Abstract
Fibroblast growth factor (FGF) receptors (FGFRs) signal to modulate diverse cellular functions, including epithelial cell morphogenesis. In epithelial cells, E-cadherin plays a key role in cell-cell adhesion, and its function can be regulated through endocytic trafficking. In this study, we investigated the location, trafficking, and function of FGFR1 and E-cadherin and report a novel mechanism, based on endocytic trafficking, for the coregulation of E-cadherin and signaling from FGFR1. FGF induces the internalization of surface FGFR1 and surface E-cadherin, followed by nuclear translocation of FGFR1. The internalization of both proteins is regulated by common endocytic machinery, resulting in cointernalization of FGFR1 and E-cadherin into early endosomes. By blocking endocytosis, we show that this is a requisite, initial step for the nuclear translocation of FGFR1. Overexpression of E-cadherin blocks both the coendocytosis of E-cadherin and FGFR1, the nuclear translocation of FGFR1 and FGF-induced signaling to the mitogen-activated protein kinase pathway. Furthermore, stabilization of surface adhesive E-cadherin, by overexpressing p120ctn, also blocks internalization and nuclear translocation of FGFR1. These data reveal that conjoint endocytosis and trafficking is a novel mechanism for the coregulation of E-cadherin and FGFR1 during cell signaling and morphogenesis.
INTRODUCTION
The fibroblast growth factor (FGF) family consists of 22 pleiotropic mammalian ligands intimately involved in early embryo patterning and development (Martin, 1998; Ornitz and Itoh, 2001). In the adult, FGFs have been implicated as key players in tumorigenesis (Dickson et al., 2000). FGFs and their cognate receptors (FGFRs) also are involved in epithelial to mesenchymal transitions (EMTs) (Thiery, 2002), and accordingly, have emerged as regulators of cell fate. Receptor activation by FGFs involves high-affinity binding to the type I transmembrane FGFRs, a family of four genes, and lower affinity binding to heparin sulfate proteoglycans (Johnson and Williams, 1993). At the cell surface, these three elements interact as heterotrimers to form the ternary FGF signaling complex (Ornitz, 2000). Complexity of signaling interactions is greatly increased by alternate splicing of some FGF ligands and of all FGFRs (Johnson and Williams, 1993; Ornitz et al., 1996; Prudovsky et al., 1996; Ornitz and Itoh, 2001).
Ligand-bound FGFRs can signal through a number of different pathways, including through mitogen-activated protein kinase (MAPK) and β-catenin, depending on the cellular context (Klint and Claesson-Welsh, 1999). On ligand-mediated activation of surface receptor, the FGF signaling complex is internalized (Sorokin et al., 1994; Prudovsky et al., 1996; Belleudi et al., 2002). The intracellular trafficking of FGFs and FGFRs is poorly defined, with a variety of fates described for different ligand and receptor combinations, as well as splice variants. In some contexts, both FGF and FGFR are translocated into a subcompartment of the nucleus (Wiedlocha et al., 1994; Maher, 1996; Reilly and Maher, 2001). Although nuclear translocation of FGFRs is dependent on cAMP and importin-β (Reilly and Maher, 2001), the pathway and mechanisms involved in this translocation are poorly understood.
The incorporation of adhesion molecules and receptor tyrosine kinases (RTKs) into functional complexes is an emerging paradigm (e.g., VE-cadherin with VEGFR2; EGFR, IGF1-R, or c-Met with E-cadherin) (reviewed in Cavallaro and Christofori, 2004). The functional consequences of such complexes are varied, having the potential to regulate both signaling and adhesion, but the relevant mechanisms are still not fully understood (Cavallaro and Christofori, 2004). A number of cell adhesion proteins, such as N-CAM and N-cadherin, can form part of a signaling complex with FG-FRs in a range of cell types (Cavallaro et al., 2001; Suyama et al., 2002). Stimulation of epithelial cells with FGF results in scattering, and in some cases, induction of EMT events (Savagner et al., 1997; Thiery, 2002), which depend directly on transcriptional down-regulation of E-cadherin levels and also involve Wnt/β-catenin signaling (Ciruna and Rossant, 2001). A direct interaction between FGFRs and N-cadherin has been demonstrated in both neuronal and epithelial cells (Doherty and Walsh, 1996; Suyama et al., 2002), but to date no evidence has arisen for any potential cellular association of FGFR with E-cadherin, which would potentially have different physiological consequences.
E-cadherin, the prototypical epithelial cadherin, is localized to the lateral surface of polarized epithelial cells, where it is concentrated in adherens junctions and mediates adhesion to adjacent cells in a Ca2+-dependent homotypic manner (Yap et al., 1997). The conserved intracellular domain of E-cadherin supports interactions with the Src substrate p120ctn, and either β-catenin or γ-catenin, and α-catenin for coupling to F-actin (Yap, 1998). β-Catenin is involved in transduction of the Wnt signaling pathway, also translocating to the nucleus where it has key roles in early embryo patterning, cell polarity, and cellular transformation during many forms of metastasis (Gottardi and Gumbiner, 2001; Nelson and Nusse, 2004). p120ctn has multiple signaling roles, as well as influencing cadherin adhesive strength and acting as a molecular “stabilizer” of E-cadherin at adherens junctions (Yap et al., 1998; Daniel and Reynolds, 1999; Anastasiadis et al., 2000; Davis et al., 2003; Xiao et al., 2003).
The endocytosis of cadherins, with subsequent recycling back to the plasma membrane, has emerged as a key mechanism to dynamically regulate cell-cell adhesion, signaling, and morphogenesis (reviewed in Bryant and Stow 2004). Cadherins are endocytosed via a number of different routes, including both clathrin-dependent and clathrin-independent pathways. Stabilization of E-cadherin at the plasma membrane by p120ctn inversely regulates levels of E-cadherin endocytosis and cadherin turnover (Davis et al., 2003; Xiao et al., 2003), as does an interplay between active Rac, Cdc42, and IQGAP (Izumi et al., 2004). Src or RTK-induced modulation of tyrosine phosphorylation regulates the integrity of the cadherin/catenin complex (Behrens and Birchmeier, 1994; Daniel and Reynolds, 1997), and subsequent ubiquitylation of tyrosine-phosphorylated E-cadherin by the E3 ubiquitin ligase Hakai can induce endocytosis of E-cadherin (Fujita et al., 2002). Because signaling from RTKs can induce the internalization of E-cadherin, there is the possibility for coendocytosis of activated RTKs with cadherins as a novel mechanism for regulating cadherin adhesive dynamics. In developmental systems, E-cadherin expression and adhesive function seems to lay downstream of FGFR signaling (Ciruna and Rossant, 2001; Chihara et al., 2003).
