ABSTRACT
The development of an effective maternal HIV-1 vaccine that could synergize with antiretroviral therapy (ART) to eliminate pediatric HIV-1 infection will require the characterization of maternal immune responses capable of blocking transmission of autologous HIV to the infant. We previously determined that maternal plasma antibody binding to linear epitopes within the variable loop 3 (V3) region of HIV envelope (Env) and neutralizing responses against easy-to-neutralize tier 1 viruses were associated with reduced risk of peripartum HIV infection in the historic U.S. Woman and Infant Transmission Study (WITS) cohort. Here, we defined the fine specificity and function of the potentially protective maternal V3-specific IgG antibodies associated with reduced peripartum HIV transmission risk in this cohort. The V3-specific IgG binding that predicted low risk of mother-to-child-transmission (MTCT) was dependent on the C-terminal flank of the V3 crown and particularly on amino acid position 317, a residue that has also been associated with breakthrough transmission in the RV144 vaccine trial. Remarkably, the fine specificity of potentially protective maternal plasma V3-specific tier 1 virus-neutralizing responses was dependent on the same region in the V3 loop. Our findings suggest that MTCT risk is associated with neutralizing maternal IgG that targets amino acid residues in the C-terminal region of the V3 loop crown, suggesting the importance of the region in immunogen design for maternal vaccines to prevent MTCT.
IMPORTANCE Efforts to curb HIV-1 transmission in pediatric populations by antiretroviral therapy (ART) have been highly successful in both developed and developing countries. However, more than 150,000 infants continue to be infected each year, likely due to a combination of late maternal HIV diagnosis, lack of ART access or adherence, and drug-resistant viral strains. Defining the fine specificity of maternal humoral responses that partially protect against MTCT of HIV is required to inform the development of a maternal HIV vaccine that will enhance these responses during pregnancy. In this study, we identified amino acid residues targeted by potentially protective maternal V3-specific IgG binding and neutralizing responses, localizing the potentially protective response in the C-terminal region of the V3 loop crown. Our findings have important implications for the design of maternal vaccination strategies that could synergize with ART during pregnancy to achieve the elimination of pediatric HIV infections.
KEYWORDS: HIV, mother-to-child transmission, V3, antibodies
INTRODUCTION
Mother-to-child-transmission (MTCT) of HIV remains a major public health problem worldwide, with more than 150,000 new infant infections continuing to occur each year (1). Antiretroviral therapy (ART) treatment during pregnancy has drastically reduced MTCT to low rates (2–4). Despite significant efforts to increase ART availability to HIV-infected pregnant women in low- and middle-income countries, as many as 49% of women do not attend the minimum antenatal care visit (ANC) as defined by the World Health Organization, and up to 65% of HIV-infected women from these areas who attend ANC visits do not receive optimal ART treatments during pregnancy (5). While ART during pregnancy has drastically reduced MTCT incidence, even under optimal prophylactic ART regimens, pediatric HIV infections can still occur (4). Furthermore, certain triple-ART combinations administered during pregnancy may have detrimental effects on infant health, such as increased adverse pregnancy outcomes, preterm delivery, and even infant death associated with protease-containing ART (50). Thus, there is a pressing need to develop immune-based strategies that can synergize with ART regimens to completely eliminate MTCT of HIV.
Established major maternal risk factors of vertical HIV transmission include plasma viral load levels, peripheral CD4+ T cell count, delivery mode (caesarean section versus vaginal delivery), and infant gestational age at ART initiation (6–10). However, despite these risk factors, in the absence of ART, only up to 40% of HIV-infected women transmit the virus to their infants. This suggests the presence of maternal immune factors that contribute to protection of the infant from HIV acquisition. Maternal IgG is passively transferred across the placenta to the fetus throughout the latter half of pregnancy and provides protection to the fetus against infections during the first year of life (11–14). Despite the known protective role of maternal passively acquired IgG against several neonatal pathogens, the ability of maternal HIV envelope (Env)-specific IgG to mediate partial protection against MTCT of HIV remains unclear (15, 16). High levels of maternal Env-specific IgG in nontransmitting women compared to transmitting women have been associated with reduced MTCT risk in some previous studies (17). However, other studies did not confirm this association (18, 19). Studies have also evaluated the association of maternal plasma neutralization breadth and MTCT risk with inconclusive results (20, 21). To gain a deeper understanding of the role of maternal protective antibody (Ab) responses during MTCT, our group recently conducted a comprehensive maternal humoral correlate of risk against MTCT of HIV using samples from the historic Women and Infant Transmission Study (WITS), a North American clade B infection cohort that was enrolled prior to the availability of ART (22). We identified an association between high levels of maternal plasma V3-specific IgG binding and tier 1 (“easy-to-neutralize”) virus-neutralizing responses with lower MTCT risk (22). Importantly, it was recently shown that Env-specific IgG antibodies that mediate tier 1 virus-neutralizing activity exert immune pressure on autologous circulating viruses and therefore select for neutralization-resistant autologous viruses that repopulate the plasma virus pool (23). Autologous virus escape from concurrent tier 1 virus-neutralizing antibodies has important implications in the setting of MTCT, as the susceptible fetus is passively immunized with maternal IgG and exposed only to viruses against which those antibodies are raised. Therefore, the ability of maternal antibodies to neutralize concurrently circulating autologous viruses may be an important feature for blocking infection against MTCT of HIV. We hypothesized that maternal plasma tier 1 virus-neutralizing activity that predicted reduced risk of MTCT of HIV could be a surrogate marker for maternal autologous virus-neutralizing antibodies (22). Therefore, defining the epitope specificities of maternal tier 1 virus-neutralizing antibodies is important for designing maternal vaccine strategies aimed at reducing MTCT risk.
