Abstract
Objective
Ovarian clear cell carcinoma (CCC) is one of histological subtypes showing poor prognosis due to chemoresistance. The association of autophagy-related proteins and clinical implementation in CCC has not been determined.
Methods
The present study investigated whether expression of autophagy-related protein, light chain 3A (LC3A), was related with prognoses in the patients with CCC using immuno-histochemical stainings, and whether inhibition of autophagy modified the sensitivity to cisplatin in CCC cells in vitro.
Results
High expression of autophagy-related protein, LC3A, was detected in 78 cases (78%) in all CCC cases. The patients with high LC3A expression showed significantly lower response rate to primary chemotherapy (17% vs. 100%, p<0.010), and had worse progression-free survival (PFS) and overall survival (OS) compared with those with LC3A low expression. Furthermore, multivariate analyses revealed that high expression of LC3A was identified as independent worse prognostic factors for PFS and OS. Inhibition of autophagy protein LC3A using hydroxychloroquine (HCQ) increased sensitivity to cisplatin in CCC cells in vitro.
Conclusion
High expression of LC3A proteins was associated with lower response to platinum therapy, leading to worse prognoses in CCC. Although further studies are needed to confirm the results, inhibition of autophagy by HCQ was associated with platinum sensitivity. Autophagy protein LC3A could be a promising target for treatment for CCC.
Keywords: Ovarian Neoplasms; Adenocarcinoma, Clear Cell; Autophagy; Prognosis
INTRODUCTION
Ovarian carcinoma is the leading cause of death among gynecologic malignancies. The incidence of the epithelial ovarian carcinoma has been increasing, and the prognosis of patients with advanced-stages is still poor despite aggressive surgery, or recent advances in chemotherapy [1,2]. Among histological subtypes, ovarian clear cell carcinomas (CCCs) is a distinct subtype showing lower response rate to platinum-based chemotherapy compared with serous subtype [3,4,5].
Autophagy is a major cellular pathway for the degradation of long-lived proteins and cytoplasmic organelles, and maintains normal cellular homeostasis [6]. In cancer cells, the role of autophagy included two different functions: a tumor-suppressor phenotype, and a tumor-promoter phenotype [7]. The different function usually depended on tumor microenvironment such as the subtype of cancer, hypoxic condition, and mutation status. Autophagy usually functions as a cell survival adaptive mechanism during stress conditions; however, persistent stress can also promote extensive autophagy, leading to cell death [7]. Similarly, inhibition of autophagy induced gynecologic cancer cell survival and/or cell death [8]. In a previous report, high expression of light chain 3A (LC3A), which was a biomarker of activation of autophagy, was identified as a poor prognostic factor in CCC of the ovary [9], although the mechanisms were not clarified.
The aim of this study was to examine whether LC3A expression was associated with response to primary chemotherapy, or prognosis in the patients with CCC, and whether inhibition of autophagy sensitized ovarian CCC cell lines to cisplatin in vitro.
MATERIALS AND METHODS
1. Patients and tissue microarray
Tissue blocks from 117 patients with CCC who received surgery at the National Defense Medical College Hospital between 1984 and 2010 were used. The patients which received neoadjuvant chemotherapy were excluded. Seventeen cases were excluded due to insufficient cancer tissues. To make tissue microarray slides, 1.5-mm cores were punched from donor blocks, and were inserted into a recipient block. All specimens were cut to 4-μm-thick slices to make sections for immunohistochemical (IHC) staining. Satisfactory IHC stainings were obtained from all 100 cases. The research protocol was approved by the Institutional Ethical Review Board Committee of the National Defense Medical College, Tokorozawa, Japan.
2. IHC staining and interpretation
For IHC staining, we used rabbit polyclonal antibody for LC3A (AP1805a, dilution 1:50; Abgent, San Diego, CA, USA). Tissue microarray slides were deparaffinized in xylene and hydrated with alcohol, boiled in an autoclave at 121°C for 15 minutes in 0.01 mol/L citrate buffer (pH 6.0), and then allowed to cool at room temperature. Endogenous peroxidase activity was blocked by 0.3% H2O2/methanol. The slides were incubated at 4°C overnight with primary antibodies and reacted with the DAKO EnVision+ system-HRP labeled polymer as secondary antibody for 30 minutes at room temperature. Specific antigen-antibody reactions were visualized with 0.2% diaminobenzine tetrahydrochloride and hydrogen peroxide, and counterstained with Mayer hematoxylin. Negative control studies were performed without the primary antibody. No significant staining was observed in the negative control sections.
