ABSTRACT
During the lytic phase of Epstein-Barr virus (EBV), binding of the transactivator Zta to the origin of lytic replication (oriLyt) and the BHLF1 transcript, forming a stable RNA-DNA hybrid, is required to initiate viral DNA replication. EBV-encoded viral DNA replication proteins form complexes to amplify viral DNA. BMRF1, the viral DNA polymerase accessory factor, is essential for lytic DNA replication and also known as a transcriptional regulator of the expression of BHLF1 and BALF2 (single-stranded DNA [ssDNA]-binding protein). In order to determine systematically how BMRF1 regulates viral transcription, a BMRF1 knockout bacmid was generated to analyze viral gene expression using a viral DNA microarray. We found that a subset of Rta-responsive late genes, including BcLF1, BLLF1, BLLF2, and BDLF3, were downregulated in cells harboring a BMRF1 knockout EBV bacmid (p2089ΔBMRF1). In reporter assays, BMRF1 appears to transactivate a subset of viral late promoters through distinct pathways. BMRF1 activates the BDLF3 promoter in an SP1-dependent manner. Notably, BMRF1 associates with the transcriptional regulator BRG1 in EBV-reactivated cells. BMRF1-mediated transactivation activities on the BcLF1 and BLLF1 promoters were attenuated by knockdown of BRG1. In BRG1-depleted EBV-reactivated cells, BcLF1 and BLLF1 transcripts were reduced in number, resulting in reduced virion secretion. BMRF1 and BRG1 bound to the adjacent upstream regions of the BcLF1 and BLLF1 promoters, and depletion of BRG1 attenuated the recruitment of BMRF1 onto both promoters, suggesting that BRG1 is involved in BMRF1-mediated regulation of these two genes. Overall, we reveal a novel pathway by which BMRF1 can regulate viral promoters through interaction with BRG1.
IMPORTANCE The cascade of viral gene expression during Epstein-Barr virus (EBV) replication is exquisitely regulated by the coordination of the viral DNA replication machinery and cellular factors. Upon lytic replication, the EBV immediate early proteins Zta and Rta turn on the expression of early proteins that assemble into viral DNA replication complexes. The DNA polymerase accessory factor, BMRF1, also is known to transactivate early gene expression through its interaction with SP1 or Zta on specific promoters. Through a global analysis, we demonstrate that BMRF1 also turns on a subset of Rta-regulated, late structural gene promoters. Searching for BMRF1-interacting cellular partners revealed that the SWI/SNF chromatin modifier BRG1 contributes to BMRF1-mediated transactivation of a subset of late promoters through protein-protein interaction and viral chromatin binding. Our findings indicate that BMRF1 regulates the expression of more viral genes than thought previously through distinct viral DNA replication-independent mechanisms.
KEYWORDS: Epstein-Barr virus, DNA polymerase accessory factor, BMRF1, chromatin regulator, BRG1, transcriptional regulation
INTRODUCTION
Epstein-Barr virus (EBV) is a gammaherpesvirus that may cause infectious mononucleosis (IM) and is highly associated with several malignancies, such as nasopharyngeal carcinoma (NPC), Hodgkin's lymphoma, and Burkitt's lymphoma (1). The life cycle of EBV consists of latent and lytic stages. During latent infection, EBV utilizes host replication components to maintain its genome as intranuclear episomes, mainly through virus-encoded nuclear antigen 1 (EBNA1)-mediated functions (2). When EBV switches from latency to the lytic phase, virus-encoded replication components amplify the viral genome for virion production (3). The lytic switch is mainly controlled by two key immediate early proteins, Zta and Rta, which coordinately bind to the origin of lytic replication (oriLyt) and recruit essential lytic replication proteins to form a core replication complex (4, 5). The viral DNA replication machinery comprises the DNA polymerase (BALF5), single-stranded DNA (ssDNA) binding protein (BALF2), DNA polymerase processivity factor (BMRF1), helicase (BBLF4), primase (BSLF1), primase accessory proteins (BBLF2/BBLF3), and uracil DNA glycosylase (BKRF3) and replicates viral DNA in a manner similar to that of the mammalian DNA replication system (4–7). In addition, the oriLyt transcript (BHLF1 RNA), which anneals to its DNA template during the early steps of lytic reactivation, is crucial for initial DNA strand separation and loading of the core replication complex (8), indicating that the coupling of the transcription and replication machineries is required for EBV lytic replication.
BMRF1 has dual functions in viral DNA replication and transcription (9–13). BMRF1 functions as a polymerase accessory factor that associates with the viral DNA polymerase BALF5 to stabilize its DNA binding (14, 15). In addition, BMRF1 also serves as a transcription activator to enhance the activity of the viral oriLyt BHLF1 promoter through a GC-rich SP1/ZBP89 binding element (10, 11), indicating that BMRF1 may promote the initiation of viral DNA replication via an increase of BHLF1 transcripts. The N-terminal 300 amino acids (aa) of BMRF1 contains two domains that are required for DNA binding, and the C-terminal amino acids 379 to 388 of BMRF1 have been characterized as a transactivation (TA) domain (16–18). Besides its transactivator activity, BMRF1 also interacts with Zta to bind to the promoter of BALF2 and enhances the transactivation activity of Zta on the BALF2 promoter (13). Given the transcriptional regulatory function of BMRF1, it remains to be determined whether BMRF1 may regulate the expression of other viral genes on viral genomes.
During herpesvirus infection, regulation of viral chromatin structure plays a major role in viral genome propagation (19). In the case of another gammaherpesvirus, several histone deacetylases (HDACs), including HDAC-1, -5, and -7, are recruited to the Kaposi's sarcoma-associated herpesvirus (KSHV) ORF50 (Rta) promoter in KSHV latently infected cells (19). Upon treatment with a lytic inducer, sodium butyrate (SB), the rapid association of the chromatin remodeler INI1/SNF5 (BAF47/Smarcb1) onto the ORF50 promoter initiates the transcription of KHSV Rta (20). In addition, CBP, BRG1, and thyroid hormone receptor-associated protein (TRAP)/mediator complexes interact with KHSV Rta to facilitate KSHV lytic reactivation (21). The recruitment of BRG1 to the KHSV Rta and ORF57 promoters is through direct interaction with Rta (21). Accordingly, we wondered whether chromatin modifiers may also participate in the BMRF1-mediated regulatory function of gene expression during EBV reactivation.
To delineate the molecular mechanism of BMRF1-mediated gene expression, we generated a BMRF1 knockout EBV bacmid (p2089ΔBMRF1) and analyzed EBV genome-wide expression with an EBV DNA microarray. In addition to previously identified BMRF1-responsive genes, we found that a number of Rta-regulated late genes were downregulated in cells harboring p2089ΔBMRF1 and that the expression of these genes was rescued by BMRF1 complementation. In luciferase (Luc) reporter assays, BMRF1 expression alone turned on the promoter activities of the BcLF1 (major capsid protein), BDLF3 (gp150), BLLF1 (gp350/220), and BLLF2 (function unknown) promoters. Knockdown of SP1 reduced BMRF1-mediated transactivation of the BDLF3 and BHLF1 promoters. Immunoprecipitation-mass spectrometry and coimmunoprecipitation analyses demonstrated that BMRF1 interacted with the SWI/SNF chromatin modifier subunit BRG1. In reactivated EBV-positive (EBV+) epithelial NA cells, knockdown of BRG1 attenuated the protein levels of BcLF1 and BLLF1, and this was accompanied by reduced secretion of virus particles. Thus, we demonstrate that, in addition to cooperation with Zta and SP1, BMRF1 interacts with BRG1 to regulate viral gene expression.
RESULTS
Construction of p2089ΔBMRF1 and selection of inducible cells.
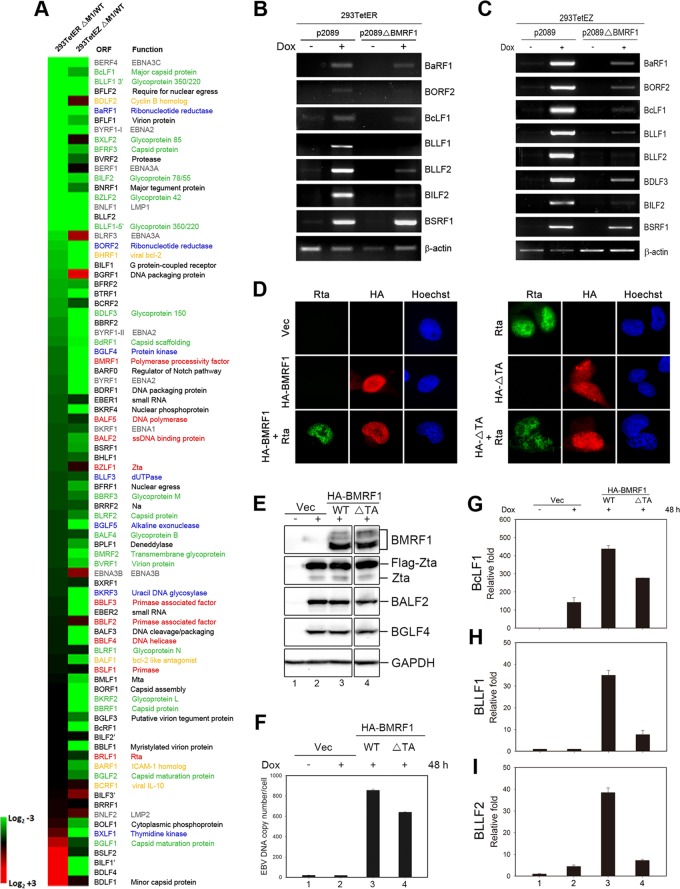
To investigate BMRF1-regulated viral gene expression in the context of a whole-virus system, we constructed p2089ΔBMRF1 by PCR targeting (Fig. 1A) (22, 23). The size of the BamHI M fragment of p2089ΔBMRF1 was increased from 7,980 bp to 8,471 bp (Fig. 1B). EBV p2089 or p2089ΔBMRF1 bacmid was transfected into 293TetEZ or 293TetER cells, the cell lines containing doxycycline (Dox)-inducible expression of Zta or Rta, respectively (24). After transfection, cells were selected with hygromycin (100 μg/ml) to establish the 293TetEZ/p2089 (designated 293TetEZ/WT), 293TetEZ/p2089ΔBMRF1 (293TetEZΔBMRF1), 293TetER/p2089 (293TetER/WT), and 293TetER/p2089ΔBMRF1 (293TetERΔBMRF1) stable cell lines. The lytic cycle of EBV was induced by doxycycline (100 ng/ml) treatment for 48 h to confirm the expression of lytic proteins in 293TetEZ/WT, 293TetEZΔBMRF1, 293TetER/WT, and 293TetERΔBMRF1 cells, including Zta, Rta, BALF2, and BGLF4. The expression of BMRF1 was not detected in 293TetEZΔBMRF1 and 293TetERΔBMRF1 cells (Fig. 1C and D). Of note, the expression level of BALF2 was slightly reduced in BMRF1-deleted bacmid-containing cells, confirming that BALF2 expression is regulated by BMRF1 (13).
FIG 1.
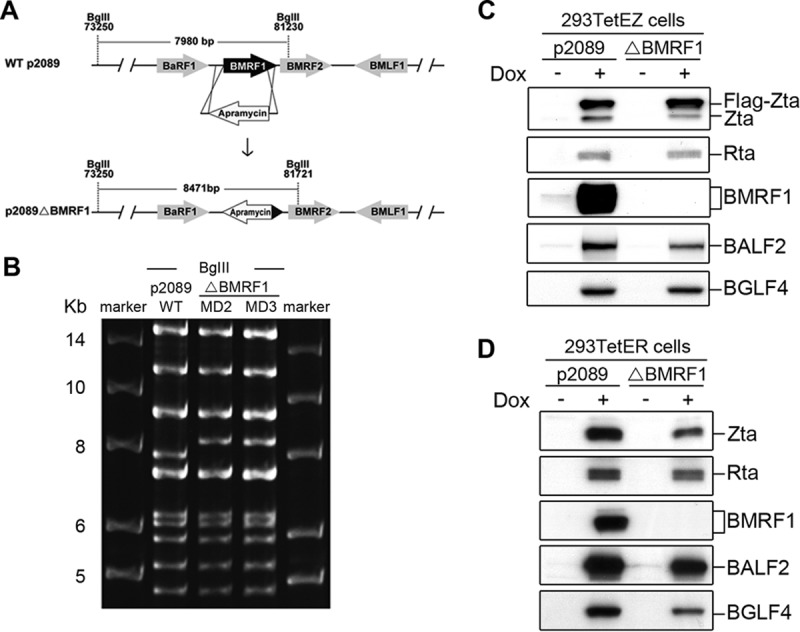
Construction of the BMRF1 knockout EBV bacmid and establishment of doxycycline-inducible bacmid 293TetEZ or 293TetER cell lines harboring WT or mutant bacmids. (A) To generate the Maxi-EBVΔBMRF1 bacmid, the BMRF1 open reading frame of Maxi-EBV p2089 was replaced with an apramycin-resistant gene by PCR targeting. After homologous recombination, the size of the BamHI M fragment was increased from 7,980 bp to 8,471 bp. (B) Maxi-EBV p2089 wild-type and p2089ΔBMRF1 bacmid DNAs were digested by BglII, and the genome patterns of the wild type and BMRF1 mutant were analyzed by pulsed-field gel electrophoresis. (C and D) The wild-type p2089 or BMRF1 knockout bacmid was transfected into 293TetEZ or 293TetER cells and selected with hygromycin (50 μg/ml) for 1 month. Selected bacmid cell lines with similar copy numbers of viral genomes were treated with doxycycline (100 ng/ml) to induce lytic cycle progression. Cell lysates from p2089- and p2089ΔBMRF1-containing cells were harvested to detect lytic protein expression by Western blotting with the antibodies indicated.
