Abstract
Objective
To evaluate the effect of elevated plasma fibrinogen levels on the prognosis of epithelial ovarian cancer (EOC).
Methods
We reviewed the data of 217 patients with advanced-stage EOC between 2000 and 2012, and investigated the prognostic role of elevated plasma fibrinogen levels compared with serum CA-125 levels, neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR). For further evaluation, we performed a meta-analysis using 5 cohort studies published to July 2015, including our cohort study after a literature review.
Results
Among the four biomarkers, only plasma fibrinogen levels >485.2 mg/dL were correlated with impaired progression-free survival (PFS) and overall survival (OS) (median, 13.9 vs. 20.3 months and 42.2 vs. 55.4 months; p<0.010). Elevated plasma fibrinogen levels were an independent factor for poor PFS with marginal significance and OS (adjusted hazard ratios [HRs]=1.389 and 1.581; 95% confidence intervals [CIs]=0.979–1.972 and 1.032–2.423, respectively). Furthermore, crude and subgroup meta-analyses demonstrated that elevated plasma fibrinogen levels were associated with impaired PFS and OS in patients with all stage EOC.
Conclusion
Elevated plasma fibrinogen levels be more important for predicting survival than serum CA-125 levels, NLR and PLR in patients with EOC, in particular, advanced-stage disease. Moreover, it may be related to poor prognosis of EOC.
Keywords: Fibrinogen; Survival; Ovarian Neoplasms, Meta-Analysis
INTRODUCTION
Epithelial ovarian cancer (EOC) exhibits the highest mortality among female genital tract cancers because of no effective screening methods for early detection [1], resulting in a diagnosis of advanced-stage disease in most patients with EOC [2]. Although serum CA-125 level is useful for early detection and prediction of survival in patients with EOC [3,4], its role as a prognostic factor remains controversial in advanced-stage disease and non-serous EOC [5,6,7]. Thus, considerable attention has been focused on the development of prognostic biomarkers of EOC for rapid application in clinical settings.
Although many studies have suggested novel biomarkers showing the relationship between prognosis of EOC and genetic alteration, only a few biomarkers such as HE-4 are considered as a prognostic factor of EOC in clinical setting [8]. On the other hand, clinical biomarkers using systemic inflammation and coagulation has been suggested to be useful to predict prognosis of EOC with the advantage of being clinically easily measured [9]. In particular, systemic inflammation leading to secondary hematological changes has been shown in various types of malignancies [10,11], and changes, such as the neutrophil to lymphocyte ratio (NLR) and the platelet to lymphocyte ratio (PLR), have been investigated as prognostic biomarkers in EOC [12,13].
Moreover, biomarkers using systemic coagulation have been also investigated because the relationship between cancer and hemostatsis-related procoagulants has been widely established [14]. Among related biomarkers suing coagulation factors, plasma fibrinogen levels have been in the limelight because they increase via the extrahepatic synthesis of tumor cells, resulting in a hypercoagulable status [15], tumor progression, and metastasis in various types of malignancies [16,17,18]. However, the role of elevated plasma fibrinogen levels as a prognostic biomarker has not been also sufficiently investigated in EOC.
Thus, we performed two-step studies due to the insufficient evidences. First, we performed a cohort study to evaluate the effect of elevated plasma fibrinogen levels for predicting the prognosis of advanced-stage EOC compared with serum CA-125 levels and systemic inflammatory biomarkers, such as NLR and PLR. Second, we evaluated the role of elevated plasma fibrinogen levels as a prognostic biomarker in EOC by a meta-analysis using relevant studies.
MATERIALS AND METHODS
1. Cohort study
We extracted clinico-pathologic data from a database of EOC patients between January 2000 and December 2012. The institutional review board at our institution approved the current study (No. 1409-154-616). We included patients with the following criteria: patients with EOC; patients with International Federation of Gynecology and Obstetrics (FIGO) stage III to IV disease; patients who received blood tests including differential white blood cells counts, serum CA-125, and plasma fibrinogen levels checked routinely within 2 weeks before the treatment; and patients with Eastern Cooperative Oncology Group performance status of 0–2. However, we excluded patients with inflammatory diseases or other malignancies and those who could not tolerate in surgery or chemotherapy due to low performance status.
Clinico-pathologic data including age, FIGO stage, grade, histology, plasma fibrinogen, serum CA-125 levels, NLR, PLR, optimal debulking surgery, neoadjuvant chemotherapy, and response to platinum and survival were collected. In this cohort study, taxane- and platinum-based chemotherapy before or after surgery was administered in all patients, and those who relapsed within 6 months of completing adjuvant chemotherapy were considered platinum-resistant. Higher levels of plasma fibrinogen, serum CA-125 levels, NLR, and PLR were defined when they were more than the median values. Optimal debulking surgery was defined as residual tumor less than 1 cm in the maximal diameter. Progression-free survival (PFS) was considered as the time that had elapsed from the treatment start date to the date of disease recurrence, and overall survival (OS) was defined as the time that had elapsed from the date of diagnosis to the date of disease-related death or end of the current study.
