ABSTRACT
We compared the HIV-1-specific immune responses generated by targeting HIV-1 envelope protein (Env gp140) to either CD40 or LOX-1, two endocytic receptors on dendritic cells (DCs), in rhesus macaques primed with a poxvirus vector (NYVAC-KC) expressing Env gp140. The DC-targeting vaccines, humanized recombinant monoclonal antibodies fused to Env gp140, were administered as a boost with poly-ICLC adjuvant either alone or coadministered with the NYVAC-KC vector. All the DC-targeting vaccine administrations with poly-ICLC increased the low-level serum anti-Env IgG responses elicited by NYVAC-KC priming significantly more (up to a P value of 0.01) than in a group without poly-ICLC. The responses were robust and cross-reactive and contained antibodies specific to multiple epitopes within gp140, including the C1, C2, V1, V2, and V3, C4, C5, and gp41 immunodominant regions. The DC-targeting vaccines also elicited modest serum Env-specific IgA responses. All groups gave serum neutralization activity limited to tier 1 viruses and antibody-dependent cytotoxicity responses (ADCC) after DC-targeting boosts. Furthermore, CD4+ and CD8+ T cell responses specific to multiple Env epitopes were strongly boosted by the DC-targeting vaccines plus poly-ICLC. Together, these results indicate that prime-boost immunization via NYVAC-KC and either anti-CD40.Env gp140/poly-ICLC or anti-LOX-1.Env gp140/poly-ICLC induced balanced antibody and T cell responses against HIV-1 Env. Coadministration of NYVAC-KC with the DC-targeting vaccines increased T cell responses but had minimal effects on antibody responses except for suppressing serum IgA responses. Overall, targeting Env to CD40 gave more robust T cell and serum antibody responses with broader epitope representation and greater durability than with LOX-1.
IMPORTANCE An effective vaccine to prevent HIV-1 infection does not yet exist. An approach to elicit strong protective antibody development is to direct virus protein antigens specifically to dendritic cells, which are now known to be the key cell type for controlling immunity. In this study, we have tested in nonhuman primates two prototype vaccines engineered to direct the HIV-1 coat protein Env to dendritic cells. These vaccines bind to either CD40 or LOX-1, two dendritic cell surface receptors with different functions and tissue distributions. We tested the vaccines described above in combination with attenuated virus vectors that express Env. Both vaccines, but especially that delivered via CD40, raised robust immunity against HIV-1 as measured by monitoring potentially protective antibody and T cell responses in the blood. The safety and efficacy of the CD40-targeted vaccine justify further development for future human clinical trials.
KEYWORDS: HIV-1, dendritic cells, vaccines
INTRODUCTION
While antiretroviral therapies have reduced morbidity and mortality from HIV-1 infection, there remains an urgent need for a vaccine to prevent infection. Current prophylactic HIV-1 vaccine development efforts are focused on optimizing prime-boost strategies, combinations of DNA/viral vectors with protein formulation, and investigating vaccination regimens for optimal long-lasting protective antibody and cytotoxic T cell responses. One approach to increasing protein antigen efficacy is direct delivery to endocytic receptors on dendritic cell (DC) surfaces, the quintessential cell type for initiating and regulating immune responses (1). Antibody responses directed to HIV-1 envelope protein (Env) are key to protective humoral responses. In a prior study (2), we investigated the efficacy in nonhuman primates (NHPs) of a humanized anti-human LOX-1 antibody fused to Env gp140 for eliciting cellular and humoral immunity against gp140 in both prime and boost settings. Based on in vitro and in vivo studies of targeting antigens to LOX-1 on antigen-presenting cells (APCs), targeting Env to LOX-1 was predicted to favor humoral immunity (3). However, it is not yet clear which DC surface receptors are the best targets for particular protective immune responses (4–6). CD40, a potently activating tumor necrosis factor (TNF) family receptor broadly expressed on APCs, has been investigated as a preferred target for eliciting cytotoxic T cell responses (7–12), but it has not been well studied for evoking humoral responses. CD40 signaling is essential for T cell-dependent humoral responses (reviewed in reference 13) and therefore seems an attractive option for concomitant delivery of antigen and activation signals to promote antibody development. In this study, we directly compare the efficacy of targeting HIV-1 Env gp140 to CD40 versus LOX-1 in combination with replication-competent vaccinia virus vector NYVAC-KC encoding Env gp140. Specifically, this study aimed to (i) establish the safety and immunogenicity of NYVAC-KC prime followed by boost via DC targeting to either LOX-1 or CD40 formulated with poly-ICLC adjuvant and either administered alone or coadministered with NYVAC-KC, (ii) compare the boosting ability of DC targeting via either LOX-1 or CD40 formulated with poly-ICLC adjuvant either administered alone or coadministered with NYVAC-KC, and (iii) test the boosting ability of DC targeting via CD40 either coadministered with poly-ICLC or administered alone.
RESULTS
HIV-1 Env gp140 targeted to either LOX-1 or CD40 elicits Env-specific B cell responses in NHPs primed with a live virus vector.
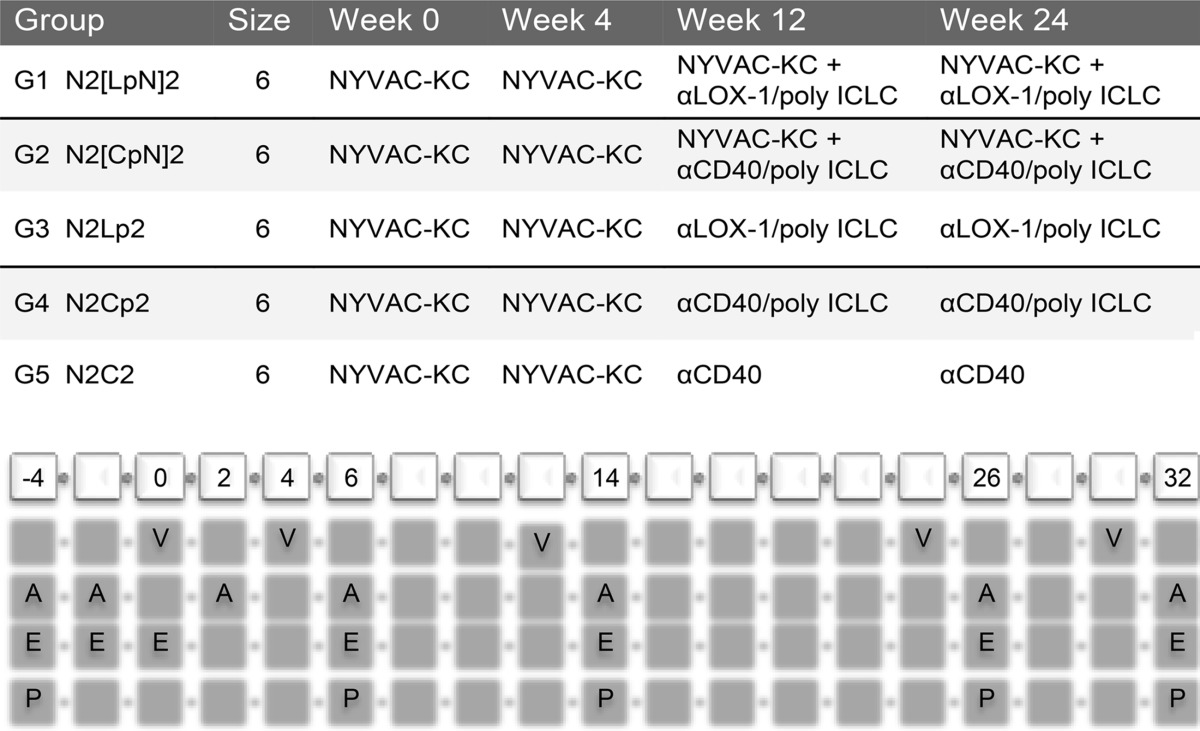
We previously described an anti-human LOX-1 recombinant human IgG4 antibody fused via a flexible linker to the heavy (H)-chain C terminus to codon optimized Env gp140 protein from the clade C HIV-1 96ZM651 (called ZM96) strain (anti-LOX-1.Env gp140 [2]). A similar fusion protein (anti-CD40.Env gp140) was generated linking gp140 to a previously described (A. L. Flamar, S. Zurawski, C. Lacabaratz, M. Montes, H. Bonnabau, L. Rickert, A. Wiedemann, L. Galmin, D. Weiss, A. Cristillo, L. Hudacik, A. Salazar, C. Peltekian, R. Thiebaut, G. Zurawski, and Y. Levy, submitted for publication) humanized anti-human CD40 recombinant human IgG4 antibody. Anti-CD40.Env gp140 retained specific binding to human and rhesus macaque CD40 (see Materials and Methods). In our previous study (2), anti-LOX-1.Env gp140 administered intradermally (i.d.) with Toll-like receptor 3 (TLR3) ligand poly-ICLC given nearby subcutaneously (s.c.) efficiently boosted both humoral and cellular responses in rhesus macaques primed with two intramuscular (i.m.) injections of replication-competent NYVAC-KC vaccinia virus vectors encoding HIV-1 Gag, Nef, Pol, and Env gp140 sequences. To this protocol we added other NHP groups to test the relative efficacy of targeting Env gp140 via CD40 versus LOX-1, to investigate potential benefits of coadministering NYVAC-KC viruses coding for Env gp140 and Gag, Nef, and Pol with the DC-targeting vaccinations, and to establish the potency of CD40 targeting in the absence of TLR3 stimulation. Table 1 shows the overall study design and tissue sampling schedule.
TABLE 1.
Study design for testing antigenicity of αLOX-1.Env gp140 and αCD40.Env gp140 fusion proteins in NHPsa
Shown are the immunization regimens used in this study. The reference codes for the five vaccination groups are as follows: N, NYVAC-KC; L, anti-LOX-1.Env gp140; p, poly-ICLC; 2, administered twice in sequence. NYVAC-KC administration was intramuscular (i.m.), DC-targeting protein administration was intradermal (i.d.), and poly-ICLC administration was subcutaneous (s.c.) in proximity to the protein. The cells at the bottom are in biweekly increments starting at week −4 through week 32. V, vaccination dates; A, serum collection for antibody response determination; E, blood collection for IFN-γ ELISPOT measurements; P, frozen PBMC preparation for antigen-specific T cell analysis via flow cytometry including intracellular cytokine staining.
