ABSTRACT
Foot-and-mouth disease virus (FMDV) is a highly contagious viral disease. Antibodies are pivotal in providing protection against FMDV infection. Serological protection against one FMDV serotype does not confer interserotype protection. However, some historical data have shown that interserotype protection can be induced following sequential FMDV challenge with multiple FMDV serotypes. In this study, we have investigated the kinetics of the FMDV-specific antibody-secreting cell (ASC) response following homologous and heterologous inactivated FMDV vaccination regimes. We have demonstrated that the kinetics of the B cell response are similar for all four FMDV serotypes tested following a homologous FMDV vaccination regime. When a heterologous vaccination regime was used with the sequential inoculation of three different inactivated FMDV serotypes (O, A, and Asia1 serotypes) a B cell response to FMDV SAT1 and serotype C was induced. The studies also revealed that the local lymphoid tissue had detectable FMDV-specific ASCs in the absence of circulating FMDV-specific ASCs, indicating the presence of short-lived ASCs, a hallmark of a T-independent 2 (TI-2) antigenic response to inactivated FMDV capsid.
IMPORTANCE We have demonstrated the development of intraserotype response following a sequential vaccination regime of four different FMDV serotypes. We have found indication of short-lived ASCs in the local lymphoid tissue, further evidence of a TI-2 response to FMDV.
KEYWORDS: B cell, FMDV, T-independent, viral immunology
INTRODUCTION
Foot-and-mouth disease virus (FMDV) is a highly contagious pathogen that has a large socioeconomic impact upon countries in which the virus is endemic. Studies of cattle and mice have shown that protection from the virus is largely mediated by antibodies (1). One of the main approaches to FMD control is through vaccination with inactivated FMDV antigen in formulation with adjuvants (2). However, the current inactivated FMDV vaccines are unable to induce long duration of immunity in cattle; regular repeated immunizations are required to maintain protective antibody titers (1).
It is generally considered that serological protection against one FMDV serotype does not confer interserotype protection and may not, in some cases, confer intraserotype protection, given the antigenic variation seen within some serotypes (1). We have recently shown that intraserotype protection is possible within the FMDV O serotype using a heterologous FMDV challenge model following a single vaccination with inactivated FMDV (3). Indeed, Cottral and Gailiunas were able to demonstrate that after three rounds of challenge with multiple FMDV serotypes, the animals were resistant to further FMDV challenges (4). These animals had clearly developed cross-reactive neutralizing antibodies that were able to protect them against further serotype challenges.
To understand the kinetics and magnitude of the bovine antibody response to multiple FMDV vaccinations, we first performed a study to understand the kinetics of the antibody-secreting cells and serological response to a homologous prime and booster vaccination regime with four different FMDV serotypes. We then used these data as the basis of a study to establish the kinetics and FMDV serotype specificity of the antibody response during a sequential vaccination regime with the same four different FMDV serotypes. For the first time, we establish that exposure to different FMDV serotypes following a prime and heterologous boost regime drives an antibody response that recognizes multiple serotypes. This has important implications to inform continuous vaccine strategies that are driven by the short duration of immunity offered by current vaccines, as well as the identification of shared epitopes that could drive cross-protection in the next generation of vaccines.
RESULTS
To understand the kinetics and magnitude of the bovine antibody response to multiple FMDV vaccinations, we performed two animal studies. The first study was designed to understand the kinetics of the antibody-secreting cells (ASCs) and serological response to a homologous prime and booster vaccination regime with four different FMDV serotypes. The second study was designed to investigate the antibody-secreting cell and serological response kinetics following sequential vaccination with inactivated antigen based on four different FMDV serotypes. The FMDV specificity of this response will also be investigated to determine whether a cross-reactive antibody response has indeed been induced.
Kinetics of the antigen-specific antibody-secreting cell response following FMDV prime and boost vaccination.
The magnitude and kinetics of the FMDV-specific IgG antibody-secreting cell response was monitored using an FMDV-specific enzyme-linked immunosorbent spot (ELISpot) assay using peripheral blood mononuclear cells (PBMCs) isolated from FMDV-vaccinated animal groups. Only the homologous FMDV-specific IgG antibody-secreting cell response was monitored for three of the prime and booster vaccination groups (A22, Asia 1 Shamir [A1S], and SAT1 groups), while for the FMDV O Pan Asia (OPA) group, which received multiple vaccinations with different FMDV serotypes, the responses to all of these serotypes were monitored. However, the initial prime and booster vaccination of the OPA group will be discussed in this section in relation to the FMDV OPA-specific response to the homologous vaccine antigen.
Following primary vaccination of the highly purified inactivated FMDV antigens, there were no FMDV-specific plasma cells detected in any of the vaccinated groups (A22, A1S, SAT1, and OPA groups) at either of the time points tested (6 and 20 days after primary vaccination [dpv], Fig. 1 and Table 1).
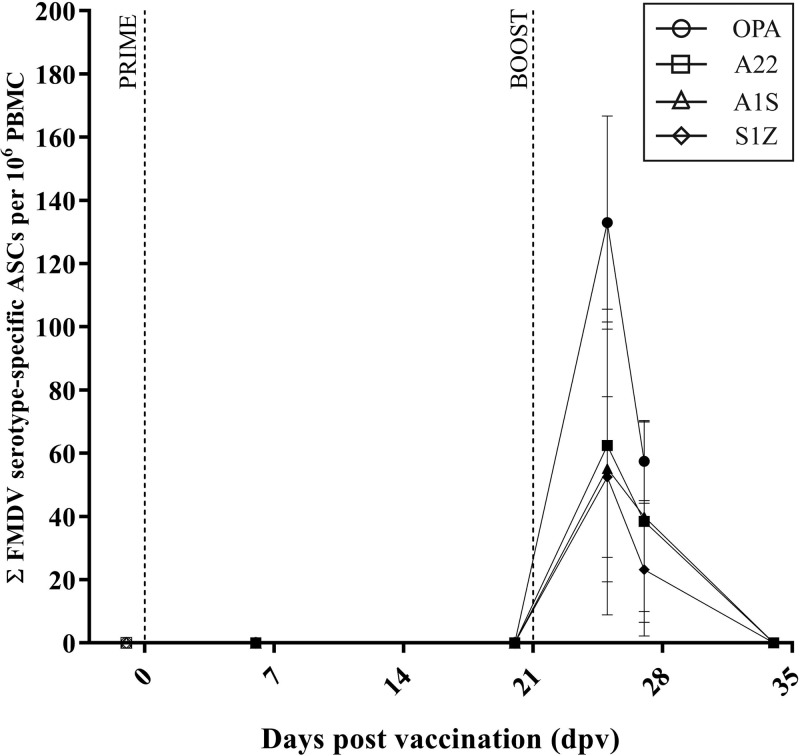
FIG 1.