2. Meta-analysis
After we completed our cohort study, we performed a meta-analysis to evaluate the effect of elevated plasma fibrinogen levels on the prognosis of EOC, consistent with the recommendation from the Preferred Reporting Items for Systematic Review and Meta-analysis guidelines [19]. We searched PubMed, EMBASE, and the Cochrane Library for related studies published to July 2015, and the MeSH search terms were used as follows: “ovarian cancer” or “ovarian neoplasm(s)” or “cancer of the ovary” or “ovarian carcinoma” or “ovarian tumor(s)”; “fibrinogen.” The inclusion criteria were as follows: EOC; calculation of plasma fibrinogen levels before the treatment; comparison of PFS or OS based on plasma fibrinogen levels. However, we excluded case reports, editorials or letters to the editor, review articles, and non-English studies.
Two of the authors (YL and HSK) evaluated the potential eligibility of all studies from the database independently, and the other author (ML) resolved any disagreements. Thus, a total of 95 studies were identified. Among these studies, we excluded six duplicates and 13 studies such as non-English studies (n=6), review articles (n=3), case reports (n=3), and letter to the editor (n=1). Moreover, we excluded 60 studies, including preclinical studies (n=37), non-ovarian cancer (n=14), other coagulation factors (n=7), and clinical materials using fibrinogen (n=2). After 12 studies were additionally excluded due to no comparison of survival based on plasma fibrinogen levels (n=8) and insufficient data to calculate survival (n=4), the remaining four studies and this cohort study were ultimately included (Supplementary Fig. 1) [20,21,22,23].
Next, the 2 authors (YL and HSK) extracted data from the 5 selected studies, and any discrepancies were resolved by the other author (ML). Consequently, we extracted data from each study as follows: first author; country; FIGO stage; cut-off values of plasma fibrinogen levels; number of patients with higher levels and those with lower levels of plasma fibrinogen according to the cut-off values; the total number of patients enrolled to evaluate the effect of an increased fibrinogen levels per 100 units if no cut-off values; PFS; and OS. For subgroup analyses, we adjusted meta-analytic results with potential confounding factors. Next, we evaluated the quality of each study by the Newcastle-Ottawa Scale (NOS) [24], and the NOS score. It was 7 (n=1) or 9 (n=4) for PFS, and 7 (n=2) or 9 (n=3) for OS (Supplementary Table 1).
3. Statistical analysis
For this cohort study, we compared plasma fibrinogen and serum CA-125 levels, NLR and PLR based on clinico-pathological characteristics using Student's t-tests and performed the Kaplan-Meier analysis with the log-rank test to evaluate PFS and OS according to plasma fibrinogen levels, and Cox's proportional hazard analysis using hazard ratios (HRs) and 95% confidence intervals (CIs) to determine whether elevated plasma fibrinogen levels can be an independent prognostic factor. For these analyses, we used SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA), and a p<0.05 was considered statistically significant.
Furthermore, survival analyses were performed by the statistical procedure described by Tierney et al. [25]. In our meta-analysis, all survival data were presented as HRs with 95% CIs. We evaluated heterogeneity using Higgins I2, measuring the percentage of total variation across studies because of heterogeneity rather than chance [26]. Thus, an I2>0.5 represented substantial heterogeneity, and the random effects model was applied, whereas the fixed effects model was used if I2≤0.5 because it suggested no heterogeneity. Moreover, we identified no publication bias by funnel plots and Egger's tests (p>0.05) in this meta-analysis (Supplementary Fig. 2). For this meta-analysis, Comprehensive Meta-Analysis version 2.0 (Biostat Inc., Englewood, NJ, USA) was used.
RESULTS
1. Cohort study
We included 217 patients with advanced-stage EOC, and Table 1 presents their clinico-pathological characteristics. The median age was 54.4 years (range, 25–84 years), and the median duration of follow up was 44.5 months (range, 7–167.2 months). Among all the patients, 3 patients (1.4%) was in stage IIIA, 15 patients (6.9%) were in stage IIIB, 149 patients (68.7%) were in stage IIIC, and 50 patients (23%) were in stage IV. Histologically, 126 (58.1%), 27 (12.4%), 37 (17.1%), 22 (10.1%), and 5 patients (2.3%) had serous, mucinous, endometrioid, clear cell, and undifferentiated carcinoma of the ovary. The mean values of plasma fibrinogen, serum CA-125 levels, NLR, and PLR were 485.2 mg/dL, 4,442.5 IU/mL, 4.46, and 293.7, respectively.