In light of the emerging evidence for interaction of RTKs and adhesion molecules, generating a range of different physiological consequences, we set out to examine possible cotrafficking of FGFR1 and E-cadherin. Moreover, we show that cotrafficking of an RTK with E-cadherin is a possible mechanism for the joint regulation of both signaling and adhesion.
MATERIALS AND METHODS
Cell Culture and Treatments
Human breast adenocarcinoma (MCF-7) cell monolayers were grown in minimal essential medium (Earle's Salts) supplemented with 10% fetal calf serum, 20 mM HEPES, 2 g/l sodium bicarbonate, 4 mM l-glutamine, 1% nonessential amino acids, and 5 μg/ml insulin at 37°C in 5% CO2, 95% air, as described previously (Nurcombe et al., 2000). Cells were plated on glass coverslips at varying densities for experiments. For some experiments, cells were incubated with either FGF-1 and 5 μg/ml heparin or FGF-2 (both at 10 ng/ml) (Sigma-Aldrich, Castle Hill, Australia) for various times in either complete medium or after incubation in serum-free medium for 18 h at 37°C. For some endocytosis assays, cells were treated with 10 μM cycloheximide at 37°C to block protein synthesis.
Antibodies and Reagents
E-cadherin was detected using mouse monoclonal (HECD-1) and rabbit polyclonal antibodies to human E-cadherin (Dr. Alpha Yap, University of Queensland, Brisbane, Australia) or a mouse monoclonal anti-E-cadherin antibody (BD Transduction Laboratories, Lexingon, CA) for Western blotting. Rabbit polyclonal β-catenin (Sigma-Aldrich), mouse monoclonal tubulin and green fluorescent protein (GFP) (Molecular Probes, Eugene, OR) antibodies also were used. Mouse monoclonal myc antibody is as described previously (Evan et al., 1985). Mouse monoclonal β-catenin, p120ctn, and EEA1 antibodies (BD Transduction Laboratories) and rabbit polyclonal FGFR1, mitogen-activated protein kinase kinase (MEK)-1, MEK-2, extracellular signal-regulated kinase (ERK)-1 and ERK-2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used. Rabbit polyclonal phospho-MEK1/2 and mouse monoclonal phospho-ERK1/2 antibodies were from Cell Signaling Technology (GeneSearch, Arundel, Australia). Secondary antibodies were Cy3-conjugated sheep antimouse and goat anti-rabbit IgGs (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa 488-conjugated goat anti-rabbit and goat anti-mouse IgGs (Molecular Probes), and horseradish peroxidase-conjugated sheep antimouse IgG and goat anti-rabbit IgG (Amrad, Victoria, Australia). Alexa 488-conjugated phalloidin, used to label F-actin, and 4,6-diamidino-2-phenylindole (DAPI), used to label nuclei, were both from Molecular Probes. Cycloheximide was purchased from Sigma-Aldrich.
cDNA Constructs and Transfection
A plasmid encoding full-length human E-cadherin-GFP (C-terminally tagged) is as described previously (Miranda et al., 2003). A GTPase-deficient mutant of Rab5 with either a GFP tag (Rab5Q79L-GFP) or a myc tag (Rab5Q79L-myc), and GFP-tagged dominant negative mutant of Rab5 (Rab5S34N-GFP), as well as GFP-tagged dominant-negative Eps15 (pEGFP-Eps15/EΔ95/295) were kindly provided by Dr. R. Parton (The University of Queensland). A plasmid encoding human FGFR1 was a generous gift from Dr. I. Prudovsky (Maine Medical Centre Research Institute, Scarborough, ME) and was cloned into the pEGFP-N1 expression vector to give C-terminally GFP-tagged FGFR1 (FGFR1-GFP). Hemagglutinin (HA)-tagged Dynamin2K44A was kindly provided by Dr. He Li (Monash University, Melbourne, Australia). HA-tagged ARF6T27N and p120-GFP were kindly provided by Dr. Julie Donaldson (National Institutes of Health, Bethesda, MD) and Dr .Alpha Yap, respectively. The empty vector pEGFP-N1 was from BD Biosciences Clontech (Palo Alto, CA).
MCF-7 cells were plated at subconfluent densities 24 h before transfection. Plasmids were transfected using the LipofectAMINE Plus system (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. Cells were typically left for 18–36 h posttransfection before use. For stable expression, transfected cells were passaged and maintained in G-418–containing medium (Geneticin; Invitrogen) for 10–14 d. Surviving cells were ring-cloned and grown to confluence before being subjected to immunofluorescence and immunoblotting to select lines with appropriate levels of recombinant protein expression.
Immunofluorescence
Cells grown on filters or glass coverslips were fixed in 4% paraformaldehyde in phosphate-buffered saline for 60 min, permeabilized using 0.1% Triton X-100 for 5 min, and then stained as described previously (Miranda et al., 2001). Cells were viewed using an Olympus Provis AX-70 microscope with 40–100× objective lenses, or a Bio-Rad Radiance 2000 confocal microscope. Images were analyzed and adjusted using Adobe Photoshop 7 and ImageJ (National Institutes of Health) as described previously (Miranda et al., 2003).
Immunoblotting
Cells were solubilized in ice-cold extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5 mM MgCl2, 0.2 mM EGTA, and 1% Triton Tx-100) on ice for 5 min and then extracted at 4°C for 45 min. Postnuclear supernatants were obtained by centrifugation at 14,000 × g for 10 min. Samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and analyzed by immunoblotting by using chemiluminescence (SuperSignal chemiluminescence kits; Pierce Chemical, Rockford, IL) (Miranda et al., 2001). Protein concentrations were determined using a BCA Protein Assay Reagent kit (Pierce Chemical); protein transfer and loading were assessed by staining with 0.1% Coomassie Brilliant Blue, and immunolabeling was assessed by densitometry using a Bio-Rad GS-800 densitometer.
RESULTS
FGF Induces Nuclear Translocation of FGFR1 and Internalization of E-Cadherin
MCF-7 cells maintain a polarized morphology, express E-cadherin, and proliferate and migrate in response to FGF (Nurcombe et al., 2000; Paterson et al., 2003). MCF-7 cells were immunolabeled to localize FGFR1 and E-cadherin over a time course of FGF stimulation. In unstimulated cells, cell surface staining of endogenous FGFR1 was barely detectable using available antibodies; however, some staining was apparent on membrane ruffles (Figure 1A, arrowheads), and there was also diffuse staining through the cytoplasm, as noted previously (Johnston et al., 1995). Stimulation with FGF-1 resulted in the accumulation of FGFR1 staining in cell nuclei (arrows); beginning at 1 h, with more intense staining noted at 4 h (Figure 1A). Nuclear staining persisted during prolonged incubation in FGF-1 (up to 24 h) (Figures 1B and 5A, bottom). The intranuclear localization of FGFR1 was confirmed by colocalization with DAPI staining (Figure 1B). Stimulation of MCF-7 cells with FGF-2 also resulted in a similar nuclear translocation of FGFR1 (Figure 4B). Thus, FGF induces trafficking of FGFR1 to the nucleus in MCF-7 cells.
