ABSTRACT
Dengue viruses (DENVs) are an emerging threat to global public health. The NS2B3 protease complex of DENV has recently been shown to cleave the antiviral protein STING and thereby subvert the innate immune signaling to facilitate virus replication. Whether host cells have a mechanism to counteract this virus-mediated immunosuppression is unclear. We discovered that the K27-linked polyubiquitination of NS3 protein facilitates its recruitment of NS2B, the formation of NS2B3, and consequently the enhanced cleavage of STING. However, an endoplasmic reticulum (ER) protein, SCAP, through binding to NS2B protein, inhibits the ubiquitination of NS3, rendering NS2B3 protease incapable of binding and cleaving STING. Importantly, ectopic expression of SCAP impaired DENV infection, whereas silencing of SCAP potentiated DENV infection. Collectively, this study uncovered a novel function of SCAP of counteracting the inhibitory action of DENV NS2B3 protease on STING signaling, suggesting that modulation of SCAP levels may have therapeutic implications.
IMPORTANCE This study reports the first ubiquitylation target protein in DENV, the NS3 protein, and the unique role of K27-linked polyubiquitylation in NS3's ability to recruit NS2B and formation of the NS2B3 protease complex. Additionally, this study identified novel functions of the ER protein SCAP: one is to compete with NS2B for binding to STING, and the other is to inhibit the ubiquitination of NS3. Both of these functions protect STING from being cleaved by the NS2B3 protease and thus contribute to host antiviral response.
KEYWORDS: dengue virus, NS2B3, STING, SCAP, K27-polyubiquitin, innate immunity
INTRODUCTION
Dengue viruses (DENVs) are single-positive-stranded RNA viruses belonging to the Flavivirus genus of the Flaviviridae family. Serologically, they are classified into four main serotypes (DENV1 to -4) (1). These viruses propagate between arthropod and mammalian species and cause dengue fever or severe dengue fever in humans (2, 3). The DENV genome is approximately 11 kb and has a single open reading frame encoding a single polyprotein that is cotranslationally or posttranslationally processed into three structural proteins (capsid [C], membrane [M], and envelope [E] glycoproteins) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (4). Similarly to most RNA viruses, the dengue virus life cycle and viral protein activities are largely dependent on the endoplasmic reticulum (ER) and the ER-derived membrane web (5).
Innate immunity is the first line of host defense against microbial invasions, including that by dengue virus. Cytoplasmic sensors (RIG-I and MDA5) and the Toll-like receptor (TLR) family members (TLR3/TLR7/TLR8) have been characterized as the most relevant DENV sensors for eliciting host immune responses (6, 7). Stimulator of interferon genes (STING, also known as MITA, MPYS, and ERIS) is an ER-resident antiviral protein that has traditionally been thought to be a hub signaling adaptor for sensing cytosolic DNA viruses (8–12). More recently, it was reported that the NS2B3 protease of DENV could specifically cleave human STING in experimental models of DENV infection, thus linking STING to RNA viruses (13, 14). However, DENV per se could not trigger the activation of the STING signaling. Therefore, it is puzzling why DENV has evolved to use NS2B3 to cleave human STING. It might be possible that other STING-dependent pathways could be important for host anti-DENV activities and that the cleavage of STING protects DENV from these other antiviral factors.
Immune activation, as well as a variety of other cellular processes, is dependent on posttranslational protein modification by ubiquitylation (15), which requires the sequential action of three different enzymes: E1 ubiquitin (Ub)-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases (16, 17). These normal cellular machineries, however, are often hijacked or subverted by microbes for their own use. For examples, the Gag protein of HIV is modified by ubiquitin to facilitate the recruitment of Tsg101 and thus enables virion budding and release (18, 19), and VP40 of Ebola virus is ubiquitylated for efficient virion release (20). Although NS3 and NS5 proteins of Japanese encephalitis virus (JEV), another mosquito-borne flavivirus, could be K48 ubiquitylated and then targeted to the proteasome for degradation (21), whether ubiquitylation has a role in DENV infection is unknown.
Another important factor in cellular metabolic processes is the sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP), which is a 1,276-amino-acid (aa)-long integral membrane protein located on the endoplasmic reticulum (ER) membrane (22, 23). We have recently reported that SCAP could interact with STING and positively modulate the STING-mediated signaling pathway to inhibit a DNA virus (24). To examine whether SCAP can modulate an RNA virus similarly, we examined DENV infection in cell culture models in the current study. We observed that NS3 is modified by the K27-linked polyubiquitin, which facilitates its recruitment by NS2B to form the NS2B3 protease complex that cleaves STING. However, SCAP is capable of reversing the inhibitory action of DENV on the STING signaling. Specifically, through binding to NS2B protein, SCAP competes with NS2B3 protease complex for binding to STING. Additionally, SCAP inhibits the K27-linked ubiquitination of NS3 and thus restricts the cleavage of STING by NS2B3 protease. Collectively, this study uncovered SCAP as a novel anti-DENV host factor, shedding new light on the dynamic interactions between host and DENV in the ER.
RESULTS
STING is critical for restricting dengue virus infection.
Two recent studies reported that DENV NS2B3 protease complex specifically cleaves the antiviral protein STING on the ER to suppress the activation of innate immune responses (13, 14). Whether this will alter DENV replication is yet to be determined. To further explore the function of STING, we measured the replication of the specific viral genes after DENV infection of A549 cells using the reverse transcription-quantitative PCR (qRT-PCR) method. As expected, the RNA levels of nonstructural protein 5 (NS5), structural protein capsid (C), and membrane protein (M) genes were increased when STING was knocked down (Fig. 1A). Consistently, the viral RNA levels were all decreased when murine STING or MAVS was ectopically expressed in Huh7 cells, but not with control PC3 (Fig. 1B). These results indicate that STING acts as an anti-DENV host factor. A number of host factors have been known to increase their levels during virus infection in order to confer protection against viral pathogenesis. Thus, we next examined the STING induction and found that its mRNA level was increased after dengue virus infection (Fig. 1C). In addition, knockdown of STING with small interfering RNA (siRNA) increased the NS3 and NS2B protein levels (Fig. 1D, left panel), whereas ectopic expression of N-terminally hemagglutinin (HA)-tagged STING reduced their levels (Fig. 1D, right panel). Using immunofluorescence staining, we confirmed that knockdown of endogenous STING with siRNA or ectopically expressed STING using HA-STING plasmid rendered cells more permissive or more resistant to DENV infection, respectively (Fig. 1E). To further test whether STING also restricts the production of infectious virus particles, we measured DENV titer in the culture supernatant using a focus formation assay. Consistently, the DENV titer was markedly increased in the STING knockdown cells (Fig. 1F). Collectively, these data confirmed that STING is an important antiviral protein against DENV.
FIG 1.