Kinetics of the FMDV-specific plasma cell response in cattle after prime and boost vaccination. The kinetics of the FMDV-specific plasma cell response after prime (0 dpv) and boost (21 dpv) vaccination (indicated by the vertical dotted lines) are depicted. Results are expressed as the group mean (four calves per group) ± standard error of the mean (SEM) (error bar) of the group with duplicate determinations for each calf.
TABLE 1.
Total number of IgG FMDV-specific antibody-secreting cells in PBMC population following prime and booster vaccination with a single inactivated FMDV serotypea
| Treatment | Day postvaccination (day post-boost vaccination) | Sample | Total no. of FMDV serotype-specific ASCs/106 PBMCsb |
|||
|---|---|---|---|---|---|---|
| A22 group (n = 4) | A1S group (n = 4) | OPA group (n = 4) | SAT1 group (n = 4) | |||
| Inactivated FMDV vaccination (prime) | −1 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0 | Peripheral blood | |||||
| 6 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 20 (−1) | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Inactivated FMDV vaccination (boost) | 21 (0) | Peripheral blood | ||||
| 25 (4) | Peripheral blood | 63 ± 21.6** | 55 ± 23.2** | 133 ± 33.8** | 53 ± 12.7* | |
| 27 (6) | Peripheral blood | 39 ± 16.0* | 40 ± 15.0* | 58 ± 12.4** | 23 ± 10.5 | |
| 34 (13) | Peripheral blood | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | |
| 39 (18) | Left PSLN | NP | NP | NP | 114 ± 45.7** | |
| Right PSLN | NP | NP | NP | 0 ± 0 | ||
| 67 (36) | Left PSLN | 0 ± 0 | 3 ± 1.7 | NP | NP | |
| Right PSLN | 0 ± 0 | 0 ± 0 | NP | NP | ||
Abbreviations: ASCs, antibody-secreting cells; PSLN, prescapular lymph node.
Results are expressed as the means ± standard errors of the means (SEMs) of the groups with duplicate determinations for each animal. Values that are statistically significantly different from the value at −1 day postvaccination (the day before vaccination) by two-way ANOVA analysis with multiple comparison are indicated by asterisks as follows: *, P < 0.05; **, P < 0.001. NP, test not performed.
A homologous booster vaccination of highly purified inactivated FMDV antigen was given 21 days after the primary vaccination (21 dpv, 0 days postboost [dpb]). A burst of FMDV-specific ASCs was detected 4 days after the booster vaccination was delivered (4 dpb) (Fig. 1 and Table 1). The magnitudes of the ASC responses were similar for the vaccinated groups (63 ± 21.6 FMDV A22-specific ASCs per 106 PBMCs for the A22 group, 55 ± 23.2 FMDV A1S-specific ASCs per 106 PBMCs for the A1S group, 53 ± 12.7 FMDV SAT1-specific ASCs per 106 PBMCs for the SAT1 group, and 133 ± 33.8 FMDV OPA-specific ASCs per 106 PBMCs for the OPA group; Fig. 1 and Table 1). The number of FMDV-specific ASCs had decreased by 6 dpb in all FMDV-vaccinated groups, but these cells were still detected. By 13 dpb, there were no FMDV-specific ASCs detected in the three vaccinated groups tested at this time point (Fig. 1 and Table 1).
Animals in the SAT1-vaccinated group were culled on 18 dpb, and the left and right prescapular lymph nodes (PSLN) were tested for the presence of FMDV-specific ASCs. Only the left PSLN had a detectable number of FMDV SAT1-specific ASCs present (114 ± 45.7 FMDV SAT1-specific ASCs per 106 PBMCs for the SAT1 group; Fig. 1 and Table 1). Animals in the A22 group and the A1S group were culled at a later time point (36 dpb), and again both the left and right PSLN were tested for the presence of FMDV-specific ASCs. The A22 group had no FMDV-specific ASCs detected in either PSLN tested. The A1S group had a small number of FMDV-specific ASCs detected in the left PSLN only (3 ± 1.7 FMDV A1S-specific ASCs per 106 PBMCs in the left PSLN of animals in the A1S group; Fig. 1 and Table 1).
The nonvaccinated control (NVC) group had no detectable FMDV-specific ASCs detectable at any of the time points tested (Table 2).
TABLE 2.