Immunoreactivity was scored according to the staining intensity as follows: weak (0), moderate (1+), or strong (2+) in parts of more than 50% of immunoreactive components. If more than 50% of observed cells 2+ staining, the cases were defined as high expression. Cases that did not reach such expression level were defined as low expression. Two observers independently evaluated and interpreted the results of IHC staining without knowledge of the clinical data of each patient. During the course of interpretation of immunohistochemistry, a multiviewer microscope was not provided, and any discrepancies between the two observers were resolved by discussion.
3. Reagents/antibodies
Hydroxychloroquine (HCQ) was purchased from Abcam (Cambridge, UK). Cisplatin was purchased from Bristol Meier's Squib Oncology (Tokyo, Japan). The primary antibodies against X-linked inhibitor of apoptosis (XIAP), polymerase (PARP), cleaved-PARP, LC3A, and β-actin were obtained from Cell Signaling Technology (Danvers, MA, USA).
4. Cell lines and culture conditions
Ovarian clear cell cancer cell line, KK [10], were used for in vitro analysis. The cell lines were grown as monolayer cultures in RPMI-1640+Glutmax™-I (Invitrogen Japan KK, Tokyo, Japan) medium supplemented with 10% fetal bovine serum (Invitrogen Japan KK), 100 U penicillin per mL, and 100 mg streptomycin per mL (Invitrogen Japan KK) in a humidified atmosphere of 5% CO2 at 37°C, and routinely tested for mycoplasma infection. Protein concentrations were determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
5. Cell proliferation and cytotoxicity assay
KK cells were seeded onto 96-well plates at approximately 1×104 or 4×104 cells cm−2 for cytotoxicity assays. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method using Tetra Color ONE (Seikagaku Corporation, Tokyo, Japan) according to the manufacturer's instructions. Cell cytotoxicity by HCQ was measured after 5 days from the treatment at different concentration. For the analysis for the additional effect of cisplatin after the pre-treatment by HCQ, KK were treated with several at different concentrations for 24 hours. Then, cisplatin was added to KK at different doses, and cell survivals were measured after 5 days from the treatment of cisplatin.
6. Preparation of cell lysate for western blot analysis
Protein lysates were extracted in RIPA buffer® according to the manufacturer's instructions (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Ten μg of cytosolic fractions were and loaded onto Mini-PROTEIN® TGX™ gel (Bio-Rad Laboratories). After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes using Trans-Blot® Turbo™ Transfer System Transfer Pack (Bio-Rad Laboratories). Subsequently, the membranes were blocked for 1 hour in 4% bovine serum albumin (BSA) in tris buffered saline (TBS) with 0.5% Tween-20 (TBS-T) and incubated overnight at 4°C in primary antibody in TBS-T with 4% BSA. The following antibodies and concentrations were used: 1/2,500 rabbit LC3A, 1/2,500 rabbit PARP, 1/2,500 rabbit cleaved-PARP, 1/5,000 rabbit β-actin. After 3 washes with TBS-T, membranes were incubated for 1 hour at room temperature using horseradish peroxidase-conjugated anti-rabbit secondary antibody as appropriate. After 3 washes with TBS-T, they were visualized using the ECL detection system (GE Healthcare UK Ltd., England, UK) by a LAS-3000 (Fujifilm, Tokyo, Japan). Protein expression was determined densitometrically and normalized against β-actin expression using Multi Gauge version 3.1 (Fujifilm).