STING is critical for restricting dengue virus infection. (A and B) A549 cells (A) or Huh7 cells (B) were transfected with indicated siRNA or plasmids and infected with DENV2 (for A549 cells, MOI of 0.5; for Huh7 cells, MOI of 1) for 72 h. The induction of indicated mRNA and viral RNA replication was measured by qRT-PCR with primers targeting indicated genes and normalized against β-actin and controls, using the ΔΔCT method. The antiviral protein MAVS was a positive control, and the mitochondrial protein TOM20 was used as a negative control. Data are presented as means ± standard deviations from three independent experiments. Student's t test was performed, and a P value of >0.05 was considered statistically not different and marked with “ns”; a P value of <0.01 was considered a highly significant difference and marked with double asterisks. (C) Huh7 cells infected with DENV2 (MOI of 1). Time course of STING mRNA level was measured by qRT-PCR. (D) A549 cells were transfected with STING siRNA or NC (left), and Huh7 cells were transfected with N-terminally HA-tagged STING or pc3 plasmid. After 8 h, the cells were infected with DENV2 or mock infected for 72 h, and then cell lysates were immunoblotted with anti-NS3 antibody, anti-NS2B antibody, anti-STING antibody, anti-HA antibody, or anti-β-actin antibody, respectively. Numbers at left of blots are molecular masses in kilodaltons. (E) The indicated siRNAs or plasmids were transfected into Huh7 cells, and the cells were infected with DENV2 for 90 h (MOI of 0.1), subsequently fixed and stained with anti-NS2B antibody and secondary anti-rabbit FITC-conjugated antibody, and then imaged by fluorescence microscopy. Bars, 20 μm. IHC, immunohistochemistry. (F) Huh7 cells were transfected with indicated siRNA, followed by DENV2 infection for 72 h. The viral titers from supernatants were determined by immune focus-forming assay.
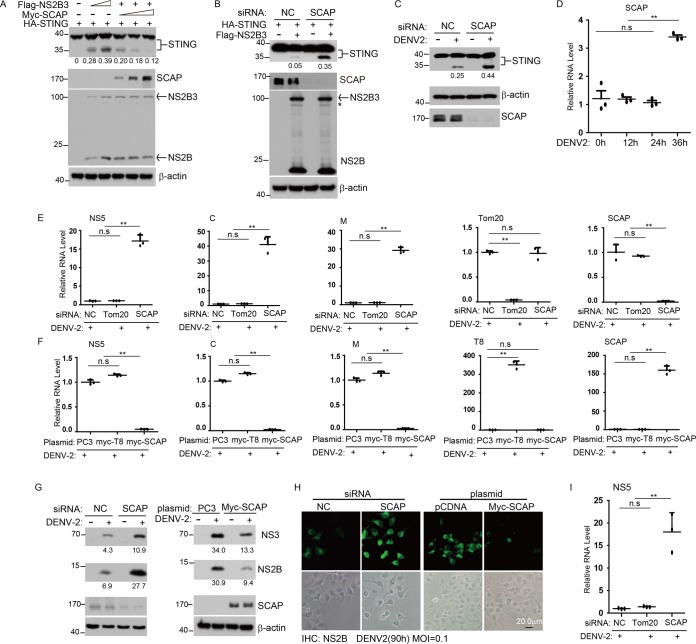
SCAP impairs the cleavage of STING and acts as a new anti-DENV protein.
Given that STING is cleaved by DENV NS2B3 protease complex and SCAP interacts with STING (13, 14, 24), we further tested whether SCAP could influence the cleavage of STING through an interaction with NS2B or NS3. Results showed that the NS2B3-mediated cleavage of STING was markedly impaired in the presence of an increased amount of SCAP in cells with STING and NS2B3 (Fig. 2A). (The N-terminally Flag-tagged NS2B3 protein cleaved itself into NS2B and NS3 protein, so after ectopic expression of this plasmid, two bands, Flag-NS2B3 and Flag-NS2B, were detected in the Flag immunoblotting assay.) In agreement with this, the cleavage of STING was increased when the endogenous SCAP was knocked down by siRNAs (mixture of two different siRNAs) (Fig. 2B). To determine whether the antagonistic action between SCAP and NS2B3 on STING is physiologically relevant, we next measured the cleavage of endogenous STING after DENV infection. Consistently, siRNA knockdown of SCAP markedly increased the cleavage of the endogenous STING after DENV infection (Fig. 2C). These data indicate that SCAP prevents the cleavage of STING by the NS2B3 protein. We further measured the SCAP mRNA induction during DENV infection and found that the mRNA level of SCAP was indeed increased at 36 h after virus infection (Fig. 2D).
FIG 2.
SCAP impairs the cleavage of STING and acts as a new anti-dengue virus protein. (A) N-terminally HA-tagged STING was transfected into HEK 293T cells together with increasing amounts of N-terminally tagged Flag-NS2B3 and Myc-SCAP, followed by Western blotting using anti-HA antibody, anti-Flag antibody, and anti-Myc antibody, respectively. (B) A549 cells were transfected with nonspecific control (NC) or SCAP siRNA for 24 h and then transfected with N-terminally tagged HA-STING and Flag-NS2B3 plasmids, and 24 h later, Western blot analysis was performed as described above. (C) A549 cells were transfected with NC or SCAP siRNA and infected with DENV2 (MOI of 0.5) or mock infected for 72 h, and then cell lysates were immunoblotted with the indicated antibodies. (D) Huh7 cells were infected with DENV2 (MOI of 1), and the time course of SCAP mRNA level was determined by qRT-PCR. (E and F) A549 cells (E) or Huh7 cells (F) were transfected with indicated siRNA or plasmids and infected with DENV2 (for A549 cells, MOI of 0.5; for Huh7 cells, MOI of 1) for 72 h. The viral RNA replication was measured by qRT-PCR using primers targeting the indicated genes. Trc8 (T8; an ER-resident protein) was a negative control. Data are presented as means ± standard deviations from three independent experiments. (G) A549 cells (left) or Huh7 cells (right) were transfected with the indicated siRNA or plasmid and infected with DENV2 or mock infected for 72 h, and then cell lysates were immunoblotted with the indicated antibodies. (H) The indicated siRNAs or plasmids were transfected into Huh7 cells, and the cells were infected with DENV2 for 90 h (MOI of 0.1), subsequently fixed and stained with anti-NS2B rabbit polyclonal antibody and a secondary anti-rabbit FITC-conjugated antibody, and then imaged by fluorescence microscopy. (I) Human monocyte-derived DCs were transfected with indicated siRNA and infected with DENV2 (MOI of 0.5) for 72 h. Then, viral RNA replication was measured by qRT-PCR with primers targeting NS5 and normalized against β-actin. Numbers at left of blots in panels A, B, C, and G are molecular masses in kilodaltons. Statistical significance is indicated as described in the legend to Fig. 1.
