15 | 40.9 | AA | GT | TT |
| 37 | F | 46 | 24.8 | 650 | 87 | 43.8 | AA | GG | TT |
| 38 | F | 34 | 32.5 | 790 | 63 | 44.5 | AA | GG | TT |
| 39 | M | 48 | 26.2 | 310 | 33 | 19.4 | AA | GT | TT |
| 40 | M | 42 | 25.8 | 1150 | 67 | 106.5 | AA | GG | TT |
| 41 | F | 56 | 30.9 | 1230 | 108 | 92.9 | AA | GG | TT |
| 42 | M | 45 | 27.2 | 450 | 13 | 60.2 | AA | GG | TT |
| 43 | M | 44 | 26.3 | 360 | 10 | 82.5 | AA | GT | TT |
| 44 | M | 35 | 23.1 | 420 | 14 | 74.9 | AA | GG | CT |
| 45 | M | 41 | 29.6 | 700 | 18 | 108.5 | AA | GT | CT |
| 46 | F | 49 | 28.7 | 850 | 66 | 359 | AA | GG | TT |
| 47 | F | 53 | 29.4 | 600 | 25 | 149.7 | AA | GT | TT |
| 48 | F | 52 | 20.4 | 280 | 13 | 27.5 | AA | GG | TT |
| 49 | M | 38 | 27.8 | 570 | 15 | 8.4 | AC | GG | TT |
| 50 | M | 52 | 25.5 | 1160 | 89 | 128.7 | AA | GG | TT |
| 51 | F | 32 | 32.5 | 1340 | 142 | 208.3 | AA | GG | TT |
| 52 | M | 31 | 29.4 | 1150 | 63 | 379.9 | AA | GG | TT |
| 53 | M | 49 | 30.8 | 1300 | 63 | 100.7 | AC | GT | CT |
| 54 | M | 53 | 27.8 | 1892 | 132 | 202.6 | AA | GG | TT |
| 55 | F | 46 | 20.8 | 930 | 80 | 191.7 | AA | GT | TT |
| 56 | F | 45 | 29.4 | 1770 | 145 | 113.3 | AA | GG | TT |
| 57 | F | 36 | 25.9 | 1000 | 102 | 95.2 | AA | GG | TT |
| 58 | M | 50 | 39.5 | 1500 | 106 | 58.6 | AA | GT | TT |
| 59 | M | 31 | 26.5 | 550 | 45 | 29.2 | AA | GT | TT |
| 60 | M | 52 | 22.5 | 470 | 13 | 25 | AA | TT | TT |
| 61 | M | 52 | 27.5 | 900 | 24 | 32.4 | AA | GG | TT |
| 62 | F | 50 | 24.3 | 1840 | 136 | 70.8 | AC | GG | TT |
| 63 | M | 47 | 23.0 | 2200 | 113 | 169.9 | AA | GG | TT |
| 64 | F | 32 | 22.1 | 1350 | 118 | 61.2 | AA | GT | TT |
| 65 | M | 45 | 21.3 | 480 | 35 | 19.7 | AC | TT | TT |
| 66 | F | 46 | 37.0 | 200 | 18 | 23.3 | AC | GG | TT |
| 67 | F | 32 | 23.2 | 440 | 40 | 43.2 | AA | GG | TT |
| 68 | M | 38 | 29.0 | 1050 | 55 | 28.2 | AC | GT | TT |
| 69 | M | 52 | 29.8 | 420 | 11 | 29.1 | AA | GT | TT |
| 70 | F | 33 | 21.2 | 700 | 45 | 25.4 | AA | GG | TT |
| 71 | M | 31 | 28.8 | 900 | 45 | 393.6 | AA | GG | TT |
| 72 | M | 55 | 20.7 | 300 | 15 | 28.8 | AA | TT | TT |
| 73 | M | 43 | 26.8 | 1050 | 80 | 41.8 | AA | GG | TT |
| 74 | M | 32 | 22.4 | 1340 | 60 | 38.7 | AA | GG | TT |
| 75 | F | 33 | 23.0 | 270 | 15 | 28.5 | AA | GG | TT |
| 76 | F | 35 | 22.0 | 1600 | 95 | 58.9 | AA | GT | TT |
| 77 | M | 34 | 20.1 | 810 | 63 | 40.6 | AA | GT | TT |
| 78 | M | 32 | 25.2 | 830 | 65 | 54.9 | AA | GG | TT |
| 79 | M | 49 | 26.6 | 1200 | 67 | 116.6 | AA | GG | TT |
| 80 | F | 49 | 28.3 | 400 | 17 | 14.2 | AA | GG | TT |
| 81 | F | 38 | 27.7 | 500 | 13 | 37.8 | AA | GT | TT |
| 82 | M | 45 | 21.1 | 250 | 10 | 8 | AA | GG | TT |
| 83 | M | 43 | 29.4 | 320 | 13 | 8.2 | AA | GG | TT |
| 84 | F | 46 | 28.9 | 240 | 13 | 30.6 | AA | GG | TT |
| 85 | M | 54 | 34.7 | 1750 | 89 | 504.1 | AA | GG | TT |
Fig. 1.