The HIV envelope has four main vulnerable neutralization sites: the CD4 binding site, the membrane-proximal external region, variable loops 1 and 2 (V1V2), and variable loop 3 (V3). Antibodies against V3 were recently found to be associated with decreased risk of HIV acquisition in the RV144 vaccine efficacy trial when other antibody responses were low (24–26). Furthermore, vaccine-elicited V3-specific responses in this efficacy trial may have imposed immune pressure on specific V3 loop amino acid residues on breakthrough viruses (27, 28). In the setting of MTCT of HIV, we also found maternal V3-specific IgG binding responses targeting amino acid residues in the C-terminal region V3 to be associated with reduced MTCT risk. In this study, we employed a multivariable logistic regression model to define the fine specificity and neutralizing function of potentially protective maternal V3-specific IgG responses using transmitting (case) and nontransmitting (control) maternal plasma samples from the WITS cohort (22). Further defining the fine specificity and function of maternal V3-specific IgG responses that are predictive of reduced MTCT risk could inform maternal immunization strategies aimed at temporarily raising the level of potentially protective V3-specific antibodies targeting autologous maternal viruses. Such immune-based prevention strategies that can synergize with current ART treatment strategies will likely be required to achieve an HIV-free generation.
RESULTS
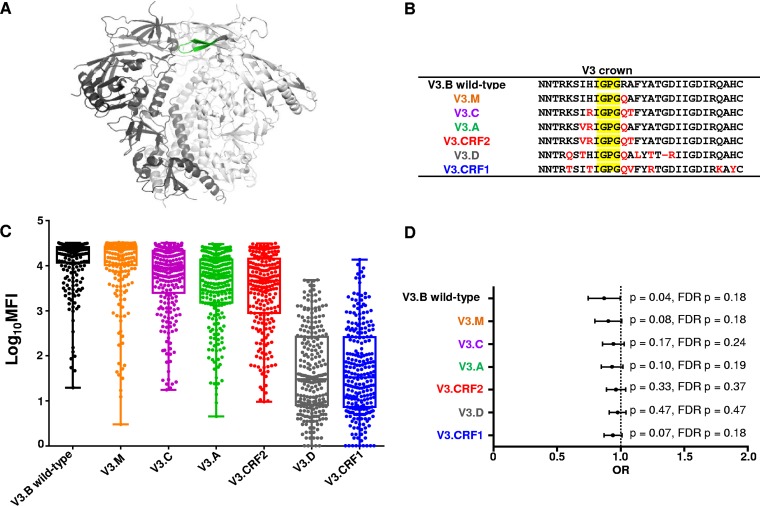
Mapping the regions in the V3 loop critical for the maternal V3-specific IgG binding responses that predict reduced MTCT of HIV in the WITS cohort.