To examine whether SCAP has a direct antiviral function, we manipulated its levels in DENV-infected cells. As expected, siRNA knockdown of SCAP resulted in elevated levels of NS5, C, and M viral RNA in A549 cells (Fig. 2E), and ectopically expressed human SCAP in Huh7 cells reduced the NS5, C, and M viral RNA levels (Fig. 2F). Consistently, siRNA knockdown of SCAP increased NS3 and NS2B protein levels in DENV-infected cells and ectopic expression of SCAP reduced the NS3 and NS2B protein levels (Fig. 2G). Using immunofluorescence staining, we confirmed that knockdown of endogenous SCAP or ectopic expression of SCAP rendered cells more permissive or more resistant to DENV infection, respectively (Fig. 2H). To demonstrate the physiological relevance, we used DENV-infected human monocyte-derived dendritic cells (DCs). Results confirmed that siRNA knockdown of SCAP increased the NS5 viral RNA level (Fig. 2I). Collectively, these data demonstrate that SCAP has anti-DENV activity, possibly through directly affecting the function of STING or indirectly acting on other factors associated with STING.
NS3 is modified by the K27-linked polyubiquitin.
It has been established that DENV NS2B3 binds and cleaves STING. Since posttranslational modifications of protein have significant effects on many biological processes, we first examined whether the ubiquitin and ubiquitin-like proteins (SUMO and ISG15) could influence the protease function of NS2B3. By cotransfecting the C-terminally HA-tagged STING and N-terminally Flag-tagged NS2B3 into HEK 293T cells, along with ubiquitin, SUMO3, or ISG15, respectively, we observed that ectopic expression of SUMO3 (Fig. 3A) or ISG15 (Fig. 3B) did not influence the cleavage of STING; however, the NS2B3-mediated cleavage of STING was dramatically enhanced by overexpression of ubiquitin (Fig. 3C). This observation led us to speculate that the DENV protein NS2B3 may be modified by ubiquitin.
FIG 3.
NS3 is modified by K27-linked polyubiquitylation. (A to C) HEK 293T cells were transfected with N-terminally tagged Flag-NS2B3 and HA-STING, along with SUMO3 (A), ISG15 (B), or ubiquitin (C), respectively, followed by Western blotting using the indicated antibodies. (D) N-terminally Flag-tagged NS2B, NS3, or NS2B3 was transfected into HEK 293T cells along with N-terminally HA-tagged Ub. At 32 h after transfection, cell lysates were denatured and immunoprecipitated with an anti-Flag antibody, followed by Western blotting using anti-HA antibody and anti-Flag antibody, respectively. Asterisks indicate a cross-reaction band. (E) A549 cells were mock infected or infected with DENV2 for 72 h; the lysates were subjected to denatured immunoprecipitation with anti-NS2B, anti-NS3, or normal IgG; and the precipitated fractions (IP) and whole-cell lysate (WCL) were then analyzed by immunoblotting with the indicated antibodies. Ab, antibody; MAb, monoclonal antibody. (F) Huh7 cells were mock infected or infected with DENV2 for 72 h, the lysates were subjected to denatured immunoprecipitation with anti-Ub or normal IgG, and the precipitated fractions (IP) and whole-cell lysate (WCL) were then analyzed by immunoblotting with the indicated antibodies. (G) Schematic presentation of WT Ub and its mutants. (H and I) HEK 293T cells were transfected with N-terminal Flag-NS3 along with WT Ub or its one-point mutants. At 32 h after transfection, cell lysates were denatured and immunoprecipitated by anti-Flag antibody and anti-HA antibody, respectively, and then immunoblotted with the indicated antibodies. (J and K) HEK 293T cells were transfected with N-terminally tagged Flag-NS3 along with WT Ub or its one-point rescued mutants. At 32 h after transfection, cell lysates were denatured and immunoprecipitated by anti-Flag antibody and anti-HA antibody, respectively, and precipitated fractions (IP) and whole-cell lysate (WCL) were then immunoblotted with the indicated antibodies. IB, immunoblotting. Numbers at right of blots are molecular masses in kilodaltons.
To further examine whether and how ubiquitin modifies DENV proteins, we cotransfected HA-ubiquitin with Flag-NS2B, -NS3, or -NS2B3, respectively, in HEK 293T cells. The cell lysates were denatured and subjected to immunoprecipitation (IP) with anti-Flag. The precipitates were analyzed by immunoblotting with anti-HA antibody. Notably, NS3 and NS2B3 were markedly polyubiquitylated in the presence of ubiquitin. In contrast, NS2B was not modified by ubiquitin (Fig. 3D), indicating that NS3 is the target of the polyubiquitylation. To examine its physiological relevance, we tested the ubiquitylation of the endogenous NS3 after DENV infection and found that the DENV NS3 was markedly polyubiquitylated (Fig. 3E). To further confirm the ubiquitylation of NS3 in vivo, we performed reverse immunoprecipitation with the ubiquitin (Ub) antibody and demonstrated the presence of elevated-molecular-weight species for NS3 after DENV infection (Fig. 3F).
To dissect the specific type of the polyubiquitin chain linkages on NS3, a panel of ubiquitin mutants were generated that included those with a point mutation at a corresponding lysine (Fig. 3G) and those with all lysines mutated to arginines except for the indicated one (Fig. 3G). As expected, NS3 could be polyubiquitylated in the presence of wild-type (WT) ubiquitin. Notably, NS3 was not polyubiquitylated when using ubiquitin K27R as demonstrated in the immunoprecipitation (IP) performed with anti-Flag (Fig. 3H) or anti-HA (Fig. 3I). In contrast, polyubiquitylation of NS3 was almost abolished when using the ubiquitins K6, K11, K29, K33, K48, and K63. This modification reappeared when K27 ubiquitin was used as demonstrated in the IP performed with anti-Flag (Fig. 3J) or anti-HA (Fig. 3K). These observations firmly established that NS3 is modified exclusively by K27-linked polyubiquitin chain.
K27-linked polyubiquitin chain on NS3 facilitates its recruitment by NS2B to form NS2B3 protease complex.
To probe the functional role of the K27-linked polyubiquitin of NS3, we compared ectopic expressions of WT and various ubiquitin mutants and found that only the K27R mutant did not enhance the cleavage of STING by NS2B3 (Fig. 4A). Consistently, only K27 ubiquitin enhanced the NS2B3 cleavage of STING, whereas K6, K11, K29, K33, K48, and K63 ubiquitin did not (Fig. 4B).These data suggest that the K27-linked polyubiquitin chain on NS3 facilitated the cleavage of STING.
FIG 4.