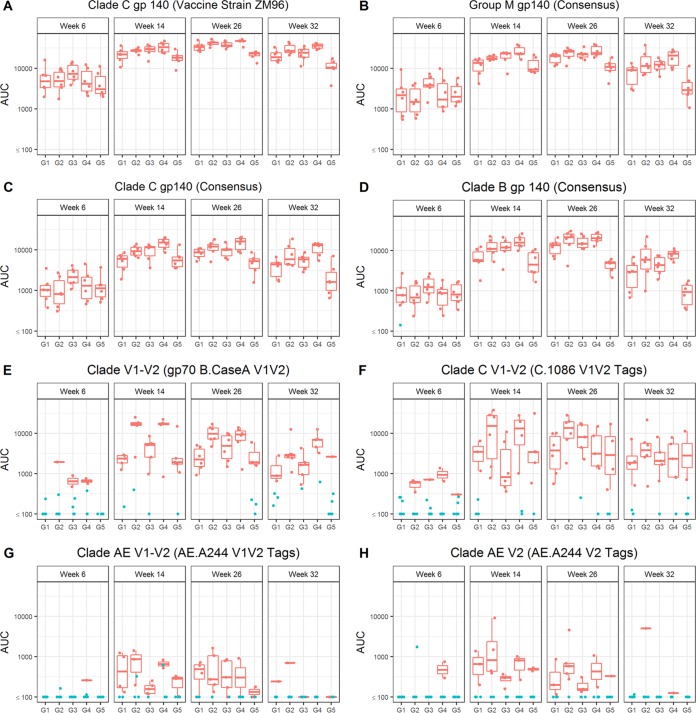
In this study, Env-specific serum IgG responses were observed in all animals at all 3 post-DC-targeting vaccination time points, including high titers against the vaccine strain ZM96 gp140 (Fig. 1A) as well as group M consensus (Con S gp140 CF [Fig. 1B]), clade C (C.con.env03 140 CF [Fig. 1C]), and clade B (B.con.env03 140 CF [Fig. 1D]) antigens. Titers in response to the two NYVAC-KC vaccinations (week 6) were modest but were boosted in all groups to almost maximal levels after one DC-targeting vaccine administration. These responses were maximal after the second DC-targeting vaccine administration (week 26, the primary immunogenicity endpoint) and waned somewhat 8 weeks after the final vaccinations (Fig. 1). IgG binding antibody responses to V1V2 region antigens (i.e., a response that correlated with decreased risk of HIV-1 infection in the RV144 human clinical trial; see references 14, 15, and 16) were observed in only a few NHPs at the early time point but were boosted or evoked to almost maximal levels by a single LOX-1 or CD40-targeting vaccination (Fig. 1E to H). Notably, IgG responses to gp70 B.CaseA V1V2 were significantly higher (P < 0.01 for week 26 and P = 0.02 for week 32) when NYVAC-KC was coadministered with CD40 but not LOX-1 targeting despite similar overall binding antibody levels between the paired groups (see G1 and G2 in Fig. 1 and Table 2). We next confirmed that the V1V2 response in this study was in part due to recognition of the V2 sequence (and not just C1-V1) contained within these antigens by demonstrating that all groups elicited responses to an antigen designed to only expose V2 region conformational and linear epitopes (V2 tags) (Fig. 1 and Table 2). In all cases, after the DC-targeting vaccinations, G4 N2Cp2 values were significantly higher than G5 N2C2 values for all gp140 antigens and the gp70 B.CaseA V1V2 antigen (P < 0.01 at week 32), indicating a significant benefit of coadministering poly-ICLC with the CD40-targeting vaccine (Table 2).
FIG 1.
Serum Env gp140-specific IgG responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. Total binding IgG antibody levels against clade C gp140 (vaccine strain ZM96) (A), group M gp140 (consensus) (B), clade C gp140 (consensus) (C), clade B gp140 (consensus) (D), clade B V1-V2 Env (gp70 B.CaseA V1V2) (E), clade C V1-V2 (C.1086C V1V2 tags) (F), clade AE V1-V2 (AE.A244 V1V2 tags) (G), and clade AE V2 (AE.A244 V2 tags) (H) induced by the different vaccination groups are shown. Individual serum samples were obtained at weeks 6, 14, 26, and 32 from each macaque (n = 6 per group) immunized as per G1 N2[LpN]2, G2 N2[CpN]2, G3 N2Lp2, G4 N2Cp2, and G5 N2C2 (Table 1). Binding antibodies were measured as indicated in Materials and Methods. The magnitudes of the antibody responses are expressed as the area under the curve (AUC) from serial dilutions of plasma. Each dot represents the value for one individual macaque. Weeks of sampling are indicated above each frame, and groups are defined below each frame. Blue points indicate nonresponders and red points responders. The midline of the box indicates the median, and the ends of the box indicate the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1.5 times the interquartile range (i.e., the height of the box) or if no value meets this criterion, to the data extremes. Table 2 shows statistical analysis of paired comparisons of serum IgG responses against various Env antigens, including those shown in this figure.
TABLE 2.
Comparison between paired groups for plasma IgG binding magnitudesa
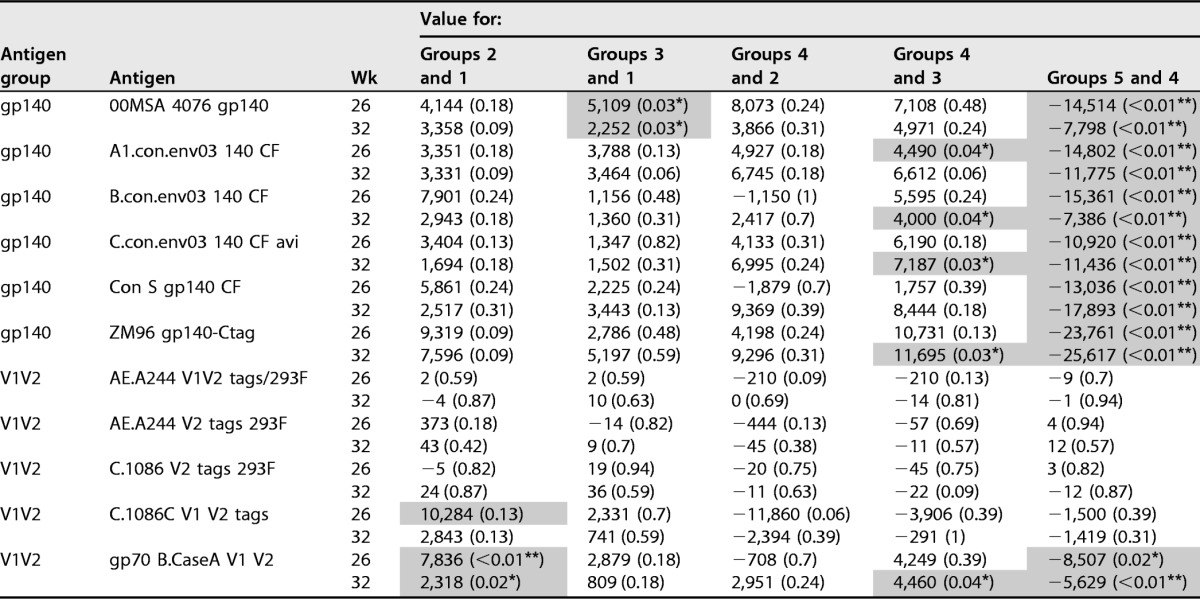
Test results shown are median differences between groups followed by P values in parentheses. P values are from Wilcoxon rank sum test. Values for comparison are AUC values as measured in binding antibody multiplex assay (BAMA) (see Materials and Methods) and shown in Fig. 1. Groups are G1 N2[LpN]2, G2 N2[CpN]2, G3 N2Lp2, G4 N2Cp2, and G5 N2C2 (Table 1). Significant differences are shaded and indicated by asterisks as follows: *, P < 0.05, and **, P < 0.01.
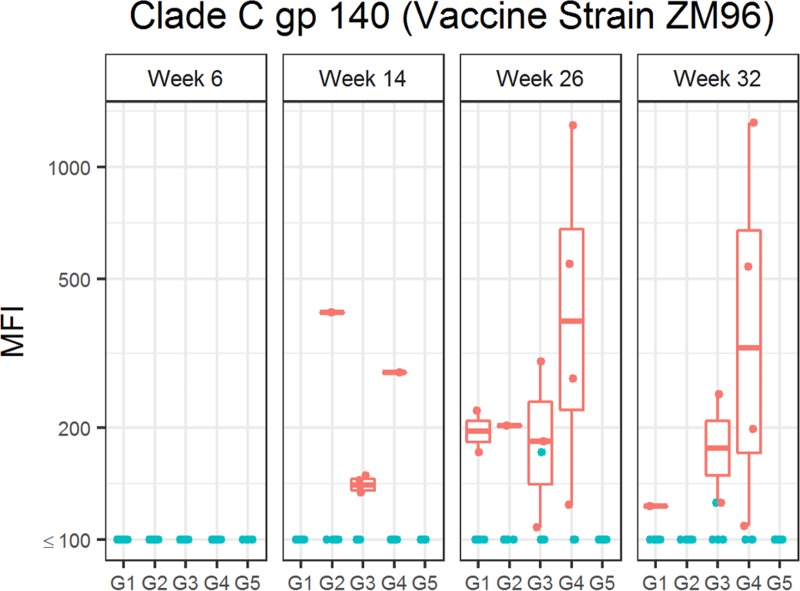
Low levels of serum IgA specific to Env gp140 were detected in a few animals after the NYVAC-KC vaccinations, but the levels were boosted most in G4 N2Cp2 (Fig. 2).
FIG 2.