Table 1. Plasma fibrinogen and serum CA-125 levels, neutrophil to lymphocyte and PLRs according to clinico-pathologic characteristics in 217 patients with advanced-stage EOC.
| Characteristics | No. (%) | Plasma fibrinogen levels (mg/dL) | Serum CA-125 levels (IU/mL) | NLR | PLR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | p-value | Mean±SD | p-value | Mean±SD | p-value | Mean±SD | p-value | |||
| Age (yr) | 0.389 | 0.193 | 0.689 | 0.914 | ||||||
| ≤54 | 112 (51.6) | 476.2±145.6 | 2,572.3±4,538.3 | 4.36±2.56 | 292.23±156.93 | |||||
| >54 | 105 (48.4) | 494.7±168.7 | 6,456.6±2,993.9 | 4.57±4.70 | 295.19±237.72 | |||||
| FIGO stage | 0.001 | 0.860 | 0.139 | 0.538 | ||||||
| III | 167 (77.0) | 466.4±155.8 | 4,303.1±2,360.1 | 4.26±3.28 | 289.08±211.99 | |||||
| IV | 50 (23.0) | 548.1±146.2 | 4,905.3±8,454.6 | 5.15±4.99 | 308.96±152.26 | |||||
| Grade | 0.721 | 0.333 | 0.333 | 0.464 | ||||||
| 1–2 | 64 (29.5) | 474.8±165.3 | 2,113.7±4,269.6 | 3.99±1.91 | 267.12±125.98 | |||||
| 3 | 153 (70.5) | 484.4±152.4 | 3,050.5±5,973.7 | 4.66±4.53 | 293.71±231.75 | |||||
| Histology | 0.758 | 0.182 | 0.080 | 0.001 | ||||||
| Serous | 162 (74.7) | 484.8±150.1 | 5,574.5±2,428.1 | 4.72±4.20 | 312.65±217.79 | |||||
| Non-serous | 54 (25.3) | 477.3±166.6 | 1,138.8±2,329.9 | 3.69±1.71 | 234.80±116.42 | |||||
| Debulking surgery | 0.186 | 0.102 | 0.367 | 0.004 | ||||||
| Optimal | 115 (53.0) | 471.9±159.4 | 2,091.4±4,393.7 | 4.25±3.93 | 257.22±145.32 | |||||
| Suboptimal | 102 (47.0) | 500.2±153.9 | 7,070.3±3,016.6 | 4.71±3.53 | 334.75±241.26 | |||||
| Neoadjuvant chemotherapy | 0.002 | 0.854 | 0.798 | 0.385 | ||||||
| No | 170 (78.3) | 468.0±154.3 | 4,582.6±2,353.1 | 4.43±4.12 | 287.45±210.87 | |||||
| Yes | 47 (21.7) | 547.3±153.1 | 3,938.9±7,203.1 | 4.59±1.86 | 316.12±152.23 | |||||
| Response to chemotherapy | 0.002 | 0.948 | 0.125 | 0.371 | ||||||
| Platinum-sensitive | 193 (88.9) | 473.8±155.3 | 4,474.9±2,197.9 | 4.21±3.05 | 289.37±204.46 | |||||
| Platinum-resistant | 24 (11.1) | 576.5±144.0 | 4,170.1±1,101.9 | 6.51±7.02 | 328.15±155.03 | |||||
EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
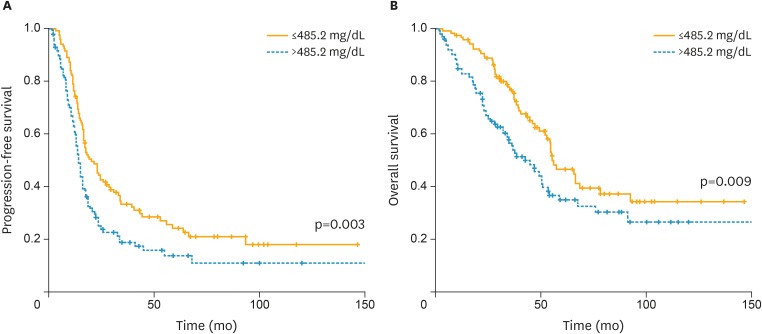
Among all 4 biomarkers, PLR was higher in patients with serous carcinoma and those who received suboptimal debulking surgery. Additionally, plasma fibrinogen levels were higher in patients with stage IV disease, those who received neoadjuvant chemotherapy or showed platinum-resistance (Table 1). Moreover, patients with plasma fibrinogen levels >485.2 mg/dL exhibited decreased PFS (median, 13.9 vs. 20.3 months; p=0.003) and OS compared with those with plasma fibrinogen levels ≤485.2 mg/dL (median, 42.2 vs. 55.4 months; p=0.009; Fig. 1). In multivariate analyses, suboptimal debulking surgery was an independent prognostic factor for PFS (adjusted HR=1.675; 95% CI=1.169–2.4; p=0.005), and plasma fibrinogen levels >485.2 mg/dL reduced PFS with marginal significance (adjusted HR=1.389; 95% CI=0.979–1.972; p=0.066). On the other hand, suboptimal debulking surgery and plasma fibrinogen levels >485.2 mg/dL were also independent prognostic factors for OS (adjusted HRs=2.124 and 1.581; 95% CIs=1.374–3.283 and 1.032–2.423; p=0.001 and 0.035; Table 2).