K27-linked polyubiquitin chain on NS3 facilitates its recruitment by NS2B. (A and B) HEK 293T cells were transfected with N-terminally tagged Flag-STING and Flag-NS2B3 along with HA-Ub and its one-point mutants (A) or its one-point rescued mutants (B), and Western blot analyses were done as described in Materials and Methods. (C) HEK 293T cells were transfected with Flag-NS3 or Flag-NS2B. Cell lysates were incubated with diubiquitin substrates, subjected to a two-step immunoprecipitation, and then immunoblotted with the indicated antibodies. Asterisks in panels A, B, and C indicate cross-reactivity. (D) HA-tagged NS3 and Flag-tagged NS2B were transfected into cells along with increasing amounts of His-tagged Ub. Cell lysates were immunoprecipitated by anti-Flag antibody and immunoblotted with the indicated antibodies. (E) HEK 293T cells were transfected with HA-STING and Flag-NS2B along with a gradient of increasing His-tagged Ub. At 24 h after transfection, the cell lysates were immunoprecipitated by anti-Flag antibody and then immunoblotted with the indicated antibodies. (F and G) Flag-tagged NS3 and Myc-tagged SCAP were introduced to the cells together with WT ubiquitin or K27 ubiquitin, respectively, at 32 h after transfection, and the cell lysates were denatured and immunoprecipitated by anti-Flag antibody and then immunoblotted with the indicated antibodies. Numbers at left of blots in panels A and B and at right of blots in other panels are molecular masses in kilodaltons. IB, immunoblotting.
The above results led us to hypothesize that the K27-linked polyubiquitin chain on NS3 creates an anchor for recruiting NS2B. To test this, chemically synthesized K63-linked diubiquitin chain (K63-Ub2) or K27-linked diubiquitin chain (K27-Ub2) was subjected to coimmunoprecipitation with NS2B or NS3, respectively. As expected, NS2B could directly interact with the K27-linked diubiquitin chains, whereas NS2B failed to interact with the K63-linked diubiquitin (Fig. 4C), indicating that this interaction is both selective and robust. Additionally, when ubiquitin was overexpressed, the interaction between NS3 and NS2B was enhanced (Fig. 4D), and the interaction between NS2B and STING was also strengthened in a dose-dependent manner (Fig. 4E). These data indicate that the K27-linked polyubiquitin chains facilitate NS3 binding to NS2B, thus promoting the formation of NS2B3 protease complex and efficient cleavage of STING.
SCAP impairs the polyubiquitylation of NS3.
Given that SCAP inhibits the cleavage of STING by NS2B3, it may also modulate the polyubiquitylation of NS3. To test whether this is the case, we cotransfected NS3 with HA-tagged ubiquitin in the presence or absence of ectopic expression of SCAP. Results showed that the NS3 protein was significantly polyubiquitylated in the absence of SCAP but almost empty of polyubiquitylation in the presence of SCAP (Fig. 4F). Using K27 ubiquitin, we further demonstrated that SCAP specifically inhibited the K27-linked polyubiquitylation of NS3 (Fig. 4G). Collectively, these data indicate that SCAP impairs the NS2B3-mediated cleavage of STING, via interfering with the polyubiquitylation of NS3.
SCAP hinders NS2B from binding to STING.
Given that NS2B and STING are both localized in the ER, we next examined whether the interaction between the NS2B3 protease complex and STING is mediated through NS2B protein. Using a coimmunoprecipitation assay, we observed that the NS2B3 complex and the NS2B3pro complex (NS2B and NS3 protease domain only) could interact with and cleave STING. Notably, NS2B alone interacted with STING, whereas NS3 alone did not. In addition, by making various truncated forms of NS2B, we mapped the 75- to 104-amino-acid (aa) or the C-terminal 10-aa motifs of NS2B to be critical regions for its interaction with STING (Fig. 5A). Interestingly, the same two motifs of NS2B also mediate its interaction with SCAP (Fig. 5B). These observations suggest that SCAP could compete with NS2B for binding to STING.
FIG 5.
SCAP directly prevents NS2B from binding to STING. (A) N-terminally Flag-tagged NS2B3 or its mutants were individually transfected into HEK 293T cells along with N-terminally HA-tagged STING. The cell lysates were immunoprecipitated with an anti-Flag antibody and then immunoblotted with the indicated antibodies. (B) N-terminally Flag-tagged NS2B3 or its mutants were individually transfected into HEK 293T cells along with Myc-tagged SCAP. The cell lysates were subjected to immunoprecipitation by anti-Flag antibody and immunoblotting with the indicated antibodies. (C) HEK 293T cells were transfected with HA-tagged STING along with increasing amounts of Flag-tagged NS2B and Myc-tagged SCAP. Then, cell lysates were immunoprecipitated by anti-Flag antibody, and precipitated fractions (IP) and whole-cell lysate (input) were immunoblotted with the indicated antibodies. The asterisk indicates a cross-reaction band. (D) A549 cells were transfected with NC or SCAP siRNA before transfection of HA-STING or Flag-NS2B plasmids. Cell lysates were immunoprecipitated by anti-Flag antibody and immunoblotted with anti-HA and anti-Flag antibodies. (E) A549 cells were transfected with NC or STING siRNA and infected with DENV2 or not for 72 h. Cell lysates were immunoprecipitated by anti-STING antibody and immunoblotted with anti-NS3, anti-NS2B, and anti-STING antibodies, respectively. (F) Huh7 cells were transfected with NC or SCAP siRNA for 8 h and transfected with green fluorescent protein (GFP)-STING plasmid. After infection with DENV2 for 48 h (MOI of 1), cells were fixed and stained with indicated antibodies and imaged by confocal microscopy. (G) NC or SCAP siRNA was transfected into Huh7 cells for 8 h, subsequently infected with DENV2 for 48 h (MOI of 0.5), and then stained with indicated antibodies and imaged by confocal microscopy. IB, immunoblotting. Numbers at right of blots are molecular masses in kilodaltons.
To further test this possibility, we performed immunoprecipitation assays and found that the interaction between STING and NS2B was dramatically diminished in a dose-dependent manner when SCAP was expressed (Fig. 5C). Consistently, siRNA knockdown of SCAP markedly promoted the interaction between STING and NS2B (Fig. 5D). To demonstrate its physiological relevance, we performed the same experiment in dengue virus-infected cells and found that siRNA knockdown of SCAP markedly enhanced the interaction between STING and NS2B in DENV-infected cells (Fig. 5E).
To further substantiate this observation, green fluorescent protein (GFP)-STING was introduced into Huh7 cells, which were then infected with DENV. Confocal immunofluorescence assay revealed that much more GFP-STING was colocalized with the endogenous NS2B when SCAP was knocked down by siRNA (Fig. 5F). Using anti-STING antibody in Huh7 cells, we further confirmed the increased colocalization of the endogenous STING and NS2B when SCAP was knocked down (Fig. 5G). Taken together, these data demonstrate that SCAP directly prevents NS2B from binding to STING and thus may interfere with the cleavage of STING by NS2B3 protease complex and potentiate the activation of innate antiviral responses.