Total number of IgG FMDV-specific antibody-secreting cells in PBMC population following sequential immunization with multiple inactivated FMDV serotypesa
| Treatment | Day postvaccination | Sample | Total no. of FMDV serotype-specific ASCs/106 PBMCsb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPA group (n = 4) |
NVC group (n = 2) |
|||||||||||
| OPA | A22 | A1S | SAT1 | C1 | A22 | A1S | OPA | SAT1 | C1 | |||
| Inactivated FMDV OPA vaccination | −1 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0 | ||||||||||||
| 6 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 20 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Inactivated FMDV OPA vaccination | 21 | |||||||||||
| 25 | Peripheral blood | 133 ± 33.8** | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 27 | Peripheral blood | 58 ± 12.4** | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 41 | Peripheral blood | 6 ± 5.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Inactivated FMDV A22 vaccination | 42 | |||||||||||
| 46 | Peripheral blood | 43 ± 17.2** | 12 ± 3.3 | 8 ± 2.8 | 6 ± 3.6 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 48 | Peripheral blood | 45 ± 20.5** | 24 ± 7.8** | 18 ± 6.2* | 8 ± 1.1 | 50 ± 7.5* | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 62 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Inactivated FMDV A1S vaccination | 63 | |||||||||||
| 67 | Peripheral blood | 14 ± 0.6 | 7 ± 1.7 | 12 ± 4.0* | 3 ± 1.5 | 4 ± 1.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 69 | Peripheral blood | 5 ± 2.9 | 3 ± 1.0 | 9 ± 3.2* | 0 ± 0 | 2 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 83 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Inactivated FMDV SAT1 vaccination | 84 | |||||||||||
| 88 | Peripheral blood | 13 ± 3.2 | 4 ± 1.7 | 6 ± 3.5 | 5 ± 2.3 | 5 ± 2.7 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 90 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 97 | Peripheral blood | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 102 | Left PSLN | 27 ± 9.1 | 27 ± 10.3** | 44 ± 15.7** | 3 ± 2.0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Right PSLN | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | NP | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||
Abbreviations: ASC, antibody-secreting cells; PSLN, prescapular lymph node.
Results are expressed as the means ± standard errors of the means (SEMs) of the groups with duplicate determinations for each animal. Values that are statistically significantly different from the value at −1 day postvaccination (the day before vaccination) by two-way ANOVA analysis with multiple comparison are indicated by asterisks as follows: *, P < 0.05; **, P < 0.001. NVC, nonvaccinated group; NP, test not performed.
Kinetics of the serological response following FMDV prime and boost vaccination.
The kinetics of the serological response to FMDV vaccination was determined via FMDV serotype-specific liquid phase blocking ELISA (enzyme-linked immunosorbent assay) (LPBE). The FMDV virus strains used for LPBE assays are listed in Table 3.
TABLE 3.
FMDV stocks used for LPBE assaya
| FMDV serotype | FMDV strain utilized in the following assay: |
|
|---|---|---|
| ELISpot | LPBE | |
| A | A22 Iraq | A22 Iraq |
| O | O Tur 5/09 | O1 Manisa |
| Asia | Asia1 Shamir | Asia1 Shamir |
| SAT1 | SAT1 Zim 22/89 | SAT105 |
| C | C1 Oberbayern | C/PHI/7/84 |
Details of the live-virus FMDV stocks used for the liquid phase blocking ELISA (LPBE).
The vaccination groups that received a homologous primary and booster vaccination of an inactivated FMDV serotype showed similar kinetics of increasing FMDV-specific LPBE titers following primary vaccination with inactivated FMDV (Fig. 2 and Table 4). However, the only groups to demonstrate a significant increase in FMDV-specific LPBE titers were the OPA and SAT1 groups by 20 dpv (P < 0.05) (Table 4).
FIG 2.
Kinetics of the FMDV-specific serological response in cattle after prime and boost vaccination. The kinetics of the FMDV-specific liquid phase blocking ELISA (LPBE) antibody response after prime (0 dpv) and boost (21 dpv) vaccination (indicated by the vertical dotted lines). Results are expressed as the group mean (four calves per group) ± SEM with duplicate determinations for each calf.
TABLE 4.
FMDV serotype-specific LPBE titers following prime and boost vaccination with a single inactivated FMDV serotypea
| Treatment | Day postvaccination (day post-boost vaccination) | FMDV serotype-specific serological response (log10 LPBE titer)b |
|||
|---|---|---|---|---|---|
| A22 group (n = 4) | A1S group (n = 4) | OPA group (n = 4) | SAT1 group (n = 4) | ||
| Inactivated FMDV vaccination | −1 | 1.20 ± 0 | 1.42 ± 0.18 | 1.20 + 0.0 | 1.20 + 0.0 |
| 0 | |||||
| 6 | 1.24 ± 0.03 | 1.50 ± 0.16 | 1.20 + 0.0 | 1.35 + 0.11 | |
| 20 (−1) | 1.73 ± 0.18 | 1.95 ± 0.12 | 1.84 + 0.14** | 1.76 + 0.19* | |
| Inactivated FMDV vaccination | 21 (0) | ||||
| 25 (4) | 1.99 ± 0.16* | 2.11 ± 0.15* | NP | NP | |
| 27 (6) | 2.56 ± 0.18** | 2.75 ± 0.18** | 2.26 + 0.17** | 2.45 + 0.14** | |
| 34 (13) | 2.78 ± 0.23** | 2.90 ± 0.16** | NP | 2.60 + 0.09** | |
LPBE, liquid phase blocking ELISA.
Results are expressed as the log10-transformed means ± standard errors of the means (SEMs) of FMDV-specific LPBE titers postvaccination of the groups with duplicate determinations for each animal. Values that are statistically significantly different from the value at −1 day postvaccination (the day before vaccination) by two-way ANOVA analysis with multiple comparison are indicated by asterisks as follows: *, P < 0.05; **, P < 0.001. NP, test not performed.
A homologous inactivated FMDV vaccination was given at 21 dpv (0 dpb); this resulted in a further increase in the homologous FMDV-specific LPBE titers from 6 dpb (27 dpv) (P > 0.001) (Table 4). These homologous FMDV-specific LPBE titers remained significantly elevated at 13 dpb (34 dpv) (P > 0.001) (Table 4).
Kinetics of the antigen-specific antibody-secreting cell response following sequential FMDV vaccination.