Knockout of BMRF1 reduced subsets of viral latent and lytic gene expression.
To explore BMRF1-regulated viral gene expression, cells harboring the BMRF1 knockout bacmid were induced into lytic cycle gene expression and analyzed with our homemade EBV microarray (25). At 48 h postinduction, the total RNA harvested from EBV wild-type ([WT] p2089) or BMRF1 knockout (p2089ΔBMRF1) cell lines was reverse transcribed into biotin-labeled cDNA for hybridization onto the EBV DNA microarrays. The fold change in lytic activation of viral genes was normalized to the level in the noninduced control and is shown as a heat map representing the log2-fold change in the expression level in the knockout virus-containing cell line versus a paired wild-type control (Fig. 2A). In the left column the map displays the fold change in gene expression in Rta-inducible cells (293TetERΔBMRF1 versus 293TetER/WT), from downregulated to upregulated genes as indicated by the color legend on the figure; for each gene, the fold change in expression in Zta-inducible cells (293TetEZΔBMRF1 versus 293TetEZ/WT) is shown next to the respective gene. In the list of the 28 most affected genes (>3-fold downregulation in ΔBMRF1 cells), some latent genes, including EBNA3C, EBNA2, EBNA3A, and LMP1, were significantly downregulated in 293TetERΔBMRF1 and 293TetEZΔBMRF1 cells (Table 1). EBV latent gene expression during viral replication is thought to delay cell death and be beneficial for cells to produce more viral progeny. The dramatically decreased expression of EBNA3C in BMRF1 knockout cells is consistent with our previous data showing that EBNA3C was not sufficiently transcribed in IgG cross-linked Akata cells in the presence of the viral DNA polymerase inhibitor phosphonoacetic acid (PAA) (25), suggesting that BMRF1-mediated viral DNA replication is required for enhanced EBNA3C expression in the viral lytic phase. We also found that two cellular homolog proteins, BDLF2 (cyclin B homolog) and BHRF1 (viral bcl-2), were downregulated in 293TetERΔBMRF1. BMRF1 may also regulate expression of certain early genes. Here, both subunits of ribonucleotide reductase (BaRF1 and BORF2) were downregulated in BMRF1 knockout cells.
FIG 2.
Effects of BMRF1 on viral gene expression in Rta- and Zta-inducible HEK293T cells. (A) At 48 h post-doxycycline treatment, the fold change in expression of EBV genes affected by knockout of BMRF1 in Rta- or Zta-inducible cell lines was converted to log2. The fold change in expression levels of the 28 genes most affected (>3-fold change of the downregulated genes) are indicated in Table 1. Proteins are indicated as follows: gray, latent protein; yellow, cellular homolog protein; red, DNA replication protein; blue, early protein; green, structural protein. Representative data of two independent experiments are shown. (B and C) After doxycycline induction, the expression of EBV lytic genes in 293TetER or 293TetEZ cells harboring p2089 WT and p2089ΔBMRF1 was detected by RT-PCR. Representative data of two independent experiments are shown. (D) Slide-cultured NA cells were cotransfected with control vector or an HA-BMRF1-expressing plasmid coupled with vector control or Rta-expressing plasmid. At 48 h posttransfection, cultured slides were examined for subcellular distribution of HA-BMRF1 and Rta in NA cells by immunofluorescence assay. (E to I) 293TetEZ/p2089ΔBMRF1 cells were transfected with vector or an HA-BMRF1 wild-type or transactivation domain deletion (ΔTA) expression plasmid and induced by doxycycline (50 ng/ml). Cells were harvested for lytic protein detection by Western blotting (E), intracellular EBV DNA copy number detection by qPCR (F), and viral transcript detection (relative fold change in expression) by RT-qPCR at 48 h postinduction (G to H), as described in Materials and Methods.
TABLE 1.
Fold change in expression of BMRF1-regulated EBV genes in Rta- and Zta-inducible cellsa
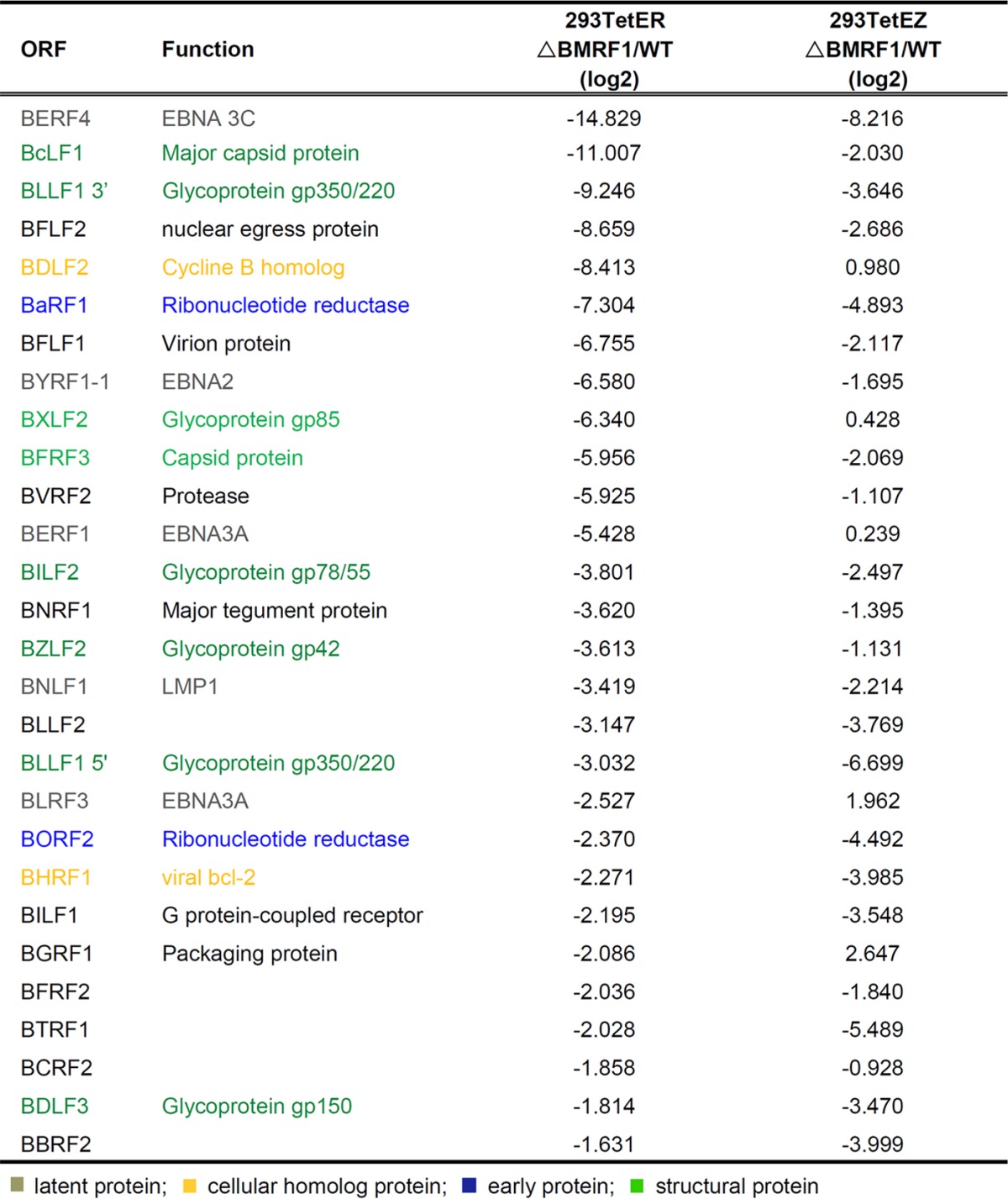
Proteins are indicated as follows: gray, latent protein; yellow, cellular homolog protein; blue, early protein; green, structural protein.
The structural proteins BcLF1 (major capsid protein) and BFRF3 (capsid protein) and glycoproteins BLLF1 (gp350/220), BILF2 (gp78/55), BZLF2 (gp42), and BDLF3 (gp150) were significantly downregulated in both 293TetERΔBMRF1 and 293TetEZΔBMRF1 cells in repeated experiments (Table 1). These late genes were also found to be only slightly decreased in PAA-treated IgG cross-linked Akata cells in our previous study (25), implying that the downregulation of these EBV late genes may due to either the failure of DNA replication or direct regulation of BMRF1. Our previous study reported that BcLF1, BLLF1, BDLF3, and BILF2 are Rta-responsive EBV late genes (26); however, the transcripts of these four genes were decreased significantly in 293TetERΔBMRF1 cells which expressed Rta abundantly, suggesting that BMRF1 may be required for their full expression. Among the 28 most affected genes in 293TetERΔBMRF1 cells, most were also downregulated in 293TetEZΔBMRF1 cells, except for BDLF2, BXLF2, BERF1, and BGRF1. EBV transactivators Zta and Rta differentially triggered vial protein expression. For example, BMRF1, which is preferentially regulated by Zta, was highly expressed in Zta-inducible cells (293TetEZ) compared to levels in 293TetER cells (Fig. 1C and D). Thus, the fold change in induction of gene expression may be different in 293TetER and 293TetEZ cells (Fig. 2A). In addition, viral lytic DNA replication in 293TetEZ/WT cells was higher than that in 293TetER/WT cells (data not shown), suggesting that some viral DNA replication-dependent gene enhancement may also be observed in current settings. Because BMRF1 regulates the promoter of oriLyt BHLF1 in an SP1-dependent manner and the ssDNA binding protein BALF2 promoter through binding to Zta, we decided to explore whether BMRF1 may function as an activator or coactivator to regulate these Rta-responsive late genes.