In the WITS cohort, maternal V3-specific IgG binding responses predicted reduced MTCT risk (odds ratio [OR] = 0.86; confidence interval [CI] = 0.75 to 1.00; P = 0.04) (22). The V3 loop, located near the apex of HIV Env gp120, has been previously described as the principal neutralizing domain (PND), as it is readily accessible to V3-specific binding and neutralizing antibodies (Fig. 1A shows the structure of the BG505 SOSIP.664 HIV-1 Env trimer [29]). To define the fine epitope specificity of the maternal plasma V3-specific IgG responses associated with reduced MTCT risk in the WITS cohort, we measured the ability of maternal plasma to bind to a multiclade panel of V3 consensus peptides. The V3 peptides from the panel differ at several amino acid residues located in regions flanking the V3 crown GPG motif (Fig. 1B). Maternal V3-specific IgG binding responses bound with the greatest strength to the V3.B peptide compared to other clade consensus V3 peptides. Maternal V3-specific IgG responses also strongly bound to the V3.M consensus peptide, which differs from V3.B only at amino acid residue position R315Q (Fig. 1C). This suggests that this amino acid residue is not important for mediating the binding of potentially protective V3-specific IgG responses. Maternal V3-specific IgG bound with moderate strength to V3.C, V3.A, and V3.CRF2 consensus peptides. V3.C, V3.A, and V3.CRF2 peptides differed from the V3.B consensus peptide at amino acid residues I307V, H308R, R315Q, and A316T. The marked decrease in binding strength against V3.C, V3.A, and V3.CRF2 suggests that these distinct amino acid residue positions are important for maternal V3-specific IgG binding. Finally, maternal V3-specific IgG bound with low strength to V3.D and V3.CRF1 consensus peptides (Fig. 1C). These clade D and CRF1 consensus V3 peptides differed from the V3.B peptide at amino acid residue positions K305Q, R315Q, F317L, A319T, and D322R. In addition, the V3.CRF1 peptide differed from the V3.B clade consensus peptide at amino acid residue positions Q329K and H331Y. Therefore, these amino acid residue positions are also important for potentially protective V3-specific IgG binding. As expected, maternal V3-specific IgG binding responses were predictive of reduced MTCT risk against V3.B (OR = 0.86; CI = 0.75 to 1.00; P = 0.04; false-discovery rate [FDR] [q] = 0.18) (Table 1). However, the association of maternal plasma V3-specific IgG binding responses and reduced MTCT risk was abrogated against other clade consensus V3 peptides, suggesting amino acid residues that differ between these peptides and V3.B are important for the binding of potentially protective maternal V3-specific IgG (Fig. 1D and Table 1).
FIG 1.
Breadth of plasma V3-specific IgG responses of HIV-1-infected mothers from the WITS cohort. Maternal plasma linear V3-specific IgG responses were tested against a multiclade consensus linear V3 peptide panel. (A) HIV Env gp120 with the V3 loop in green (29). (B) Differences in amino acid residues across the multiclade consensus V3 peptides compared to the V3.B consensus peptide (red); the V3 loop crown is shaded in yellow. (C) Clade-specific maternal V3-specific IgG responses (n = 248) measured by BAMA. The horizontal lines indicate the medians, and the boxes depict 25 to 75% MFI against each peptide. The error bars depict ranges. The V3-specific MAb CH22 was used as a positive control (mean MFIs at 5 μg/ml, 27,808, 28,309, 25,991, 23,476, 26,317, 2,301, and 826 for V3.B, V3.M, V3.C, V3.A, V3.CRF2, V3.D, and V3.CRF1, respectively). Normal human serum was used as a negative control (mean MFI at 1:500 dilution, 100). (D) Odds ratio plot of maternal clade-specific V3-specific IgG binding responses and MTCT risk. Raw (p) and false-discovery rate (FDR p) P values are reported.
TABLE 1.