Following the homologous prime and boost vaccination with highly purified FMDV O Pan Asia, the OPA group then received three further vaccinations of three different FMDV serotypes at 21-day intervals; the three different FMDV serotypes were FMDV A22 Iraq, FMDV Asia 1 Shamir, and finally SAT1 Zimbabwe (S1Z). The FMDV-specific ASC response was monitored against all FMDV serotypes used for vaccination (OPA, A22, A1S, and SAT1) and also a FMDV serotype C (C1 Oberbyern) using an FMDV-specific ELISpot assay.
After the primary vaccination with inactivated FMDV OPA, there were no FMDV-specific ASCs detected in the peripheral blood (Fig. 3 and Table 2). A booster vaccination of inactivated FMDV OPA was administered at 21 dpv. This resulted in a burst of FMDV OPA-specific ASCs at 25 dpv (133 ± 33.8 FMDV OPA ASCs per 106 PBMCs in the OPA group; Fig. 3 and Table 2). The FMDV OPA-specific plasma cell burst was still detected at 27 and 41 dpv, albeit at a lower magnitude (Fig. 3 and Table 2). There were no other FMDV serotype-specific ASCs detected following the boost vaccination with inactivated FMDV OPA.
FIG 3.
Kinetics of the FMDV-specific plasma cell response in cattle after sequential vaccination. The kinetics of the FMDV-specific plasma cell response after OPA prime (0 dpv), OPA boost (21 dpv), A22 (42 dpv), A1S (63 dpv), and S1Z (84 dpv) vaccination (indicated by the vertical dotted lines) are depicted. Results are expressed as the group mean (n = 4) ± SEM with duplicate determinations for each calf.
At 42 dpv, the OPA group was vaccinated with inactivated FMDV A22. This induced a burst of FMDV A22-specific ASCs from 46 to 48 dpv (24 ± 7.8 FMDV A22-specific ASCs per 106 PBMCs at 48 dpv in the OPA group; Fig. 3 and Table 2). This was also coupled with an increase in FMDV-OPA-specific ASCs which again peaked at 48 dpv (45 ± 20.5 FMDV OPA-specific ASCs per 106 PBMCs at 48 dpv in the OPA group; Fig. 3 and Table 2). A burst of FMDV A1S-specific ASCs seen at both 46 and 48 dpv (18 ± 6.2 FMDV A1S-specific ASCs per 106 PBMCs at 48 dpv in the OPA group; Fig. 3 and Table 2). There was also a burst of FMDV SAT1-specific ASCs at both 46 and 48 dpv (8 ± 1.1 FMDV SAT1-specific ASCs per 106 PBMCs at 48 dpv in the OPA group; Fig. 3 and Table 2) and FMDV-C1-specific ASCs at 48 dpv (50 ± 7.5 FMDV C1-specific ASCs per 106 PBMCs in the OPA group; Fig. 3 and Table 2).
The OPA group of animals was vaccinated with a third FMDV serotype (A1S) at 63 dpv. This induced a burst of ASCs that were specific for all five of the FMDV serotypes tested by ELISpot assay. The FMDV-specific ASC burst was detected for all five of the FMDV serotypes tested at 67 and 69 dpv, although the magnitude of the response was reduced compared to the FMDV-specific ASC responses at 46 and 48 dpv (Fig. 3 and Table 2). The FMDV-specific ASC response was not detected by 62 dpv for all serotypes tested (Fig. 3 and Table 2).
A final vaccination of a fourth FMDV serotype (SAT1) was given at 84 dpv to all the animals in the OPA group. This resulted in a small burst of ASCs that were specific for all five FMDV serotypes tested at 88 dpv only (Fig. 3 and Table 2).
On 102 dpv, the animals were culled and mononuclear cells were extracted from both the left and right prescapular lymph nodes. The presence of FMDV-specific ASCs was again tested by ELISpot assay for the four FMDV serotypes used in the sequential vaccination regime (Fig. 4). Only the left PSLN, which was directly below the vaccination site, had a FMDV-specific ASC response detected against all four FMDV serotypes (Table 2). There were no FMDV-specific ASCs detected in the right PSLN (Table 2).
FIG 4.
Schematic of the vaccination regime. (A and B) Schematic diagram of the schedule (from day 0 to day 70 or 105) of the prime and boost vaccination regime (A) or sequential serotype vaccination regime (B) used in this study. The vaccination time points (black arrows), blood sampling time points (red diamonds), and subscapular left (L) and right (R) lymph node sampling (gray diamonds) are indicated.
The NVC group showed no detectable FMDV-specific ASCs at any time point throughout the study (Table 2).
Kinetics of the serological response following sequential FMDV vaccination.
The serological response of the OPA group (which received immunizations of three different FMDV serotypes at 21-day intervals) to the four FMDV serotypes that were used as vaccine antigens were tested by LPBE, along with a fifth serotype (FMDV serotype C) to which the animals had not been exposed.
Following a primary vaccination with FMDV OPA, the animals showed a significant increase in FMDV O-serotype-specific LPBE titer at 20 dpv (P < 0.05) (Fig. 5 and Table 5). There was no increase in the LPBE titers of the other FMDV serotypes tested (Fig. 5 and Table 5). After an additional vaccination with inactivated FMDV OPA at 21 dpv, there was a significant increase in the FMDV O-serotype-specific LPBE titers from 27 dpv (P < 0.001) (Fig. 5 and Table 5). Again, there was no further increase in LPBE of the other FMDV serotypes tested (Fig. 5 and Table 5).
FIG 5.
Kinetics of the FMDV-specific serological response in cattle after sequential vaccination. The kinetics of the FMDV-specific LPBE cell response after OPA prime (0 dpv), OPA boost (21 dpv), A22 (42 dpv), A1S (63 dpv), and S1Z (84 dpv) vaccination (indicated by the vertical dotted lines). Results are expressed as the group mean (n = 4) ± SEM with duplicate determinations for each calf.