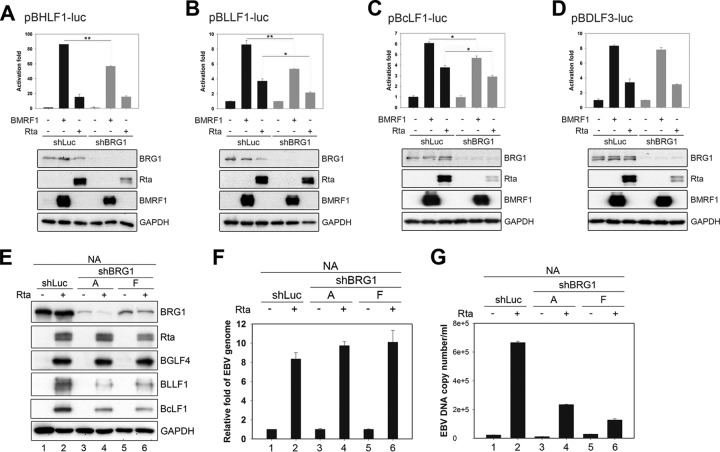
To confirm the microarray data, the RNA expression levels of Rta-mediated EBV lytic genes, including BLLF1, BLLF2, BcLF1, BDLF3, and BILF2, in 293TetEZΔBMRF1 and 293TetERΔBMRF1 cells were detected by reverse transcription-PCR (RT-PCR) analysis (Fig. 2B and C). Because BMRF1 is required for DNA replication, we confirmed the EBV DNA copy number of BMRF1 knockout cells after lytic induction. In comparison with expression in wild-type cells, the expression levels of Flag-Zta, Rta, and BGLF4 are similar while viral DNA replication was defective in BMRF1 knockout cells (Fig. 2E and F, lanes 2). Furthermore, complementation of BMRF1 rescued viral DNA replication in BMRF1 knockout cells (Fig. 2E and F, lanes 3), similar to the findings of previous studies (9, 14, 17). It has been reported that most EBV late gene expression is repressed when viral DNA replication is inhibited (27, 28). In the RT-PCR analysis, some of late transcripts such as BcLF1 (major capsid protein) and BLLF1 (gp350/220) were weakly expressed in ΔBMRF1 cells compared with levels in wild-type cells, which expressed abundant viral late transcripts (Fig. 2B and C). In order to dissect the transactivation function of BMRF1 from the effects of amplified viral DNA templates, a transactivation domain-truncated version was generated (BMRF1ΔTA, with a deletion of residues 379 to 383) as demonstrated in a previous study (16). The nuclear localization signal of BMRF1 (aa 379 to 388) overlaps the transactivation domain of BMRF1; however, it was shown that BMRF1ΔTA (deletion of aa 379 to 383) distributes in the HeLa cell nucleus without transactivation activity (16). Because 293TetER(Z) cells have relatively less cytoplasm, we also confirmed the subcellular localization of BMRF1ΔTA in EBV-positive naso-epithelial NA cells, which display more distinguishable cytoplasmic parts. When transfected alone, BMRF1ΔTA was detected to some extent in the cytoplasm and was significantly expressed in the nucleus. Moreover, BMRF1ΔTA was detected almost completely in the nucleus of NA cells when Rta was cotransfected, suggesting that other viral DNA replication components may ensure its proper nuclear targeting (Fig. 2D). In doxycycline-treated 293TetEZΔBMRF1 cells, the viral genome DNA copy number increased dramatically with the complementation of WT BMRF1, while BMRF1ΔTA complementation gave a replication efficiency of about 80% of that of the WT BMRF1, suggesting that this mutant is capable of supporting viral DNA replication (Fig. 2E and F, lanes 4). By reverse transcription-quantitative PCR (RT-qPCR) analysis, viral late gene transcripts BcLF1 (major capsid protein), BLLF1 (gp350/220), and BLLF2 (function unknown) were increased 142-, 0.5-, and 4.5-fold, respectively, in doxycycline-treated 293TetEZΔBMRF1 cells (Fig. 2G to I, lanes 1 and 2), and the complementation of WT BMRF1 induced expression of these genes 437-, 35-, and 38-fold, respectively, compared with levels in the vector control (Fig. 2G to I, lanes 1 and 3). In 293TetEZΔBMRF1 cells complemented with the BMRF1ΔTA mutant, the amounts of BcLF1, BLLF1, and BLLF2 transcripts were significantly decreased by 40%, 78%, and 82%, respectively, compared with levels in BMRF1 WT compensated cells. These results thus hint that the transactivation domain of BMRF1, rather than its DNA replication-enhancing function, is critical for the expression of these genes, especially BLLF1 and BLLF2 (Fig. 2G to I, lanes 4). Furthermore, we also confirmed the expression of the Rta-mediated late gene BSRF1 (an uncharacterized protein), which was not affected in either 293TetERΔBMRF1 or 293TetEZΔBMRF1 cells, as determined by RT-PCR (Fig. 2B and C), suggesting that BMRF1 regulates only a subset of viral late gene expression.
BMRF1 functions as a transactivator or a coactivator to turn on a subset of Rta-responsive late gene promoters.
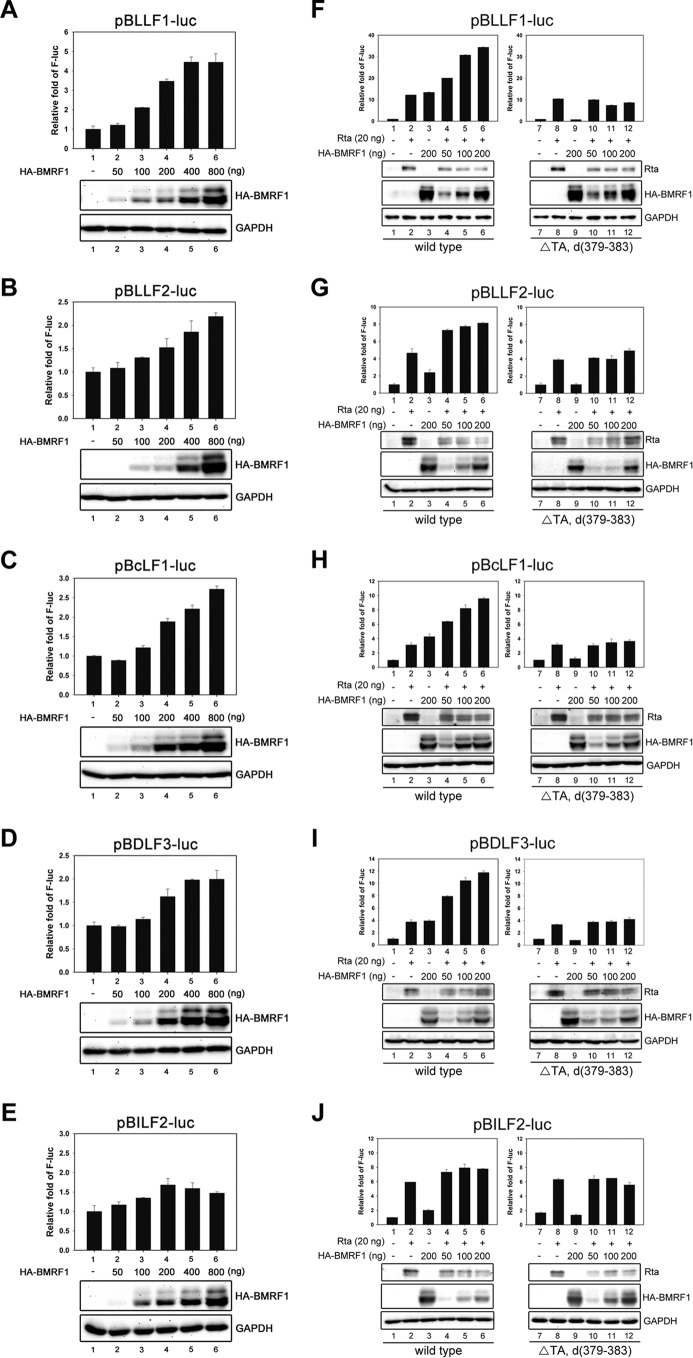
To determine whether BMRF1 can directly turn on promoter activities of a subset of Rta-responsive late genes, the transactivation activities of BMRF1 on the BcLF1, BDLF3, BLLF1, BLLF2, and BILF2 promoters were measured by luciferase reporter assays. We found that BMRF1 directly turned on the promoter activities of BcLF1, BLLF1, BLLF2, and BDLF3 in a dose-dependent manner, suggesting that BMRF1 functions as an activator of these late promoters (Fig. 3A to D). Previously, it was found that Rta, but not Zta, turns on the promoter activities of certain late genes, including BcLF1, BLLF1, BDLF3, and BILF2 (26). Therefore, we investigated whether BMRF1 may stimulate Rta-mediated transactivation activities on these late gene promoters. In the cotransfection experiments, the expression of BMRF1 additively enhanced Rta-mediated luciferase activities in a dose-dependent manner on the BcLF1, BLLF1, BDLF3, and BLLF2 promoters (left panels in Fig. 3F to I), and the enhancement was abolished when a transactivation domain-deleted BMRF1 (BMRF1ΔΤΑ, with a deletion of aa 379 to 383) was expressed (right panels in Fig. 3F to I). However, BMRF1 expression did not activate the promoter activity of BILF2 (Fig. 3E) or enhance the Rta-mediated activation of the BILF2 promoter (Fig. 3J). It is possible either that BMRF1 may regulate the BILF2 promoter indirectly or that the BMRF1-responsive element was not included in the BILF2 reporter construct (nucleotides [nt] 150415 to 151571 of the B95.8 EBV genome, −1046 bp to +110 bp of the transcription start site of BILF2). Together, these results indicate that BMRF1 not only facilitates late gene expression through its DNA polymerase accessory function but also serves as an activator through its carboxyl-terminal transactivation domain on a subset of late gene promoters in an Rta-independent manner.
FIG 3.
BMRF1 functions as an activator to turn on a subset of EBV late gene promoters in HEK293T cells. HEK293T cells (1 × 105 cells) were seeded into a 12-well culture plate and cotransfected with the doses of BMRF1 or BMRF1ΔTA indicated, with or without Rta-expressing plasmids and with the reporter plasmids pGL2-BcLF1 (A and F), pGL2-BLLF1 (B and G), pGL2-BLLF2 (C and H), pGL2-BDLF3 (D and I), and pGL2-BILF2 (E and J) by BBS for 48 h. In all reporter assays, the Renilla Luc plasmid (pRL-null; 250 ng/well) was cotransfected as a transfection control. The luciferase activities were detected by a luminometer using a Dual-Glo Luciferase Assay kit. The promoter upstream region of the indicated gene construct in the reporter plasmid was described in Materials and Methods. Representative data of three independent experiments are presented.
BMRF1 turns on the promoter of BDLF3 through an SP1-dependent pathway.
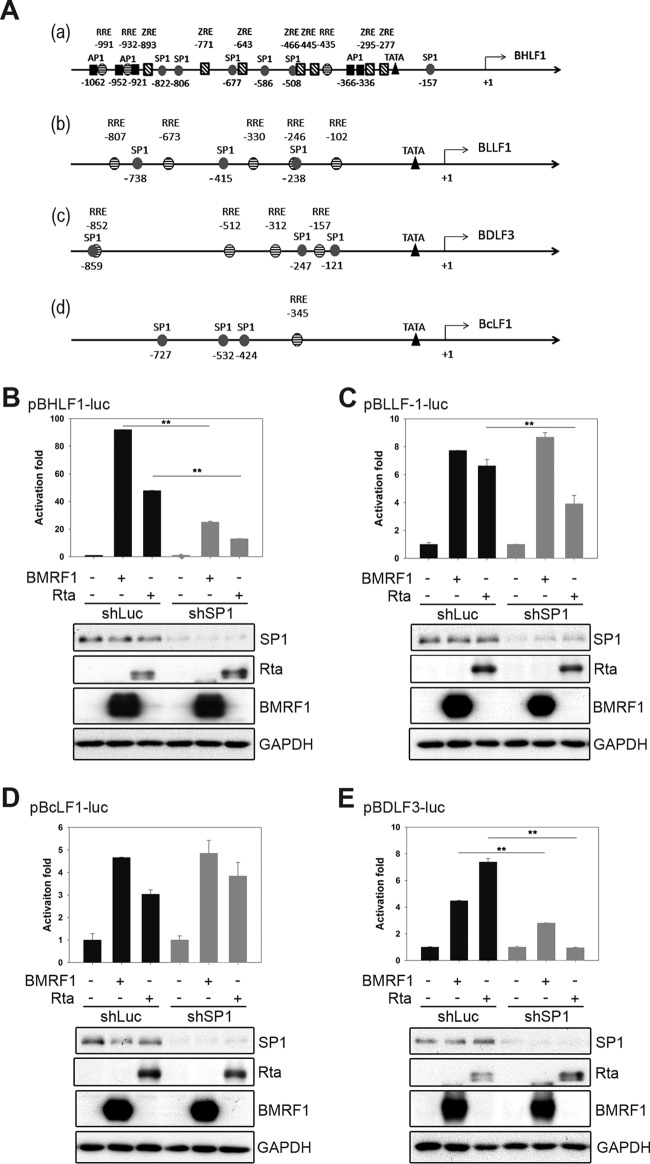
Because previous studies have shown that BMRF1 regulates viral BHLF1 transcription at the oriLyt and host gene gastrin promoters through SP1/ZBP89 binding elements (10, 29), the promoter regions of BHLF1, BLLF1, BDLF3, and BcLF1 were examined for putative SP1, Rta, and Zta binding sites (Fig. 4A). We then examined possible SP1-dependent BMRF1 transactivation activities on these promoters using an SP1 knockdown approach. In SP1 knockdown HEK293T cells, BMRF1 activation of the BHLF1 and BDLF3 promoters dropped significantly, whereas transactivation of the BLLF1 and BcLF1 promoters was not affected (Fig. 4B to E). In a parallel analysis, knockdown of SP1 also reduced the Rta transactivation activities on the BHLF1, BLLF1, and BDLF3 promoters to various extents (Fig. 4B, C, and E). The SP1-dependent Rta regulation of BHLF1 was reported previously (10); thus, we found that SP1 is also required for Rta-mediated transactivation of the BLLF1 and BDLF3 promoters. Data here suggest that BMRF1 regulates the BDLF3 promoter through an SP1-dependent pathway, similar to that of the BHLF1 promoter, whereas BMRF1 may coordinate with a cellular factor(s) other than SP1 to regulate the expression of BcLF1 and BLLF1. Alternatively, SP1 binding on viral promoter upstream regions can be used selectively by Rta or BMRF1.