Fig. 1.
Kaplan-Meier curves for (A) PFS and (B) OS broken down by the mean value (485.2 mg/dL) of plasma fibrinogen levels.
OS, overall survival; PFS, progression-free survival.
Table 2. Unfavorable factors affecting PFS and OS in 217 patients with advanced-stage EOC.
| Factors | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | ||
| PFS | |||||||
| Age ≤54 (yr) | 0.909 | 0.670–1.233 | 0.540 | - | - | - | |
| FIGO stage IV disease | 1.476 | 1.034–2.106 | 0.032 | - | - | - | |
| Grade 3 disease | 0.827 | 0.564–1.213 | 0.331 | - | - | - | |
| Non-serous type | 1.067 | 0.747–1.524 | 0.721 | - | - | - | |
| Suboptimal debulking surgery | 1.710 | 1.260–2.322 | 0.001 | 1.675 | 1.169–2.400 | 0.005 | |
| No neoadjuvant chemotherapy | 0.781 | 0.545–1.119 | 0.178 | - | - | - | |
| Fibrinogen >485.2 (mg/dL) | 1.590 | 1.172–2.157 | 0.003 | 1.389 | 0.979–1.972 | 0.066 | |
| CA-125 >4,442.5 (U/mL) | 1.373 | 0.936–2.015 | 0.105 | - | - | - | |
| NLR >4.46 | 0.910 | 0.665–1.246 | 0.557 | - | - | - | |
| PLR >293.66 | 1.164 | 0.851–1.592 | 0.341 | - | - | - | |
| OS | |||||||
| Age ≤54 (yr) | 0.813 | 0.561–1.178 | 0.274 | - | - | - | |
| FIGO stage IV disease | 1.493 | 0.982–2.270 | 0.061 | - | - | - | |
| Grade 3 disease | 1.586 | 1.003–2.508 | 0.048 | - | - | - | |
| Non-serous type | 1.387 | 0.924–2.083 | 0.115 | - | - | - | |
| Suboptimal debulking surgery | 2.105 | 1.439–3.080 | <0.001 | 2.124 | 1.374–3.283 | 0.001 | |
| No neoadjuvant chemotherapy | 1.009 | 0.633–1.609 | 0.968 | - | - | - | |
| Fibrinogen >485.2 (mg/dL) | 1.627 | 1.124–2.354 | 0.010 | 1.581 | 1.032–2.423 | 0.035 | |
| CA-125 >4,442.5 (U/mL) | 1.064 | 0.668–1.696 | 0.794 | - | - | - | |
| NLR >4.46 | 1.198 | 0.824–1.741 | 0.345 | - | - | - | |
| PLR >293.66 | 1.544 | 1.065–2.237 | 0.022 | - | - | - | |
EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; NLR, neutrophil to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio.
When plasma fibrinogen levels >400 mg/dL were defined as elevated values like previous studies [21,22,23], patients with plasma fibrinogen levels >400 mg/dL showed poorer PFS (median, 16 vs. 23.1 months; p=0.046) than those with plasma fibrinogen levels ≤400 mg/dL in spite of no difference in OS (Supplementary Fig. 3). However, plasma fibrinogen levels >400 mg/dL was not a prognostic factor for PFS and OS in multivariate analysis (Supplementary Table 2).
2. Meta-analysis
The clinic-pathological characteristics of five relevant studies including 1,154 patients with EOC are presented in Table 3. Among all 5 studies, 3 studies revealed the effect of elevated plasma fibrinogen levels on prognosis using the cut-off value of 400 mg/dL [20,21,22,23], whereas one study demonstrated the association between an increase in plasma fibrinogen levels per 100 units and survival in the patients [20]. However, this cohort study used 2 cut-off values of 400 mg/dL and per 100 units of plasma fibrinogen levels according to 2 designs of relevant studies in this meta-analysis. Potential confounding factors such as age, adjuvant or neoadjuvant chemotherapy, albumin, ascites, CA-125, C-reactive protein (CRP), FIGO stage, grade, histology, NLR, PLR, residual tumor size, thrombocytosis, and venous thromboembolism were adjusted.
Table 3. Characteristics of 5 included studies for evaluating the impact of elevated plasma fibrinogen levels on prognosis of EOC.