TABLE 5.
FMDV serotype-specific LPBE titers following sequential immunization with multiple inactivated FMDV serotypesa
| Treatment | Day postvaccination | FMDV serotype-specific serological response (log10 LPBE titer) in the OPA group (n = 4)b |
||||
|---|---|---|---|---|---|---|
| OPA | A22 | A1S | SAT1 | C1 | ||
| Inactivated FMDV OPA vaccination | −1 | 1.20 ± 0.00 | 1.20 ± 0.00 | 1.24 ± 0.03 | 1.20 ± 0.00 | 1.27 ± 0.04 |
| 0 | ||||||
| 6 | 1.20 ± 0.00 | 1.20 ± 0.00 | 1.20 ± 0.00 | 1.20 ± 0.00 | 1.20 ± 0.00 | |
| 20 | 1.84 ± 0.14** | 1.24 ± 0.03 | 1.20 ± 0.00 | 1.24 ± 0.03 | 1.20 ± 0.00 | |
| Inactivated FMDV OPA vaccination | 21 | |||||
| 27 | 2.26 ± 0.17** | 1.31 ± 0.03 | 1.20 ± 0.00 | 1.24 ± 0.03 | 1.28 ± 0.08 | |
| 41 | 2.52 ± 0.13** | 1.39 ± 0.09 | 1.20 ± 0.00 | 1.24 ± 0.03 | 1.50 ± 0.09 | |
| Inactivated FMDV A22 vaccination | 42 | |||||
| 48 | 2.63 ± 0.13** | 1.69 ± 0.09* | 1.20 ± 0.00 | 1.32 ± 0.11 | 1.34 ± 0.00 | |
| 62 | 2.56 ± 0.21** | 2.14 ± 0.13** | 1.20 ± 0.00 | 1.28 ± 0.04 | 1.54 ± 0.07 | |
| Inactivated FMDV A1S vaccination | 63 | |||||
| 69 | 2.71 ± 0.15** | 2.14 ± 0.14** | 2.22 ± 0.29** | 1.58 ± 0.08 | 1.69 ± 0.04 | |
| 83 | 2.63 ± 0.13** | 1.95 ± 0.12** | 2.60 ± 0.34** | 1.81 ± 0.27* | 1.73 ± 0.04* | |
| Inactivated FMDV S1Z vaccination | 84 | |||||
| 90 | 2.63 ± 0.10** | 2.11 ± 0.14** | 2.75 ± 0.31** | 2.14 ± 0.22** | 1.73 ± 0.07* | |
| 97 | 2.67 ± 0.14** | 2.07 ± 0.14** | 2.63 ± 0.34** | 2.33 ± 0.26** | 1.69 ± 0.04 | |
LPBE, liquid phase blocking ELISA.
Results are expressed as the log10-transformed means ± standard errors of the means (SEMs) of FMDV-specific LPBE titers postvaccination of the groups with duplicate determinations for each animal. Values that are statistically significantly different from the value at −1 day postvaccination (the day before vaccination) by two-way ANOVA analysis with multiple comparison are indicated by asterisks as follows: *, P < 0.05; **, P < 0.001.
At 42 dpv, the animals in the OPA group were vaccinated with inactivated FMDV A22, resulting in a significant increase in FMDV A22-specific LPBE titer from 48 dpv (P < 0.05) (Fig. 5 and Table 5), which further increased at 62 dpv (P < 0.001) (Fig. 5 and Table 5). The FMDV OPA-specific LPBE titers remained significantly elevated following vaccination with FMDV A22 (P < 0.001) (Fig. 5 and Table 5). There was no significant increase in the other FMDV-specific serotype LPBE titers tested (Fig. 5 and Table 5).
At 63 dpv, the animals in the OPA group were vaccinated with inactivated FMDV A1S, which resulted in a significant increase in the FMDV A1S-specific LPBE titers from 69 dpv (P < 0.05) (Fig. 5 and Table 5). The LPBE titer specific for the previous FMDV vaccine antigens (FMDV OPA and A22) also remained significantly elevated (P < 0.001) (Fig. 5 and Table 5). The LPBE titers specific for FMDV serotype SAT1 and C also showed an increase following immunization with FMDV A1S, reaching a significant elevation by 83 dpv (P < 0.05) (Fig. 5 and Table 5).
The final vaccination of inactivated FMDV SAT1 was administered on 84 dpv and resulted in a further significant increase in FMDV SAT1-specific LPBE titer at 90 dpv, which remained elevated at 97 dpv (P < 0.001) (Fig. 5 and Table 5). The LPBE FMDV A1S- and OPA-specific titers also remained significantly elevated after FMDV SAT1 vaccination. The LPBE titer specific for FMDV-A22 was also significantly elevated at 90 and 97 dpv (P < 0.001) (Fig. 5 and Table 5). The FMDV serotype C LPBE titers were significantly elevated at 90 dpv (P < 0.05) (Fig. 5 and Table 5).
DISCUSSION
This is the first study to assess the kinetics and magnitude of the FMDV-specific plasma serological response following sequential vaccination with multiple FMDV serotypes and also homologous prime and boost FMD vaccination.
Following homologous primary and boost vaccination with inactivated FMDV, a burst of FMDV-specific ASCs 4 to 6 days after the boost vaccination was detected in the peripheral blood of the vaccinated cattle groups. The timing of this antigen-specific response after the booster vaccination is in keeping with previously published data for cattle (3, 5, 6) and humans (7, 8). The antigen-specific ASC response was coupled with a significant increase in LPBE titers after the boost vaccination. The kinetics of the antibody-secreting cell and serological responses were similar in the vaccine groups. The lack of FMDV-specific ASCs detected after primary vaccination but coupled with an increase in FMDV antibody by day 20 is in keeping with a type II T-independent antigen B cell response in cattle which showed antigen-specific plasma cells not detected in the periphery following primary vaccination but an increase in the antigen-specific IgG response by 21 days after primary vaccination (5).