FIG 4.
BMRF1 regulates the activities of the BDLF3 and BHLF1 promoters through an SP1-dependent mechanism. (A) The SP1 [5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′] or AP1 (ATGAGTCAT) binding sites in the BHLF1, BLLF1, BDLF3, and BcLF1 promoter upstream regions were predicted, as indicated, by TFSEARCH (version 1.3). The Zta-responsive elements (ZREs; TG/T-T/A-G-T/C-G/C/A-A) or Rta-responsive elements (RREs; GNCCN9GGNG, where N stands for any nucleotide) are shown above the binding sites. The TATA boxes are indicated. (B to E) HEK293T cells were transduced with shLuc- or shSP1-expressing lentivirus for 2 days and selected with puromycin (3 μg/ml) for 5 days. The shLuc control or shSP1 knockdown cells were cotransfected with indicated effector plasmids and reporter plasmids, including pGL2-BHLF1, pGL2-BLLF1, pGL2-BcLF1, and pGL2-BDLF3 for luciferase reporter assays. Representative data of two independent experiments are presented.
BMRF1 associates with cellular factors involved in DNA replication, chromatin remodeling, and RNA splicing.
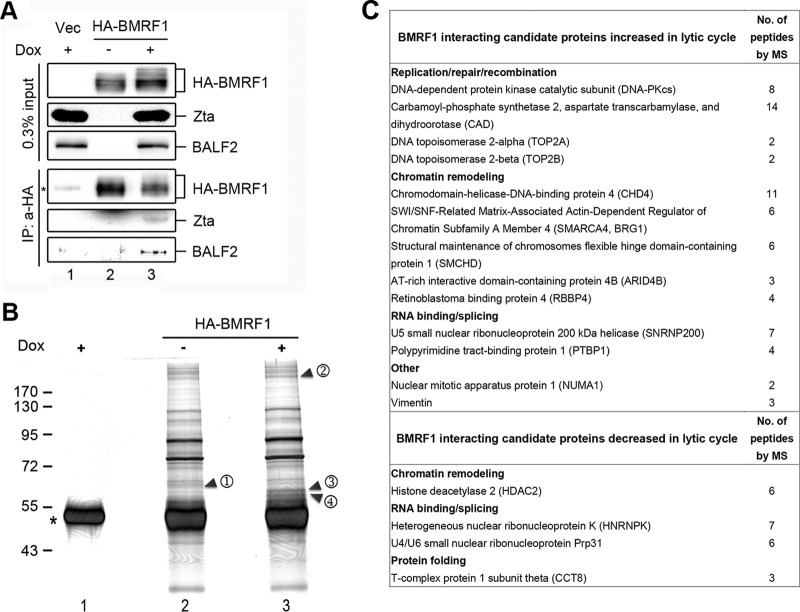
To explore the mechanism(s) that may be involved in BMRF1-mediated transcriptional regulation of viral promoters, we searched for BMRF1-interacting proteins upon lytic replication. To this end, hemagglutinin (HA)-tagged BMRF1-expressing 293TetEZΔBMRF1 cells were treated with doxycycline to induce lytic reactivation. Subsequently, HA-BMRF1-associated complexes were immunoprecipitated and separated by SDS-PAGE. As reported previously (13, 30), BMRF1 associated with Zta and BALF2 after lytic induction was detected by coimmunoprecipitation (Fig. 5A, lane 3). The silver-stained gel showed BMRF1 associated with a number of proteins in BMRF1 knockout cells, with or without lytic induction (Fig. 5B, lanes 2 and 3). We found that the intensity of the indicated band 1 pulled down by BMRF1 was decreased (Fig. 5B, lane 2), whereas the intensities of bands 2, 3, and 4 were enhanced after doxycycline induction (Fig. 5B, lane 3), suggesting that BMRF1 may be recruited to various protein complexes upon lytic cycle induction. The bands with increased or decreased intensities after lytic induction were sliced and analyzed by mass spectrometry (Fig. 5B). The major BMRF1-interacting proteins in band 3 and 4 were hyper- and hypophosphorylated BMRF1, and interacting proteins sorted from bands 1 and 2 are summarized in Fig. 5C. The proteins identified in band 2 include DNA replication/repair-associated factors, chromatin remodeling factors, and RNA binding proteins. In contrast, the BMRF1-interacting protein candidates in band 1 include histone deacetylase 2, RNA binding proteins, and the protein folding-related protein CCT8 (31) (Fig. 5C). Previous studies demonstrated that several chromatin remodelers participate in regulating herpesvirus infection (20, 21, 32). We found that one of the BMRF1-associated chromatin remodelers, BRG1, is important for KSHV reactivation through direct interaction with KSHV Rta, and ablation of that interaction abolishes KSHV lytic replication (20). Because BRG1 is a subunit of the SWI/SNF complex that regulates chromatin structure to activate or repress gene transcription (33), we decided to determine whether BRG1 is involved in BMRF1-mediated regulation of gene expression.
FIG 5.
Identification of BMRF1-interacting proteins. Cell lysates harvested from 293TetEZ/p2089ΔBMRF1 cells that were transfected with the HA-BMRF1-expressing plasmid and untreated or treated with doxycycline (Dox; 50 ng/ml) for 48 h were used for immunoprecipitation (IP) with anti-HA antibody. One-tenth of the captured immunocomplexes was analyzed by Western blotting (A), and 90% of captured products were subjected to silver staining (B). The bands (1 to 4) indicated on the silver-stained gel were subjected to mass spectrometry analysis (see text). *, Ig heavy chain. (C) BMRF1-interacting proteins identified in mass spectrometry (MS) analysis.
BMRF1 interacts with BRG1 in a transactivation domain-dependent manner.
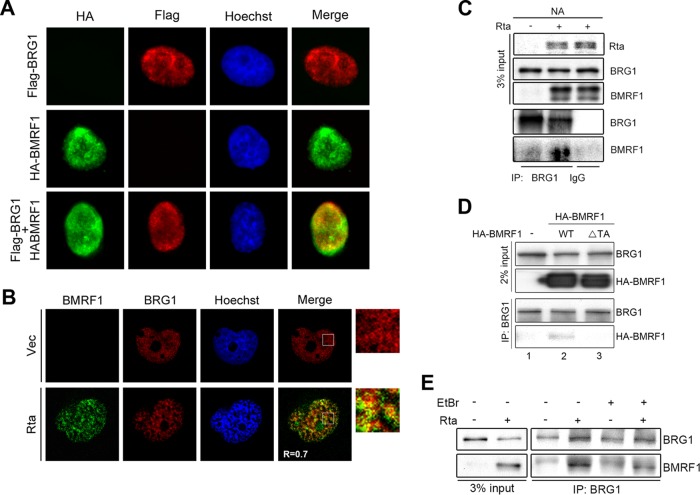
To confirm the association of BMRF1 with BRG1, colocalization of HA-BMRF1 and Flag-BRG1 was observed by immunofluorescence staining of transiently transfected HeLa cells. The nuclear pattern of Flag-BRG1 partially colocalized with that of HA-BMRF1 (Fig. 6A). We then confirmed the colocalization and interaction of BMRF1 and BRG1 in the EBV-positive NPC cell line, NA. Indeed, endogenous BRG1 partially colocalized with BMRF1 during EBV reactivation in NA cells was observed by confocal microscopy (Pearson's correlation coefficient, r = 0.7) (Fig. 6B). After lytic induction by Rta transfection, some of BMRF1 was detected in BRG1-associated immunocomplexes in NA cells (Fig. 6C). Moreover, deletion of the transactivation domain of BMRF1 abolished its interaction with BRG1, indicating that the transactivation domain of BMRF1 is required for this interaction (Fig. 6D). To determine whether the interaction of BMRF1 and BRG1 is DNA dependent, the cell lysates harvested from Rta-reactivated NA cells were pretreated with ethidium bromide (EtBr) to disrupt DNA-dependent protein associations before immunoprecipitation. The coimmunoprecipitation result indicated that the interaction of BMRF1 and BRG1 was attenuated after EtBr pretreatment, suggesting that the interaction between BMRF1 and BRG1 is at least partially dependent on DNA binding (Fig. 6E).
FIG 6.
BMRF1 associates with BRG1 in Rta-reactivated EBV-positive NA cells. (A) The Flag-BRG1-expressing plasmid was cotransfected with a vector control or HA-BMRF1 into HeLa cells for immunofluorescence analysis. The distributions of BRG1 and BMRF1 were detected by mouse anti-Flag and rabbit anti-HA antibody, respectively. Representative images of two independent experiments are shown. (B) At 48 h postinduction, slide-cultured NA cells were harvested for immunofluorescence analysis using mouse anti-BMRF1 and rabbit anti-BRG1 antibody. The distributions of BMRF1 and BRG1 were observed by confocal microscopy. Quantification of colocalization was measured by Pearson's correlation coefficient (r) using ImageJ Coloc2. Representative images of two independent experiments are shown. (C) NA cells transfected with a vector control or Rta expression plasmid were harvested for immunoprecipitation assays. Representative data of two independent experiments are shown. (D) The vector control or an HA-BMRF1 wild-type or transactivation domain mutant (ΔTA) plasmid was transfected into 293T cells for 48 h. The transfected cells lysates were harvested and subjected to immunoprecipitation assay using mouse anti-BRG1 antibody. The representative data of two independent experiments are shown. (E) Cell lysates harvested from control vector or Rta transfected NA cells were pretreated with or without EtBr (10 μg/ml) on ice for 30 min. Cell lysates were incubated with anti-BRG1 or control IgG to precipitate BRG1-associated complexes and subjected to Western blotting.
BRG1 contributes to BMRF1-mediated transactivation of the BHLF1, BLLF1, and BcLF1 promoters.
Because the data showed that BMRF1 interacts with BRG1 during EBV lytic reactivation, we sought to determine whether BRG1 is involved in BMRF1-mediated activation of various viral promoters. In reporter assays, knockdown of BRG1 attenuated the transactivation activities of BMRF1 on the BHLF1, BLLF1, and BcLF1, but BDLF3, promoters (Fig. 7A to D). Data here indicate that BRG1 regulates not only the late BLLF1 and BcLF1 promoters but also the early oriLyt BHLF1 promoter. To verify that BRG1 plays an important role in viral late gene expression during virus replication, EBV-positive NA cells were transduced with two different clones of a lentivirus expressing a short hairpin RNA targeting BRG1 (shBRG1) and subsequently induced into the lytic cycle by Rta transfection. At 60 h posttransfection, knockdown of BRG1 downregulated the expression of late protein products BcLF1 and BLLF1 in Rta-reactivated NA cells (Fig. 7E, lanes 2, 4, and 6). Because the major capsid protein BcLF1 and glycoprotein BLLF1 are required for virus maturation, the virion production from BRG1-depleted NA cells was reduced, and this was accompanied by a slight increase of intracellular EBV DNA accumulation (Fig. 7F and G). This indicates that BRG1 modulates the expression levels of BcLF1 and BLLF1, which are critical for viral assembly.
FIG 7.
Knockdown of BRG1 reduced the transactivation activities of BMRF1 on the promoters of BHLF1, BLLF1, and BcLF1 in reporter assays in HEK293T cells and reduced the expression of late proteins BLLF1 and BcLF1 in Rta-reactivated NA cells. (A to D) HEK293T cells were transduced with shLuc or shBRG1 lentivirus for 48 h and selected with puromycin for 5 days. The shLuc control and BRG1 knockdown cells were cotransfected with the indicated effector and reporter plasmids pGL2-BHLF1, pGL2-BLLF1, pGL2-BcLF1, and pGL2-BDLF3 by the calcium phosphate-BES method. At 48 h posttransfection, luciferase activities and protein expression were detected by reporter assay and Western blotting. Representative data of two independent experiments are shown. (E to G) The shLuc control and BRG1 knockdown NA cells were transfected with a vector control or Rta-expressing plasmid for 48 h. The transfected cells and culture supernatant were harvested for detection of lytic proteins (E), intracellular EBV DNA copy number (F), and extracellular virion production (G). Representative data of two independent experiments are presented.
BMRF1 and BRG1 bind to the BLLF1 and BcLF1 promoters in lytic-reactivated NA cells and Akata EBV+ cells.
