Abstract
Objective
To evaluate if uterine myoma is associated with breast cancer.
Methods
This case-control study used a nationwide database in Taiwan. We identified 24,315 patients with newly diagnosed breast cancer as cases and matched them with 24,281 patients without breast cancer on age, sex, urbanization, income, and initial diagnosis date. Patients with prior mastectomy were excluded. We used logistic regression analysis to assess the association between uterine myoma and breast cancer while adjusting for confounders. We evaluated the impact of surgical removal of uterine myoma on subsequent breast cancer among patients with uterine myoma.
Results
We found that 2,892 (11.9%) patients with newly diagnosed breast cancer and 2,541 (10.5%) patients without breast cancer had a history of uterine myoma. The association between breast cancer and uterine myoma was significant (adjusted odds ratio [aOR]=1.14; 95% confidence interval [CI]=1.07–1.21; p<0.001). This association remained in patients who used hormone (aOR=1.20; 95% CI=1.08–1.33; p=0.001) or who did not use hormone (aOR=1.11; 95% CI=1.03–1.19; p=0.005) within 5 years prior to the index date. Surgical removal of uterine myoma was not associated with a decreased risk of breast cancer (aOR=0.99; 95% CI=0.88–1.10; p=0.795).
Conclusion
A minor increased risk of breast cancer was found in women with a history of uterine myoma. This association remained in patients with recent hormone use. Removal of uterine myoma was not associated with decreased risk of breast cancer.
Keywords: Uterus, Leiomyoma, Breast Neoplasms, Hormone Replacement Therapy, Case-Control Studies, National Health Programs
INTRODUCTION
Uterine myoma is a benign tumor originating from the myometrial compartment of the uterus. It is the most common benign tumor in women, occurring in >70% of women at reproductive age [1]. Most patients with uterine myoma are asymptomatic and often detected incidentally by sonographic or pelvic examinations. The precise causes remain obscure, but advances have been made by the understanding of hormonal factors, genetic factors, growth factors, and molecular biology [2,3]. Both estrogen and progesterone have been linked to the development of uterine myoma. Researchers have found that the main inducing factor of uterine myoma development is associated with estrogen and estrogen receptor [4,5,6]. Early menarche and obesity are also considered to increase the incidence of uterine myoma [7].
Breast cancer is one of the leading causes of cancer death in women worldwide. Sex hormones are considered to be involved in the etiology of breast cancer and uterine myoma [8]. Early age of first menstruation, having no or few children, choosing not to breastfeed, later age of last menstruation, and hormone replacement therapy increase the estrogen exposure and thus increase the development of breast cancer [8]. In Taiwan, the epidemiology of breast cancer is somewhat different from Western countries. The incidence of breast cancer is the highest among women aged 45–54 years, which is about 10 years younger than the peaked age group in Western countries (60–70 years) [9,10,11].
Patients with uterine myoma may have an increased risk of developing breast cancer because the uterine myoma and breast cancer share some of the risk factors such as obesity and estrogen exposure. In addition, studies have shown that women with uterine myoma are at increased risk for developing meningioma [12] and there was an association between meningioma and breast cancer [13].
We conducted this nationwide, population-based, case-control study to evaluate the relationship between a history of uterine myoma and the risk of subsequent breast cancer in Taiwanese women.
MATERIALS AND METHODS
1. Data sources
The National Health Insurance (NHI) program in Taiwan covers over 99% of the population [14]. We obtained data from the National Health Insurance Research Database (NHIRD), which contains comprehensive information for each insurant's hospital visit and admission, including demographic data, date of visits, diagnostic codes according to the International Classification of Diseases, 9th revision, clinical modification (ICD-9-CM), and prescriptions. Details of NHIRD are described in previous study [15].
This study was approved by the Institutional Review Board (IRB No. CE1315B-1) at Taichung Veterans General Hospital.
2. Study samples
Cases were defined as patients with newly diagnosed breast cancer (ICD-9-CM codes 174, 175) who had a catastrophic illness certificate between 2006 and 2010. At least 2 specialists have to review medical records, laboratory data, and imaging findings to validate the breast cancer diagnosis before they approve a catastrophic illness certificate for a patient with breast cancer. We excluded patients if they had mastectomy or a diagnosis of other cancers before the index date or if they were <25 years old.