Characteristics of the propofol metabolisers group with mean retention time and standard deviation
On the basis of the Chi-squared and Fischer’s tests, we observed that homozygotes c.516T/T were statistically more often present in the rapid metabolisers group (p < 0.05) (Table 4). Furthermore, propofol MRT was correlated with the patient’s BMI (p < 0.05). The MRT was significantly longer in the case of individuals with a higher BMI. Moreover, we have observed that infusion time determines the MRT (p < 0.05). However, we did not report a correlation between Cmax and the MRT (p > 0.05). We also did not find the patient’s age to affect the pharmacokinetic marker MRT (p > 0.05). The infusion time did not influence the Cmax value (p > 0.05).
Table 4.
Comparison of genotypes distribution among the patients group with different pharmacokinetic profiles
| Sequence change | Genotype | Poor metabolisers | Intermediate metabolisers | Rapid metabolisers | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| CYP2C9 c.1075A>C (p.Ile359Leu) | AA | 18 | 90 | 39 | 93 | 20 | 87 | 0.99 |
| AC | 2 | 10 | 3 | 7 | 3 | 13 | ||
| CC | 0 | 0 | 0 | 0 | 0 | 0 | ||
| CYP2B6 c.516G>T (p.Gln172His) | GG | 16 | 80 | 27 | 64 | 14 | 61 | 0.03* |
| GT | 4 | 20 | 15 | 36 | 6 | 26 | ||
| TT | 0 | 0 | 0 | 0 | 3 | 13 | ||
| UGT1A9 c.98T>C (p.Met33Thr) | TT | 18 | 90 | 40 | 95 | 23 | 100 | 0.35 |
| TC | 2 | 10 | 2 | 5 | 0 | 0 | ||
| CC | 0 | 0 | 0 | 0 | 0 | 0 | ||
*Statistically significant
Discussion
Understanding the factors, especially genetic polymorphism, that influence the required personalised dose of propofol in general anaesthesia was the goal of the present study. Justification for our investigation was provided by ambiguous literature data concerning the participation of CYP2B6 and UGT1A9 polymorphisms in propofol metabolism. We have analysed the plasma pharmacokinetic profile of propofol in 85 patients after a stopped infusion of anaesthetic with an average dose of 2.5 mg/kg. As a parameter describing the pharmacokinetics in each patient, the MRT was finally calculated. A high inter-individual variability of the MRT has allowed for the identification of poor, intermediate and rapid metabolisers (Fig. 1).
Analysis of the genotype distribution (for positions c.516 in the CYP2B6, c.98 in the UGT1A9 and c.1075 in the CYP2C9 genes) in all pharmacokinetic profiles showed that only the change c.516G>T correlates with the propofol biotransformation rate. Homozygotes c.516T/T were statistically more often identified in rapid-metabolising individuals.