7. Statistical analysis
The Stat View software ver. 5.0 (SAS Institution Inc., Cary, NC, USA) was used for statistical analysis. Progression-free survival (PFS) was defined as the interval between first treatment and death or the date of disease progression. Overall survival (OS) was defined as the interval between first treatment and death. The stage of cancer was determined according to International Federation of Gynecology and Obstetrics (FIGO) system. Performance status (PS) was evaluated by World Health Organization (WHO) criteria. Response rate was evaluated by using Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Optimal surgery was defined as the cytoreductive surgery achieving residual tumor less than 1 cm in diameter, and suboptimal surgery was defined as the surgery with residual tumor equal to or more than 1 cm in diameter. The χ2 test, Fisher's exact test and Mann-Whitney U test were used to evaluate differences in the correlation between expression of LC3A and clinic-pathological parameters. PFS and OS curves were generated using the method of Kaplan-Meier. The comparison of the survival distributions was made with a log-rank test. Cox's proportional hazards model was used for multivariate analysis of PFS and OS. All experiments were repeated independently at least 3 times. All values are presented as mean±standard deviation (SD). Statistical significance between two groups was determined by use of a two-tailed t-test, the χ2 test, Fisher's exact test, and Mann-Whitney U test. Statistical significance was defined as a p<0.05.
RESULTS
1. The results of IHC analysis
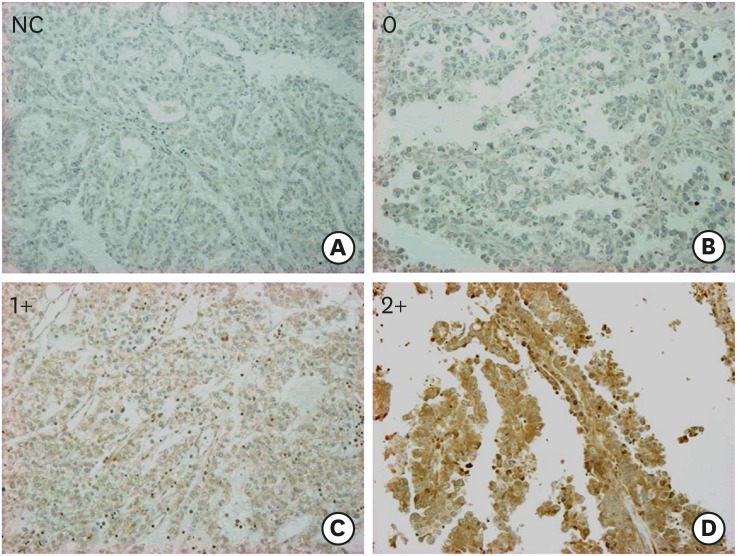
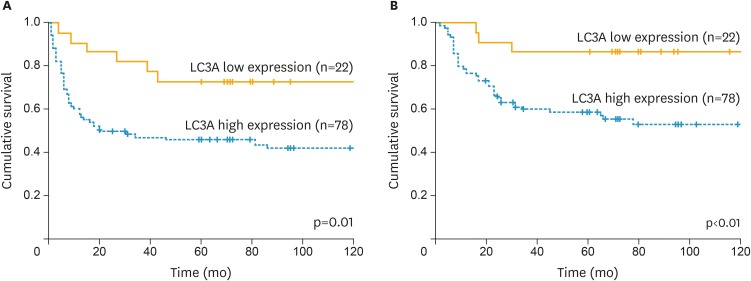
This study included 100 cases with CCC. The median age was 52 (32–75) years. The distribution of FIGO stage was as follows: 52 cases (52%) in stage I, 11 (11%) in stage II, 33 (33%) in stage III, and 4 (4%) in stage IV. Eighty-two (82%) cases received optimal surgery and 18 (18%) received suboptimal surgery. IHC analysis of LC3A revealed that score 0 was observed in 5 cases, score 1 was in 17 cases, and score 2 was in 78 cases, respectively. Representative images of LC3A stainings were shown in Fig. 1. Seventy-eights cases had LC3A high expression (score 2+) and 22 cases (score 0 and 1) had LC3A low expression. The characteristics of the patients with CCC according to LC3A expression levels were shown in Table 1. The cases with high LC3A expression were associated with advanced stages, although the significance was not observed (p=0.060), and significantly lower response to the first-line platinum-based chemotherapy (17% vs. 100%, p<0.010). Also, the patients with high LC3A expression had significantly worse PFS (p=0.010) and OS (p<0.010) (Fig. 2). Furthermore, multivariate analyses revealed that high expression of LC3A was identified as independent poor prognostic factors for PFS and OS, in addition to FIGO stage and residual tumor diameter at primary surgery (Table 2).