The controls were selected from a random sample of one million individuals from NHIRD. We excluded individuals with mastectomy before the index date or patients with a diagnosis of breast cancer or other cancer that was recorded between 1996 and 2010. The remaining individuals from the registry of beneficiaries were then randomly extracted as controls and were 1:1 matched with cases by age (25–39, 40–54, 55–64, ≥65 years), sex, urbanization, income, and the index date of breast cancer diagnosis. For controls, the date of the first ambulatory visit during the matched index year was selected as the index date.
3. Uterine myoma
In Taiwan, the Administration of NHI (NHIA) encourages women to receive annual gynecological check-ups (Papanicolaou smear and pelvic examination). Gynecologists may perform pelvic ultrasonography for women with clinical symptoms and positive pelvic findings. In this study, patients with a history of uterine myoma were defined as patients who had uterine myoma diagnosis (ICD-9-CM codes 218, 219.9) during ≥3 outpatient visits or 1 inpatient admission before the index date.
4. Hormone use
Patients who used hormones of estrogen, estradiol, or progesterone within 5 years prior to their index date were defined as patients with recent hormone use. We calculated the cumulative exposure time of hormone use by summing up the number of days of hormone use within 5 years prior to the index date.
5. Comorbidities
We used inpatient diagnosis to ascertain the existence of comorbidities, such as hypertension (ICD-9 codes 401–405), diabetes (ICD-9 code 250), hyperlipidemia (ICD-9 code 272), and endometriosis (ICD-9 codes 617.0, 617.1, 617.9), that occurred within 1 year before the index date.
6. Statistical analysis
We compared characteristics between cases and controls using Student's t-test and χ2 test when appropriate. Multivariable logistic regression analysis was used to determine the odds ratio (OR) and 95% confidence interval (CI) of the risk of breast cancer in relation to a history uterine myoma, adjusting for potential confounders. We adjusted for the frequency of inpatient or outpatient visit when analyzing the association between breast cancer and uterine myoma. Patients with breast cancer possibly utilize healthcare services more often than those without breast cancer, which can result in increased likelihood to identify uterine myoma during hospital visits. We assessed the association between breast cancer and uterine myoma in all patients and in patients stratified by age group or by hormone use. Additionally, we examined the impact of surgery treatment for uterine myoma on subsequent breast cancer in patients with a history of uterine myoma.
A 2-tailed p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA).
RESULTS
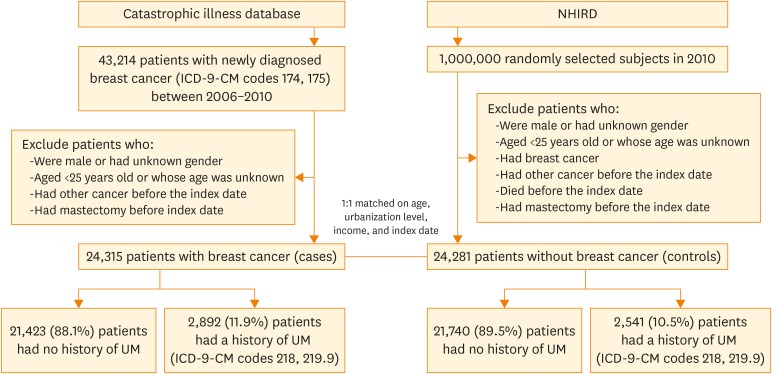
We identified 24,315 adult patients with newly diagnosed breast cancer as cases and 24,281 matched controls (Fig. 1; Table 1). Cases and controls were similar with respect to age, gender, urbanization level, and family income because of matching. Compared with controls, patients with breast cancer were more likely to have hypertension (24.0% vs. 23.1%), diabetes (11.3% vs. 10.6%), and more hospital visits or admissions (19.7 times vs. 16.7 times) within 1 year prior to the index date. Patients with breast cancer were slightly less likely to have hormone use within 5 years prior to the index date (19.0% vs. 20.2%), compared with controls. The cumulative exposure time for hormone use was 136.7±245.0 days for cases and 128.0±237.5 for controls (p=0.079). Neither hyperlipidemia nor endometriosis was associated with breast cancer.