| Study | Country | Duration of Study | FIGO stage | Cut-off values of plasma fibrinogen levels | No. of patients | Outcomes | Adjustment of potential confounding factors | |
|---|---|---|---|---|---|---|---|---|
| Higher levels of plasma fibrinogen | Lower levels of plasma fibrinogen | |||||||
| Polterauer et al. [20] | Austria | Not mentioned | I–IV | Per 100 units | 422 | PFS, OS | Age, CA-125, CRP, FIGO stage, grade, histology, residual tumor size | |
| Qiu et al. [21] | China | 2002–2005 | I–IV | 400 mg/dL | 49 | 87 | PFS, OS | Adjuvant chemotherapy, age, CA-125, FIGO stage, grade, histology, residual tumor size, thrombocytosis |
| Man et al. [22] | China | 2000–2010 | I–IV | 400 mg/dL | 80 | 110 | PFS, OS | Adjuvant chemotherapy, age, FIGO stage, grade, neoadjuvant chemotherapy, residual tumor size, VTE |
| Zhang et al. [23] | China | 2000–2012 | I–IV | 400 mg/dL | 89 | 100 | PFS, OS* | Age, albumin, ascites, CA-125, CRP, FIGO stage, grade, histology, NLR, PLR, residual tumor size |
| The current study | Korea | 2000–2012 | III–IV | Per 100 units | 217 | PFS, OS | Adjuvant chemotherapy, age, CA-125, FIGO stage, grade, histology, neoadjuvant chemotherapy, NLR, PLR, residual tumor size | |
| 400 mg/dL | 142 | 75 | ||||||
CRP, C-reactive protein; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; NLR, neutrophil to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet to lymphocyte ratio; VTE, venous thromboembolism.
*Not adjusted by potential confounding factors.
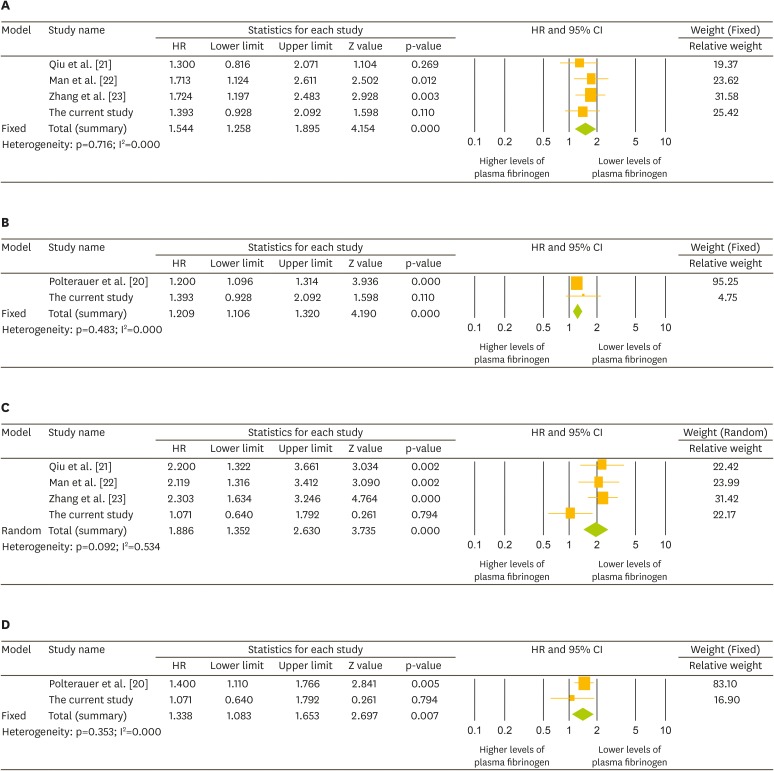
Crude analyses demonstrated that plasma fibrinogen levels >400 mg/dL were correlated with poor PFS (HR=1.544; 95% CI=1.258–1.895) and OS (HR=1.886; 95% CI=1.352–2.630), whereas an increase in plasma fibrinogen levels per 100 units was also associated with poor PFS (HR=1.209; 95% CI=1.106–1.320) and OS (HR=1.338; 95% CI=1.083–1.653; Fig. 2). When we performed subgroup analyses according to quality of study (NOS) and potential confounding factors, we also found that elevated plasma fibrinogen levels were related with poor PFS and OS (Table 4).
Fig. 2.
Forest plots for HRs with 95% CIs for the effect of elevated plasma fibrinogen levels on the prognosis of EOC. (A) Effect of plasma fibrinogen levels of >400 mg/dL; (B) increase in plasma fibrinogen levels per 100 units on PFS; (C) effect of plasma fibrinogen levels of >400 mg/dL; and (D) increase in plasma fibrinogen levels per 100 units on OS.
CI, confidence interval; EOC, epithelial ovarian cancer; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Table 4. Subgroup analyses for the impact of elevated plasma fibrinogen levels on prognosis of EOC.