Figure 1.
FGF induces nuclear translocation of FGFR1. (A) MCF-7 cells incubated with FGF-1 for 0–4 h at 37°C were fixed and stained by immunofluorescence by using a polyclonal antibody against endogenous FGFR1. Arrowheads indicate membrane labeling that was only evident on membrane ruffles, whereas arrows denote nuclear FGFR1 labeling. At higher magnification the FGFR1 can be seen inside cell nuclei (inset). (B) MCF-7 cells were incubated with FGF-1 for 24 h at 37°C were fixed and triple labeled using an antibody against endogenous FGFR1, phalloidin to label F-actin in the cell periphery, and DAPI to label cell nuclei. FGFR1 staining overlaps with DAPI-stained nuclei. Bar, 50 μm.
Figure 5.
Coendocytosis of E-cadherin is required for nuclear translocation of FGFR1. (A) Immunofluorescence labeling of MCF-7 cells by using antibodies to E-cadherin (red) and FGFR1 (green), either without (top) or with (bottom) prior incubation with FGF-1 for 24 h at 37°C. FGF induces changes in the morphology and staining in regions of the monolayer. Outline separates cells with (outside dotted outline) and without (inside dotted outline) nuclear FGFR1 labeling. (B) MCF-7 cells were transiently transfected with E-cadherin-GFP, incubated with FGF-1 for 4 h at 37°C, fixed, and stained for immunofluorescence by using an anti-FGFR1 antibody. Arrow depicts particularly strong cell surface labeling. (C) MCF-7 cells stably overexpressing E-cadherin-GFP were incubated with FGF-1 for 2 h, fixed, and stained for endogenous FGFR1. (D) Quantification of cells expressing nuclear FGFR1 labeling after transfection with E-cadherin-GFP in either serum-grown control cells or in cells stimulated with FGF-1 or FGF-2 for 4 h. Control cells represent untransfected cells from the same experiment. (E) Extracts of wild-type MCF-7 cells (wt-MCF-7) and MCF-7 cells stably overexpressing E-cadherin-GFP (hE-GFP-MCF-7) in serum, or with FGF-1 for 2 h, and immunoblotted for E-cadherin, FGFR1 and tubulin.
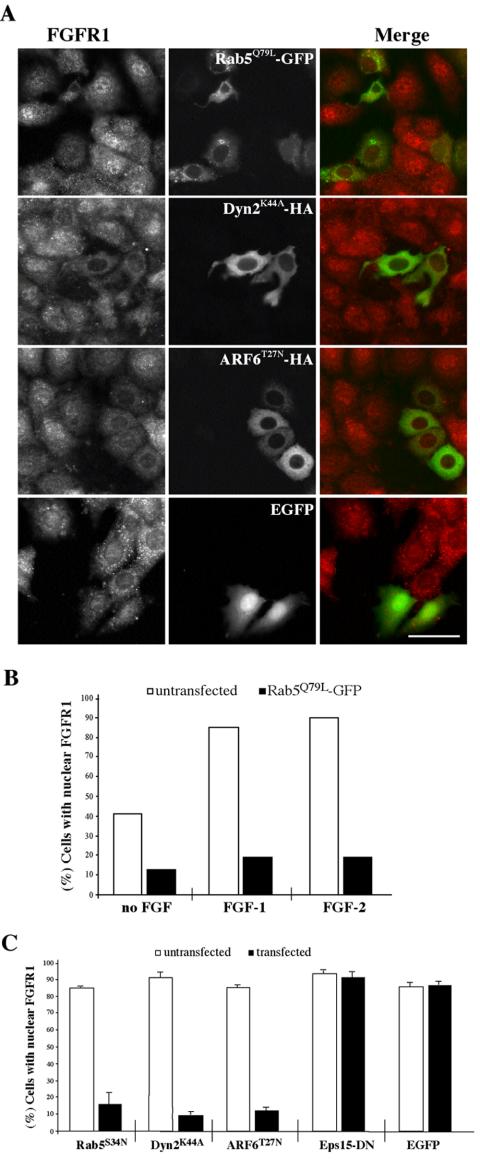
Figure 4.
Nuclear translocation of FGFR1 requires endocytosis of surface FGFR1. (A) MCF-7 cells transiently transfected with either GFP-Rab5Q79L (top), Dyn2K44A-HA (second row), ARF6T27N-HA (third row), or EGFP alone (bottom) were incubated with FGF-1 for 4 h at 37°C, fixed, and stained for FGFR1 (red) and HA (second and third rows). Bar, 50 μm. (B) Quantification of nuclear FGFR1 labeling (percentage of cells) after transfection with Rab5Q79L-GFP in either serumgrown control cells or after stimulation with FGF-1 or FGF-2 for 4 h. Control cells represent untransfected cells from the same experiment, with values representing >100 cells from triplicate samples. (C) Quantification of the percentage of cells expressing nuclear FGFR1 labeling after transfection with Rab5S34N-GFP, Dyn2K44A-HA, ARF6T27N-HA, DN-Eps15-GFP, or EGFP alone and stimulation with FGF-1 for 4 h. Control cells represent untransfected cells from the same experiment as mutant transfectants. The error bars represent mean ± SD from triplicate samples.
We next examined the localization of endogenous E-cadherin in ligand-stimulated cells. In unstimulated MCF-7 cells, E-cadherin was found predominantly on lateral cell membranes (Figure 2A). Soon after incubation with FGF-1, punctate intracellular staining of E-cadherin occurred (Figure 2A). FGF-1 caused a progressive loss of E-cadherin staining at the cell surface, concomitant with morphological changes, signified by a loss of the cobblestone appearance of the cell monolayer and the spreading and movement of cells. E-cadherin staining progressively occurred in the punctate intracellular pattern (Figure 2A), consistent with its endocytosis (Le et al., 1999; Paterson et al., 2003). Similar results were obtained by incubation of cells with FGF-2 (our unpublished data). Thus, FGF induces translocation of E-cadherin staining from the cell surface to an intracellular pool. To confirm the endocytic nature of this FGF-induced internal pool of E-cadherin, MCF-7 cells were preincubated with cycloheximide. Blocking protein synthesis did not eliminate the vesicular labeling of E-cadherin induced by FGF stimulation (Figure 2B), indicating that it is not newly synthesized E-cadherin. Furthermore, marked accumulation of E-cadherin in intracellular vesicles occurred after repeating these treatments at 18°C instead of 37°C, a technique previously shown to accumulate E-cadherin in early or sorting endosomal compartments (Le et al., 1999). Finally, intracellular E-cadherin was colocalized with an early endosomal marker, EEA1, in some vesicles in FGF-stimulated cells (Figure 2C). Thus, in MCF-7 cells, FGF disrupts epithelial cell-cell contacts and induces endocytosis of surface E-cadherin.