Fig. 1.
Flowchart of selection process of study population.
ICD-9-CM, International Classification of Diseases, 9th revision, clinical modification; NHIRD, National Health Insurance Research Database; UM, uterine myoma.
Table 1. Characteristics of patients with breast cancer and matched controls.
| Variable* | All patients (n=48,596) | Without breast cancer (n=24,281) | With breast cancer (n=24,315) | p-value† | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Age (yr) | 54.2±12.0 | 54.1±12.1 | 54.3±12.0 | 0.307 | ||||
| Urbanization | 1.000 | |||||||
| 1 (highest) | 17,350 | 36.5 | 8,675 | 36.5 | 8,675 | 36.5 | ||
| 2 | 14,924 | 31.4 | 7,462 | 31.4 | 7,462 | 31.4 | ||
| 3 | 6,650 | 14.0 | 3,325 | 14.0 | 3,325 | 14.0 | ||
| 4 (lowest) | 8,547 | 18.0 | 4,273 | 18.0 | 4,274 | 18.0 | ||
| Family income (NTD) | 0.999 | |||||||
| 0–15,840 | 16,924 | 34.9 | 8,462 | 34.9 | 8,462 | 34.8 | ||
| 15,841–45,800 | 27,658 | 57.0 | 13,829 | 57.0 | 13,829 | 57.0 | ||
| ≥45,801 | 3,979 | 8.2 | 1,988 | 8.2 | 1,991 | 8.2 | ||
| Comorbidites‡ | ||||||||
| Hypertension | 11,444 | 23.5 | 5,599 | 23.1 | 5,845 | 24.0 | 0.011 | |
| Diabetes | 5,326 | 11.0 | 2,574 | 10.6 | 2,752 | 11.3 | 0.011 | |
| Hyperlipidemia | 6,734 | 13.9 | 3,329 | 13.7 | 3,405 | 14.0 | 0.349 | |
| Endometriosis | 590 | 1.2 | 285 | 1.2 | 305 | 1.3 | 0.417 | |
| Hormone therapy§ | 9,527 | 19.6 | 4,915 | 20.2 | 4,612 | 19.0 | <0.001 | |
| Cumulative exposure time for hormone use (day) | 128.0±237.5 | 136.7±245.0 | 0.079 | |||||
| No. of inpatient or outpatient visit | 18.2±15.5 | 16.7±15.5 | 19.7±15.3 | <0.001 | ||||
NTD, New Taiwan Dollars.
*Data are shown as number (%) or mean±standard deviation; †We compared continuous variables were analyzed by Student's t-test; categorical variables were analyzed by χ2 test; ‡Reference group is patients without the corresponding condition; §Use of estrogen or progesterone within 5 years prior to the index date.
We found 11.9% of cases and 10.5% of controls had a history of uterine myoma (Table 2). The association between a history of uterine myoma and newly diagnosed breast cancer was significant (adjusted OR [aOR]=1.14; 95% CI=1.07–1.21) after adjusting for potential confounders. This association remained in patients who received hormone recently (aOR=1.20; 95% CI=1.08–1.33) or patients who did not receive hormone recently (aOR=1.11; 95% CI=1.03–1.19) (Table 2). Of 5,433 women with a history of uterine myoma, surgical intervention for uterine myoma (myomectomy or hysterectomy) was not associated with decreased risk of subsequent breast cancer (Table 3).