Serum Env gp140-specific IgA responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. Total binding IgA antibody levels against clade C gp140 (vaccine strain ZM96) induced by the different vaccination groups are shown. Individual serum samples were obtained at weeks 6, 14, 26, and 32 from each macaque (n = 6 per group) immunized as per G1 N2[LpN]2, G2 N2[CpN]2, G3 N2Lp2, G4 N2Cp2, and G5 N2C2 (Table 1). Binding antibodies were measured as indicated in Materials and Methods. Each panel shows anti-Env IgA binding units (MFI) of individual animals. Plotting details are as described for Fig. 1, except that IgA levels were log10 transformed.
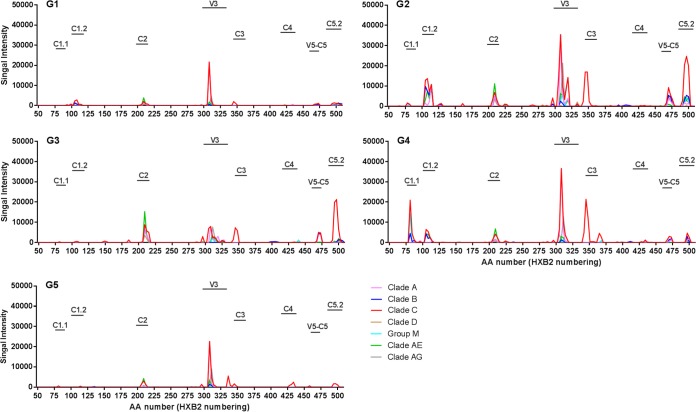
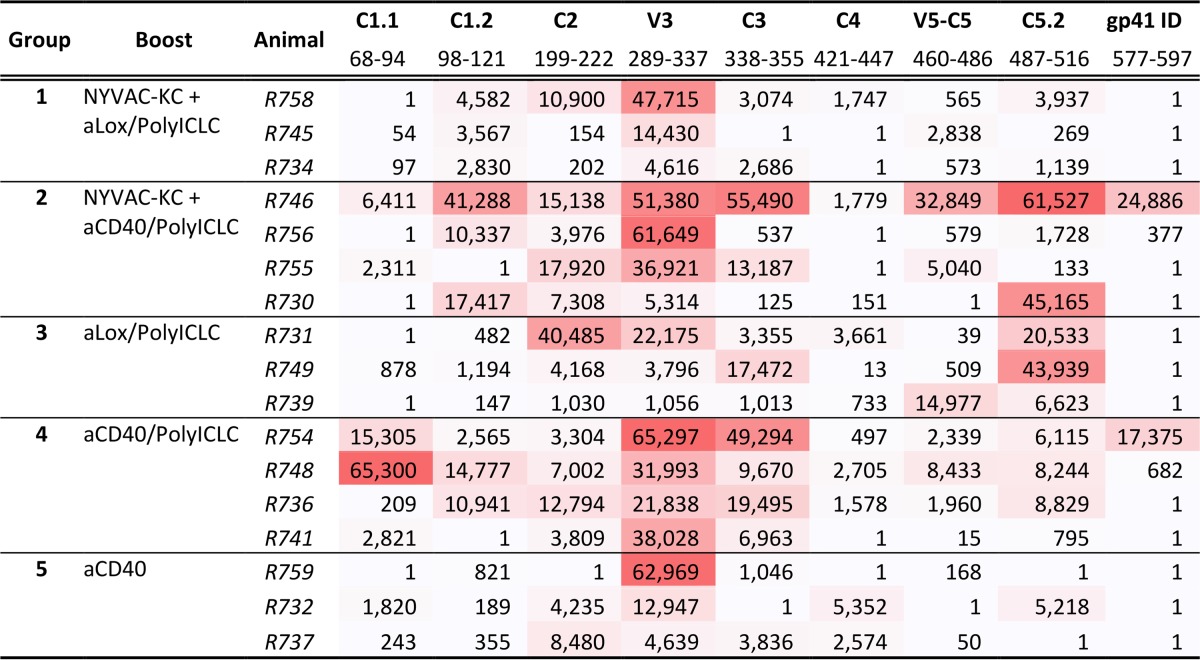
The breadth and magnitude of binding antibodies to linear epitopes were evaluated by peptide microarray mapping. Overall, the animals developed binding antibodies against gp120 linear epitopes C1, C2, V3, C3, C4, and C5, with V3 dominating the responses (Fig. 3). Linear binding responses were overall cross-clade responses, with a preference for clade C sequences. The C5.2 response was focused on clade C and the C3 response was restricted to C.ZM651, while the V3 and C1.2 binding was broader across clades (Table 3). The magnitude of binding trended higher in groups G2 N2[CpN]2 and G4 N2Cp2 than in groups G1 N2[LpN]2, G3 N2Lp2, and G5 N2C25 (Table 3).
FIG 3.
Env gp120 epitope-specific IgG responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. A gp160 peptide array was probed with week −4 (for baseline) and week 26 (at peak response) plasma from 17 animals representing all 5 immunization groups, selected based on higher binding (per group) to ZM96 gp140 and/or 1086 V1V2 tags. Three animals each were tested from groups 1, 3, and 5, and four animals each were tested from group 2 and 4. Mean binding values for each gp120 peptide are shown for group 1 to 5 (G1 to G5). Different colors of lines represent different clades. Data from vaccine strains for clade B (MN), clade C (TV1, 1086C, and ZM651), and clade AE (A244 and TH023) are combined with the consensus of each to represent clades B, C, and AE, respectively. Peptide positions are indicated on the x axis as the amino acid (aa) numbers, based on HXB2 numbering, of the center amino acids of the overlapping peptides (15-mer overlapping by 12). Epitope regions identified in the study are labeled over the horizontal bars. Summary data for each epitope shown here are presented in Table 3.
TABLE 3.
Binding magnitude to linear epitopes by plasma IgG from animals in groups 1 to 5 at the peak response time point (week 26)a
Epitope regions for gp120 are as shown graphically in Fig. 3 and here include data for the gp41 immunodominant (ID) region. Magnitude shown here are maximum binding per epitope, which is calculated as the highest signal intensity to a single peptide within a given epitope region. Shown below each epitope name is the location of each epitope, defined as the HXB2 amino acid number of the first amino acid of the first peptide in the epitope to the last amino acid of the last overlapping peptide in the epitope. Data are shaded with higher values in darker red. The rank order in higher values was G4 N2Cp2, G2 N2[CpN]2, G3 N2Lp2, and G1 N2[LpN]2/G5 N2C2.
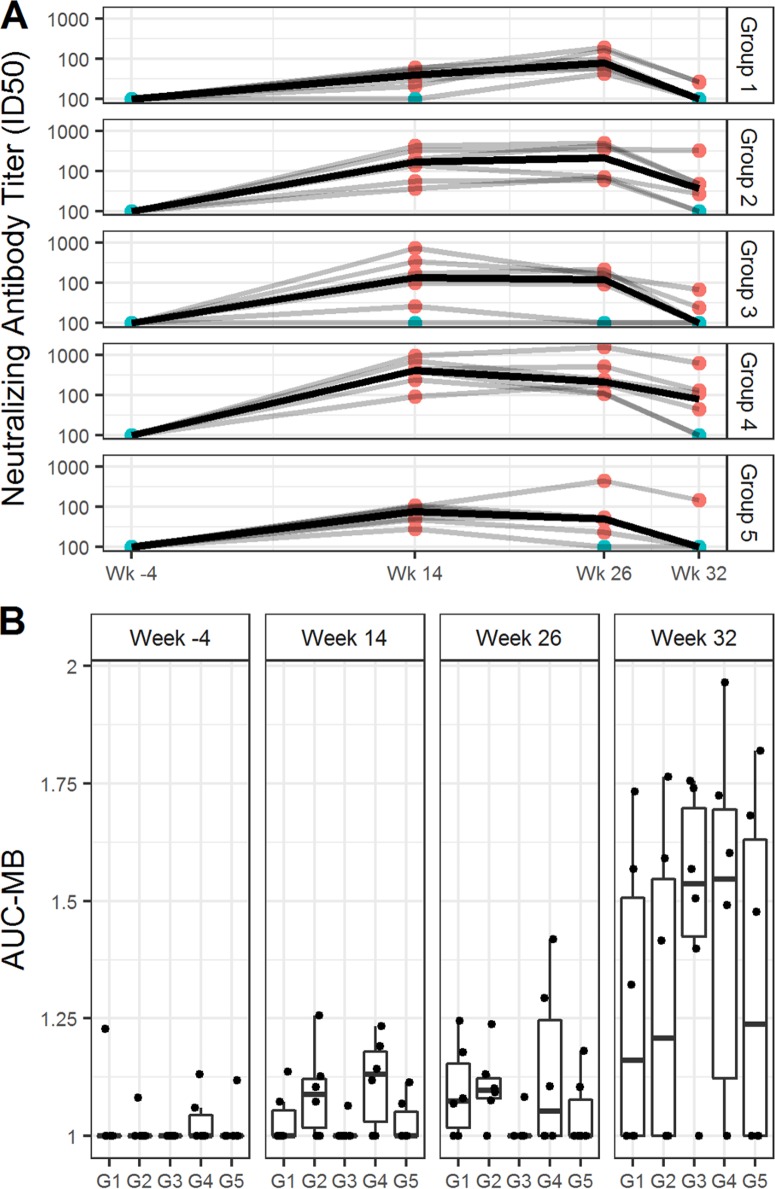
Analysis of neutralizing activity in week 14, week 26, and week 32 plasma samples was performed via TZM.bl and A3R5 assays against a panel of tier 1 and tier 2 HIV-1 Env-pseudotyped viruses. Neutralizing activity was detected against MW965.26 (clade C, tier 1A [Fig. 4A]) and TH023.6 (CRF01_AE, tier 1A [data not shown]). Little or no neutralization against Ce1086_B2, Ce1176_A3, C32010_F5, and DU151.2 viruses was detected (data not shown). Also, no neutralization was detected against 96ZM651.2, Bal.26, CE1086_B2, MN.3, SF162.LS, TV1.21 or murine leukemia virus (MLV; the control for nonspecific activity) (data not shown). Titers of neutralizing antibody (NAb) against the sensitive viruses (MW965.26 and TH023.6) decreased somewhat from week 26 to week 32 in all groups. Group 4 N2Cp2 had significantly higher 50% inhibitory dose (ID50) titers against MW965.26 than groups G1 N2(LpN)2 and G5 (N2C2) at weeks 14 and 26 (Table 4). At week 32, 8 weeks after completion of all vaccinations, G3 N2Lp2 and G4 N2Cp2 had the highest neutralizing scores based on individual-specific and group-specific magnitude-breadth (MB) analysis, which measures the overall magnitude and breadth of NAb activities for each individual time point (Fig. 4B).