Our results confirm the significance of this non-synonymous substitution c.516G>T of the CYP2B6 gene in the propofol metabolic rate and further dosing, which was proved in several previous studies (Kansaku et al. 2011; Mastrogianni et al. 2014; Mourão et al. 2016). Kansaku et al. (2011) has proved this change as a genetic factor determining the pharmacokinetics and pharmacodynamics of propofol. They correlated a high maximum blood concentration (Cmax) of anaesthetic with genotype c.516T/T. It may suggest, in contrast to our study, a poor metabolism of propofol. This sequence variation c.516G>T was also the subject of pharmacokinetic research on a group of Greek women. Allele c.516T determined a high blood level of propofol, and its frequency was 29.5% (Mastrogianni et al. 2014). A recent study conducted by Mourão et al. (2016) shared the conclusions formulated by Kansaku et al. (2011) and Mastrogianni et al. (2014), suggesting that allele c.516T determines a lower dose of propofol administered to patients undergoing intravenous general anaesthesia.
On the other hand, Iohom et al. (2007) was the first to suggest an important role of the CYP2B6 gene in the individual pharmacokinetic and pharmacodynamic profiles of propofol. However, they did not demonstrate a correlation between change p.Q172H and clearance of propofol. Similar conclusions were reached in studies performed by Khan et al. (2014); none of the analysed polymorphisms in CYP2B6 were associated with a propofol response. Also, Loryan et al. (2012) did not prove a significant linkage between CYP2B6 and UGT1A9 allelic variants and blood propofol concentration. As they explained, for some of the rare genetic polymorphisms, the study group size was probably too small.
Among the clinical parameters collected in our study, only BMI was significantly correlated with the pharmacokinetic profiles of propofol. A longer retention time observed in patients with higher BMI explains the lipophilic nature of the anaesthetic (Lotia and Bellamy 2008). However, we did not confirm the conclusion propounded by Loryan et al. (2012) concerning the impact of sex on propofol metabolism.
The analysed allele CYP2C9*3 (p.I359L), although it is known as being associated with altered enzyme activity, did not have a significant effect on the biotransformation rate of propofol in our study group. We demonstrated this allele frequency of 4.7 %, which corresponds to the range reported in Caucasians. Global studies proved the allele CYP2C9*3 to be correlated with the overdose risk of warfarin and phenytoin (Lindh et al. 2009). Because, so far, there are no data regarding the role of p.I359L change in the CYP2C9 gene in propofol metabolism in anaesthetised patients, it is difficult to discuss the outcome. Certainly, an important explanation for our results may constitute suggested substrate dependence of the CYP2C9 polymorphism.
The effect of the CYP2B6 p.Q172H change on the propofol pharmacokinetic profile reported in the available studies is not fully elucidated. Nevertheless, CYP2B6 plays an important role in the biotransformation process of this anaesthetic by the hydroxylation pathway. Possibly, in our study group, glucuronidation may be the main reaction in anaesthetic metabolism, which would minimise a significant influence of CYP2B6 gene polymorphism in the propofol response. On the other hand, there are certain differences between parameters in our study and opposed research performed by Kansaku et al. (2011). The average age of patients, as well as the infusion time of propofol, was higher in the Japanese investigation (65 years; an average of 250 min), which may partly explain the significant divergences in the obtained results. Moreover, the analysis time of propofol clearance in our research was limited to the first 30 min after the end of propofol infusion, while in the Japanese study, it reached 60 min. A clearer demonstration of the influence of the CYP2B6 c.516G>T mutation on propofol concentration in patient plasma would probably be possible with the use of the determination of propofol’s metabolites; for example, propofol glucuronide and 4-hydroxypropofol. Additionally, the low frequency of the c.516G>T variant of the CYP2B6 gene may be a source of discrepancies between the studies. Kansaku et al. (2011) found two patients as c.516T homozygotes (of the group of 61 patients) and classified them as poor metabolisers, whereas in our study, three patients were identified as homozygotes TT; however, they were all classified as rapid metabolisers. The statistical analysis has shown the significant correlation of this genotype with a high rate of propofol metabolism.