To define the BRG1-responsive elements, the upstream regions of the BcLF1 and BLLF1 promoters were serially deleted. We found that the presence of BMRF1 alone enhanced the basic luciferase activities for 4-fold, probably because its DNA binding activity helped the stability of transiently transfected DNA. With the BcLF1 promoter upstream regions (bp −625 to +1), BMRF1 activated BcLF1-Luc 7-fold compared to the level of the vector control. Deletion of bp −425 to −210 of the BcLF1 promoter region reduced BMRF1 transactivation activity significantly (Fig. 8A). In comparison with the vector control, BMRF1 activated the upstream promoter region of BLLF1 (bp −980 to +100) by 15-fold and, activation was reduced to 6-fold with the region of bp −627 to +100 of BLLF1, suggesting that the region of bp −980 to −627 of BLLF1 contains BMRF1-responsive elements (Fig. 8B). Here, we used the upstream regions (bp −425 to −210) of BcLF1 and the region of bp −980 to −627 of BLLF1 to verify the specific binding of BMRF1 to the promoter regions by chromatin immunoprecipitation (ChIP) assay. BMRF1 showed basal levels of binding on BLLF1, BcLF1, BDLF3, and BILF2 promoters in vector transfected NA cells, possibly because of spontaneous lytic gene expression. After transfection of the Rta-expressing plasmid, BMRF1 binding signals were enhanced on promoters of BLLF1, BcLF1, and BDLF3 but not on the BILF2 promoter (Fig. 8C). These binding preferences are consistent with the luciferase reporter analysis of BILF2 (Fig. 3). Because BRG1 knockdown significantly reduced the BMRF1-mediated activities on BLLF1 and BcLF1 promoters (Fig. 7B and C), it is possible that BRG1 cooperates with BMRF1 to regulate the BLLF1 and BcLF1 promoters. Indeed, BRG1 associated with the BMRF1-regulated promoter upstream regions of only BLLF1 and BcLF1, not of BDLF3 and BILF2, in the ChIP assay, indicating that BRG1 may help the recruitment of BMRF1 on BLLF1 and BcLF1 promoters (Fig. 8D). In EBV-positive B cells, we found that the binding activities of BMRF1 on BLLF1, BcLF1, and BDLF3 promoter regions in IgG cross-linked Akata EBV+ cells were also enhanced (Fig. 8E). The recruitment of BRG1 onto the BLLF1 and BcLF1 promoters was also detected in IgG cross-linked Akata EBV+ cells with similar patterns (Fig. 8F). Here, BRG1 was not detected in a ChIP of the BDLF3 promoter in lytic, reactivated NA or Akata EBV+ cells. It is possible that the BDLF3 promoter binding of BMRF1 may be regulated by other cellular factors, such as SP1. Indeed, knockdown of BRG1 reduced the recruitment of BMRF1 onto the BcLF1 and BLLF1, but not BDLF3, promoter regions (Fig. 9A), suggesting that BRG1 may promote BMRF1 binding onto a subset of viral promoters. Taking these results together, we identify a novel mechanism by which BMRF1 may regulate the BLLF1 and BcLF1 promoters through recruitment of BRG1.
FIG 8.
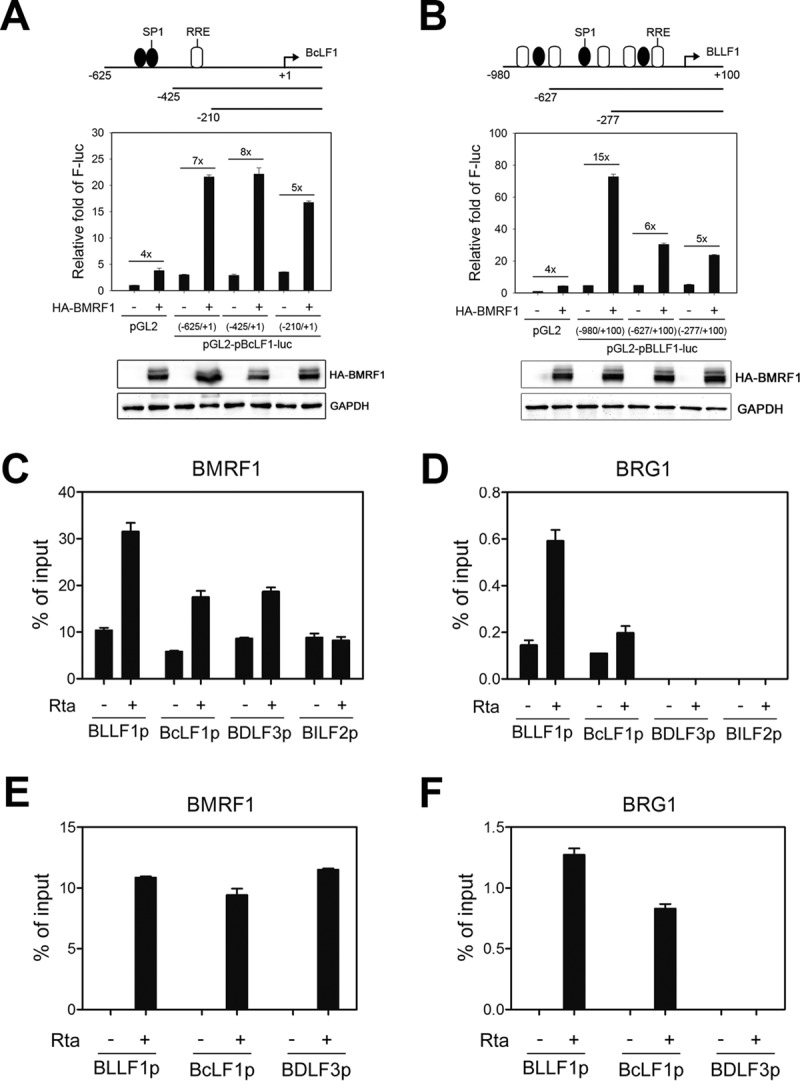
BMRF1 and BRG1 bind to the viral late gene promoters BcLF1p and BLLF1p. HEK293T cells were transfected with a plasmid expressing pGL2-basic Luc or serially deleted BcLF1 promoter (A) or BLLF1 promoter (B) Luc-expressing plasmids coupled with a vector control or HA-BMRF1-expressing plasmid for 48 h. Luciferase activities were detected using a Dual-Glo Luciferase Assay kit. Representative data of two independent experiments are presented. (C to F) At 36 h postinduction, NA cells (1 × 107 cells) (Cand D) and Akata EBV-positive cells (2 × 107 cells) (E and F) were harvested for ChIP-qPCR analysis with antibody against BMRF1, BRG1, or control IgG on promoter regions of BLLF1, BcLF1, BDLF3, and BILF2. To normalize the ChIP-qPCR data, the signals obtained from the ChIP assay were divided by the signals obtained from an input sample, and the data are presented as the percentage of input. Representative data of two independent experiments are presented.
FIG 9.
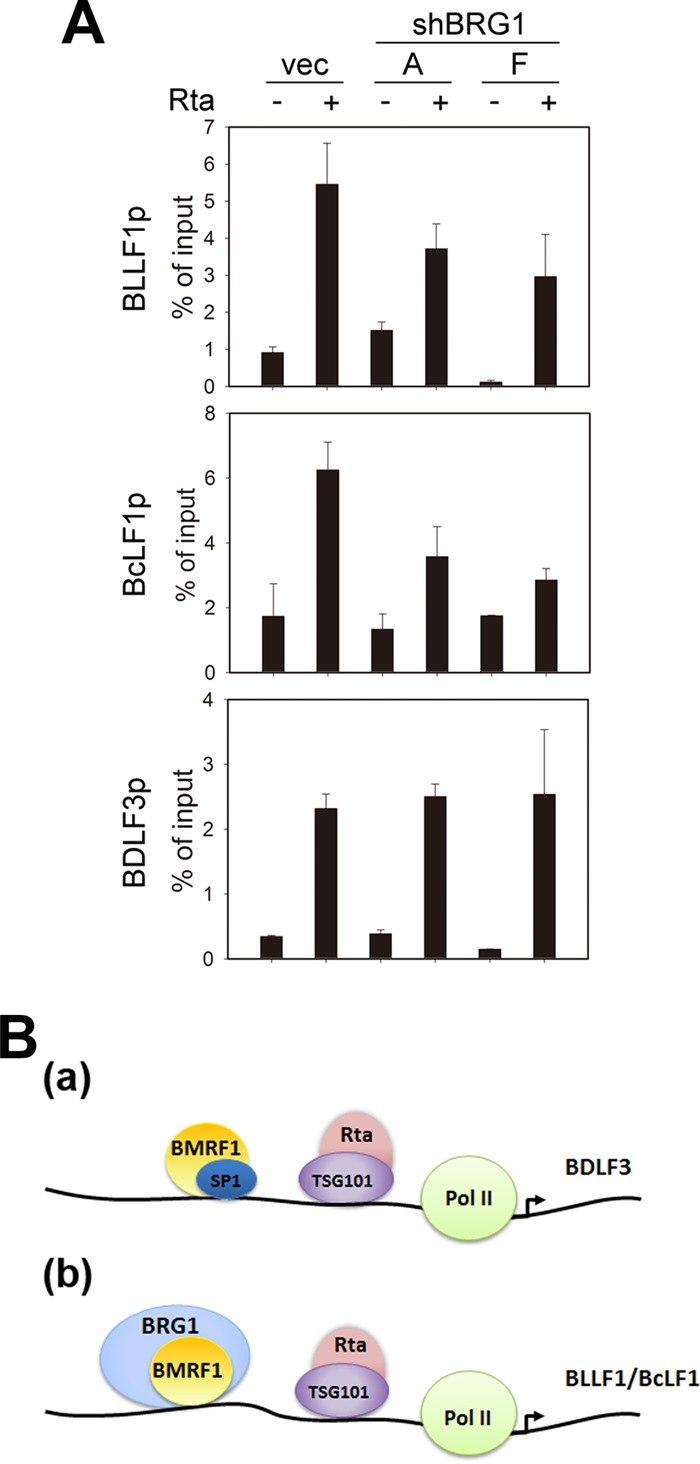
BRG1 promotes the recruitment of BMRF1 onto the BLLF1 and BcLF1 promoters. (A) For lytic reactivation, BRG1-depleted NA cells were transfected with control vector or Rta expression plasmid. At 36 h posttransfection, the cross-linked chromatin was harvested for ChIP-qPCR analysis with antibody against BMRF1. Representative data of two independent experiments are presented. (B) A hypothetical model of BMRF1-regulated expression of a subset of EBV late genes. Upon lytic replication, the immediate protein Rta regulates the late genes BDLF3, BLLF1, and BcLF1 by interacting with TSG101 and binding to the proximal region of those gene promoters (26). In addition, BMRF1 cooperates with SP1 (A) or BRG1 (B) to regulate BDLF3, BLLF1, and BcLF1 gene expression by binding to the upstream regions of their promoters.
DISCUSSION
Before this study, the gene expression regulatory functions of BMRF1 were assigned to two mechanisms. First, BMRF1 was considered to act as a transcriptional activator to activate the viral oriLyt promoter BHLF1 and cellular gastrin promoter in an SP1-dependent manner (10, 29). Alternatively, it was suggested that BMRF1 serves as a coactivator by interacting with Zta to enhance the transactivation of the early gene BALF2 promoter (13). In addition to the regulation of early gene promoters, here we found that knockout of BMRF1 reduced the expression of a subset of late genes that are expressed independently of DNA replication (Fig. 2 and Table 1). We also showed that BMRF1 expression alone activated a subset of Rta-responsive late gene promoters, including those of the major capsid protein (BcLF1), glycoprotein gp350/220 (BLLF1), a protein of unknown function (BLLF2), and glycoprotein gp150 (BDLF3). In addition, BMRF1 also enhanced Rta-regulated activation of these late promoters additively (Fig. 3). The transcriptional regulatory function of BMRF1 appears to depend on its carboxyl transactivation domain. Similar to the BMRF1-mediated regulation of the BHLF1 promoter, BMRF1 regulated the BDLF3 promoter in an SP1-dependent manner (Fig. 4). Moreover, we found that BMRF1 interacted with the cellular SWI/SNF chromatin modifier subunit BRG1 through a partially DNA-dependent mechanism (Fig. 5 and 6). Knockdown of BRG1 affected the transactivation activities of BMRF1 on the BHLF1, BLLF1, and BcLF1 promoters and reduced the expression levels of the structural proteins BcLF1 and BLLF1 in reactivated NA cells (Fig. 7). Furthermore, BMRF1 and BRG1 bound to the same upstream regions of the BLLF1 and BcLF1 promoters, and the recruitment of BRG1 onto these promoters assisted the subsequent binding of BMRF1 (Fig. 8 and 9A). Hence, we propose two novel pathways through which BMRF1 may coordinate with SP1 or BRG1 to regulate a subset of Rta-responsive late genes (Fig. 9B). In the case of the BDLF3 promoter, BMRF1 functions through interaction with SP1, whereas BRG1- or BRG1-associated complexes are required for BMRF1 transactivation of the BLLF1 and BcLF1 promoters.