FIG 4.
Serum neutralizing antibody responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. (A) Neutralizing antibodies against HIV-1 were measured as a function of reduction in Tat-regulated reporter gene expression in TZM-bl cells infected with HIV-1 isolate MW965. Neutralizing antibody titer values are ID50s. Samples were tested from sera collected at weeks −4, 14, 26, and 32. Groups are indicated beside the graph. Each dot represents the value for one individual macaque, responders in red, nonresponders in blue. (B) The graph shows the area under MB curves (see Materials and Methods) used to summarize overall breadth and magnitude of neutralizing antibody activities against A3R5 virus for each individual time point. Samples were tested from sera collected at weeks −4, 14, 26, and 32. Groups are indicated below the graph. The midline of the box indicates the median, and the ends of the box indicate the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1.5 times the interquartile range (i.e., the height of the box) or if no value meets this criterion, to the data extremes. Table 4 shows analysis of paired comparisons of ID50 values for isolates MW965 and Th023.6.
TABLE 4.
Comparison between paired groups for serum neutralizing responsesa
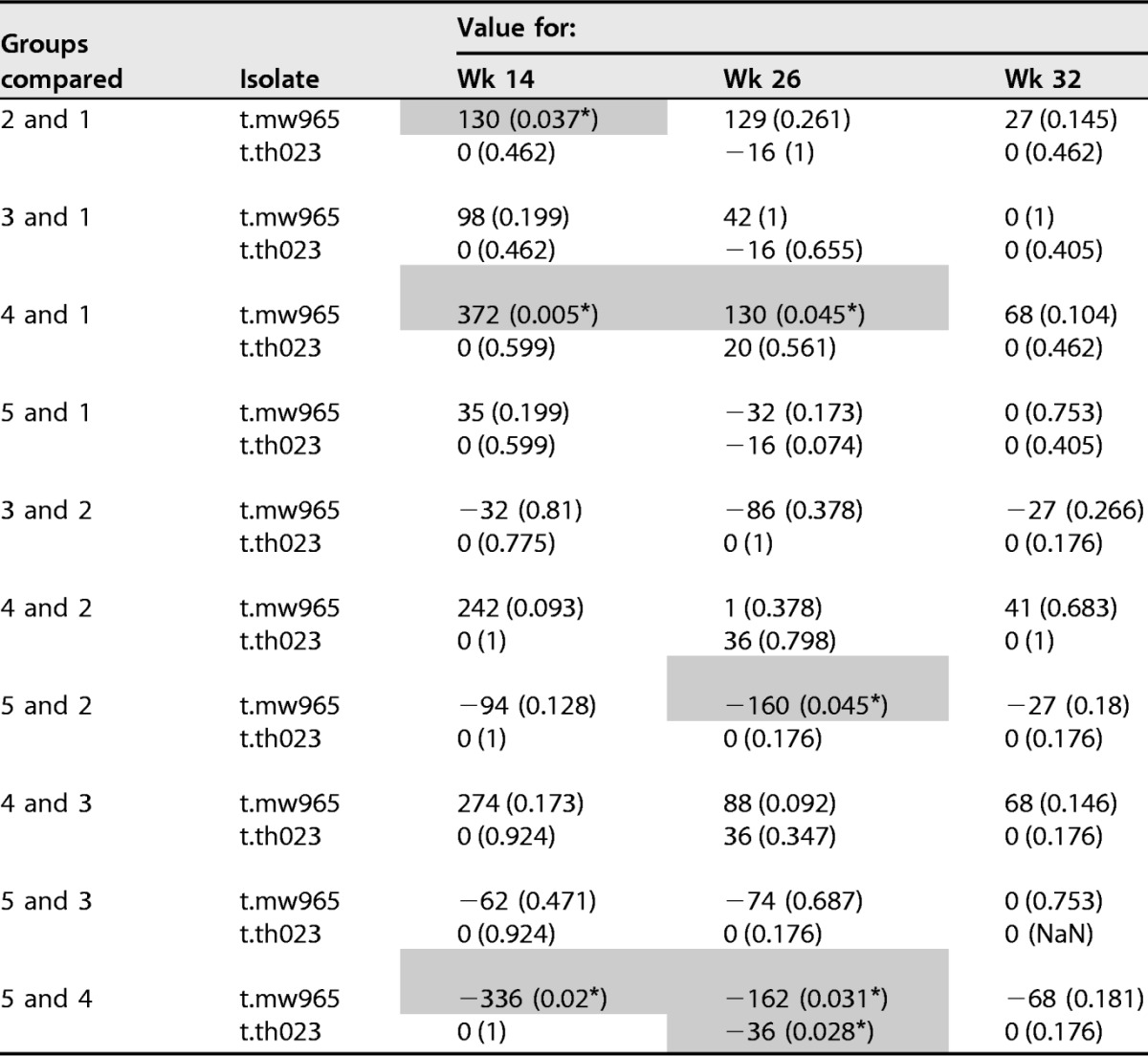
Neutralizaiton assay was performed with TZM-bl cells. Test results shown are median difference between groups followed by P value in parentheses. Analysis was done by Wilcoxon rank sum test. Values used for comparison are serum ID50 titers as shown in Fig. 4. Significant differences (P < 0.05) are shaded and indicated by an asterisk. Groups are G1 N2[LpN]2, G2 N2[CpN]2, G3 N2Lp2, G4 N2Cp2, and G5 N2C2.
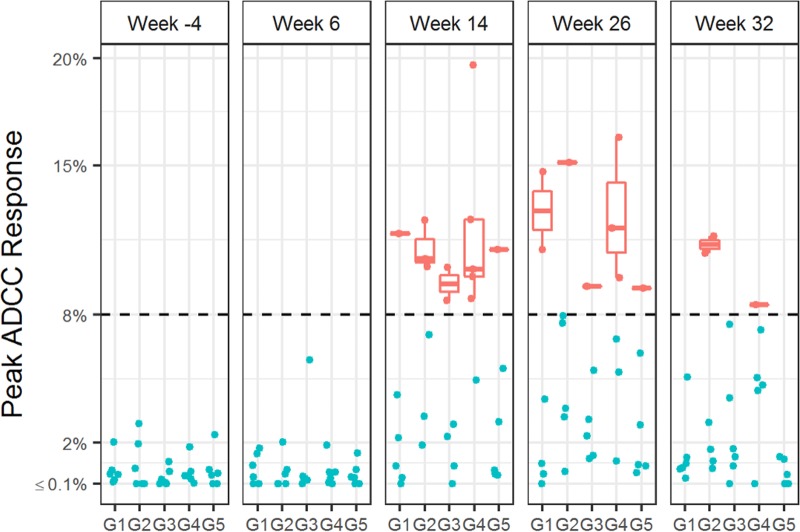
Antibody-dependent cytotoxicity response (ADCC)-mediated antibody responses in plasma samples collected at weeks −4, 14, 26, and 32 were measured via GranToxLux (GTL) assay. In all groups, positive GTL responses were observed at week 14 and week 26, 2 weeks after the DC-targeting vaccine boosts. Group 4 (G4 N2Cp2) included more positive responses than other groups, and positive GTL responses at week 32 were observed only in groups G2 N2[CpN]2 and G4 N2Cp2 (Fig. 5).
FIG 5.
ADCC-mediated antibody responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. ADCC-mediating serum antibodies were measured by the GranToxLux assay, which measures percent granzyme B activity against HIV-Env protein-coated cells. Positive responses, as defined in Materials and Methods, are shown in red; nonresponders are shown in blue. The midline of the box indicates the median, and the ends of the box indicate the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1.5 times the interquartile range (i.e., the height of the box) or, if no value meets this criterion, to the data extremes.
HIV-1 Env gp140 targeted to either LOX-1 or CD40 elicits Env-specific T cell responses in NHPs primed with a live virus vector.
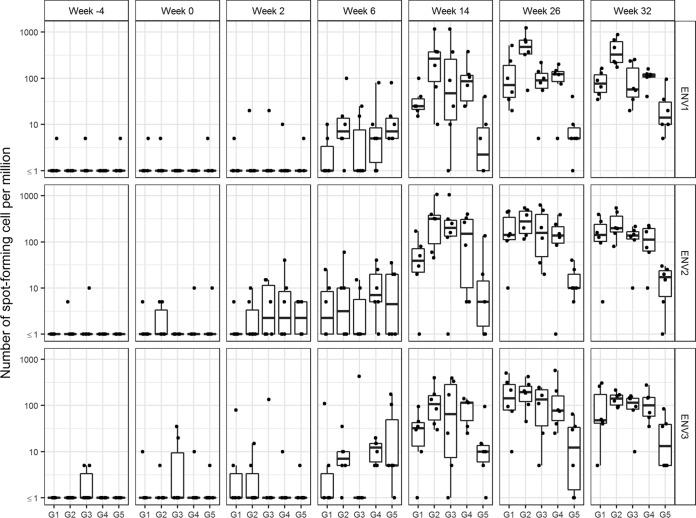
Peripheral HIV-1 antigen-specific T cell responses to the vaccinations were evaluated by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) analysis of peripheral blood mononuclear cells (PBMCs). Few or no responses were detected against Env gp140 antigen after the NYVAC-KC administrations; however, the DC-targeting vaccine boost raised significant responses against Env gp140 in all animals in all groups except two of the six animals in G5 N2C2, and this included responses against all three Env subdomains sampled (Fig. 6). No significant responses were detected against other HIV-1 antigens represented in the NYVAC-KC Gag Nef Pol vector at any time point (data not shown). In pairwise comparisons of G1 N2[LpN]2 versus G3 N2Lp2 (i.e., LOX-1 targeting with and without coadministered NYVAC-KC), there were no significant differences in anti-Env T cell responses at week 26 or 32 (Fig. 6). However, in pairwise comparisons of G2 N2[CpN]2 versus G4 N2Cp2 (i.e., CD40 targeting with and without coadministered NYVAC-KC), there was a trend toward higher responses with coadministered NYVAC-KC at week 26 (P = 0.065), which became a significant difference (P = 0.009) at week 32 (Fig. 6). In the pairwise comparison of G4 N2Cp2 versus G5 N2C2 (i.e., CD40 targeting with and without poly-ICLC adjuvant), there was a trend to higher responses with poly-ICLC (P = 0.143 with 6/6 versus 2/6 responders) at week 26, which became significant at week 32 (P = 0.010 with 6/6 versus 4/6 responders) (Fig. 6).