We can conclude that polymorphism c.516G>T in the CYP2B6 gene and BMI affect the metabolism rate of propofol. Our results constitute an inspiration for further extensive studies including metabolites measurements and larger groups of patients. It is suggested that there are more candidate genes as genetic determinants of individual propofol response, such as genes coding for transporters and receptor proteins (Iohom et al. 2007). By using a valuable tool of molecular biology, high-throughput sequencing techniques, which enable efficient and deep multi-gene analysis, it seems possible to be able to deliver to clinicians the outline for optimal anaesthesia with propofol to avoid the risk of adverse reactions (Pareek et al. 2011).
Acknowledgements
This work was supported by the Polish Ministry of Science and Higher Education (grant no. N N401 037838). Oliwia Zakerska-Banaszak holds a scholarship from the European Regional Development Fund (ERDF), the European Fund for Innovative Economy and the Foundation for Polish Science, and a scholarship as part of the project “Scholarship support for Ph.D. students specializing in majors strategic for Wielkopolska’s development”, Sub-measure 8.2.2 Human Capital Operational Programme, co-financed by the European Union under the European Social Fund.
Statement of author contributions
Adam Mikstacki: Designed the study, classified patients, collected and analysed clinical data, participated in writing the paper.
Oliwia Zakerska-Banaszak: Performed research (molecular part), analysed data and wrote the paper.
Marzena Skrzypczak-Zielinska: Designed the study (molecular part) and analysed molecular data.
Barbara Tamowicz: Performed anaesthesia, collected and analysed clinical data.
Michał Prendecki: Performed research concerning the pharmacokinetic part of the study.
Jolanta Dorszewska: Established the pharmacokinetic research and analysed data.
Marta Molinska-Glura: Performed statistical analyses of the obtained results.
Malgorzata Waszak: Participated in data analysis.
Ryszard Slomski: Participated in study design, critically revised the manuscript.
Compliance with ethical standards
Funding
This study was funded by the Polish Ministry of Science and Higher Education (grant no. N N401 037838) and by grant from Poznan University of Medical Sciences awarded to Adam Mikstacki.
Conflict of interest
All authors of this manuscript declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of the Poznan University of Medical Sciences in Poznan, Poland (resolution no. 653/09) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Communicated by: Michal Witt
Adam Mikstacki and Oliwia Zakerska-Banaszak contributed equally to this work.
References
- Iohom G, Ni Chonghaile M, O’Brien JK, Cunningham AJ, Fitzgerald DF, Shields DC. An investigation of potential genetic determinants of propofol requirements and recovery from anaesthesia. Eur J Anaesthesiol. 2007;24(11):912–919. doi: 10.1017/S0265021507000476. [DOI] [PubMed] [Google Scholar]
- Kansaku F, Kumai T, Sasaki K, et al. Individual differences in pharmacokinetics and pharmacodynamics of anesthetic agent propofol with regard to CYP2B6 and UGT1A9 genotype and patient age. Drug Metab Pharmacokinet. 2011;26(5):532–537. doi: 10.2133/dmpk.DMPK-11-RG-039. [DOI] [PubMed] [Google Scholar]
- Karwacki Z, Niewiadomski S, Rzaska M, Witkowska M. The effect of bispectral index monitoring on anaesthetic requirements in target-controlled infusion for lumbar microdiscectomy. Anaesthesiol Intensive Ther. 2014;46:284–288. doi: 10.5603/AIT.2014.0046. [DOI] [PubMed] [Google Scholar]