The group of animals that received a prime and booster vaccination of inactivated FMDV OPA then went on to receive a sequential vaccination regime of different inactivated FMDV serotypes at 21-day intervals. After the initial prime and booster vaccination with inactivated FMDV OPA, only FMDV OPA-specific antibody-secreting cells were detected in the peripheral blood of the vaccinated animals 4 to 6 days after the boost. This was coupled with a significant increase in FMDV O-serotype-specific LPBE titers. There were no ASCs specific for any other FMDV serotype detected after homologous prime and boost vaccination with inactivated FMDV OPA.
A second inactivated FMDV serotype (A22) was administered 21 days after the inactivated FMDV OPA booster vaccination. This resulted in a burst of FMDV-specific ASCs 4 to 6 days after the vaccine was given, and these ASCs were specific for all FMDV serotypes tested, including the three “unseen” FMDV serotypes (A1S, SAT1Z, and C1). This was coupled with a significant increase in the LPBE titer for both the previous inactivated FMDV vaccine antigens (FMDV OPA and A22) that had been administered. The difference in antigen specificity seen between the ASC response and the antibody response is likely to be due to the detection methodologies used in this study. The ASC detection method is a membrane-based assay which is used to detect secreted FMDV-specific antibody from ASCs, whereas the LPBE assay used to define the antibody response is based upon the specific blocking of FMDV antigen in the liquid phase by antibodies in the test serum in competition with FMDV-specific rabbit sera. Thus, if the FMDV-specific response to an “unseen” antigen is of a very weak avidity, then this response would not be detected in the LPBE assay, but it would be detected in a membrane-based assay such as the ELISpot assay.
Sixty-three days after the primary OPA vaccination, a third inactivated FMDV serotype vaccination was given (FMDV A1S). This resulted in a detected ASC response that was specific for the three FMDV serotypes that had been administered (FMDV OPA, A22, and A1S) and also the “unseen” FMDV SAT1 and C1 4 to 6 days after the inactivated FMDV A1S vaccine was given. This was coupled with a significant increase in the LPBE titers specific for all five FMDV serotypes tested.
The final inactivated FMDV serotype to be administered was SAT1, which was given 84 days after the primary vaccination. Antibody-secreting cells specific for all five FMDV serotypes tested were detected 4 days after the vaccine was given. Again, this was coupled with a significantly elevated LPBE titer specific for all five FMDV serotypes tested.
The presence of specific ASCs and significantly elevated LPBE titers specific for serotype C following sequential vaccination with three different inactivated FMDV serotypes and in the absence of vaccination with inactivated FMDV serotype C antigen indicates the development of a cross-reactive FMDV-specific response. This is in keeping with the findings of Cottral and Gailiunas who described the induction of a cross-protective response to FMDV following a sequential FMDV challenge model with three different FMDV serotypes (4). Indeed, in silico modeling of the antibody response to HIV infection also suggests that a sequential vaccination regime is likely to better promote the development of a broadly neutralizing response to the immunizing antigen compared to immunization with the same cocktail of antigens at one time point (9).
The animals from all of the study groups were culled at the end of their respective studies, the prescapular lymph nodes were removed, and the presence of FMDV-specific ASCs was tested by an ELISpot assay. The groups that received the homologous prime and booster vaccination of inactivated FMDV A22 and A1S were culled 67 days after the initial vaccination was administered. There was a small number of FMDV A1S-specific ASCs present in the left prescapular lymph node, which was directly below the vaccination site. The group that received the inactivated FDMV SAT1 were culled at an earlier time point after the initial vaccination (39 dpv). There was a large number of FMDV SAT1-specific ASCs detected in these animals, again in the left prescapular lymph node only.
The animals that went on to receive the sequential immunization regime of different FMDV serotypes were culled 18 days (102 dpv) after the final inactivated FMDV SAT1 vaccine was administered (84 dpv). These animals also had ASCs detected that were specific for all four inactivated vaccine antigens that were administered to the animals (FMDV OPA, A22, A1S, and SAT1). Again, these were present only in the left prescapular lymph nodes.
These data suggest that there is very little migration of antigen-specific plasma cells from the local draining lymph node after vaccination with inactivated FMDV. This is in keeping with the findings of Pega et al. who have previously shown that the FMDV-specific ASC response to live-virus challenge is largely restricted to the local lymphoid tissues draining the infection site, where FMDV undergoes proliferation (10). These data also indicate that the ASCs generated after FMDV vaccination are likely to be short-lived extrafollicular plasma cells which remain at the site of induction (11), as shown by the lack of FMDV-specific ASCs detected in the circulation systems of these animals 3 days prior to removal of the prescapular lymph node. A further indication of the short-lived nature of the ASCs induced following vaccination with inactivated FMDV antigen is the very low number or absence of FMDV-specific ASCs detected in the left PSLN of the prime and boost groups that were culled 39 days after the booster vaccination was given. In contrast, animals in the group vaccinated with inactivated FMDV SAT1 that were culled 18 days after the boost vaccination had a large number of FMDV-specific ASCs present in the left PSLN.
These findings add further evidence to the hypothesis that FMDV is a largely T-independent 2 (TI-2) antigen, which is able to stimulate B cells in the absence of CD4+ T cell help (12). The structure of the FMDV capsid has regular repeating epitopes likely to stimulate B cells via a TI-2 mechanism. The ASCs that are induced via a TI-2 mechanism are short-lived extrafollicular ASCs which remain at the site of induction, the local draining lymphoid tissue (11).