FIG 6.
Blood HIV-1 antigen-specific T cell responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. PBMCs were analyzed by IFN-γ ELISPOT assay for responses to three Env gp140 peptide pools. Sample collection times are shown in weeks below the graph. Dots are results for individual animals, and the box plots represent the distribution of values, with the bars indicating the median values for the group. No significant responses were detected against non-Env HIV peptides (data not shown). Statistical analysis of blood HIV-1 antigen-specific T cell responses elicited at week 26 by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration, according to IFN-γ ELISPOT assay, summed over all antigens by the Wilcoxon rank sum test comparing G1 versus G3 (P = 0.699), G2 versus G4 (P = 0.065), G1 versus G2 (P = 0.180), G3 versus G4 (P = 0.818), and G4 versus G5 (P = 0.143). For the week 32 data, the analysis compared G1 versus G3 (P = 0.699), G2 versus G4 (P = 0.009), G1 versus G2 (P = 0.015), G3 versus G4 (P = 1.000), and G4 versus G5 (P = 0.010).
Peripheral HIV-1 antigen-specific CD4+ T cell responses to the DC-targeting boost vaccinations (week 26 and 32) were also evaluated by intracellular cytokine staining (ICS) analysis of PBMCs. Low-level responses were detected in some animals against HIV-1 antigens represented in the NYVAC-KC Gag Nef Pol vector at these sample times, but responses against Env antigens were higher in all groups and were positive in almost all animals (Fig. 7). In pairwise comparisons, there was a significant difference among responders to Env only between G4 (N2Cp2) and G5 (N2C2) (i.e., CD40 targeting with versus without poly-ICLC adjuvant) at week 26 but not week 32 (P = 0.004 and P = 0.052; Mann-Whitney U test). The CD4+ T cell responses elicited by the DC-targeting vaccines were of a high quality, as IFN-γ, interleukin 2 (IL-2), and TNF-α were detected in response to Env antigens (Fig. 7).
FIG 7.
Specificity and magnitude of blood HIV-1 antigen-specific CD4+ T cell responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. PBMCs from blood collected at weeks 26 and 32 were analyzed by ICS for CD4+ T cell responses to HIV-1 antigen peptide pools (indicated above the graph). In contrast to Fig. 6, these data combine IFN-γ, IL-2, and TNF-α cytokine secretion responses as well as integrating responses across all Env proteins (the response patterns represented with combined cytokine responses were similar to those seen when the individual cytokine responses were plotted). Responders are shown in blue and nonresponders in red. Dots are results for individual animals, and the box plots represent the distribution of values, with the bars indicating the median values for the responders in the group (indicated below each panel).
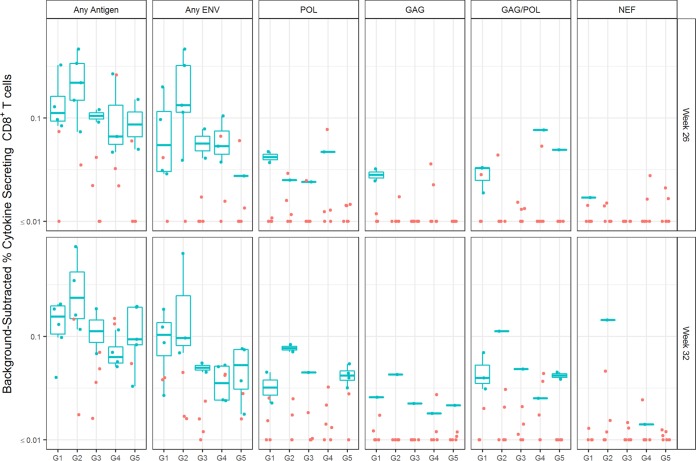
Peripheral HIV-1 antigen-specific CD8+ T cell responses to the DC-targeting boost vaccinations (week 26 and 32) were also evaluated by ICS analysis of PBMCs. Low-level responses were detected in some animals against HIV-1 antigens represented in the NYVAC-KC Gag Nef Pol vector at these sample times, but responses against Env antigens were generally higher for all groups (Fig. 8). In pairwise comparisons, there was a significant difference among responders to any protein only, with G2 N2[CpN]2 > G4 N2Cp2 at week 32 (P = 0.029; Mann-Whitney U test). High-quality CD8+ T cell responses (as measured by positive responders for IFN-γ, IL-2, and TNF-α) were especially evident in G2 N2[CpN]2 (Fig. 8). Unlike for CD4+ T cell responses, poly-ICLC adjuvant did not benefit the development of Env-specific CD8+ T cell responses (see Fig. 8, G4 N2Cp2 versus G5 N2C2).
FIG 8.
Specificity and magnitude of blood HIV-1 antigen-specific CD8+ T cell responses elicited by anti-LOX-1.Env gp140 and anti-CD40.Env gp140 fusion protein administration. PBMCs from blood collected at weeks 26 and 32 were analyzed by ICS for CD8+ T cell responses to HIV-1 antigen peptide pools (indicated above the graph). In contrast to Fig. 6, these data combine IFN-γ, IL-2, and TNF-α cytokine secretion responses as well as integrating responses across all Env proteins (the response patterns represented with combined cytokine responses were similar to those seen when the individual cytokine responses were plotted). Responders are shown in blue and nonresponders in red. Dots are results for individual animals, and the box plots represent the distribution of values, with the bars indicating the median values for the responders in the group (indicated below each panel).
Anti-CD40.Env gp140 fusion protein elicits a more durable binding antibody response than anti-LOX-1.Env gp140 fusion protein.
Durability of binding and neutralizing antibody responses was assessed by proportion of change per week, which was calculated as [(response at durability time point minus response at peak time point)/response at peak time point]/number of weeks between time points, where the durability time point is week 32 and the peak time point is week 26. Durability was not evaluated for ADCC and T cell response (ICS) due to the limited number of responders at the durability time point. Significant differences were observed in the rate of binding antibody response decline observed among the 5 groups (Fig. 9). Both groups with the anti-CD40.Env gp140 fusion protein trended for better binding response durability than did the groups with anti-LOX-1.Env gp140 fusion protein, i.e., G2 N2[CpN]2 > G1 N2[LpN]2 (trend) and G4 N2Cp2 > G3 N2Lp2 (trend) for proportion of change per week (Fig. 9A). G4 N2Cp2 showed the best durability among the groups, with significantly slower decline of responses than for G1 N2[LpN]2 and G5 N2C2 for binding to most gp140s, including Con S gp140 CF and C.con.env03 140 CF (P values of 0.0056 to 0.018; 2-tailed t test) (Table 5). Durability for neutralization response was evaluated only for neutralization of MW965.26 (in TZM-bl cells), due to limited positive responders for other viruses at the durability time point. G4 N2Cp2 also trended for better neutralizing response durability than that of G3 N2Lp2 (Fig. 9B). No significant difference was observed among the groups for the rate of neutralization response decline (Table 5).
FIG 9.
Durability of plasma IgG binding (A) and serum neutralizing (B) antibody responses evaluated as proportion of change per week. Proportion of change per week was calculated as [(response at durability time point − response at peak time point)/response at peak time point]/number of weeks between peak and durability time points and was calculated only for animals that showed a positive response at the peak immunity time point. The midline of the box indicates the median, and the ends of the box indicate the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1.5 times the interquartile range (i.e., the height of the box) or, if no value meets this criterion, to the data extremes. For binding antibody response, AUCs from titration data in BAMA were used for the calculation. ID50 titers were used for neutralization response. Descriptive statistics of these values and results for between-group comparisons are in Table 5.
TABLE 5.
Analysis of paired comparisons of proportion of decline per week of binding and neutralizing antibody responsesa

Test results shown are median difference between groups followed by P value in parentheses. Analysis was by t test assuming unequal variance between groups. Values used for comparison are plasma binding AUC (as shown in Fig. 3) and serum neutralization ID50 titers measured on TZM-bl cells (as shown in Fig. 4). Groups are G1 N2[LpN]2, G2 N2[CpN]2, G3 N2Lp2, G4 N2Cp2, and G5 N2C2 (Table 1). Significant differences are shaded and indicated by asterisks as follows: *, P < 0.05, and **, P < 0.01.
DISCUSSION
To inform future human clinical trial design, our goal was to compare the efficacy of targeting Env gp140 to LOX-1 versus CD40 for boosting immunity primed by NYVAC-KC vaccine, as well as to ascertain if coadministration of NYVAC-KC and DC-targeting vaccines was beneficial. Analysis of serum anti-Env IgG responses revealed that the DC-targeting vaccines strongly boosted the modest responses raised by NYVAC-KC priming. Responses after the DC-targeting protein administration were robust, were broadly reactive across HIV-1 clades, represented multiple epitopes across gp140, and had ADCC activity but neutralized only the sensitive HIV-1 strains. Importantly, following priming with NYVAC-KC, coadministering NYVAC-KC and DC-targeting agents had no benefit over DC targeting alone, although fewer animals raised positive IgA responses in the coadministered groups. It is uncertain if IgA responses are desirable, since binding IgA Env antibodies were modestly associated with decreased vaccine efficacy in the RV144 study (14, 17). Poly-ICLC raised the potency of targeting Env to CD40, although a single administration of anti-CD40.Env gp140 without adjuvant significantly boosted serum anti-Env IgG levels. Targeting Env to CD40 was largely comparable to targeting to LOX-1; however, based on development of responses with higher overall binding and durability, together with earlier and higher neutralizing serum titers, G4 N2Cp2 (i.e., poly-ICLC-adjuvanted CD40 targeting without NYVAC-KC coadministration) was most efficacious for antibody responses.