Table 2. Association between a history of uterine myoma and subsequent occurrence of breast cancer, stratified by age group and recent hormone use.
| Stratified group | UM | All patients (n=48,596) | Without breast cancer (n=24,281) | With breast cancer (n=24,315) | Adjusted OR (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| All patients* | No UM | 43,163 | 88.8 | 21,740 | 89.5 | 21,423 | 88.1 | Reference | <0.001 | |
| UM | 5,433 | 11.2 | 2,541 | 10.5 | 2,892 | 11.9 | 1.14 (1.07–1.21) | |||
| Used of hormone† | ||||||||||
| Not used | No UM | 35,534 | 91.0 | 17,731 | 91.6 | 17,803 | 90.4 | Reference | 0.005 | |
| UM | 3,535 | 9.0 | 1,635 | 8.4 | 1,900 | 9.6 | 1.11 (1.03–1.19) | |||
| Used | No UM | 7,629 | 80.1 | 4,009 | 81.6 | 3,620 | 78.5 | Reference | 0.001 | |
| UM | 1,898 | 19.9 | 906 | 18.4 | 992 | 21.5 | 1.20 (1.08–1.33) | |||
CI, confidence interval; OR, odds ratio; UM, uterine myoma.
*Adjusted for age, urbanization, family income, hypertension, diabetes, hyperlipidemia, hormone use, endometriosis, and number of hospital inpatient/outpatient visit; †Adjusted for urbanization, family income, hypertension, diabetes, hyperlipidemia, hormone use, endometriosis, and number of hospital inpatient/outpatient visit.
Table 3. Surgical interventions for uterine myoma and subsequent occurrence of breast cancer risk among patients with a history of uterine myoma.
| Variable | Patients with UM (n=5,433) | Without breast cancer (n=2,541) | With breast cancer (n=2,892) | Adjusted OR (95% CI)* | p-value | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| No operation | 3,332 | 61.3 | 1,544 | 60.8 | 1,788 | 61.8 | Reference | - |
| Operation | 2,101 | 38.7 | 997 | 39.2 | 1,104 | 38.2 | 0.99 (0.88–1.10) | 0.795 |
| Myomectomy | 499 | 9.2 | 229 | 9.0 | 270 | 9.3 | 1.07 (0.88–1.30) | 0.474 |
| Hysterectomy | 1,602 | 29.5 | 768 | 30.2 | 834 | 28.8 | 0.96 (0.85–1.08) | 0.497 |
CI, confidence interval; OR, odds ratio; UM, uterine myoma.
*Adjusted for age, urbanization, family income, hypertension, diabetes, hyperlipidemia, hormone use, endometriosis, and number of inpatient/outpatient visit.
DISCUSSION
This nationwide, population-based, case-control study used administrative data to evaluate the association between a history of uterine myoma and breast cancer development in Taiwan. We found that women with a history of uterine myoma had a slightly increased risk of developing breast cancer than women without a history of uterine myoma (aOR=1.14). Stratified analyses revealed that women with uterine myoma had a higher risk for later breast cancer development in patients who received hormone recently or patients who did not receive hormone recently. Further, women with a history of uterine myoma and underwent myomectomy or hysterectomy to remove their uterine myoma or uterine did not have a decreased risk of breast cancer.
Two recent studies reported conflicting results. Chuang et al. [16] used the NHIRD in Taiwan and found that uterine myoma was associated with breast cancer (aOR=1.20; 95% CI=1.03–1.40). However, the selection method in our study is more robust than their study. The breast cancer cases in our study represent better than the cases in their study because their cases were selected from a random sample of subjects who were covered by NHI while our cases included all the patients who had breast cancer in Taiwan. In addition, Chuang et al.'s study [16] did not exclude patients who had undergone mastectomy before the index date. These patients should be excluded because they were no longer at risk of acquiring breast cancer. Wise et al. [17] assessed association between history of uterine myoma and breast cancer incidence in the Black Women's Health Study and they did not find significant association (incidence rate ratio=0.99; 95% CI=0.90–1.08). The discrepancy could be due to the differences in genetic, environmental, and life style factors between Asian population and Black population.
Sex hormones such as estrogen and progesterone are considered to involve in the etiology of both breast cancer and uterine myoma [8]. However, in our study, recent hormone use was not associated with breast cancer development and the association between uterine myoma and breast cancer persisted when stratifying patients by recent hormone use. We did not find that hormone use modifies the association between uterine myoma and breast cancer. We think that defining recent hormone use as any hormone use within 5 years prior to the index date should be reasonable because 5 years should be long enough for patients with hormone use to develop breast cancer. A large collaborative study of 51 epidemiological studies found that among current users of hormone replacement therapy or those who ceased use 1–4 years previously, the relative risk of breast cancer increased by a factor of 1.023 (95% CI=1.011–1.036; p<0.001) for each year of use [18]. In addition, this risk effect was reduced after ceasing use of hormone and had largely disappeared after about 5 years.