Figure 2.
FGF induces internalization of E-cadherin into early endosomes. (A) MCF-7 cells were incubated with FGF-1 at 37°C for 0–4 h, fixed, and stained by immunofluorescence by using an antihuman E-cadherin antibody. Staining is seen on lateral cell membranes and increasingly in intracellular vesicles. (B) MCF-7 cells were incubated with 10 mM cycloheximide (CHX) for 2.5 h to halt new protein synthesis, fixed, and stained for endogenous E-cadherin. Internal vesicular E-cadherin labeling was depleted after CHX treatment at 37°C (left) but reappeared after addition of FGF-1 during the last 2 h of CHX treatment (middle). Insets demonstrate higher magnification of dotted regions. Incubation of cells at 18°C under the same conditions resulted in a marked accumulation of endosomal E-cadherin staining (right). (C) Preconfluent MCF-7 cells stimulated with FGF-1 for 2 h at 37°C were fixed and double stained by using antibodies against E-cadherin (green) and EEA1 (red). Arrows denote examples of E-cadherin and EEA1 colocalized in early endosomes. Inset depicts higher magnification of boxed region to emphasize colocalization. Bar, 50 μm.
The internalization of both FGFR1 and E-cadherin in response to FGF led us to examine possible coendocytosis and trafficking of both proteins. Because endogenous FGFRs are present at relatively low levels in MCF-7 cells (Johnston et al., 1995; Nurcombe et al., 2000) and are difficult to immunolabel at cell membranes (Figure 1; Maher, 1996), MCF-7 cells were transiently transfected with a GFP-tagged FGFR1 (FGFR1-GFP). FGFR1-GFP was expressed both at the cell surface of serum-grown MCF-7 cells (Figure 3A, top), and in intracellular puncta. Stimulation with FGF-1 resulted in the loss of membrane labeling of FGFR1-GFP (Figure 3A, bottom). Expression of FGFR1-GFP in serum-grown cells resulted in a decrease of total and plasma membrane-associated E-cadherin labeling, concomitant with the appearance of exaggerated internal punctate labeling (Figure 3A), consistent with endocytosis of E-cadherin in response to FGFR1 activation (Figure 2). Internalized FGFR1-GFP was found to localize with endogenous E-cadherin in a proportion of endosomes, in both serum-grown cells (Figure 3A, top) and cells treated with FGF-1 (Figure 3A, bottom). Confocal imagining of FGFR1-GFP-transfected cells treated with FGF-1 again confirmed the coincident labeling of E-cadherin in a subset of endosomes (Figure 3A, bottom insets). The internalization of E-cadherin into Rab5- and EEA-1–positive early endosomes has been well documented in both Madin-Darby canine kidney and MCF-7 cells (Le et al., 1999; Paterson et al., 2003) and also was seen in response to FGF stimulation in MCF-7 cells transfected with a constitutively active mutant GFP-Rab5Q79L (Figure 2 and 3B, top, arrows). When MCF-7 cells were cotransfected with a myc-tagged Rab5Q79L and FGFR1-GFP and stimulated with FGF-1, the FGFR1-GFP also was found in enlarged early endosomes induced by Rab5Q79L (Figure 3B, top, arrows), characteristic of longer duration expression of this mutant (Stenmark et al., 1994). Distinct localization of internal FGFR1-GFP to these enlarged Rab5-positive endosomes is demonstrated in Figure 3B (insets). Thus, surface E-cadherin and FGFR1 are cointernalized into early endosomal compartments in response to FGF stimulation.
Figure 3.
Colocalization of E-cadherin and FGFR1 on lateral membranes and in endosomes. (A) MCF-7 cells were transiently transfected with FGFR1-GFP (green) and either grown in serum without (top) or with (bottom) exogenous FGF-1, fixed, and stained for endogenous E-cadherin (red). Insets depict confocal imaging of E-cadherin and FGFR1-GFP. Note colocalization with E-cadherin in endosomes. (B) MCF-7 cells were transiently transfected with GFP-Rab5Q79L alone (green, top) or cotransfected with FGFR1-GFP (green) and myc-Rab5Q79L (red, bottom). Cells were incubated with FGF-1 for 2 h at 37°C, fixed, and stained using a myc (bottom) or E-cadherin antibody (top) and imaged for GFP and immunofluorescence. Insets depict cropped, higher-magnification examples of colocalization of FGFR1-GFP with Rab5Q79L. Bar, 50 μm.
Endocytosis Is an Essential, Early Step in Nuclear Translocation of FGFR1
Our data suggest that ligand-activated FGFR1 is internalized from the cell surface in what could be the initial step in nuclear accumulation of FGFR1. To test this, MCF-7 cells were transfected with functional mutants of endocytic machinery proteins to block endocytosis by different routes. Overall, nuclear staining of FGFR1 was recorded in ∼40% of serum-grown MCF-7 cells, and this incidence increased to >90% after stimulation with FGF-1 or FGF-2 (Figure 4B), whereas serum-starved cells had virtually no nuclear staining (Figure 5A, top). Expression of Rab5Q79L-GFP resulted in a more than threefold reduction in FGF-1– or FGF-2–stimulated cells with nuclear FGFR1. After longer incubations in FGF-1, cells expressing Rab5Q79L-GFP had no nuclear staining of FGFR1 in contrast to surrounding cells with prominent nuclear FGFR1 (Figure 4A, top). Rab5Q79L-GFP expression experiments also served to highlight that both FGF-1 and FGF-2 produce equivalent internalization of FGFR1 (Figure 4B). Consistent with this, a dominant-negative mutant of Rab5 (Rab5S34N-GFP) also blocked nuclear translocation of FGFR1 in response to FGF-1 stimulation, at similar levels (Figure 4C). Expression of a dominant-negative mutant of dynamin 2 (HA-Dyn2K44A) (which functions at both the plasma membrane and Golgi network) (Praefcke and McMahon, 2004) additionally was able to block nuclear translocation of FGFR1 (Figure 4A, middle), resulting in only 15% of transfected cells with nuclear staining (Figure 4C), whereas surrounding untransfected cells had consistent nuclear labeling of FGFR1. Dominant-negative ARF6 (HA-ARF6T27N), which we have previously shown to block E-cadherin endocytosis in MCF-7 cells (Paterson et al., 2003), also reduced nuclear FGFR1 labeling to 10% of transfected cells (Figure 4C). Expression of either a dominant-negative mutant of the clathrin endocytosis machinery component, Eps15 (DN-Eps15-GFP), or of EGFP alone, did not significantly reduce the incidence of nuclear translocation of FGFR1 in response to FGF (Figure 4, B and C). Interestingly, this mutant is also unable to block internalization of E-cadherin (Paterson et al., 2003). These data confirm that similar endocytic machinery is responsible for the uptake of FGFR1 and E-cadherin (Paterson et al., 2003) in MCF-7 cells. Importantly, these experiments also show that traffic into an early endosome is an essential first step in the nuclear translocation of FGFR1.