Peripheral HIV-1-specific T cell responses were very weak after the NYVAC-KC administrations, but Env-specific T cell responses were boosted by both anti-CD40.Env gp140 and anti-LOX-1.Env gp140 DC-targeting vaccinations given with poly-ICLC, except for adjuvant-free anti-CD40.Env gp140 G5 N2C2. The significantly lower T cell responses observed in G5 N2C2, the only group without poly-ICLC adjuvant, is consistent with the ability of poly-ICLC to enhance T cell responses (18). These responses were directed against multiple Env regions and contained high-quality CD4+ and CD8+ T cells. Coadministration of NYVAC-KC with the DC-targeting vaccines plus poly-ICLC tended to improve the T cell responses. Overall, high-quality CD8+ T cell responses were especially evident in G2 N2[CpN]2, i.e., poly-ICLC-adjuvanted CD40 targeting with NYVAC-KC coadministration.
Studies in vitro (12) show that targeting influenza virus nucleoprotein (NP) and hemagglutinin (HA) antigens to either LOX-1 or CD40 in the absence of TLR stimulation in human PBMC cultures expanded antigen-specific T cells where LOX-1 targeting elicited greater CD4+ T cell expansion than targeting CD40 and, conversely, CD40 targeting expanded CD8+ T cells more effectively than targeting LOX-1. Thus, the superiority of CD40 targeting over LOX-1 targeting for CD4+ responses seen in G3 N2Lp2 and G4 N2Cp2 could be due to the effect of adjuvant or could reflect a difference between in vitro and in vivo conditions, possibly due to different types of resident DC types being targeted. A similar augmentation of T cell responses via CD40 targeting plus poly-IC has been shown recently in vivo in a mouse model (12). The low CD8+ T cell responses in group 5 may also reflect differences in vitro without TLR stimulation versus in vivo with TLR stimulation, which clearly drives the more robust responses seen in G3 N2Lp2 and G4 N2Cp2. Also, while weak, the T cell responses in G5 N2C2 were positive for most animals. This is in contrast to previous observations with nonadjuvanted protein vaccination in NHPs (see references 19 and 20). These differences highlight the difficulties in correlating in vitro and in vivo data.
Durability analysis based on a relatively short follow-up period, 8 weeks, revealed the highest durability of binding antibody responses in the G4 N2Cp2 group, suggesting a beneficial effect of CD40 targeting on durability. This is further supported by the consistent trend of higher durability of G4 N2Cp2 than for G3 N2Lp2 and of G2 N2[CpN]2 than for G1 N2[LpN]2 for binding to all antigens tested (Fig. 9 and Table 5). Another observation, that G4 N2Cp2 trended for better durability than G2 N2[CpN]2 and G3 N2Lp2 trended for better durability than G1 N2[LpN]2, in both cases the only difference being that the latter group had coadministration of NYVAC-KC, indicates that coadministration of NYVAC-KC may have negatively affected durability of binding antibody response in these DC-targeting regimens. For all antigens tested, G5 N2C2 showed the lowest durability of response, suggesting improvement of durability with poly-ICLC adjuvant.
Regarding our specific aims, the DC-targeting vaccines were well tolerated, with no reported adverse events with or without NYVAC-KC coadministration; coadministration of the NYVAC-KC with poly-ICLC-adjuvanted DC-targeting vaccines did not improve the magnitude of humoral responses but benefited the development of high-quality Env-specific CD8+ T cells, and poly-ICLC adjuvant improved the humoral and cellular response to CD40-targeted Env. As noted, CD40 and LOX-1 targeting responses were very similar, with CD40 targeting showing advantages for magnitude and breadth of antibody response, in raising ADCC and CD8+ T cell responses, and in improving the durability of potentially protective antibody response. This proof-of-concept study did not incorporate a control non-DC-targeting group, and resources were not available for probing mucosal immunity or for following Tfh responses. A follow-up study to specifically address these key issues is planned. Lastly, we expect that the NHPs will raise antibody responses directed to the DC-targeting antibody vehicle portion of the vaccine and that this well may attenuate the potential potency of this class of vaccines. Since the DC-targeting antibody vehicles are fully humanized, this should not be a problem for human vaccination.
MATERIALS AND METHODS
Production and quality assurance of anti-CD40.Env gp140 and anti-LOX-1.Env gp140.
For the DC-targeting vaccines, vectors, cloning strategies, methods for deriving stably transfected CHO-S cells, their culturing, purification of secreted recombinant antibodies and fusion proteins, and quality assurance tests have been described previously (9, 21). Specifically, anti-CD40.Env gp140 was derived from the parental anti-human CD40 12E12 recombinant human IgG4 monoclonal antibody (MAb) (GenBank accession numbers HQ738667.1 and HQ738666.1 [9]) via humanization of the mouse variable regions (Antitope, Ltd.) as defined by the variable regions in the sequences under GenBank accession numbers KM660791 and KM660792. Env gp140 sequence derived from the codon-optimized HIV-1 96ZM651 synthetic construct (NIH AIDS reagent program, GenBank accession number AY181197.1 residues 94 to 2064) was inserted at the IgG4 heavy-chain C-terminal codon distal to a flexV1 flexible linker spacer (9) and proximal to 6 His codons. Anti-LOX-1.Env gp140 (2) was derived from the parental anti-human LOX-1 15C4 recombinant human IgG4 MAb (3, 22) (GenBank accession numbers KM246787 and KM246788) via humanization of the mouse variable regions (Antitope, Ltd.). This was fused to Env gp140 sequence as described above. Specificity and relative binding affinity of anti-CD40.Env gp140 and anti-LOX-1.Env gp140 to human and rhesus macaque CD40 and LOX-1 ectodomains (corresponding to, respectively, by GenBank accession numbers AAO43990.1 residues 22 to 193, and NP_001252791.1 residues 21 to 193, AB102861.1 residues 169 to 918, and NM_001194668.1 residues 295 to 945) fused via the cohesin C terminus (GenBank CP000568.1 residues 3622666 to 3623172 with an NheI site linker) was tested by an adaption of multiplexed bead-based assay (23) for equilibrium competition binding analysis of anti-LOX-1 Env gp140 and anti-CD40 Env gp140 interaction with human and NHP LOX-1 and CD40 ectodomains. In this assay, beads were coated with human LOX-1, NHP LOX-1, human CD40, or NHP CD40 ectodomains and incubated overnight with 10 ng/ml of the parental mouse anti-LOX-1 15C4 or anti-CD40 12E12 MAbs and various concentrations of humanized anti-LOX-1, anti-LOX-1 Env gp140, anti-CD40, or anti-CD40 Env gp140, then probed with phycoerythrin (PE)-labeled anti-mouse IgG, and analyzed with a Bio-Plex 200 instrument. There was no significant difference between the binding of humanized anti-LOX-1 versus anti-LOX-1 Env gp140 to human (50% inhibitory concentration [IC50], 40 pM) or NHP (IC50, 35 pM) LOX-1-coated beads, and the levels of binding of humanized anti-CD40 versus anti-CD40 Env gp140 to human (IC50, 0.25 nM versus 0.1 nM) and NHP (IC50, 0.2 nM versus 0.1 nM) CD40-coated beads were similar (data not shown). The vaccines were formulated and administered exactly as described below. A schematic representation, SDS-PAGE analysis, and binding verification for anti-LOX-1.Env gp140 can be found in reference 2.
Animals and assays.
Thirty male rhesus macaques ranging in age from 3 to 6 years and weighing at least 4 kg were procured from Harlan Laboratories and housed at the Advanced Biosciences Laboratories (ABL) animal facility in Rockville, MD. The ABL in vivo facility is USDA registered and is accredited by the American Association for the Accreditation of Laboratory Animal Care International (AAALAC). ABL's veterinary practices comply with all policies of the Guide for the Care and Use of Laboratory Animals; DHHS (NIH 85-23); Animal Welfare (DHHS-TN 73-2); NIH Manual Issuances 4206 and 6000-3-4-58; Responsibility for Care and Use of Animals, CDC/NIH, 4th edition; Biosafety in Microbiological and Biomedical Laboratories; and Public Health Service Policy on Humane Care and Use of Laboratory Animals under a category 1 assurance from the Office of Laboratory Animal Welfare (OLAW). All procedures were carried out under ketamine anesthesia by trained personnel under the supervision of veterinary staff, and all efforts were made to ameliorate the welfare and to minimize animal suffering in accordance with the recommendations of the Weatherall report (39) for the use of nonhuman primates. Other than for a 7-day postinoculation follow-up observation period, animals were pair-housed in adjoining primate cages allowing social interactions, under controlled conditions of humidity, temperature, and light (12-h light/12-h dark cycles). Food and water were available ad libitum. Animals were monitored and fed standard laboratory rations twice daily. Trained personnel offered dietary supplements with fresh fruit and occasional treats at least once a day. Early endpoint criteria, as proposed by the project team and approved by the ABL's Institutional Animal Care and Use Committee (IACUC), were used to determine when animals should be humanely euthanized. The ABL veterinarian was authorized to determine whether animals met such criteria and, if necessary, was tasked to stabilize any affected animals prior to consulting with the lead investigators. The IACUC approved of the proposed study protocol prior to the initiation of any in vivo work. The protocol number assigned by the IACUC/ethics committee that approved this study is AUP567.
Vaccination protocols.