The cascade of gene expression regulation of herpesviruses during viral lytic replication was once quite mysterious. It was always considered that immediate early gene can turn on early genes to promote viral DNA replication. The regulation of late gene expression may be more complicated: either the replicated viral DNA template is differentially modified, or the viral or cellular factors involved in controlling the transcription of various late genes are differentially expressed upon viral DNA replication. As with other beta- and gammaherpesviruses, EBV late gene expression could be regulated in either a DNA replication-dependent or -independent manner (34). Similar to the findings in KSHV (35, 36), recent studies showed that in the presence of replicated viral DNA and the viral preinitiation complex (vPIC; containing BcRF1, BFRF2, BGLF3, BVLF1, BDLF4, and BDLF3.5), BcRF1 can function as a TATA box binding protein to regulate TATA box-containing late promoters, such as the BcLF1 promoter (37–40). Our microarray data showed that the expression of a member of the vPIC, BFRF2, was downregulated in both 293TetER/p2089ΔBMRF1 and 293TetEZ/p2089ΔBMRF1 cells. Here, we cannot exclude the possibility that some of the downregulated genes observed in a BMRF1 knockout microarray may be mediated through some early genes that are important for late gene expression (Fig. 2). In contrast, the replication of the transcriptional template is not essential for the expression of late genes such as the major capsid protein BcLF1 and capsid protein BFRF3 (41). In 293 cells, Rta alone induces glycoprotein 350/220 BLLF1 expression in a Zta-deleted virus, without EBV DNA replication (42). Our microarray data showed that the expression of the Rta-responsive genes BcLF1, BLLF1, and BFRF3 was reduced in cells harboring viral DNA replication-deficient viruses (293TetERΔBMRF1 or 293TetEZΔBMRF1 cells) (Table 1). As a DNA polymerase processivity factor, BMRF1 should also be able to enhance gene expression through enrichment of viral DNA templates. Here, we showed that a subset of late transcripts was significantly downregulated by complementation of BMRF1ΔTA, which still supports viral DNA replication (Fig. 2E to I). In addition, BMRF1 expression alone transactivates certain late promoters in HEK293T cells without the endogenous EBV genome (Fig. 3). Together, these results indicate that BMRF1 regulates the transcription of a subset of EBV late genes in both DNA replication-dependent and -independent manners.
To date, several cellular molecules have been shown to be involved in BMRF1-mediated transactivation of viral and cellular promoters. BMRF1 activates the BHLF1 and gastrin promoters through SP1/ZBP89 binding sites (11, 29). As shown in Fig. 4E, BMRF1-induced activation of the BDLF3 promoter was diminished in SP1 knockdown cells. Therefore, we propose that BMRF1 regulates the BDLF3 promoter through SP1. The fact that BMRF1 still turned on the promoter activities of BLLF1 and BcLF1 in SP1 knockdown cells in reporter assays thus prompted us to search for another cellular factor(s) that may coordinate with BMRF1 to stimulate viral gene transcription. Here, we demonstrate that a member of the SWI/SNF family, BRG1, is involved in BMRF1 activation of viral gene expression. BRG1 has helicase and ATPase activities to alter chromatin structure and activate or repress gene expression (43, 44). We showed that BMRF1 interacts with BRG1 in vivo and that the transactivation domain of BMRF1 is crucial for this interaction (Fig. 6), suggesting that the transcription regulatory function of BMRF1 may be controlled by BRG1, at least on the BLLF1 and BcLF1 promoter regions.
As a chromatin structural modifier, BRG1 selectively regulates gene expression via its recruitment to cellular promoters through interaction with specific transcription factors (45–48). For example, BRG1 associates with phosphorylated Stat1 to activate the expression of heat shock protein 90 (HSP90) upon exposure to gamma interferon (IFN-γ) (49). In addition, BRG1 interacts with microphthalmia-associated transcription factor (MITF) and regulates the dynamics of MITF genomic occupancy to activate melanocyte lineage gene expression (50). Here, we showed that BRG1 selectively regulates the BMRF1-mediated promoter activities of BHLF1, BLLF1, and BcLF1 but not that of BDLF3, implying that protein interaction with BMRF1 is critical for its transcriptional gene activation (Fig. 7 and 8). In the experiment shown in Fig. 7A, the BMRF1-mediated transactivation of BHLF1 was reduced by about 15%; thus, the increase of viral DNA copy number per cell probably reflects that BHLF1 transcripts can still be stimulated by the immediate early transactivators Zta and Rta (Fig. 7F). However, the reduced expression of structural gene products, including capsid protein BcLF1 and glycoprotein BLLF1, significantly affects the amounts of secreted virus (Fig. 7G). Indeed, BRG1 knockdown influenced recruitment of BMRF1 to the BLLF1 and BcLF1 promoters, suggesting that BMRF1 interacts with various transcription regulators to mediate viral gene expression (Fig. 9). The use of different combinations of cellular factors to modulate BMRF1-mediated regulation of viral promoters again reveals how viruses can use the limited array of viral proteins to perform various regulatory activities. We noticed that the promoter regions of BRG1-dependent BMRF1-regulated genes on the EBV genome did not cluster in a particular region; therefore, the binding preference of the BMRF1 interactome needs to be further elucidated.
In addition to BRG1, there is a possibility that BMRF1 may regulate gene expression through other chromatin remodelers, including CHD4, RBBP4 (retinoblastoma binding protein 4), and HDAC-2, which were detected in BMRF1-associated complexes in this study (Fig. 5). CHD4 and RBBP4 are components of the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex (51). The Mi-2/NuRD complex is thought to play a role in modifying chromatin structure to repress gene expression (51). A previous study reported that human cytomegalovirus (HCMV) UL29 and UL28 associate with the NuRD complex for efficient HCMV replication through enhancing the activity of the HCMV major immediate early promoter (52). The possibility that the Mi-2/NuRD complex may be required for BMRF1-mediated gene regulation needs to be clarified.
Overall, BMRF1 seems a good example of a virus using a single gene product to carry out multiple tasks during replication. Upon the initiation of EBV replication, BMRF1 synergistically activates the Zta-responsive promoters of the BHLF1 upstream sequence, through SP1, and the BALF2 upstream regions, through interaction with Zta. BMRF1 then interacts with the viral DNA polymerase BALF5 to promote the synthesis of progeny viral genomes. In this study, we found that BMRF1 also activates the Rta-responsive late promoter of BDLF3 in an additive manner with Rta through SP1. Notably, we reveal a mechanism whereby BMRF1 regulates the late genes BLLF1 and BcLF1 through interaction with the cellular SWI/SNF chromatin regulator BRG1. It is suggested that either posttranslational modification of BMRF1 may regulate its binding preference or the coordinated protein expression kinetics may contribute to the regulation of BMRF1 function at different stages of virus replication. On the other hand, because BRG1 is important not only for cellular gene expression control but also for the DNA repair machinery to function properly, whether BMRF1 may also affect the cellular function of BRG1 is worth studying.
MATERIALS AND METHODS
Cell lines and transfection.
The HEK293T cell line is a derivative of a human kidney epithelial cell line (ATCC CRL-1573). The HeLa cell line was derived from human cervical epithelial cells (ATCC CCL-2). The EBV producer cell line, Akata EBV+, was established from a Japanese patient with Burkitt's lymphoma (53). NA is an NPC-W01 cell line latently infected with the recombinant EBV Akata strain (54). The EBV lytic cycle can be induced in NA cells by transfection with an Rta-expressing plasmid. 293TetER cells are a doxycycline-inducible Flag-Rta-expressing cell line generated from T-REx 293 cells (Invitrogen) (24). The 293TetEZ cell line, a Zta-inducible T-REx 293 cell line, was established by transfection with pLenti4-Flag-Zta and selected by zeocin (400 μg/ml). DNA transfection was performed using Lipofectamine 2000 transfection reagent (Invitrogen) according to the protocol suggested by the manufacturer. Cells were incubated for the periods of time indicated in the figure legends at 37°C with 5% CO2. Introduction of BMRF1 plasmids into 293TetER/p2089ΔBMRF1 or 293TetEZ/p2089ΔBMRF1 cells was performed with a transfection protocol the calcium using phosphate–N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES)-buffered solution (BBS) (55).
Plasmids.
The pCR3.1-BMRF1 expression plasmid, pYPW88, was cloned by PCR from a Flag-BMRF1 plasmid using primers LMRC439/440 with BamHI and EcoRI sites (56). To construct the transactivation domain-deleted BMRF1 clone (BMRF1ΔTA; deletion of aa 379 to 383) (plasmid pMTS9), single-primer mutagenesis (57) was performed using the primer LMRC772, as shown in Table 2. HA-tagged BMRF1- and BMRF1ΔTA-expressing plasmids (pMT8 and pMT10) were generated by inserting the HA sequence (YPYDVPDYA) into pYPW88 and pMTS9 by single-primer mutagenesis with primer LMRC912. The Rta expression plasmid (pRTS15) and BALF5 expression plasmid (pDH312) were gifts from Diane Hayward (58, 59). The luciferase reporter plasmids containing promoter regions of BcLF1, BDLF3, BILF2, and BLLF1 were amplified by PCR, and the fragments were cloned into the pGL2-basic vector as described previously (26). The pGL2-BLLF2 reporter plasmid was generated by ligating the PCR products of the promoter region of BLLF2 (pBLLF2, nt 89951 to 91051 of the EBV B95.8 genome; GenBank accession number V01555.2) using primers LMRC955/956. For serial deletions, constructs of the promoter region of BLLF1, including nt 92053 to 92430 and nt 92053 to 92780 of B95.8 EBV, were cloned into the pGL2 reporter plasmid using primers LMRC1000/999 and LMRC1001/999. For serial deletions, constructs of the promoter region of BcLF1, including nt 137467 to 137676 and nt 137467 to 137891 of B95.8 EBV, were cloned into the pGL2 reporter plasmid using primers LMRC1003/1002 and LMRC1004/1002.
TABLE 2.
List of the primers used in this study
| Primer function and name | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| Plasmid and bacmid construction | ||
| LMRC439/440 | CCGGGATCCATGGAAACCACTCAGACTCT | CGCGAATTCTTAAATGAGGGGGTTAAAGGC |
| LMRC718/719 | TCTGCTCTGGTACGTTGGCTTCTGCTGCTGCTTGTGATCTGTAGGCTGGAGCTGCTTC | CCGTACTGGCGGCCGCCTCTTCGGAGGCGTGGTTAAATAATTCCGGGGATCCGTCGACC |
| LMRC772 | TGGCTGTTCAGCTCGCGTCGGAGGCCAGGCAGAA | |
| LMRC912 | TGGTACCGAGCTCGGATCCATGTACCCATACGATGTTCCAGATTACGCTATGGAAACCACTCAGACTC | |
| LMRC955/956 | ATCGGTACCTTTCTGGTGCATTTGCGAGC | CTGAGATCTCTGGTGGACACATGATGTGT |
| LMRC1000/999 | TGGGTACCTTCGTACCCTTCTGGGCCGG | GCTCTAGAGGGAATTCCGGAATCTCAAC |
| LMRC1001/999 | TGGGTACCTCTCCGACGATCTCTAATGG | GCTCTAGAGGGAATTCCGGAATCTCAAC |
| LMRC1003/1002 | TGGGTACCTTCGGATAACGCCGAGTAGA | GCTCTAGAGACACAAGGTAAGAGGGAGATGG |
| LMRC1004/1002 | TGGGTACCGATGATAAGGCGAAACATTG | GCTCTAGAGACACAAGGTAAGAGGGAGATGG |
| RT-PCR and RT-qPCR | ||
| BcLF1 | GTTGAGGCTGTTTAGGGTATG | CAATCCCAAGTACACGACC |
| BLLF1 | TACTGGGGTGGGACTTGTT | GAGCGGGGGAGATTACTG |
| BLLF2 | TGACCAGCAGCAGCAGAAG | ATGTGTCCACCAGTTCGCC |
| BILF2 | ACTGGTGCTTGTGGTGTG | CTGTGTGGGGTCTGTTTGT |
| BSRF1 | GGCGGGCTAAACAGAACGA | AAGGCGGAGTTGATGAAAG |
| BaRF1 | TCCTTCTACAGCATAGCCCT | AGGTCATCTACCACCAGCAT |
| BORF2 | GGACACCCACCACACAGCA | TCAAACTCCTCCCCGTAGA |
| EBV genome copy number detection | ||
| BamHI W | CCCTGGTATAAAGTGGTCCT | AAGTCCACTTACCTCTGG |
| HBG | GCTTCTGACACAACTGTGTTCACTAGC | CACCAACTTCATCCACGTTCACC |
| ChIP-qPCR | ||
| BcLF1p | ATGAATGACTGCCAGGAGCT | GGATGATAAGGCGAAACATTG |
| BILF2p | ATTCACTCTAGCACCTGC | GCTGTTGTTGCAGAATATGA |
| BLLF1p | AATCCAGCTTCTCGTAGAGAT | AAAGGTCACGTGACACCTAC |
| BDLF3p | CGCGTCTGGACGCAGA | CGGAAGCAATGTCGTGTTG |
Construction of the BMRF1 knockout EBV bacmid and selection of doxycycline-inducible cells containing EBV bacmid DNA.