SCAP promotes host anti-DENV responses.
Having shown that SCAP can affect dengue virus infection through liberating its immunosuppression on STING, we went on to investigate the antiviral function of SCAP by performing DENV infection in the presence of varied forms of manipulation of SCAP levels. As shown previously, immunofluorescence staining revealed that knockdown of endogenous SCAP enhanced virus production detected in the supernatant (Fig. 6A, left panel), whereas ectopic expression of SCAP diminished the virus production (Fig. 6A, right panel). In flow cytometry analysis, there was a 9-fold increase of DENV-positive cells when SCAP was knocked down but a 7-fold decrease of DENV-positive cells when SCAP was ectopically expressed (Fig. 6B). To test whether SCAP also restricts virus particle production, DENV titer in the culture supernatant was measured by immune focus formation assay. Consistently, DENV titer was markedly increased in the SCAP-knockdown cells, whereas ectopic expression of SCAP decreased virus titer (Fig. 6C, left panel). The relevant virus titers under each condition were quantified. Knockdown of SCAP resulted in a more-than-4-fold increase in virus titer compared with the controls, whereas overexpression of SCAP led to a 6-fold decrease in virus titer compared with the controls (Fig. 6C, right panel). Then, the supernatants were further employed for a second infection assay. The cells were harvested for qRT-PCR and Western blotting assays. As expected, knockdown of SCAP resulted in an increase in NS5, C, and M virus RNA levels (Fig. 6D) and NS3 and NS2B protein levels (Fig. 6E). Furthermore, in a one-step virus growth experiment, it was clearly observed that the virus amplification was augmented when SCAP was knocked down and virus amplification was reduced when SCAP was overexpressed (Fig. 6F). Collectively, these data demonstrated that SCAP has anti-DENV activities, and such functions may be mediated through direct or indirect mechanisms.
FIG 6.
SCAP promotes host anti-dengue virus responses. (A) The indicated siRNAs or plasmids were transfected into Huh7 cells, the cells were infected with DENV2 for 90 h (MOI of 0.1), the supernatant was collected to infect Vero cells for 90 h, and cells were subsequently fixed and stained with rabbit anti-NS2B antibody and secondary anti-rabbit FITC-conjugated antibody and then imaged by fluorescence microscopy. (B) The FITC-positive Vero cells from panel A were detected by flow cytometry as described in Materials and Methods. Positive cells were analyzed and counted with FlowJo software. (C) Huh7 cells were transfected with the indicated siRNA or plasmid, followed by DENV2 infection for 72 h. The viral titers from the supernatants were determined by immune focus-forming assay. (D and E) A549 cells were transfected with indicated siRNA and treated with or without DENV2 for 72 h. Equal volumes of culture supernatants from these treatments were used to infect naive A549 cells and Huh7 cells, and the replication of viral RNA was measured by qRT-PCR using A549 cells (D). The protein level of viral protein NS2B and NS3 was measured by immunoblotting (E). Numbers at right of blots are molecular masses in kilodaltons. (F) A549 cells (top) were transfected with the indicated siRNA, Huh7 cells (bottom) were transfected with the indicated plasmid, and both sets of cells were subsequently infected with dengue virus. Viral RNA levels (NS5) were measured by qRT-PCR at indicated time points to plot virus growth curves.
DISCUSSION
Dengue virus infection is one of the most significant emerging diseases that challenges the global public health system (4, 25) and one for which there are no effective preventative or therapeutic measures. Investigation into the host-virus interactions may lead to new ideas for the development of therapeutics. In this study, we have identified an ER protein, SCAP, as a novel anti-DENV host factor and revealed possible mechanisms of its action. Our overall results are consistent with a model sketched in Fig. 7. Briefly, during dengue virus replication, the NS3 interacts with the ER-resident NS2B to form the NS2B3 protease complex. The NS2B subunit could bind to the ER-resident antiviral protein STING, resulting in the cleavage of STING and subversion of host innate antiviral signaling. The NS3 subunit could be modified by the K27-linked polyubiquitin chains. This modification facilitates the interaction between NS2B and NS3 and improves the efficiency of NS2B3-mediated cleavage of STING. To counteract this, the ER protein SCAP can prevent NS2B from binding to STING, probably via steric hindrance. In addition, SCAP could directly impair the polyubiquitylation of NS3, and consequently the formation of NS2B3, and thus protect STING from its cleavage.
FIG 7.
A model for SCAP's anti-DENV action. In DENV-infected cells, the cytosolic viral protein NS3 interacts with the ER-resident viral protein NS2B to form the NS2B3 protease complex. This complex could bind to the ER-resident antiviral protein STING through its NS2B subunit and cleave STING with its NS3pro protease activities, leading to the attenuation of host innate antiviral signaling, facilitating virus replication. NS3 can be modified by the K27-linked polyubiquitin chain, which facilitates the recruitment of NS3 to NS2B and further promotes the cleavage of STING (as shown in SCAP knockdown [KD] panel). In the wild-type cells (WT), ER-resident protein SCAP can counteract the above processes by directly competing with NS2B for binding to STING, probably via steric hindrance. Additionally, SCAP could also reduce STING cleavage by impairing the polyubiquitylation of NS3. These functions make SCAP a novel anti-dengue virus host protein.
Protein modifications are intimately linked to the protein's function. The N-terminal third of NS3 is the catalytic domain of the protease (NS3pro), and the C terminus of NS3 carries the functions of helicase and nucleoside triphosphatase (NTPase) (26, 27). NS2B is a cofactor of NS3pro that stabilizes NS3 through its N terminus and also completes the substrate binding site of NS3pro with its C-terminal region, especially residues 78 to 87 (28). In agreement with this, our study indicated that the C-terminal 10 amino acids and residues 75 to 104 of NS2B are essential for binding to STING and SCAP. Although structural analysis has demonstrated that interaction and folding within NS2B3 complex are finally stabilized by hydrogen bonds, our study infers that the K27 ubiquitin chains on NS3 may facilitate the initial recruitment of NS2B. The absence of NS2B leads to a 3,300- to 6,600-fold decrease in viral protease activity (29), which is critical for viral protein processing and dengue virus life cycle. Thus, it would be interesting to further clarify whether SCAP also inhibits viral replication independently of protecting STING. The identity of the ubiquitin E3 ligase for NS3 is yet to be determined. Such future investigation will likely provide a better understanding of how SCAP specifically impairs the K27-linked polyubiquitylation of NS3. Although our results indicate that NS3 is modified preferentially by K27 ubiquitin chains, we cannot rule out the possibility that NS3 is modified by linear ubiquitin chains at this moment.