The NYVAC-KC virus (described in references 2 and 24) inoculum was provided in 0.2-ml aliquots as NYVAC-KC expressing Env gp140 (ZM96) and ZM96 Gag plus CN54 PolNef at 0.1 ml of each virus in 1.2 × 108 PFU per vial. The immunogen mixture according to molar ratios was Env/GagPolNef at 1:1. Prior to administration, the required number of vaccine vials containing the virus mixture were thawed at 37°C and put on ice immediately after thawing. One milliliter of 1× Tris-buffered saline (TBS) was added to each vial and briefly vortexed. Sonication with a Bransonic 1210 unit was performed according to the following procedure: fill water bath of the sonicator with water and ice; sonicate sample 3 times for 10 s each time; and, after the sonication steps, vortex sample. For each animal, 1 ml of the vaccine preparation was drawn up from the vials by a 1-ml syringe and kept on ice until administration. Before immunization, the skin of the upper arm (deltoid) was shaved and cleaned with alcohol. Animals received the vaccine preparation via intramuscular injection of 1 ml of the vaccine preparation at 2 × 108 PFU/ml total of 2 viruses in the deltoid. The poly-ICLC was provided in 1-ml vials at a concentration of 2 mg/ml. Vials (Hiltonol lot PJ215-1-10-01) were stored at 2 to 8°C. Administration was 1 mg (subcutaneous) in two injections of 250 μl in the center of each circular injection pattern (3- to 4-cm diameter) formed by the intradermal administrations of anti-LOX-1.Env gp140 or anti-CD40.Env gp140 vaccine components which were stored in 1 M arginine plus 100 mM Tris-HCl buffer, pH 6 to 8. Protein vaccine administrations were at a 200-μg dose, intradermal, in a total of eight injections of 250 μl each (2-ml total injection); four injections were performed in each side of the back, placed in a circular pattern 3 to 4 cm in diameter. To avoid toxicity of 1 M arginine buffer, the concentrated protein was diluted approximately 1:4 in phosphate-buffered saline (PBS) before use. Skin was shaved before injection and cleaned with 70% alcohol solution. Intradermal injections were performed using an insulin syringe. The injection of poly-ICLC was performed after the i.d. injections of the proteins and in the middle of each circle with 500 μl injected s.c. This administration procedure was designed to promote drainage of antigen and adjuvant to the same lymph node site and at the recommendation of ABL's head veterinarian. The supervising veterinarian reported no adverse events from the vaccinations.
Neutralization assay.
Neutralizing antibodies were measured as a function of reductions in luciferase (Luc) reporter gene expression after a single round of infection in TZM-bl cells (25). TZM-bl cells (also called JC57BL-13 cells) and A3R5 cells were obtained from the NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. Briefly, a pretitrated dose of virus was incubated with serial 3-fold dilutions of test sample in duplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg/ml of DEAE dextran) were added to each well. One set of 8 control wells received cells plus virus (virus control) and another set received cells only (background control). After 48 h of incubation, 100 μl of cells was transferred to a 96-well black solid plate (Costar) for measurements of luminescence using the Britelite luminescence reporter gene assay system (PerkinElmer Life Sciences). Assay stocks of molecularly cloned Env-pseudotyped viruses were prepared by transfection in 293T/17 cells (American Type Culture Collection) and titrated in TZM-bl cells as described previously (25). This assay has been formally optimized and validated (26) and was performed in compliance with good clinical laboratory practices (GCLP), including participation in a formal proficiency testing program (27). Additional information on the assay and all supporting protocols may be found at http://www.hiv.lanl.gov/content/nab-reference-strains/html/home.htm. The method for MB analysis of serum neutralization assays as shown in Fig. 4 has been described previously (28).
HIV-1 specific binding antibody assay.
HIV-1 specific IgG antibodies to gp120/gp140 proteins and V1/V2 scaffolds were measured by an HIV-1 binding antibody multiplex assay (29). All assays were run under GCLP-compliant conditions, including tracking of positive controls by Levy-Jennings charts using 21CFR part 11-compliant software. Positive controls included an HIVIG and CH58 MAb IgG titration. Negative controls included in every assay were blank (uncoupled) and MulVgp70_His6 (empty gp70 scaffold)-coupled beads, a blank well on each assay plate, and HIV-1-negative sera. To control for antigen performance, we used the preset criterion that the positive-control titer (HIVIG) included on each assay (and for assays with V1V2 antigens, CH58 MAb [30]) had to be within ±3 standard deviations of the mean for each antigen (tracked with a Levy-Jennings plot with preset acceptance of titer and calculated with a four-parameter logistic equation, SigmaPlot, Systat Software). Antibody measurements were acquired on a Bio-Plex instrument (Bio-Rad, Hercules, CA) using 21CFR part 11-compliant software, with the readout as mean fluorescence intensity (MFI). The following antigens were examined: ZM96 gp140-C tag (Baylor Health), Con S gp140 CF (a group M consensus envelope gp140 [31, 32]), A1.con.env03 140 CF (clade A consensus), B.con.env03 140 CF (clade B consensus), C.con.env03 140 CF (clade C consensus), clade A 00MSA 4076 gp140, gp70 control, gp70_B.CaseA2 V1/V2 (a recombinant clade B gp70 scaffold protein with the V1V2 variable region), C.1086 V1V2 tags and C.1086 V2 tags (a transmitted clade C isolate provided by H. X. Liao and B. F. Haynes, Duke University), and AE.A244 V1/V2 tags and AE.A244 V2 tags (clade AE sequence of RV144 vaccine immunogen (14).
Serum antibody linear epitope mapping.
Serum epitope mapping of heterologous strains was performed as previously described (33–35), with minor modifications. Briefly, array slides were provided by JPT Peptide Technologies GmbH (Germany) by printing a library designed by B. Korber, Los Alamos National Laboratory, onto epoxy glass slides (PolyAn GmbH, Germany). The library contains overlapping peptides (15-mers overlapping by 12) covering 6 full-length gp160 consensus sequences (clade A, B, C, and D, group M, CRF1, and CRF2) and gp120 sequences of 6 vaccine strains (MN, A244, Th023, TV-1, ZM651, 1086C). Three identical subarrays, each containing the full peptide library, were printed on each slide. All array slides were blocked for 1 h, followed by a 2-h incubation with 1:50-diluted serum samples and a subsequent 45-min incubation with goat anti-human IgG conjugated with AF647 (Jackson ImmunoResearch, PA). Array slides were scanned at a wavelength of 635 nm using an Axon Genepix 4300 scanner (Molecular Devices, Sunnyvale, CA). Images were analyzed using Genepix Pro 7 software (Molecular Devices) to obtain binding intensity values for all peptides. Baseline value for each peptide, which was defined as the median signal intensity of the triplicates of the peptide for the matched prebleed serum plus 3 times the standard error of the triplicates, was subtracted from the signal intensity of postimmunization serum to each peptide. The magnitude of binding to each identified epitope was defined as the highest binding by a single peptide within the epitope region.
ICS.
Simulations were performed as described previously (36). In short, cryopreserved PBMCs were thawed and rested overnight in R10/RPMI 1640 (BioWhittaker, Walkersville, MD) containing 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml of penicillin G, and 100 μg/ml of streptomycin with 50 U/ml of Benzonase (Novagen, Madison, WI) in a 37°C and 5% CO2 incubator. The following morning, cells were stimulated with peptide pools (2 μg/ml; described in reference 2) in the presence of GogiPlug (10 μg/ml; BD Biosciences, San Jose, CA) for 6 h. Negative controls received an equal concentration of dimethyl sulfoxide (DMSO) instead of peptides. Subsequently, intracellular cytokine staining (ICS) was performed as described previously (37). The following monoclonal antibodies were used: CD4-BV421 (clone OKT4; BioLegend), CD8-BV570 (clone RPA-T8; BioLegend), CD69-ECD (clone TP1.55.3; Beckman Coulter), CD3-Cy7-allophycocyanin (APC) (clone SP34.2; BD Biosciences), IFN-γ–APC (clone B27; BD Biosciences), IL-2–PE (clone MQ1-17H12; BD Biosciences), and TNF-fluorescein isothiocyanate (FITC) (clone Mab11; BD Biosciences). The Aqua LIVE/DEAD kit (Invitrogen, Carlsbad, CA) was used to exclude dead cells. All antibodies were previously titrated to determine the optimal concentration. Samples were acquired on an LSR II flow cytometer and analyzed using FlowJo version 9.8 (Treestar, Inc., Ashland, OR).
ADCC.
ADCC activity against MW965.1 gp120 (provided by the Duke CAVD repository)-coated CEM.NKRCCR5 (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH; from Alexandra Trkola) target cells was measured using the ADCC-GranToxiLux (GTL) assay as previously described (38). The peak activity is the maximum activity observed at any dilution and considered positive if above the 8% granzyme B activity cutoff.
ELISPOT.
Millipore 96-well filtration plates were pretreated with 70% ethyl alcohol (EtOH), washed 5 times with 1× PBS, and then coated with 5 μg/ml of mouse anti-human IFN-γ antibody (BD Pharmingen) overnight at 4°C. After blocking with complete RPMI for 2 h at 37°C, 2 × 105 PBMCs (prepared as for ICS) were stimulated in triplicate with peptide pools at 1 μg/ml, or phytohemagglutinin (PHA; 2.5 μg/ml) as a positive control, while addition of medium only served as a negative control. The peptides and peptide pools used are those described in reference 36. Plates were incubated at 37°C for 18 to 24 h before being washed with cold H2O twice and PBS-Tween five times. Biotinylated anti-IFN-γ antibody (Mabtech) was added at 1 μg/ml for 1 h at 37°C and, after a washing, a 1:2,000 dilution of avidin-horseradish peroxidase (HRP) (Vector Laboratories) was added, again for 1 h at 37°C. After a final washing, stable diaminobenzidine (Invitrogen) was added for 2 min, and then the reaction was stopped with water washing. After drying, the spots in each well were counted with an automated ELISPOT reader (CTL Immunospot).
Statistical methods.