| Category | No. of studies with references | HR | 95% CI | Heterogeneity | Model | ||
|---|---|---|---|---|---|---|---|
| p-value | I2 | ||||||
| PFS | |||||||
| Adjustment for potential confounding factors | |||||||
| Adjuvant chemotherapy, age, FIGO stage, grade, residual tumor size | 3 | 1.467 | 1.145–1.880 | 0.657 | 0.000 | Fixed effect | |
| Age, CA-125, FIGO stage, grade, histology, NLR, PLR, residual tumor size | 2 | 1.568 | 1.195–2.056 | 0.444 | 0.000 | Fixed effect | |
| Adjuvant chemotherapy, age, FIGO stage, grade, histology, neoadjuvant chemotherapy, residual tumor size | 2 | 1.352 | 1.005–1.836 | 0.827 | 0.000 | Fixed effect | |
| OS | |||||||
| Quality of study (NOS) | |||||||
| 9 | 3 | 1.718 | 1.094–2.698 | 0.087 | 0.591 | Random effects | |
| Adjustment for potential confounding factors | |||||||
| Adjuvant chemotherapy, age, FIGO stage, grade, residual tumor size | 3 | 1.718 | 1.094–2.698 | 0.087 | 0.591 | Random effects | |
| Age, CA-125, FIGO stage, grade, histology, NLR, PLR, residual tumor size | 2 | 1.610 | 1.238–3.405 | 0.015 | 0.830 | Random effects | |
| Adjuvant chemotherapy, age, FIGO stage, grade, histology, neoadjuvant chemotherapy, residual tumor size | 2 | 1.537 | 1.231–3.111 | 0.051 | 0.737 | Random effects | |
CI, confidence interval; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; NOS, the Newcastle-Ottawa Scale; PFS, progression-free survival; PLR, platelet to lymphocyte ratio.
DISCUSSION
In this study, we examined the impact of elevated plasma fibrinogen levels on the prognosis of EOC using a two-step approach of our cohort study and meta-analysis. When we consider some limitations, such as the relative lack of literature, our cohort study indicated that elevated plasma fibrinogen levels may be related with poor prognosis of EOC, and our meta-analysis supported this hypothesis.
In particular, we investigated the prognostic value of plasma fibrinogen levels compared with serum CA-125 levels, NLR, and PLR in patients with advanced-stage EOC. As a result, we found that elevated plasma fibrinogen levels were exclusively correlated with poor PFS and OS, whereas the three biomarkers had no prognostic values in the patients. In previous studies, serum CA-125 levels, NLR, and PLR are prognostic factors for early-stage EOC because three factors are associated with potential implications of distant metastasis [12,27,28,29]. However, serum CA-125 levels cannot efficiently predict survival because these levels increase with tumor burden in advanced-stage EOC, which makes it difficult to predict prognosis [30,31]. Moreover, recent studies have focused on the effect of debulking surgery, which can alter post-treatment levels, affecting prognosis in advanced-stage EOC [6,32].
Although there are few studies about the effect of NLR and PLR on the prognosis of advanced-stage EOC, we demonstrated that the role of NLR and PLR as biomarkers to predict prognosis was small in advanced-stage EOC. One explanation is that NLR and PLR may also increase secondarily as systemic inflammation increases [7]. These factors are expected to detect advanced-stage disease and thereby predict prognosis similar to serum CA-125 levels in early-stage EOC [13,33].
However, elevated plasma fibrinogen levels can activate tumor progression in addition to the increase, resulting from systemic inflammation by cancer. Previous studies have revealed that fibrinogen may have the metastatic potential of circulating tumor cells [34]. Furthermore, pre-treatment plasma fibrinogen levels may correlate with tumor burden, and elevated plasma fibrinogen levels may provide favorable conditions for circulating tumor cells to metastasize via the lymphatic and hematogenous systems [35,36]. In a preclinical study, fibrinogen-deficient mice showed markedly decreased lymphatic and hematogenous metastases compared with wild-type mice, suggesting the active role of elevated plasma fibrinogen levels on the metastatic potential of cancer [34]. This cohort study supports this hypothesis that plasma fibrinogen levels may be more effective to predict the prognosis of advanced-stage EOC than serum CA-125 levels, NLR, and PLR.
However, we failed to show the clear association between elevated plasma fibrinogen levels and poor PFS. In spite of no definite evidence, we could consider the relatively weak role of elevated plasma fibrinogen levels in this cohort because we enrolled only patients with advanced-stage EOC, which was related with the increased rate of suboptimal cytoreduction affecting disease recurrence when compared with previous studies where patients with early-stage EOC were also included [20,21,22,23]. Thus, intra-abdominal tumor volume in addition to circulating tumor cells affecting plasma fibrinogen levels could act as a bias related with poor PFS unlike the previous studies, and thereby led to the weak role of elevated plasma fibrinogen levels. However, the role of elevated plasma fibrinogen levels was clear in terms of OS because response to cytotoxic drugs may affect tumor burden related with elevated plasma fibrinogen levels finally in patients with EOC.
Furthermore, this meta-analysis also supports the effect of elevated plasma fibrinogen levels for predicting PFS and OS in patients with EOC. In particular, subgroup analyses for adjusting potential confounding factors showed a similar effect, suggesting that elevated plasma fibrinogen levels in EOC may be considered for use in clinical settings in a manner similar to NLR and PLR, which have been investigated as clinical biomarkers similar to serum CA-125 levels in relevant meta-analyses [37,38]. Although the cut off value of 400 mg/dL was related with prognosis of EOC in this meta-analysis, we failed to show that the cut off value of 400 mg/dL was a meaningful association with survival in our cohort. The reason is that we included only patients with advanced-stage EOC, who could show elevated plasma fibrinogen levels compared with those with early-stage EOC. For supporting this hypothesis, we demonstrated that plasma fibrinogen levels were higher in patients with stage IV disease than in those with stage III disease (mean, 546.1 mg/dL vs. 466.4 mg/dL). Thus, we thought that the meaningful cut off value of plasma fibrinogen levels might be higher than 400 mg/dL in patients with advanced-stage EOC. However, we did not include patients with early-stage EOC because they showed very low rates of recurrence and death, which made the analysis of correlation between elevated plasma fibrinogen levels and survival difficult. Thus, it can act as a limitation for the current study.