E-Cadherin Modulates FGFR1 Endocytosis and Nuclear Translocation
Based on the endocytosis of both E-cadherin and FGFR1 in response to FGF stimulation, we next examined a possible direct role for E-cadherin in regulating the internalization and nuclear translocation of FGFR1. MCF-7 cells were grown to ∼80% confluence, serum-starved for 18 h, and stimulated with FGF-1 for 24 h to induce both nuclear translocation of FGFR1 and extensive endocytosis of surface E-cadherin. Overall, there was a significant change in the morphology of cells, including disruption of the epithelial monolayer (as noted in Figure 1), cell spreading, and cell motility (Figure 5A). E-cadherin staining revealed the appearance of irregular, jagged cell-cell contacts. Interestingly, nuclear labeling of FGFR1 was inversely correlated to retention of adherens junctions and epithelial polarity, with nuclear FGFR1 only observed in cells with disrupted E-cadherin-based cell-cell contacts (Figure 5A, outside outline).
MCF-7 cells were then transiently transfected to overexpress E-cadherin-GFP, stimulated with FGF-1, and then fixed and stained for endogenous FGFR1. In nontransfected cells, FGF-1 stimulation induced nuclear translocation of FGFR1 (Figure 5B), whereas in contrast, cells overexpressing E-cadherin-GFP had no nuclear FGFR1 labeling (Figure 5B). Quantification of this effect revealed a marked suppression by E-cadherin (>8-fold) on the incidence of nuclear translocation of FGFR1 induced by either serum, FGF-1, or FGF-2 (Figure 5D). MCF-7 cell lines stably overexpressing E-cadherin-GFP (to >2-fold) (Figure 5E) also were treated with FGF-1 for 2 h and then fixed and stained for localization of endogenous FGFR1. In these cells where overexpressed E-cadherin-GFP was largely at the cell surface, there was no nuclear translocation of FGFR1 (Figure 5C), further confirming the results in transiently transfected cells. A major finding of these experiments is thus that E-cadherin levels and intact adherens junctions directly and inversely regulate FGFR1 nuclear translocation. To examine the possibility that E-cadherin levels might influence the expression of FGFR1, immunoblotting was performed on extracts of wild-type and E-cadherin-GFP stably overexpressing cells. Total FGFR1 levels were unchanged by overexpression of E-cadherin and were also not influenced by treatment with FGF-1 (2 h) (Figure 5E). Thus, the effects of E-cadherin on FGF-induced nuclear translocation of FGFR1 are purely attributable to suppressing its trafficking to this compartment.
E-Cadherin Attenuates FGF Signaling to the MAPK Pathway
Translocation of FGF ligand to the nucleus is involved in signaling to the MAPK pathway (Wiedlocha et al., 1994). Thus, inhibition of FGFR1 nuclear translocation by E-cadherin overexpression has the potential to influence such signaling. To test this, wild-type MCF-7 cells (wt-MCF-7) and clonal populations of MCF-7 cells stably overexpressing E-cadherin-GFP (hE-GFP-MCF-7) were used to compare MEK and ERK activation. Cells were initially grown to confluence, serum-starved for 18 h, and then stimulated with FGF-1 for various times. In wild-type MCF-7 cells, stimulation with FGF-1 resulted in activation of MEK1/2 in the first 10 min, followed by reduced but still strong levels at 2 and 18 h after treatment, even increasing slightly at the latter time point (Figure 6). Robust activation of ERK1/2 occurred by 10 min of stimulation; thereafter activation was sustained at a reduced level by 18 h (Figure 6). hE-GFP-MCF-7 cells displayed markedly reduced MEK1/2 activation across the time course of FGF stimulation (Figure 6). Interestingly, densitometry of immunoblots revealed the extent of this inhibition (20-fold reduction at 10 min) but also revealed an activation pattern for MEK1/2 in hE-GFP-MCF-7 cells, similar to wild-type MCF-7 cells, albeit at a much less robust level (Figure 6). In hE-GFP-MCF-7 cells, there was a severe reduction in the levels of p-ERK and its persistence over the time course, with no detectable signal by 18 h (Figure 6). Equal amounts of protein were loaded for all conditions, as detected by immunoblotting for total MEK1/2 and ERK1/2. These results show that E-cadherin overexpression attenuated signaling of FGFR1 to both MEK and particularly ERK. Because MCF-7 cells already express endogenous E-cadherin, we repeated this same experiment in wild-type Chinese hamster ovary cells, which do not express E-cadherin. Overexpression of E-cadherin in this context also attenuated MAPK signaling (as measured by ERK activation) in response to FGF (our unpublished data). Together, our results indicate that E-cadherin has a direct and suppressive effect on signaling to the MAPK pathway by FGF/FGFR, with E-cadherin attenuating both the level and duration of MAPK activation. This stands as further evidence that E-cadherin can directly affect both the trafficking and function of FGFR.
Figure 6.
E-cadherin attenuates FGF signaling to the MAPK pathway. Wild-type MCF-7 cells (wt-MCF-7) or MCF-7 cells stably overexpressing high levels of human E-cadherin-GFP (hE-GFP-MCF-7) were grown to confluence, serum-starved for 18 h, and either unstimulated (control) or incubated with FGF-1 for 10 min, 2 h, or 18 h and analyzed by SDS-PAGE and immunoblotting by using antibodies against total MEK1/2 (t-MEK) and ERK1/2 (t-ERK) or phosphorylated MEK1/2 (p-MEK) and ERK1/2 (p-ERK). Densitometric analysis of MEK1/2 and ERK1/2 activation in response to FGF-1, compared with control, unstimulated cells also was performed. Phosphorylated pools in wt-MCF-7 cells, uncolored; phosphorylated pools in hE-GFP-MCF-7 cells, black columns.