Wilcoxon signed-rank tests or 2-tailed t tests were used to compare change of marker value at specific time points with the baseline within each group. Comparison between groups at specific time points was made using the Wilcoxon rank sum test. Response rates were compared between groups using Fisher's exact test. Overall magnitudes of ELISPOT response were compared between groups G1 and G2 by fitting a random-effect model using log-transformed data measured after week 14. A P value of less than or equal to 0.05 is considered statistically significant. Statistical analyses were done with R (version 3.1.2; The R Foundation for Statistical Computing, Vienna, Austria). For the antigen-specific antibody measurements, several criteria are used to determine if data from an assay are acceptable and can be statistically analyzed. The blood draw rate must be within the allowable visit window as determined by the protocol. Second, if the blank-bead negative control exceeds an MFI of 5,000, the sample will be repeated. If the repeat value exceeds an MFI of 5,000, the sample will be excluded from analysis due to high background. Quality control (QC) and standard curve titers must fall within ±3 standard deviations of the historical mean plotted on Levy-Jennings charts. Sample and control replicates must also be within a coefficient of variation (CV) of 20%. Samples are declared to have positive responses if they meet three conditions: (i) the MFI minus blank bead or MulVgp70 His6 values are greater than or equal to antigen-specific cutoff (based on the average plus 3 standard deviations of 60 seronegative plasma samples), (ii) the MFI minus blank values are greater than 3 times the baseline MFI minus blank values, and (iii) the MFI values are greater than 3 times the baseline MFI values. For each antigen and visit, the magnitude of binding response among both responders and nonresponders is compared between groups using the Wilcoxon rank sum test. Response rates were compared between groups using Fisher's exact test. No adjustments were made for multiple comparisons, as these were exploratory analyses for which increased type 1 error is tolerated for better sensitivity to detect effects. A P value of less than or equal to 0.05 is considered statistically significant.
ACKNOWLEDGMENTS
Mireille Centlivre (INSERM U955) helped edit the manuscript. Matthew Baker (Antitope, Ltd.) provided humanization of the anti-CD40 12E12 MAb. Lauren Hudacik (ABL), Cécile Peltekian (INSERM U955), and Song Ding (EuroVacc Foundation) are thanked for project management. Xua-Xin Liao is thanked for providing reagents. We thank Hongmei Gao and Kelli Greene (Duke University) for CAVIMC program management and Ryan Duffy, Tara McNair, and William T. Williams (Duke University) for technical expertise.
G.Z., S.Z., and Y.L. are named inventors on LOX-1 and CD40 targeting vaccine patents and patent filings held by the Baylor Research Institute. G.P. is a named inventor on NYVAC-KC-related patents held by Centre Hospitalier Universitaire Vaudois Lausanne and others.
This study was funded within the Vaccine Research Institute HIV vaccine program (ANRS/INSERM) and the project A Novel Replication Competent Flavivirus-based HIV Vaccine Platform, i.e., RepliVax, as a Priming Component for Improving Antibody Response (OPP1040705), funded by the Bill & Melinda Gates Foundation, and was also supported by the Investissements d'Avenir program managed by the ANR under reference ANR-10-LABX-77. Support from the Poxvirus T-cell Vaccine Consortium funded by the Bill & Melinda Gates Foundation (OPP38599) was for the development of the NYVAC-KC vector. Support for the ICS and antibody immune monitoring assays was provided by the Vaccine Immune Monitoring Centers (OPP1032325 and OPP1032144), and support for the statistical analysis came from the Vaccine Immunology Statistical Center (OPP1032317), all part of the Collaboration for AIDS Vaccine Discovery (CAVD), funded by the Bill & Melinda Gates Foundation.
The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Steinman RM. 2012. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Zurawski G, Zurawski S, Flamar AL, Richert L, Wagner R, Tomaras GD, Montefiori DC, Roederer M, Ferrari G, Lacabaratz C, Bonnabau H, Klucar P, Wang Z, Foulds KE, Kao SF, Yates NL, LaBranche C, Jacobs BL, Kibler K, Asbach B, Kliche A, Salazar A, Reed S, Self S, Gottardo R, Galmin L, Weiss D, Cristillo A, Thiebaut R, Pantaleo G, Levy Y. 2016. Targeting HIV-1 Env gp140 to LOX-1 elicits immune responses in rhesus macaques. PLoS One 11:e0153484. doi: 10.1371/journal.pone.0153484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, Xue Y, Zurawski S, Le Grand R, Liu YJ, Kuroda M, Zurawski G, Oh S. 2014. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity 41:592–604. doi: 10.1016/j.immuni.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastenmüller W, Kastenmüller K, Kurts C, Seder RA. 2014. Dendritic cell-targeted vaccines—hope or hype? Nat Rev Immunol 14:705–711. doi: 10.1038/nri3727. [DOI] [PubMed] [Google Scholar]
- 5.Cohn L, Delamarre L. 2014. Dendritic cell-targeted vaccines. Front Immunol 5:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Stojanovska L, McKenzie IF, Vassilaros S. 2014. Dendritic cell immunotherapy: clinical outcomes. Clin Transl Immunol 3:e21. doi: 10.1038/cti.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schjetne KW, Fredriksen AB, Bogen B. 2007. Delivery of antigen to CD40 induces protective immune responses against tumors. J Immunol 178:4169–4176. doi: 10.4049/jimmunol.178.7.4169. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. 2012. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 9.Flamar AL, Xue Y, Zurawski SM, Montes M, King B, Sloan L, Oh S, Banchereau J, Levy Y, Zurawski G. 2013. Targeting concatenated HIV antigens to human CD40 expands a broad repertoire of multifunctional CD4+ and CD8+ T cells. AIDS 27:2041–2051. doi: 10.1097/QAD.0b013e3283624305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zurawski G, Zurawski S, Wang Z, Akagawa K, Oh S, Hideki U, Fay J, Banchereau J, Song W, Palucka AK. 2015. A novel vaccine for mantle cell lymphoma based on targeting cyclin D1 to dendritic cells via CD40. J Hematol Oncol 8:35. doi: 10.1186/s13045-015-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, de Gruijl T, Lowik C, Oostendorp J, van der Burg SH, Ossendorp F. 2015. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Yin W, Gorvel L, Zurawski S, Li D, Ni L, Duluc D, Upchurch K, Kim JR, Gu C, Ouedraogo R, Wang Z, Xue Y, Joo HM, Gorvel JP, Zurawski G, Oh SK. 2016. Functional specialty of CD40 and dendritic cell surface lectins for exogenous antigen presentation to CD8+ and CD4+ T cells. EBioMedicine 5:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. 2009. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O'Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, Esteban M, Park CG, Trumpfheller C, Keler T, Pantaleo G, Steinman RM, Seder R. 2011. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A 108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 213:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H, Adamson L, Ha T, Mullen K, Hagen SI, Nogueron A, Sylwester AW, Axthelm MK, Legasse AI, Piatak M Jr, Lifson JD, McElrath JM, Picker LJ, Seder RA. 2013. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. J Immunol 190:4103–4115. doi: 10.4049/jimmunol.1202958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamar AL, Zurawski S, Scholz F, Gayet I, Ni L, Li XH, Klechevsky E, Quinn J, Oh S, Kaplan DH, Banchereau J, Zurawski G. 2012. Noncovalent assembly of anti-dendritic cell antibodies and antigens for evoking immune responses in vitro and in vivo. J Immunol 189:2645–2655. doi: 10.4049/jimmunol.1102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, O'Garra A, Zurawski G, Banchereau J, Oh S. 2012. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med 209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner JA, Zurawski SM, Sugimoto C, Vinet-Oliphant H, Vinod P, Xue Y, Russell-Lodrigue K, Albrecht RA, Garcia-Sastre A, Salazar AM, Roy CJ, Kuroda MJ, Oh S, Zurawski G. 2014. Immunologic characterization of a rhesus macaque H1N1 challenge model for candidate influenza virus vaccine assessment. Clin Vaccine Immunol 21:1668–1680. doi: 10.1128/CVI.00547-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kibler KV, Gomez CE, Perdiguero B, Wong S, Huynh T, Holechek S, Arndt W, Jimenez V, Gonzalez-Sanz R, Denzler K, Haddad EK, Wagner R, Sekaly RP, Tartaglia J, Pantaleo G, Jacobs BL, Esteban M. 2011. Improved NYVAC-based vaccine vectors. PLoS One 6:e25674. doi: 10.1371/journal.pone.0025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 26.Sarzotti-Kelsoe M, Daniell X, Todd CA, Bilska M, Martelli A, LaBranche C, Perez LG, Ochsenbauer C, Kappes JC, Rountree W, Denny TN, Montefiori DC. 2014. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J Immunol Methods 409:147–160. doi: 10.1016/j.jim.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd CA, Greene KM, Yu X, Ozaki DA, Gao H, Huang Y, Wang M, Li G, Brown R, Wood B, D'Souza MP, Gilbert P, Montefiori DC, Sarzotti-Kelsoe M. 2012. Development and implementation of an international proficiency testing program for a neutralizing antibody assay for HIV-1 in TZM-bl cells. J Immunol Methods 375:57–67. doi: 10.1016/j.jim.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Gilbert PB, Montefiori DC, Self SG. 2009. Simultaneous evaluation of the magnitude and breadth of a left and right censored multivariate response, with application to HIV vaccine development. Stat Biopharm Res 1:81–91. doi: 10.1198/sbr.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 32.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol 85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen X, Duffy R, Howington R, Cope A, Sadagopal S, Park H, Pal R, Kwa S, Ding S, Yang OO, Fouda GG, Le Grand R, Bolton D, Esteban M, Phogat S, Roederer M, Amara RR, Picker LJ, Seder RA, McElrath MJ, Barnett S, Permar SR, Shattock R, DeVico AL, Felber BK, Pavlakis GN, Pantaleo G, Korber BT, Montefiori DC, Tomaras GD. 2015. Vaccine-induced linear epitope-specific antibodies to simian immunodeficiency virus SIVmac239 envelope are distinct from those induced to the human immunodeficiency virus type 1 envelope in nonhuman primates. J Virol 89:8643–8650. doi: 10.1128/JVI.03635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donaldson MM, Kao SF, Eslamizar L, Gee C, Koopman G, Lifton M, Schmitz JE, Sylwester AW, Wilson A, Hawkins N, Self SG, Roederer M, Foulds KE. 2012. Optimization and qualification of an 8-color intracellular cytokine staining assay for quantifying T cell responses in rhesus macaques for pre-clinical vaccine studies. J Immunol Methods 386:10–21. doi: 10.1016/j.jim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foulds KE, Donaldson M, Roederer M. 2012. OMIP-005: quality and phenotype of antigen-responsive rhesus macaque T cells. Cytometry A 81:360–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weatherall D. (ed). 2006. The use of non-human primates in research. https://royalsociety.org/topics-policy/publications/2006/weatherall-report/.