The BMRF1 knockout bacmid was constructed by PCR targeting, as described previously (22). In brief, the apramycin resistance cassette was amplified by PCR from pIJ773 using primers LMRC718/719. The apramycin gene products were electroporated into Escherichia coli DH10B containing the Maxi-EBV bacmid p2089 and red recombinase plasmid pKD46. The recombination of PCR products with a wild-type EBV genome resulted in an exchange between the BMRF1 gene region of nt 79899 to 808778 of EBV B95.8 and the apramycin resistance cassette. The p2089ΔBMRF1 bacmid was selected by apramycin and confirmed by BamHI fragmentation analysis. To select doxycycline-inducible EBV bacmid-positive cells, 293TetER or 293TetEZ cells were transfected with EBV p2089 wild-type or mutant bacmids as described previously (6). At 72 h posttransfection, transfected cells were split into two 10-cm culture dishes and selected with hygromycin B (100 μg/ml) for 1 month. Four to five green fluorescent protein (GFP)-positive cell colonies were picked to obtain pool clones. The selected cells containing EBV wild-type or mutant bacmids were treated with doxycycline (50 ng/ml) to confirm lytic cycle progression by Western blotting. Cell pools with similar viral DNA copy numbers were picked for further analysis.
Western blot analysis.
Western blotting was performed as described previously (60). The primary antibodies, including mouse anti-BMRF1 88A9, mouse anti-Zta 1B4, anti-Rta 467, anti-BGLF4 2616, anti-gp350/220 (72A1; ATCC), and anti-BcLF1 (viral capsid antigen [VCA]), were used to detect EBV lytic proteins (6). The mouse BALF2 (OT13B) antibody was a gift from Jaap M. Middeldorp (30). Other primary antibodies used included anti-HA antibody (HA.11; Covance), mouse anti-Flag antibody (M2; Sigma), anti-BRG1 (EPNCIR111A; Abcam), anti-SP1 (PEP2; Santa Cruz Biotechnology), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Biodesign), and anti-actin (Sigma-Aldrich).
EBV DNA microarray analysis.
Twenty micrograms of total RNA from 293TetEZ/p2089, 293TetEZ/p2089ΔBMRF1 , 293TetER/p2089, or 293TetER/p2089ΔBMRF1 cells was reverse transcribed and labeled with biotin-dUTP, as described previously (61). Hybridization of biotin-labeled cDNA with the duplicated EBV DNA microarray was performed according to a previously described protocol (25). In brief, the microarray membrane was prehybridized with salmon sperm DNA at 60°C for 90 min. After prehybridization, the microarray membrane was incubated with biotin-labeled cDNA at 63°C for 15 h. Following hybridization with a biotin-labeled cDNA probe, the microarray membrane was washed with 2× SSC washing buffer (0.3 M NaCl, 30 mM sodium citrate, 0.1% SDS), and the membrane was subjected to color development. The microarray chromogenic image was scanned and converted to digital output by ImageJ, version 1.48.
RT-PCR and RT-qPCR.
Cells were harvested for RNA purification using TRIzol reagent (Life technologies). Before reverse transcription, 10 μg of total RNA sample was pretreated with 10 U of DNase I (Life technologies). Subsequently, RNA was reverse transcribed into cDNA for viral gene detection by PCR or qPCR, as described previously (25). The primers used for detection of viral transcripts are listed Table 2.
Luciferase assay.
The promoter upstream sequences used in this study, including pBLLF1 (upstream bp 1000 to downstream bp 100 of the transcription start site of BLLF1 [−1000 bp to +100 bp], nt 92053 to 93153 of the B95.8 EBV genome), pBLLF2 (−1038 bp to +62 bp, nt 89951 to 91051 of EBV B95.8), pBcLF1 (−626 bp to +1 bp, nt 137467 to 138092 of B95.8 EBV), pBDLF3 (−180 bp to +1 bp, nt 131067 to 131246 of B95.8 EBV), and BILF2 (−1046 bp to +110 bp, nt 150415 to 151571 of B95.8 EBV) were cloned into the pGL-2 basic reporter plasmid. HEK293T cells were transfected with pBLLF1-Luc, pBLLF2-Luc, pBcLF1-Luc, pBDLF3-Luc, or pBILF2-Luc, the effector plasmids indicated on the figures, and a Renilla luciferase control reporter vector (pRL-null). At 48 h posttransfection, the cells were harvested and assayed for firefly and Renilla luciferase activities using a Dual-Glo Luciferase Assay system (Promega). Promoter activities were determined from firefly luciferase activities and normalized to Renilla luciferase activities. Relative fold activation indicates the ratio of reporter activity to that of vector-transfected cells.
Coimmunoprecipitation assay.
Approximately 1 × 107 cells were lysed with 1 ml of NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl, PH 8.0, 1× protease inhibitor) with gentle shaking at 4°C for 2 h. Cell debris was precipitated by centrifugation at 16,000 × g at 4°C for 10 min. The supernatant was precleaned with 10 μl of 10% protein A Mag Sepharose magnetic beads (GE Healthcare) at 4°C for 1 h. The precleaned lysate was then incubated with 2 μg of anti-HA or anti-BRG1 antibody for 12 h at 4°C, followed by incubation with 20 μl of 10% protein A Mag Sepharose beads at 4°C for 2 h. Immunocomplexes were collected and washed with NP-40 lysis buffer once and twice with cold phosphate-buffered saline (PBS) and then subjected to Western blot analysis.
Immunofluorescence assay.
An immunofluorescence assay was performed as described previously (6). Briefly, the cultured slides were fixed with 4% paraformaldehyde and incubated with anti-Flag (M2; Sigma-Aldrich), anti-HA (HA.11; Covance), or anti-BRG1 (EPNCIR111A; Abcam) at 4°C overnight. Following three washes with PBS, the slides were incubated with a fluorescein isothiocyanate (FITC)- or a rhodamine-conjugated secondary antibody at 37°C for 1 h. Finally, the slides were stained with 100 ng/ml Hoechst 33258 (Sigma-Aldrich) for 5 min at room temperature. The stained slides were covered with mounting medium (H1000; Vector Laboratories) and observed by fluorescence microscopy (Axioskop 40 FL; Zeiss) or confocal microscopy (LSM 510 META; Zeiss).
Genomic DNA extraction, isolation of EBV virions, and qPCR for EBV copy number detection.
The genomic DNA purification protocol was described previously (6). The EBV DNA from cell-free culture medium was purified by a QIAamp MinElute Virus Spin kit (Qiagen) according to the manufacturer's instructions. The EBV DNA copy number and human β-globin (HBG) were determined using BamHI W and HBG primers as shown in Table 2. For qPCR, EBV BamHI W DNA and HBG were quantified using a SensiFAST SYBR No-ROX kit (Bioline). The standard curve for qPCR was generated by a 10-fold serial dilution of a mixture of 104 copies of genomic DNA of 293TetER cells and 5 × 106 copies of purified EBV bacmid DNA.
Immunoprecipitation-spectrometry assay.
To immunoprecipitate BMRF1-associated complexes, 293TetEZ/p2089ΔBMRF1 cells were transfected with an HA-tagged BMRF1 (pMT8) and induced into the lytic cycle for 48 h. After lytic induction, the cells were lysed by NP-40-lysis buffer, and Flag-BMRF1 was precipitated by anti-Flag M2 antibody (Sigma-Aldrich). The BMRF1-containing complexes were captured by protein A-Sepharose beads (GE Healthcare), and the captured complexes were analyzed by SDS-PAGE and silver staining. The bands of interest were excised and digested with trypsin. Resulting peptide fragments were extracted from the gel for mass spectrometry in the Proteomics and Protein Function Core Laboratory of the Center of Genomic Medicine, National Taiwan University. The BMRF1-interacting proteins were identified via peptide mass fingerprint and by searching matches of all entries in the National Center for Biotechnology Information and Swiss-Prot databases.
shRNA lentivirus production.
Short hairpin RNA (shRNA) plasmids pLKO.1-SP1, pLKO.1-BRG1, and pLKO.1-Luc were obtained from the National RNAi Core Facility (Academia Sinica, Taiwan [http://rnai.genmed.sinica.edu.tw]). The shRNA target sequence for SP1 is CCAGGTGCAAACCAACAGATT (TRCN00000020447). The shRNA target sequences for BRG1 are CTTTGCGTATCGCGGCTTTAA (shRNA TRCN0000231101), CGGCAGACACTGTGATCATTT (TRCN0000015552), and CCCGTGGACTTCAAGAAGATA (TRCN0000015549). The pLKO.1-shLuc plasmid is an shRNA control. For lentiviral packaging, HEK293T packing cells were transfected with shRNA-pLKO.1, pCMV-dR8.91 (Delta 8.9), and pMD.G plasmids at a ratio of 10:9:1 by BBS transfection (55). The transfected cells were incubated at 30°C with 3% CO2. At 18 h posttransfection, cells were refed with fresh medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 1% bovine serum albumin (BSA). The culture supernatants containing lentiviruses were harvested at 36 h after transfection.
Chromatin immunoprecipitation-qPCR assay.
The chromatin immunoprecipitation protocol was performed as described previously (62). In brief, NA cells (1 × 107 cells) were transfected with or without the Rta-expressing plasmid for 36 h and then treated with 1% formaldehyde for 15 min at room temperature to cross-link DNA to protein. The cross-linking reaction was terminated by the addition of glycine to a final concentration of 0.125 M. Cross-linked cell lysates were harvested in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0, 1× protease inhibitor cocktail [Roche]), followed by shearing of the DNA into 300- to 500-bp fragments by sonication. The sonicated chromatin lysates were centrifuged at 13,200 rpm for 10 min at 4°C to remove cellular debris. The BMRF1- or BRG1-associated chromatin was immunoprecipitated by homemade mouse anti-BMRF1 antibody (88A9) or rabbit anti-BRG1 antibody (H-88; Santa Cruz Biotechnology). In addition, mouse or rabbit IgG was used as a negative control. The immunoprecipitated DNA was extracted, and the quantitative real-time PCR was performed using specific primers, as shown in Table 2.
ACKNOWLEDGMENTS
We thank H. J. Delecluse (German Cancer Research Centre [DKFZ]) for the EBV bacmid p2089, PCR template plasmid PIJ773, and λ Red recombination plasmid. We thank the Center of Genomic Medicine, National Taiwan University, for the mass spectrometry analysis. We thank the Cell Imaging Core of the First Core Labs in the National Taiwan University College of Medicine for confocal image analysis. We thank Tzu-Lung Lin (National Taiwan University) and Chia-Wei Wu (Oak Ridge National Laboratory) for the microarray analysis. We are grateful to Tim J. Harrison of the University College London for critical reading and editing of the manuscript.
This study was funded by the Ministry of Science and Technology, Taiwan (MOST) (NSC 101-2320-B-002-031-MY3 and MOST-104-2320-B-002-054-MY3) and partially supported by the National Health Research Institutes (NHRI) and National Taiwan University College of Medicine through grants NHRI-EX105-10201BI, 105C101-A2, and 106C101-F2. Mei-Tzu Su was supported by grants MOST-104-2811-B-002-115 and MOST-105-2811-B-002-085.