It is possible that the duration of hormone use has to be long enough to affect the risk of breast cancer. In our study, the cumulative exposure time of hormone use within 5 years prior to the index date was less than 6 months (mean 136.7 days for cases and 128.0 days for controls), which could be too short. Also, hormone use does not represent high hormone level in the body. We were unable to obtain hormone level data because the NHIRD does not collect that information.
We did not find a significant association between endometriosis and breast cancer (1.3% of cases and 1.2% of controls; p=0.417). Unlike our study, Chuang et al. [16] also used NHIRD data but they found a higher proportion of endometriosis diagnosis (2.8% of cases and 1.9% of controls) and a significant association between endometriosis and breast cancer (OR=1.51; 95% CI=1.24–1.85). This discrepancy is possibly caused by different endometriosis diagnosis time. We included only the endometriosis diagnosis that occurred within 1 year prior to the index date, while Chuang et al. [16] included any endometriosis diagnosis occurring before the index date. Therefore, we may not have enough sample size and power to detect a significant association between endometriosis and breast cancer.
Further analysis of patients with a history of uterine myoma revealed that women who undergone myomectomy or hysterectomy to remove their uterine myoma did not have a decreased risk of developing breast cancer. This implies that the etiology of breast cancer is multifactorial and uterine myoma is not a sufficient predictor of breast cancer development.
The limitations of this study come from the nature of an administrative database and an observational study. The NHIRD does not collect data on all potential confounders that are associated with both uterine myoma and breast cancer, such as obesity, early menarche, and late childbirth. In addition, the accuracy of diagnosis based on ICD-9 codes could be an issue of concern [15]. However, the accuracy and validity of breast cancer diagnosis should be good in our study because the NHIA requires at least 2 experienced and qualified specialists to validate breast cancer diagnosis by reviewing patients' medical charts, laboratory data, and x-ray images before issuing a catastrophic illness certificate of breast cancer. The accuracy of uterine myoma diagnosis is possible. However, we optimized the accuracy by defining a history of uterine myoma as having uterine myoma diagnosis for at least 3 outpatient visits or 1 inpatient admission before the index date. If misclassification bias exists, it would be non-differential and would only underestimate the magnitude of the association between uterine myoma and breast cancer. We excluded patients who underwent mastectomy before the index date because these patients should not be at risk of breast cancer development. However, we could not differentiate the side of mastectomy and the side of breast cancer because that data was not collected in NHIRD. We might have excluded patients who underwent right mastectomy but acquired breast cancer on the left breast. This selection bias should be non-differential and should cause underestimation of our findings.
This nationwide population-based study found that women with a history of uterine myoma had an increased risk of breast cancer. Although it might not be causal, from the perspective of public health, our study recommends that women with uterine myoma should be aware of potential breast cancer development in their lifetime. Early diagnosis and management of breast cancer should improve the outcome substantially. Further robust study is necessary to confirm our finding.
ACKNOWLEDGMENTS
This study is based in part on data from the National Health Insurance Research Database (NHIRD) provided by the National Health Insurance Administration (NHIA), Ministry of Health and Welfare and managed by National Health Research Institutes (registered No. 101095, 102148). The interpretation and conclusions contained herein do not represent those of NHIA, Ministry of Health and Welfare or National Health Research Institutes. We thank the Healthcare Service Research Center of Taichung Veterans General Hospital for assistance with statistical analysis.
Footnotes
Funding: This study was supported in part by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-1057329D, TCVGH-105G213, TCVGH-NHRI10505, and TCVGH-1057308C).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: T.J.J., L.C.H.
- Formal analysis: C.Y.H., C.H.Y.
- Funding acquisition: L.C.H.
- Investigation: T.J.J., L.C.H.