The replication and assembly of DENV are tightly linked to the host ER membrane system. DENV infection is known to induce host ER stress responses which help to complete its life cycle (30). NS4A of DENV can induce the rearrangement of the ER membrane, leading to the formation of vesicle pockets and convoluted membranes that assist virion budding (31). DENV also adapts the autophagy processes to enhance its replication. siRNA knockdown of autophagy-related genes significantly reduced the DENV load in infected cells (32). A recent study of HSP70 networks also elucidated that the dengue virus life cycle is highly dependent on the ER and ER-related membrane-resident chaperones the HSP70 family of proteins and their cofactors, DNAJs (33). Thus, ER may represent a major battlefield for the host and DENV. Recently, York et al. reported that SCAP could modulate STING signaling in response to cholesterol metabolic perturbation (34). Our current study further identified the novel anti-DENV function of SCAP.
STING is critical for the cytosolic DNA-triggered innate immune responses (8), whereas its function in cytosolic RNA-triggered innate immune responses remains controversial. Recently, STING was shown to mediate innate antiviral signaling stimulated by RNA viruses such as Sendai virus (SeV) and vesicular stomatitis virus (VSV) (12, 36). It is intriguing that DENV effectively cleaves STING to terminate this pathway of antiviral signaling (13, 14). Unexpectedly, NS2B3 selectively cleaves only human STING but not mouse STING (14). This is consistent with the observation that DENV could not propagate in mouse embryonic fibroblast (MEF) cells (37). Different human cell lines have different susceptibilities to DENV infection. This may be related to different SCAP gene expression levels, different SCAP protein levels, or different degrees of STING cleavage. Indeed, ectopic expression of mouse STING in human cells makes them more resistant to DENV infection. Taken together, these observations further support the idea that STING is a central antiviral protein. Therefore, it is essential to secure its integrity by preventing its cleavage by microbial protease.
Some microbial proteins could be modified by ubiquitin or ubiquitin-like proteins such as ISG15 and SUMO. In flavivirus, the role of the ubiquitin system is complex. The ubiquitin ligase CBLL1 and the ubiquitin/proteasome pathway are thought to be essential for West Nile virus replication (38, 39), whereas ubiquitylation of JEV NS3 and NS5 results in viral protein degradation (21). Previously, it was observed that inhibitors of the cellular ubiquitylation could dramatically decrease DENV replication (40). However, the target(s) of ubiquitylation and the underlying molecular mechanisms of why inhibition of ubiquitylation affects DENV replication were largely unknown. To our knowledge, the current study identified the first DENV ubiquitylation target protein, NS3 protein, and uncovered the mechanism of how the K27-linked polyubiquitylation of NS3 augments DENV replication. Because the protease activity of NS3 is indispensable for processing the DENV polyprotein at the junction of NS2A/NS2B, NS2B/NS3, NS3/NS4A, and NS4B/NS5 (41, 42), targeting this activity of NS3 could be a novel route for antiviral development.
MATERIALS AND METHODS
Cells and viruses.
All cell lines used except for C6/36 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). HEK 293T, Huh7, A549, and Vero cell lines were obtained from the American Type Culture Collection (ATCC). The cells were maintained in a humidified 5% CO2 incubator at 37°C. Human monocyte-derived DCs were prepared as described previously (43, 44).
Dengue virus serotype 2 (strain 16681) (DENV2) was a generous gift of Claire Huang (CDC, Fort Collins, CO, USA). High-titer DENV stocks were obtained by passage in C6/36 mosquito cells that were cultured at 28°C in minimum essential medium (MEM; Gibco, Grand Island, NY, USA) supplemented with nonessential amino acids and 10% FBS in a 5% CO2 incubator. The cells were preseeded in cell culture flasks and infected by DENV (multiplicity of infection [MOI] of 0.1) when they reached 90% confluence. After incubation for 4 to 7 days, the cell culture supernatant was collected for viral titration and preparation of viral stocks.
The viral infection experiment was performed when cells reached 80% confluence. The culture medium was replaced by serum-free DMEM, and then DENV2 was added to the cultured cells at an MOI of 0.1 to 1, according to specific experimental requirements. After 2 h, the medium was removed and the cells were fed with DMEM containing 2% FBS and then cultured until they were ready for harvesting.
Virus titration.
Virus titer was determined by immunofocus assay on Vero cells. Briefly, the virus was 10-fold serially diluted in 100 μl DMEM and then added to Vero cells plated in a 48-well cell culture plate for 1 h at 37°C. Virus solutions were then replaced with 600 μl overlay medium containing 50% (vol/vol) DMEM, 1.5% (vol/vol) FBS, 0.45% (wt/vol) NaCl, and 1.5% (wt/vol) carboxylmethyl cellulose (CMC). Infected cells were cultured at 37°C for another 96 h. The CMC overlay was gently removed, and the cell layer was washed with phosphate-buffered saline (PBS) and then fixed with 4% paraformaldehyde. The fixed cells were stained with dengue virus-specific primary antibody D1-11 (sc-65659; Santa Cruz Biotechnology) for 3 h at room temperature, washed three times with PBS, and then stained with a secondary biotinylated goat anti-mouse antibody for 3 h. After the cells were washed three times with PBS, streptavidin-alkaline phosphatase was added for another 3 h. After three washes with PBS, foci of infected cells were visualized with the addition of BCIP (5-bromo-4-chloro-3-indolylphosphate)/NBT (nitroblue tetrazolium) substrates. Virus titer was calculated as focus-forming units (FFU) of virus per milliliter according to the dilutions of input virus and the focus number in each well.
Antibodies and reagents.
The K27-linked and K63-linked diubiquitin was purchased from Boston Biochem (Cambridge, MA), and 4′,6-diamidino-2-phenylindole (DAPI) was obtained from Life Technologies (Thermo Fisher Scientific, Waltham, MA). The following antibodies were used for Western blotting or immunoprecipitation: anti-β-actin (A5316; Sigma), normal mouse IgG (sc-2025; Santa Cruz Biotechnology, Dallas, TX), normal rabbit IgG (sc-2027; Santa Cruz Biotechnology), antihemagglutinin (anti-HA) (sc-7392; Santa Cruz Biotechnology), anti-Flag (F1804; Sigma-Aldrich), anti-Myc (sc-40; Santa Cruz Biotechnology), anti-His (H1029; Sigma), antiubiquitin (sc-8017; Santa Cruz Biotechnology), anti-SCAP (sc-9675; Santa Cruz Biotechnology), anti-STING (generated by this laboratory and also purchased from Cell Signaling, Danvers, MA [catalog no. 3337S]), anti-NS2B of dengue virus (GTX124246; Gene Tex, Taiwan), anti-NS3 of dengue virus (GTX629477; Gene Tex, Taiwan), and mouse anti-dengue virus antibody D1-11 (sc-65659; Santa Cruz Biotechnology).