Primary and secondary maternal V3-specific IgG responses and MTCT risk-immune correlate analyses
| Response | Antigen | OR | CI |
P valuea |
Assay | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Raw | FDR | ||||
| Primary | V3.B wild type | 0.86 | 0.75 | 1.00 | 0.04 | 0.18 | BAMA |
| V3.M | 0.90 | 0.80 | 1.01 | 0.08 | 0.18 | ||
| V3.C | 0.94 | 0.86 | 1.03 | 0.17 | 0.24 | ||
| V3.A | 0.93 | 0.85 | 1.02 | 0.10 | 0.19 | ||
| V3.CRF2 | 0.96 | 0.89 | 1.04 | 0.33 | 0.37 | ||
| V3.D | 0.98 | 0.91 | 1.04 | 0.47 | 0.47 | ||
| V3.CRF1 | 0.94 | 0.87 | 1.01 | 0.07 | 0.18 | ||
| V3.B mutant K305Q I307T H308T | 0.88 | 0.80 | 0.97 | 0.01 | 0.11 | ||
| V3.B/D F317L A319T D322R | 0.94 | 0.86 | 1.03 | 0.19 | 0.24 | ||
| Secondary | V3.B K305A | 0.68 | 0.56 | 0.82 | 0.0001 | 0.07 | |
| V3.B I307A | 0.79 | 0.64 | 0.97 | 0.02 | 0.01 | ||
| V3.B H308A | 0.85 | 0.69 | 1.05 | 0.13 | 0.18 | ||
| V3.B F317A | 0.87 | 0.74 | 1.03 | 0.10 | 0.14 | ||
| V3.B A319K | 0.83 | 0.70 | 0.99 | 0.04 | 0.18 | ||
| V3.B D322A | 0.77 | 0.65 | 0.92 | 0.004 | 0.05 | ||
| V3.B F317A A319K D322A | 0.90 | 0.77 | 1.04 | 0.15 | 0.19 | ||
| gp70MNV3 IgG1 subclass | 0.85 | 0.66 | 1.09 | 0.20 | 0.23 | ||
| V3.B wild type | 0.84 | 0.75 | 0.95 | 0.005 | 0.01 | ELISA | |
| V3.B mutant K305Q I307T H308T | 0.90 | 0.80 | 1.01 | 0.07 | 0.12 | ||
| V3.B/D F317L A319T D322R | 0.94 | 0.84 | 1.06 | 0.31 | 0.31 | ||
| V3.B wild-type peptide | 0.58 | 0.39 | 0.86 | 0.007 | 0.01 | Competition neutralization | |
| Scrambled peptide | 0.49 | 0.32 | 0.75 | 0.001 | 0.005 | ||
| No peptide | 0.50 | 0.33 | 0.73 | 0.0004 | 0.004 | ||
| HIV-2 V3.B wild-type A319T D322N | 0.69 | 0.40 | 1.17 | 0.16 | 0.29 | ||
| HIV-2 V3.B wild-type | 0.49 | 0.30 | 0.79 | 0.004 | 0.01 | ||
Bold font indicates significant P values.
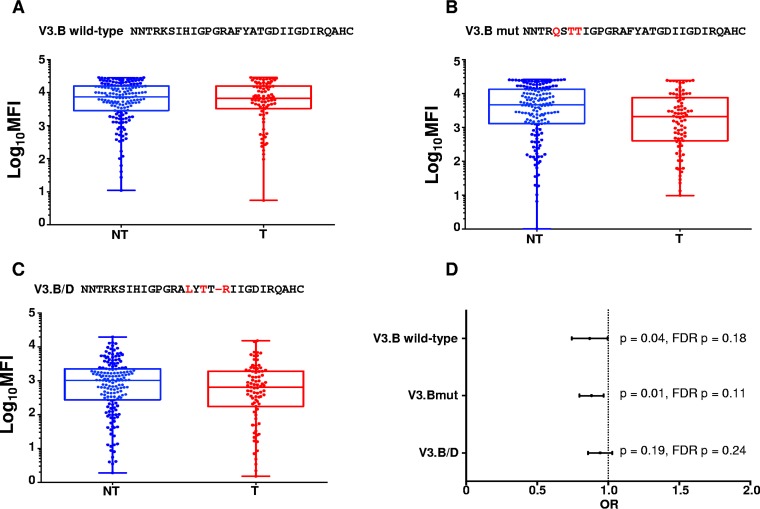
To test the relative importance of these identified amino acid residues in the flanking regions of the V3 loop, we generated two mutant V3 peptides and measured maternal V3-specific IgG binding. We generated an N-terminal-region V3.B K305Q I307T H308T mutant peptide and a C-terminal-region V3.B F317L A319T D322R mutant peptide, which were designed based on the most frequently occurring amino acid residue at each position. Despite a marked decrease in binding strength of the maternal V3-specific binding responses to the N-terminal region V3.B K305Q I307T H308T mutant, maternal V3-directed IgG responses to the peptide were still predictive of reduced MTCT risk (OR = 0.88; CI = 0.80 to 0.97; P = 0.01) by binding antibody multiplex assays (BAMA), suggesting that V3 amino acid residues K305, I307, and H308, while important for V3 binding, are not the targets of potentially protective V3-specific IgG responses associated with reduced MTCT risk (Fig. 2). In contrast, maternal V3-specific IgG responses to the C-terminal-region V3.B F317L A319T D322R mutant peptide were found to no longer be predictive of reduced MTCT risk (OR = 0.94; CI = 0.86 to 1.03; P = 0.19) (Fig. 2 and Table 1). These findings were confirmed in a linear peptide enzyme-linked immunosorbent assay (ELISA), with the V3.B K305Q I307T H308T mutant trending toward being predictive of MTCT risk, yet V3-specific IgG responses to the V3.B F317L A319T D322R mutant peptide were not predictive of reduced MTCT risk (Table 1). Altogether, these findings confirm that the V3 loop is a target of potentially protective maternal responses and suggest that amino acid residues C terminal to the V3 crown mediate the binding of potentially protective maternal V3-specific IgG.