Previous studies have shown that FMDV live-virus challenge and/or vaccination is able to rapidly induce FMDV-specific ASCs in the local lymphoid tissue (10, 13) but unable to induce FMDV-specific memory B cells (3, 13) which are the hallmarks of a TI-2 antigenic response (5) and consistent with findings in humans as well (7). The findings of this study indicate that FMDV-specific ASCs are largely restricted to the local draining lymphoid tissue but are also relatively short-lived in nature, indicating the development of short-lived extrafollicular ASCs. FMDV vaccines must be administered every 6 months in the field to maintain vaccine efficacy against a single serotype.
To conclude, this is the first FMDV vaccination study to demonstrate that a serotype cross-reactive serological response can be generated after sequential vaccination with at least three different inactivated FMDV serotypes. While these results may not represent an optimal or economically viable vaccine strategy, they do clearly illustrate both the potential to develop a heterologous vaccine regime that could improve the breadth of protection and also that conserved epitopes exist between serotypes that could be exploited.
MATERIALS AND METHODS
Calves and vaccination protocols.
Eighteen 6-month-old conventionally reared Holstein-Friesian male calves (Bos taurus) (The Pirbright Institute, Woking, United Kingdom) were split into four groups of four and a control group of two animals. Each calf was immunized with 10 μg of inactivated and highly purified FMDV antigen, with the exception of the nonvaccinated control (NVC) groups. The first cohort (A22 group [n = 4]) received inactivated FMDV A22 Iraq (FMDV A22/Iraq [A22]). The second cohort (A1S group [n = 4]) was immunized with inactivated Asia 1 Shamir (FMDV Asia 1/Shamir [A1S]). The third group (S1Z group [n = 4]) was immunized with inactivated SAT 1 Zim (S1Z) (FMDV SAT1 ZIM 22/89 [SAT1]). The fourth group (OPA group [n = 4]) was given inactivated O Pan Asia (OPA) FMDV (FMDV O TUR/5/2009 [OPA]). The nonvaccinated controls (NVC group [n = 2]) were given an adjuvant-only vaccination. A booster vaccination of the same FMDV vaccine was given to each cohort 21 days after the primary vaccination was administered. All vaccines were formulated in Montanide ISA 201 VG adjuvant (Seppic, Paris, France) and were given intramuscularly above the left prescapular lymph node of each animal.
The OPA group (n = 4) then went on to receive further vaccinations given at 21-day intervals in the following order: A22 Iraq (FMDV A22/Iraq), Asia I Shamir (FMDV Asia1/Shamir), and SAT 1 Zim (FMDV SAT1 ZIM 22/89).
Heparinized peripheral blood and serum samples were taken from all animals at various time points following all immunizations (Fig. 4). On the final day of the study, the animals were culled and both the left and right subscapular lymph node were removed (Table 1). All experiments were approved by The Pirbright Institute's ethical review process and were in accordance with national guidelines on animal use.
Purification of inactivated FMDV vaccine antigens.
Crude PEG precipitate containing inactivated FMDV vaccine antigens for four different serotypes, A22/Iraq, Asia1/Shamir, SAT1 ZIM 22/89, and O TUR/5/2009, were received as frozen pellets from MSD Animal Health (Boxmeer, Netherlands). The particles were purified as described in reference 14. Briefly, the pellets were resuspended in 50 mM HEPES (pH 8.0) containing 200 mM NaCl and 1% (vol/vol) NP-40 and clarified, and the particles were enriched over a 3-ml, 30% sucrose cushion. The particles were further purified by two consecutive 15 to 45% sucrose density gradients. The purity of the particles was verified by SDS-PAGE, negative staining, and transmission electron microscopy.
FMDV-specific ELISpot assay for the detection of ASCs.
Bovine peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples, using the methodology of Grant et al. (5). The FMDV-specific ELISpot assay was performed using freshly isolated PBMCs. The methodology for the FMDV-specific ELISpot assay was adapted from that of Pega et al. (10). Briefly, MultiScreenHA plates (Millipore, Watford, United Kingdom) were coated with 2.2 μg/well of inactivated purified FMDV antigens (FMDV A22/Iraq, FMDV Asia1/Shamir, FMDV SAT1 ZIM 22/89, FMDV O TUR/5/2009, and FMDV C1/Obb/73/Tüb; MSD Animal Health) diluted in 0.1 M carbonate buffer (pH 9.6) for 2 h at 37°C. The plates were then washed five times in phosphate-buffered saline (PBS) and then blocked using 4% dried milk (Marvel milk powder; Premier Foods, St. Albans, United Kingdom) in PBS for 2 h at 37°C. Following blocking, the plates were washed five times in PBS. The plates were then washed and stored at 4°C until required. A total bovine IgG control (clone BG-18; Sigma-Aldrich, Gillingham, United Kingdom) ELISpot assay was also performed by the method of Grant et al. (5).
Freshly isolated PBMCs were suspended at 5 × 106 cells/ml, and 1:4 serial dilutions were performed in ELISpot medium (5) down to 3.125 × 105 cells/ml. One hundred microliters of each cell suspension (in duplicate) was place into each well, and the coated plates were incubated overnight at 37°C in a 5% CO2 incubator. Subsequently, the plates were washed five times to remove the PBMCs using PBS. One hundred microliters of a 1/1,000 dilution of sheep anti-bovine IgG conjugated to horseradish peroxidase (HRP) (AbD Serotec) was then added to each well on the plate for 3 h at room temperature. Following the incubation step, the plates were again washed five times. Detection was performed by the addition of 100 μl of 3-amino-9-ethylcarbonate substrate (AEC) (Merck, Darmstadt, Germany) to each well and subsequent incubation at room temperature for 1 h. The reaction was then stopped by washing the plates with tap water and allowing them to dry overnight at room temperature.
Enumeration of the red colored spots on the dried ELISpot plates was performed using the automated AID ELISpot reader (Autoimmun Diagnostika GmbH, Strasbourg, Germany). Each individual “spot” equates to an ASC. The ELISpot assay results were manually validated for false-positive results and expressed as the number of ASCs r per 106 PBMCs for plasma cells (mean of duplicates). During the ELISpot data analysis, any well with less than 2 spots was considered to be negative (minimum sensitivity, 4 ASCs per 1 × 106 PBMCs). There was very little variance (<20%) shown between the duplicate observations for each animal.