Plasmids, siRNA oligonucleotides, and cell transfection.
The NS2B3 of DENV2 protein-encoding plasmid was kindly provided by John Hiscott and Rongtuan Lin (McGill University, Canada). NS3 and NS2B and its truncation were generated in this laboratory. Human STING and SCAP cDNAs were cloned from a human thymus plasmid cDNA library (Clontech) using standard PCR techniques and then subcloned into the indicated vectors. The siRNA oligonucleotides were synthesized by Gene Pharma (Shanghai, China): STING 1231, 5′-GCAUCAAGGAUCGGGUUUTT-3′; SCAP 1, 5′-CCUACCUUGUGGUGGUUAUTT-3′; SCAP 2, 5′-GCUUAAUGGUUCCCUUGAUTT-3′; TOM20, 5′-CCUUUUCAUUGGGUACUGUTT-3′; negative-control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were transfected with siRNA oligonucleotides using Lipofectamine 2000 and then incubated for 48 h before further analysis. The plasmids were introduced into cells using Lipofectamine 2000. The transfected cells were cultured for 24 h before further analysis.
Real-time PCR.
Total cellular RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription of purified RNA was performed using oligo(dT) primers and random hexamers together. The quantification of gene transcripts was performed by real-time PCR using SYBR green PCR mix (Applied Biosystems). All values were normalized to the level of β-actin mRNA, and the fold change was presented as a level relative to the corresponding control, using the threshold cycle (ΔΔCT) method. The primers used were as follows: β-actin, sense (AAAGACCTGTACGCCAACAC) and antisense (GTCATACTCCTGCTTGCTGAT); capsid of DENV2, sense (CAATATGCTGAAACGCGAGA) and antisense (TGCTGTTGGTGGGATTGTTA); membrane protein of DENV2, sense (AAAGATCAGTGGCACTCGTTC) and antisense (TGTCATTGAAGGAGTGACAGC); NS5 protein of DENV2, sense (ACAAGTCGAACAACCTGGTCCAT) and antisense (GCCGCACCATTGGTCTTCTC); STING, sense (ATATCTGCGGCTGATCCTGC) and antisense (GGTCTGCTGGGGCAGTTTAT); SCAP, sense (GTATTTCGTTCACCTTTGTCC) and antisense (ATAACAAGTCTTTGAGTGTGGC).
Flow cytometry.
Huh7 cells were transfected with specific plasmids or siRNA and then infected with DENV (MOI of 1). Seventy-two hours later, the infected cells were harvested, washed once with PBS, and fixed in 3.7% formaldehyde in PBS for 15 min. After permeabilization with Triton X-100 (0.25%) in PBS for 15 min, cells were blocked for 1 h with PBS containing 5% bovine serum albumin (BSA) and then stained with anti-NS2B antibody at 4°C for 4 h. After three washes with PBS, cells were incubated with a secondary fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody for 1 h. After being washed once with PBS, the cells were finally resuspended in 200 μl PBS and analyzed using a FACSCalibur (BD Biosciences) flow cytometer in combination with FlowJo software.
Western blotting and immunoprecipitation.
Cell pellets were collected and resuspended in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.25% sodium deoxycholate, 1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], Roche complete protease inhibitor set) for immunoprecipitation or in RIPA buffer plus 0.1% SDS for Western blot analysis. The resuspended cell pellet was vortexed for 20 s and then incubated on ice for 20 min, followed by centrifugation at 20,000 × g for 20 min. Supernatants were collected for subsequent immunoprecipitation or Western blot analysis.
For immunoprecipitation, cell lysates were precleared with Protein A/G Plus agarose (Santa Cruz Biotechnology) at 4°C for 2 h, and then specific antibody or control IgG was added and incubated overnight. Next, cell lysates were incubated for an additional 2 h after the Protein A/G Plus agarose beads were added. The beads were washed with Tris-buffered saline (TBS) buffer containing 0.5% NP-40, the samples were boiled in 2× SDS loading buffer, and the supernatants were used for Western blot analysis.
For denaturing immunoprecipitation, cells were lysed in 1% SDS buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% SDS, 10 mM dithiothreitol [DTT]) and boiled for 30 min. The lysates were centrifuged and diluted 1:10 with lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100). The diluted lysates were immunoprecipitated with the specific antibodies for 4 h or overnight at 4°C. The protein A/G agarose beads were added for 2 h. After extensive washing, the immunoprecipitates were collected and subjected to immunoblot analysis.
Quantification of the Western blot bands with Image J.
Using the Image J software, we quantified the relative intensity of Western blot bands. For the STING cleavage, the value was expressed as a ratio between the cleaved and full-length STING.
Confocal microscopy imaging.
Cells were plated on coverslips in 12-well plates and transfected with the indicated plasmids or siRNA. Eight hours later, cells were infected with DENV (MOI of 1) for 48 h. Coverslips seeded with cells were washed once with PBS and fixed in 3.7% formaldehyde in PBS for 15 min. After permeabilization with Triton X-100 (0.25%) in PBS for 15 min, cells were blocked with PBS containing 5% BSA for 1 h and then incubated with selected primary antibodies for 2 h. After three washes with PBS, cells were incubated with a secondary antibody for 1 h and then stained with DAPI for 5 min. The coverslips were washed extensively with PBS and then fixed on slides. Images were captured using a Leica laser scanning confocal microscope (Leica TCS SP2 acousto-optical beam splitter [AOBS]).
Statistics.
All experiments were performed independently for at least three times. Either one representative result or the summary of the three experiments was presented. Student's t test was used for comparison between independent treatments. For all tests, a P value of <0.05 was considered statistically significant, and a P value of <0.01 was considered highly significant.
ACKNOWLEDGMENTS
We thank Claire Huang (CDC) for providing dengue virus 2 (strain 16681). We thank John Hiscott and Rongtuan Lin (McGill University, Canada) for providing dengue virus cDNA. We thank Jianhua Wang (Institut Pasteur of Shanghai, China) for providing human monocyte-derived DCs.
This work was supported by grants from the National Natural Science Foundation of China (3140010633 and 81161120542) and the Ministry of Science and Technology of China (2016YFA0501800, 2016YFD0500300, and 2016 YFC1200200).
We declare no competing financial interests.
H.L., L.Z., and J.S. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. W.C., S.L., and Q.W. provided technical and material support and critically revised the manuscript. H.Y. and Z.X. provided intellectual input and critically revised the manuscript. X.J. and C.W. conceived, designed, and supervised the study and drafted and critically revised the manuscript.