In summary, our cohort study showed that elevated plasma fibrinogen levels were more useful for predicting PFS and OS than serum CA-125 levels, NLR, and PLR in patients with EOC, in particular, advanced-stage disease. Moreover, we demonstrated the effect of elevated plasma fibrinogen levels on the prognosis of EOC in this meta-analysis. However, the relative lack of prior studies on the role of elevated plasma fibrinogen levels still presents major limitations to a complete understanding of this biomarker. Thus, its role should be further investigated to predict prognosis based on the extent of disease as well as suboptimal debulking surgery as a marker of unresectable disease, thereby enhancing our ability to select candidates for neoadjuvant chemotherapy followed by interval surgery in EOC patients with elevated plasma fibrinogen levels [20,21,22,23].
Footnotes
Funding: This research was supported by grants (No. 04-2012-0890, 03-2012-0170, 23-2015-0180, and 23-2015-0140) from the Seoul National University Hospital research fund and the Korean Health Technology R & D Project, Ministry of Health and Welfare (HI14C2404).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.Y., K.H.S., S.Y.S.
- Data curation: L.Y., K.H.S.
- Formal analysis: L.Y., K.H.S., K.M., L.M.
- Funding acquisition: K.H.S.
- Investigation: L.Y., K.H.S.
- Methodology: L.Y., K.H.S., S.Y.S.
- Resources: K.H.S., L.M.
- Software: K.H.S., S.Y.S.
- Supervision: K.M.
- Validation: K.H.S., S.Y.S.
- Visualization: K.H.S.
- Writing - original draft: L.Y., K.H.S.
- Writing - review & editing: K.H.S., K.M., L.M., S.Y.S.
Supplementary Materials
NOS for assessing qualities of 5 cohort studies for this meta-analysis
Unfavorable factors affecting PFS and OS in 217 patients with advanced-stage EOC
The search strategy and number of studies identified for inclusion in this meta-analysis.
Funnel plots with Egger's test indicating no publication bias in this meta-analysis for the effect of elevated plasma fibrinogen levels on (A) PFS and (B) OS in patients with EOC.
EOC, epithelial ovarian cancer; OS, overall survival; PFS, progression-free survival.
Kaplan-Meier curves for (A) PFS and (B) OS broken down by the value of 400 mg/dL of plasma fibrinogen levels.
OS, overall survival; PFS, progression-free survival.
References
- 1.Suh DH, Lee KH, Kim K, Kang S, Kim JW. Major clinical research advances in gynecologic cancer in 2014. J Gynecol Oncol. 2015;26:156–167. doi: 10.3802/jgo.2015.26.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JY, Ngan HY, Park W, Cao Z, Wu X, Ju W, et al. Asian Society of Gynecologic Oncology International Workshop 2014. J Gynecol Oncol. 2015;26:68–74. doi: 10.3802/jgo.2015.26.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermsen BB, von Mensdorff-Pouilly S, Berkhof J, van Diest PJ, Gille JJ, Menko FH, et al. Serum CA-125 in relation to adnexal dysplasia and cancer in women at hereditary high risk of ovarian cancer. J Clin Oncol. 2007;25:1383–1389. doi: 10.1200/JCO.2006.06.7884. [DOI] [PubMed] [Google Scholar]
- 4.Han LY, Karavasilis V, Hagen T, Nicum S, Thomas K, Harrison M, et al. Doubling time of serum CA125 is an independent prognostic factor for survival in patients with ovarian cancer relapsing after first-line chemotherapy. Eur J Cancer. 2010;46:1359–1364. doi: 10.1016/j.ejca.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Barlow TS, Przybylski M, Schilder JM, Moore DH, Look KY. The utility of presurgical CA125 to predict optimal tumor cytoreduction of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16:496–500. doi: 10.1111/j.1525-1438.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 6.Mury D, Woelber L, Jung S, Eulenburg C, Choschzick M, Witzel I, et al. Prognostic and predictive relevance of CA-125 at primary surgery of ovarian cancer. J Cancer Res Clin Oncol. 2011;137:1131–1137. doi: 10.1007/s00432-011-0977-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Choi HY, Lee M, Suh DH, Kim K, No JH, et al. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: a two center cohort study. Cancer Res Treat. 2016;48:250–258. doi: 10.4143/crt.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Kim HS, Suh DH, Kim MK, Chung HH, Song YS. Ovarian cancer biomarker discovery based on genomic approaches. J Cancer Prev. 2013;18:298–312. doi: 10.15430/JCP.2013.18.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan W, Yang G, Liu J. The inflammatory network: bridging senescent stroma and epithelial tumorigenesis. Front Biosci (Landmark Ed) 2009;14:4044–4057. doi: 10.2741/3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Ouden M, Ubachs JM, Stoot JE, van Wersch JW. Whole blood cell counts and leucocyte differentials in patients with benign or malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol. 1997;72:73–77. doi: 10.1016/s0301-2115(96)02662-0. [DOI] [PubMed] [Google Scholar]