- Methodology: T.J.J., C.Y.H., C.H.Y., L.C.H.
- Project administration: C.Y.H.
- Resources: L.C.H.
- Software: C.Y.H.
- Supervision: T.J.J., C.H.Y., L.C.H.
- Validation: C.Y.H., C.H.Y.
- Visualization: C.Y.H., C.H.Y.
- Writing - original draft: T.J.J.
- Writing - review & editing: C.Y.H., C.H.Y., L.C.H.
References
- 1.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 2.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 4.Strissel PL, Swiatek J, Oppelt P, Renner SP, Beckmann MW, Strick R. Transcriptional analysis of steroid hormone receptors in smooth muscle uterine leiomyoma tumors of postmenopausal patients. J Steroid Biochem Mol Biol. 2007;107:42–47. doi: 10.1016/j.jsbmb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Bakas P, Liapis A, Vlahopoulos S, Giner M, Logotheti S, Creatsas G, et al. Estrogen receptor alpha and beta in uterine fibroids: a basis for altered estrogen responsiveness. Fertil Steril. 2008;90:1878–1885. doi: 10.1016/j.fertnstert.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty D, Srinivasan R, Ghosh S, Rajwanshi A, Gopalan S. Estrogen receptor beta (ERbeta) in endometrial simple hyperplasia and endometrioid carcinoma. Appl Immunohistochem Mol Morphol. 2008;16:535–542. doi: 10.1097/PAI.0b013e31816755a9. [DOI] [PubMed] [Google Scholar]
- 7.Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjöblom P, Norgren A, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83:4092–4096. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- 8.Evans JM. An integrative approach to fibroids, endometriosis, and breast cancer prevention. Integr Med. 2008;7:28–31. [Google Scholar]
- 9.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Promotion Administration, Ministry of Health and Welfare (TW) Cancer registry annual report, 2005 [Internet] Taipei: Health Promotion Administration, Ministry of Health and Welfare; [cited 2015 Jan 21]. Available from: http://www.hpa.gov.tw/DOWNLOAD/Statistics/ [Google Scholar]
- 11.Health Promotion Administration, Ministry of Health and Welfare (TW) Cancer registry annual report, 2010 [Internet] Taipei: Health Promotion Administration, Ministry of Health and Welfare; [cited 2015 Jan 21]. Available from: http://www.hpa.gov.tw/DOWNLOAD/Statistics/ [Google Scholar]
- 12.Yen YS, Sun LM, Lin CL, Chang SN, Sung FC, Kao CH. Higher risk for meningioma in women with uterine myoma: a nationwide population-based retrospective cohort study. J Neurosurg. 2014;120:655–661. doi: 10.3171/2013.10.JNS131357. [DOI] [PubMed] [Google Scholar]
- 13.Lee E, Grutsch J, Persky V, Glick R, Mendes J, Davis F. Association of meningioma with reproductive factors. Int J Cancer. 2006;119:1152–1157. doi: 10.1002/ijc.21950. [DOI] [PubMed] [Google Scholar]
- 14.Cheng TM. Taiwan's national health insurance system. In: Okma KG, Crivelli L, editors. Six countries, six reform models: the healthcare reform experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland, and Taiwan. Hackensack (NJ): World Scientific; 2010. pp. 171–204. [Google Scholar]
- 15.Chen HH, Huang N, Chen YM, Chen TJ, Chou P, Lee YL, et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: a nationwide, population-based, case-control study. Ann Rheum Dis. 2013;72:1206–1211. doi: 10.1136/annrheumdis-2012-201593. [DOI] [PubMed] [Google Scholar]
- 16.Chuang SC, Wu GJ, Lu YS, Lin CH, Hsiung CA. Association between medical conditions and breast cancer risk in Asians: a national population-based study in Taiwan. PLoS One. 2015;10:e0143410. doi: 10.1371/journal.pone.0143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise LA, Radin RG, Rosenberg L, Adams-Campbell L, Palmer JR. History of uterine leiomyomata and incidence of breast cancer. Cancer Causes Control. 2015;26:1487–1493. doi: 10.1007/s10552-015-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]