REFERENCES
- 1.Guzman MG, Harris E. 2015. Dengue. Lancet 385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 2.Henchal EA, Putnak JR. 1990. The dengue viruses. Clin Microbiol Rev 3:376–396. doi: 10.1128/CMR.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mairuhu AT, Wagenaar J, Brandjes DP, van Gorp EC. 2004. Dengue: an arthropod-borne disease of global importance. Eur J Clin Microbiol Infect Dis 23:425–433. doi: 10.1007/s10096-004-1145-1. [DOI] [PubMed] [Google Scholar]
- 4.Guzman MG, Halstead SB, Artsob H, Buchy P, Jeremy F, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Cameron S, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8(12 Suppl):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. 2011. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis 5:e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai YT, Chang SY, Lee CN, Kao CL. 2009. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell Microbiol 11:604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–U674. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. 2008. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol 28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Li C, Xue P, Zhong B, Mao AP, Ran Y, Chen H, Wang YY, Yang FQ, Shu HB. 2009. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci U S A 106:7945–7950. doi: 10.1073/pnas.0900818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun WX, Li Y, Chen L, Chen HH, You FP, Zhou X, Zhou Y, Zhai ZH, Chen DY, Jiang ZF. 2009. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A 106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao FC, Lei CQ, He X, Zhang L, Tien P, Shu HB. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, Mulder LCF, Barber GN, Fernandez-Sesma A. 2012. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog 8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CY, Chang TH, Liang JJ, Chiang RL, Lee YL, Liao CL, Lin YL. 2012. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog 8:e1002780. doi: 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang XM, Chen ZJJ. 2011. The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol 12:35–48. doi: 10.1038/nri3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickart CM. 2001. Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 17.Kerscher O, Felberbaum R, Hochstrasser M. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 18.Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, Myszka DG, Sundquist WI. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55–65. doi: 10.1016/S0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 19.Demirov DG, Ono A, Orenstein JM, Freed EO. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci U S A 99:955–960. doi: 10.1073/pnas.032511899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuda J, Nakao M, Kawaoka Y, Shida H. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J Virol 77:9987–9992. doi: 10.1128/JVI.77.18.9987-9992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye J, Chen Z, Zhang B, Miao H, Zohaib A, Xu QP, Chen HC, Cao SB. 2013. Heat shock protein 70 is associated with replicase complex of Japanese encephalitis virus and positively regulates viral genome replication. PLoS One 8:e75188. doi: 10.1371/journal.pone.0075188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson RB. 2003. The SREBP pathway. Insights from Insigs and insects. Nat Rev Mol Cell Biol 4:631–640. [DOI] [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Li S, Yu H, Liu X, Huang L, Wang Q, Liu H, Cui Y, Tang Y, Zhang P, Wang C. 2016. ER adaptor SCAP translocates and recruits IRF3 to perinuclear microsome induced by cytosolic microbial DNAs. PLoS Pathog 12:e1005462. doi: 10.1371/journal.ppat.1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers TJ, Weir RC, Grakoui A, McCourt DW, Bazan JF, Fletterick RJ, Rice CM. 1990. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc Natl Acad Sci U S A 87:8898–8902. doi: 10.1073/pnas.87.22.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu T, Sampath A, Chao A, Wen DY, Nanao M, Chene P, Vasudevan SG, Lescar J. 2005. Structure of the dengue virus helicase/nucleoside triphosphatase catalytic domain at a resolution of 2.4 A. J Virol 79:10278–10288. doi: 10.1128/JVI.79.16.10278-10288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erbel P, Schiering N, D'Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U. 2006. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol 13:372–373. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- 29.Yusof R, Clum S, Wetzel M, Murthy HMK, Padmanabhan R. 2000. Purified NS2B/NS3 serine protease of dengue virus type 2 exhibits cofactor NS2B dependence for cleavage of substrates with dibasic amino acids in vitro. J Biol Chem 275:9963–9969. doi: 10.1074/jbc.275.14.9963. [DOI] [PubMed] [Google Scholar]
- 30.Klomporn P, Panyasrivanit M, Wikan N, Smith DR. 2011. Dengue infection of monocytic cells activates ER stress pathways, but apoptosis is induced through both extrinsic and intrinsic pathways. Virology 409:189–197. doi: 10.1016/j.virol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Tang WC, Lin RJ, Liao CL, Lin YL. 2014. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol 88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateo R, Nagamine CM, Spagnolo J, Mendez E, Rahe M, Gale M, Yuan JY, Kirkegaard K. 2013. Inhibition of cellular autophagy deranges dengue virion maturation. J Virol 87:1312–1321. doi: 10.1128/JVI.02177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taguwa S, Maringer K, Li X, Bernal-Rubio D, Rauch JN, Gestwicki JE, Andino R, Fernandez-Sesma A, Frydman J. 2015. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in flavivirus infection. Cell 163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.York AG, Williams KJ, Argus JP, Zhou QD, Brar G, Vergnes L, Gray EE, Zhen A, Wu NC, Yamada DH, Cunningham CR, Tarling EJ, Wilks MQ, Casero D, Gray DH, Yu AK, Wang ES, Brooks DG, Sun R, Kitchen SG, Wu TT, Reue K, Stetson DB, Bensinger SJ. 2015. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163:1716–1729. doi: 10.1016/j.cell.2015.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reference deleted.
- 36.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–U740. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zompi S, Harris E. 2012. Animal models of dengue virus infection. Viruses 4:62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Garcia MD, Meertens L, Bonazzi M, Cossart P, Arenzana-Seisdedos F, Amara A. 2011. Appraising the roles of CBLL1 and the ubiquitin/proteasome system for flavivirus entry and replication. J Virol 85:2980–2989. doi: 10.1128/JVI.02483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, Montgomery RR, Lev S, Mason PW, Koski RA, Elledge SJ, Xavier RJ, Agaisse H, Fikrig E. 2008. RNA interference screen for human genes associated with West Nile virus infection. Nature 455:242–U267. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. 2010. The ubiquitin-proteasome pathway is important for dengue virus infection in primary human endothelial cells. J Proteome Res 9:4960–4971. doi: 10.1021/pr100219y. [DOI] [PubMed] [Google Scholar]
- 41.Preugschat F, Yao CW, Strauss JH. 1990. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J Virol 64:4364–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Mohan PM, Padmanabhan R. 1992. Processing and localization of dengue virus type 2 polyprotein precursor NS3-NS4A-NS4B-NS5. J Virol 66:7549–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. 2015. Human blood-circulating basophils capture HIV-1 and mediate viral trans-infection of CD4+ T cells. J Virol 89:8050–8062. doi: 10.1128/JVI.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang AP, Jiang JF, Wei JF, Guo MG, Qin Y, Guo QQ, Ma L, Liu BC, Wang X, Veazey RS, Ding YB, Wang JH. 2015. Human mucosal mast cells capture HIV-1 and mediate viral trans-infection of CD4+ T cells. J Virol 90:2928–2937. doi: 10.1128/JVI.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]