Conjoint Regulation of E-Cadherin Endocytosis and FGFR1 Nuclear Translocation by p120ctn
p120ctn has demonstrated roles in regulating stability and endocytosis of cadherin at the plasma membrane (Davis et al., 2003; Xiao et al., 2003); in particular, p120ctn overexpression was found to block VE-cadherin endocytosis in endothelial cells. Thus, p120ctn (p120-GFP) was transiently overexpressed in MCF-7 cells, as an independent means of regulating E-cadherin endocytosis. Cells were then stimulated with FGF-1 for 2 h to induce internalization of both E-cadherin and FGFR1. Our results show that overexpression of p120-GFP also effectively inhibits E-cadherin internalization in epithelial cells, and moreover, FGFR1 nuclear translocation is simultaneously blocked. Interestingly, these effects were confluence dependent. In patches of adhesive, polarized cells, p120-GFP was distinctly localized at points of cell-cell contact as well as giving cytoplasmic staining. In these cells, p120ctn overexpression resulted in a complete inhibition of the internalization of E-cadherin in response to FGF (Figure 7, a–c, arrow), and a complete absence of nuclear FGFR1 staining (Figure 7, g–i). In contrast, internalization of E-cadherin in response to FGF was not inhibited in preconfluent, poorly adherent transfected cells, which displayed internal puncta as the predominant localization of p120-GFP (Figure 7, d–f). Nuclear translocation of FGFR1 followed a similar phenotype in preconfluent cells where p120-GFP was unable to fully inhibit nuclear translocation of FGFR1 (Figure 7, j–l), as was expression of EGFP alone (Figure 4, B and C). Modulation of E-cadherin internalization by p120ctn in response to FGF is not therefore due to levels of p120ctn expression per se, but instead reflects a functional engagement of p120ctn as part of active adhesive E-cadherin/catenin complexes at adherens junctions in polarized cells. Similarly, nuclear translocation of FGFR1 is further coupled to the E-cadherin/catenin complex, whereby stabilization of the complex by p120ctn overexpression also mitigates nuclear trafficking of FGFR1 in response to FGF. These results provide further evidence that E-cadherin, through both stability of adherens junctions and its trafficking, regulates FGFR1 nuclear translocation and signaling of FGFR1.
Figure 7.
p120ctn regulates endocytosis of E-cadherin and nuclear translocation of FGFR1. Immunofluorescence images of MCF-7 cells transiently transfected with p120-GFP, incubated with FGF-1 for 2 h at 37°C, fixed, and stained for endogenous E-cadherin (a and d, red) and FGFR1 (g and j, green). In polarized cells, a–c and g–i, transfected cells have no nuclear labeling of FGFR1, whereas in preconfluent cells, d–f and j–l, FGFR1 is still found in the nucleus of transfected cells. Bar, 50 μm.
DISCUSSION
In this article, we have studied FGFR1 and the E-cadherin/catenin complex, revealing a novel, conjoint regulation of the two systems, which is governed by endocytic trafficking. FGF stimulation revealed two distinct features—nuclear translocation of surface FGFR1 and endocytosis of surface E-cadherin—that occur concurrently, are governed by the same molecular mechanisms, and impact on the signaling and adhesive status of MCF-7 cells. Specifically, the retention of E-cadherin at cell-cell contacts directly affects the trafficking and function of FGFR1.
Previous studies have identified developmental and genetic links between the FGF and E-cadherin systems (Ciruna and Rossant, 2001; Chihara et al., 2003), with a focus primarily on transcriptional regulation of E-cadherin (Thiery, 2002). However, regulation of E-cadherin endocytosis and recycling presents an equally powerful mechanism for modulation of E-cadherin function (Le et al., 1999; Jarrett et al., 2002). We set out here to examine the role of endocytosis in regulation of the functional interaction between the FGF and E-cadherin systems.
The opportunity for conjoint regulation of E-cadherin with FGFR1 is provided at the outset by six lines of evidence in the current study: 1) E-cadherin and FGFR1 both internalize in response to FGF stimulation, confirming that E-cadherin lays downstream or at the level of FGFR1 activation; 2) both proteins internalize into Rab5/EEA1-positive endosomes and colocalize in intracellular compartments, revealing that trafficking of both proteins is influenced by receptor activation; 3) initial internalization and trafficking are governed by the same molecular mechanisms; 4) FGF signaling results in deregulation of E-cadherin function, suggesting an inverse regulatory action of FGF on E-cadherin; 5) overexpression of E-cadherin blocks nuclear translocation of FGFR1 and attenuates MAPK activation by FGF, revealing a reciprocal negative regulation of FGFR1 by E-cadherin; and finally, 6) inhibition of E-cadherin internalization (by p120ctn) also blocks nuclear translocation of FGFR1.
Since the demonstration that at least some of the nuclear-localized pool of FGFR1 derives from the cell surface (Maher, 1996), the mechanisms for this translocation have remained elusive. Curiously, FGFR1 contains no detectable nuclear localization sequence, but it is nonetheless reliant on importin β for nuclear translocation (Reilly and Maher, 2001). Here, we demonstrate the internalization of FGFR1 into the endocytic pathway is requisite for this nuclear translocation. In contrast to previous studies, we also reveal that both FGF-1 and FGF-2 are able to induce trafficking of FGFR1 into the nucleus, with equal affinity and over a similar time course in MCF-7 cells (Maher, 1996; Prudovsky et al., 1996). Much of the nuclear function of FGFR1 has been attributed to initiation of proliferation (Reilly and Maher, 2001; Stachowiak et al., 2003), a function that is dependent on the FGFR tyrosine kinase region (Reilly and Maher, 2001; Peng et al., 2002). Because interaction of FGFR1 with cAMP-responsive element complexes is able to regulate the transcription of diverse sets of genes (Stachowiak et al., 2003), it will be thus of interest to delineate whether this is the sole role for nuclear-localized FGFR1, or whether FGFR1 may fulfill other cell type-specific functions.
In our studies, nuclear translocation of FGFR1 occurred only in those cells with endocytosed surface E-cadherin, or with extensive disruption of cell-cell contacts. Importantly then, the molecular machinery components found to regulate nuclear translocation of FGFR1 are also known regulators of E-cadherin internalization (Paterson et al., 2003). Furthermore, protein kinase C is a known inducer of both E-cadherin internalization (Le et al., 1999) and of nuclear translocation of FGFR1 (Peng et al., 2002). Conjoint internalization of cell surface E-cadherin and FGFR1 in response to FGF provides a functional mechanism for coregulation of adhesion and signaling. And although we show that both proteins localize to early endosomes, this does not exclude the possibility that they may also cotraffic to other subsequent compartments, such as recycling or late endosomes.
Interestingly, in the short times studied, the transforming function of FGF did not result from a marked loss of E-cadherin expression, but instead resulted from trafficking of E-cadherin to early endosomes, potentially into a recycling pool. This highlights the potential for endocytosis to be an additional, and perhaps early, mechanism for down-regulating E-cadherin function downstream of RTKs, separate or in addition to transcriptional repression over longer times (Ciruna and Rossant, 2001; Montero et al., 2001; Lu et al., 2003).