- 12.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Liu P, Xu Y, Zhang W, Tong L, Guo Z, et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother Pharmacol. 2015;75:255–262. doi: 10.1007/s00280-014-2622-6. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence SO, Simpson-Haidaris PJ. Regulated de novo biosynthesis of fibrinogen in extrahepatic epithelial cells in response to inflammation. Thromb Haemost. 2004;92:234–243. doi: 10.1160/TH04-01-0024. [DOI] [PubMed] [Google Scholar]
- 16.von Tempelhoff GF, Nieman F, Heilmann L, Hommel G. Association between blood rheology, thrombosis and cancer survival in patients with gynecologic malignancy. Clin Hemorheol Microcirc. 2000;22:107–130. [PubMed] [Google Scholar]
- 17.Takeuchi H, Ikeuchi S, Kitagawa Y, Shimada A, Oishi T, Isobe Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol. 2007;22:2222–2227. doi: 10.1111/j.1440-1746.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147. doi: 10.1186/1471-2407-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C, et al. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist. 2009;14:979–985. doi: 10.1634/theoncologist.2009-0079. [DOI] [PubMed] [Google Scholar]
- 21.Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38:651–657. doi: 10.1111/j.1447-0756.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 22.Man YN, Wang YN, Hao J, Liu X, Liu C, Zhu C, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer. 2015;25:24–32. doi: 10.1097/IGC.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WW, Liu KJ, Hu GL, Liang WJ. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol. 2015;36:8831–8837. doi: 10.1007/s13277-015-3533-9. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] Ottawa: Ottawa Hospital Research Institute; 2011. [cited 2016 Jun 16]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powless CA, Aletti GD, Bakkum-Gamez JN, Cliby WA. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: implications for surgical staging. Gynecol Oncol. 2011;122:536–540. doi: 10.1016/j.ygyno.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Kim JW, Cho JY, Chung HH, Park NH, Song YS, et al. The role of serum CA-125 levels in early-stage epithelial ovarian cancer on preoperative CT and MRI. Eur J Surg Oncol. 2009;35:870–876. doi: 10.1016/j.ejso.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kokcu A, Kurtoglu E, Celik H, Tosun M, Malatyalıoglu E, Ozdemir AZ. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac J Cancer Prev. 2014;15:9781–9784. doi: 10.7314/apjcp.2014.15.22.9781. [DOI] [PubMed] [Google Scholar]
- 30.Tas F, Kilic L, Bilgin E, Keskin S, Sen F, Ciftci R, et al. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23:276–281. doi: 10.1097/IGC.0b013e31827b8796. [DOI] [PubMed] [Google Scholar]
- 31.Kim SI, Kim HS, Kim TH, Suh DH, Kim K, No JH, et al. Impact of underweight after treatment on prognosis of advanced-stage ovarian cancer. J Immunol Res. 2014;2014:349546. doi: 10.1155/2014/349546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HS, Park NH, Chung HH, Kim JW, Song YS, Kang SB. Serum CA-125 level after 6 cycles of primary adjuvant chemotherapy is a useful prognostic factor for complete responders' survival in patients with advanced epithelial ovarian cancer. Onkologie. 2008;31:315–320. doi: 10.1159/000131270. [DOI] [PubMed] [Google Scholar]
- 33.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 35.Lee JH, Ryu KW, Kim S, Bae JM. Preoperative plasma fibrinogen levels in gastric cancer patients correlate with extent of tumor. Hepatogastroenterology. 2004;51:1860–1863. [PubMed] [Google Scholar]
- 36.Yamashita H, Kitayama J, Nagawa H. Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol. 2005;35:595–600. doi: 10.1093/jjco/hyi150. [DOI] [PubMed] [Google Scholar]
- 37.Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 38.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NOS for assessing qualities of 5 cohort studies for this meta-analysis
Unfavorable factors affecting PFS and OS in 217 patients with advanced-stage EOC
The search strategy and number of studies identified for inclusion in this meta-analysis.
Funnel plots with Egger's test indicating no publication bias in this meta-analysis for the effect of elevated plasma fibrinogen levels on (A) PFS and (B) OS in patients with EOC.
EOC, epithelial ovarian cancer; OS, overall survival; PFS, progression-free survival.
Kaplan-Meier curves for (A) PFS and (B) OS broken down by the value of 400 mg/dL of plasma fibrinogen levels.
OS, overall survival; PFS, progression-free survival.