- Khan MS, Zetterlund EL, Gréen H, et al. Pharmacogenetics, plasma concentrations, clinical signs and EEG during propofol treatment. Basic Clin Pharmacol Toxicol. 2014;115(6):565–570. doi: 10.1111/bcpt.12277. [DOI] [PubMed] [Google Scholar]
- Korprasertthaworn P, Rowland A, Lewis BC, Mackenzie PI, Yoovathaworn K, Miners JO. Effects of amino acid substitutions at positions 33 and 37 on UDP-glucuronosyltransferase 1A9 (UGT1A9) activity and substrate selectivity. Biochem Pharmacol. 2012;84(11):1511–1521. doi: 10.1016/j.bcp.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Kübler A (2005) Postępy w anestezjologii i intensywnej terapii w 2004 roku. Medycyna Praktyczna. Available online at: http://www.mp.pl/artykuly/27216
- Landau R, Bollag LA, Kraft JC. Pharmacogenetics and anaesthesia: the value of genetic profiling. Anaesthesia. 2012;67(2):165–179. doi: 10.1111/j.1365-2044.2011.06918.x. [DOI] [PubMed] [Google Scholar]
- Lindh JD, Holm L, Andersson ML, Rane A. Influence of CYP2C9 genotype on warfarin dose requirements—a systematic review and meta-analysis. Eur J Clin Pharmacol. 2009;65(4):365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- Loryan I, Lindqvist M, Johansson I, et al. Influence of sex on propofol metabolism, a pilot study: implications for propofol anesthesia. Eur J Clin Pharmacol. 2012;68(4):397–406. doi: 10.1007/s00228-011-1132-2. [DOI] [PubMed] [Google Scholar]
- Lotia S, Bellamy MC. Anaesthesia and morbid obesity. Contin Educ Anaesth Crit Care Pain. 2008;8(5):151–156. doi: 10.1093/bjaceaccp/mkn030. [DOI] [Google Scholar]
- Martignoni E, Cosentino M, Ferrari M, et al. Two patients with COMT inhibitor-induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology. 2005;65:1820–1822. doi: 10.1212/01.wnl.0000187066.81162.70. [DOI] [PubMed] [Google Scholar]
- Mastrogianni O, Gbandi E, Orphanidis A, et al. Association of the CYP2B6 c.516G>T polymorphism with high blood propofol concentrations in women from northern Greece. Drug Metab Pharmacokinet. 2014;29(2):215–218. doi: 10.2133/dmpk.DMPK-13-NT-092. [DOI] [PubMed] [Google Scholar]
- Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9–14. doi: 10.2478/v10039-012-0065-z. [DOI] [PubMed] [Google Scholar]
- Mourão AL, de Abreu FG, Fiegenbaum M. Impact of the cytochrome P450 2B6 (CYP2B6) gene polymorphism c.516G>T (rs3745274) on propofol dose variability. Eur J Drug Metab Pharmacokinet. 2016;41(5):511–515. doi: 10.1007/s13318-015-0289-y. [DOI] [PubMed] [Google Scholar]
- Pareek CS, Smoczynski R, Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52(4):413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasin L, Landoni G, Cabrini L, et al. Propofol and survival: a meta-analysis of randomized clinical trials. Acta Anaesthesiol Scand. 2015;59(1):17–24. doi: 10.1111/aas.12415. [DOI] [PubMed] [Google Scholar]
- Restrepo JG, Garcia-Martín E, Martínez C, Agúndez JA. Polymorphic drug metabolism in anaesthesia. Curr Drug Metab. 2009;10(3):236–246. doi: 10.2174/138920009787846305. [DOI] [PubMed] [Google Scholar]
- Villeneuve L, Girard H, Fortier LC, Gagné JF, Guillemette C. Novel functional polymorphisms in the UGT1A7 and UGT1A9 glucuronidating enzymes in Caucasian and African-American subjects and their impact on the metabolism of 7-ethyl-10-hydroxycamptothecin and flavopiridol anticancer drugs. J Pharmacol Exp Ther. 2003;307(1):117–128. doi: 10.1124/jpet.103.054072. [DOI] [PubMed] [Google Scholar]
- Zakerska O, Skrzypczak-Zielińska M, Mikstacki A, et al. Genotype and allele frequencies of polymorphic UGT1A9 in the Polish population. Eur J Drug Metab Pharmacokinet. 2013;38(3):217–221. doi: 10.1007/s13318-012-0110-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]