Microdomain organization at the plasma membrane is of emerging importance to the individual and perhaps joint functions of E-cadherin and FGFR1. E-cadherin is present in both stable adhesive complexes and in a separate pool that is available for endocytosis and constitutive recycling (Le et al., 1999; Izumi et al., 2004). The microdomain organization of FGF receptors at the cell surface, for instance into glycosphingolipid-enriched microdomains, directly regulates their signaling capabilities to both MAPK and c-Src (Toledo et al., 2004), in an adhesion-dependent manner. Thus, a model supported by our observations is that, upon FGF ligation, FGFR1 and E-cadherin move into a membrane microdomain from which they can both be endocytosed. Whether this is due to a more direct, physical interaction between E-cadherin and FGFR1 is a controversial issue (El-Hariry et al., 2001; Suyama et al., 2002) and awaits further investigation in our system. This reorganization precedes their coendocytosis, which in turn is necessary for nuclear translocation and signaling by FGFR1. Overexpression of E-cadherin or the function of p120ctn act to suppress this phenomenon by stabilizing E-cadherin in adhesive junctional complexes (Fujita et al., 2002; Davis et al., 2003; Xiao et al., 2003). Indeed, levels of E-cadherin have been shown to regulate EGFR and c-Met signaling (Qian et al., 2004), and it will be of interest to see whether p120ctn can similarly regulate the function of other RTKs.
A number of recent studies have suggested that E-cadherin–mediated PI3K/Akt signaling can, in some instances, account for attenuation of the MAPK pathway (Rommel et al., 1999; Zimmermann and Moelling, 1999; Laprise et al., 2004); however, discrepancies exist as to the level at which E-cadherin attenuates RTK signaling (Qian et al., 2004). We here provide the first evidence that internalization and cotrafficking of both complexes is a further mechanism for coregulation of such processes. Suyama et al. (2002) suggest modulation of FGFR internalization by N-cadherin as a potential mechanism for regulation of cadherin/RTK function, and we here show directly that FGFR1 internalization and signaling to MAPK are modulated by E-cadherin. It will be of interest to see whether the cross talk demonstrated between E-cadherin and other receptor molecules (e.g., EGFR, Qian et al., 2004; αVβ1 integrin, Avizienyte et al., 2002) also involves modulation of intracellular trafficking.
Overall, this study provides the first example of comodulation of a cadherin and RTK by endocytic trafficking, demonstrating modulation of RTK signaling and function tightly coupled to cell adhesive status and morphology. Importantly, we show that E-cadherin attenuates FGF signaling. It is now well recognized that a critical event during metastatic transformation of some epithelial cells is the switch from E-cadherin to N-cadherin expression (Cavallaro and Christofori, 2004). Indeed, in MCF-7 cells, ectopically expressed N-cadherin binds to FGFRs to enhance signaling and metastatic behavior (Suyama et al., 2002). In contrast to N-cadherin, E-cadherin may conversely act to suppress FGF-induced transformation through direct regulation of FGFR signaling and intracellular trafficking.
Acknowledgments
We thank I. Prudovsky, R. Parton, J. Hancock, J. Donaldson, H. Li, A. Yap, S. Cool, and V. Nurcombe for generous gifts of vectors and reagents and for helpful advice and discussions. We thank members of the Stow laboratory for invaluable support and technical assistance. J.L.S. is supported by a fellowship and grants from the National Health and Medical Research Council of Australia.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–09–0845. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0845.
References
- Anastasiadis, P. Z., Moon, S. Y., Thoreson, M. A., Mariner, D. J., Crawford, H. C., Zheng, Y., and Reynolds, A. B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637-644. [DOI] [PubMed] [Google Scholar]
- Avizienyte, E., Wyke, A. W., Jones, R. J., McLean, G. W., Westhoff, M. A., Brunton, V. G., and Frame, M. C. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4, 632-638. [DOI] [PubMed] [Google Scholar]
- Behrens, J., and Birchmeier, W. (1994). Cell-cell adhesion in invasion and metastasis of carcinomas. Cancer Treat. Res. 71, 251-266. [DOI] [PubMed] [Google Scholar]
- Belleudi, F., Ceridono, M., Capone, A., Serafino, A., Marchese, C., Picardo, M., Frati, L., and Torrisi, M. R. (2002). The endocytic pathway followed by the keratinocyte growth factor receptor. Histochem. Cell Biol. 118, 1-10. [DOI] [PubMed] [Google Scholar]
- Bryant, D. M., and Stow, J. L. (2004). The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14, 427-434. [DOI] [PubMed] [Google Scholar]
- Cavallaro, U., and Christofori, G. (2004). Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 4, 118-132. [DOI] [PubMed] [Google Scholar]
- Cavallaro, U., Niedermeyer, J., Fuxa, M., and Christofori, G. (2001). N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 3, 650-657. [DOI] [PubMed] [Google Scholar]
- Chihara, T., Kato, K., Taniguchi, M., Ng, J., and Hayashi, S. (2003). Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 130, 1419-1428. [DOI] [PubMed] [Google Scholar]
- Ciruna, B., and Rossant, J. (2001). FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37-49. [DOI] [PubMed] [Google Scholar]
- Daniel, J. M., and Reynolds, A. B. (1997). Tyrosine phosphorylation and cadherin/catenin function. Bioessays 19, 883-891. [DOI] [PubMed] [Google Scholar]
- Daniel, J. M., and Reynolds, A. B. (1999). The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell. Biol. 19, 3614-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. A., Ireton, R. C., and Reynolds, A. B. (2003). A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163, 525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, C., Spencer-Dene, B., Dillon, C., and Fantl, V. (2000). Tyrosine kinase signalling in breast cancer: fibroblast growth factors and their receptors. Breast Cancer Res. 2, 191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, P., and Walsh, F. S. (1996). CAM-FGF receptor interactions: a model for axonal growth. Mol. Cell Neurosci. 8, 99-111. [DOI] [PubMed] [Google Scholar]
- El-Hariry, I., Pignatelli, M., and Lemoine, N. R. (2001). FGF-1 and FGF-2 modulate the E-cadherin/catenin system in pancreatic adenocarcinoma cell lines. Br. J. Cancer 84, 1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan, G. I., Lewis, G. K., Ramsay, G., and Bishop, J. M. (1985). Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H. E., Behrens, J., Sommer, T., and Birchmeier, W. (2002). Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222-231. [DOI] [PubMed] [Google Scholar]
- Gottardi, C. J., and Gumbiner, B. M. (2001). Adhesion signaling: how betacatenin interacts with its partners. Curr. Biol. 11, R792-R794. [DOI] [PubMed] [Google Scholar]
- Izumi, G., Sakisaka, T., Baba, T., Tanaka, S., Morimoto, K., and Takai, Y. (2004). Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 166, 237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett, O., Stow, J. L., Yap, A. S., and Key, B. (2002). Dynamin-dependent endocytosis is necessary for convergent-extension movements in Xenopus animal cap explants. Int. J. Dev. Biol. 46, 467-473. [PubMed] [Google Scholar]