Fig. 1.
Representative IHC stains of LC3A in tissue microarray-based samples of ovarian CCCs (×10). (A) NC, (B) score 0, (C) score 1+, and (D) score 2+.
CCC, clear cell carcinoma; IHC, immunohistochemical; LC3A, light chain 3A; NC, negative control.
Table 1. Characteristics of the patients with ovarian CCCs according to LC3A expression levels.
| Variables | LC3A high expression (n=78) | LC3A low expression (n=22) | p-value | |
|---|---|---|---|---|
| Age (yr) | 52.5 (32–72) | 50.5 (36–71) | 0.620 | |
| FIGO stage | 0.060 | |||
| I | 36 (46) | 16 (73) | ||
| II | 8 (10) | 3 (14) | ||
| III | 31 (40) | 2 (9) | ||
| IV | 3 (4) | 1 (4) | ||
| Lymph node metastasis | 0.500 | |||
| Positive | 13 (17) | 2 (9) | ||
| Negative | 41 (53) | 15 (68) | ||
| Not evaluated | 24 (30) | 5 (23) | ||
| Residual tumor at primary surgery | 0.220 | |||
| Optimal | 62 (80) | 20 (91) | ||
| Suboptimal | 16 (20) | 2 (9) | ||
| Response to primary chemotherapy in patients with evaluable disease | <0.010 | |||
| CR | 3 (13) | 3 (75) | ||
| PR | 1 (4) | 1 (25) | ||
| SD | 1 (4) | 0 (0) | ||
| PD | 19 (79) | 0 (0) | ||
| Response rate (%) | 17 | 100 | ||
Values are presented as median (range) or number (%).
CCC, clear cell carcinoma; CR, complete response; FIGO, the International Federation of Gynecology and Obstetrics; LC3A, light chain 3A; PD, progressive disease; PR, partial response; SD, stable disease.
Fig. 2.
PFS and OS of the patients with ovarian CCCs according to LC3A expressions. (A) PFS. (B) OS.
CCC, clear cell carcinoma; LC3A, light chain 3A; OS, overall survival; PFS, progression-free survival.
Table 2. Multivariate analyses for PFS and OS in the patients with ovarian CCCs.
| Variables | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age (yr) | >51 vs. ≤50 | 1.11 | 0.61–2.09 | 0.730 | 0.85 | 0.44–1.65 | 0.620 |
| FIGO stage | III/IV vs. I/II | 4.58 | 2.44–8.77 | <0.010 | 3.84 | 1.82–8.42 | <0.010 |
| Residual tumor at primary surgery | Suboptimal vs. optimal | 3.51 | 1.67–7.11 | <0.010 | 5.02 | 2.28–10.80 | <0.010 |
| LC3A expression | High expression vs. low expression | 2.64 | 1.17–7.12 | 0.020 | 4.45 | 1.53–19.00 | <0.010 |
CCC, clear cell carcinoma; CI, confidence interval; FIGO, the International Federation of Gynecology and Obstetrics; HR, hazard ratio; LC3A, light chain 3A; OS, overall survival; PFS, progression-free survival.