FMDV-specific liquid phase blocking ELISA.
Serum samples were prepared from clotted blood samples taken at multiple time points following vaccination from all animals. The virus liquid phase blocking ELISA (LPBE) was performed by the FMDV World Reference Laboratory (WRLFMD, The Pirbright Institute).
The LPBE assay was performed using the same virus stock or a virus stock similar to the vaccine antigen (see Table 3 for FMDV virus stocks used) according to the methodology described by Hamblin et al. (15). Results for the LPBE were expressed as the group mean titer ± standard deviation (SD).
Statistical analysis of the FMDV-specific response following vaccination.
Statistically significant increases in the number of FMDV-specific ASCs and LPBE titer were calculated by comparing −1 dpv with the time point of interest using a two-way analysis of variance (ANOVA) followed by Dunnett's multiple-comparison test using GraphPad Prism software (GraphPad Prism version 7.00 for Windows; GraphPad Software, La Jolla, CA, USA).
ACKNOWLEDGMENTS
We thank the animal technicians at both The Pirbright Institute and Compton Laboratory, in particular Andy Caines. We also thank the WRLFMD at The Pirbright Institute for performing the FMDV virus neutralization test (VNT) and LPBE immunoassays.
Financial support for this work was provided by a Wellcome Trust Strategic award (grant WT-101122Z13Z).
We declare that we have no financial competing interests. Erwin van den Born is an employee of MSD Animal Health and had no role in the study design or the interpretation of the results.
C.F.J.G. performed immunoassays, interpreted data, and wrote a draft of the manuscript. B.V.C. also performed immunoassays and was involved in the study coordination. A.K. purified the vaccination and assay antigens. E.V.D.B. was involved in the study coordination. D.I.S. was involved in the conception and study design. J.A.H. was involved in the study coordination and revised the manuscript. B.C. was involved in the study conception and design and helped draft the manuscript.
REFERENCES
- 1.Doel TR. 1996. Natural and vaccine-induced immunity to foot and mouth disease: the prospects for improved vaccines. Rev Sci Tech 15:883–911. [DOI] [PubMed] [Google Scholar]
- 2.Brehm KE, Kumar N, Thulke HH, Haas B. 2008. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 26:1681–1687. doi: 10.1016/j.vaccine.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Grant CF, Carr BV, Singanallur NB, Morris J, Gubbins S, Hudelet P, Ilott M, Charreyre C, Vosloo W, Charleston B. 2016. The B cell response to foot-and-mouth disease virus in cattle following vaccination and live-virus challenge. J Gen Virol 97:2201–2209. doi: 10.1099/jgv.0.000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottral GE, Gailiunas P. 1972. Experimental multiple infection of animals with foot-and-mouth disease viruses. Proc Annu Meet U S Anim Health Assoc 75:441–465. [Google Scholar]
- 5.Grant CF, Lefevre EA, Carr BV, Prentice H, Gubbins S, Pollard AJ, Charreyre C, Charleston B. 2012. Assessment of T-dependent and T-independent immune responses in cattle using a B cell ELISPOT assay. Vet Res 43:68. doi: 10.1186/1297-9716-43-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefevre EA, Carr BV, Prentice H, Charleston B. 2009. A quantitative assessment of primary and secondary immune responses in cattle using a B cell ELISPOT assay. Vet Res 40:3. doi: 10.1051/vetres:2008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu LM, Borkowski A, Moxon ER, Pollard AJ. 2006. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood 108:2642–2647. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 8.Bernasconi NL, Traggiai E, Lanzavecchia A. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Mata-Fink J, Kriegsman B, Hanson M, Irvine DJ, Eisen HN, Burton DR, Wittrup KD, Kardar M, Chakraborty AK. 2015. Manipulating the selection forces during affinity maturation to generate cross-reactive HIV antibodies. Cell 160:785–797. doi: 10.1016/j.cell.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pega J, Bucafusco D, Di Giacomo S, Schammas JM, Malacari D, Capozzo AV, Arzt J, Pérez-Beascoechea C, Maradei E, Rodríguez LL, Borca MV, Pérez-Filgueira M. 2013. Early adaptive immune responses in the respiratory tract of foot-and-mouth disease virus-infected cattle. J Virol 87:2489–2495. doi: 10.1128/JVI.02879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHeyzer-Williams MG. 2003. B cells as effectors. Curr Opin Immunol 15:354–361. doi: 10.1016/S0952-7915(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann M, Easten A. 1971. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med 134:103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pega J, Di Giacomo S, Bucafusco D, Schammas JM, Malacari D, Barrionuevo F, Capozzo AV, Rodríguez LL, Borca MV, Pérez-Filgueira M. 2015. Systemic foot-and-mouth disease vaccination in cattle promotes specific antibody-secreting cells at the respiratory tract and triggers local anamnestic responses upon aerosol infection. J Virol 89:9581–9590. doi: 10.1128/JVI.01082-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotecha A, Seago J, Scott K, Burman A, Loureiro S, Ren J, Porta C, Ginn HM, Jackson T, Perez-Martin E, Siebert CA, Paul G, Huiskonen JT, Jones IM, Esnouf RM, Fry EE, Maree FF, Charleston B, Stuart DI. 2015. Structure-based energetics of protein interfaces guides foot-and-mouth disease virus vaccine design. Nat Struct Mol Biol 22:788–794. doi: 10.1038/nsmb.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamblin C, Barnett IT, Crowther JR. 1986. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus. II. Application. J Immunol Methods 93:123–129. doi: 10.1016/0022-1759(86)90442-4. [DOI] [PubMed] [Google Scholar]