- Johnson, D. E., and Williams, L. T. (1993). Structural and functional diversity in the FGF receptor multigene family. Adv. Cancer Res. 60, 1-41. [DOI] [PubMed] [Google Scholar]
- Johnston, C. L., Cox, H. C., Gomm, J. J., and Coombes, R. C. (1995). Fibroblast growth factor receptors (FGFRs) localize in different cellular compartments. A splice variant of FGFR-3 localizes to the nucleus. J. Biol. Chem. 270, 30643-30650. [DOI] [PubMed] [Google Scholar]
- Klint, P., and Claesson-Welsh, L. (1999). Signal transduction by fibroblast growth factor receptors. Front. Biosci. 4, D165-D177. [DOI] [PubMed] [Google Scholar]
- Laprise, P., Langlois, M. J., Boucher, M. J., Jobin, C., and Rivard, N. (2004). Down-regulation of MEK/ERK signaling by E-cadherin-dependent PI3K/Akt pathway in differentiating intestinal epithelial cells. J. Cell Physiol. 199, 32-39. [DOI] [PubMed] [Google Scholar]
- Le, T. L., Yap, A. S., and Stow, J. L. (1999). Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146, 219-232. [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., Ghosh, S., Wang, Z., and Hunter, T. (2003). Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4, 499-515. [DOI] [PubMed] [Google Scholar]
- Maher, P. A. (1996). Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J. Cell Biol. 134, 529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, G. R. (1998). The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 12, 1571-1586. [DOI] [PubMed] [Google Scholar]
- Miranda, K. C., Joseph, S. R., Yap, A. S., Teasdale, R. D., and Stow, J. L. (2003). Contextual binding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J. Biol. Chem. 278, 43480-43488. [DOI] [PubMed] [Google Scholar]
- Miranda, K. C., Khromykh, T., Christy, P., Le, T. L., Gottardi, C. J., Yap, A. S., Stow, J. L., and Teasdale, R. D. (2001). A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J. Biol. Chem. 276, 22565-22572. [DOI] [PubMed] [Google Scholar]
- Montero, J. A., Ganan, Y., Macias, D., Rodriguez-Leon, J., Sanz-Ezquerro, J. J., Merino, R., Chimal-Monroy, J., Nieto, M. A., and Hurle, J. M. (2001). Role of FGFs in the control of programmed cell death during limb development. Development 128, 2075-2084. [DOI] [PubMed] [Google Scholar]
- Nelson, W. J., and Nusse, R. (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe, V., Smart, C. E., Chipperfield, H., Cool, S. M., Boilly, B., and Hondermarck, H. (2000). The proliferative and migratory activities of breast cancer cells can be differentially regulated by heparin sulfates. J. Biol. Chem. 275, 30009-30018. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M. (2000). FGFs, heparin sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108-112. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M., and Itoh, N. (2001). Fibroblast growth factors. Genome Biol. 2, REVIEWS3005. 1-3005.12; http://genomebiology.com/2001/2/3/reviews/3005. Accessed June 18, 2003. [DOI] [PMC free article] [PubMed]
- Ornitz, D. M., Xu, J., Colvin, J. S., McEwen, D. G., MacArthur, C. A., Coulier, F., Gao, G., and Goldfarb, M. (1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297. [DOI] [PubMed] [Google Scholar]
- Paterson, A. D., Parton, R. G., Ferguson, C., Stow, J. L., and Yap, A. S. (2003). Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 278, 21050-21057. [DOI] [PubMed] [Google Scholar]
- Peng, H., Myers, J., Fang, X., Stachowiak, E. K., Maher, P. A., Martins, G. G., Popescu, G., Berezney, R., and Stachowiak, M. K. (2002). Integrative nuclear FGFR1 signaling (INFS) pathway mediates activation of the tyrosine hydroxylase gene by angiotensin II, depolarization and protein kinase C. J. Neurochem. 81, 506-524. [DOI] [PubMed] [Google Scholar]
- Praefcke, G. J., and McMahon, H. T. (2004). The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell. Biol. 5, 133-147. [DOI] [PubMed] [Google Scholar]
- Prudovsky, I. A., Savion, N., LaVallee, T. M., and Maciag, T. (1996). The nuclear trafficking of extracellular fibroblast growth factor (FGF)-1 correlates with the perinuclear association of the FGF receptor-1alpha isoforms but not the FGF receptor-1beta isoforms. J. Biol. Chem. 271, 14198-14205. [DOI] [PubMed] [Google Scholar]
- Qian, X., Karpova, T., Sheppard, A. M., McNally, J., and Lowy, D. R. (2004). E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 23, 1739-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, J. F., and Maher, P. A. (2001). Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J. Cell Biol. 152, 1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel, C., Clarke, B. A., Zimmermann, S., Nunez, L., Rossman, R., Reid, K., Moelling, K., Yancopoulos, G. D., and Glass, D. J. (1999). Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286, 1738-1741. [DOI] [PubMed] [Google Scholar]
- Savagner, P., Yamada, K. M., and Thiery, J. P. (1997). The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J. Cell Biol. 137, 1403-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin, A., Mohammadi, M., Huang, J., and Schlessinger, J. (1994). Internalization of fibroblast growth factor receptor is inhibited by a point mutation at tyrosine 766. J. Biol. Chem. 269, 17056-17061. [PubMed] [Google Scholar]
- Stachowiak, M. K., Fang, X., Myers, J. M., Dunham, S. M., Berezney, R., Maher, P. A., and Stachowiak, E. K. (2003). Integrative nuclear FGFR1 signaling (INFS) as a part of a universal “feed-forward-and-gate” signaling module that controls cell growth and differentiation. J Cell Biochem. 90, 662-691. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Parton, R. G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama, K., Shapiro, I., Guttman, M., and Hazan, R. B. (2002). A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2, 301-314. [DOI] [PubMed] [Google Scholar]
- Thiery, J. P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442-454. [DOI] [PubMed] [Google Scholar]
- Toledo, M. S., Suzuki, E., Handa, K., and Hakomori, S. (2004). Cell growth regulation through GM3-enriched microdomain (glycosynapse) in human embryonal fibroblast WI38 and its oncogenic transformant VA13. J. Biol. Chem. 279, 34655-34664. [DOI] [PubMed] [Google Scholar]
- Wiedlocha, A., Falnes, P. O., Madshus, I. H., Sandvig, K., and Olsnes, S. (1994). Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell 76, 1039-1051. [DOI] [PubMed] [Google Scholar]
- Xiao, K., Allison, D. F., Buckley, K. M., Kottke, M. D., Vincent, P. A., Faundez, V., and Kowalczyk, A. P. (2003). Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A. S. (1998). The morphogenetic role of cadherin cell adhesion molecules in human cancer: a thematic review. Cancer Investig. 16, 252-261. [DOI] [PubMed] [Google Scholar]
- Yap, A. S., Brieher, W. M., and Gumbiner, B. M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119-146. [DOI] [PubMed] [Google Scholar]
- Yap, A. S., Niessen, C. M., and Gumbiner, B. M. (1998). The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 141, 779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, S., and Moelling, K. (1999). Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286, 1741-1744. [DOI] [PubMed] [Google Scholar]