2. Inhibition of autophagy increased sensitivities to cisplatin in KK cells
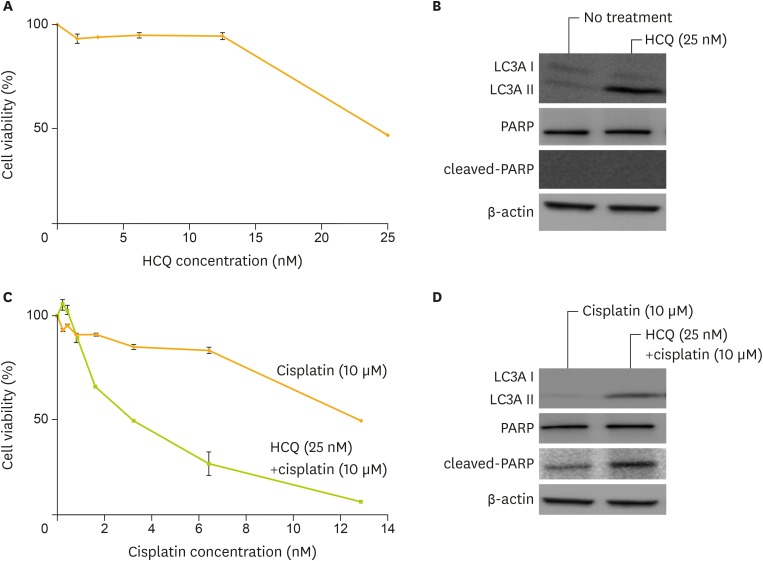
HCQ had an anti-tumor activity as a single agent treatment for CCC cell lines (Fig. 3A). After HCQ treatment for 24 hours at a dose of 25 μM, LC3A protein levels increased (Fig. 3B). The combination treatment with HCQ and cisplatin increased sensitivities to cisplatin (Fig. 3C). After HCQ treatment at a dose of 25 μM and cisplatin treatment at a dose of 10 μM for 24 hours, cleaved-PARP protein levels increased (Fig. 3D). Therefore, inhibition of autophagy protein LC3A sensitized KK cells to cisplatin.
Fig. 3.
Sensitization to cisplatin by autophagy inhibition using HCQ sulfate in ovarian clear cell cancer cell lines. (A) The activity of HCQ as a single agent was measured. (B) Western blotting analysis after treatment by 0 and 25 μM of HCQ for 24 hours revealed up-regulation of LC3AII, indicating inhibition of autophagy. (C) Cell viability by cisplatin treatment after pre-treatment using 25 μM of HCQ was measured. (D) Western blotting analysis revealed up-regulation of LC3AII and cleaved-PARP after treatment of 10 nM of cisplatin, with or without 25 μM of HCQ. Equivalent amounts (10 μg) of proteins were subjected to SDS-PAGE and blotted with anti-LC3A, anti-PARP, anti-cleaved-PARP, or anti-β-actin antibodies. Cell viability was assessed at 5 days after treatment by MTT assay.
HCQ, hydroxychloroquine sulfate; LC3A, light chain 3A; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PARP, polymerase; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
DISCUSSION
Our study clearly demonstrated high expression of LC3A was associated with worse prognosis in CCC using multivariate analyses. With regards to the association between LC3A expression and prognosis, a report revealed high expression of LC3A was associated with advanced stage and worse prognosis in CCC [8]. Although multivariate analysis was conducted in this study, variables did not include a factor of residual tumor at primary surgery [8]. It is well known that the important prognostic factors in ovarian cancers included FIGO stage, residual tumor diameters, and response to chemotherapy [11,12,13]. Particularly in CCC, not only FIGO stage, but also residual tumor diameter was extremely important for prognoses [4]. The present study included the factor of residual tumor diameters in addition to the FIGO stage, and confirmed that high LC3A expression was an independent prognostic factor.
In our study, LC3A expression was related with response to primary chemotherapy in the patients with CCC. Additionally, inhibition of autophagy by HCQ showed anti-tumor effect, and increased sensitivity to cisplatin in CCC cancer cells in vitro. In a previous report, HCQ as a single agent induced ovarian clear cell lines to cell death [9]. However, it still remains undetermined whether inhibition of autophagy modulated the sensitivity to cisplatin in CCC cells. Our report seems to be the first report documenting that modulation of autophagy protein expression could alter platinum-sensitivity in CCC cells, and that combination with HCQ and cisplatin could be a candidate therapeutic modality. In several cancers such as breast cancer, prostate cancer, colon cancer, and renal cancer, the clinical trial incorporating HCQ as a single agent or in combination with anti-cancer drugs were on-going [14]. The agents combined with HCQ included not only cytotoxic drugs such as carboplatin, paclitaxel, and gemcitabine, but also molecularly-targeted drugs such as gefitinib and erlotinib. Thus, the present study suggested the combination with HCQ and cisplatin could be a candidate for the treatment of CCC. Additionally, molecular targeting agents acting as suppression of CCC-specific proteins, such as mammalian target of rapamycin (mTOR) and hepatocyte nuclear factor 1β (HNF-1β) in combination with HCQ might be another candidate combination for CCC [15,16].