REFERENCES
- 1.Richard M, Longnecker EK, Jeffrey I. Cohen. 2013. Epstein-Barr virus, p 1898–1959. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Rancaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Kempkes B, Robertson ES. 2015. Epstein-Barr virus latency: current and future perspectives. Curr Opin Virol 14:138–144. doi: 10.1016/j.coviro.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie J, El-Guindy A. 2015. Epstein-Barr virus lytic cycle reactivation. Curr Top Microbiol Immunol 391:237–261. doi: 10.1007/978-3-319-22834-1_8. [DOI] [PubMed] [Google Scholar]
- 4.Fixman ED, Hayward GS, Hayward SD. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J Virol 69:2998–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Guindy A, Ghiassi-Nejad M, Golden S, Delecluse HJ, Miller G. 2013. Essential role of Rta in lytic DNA replication of Epstein-Barr virus. J Virol 87:208–223. doi: 10.1128/JVI.01995-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su MT, Liu IH, Wu CW, Chang SM, Tsai CH, Yang PW, Chuang YC, Lee CP, Chen MR. 2014. Uracil DNA glycosylase BKRF3 contributes to Epstein-Barr virus DNA replication through physical interactions with proteins in viral DNA replication complex. J Virol 88:8883–8899. doi: 10.1128/JVI.00950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fixman ED, Hayward GS, Hayward SD. 1992. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol 66:5030–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennekamp AJ, Lieberman PM. 2011. Initiation of Epstein-Barr virus lytic replication requires transcription and the formation of a stable RNA-DNA hybrid molecule at oriLyt. J Virol 85:2837–2850. doi: 10.1128/JVI.02175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhierl B, Delecluse HJ. 2006. The Epstein-Barr virus BMRF1 gene is essential for lytic virus replication. J Virol 80:5078–5081. doi: 10.1128/JVI.80.10.5078-5081.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Holley-Guthrie E, Ge JQ, Dorsky D, Kenney S. 1997. The Epstein-Barr virus (EBV) DNA polymerase accessory protein, BMRF1, activates the essential downstream component of the EBV oriLyt. Virology 230:22–34. doi: 10.1006/viro.1997.8470. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Hong Y, Dorsky D, Holley-Guthrie E, Zalani S, Elshiekh NA, Kiehl A, Le T, Kenney S. 1996. Functional and physical interactions between the Epstein-Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J Virol 70:5131–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsurumi T, Kobayashi A, Tamai K, Daikoku T, Kurachi R, Nishiyama Y. 1993. Functional expression and characterization of the Epstein-Barr virus DNA polymerase catalytic subunit. J Virol 67:4651–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama S, Murata T, Murayama K, Yasui Y, Sato Y, Kudoh A, Iwahori S, Isomura H, Kanda T, Tsurumi T. 2009. Epstein-Barr virus polymerase processivity factor enhances BALF2 promoter transcription as a coactivator for the BZLF1 immediate-early protein. J Biol Chem 284:21557–21568. doi: 10.1074/jbc.M109.015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsurumi T, Daikoku T, Kurachi R, Nishiyama Y. 1993. Functional interaction between Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit in vitro. J Virol 67:7648–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsurumi T. 1993. Purification and characterization of the DNA-binding activity of the Epstein-Barr virus DNA polymerase accessory protein BMRF1 gene products, as expressed in insect cells by using the baculovirus system. J Virol 67:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Holley-Guthrie E, Dorsky D, Kenney S. 1999. Identification of transactivator and nuclear localization domains in the Epstein-Barr virus DNA polymerase accessory protein, BMRF1. J Gen Virol 80:69–74. doi: 10.1099/0022-1317-80-1-69. [DOI] [PubMed] [Google Scholar]
- 17.Kiehl A, Dorsky DI. 1995. Bipartite DNA-binding region of the Epstein-Barr virus BMRF1 product essential for DNA polymerase accessory function. J Virol 69:1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LW, Lin LS, Chang YS, Liu ST. 1995. Functional analysis of EA-D of Epstein-Barr virus. Virology 211:593–597. doi: 10.1006/viro.1995.1443. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman PM. 2006. Chromatin regulation of virus infection. Trends Microbiol 14:132–140. doi: 10.1016/j.tim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. 2003. Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol 77:11425–11435. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwack Y, Baek HJ, Nakamura H, Lee SH, Meisterernst M, Roeder RG, Jung JU. 2003. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi's sarcoma-associated herpesvirus RTA-mediated lytic reactivation. Mol Cell Biol 23:2055–2067. doi: 10.1128/MCB.23.6.2055-2067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci U S A 95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YL, Chen YJ, Tsai WH, Ko YC, Chen JY, Lin SF. 2009. The Epstein-Barr virus replication and transcription activator, Rta/BRLF1, induces cellular senescence in epithelial cells. Cell Cycle 8:58–65. doi: 10.4161/cc.8.1.7411. [DOI] [PubMed] [Google Scholar]
- 25.Lu CC, Jeng YY, Tsai CH, Liu MY, Yeh SW, Hsu TY, Chen MR. 2006. Genome-wide transcription program and expression of the Rta responsive gene of Epstein-Barr virus. Virology 345:358–372. doi: 10.1016/j.virol.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 26.Chua HH, Lee HH, Chang SS, Lu CC, Yeh TH, Hsu TY, Cheng TH, Cheng JT, Chen MR, Tsai CH. 2007. Role of the TSG101 gene in Epstein-Barr virus late gene transcription. J Virol 81:2459–2471. doi: 10.1128/JVI.02289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rickinson AB, Epstein MA. 1978. Sensitivity of the transforming and replicative functions of Epstein–Barr virus to inhibition by phosphonoacetate. J Gen Virol 40:409–420. doi: 10.1099/0022-1317-40-2-409. [DOI] [PubMed] [Google Scholar]
- 28.Summers WC, Klein G. 1976. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol 18:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holley-Guthrie EA, Seaman WT, Bhende P, Merchant JL, Kenney SC. 2005. The Epstein-Barr virus protein BMRF1 activates gastrin transcription. J Virol 79:745–755. doi: 10.1128/JVI.79.2.745-755.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y, Middeldorp J, Madjar JJ, Ooka T. 1997. A major DNA binding protein encoded by BALF2 open reading frame of Epstein-Barr virus (EBV) forms a complex with other EBV DNA-binding proteins: DNAase, EA-D, and DNA polymerase. Virology 239:285–295. doi: 10.1006/viro.1997.8891. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Wang X, Cheng C, Cai J, He S, Wang H, Liu F, Zhu C, Ding Z, Huang X, Zhang T, Zhang Y. 2014. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. APMIS 122:1070–1079. doi: 10.1111/apm.12258. [DOI] [PubMed] [Google Scholar]
- 32.Wu DY, Kalpana GV, Goff SP, Schubach WH. 1996. Epstein-Barr virus nuclear protein 2 (EBNA2) binds to a component of the human SNF-SWI complex, hSNF5/Ini1. J Virol 70:6020–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson BG, Roberts CW. 2011. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 34.Gruffat H, Marchione R, Manet E. 2016. Herpesvirus late gene expression: a viral-specific pre-initiation complex is key. Front Microbiol 7:869. doi: 10.3389/fmicb.2016.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong-Ho E, Wu TT, Davis ZH, Zhang B, Huang J, Gong H, Deng H, Liu F, Glaunsinger B, Sun R. 2014. Unconventional sequence requirement for viral late gene core promoters of murine gammaherpesvirus 68. J Virol 88:3411–3422. doi: 10.1128/JVI.01374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong D, Wu NC, Xie Y, Feng J, Tong L, Brulois KF, Luan H, Du Y, Jung JU, Wang CY, Kang MK, Park NH, Sun R, Wu TT. 2014. Kaposi's sarcoma-associated herpesvirus ORF18 and ORF30 are essential for late gene expression during lytic replication. J Virol 88:11369–11382. doi: 10.1128/JVI.00793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djavadian R, Chiu YF, Johannsen E. 2016. An Epstein-Barr virus-encoded protein complex requires an origin of lytic replication in cis to mediate late gene transcription. PLoS Pathog 12:e1005718. doi: 10.1371/journal.ppat.1005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aubry V, Mure F, Mariame B, Deschamps T, Wyrwicz LS, Manet E, Gruffat H. 2014. Epstein-Barr virus late gene transcription depends on the assembly of a virus-specific preinitiation complex. J Virol 88:12825–12838. doi: 10.1128/JVI.02139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruffat H, Kadjouf F, Mariame B, Manet E. 2012. The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression. J Virol 86:6023–6032. doi: 10.1128/JVI.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isomura H, Stinski MF, Murata T, Yamashita Y, Kanda T, Toyokuni S, Tsurumi T. 2011. The human cytomegalovirus gene products essential for late viral gene expression assemble into prereplication complexes before viral DNA replication. J Virol 85:6629–6644. doi: 10.1128/JVI.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serio TR, Kolman JL, Miller G. 1997. Late gene expression from the Epstein-Barr virus BcLF1 and BFRF3 promoters does not require DNA replication in cis. J Virol 71:8726–8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse HJ. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J 19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang L, Nogales E, Ciferri C. 2010. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol 102:122–128. doi: 10.1016/j.pbiomolbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudarsanam P, Winston F. 2000. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet 16:345–351. doi: 10.1016/S0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- 45.Trotter KW, Archer TK. 2008. The BRG1 transcriptional coregulator. Nucl Recept Signal 6:e004. doi: 10.1621/nrs.06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Fang H, Zhou J, Herring BP. 2007. A novel role of Brg1 in the regulation of SRF/MRTFA-dependent smooth muscle-specific gene expression. J Biol Chem 282:25708–25716. doi: 10.1074/jbc.M701925200. [DOI] [PubMed] [Google Scholar]
- 47.Ross S, Cheung E, Petrakis TG, Howell M, Kraus WL, Hill CS. 2006. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J 25:4490–4502. doi: 10.1038/sj.emboj.7601332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Serna IL, Ohkawa Y, Berkes CA, Bergstrom DA, Dacwag CS, Tapscott SJ, Imbalzano AN. 2005. MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol Cell Biol 25:3997–4009. doi: 10.1128/MCB.25.10.3997-4009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Cheng MB, Zhang YJ, Zhong X, Dai H, Yan L, Wu NH, Zhang Y, Shen YF. 2010. A switch from hBrm to Brg1 at IFNγ-activated sequences mediates the activation of human genes. Cell Res 20:1345–1360. doi: 10.1038/cr.2010.155. [DOI] [PubMed] [Google Scholar]
- 50.Laurette P, Strub T, Koludrovic D, Keime C, Le Gras S, Seberg H, Van Otterloo E, Imrichova H, Siddaway R, Aerts S, Cornell RA, Mengus G, Davidson I. 2015. Transcription factor MITF and remodeller BRG1 define chromatin organisation at regulatory elements in melanoma cells. eLife 4:e06857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denslow SA, Wade PA. 2007. The human Mi-2/NuRD complex and gene regulation. Oncogene 26:5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- 52.Terhune SS, Moorman NJ, Cristea IM, Savaryn JP, Cuevas-Bennett C, Rout MP, Chait BT, Shenk T. 2010. Human cytomegalovirus UL29/28 protein interacts with components of the NuRD complex which promote accumulation of immediate-early RNA. PLoS Pathog 6:e1000965. doi: 10.1371/journal.ppat.1000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 54.Chang Y, Tung CH, Huang YT, Lu J, Chen JY, Tsai CH. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J Virol 73:8857–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Okayama H. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752. doi: 10.1128/MCB.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang PW, Chang SS, Tsai CH, Chao YH, Chen MR. 2008. Effect of phosphorylation on the transactivation activity of Epstein-Barr virus BMRF1, a major target of the viral BGLF4 kinase. J Gen Virol 89:884–895. doi: 10.1099/vir.0.83546-0. [DOI] [PubMed] [Google Scholar]
- 57.Makarova O, Kamberov E, Margolis B. 2000. Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques 29:970–972. [DOI] [PubMed] [Google Scholar]
- 58.Ragoczy T, Heston L, Miller G. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol 72:7978–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Z, Krithivas A, Finan JE, Semmes OJ, Zhou S, Wang Y, Hayward SD. 1998. The Epstein-Barr virus lytic transactivator Zta interacts with the helicase-primase replication proteins. J Virol 72:8559–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen MR, Chang SJ, Huang H, Chen JY. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol 74:3093–3104. doi: 10.1128/JVI.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JJ, Wu R, Yang PC, Huang JY, Sher YP, Han MH, Kao WC, Lee PJ, Chiu TF, Chang F, Chu YW, Wu CW, Peck K. 1998. Profiling expression patterns and isolating differentially expressed genes by cDNA microarray system with colorimetry detection. Genomics 51:313–324. doi: 10.1006/geno.1998.5354. [DOI] [PubMed] [Google Scholar]
- 62.Truax AD, Greer SF. 2012. ChIP and re-ChIP assays: investigating interactions between regulatory proteins, histone modifications, and the DNA sequences to which they bind. Methods Mol Biol 809:175–188. doi: 10.1007/978-1-61779-376-9_12. [DOI] [PubMed] [Google Scholar]