Several reports demonstrated the mechanism how inhibition of autophagy increased sensitivity to platinum in serous ovarian cancers. A report suggested that extracellular signal-regulated kinase (ERK)-mediated autophagy could lead to resistance to cisplatin in vitro [17]. Nucleus accumbens-1 (NAC1), a transcription receptor belonging to BTB/POZ gene family, has been reported to alter sensitivity to cisplatin via autophagic response through high mediation by morbidity group box 1 (HMGB1) [18]. Moreover, yes associated protein 1 (YAP1), a transcription coactivator, had been show to alter sensitivity to cisplatin via augmenting cellular autophagic flux [19]. These factors might be associated with upregulation of autophagy and modulation of cisplatin sensitivity in our systems. Further studies to investigate how cisplatin sensitivity was altered in ovarian CCCs are needed. The limitation of our study included a retrospective analysis using single-institutional medical information, and in vitro results using single ovarian cancer cells.
In conclusion, high expression of LC3A protein was associated with chemoresistance to primary chemotherapy, and identified as an independent prognostic factor in CCC patients. Although further studies are needed, inhibition of autophagy protein LC3A could be a promising target for the treatment of CCC.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: M.M., H.T.
- Data curation: M.M., H.T.
- Formal analysis: M.M., T.A., H.S., H.T.
- Funding acquisition: M.T., H.T., K.F.
- Investigation: M.M., T.A., H.S., T.Y.
- Methodology: M.M., H.T.
- Project administration: M.T.
- Resources: M.M., M.T., T.A., H.S., T.Y., H.T., K.F.
- Software: M.M., T.A., H.T.
- Supervision: M.T., H.T., K.F.
- Validation: M.M., M.T., T.A., H.S., H.T.
- Visualization: M.M., H.T.
- Writing - original draft: M.M., H.T.
- Writing - review & editing: M.M., M.T., T.A., H.S., T.Y., H.T., K.F.
References
- 1.Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250:3072–3076. [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 4.Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, et al. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24:37–43. doi: 10.3802/jgo.2013.24.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Notte A, Leclere L, Michiels C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem Pharmacol. 2011;82:427–434. doi: 10.1016/j.bcp.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Orfanelli T, Jeong JM, Doulaveris G, Holcomb K, Witkin SS. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int J Cancer. 2014;135:519–528. doi: 10.1002/ijc.28524. [DOI] [PubMed] [Google Scholar]
- 9.Spowart JE, Townsend KN, Huwait H, Eshragh S, West NR, Ries JN, et al. The Autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J Pathol. 2012;228:437–447. doi: 10.1002/path.4090. [DOI] [PubMed] [Google Scholar]
- 10.Sasa H, Ishii K, Hirata J, Kikuchi Y, Nagata I, Kawai T, et al. Establishment and characterization of a CA125-producing human ovarian clear cell carcinoma cell line. Hum Cell. 1993;6:279–286. [PubMed] [Google Scholar]
- 11.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 12.Chan JK, Tian C, Monk BJ, Herzog T, Kapp DS, Bell J, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008;112:2202–2210. doi: 10.1002/cncr.23390. [DOI] [PubMed] [Google Scholar]
- 13.Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 14.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Huang Z, Matsumura N, Mandai M, Okamoto T, Baba T, et al. Epigenetic determinants of ovarian clear cell carcinoma biology. Int J Cancer. 2014;135:585–597. doi: 10.1002/ijc.28701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem. 2014;289:17163–17173. doi: 10.1074/jbc.M114.558288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, et al. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao L, Shi XY, Zhang Y, Zhu Y, Zhu L, Tian W, et al. YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. Onco Targets Ther. 2016;9:1105–1114. doi: 10.2147/OTT.S102837. [DOI] [PMC free article] [PubMed] [Google Scholar]