FIG 2.
Maternal plasma V3-specific IgG responses to linear V3.B wild-type and V3.B K305Q I307T H308T and V3.B/D F317L A319T D322R mutant peptides and their association with MTCT risk. (A) Maternal plasma linear V3-specific IgG binding responses to V3.B wild-type peptide in nontransmitting (NT; blue) and transmitting (T; red) women. (B) Maternal plasma V3-specific IgG responses against triple-mutant V3.B K305Q I307T H308T peptide in NT and T mothers. (C) Maternal plasma V3-specific IgG responses against V3.B/D F317L A319T D322R in NT and T mothers. The horizontal lines represent the median MFIs, and the boxes depict 25 to 75% MFI against each peptide. The error bars depict ranges. The V3-specific MAb CH22 was used as a positive control (mean MFIs at 5 μg/ml, 25,711, 8,713, and 25,240 for V3.B, the V3.B K305Q I307T H308T mutant, and the V3.B/D F317L A319T D322R mutant, respectively). Normal human serum was used as a negative control (mean MFI at 1:500 dilution, 100). (D) Odds ratio plot depicting maternal linear V3-specific IgG binding responses against each V3 peptide and association with MTCT risk. Raw (p) and FDR-corrected (FDR p) P values are shown.
Mapping single amino acid residues within the V3 loop C-terminal region that are the targets of potentially protective maternal V3-specific IgG responses.
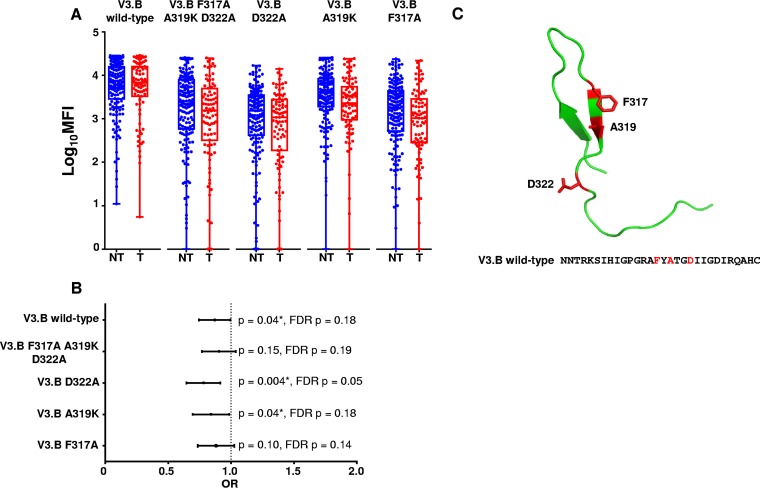
As maternal V3-specific IgG responses are dependent on amino acid residues that flank the V3 loop crown (Fig. 1), we sought to define the importance of each residue in mediating the binding of protective maternal V3 IgG responses. We designed V3 peptides with single alanine substitutions at each position identified as important for binding in the multiclade V3 peptide panel binding analysis, except for amino acid residue position 319, in which a lysine was inserted in place of a naturally occurring alanine (30). We tested whether we could eliminate the protective association of maternal plasma V3-specific IgG binding antibody responses and reduced MTCT risk against each specific V3.B mutant peptide. Intriguingly, maternal plasma V3-specific IgG binding responses to the V3.B F317A A319K D322A triple mutant was no longer associated with reduced MTCT risk (OR = 0.90; CI = 0.77 to 1.04; P = 0.15), suggesting at least one of these residues was key to the binding of potentially protective V3-specific IgG responses (Fig. 3). Maternal V3-specific IgG binding responses to the single mutants V3.B A319K and V3.B D322A were still predictive of reduced MTCT risk, ruling out these amino acid residues as the targets of potentially protective maternal V3-specific IgG binding responses (OR = 0.83, CI = 0.77 to 0.99, P = 0.04 and OR = 0.77, CI = 0.65 to 0.92, P = 0.004, respectively) (Fig. 3). However, maternal V3-specific IgG binding responses to V3.B F317A were no longer predictive of reduced MTCT risk (OR = 0.87; CI = 0.74 to 1.03; P = 0.10), suggesting that amino acid residue F317, which is located C terminal to the V3 crown, is an important target of potentially protective maternal V3-specific IgG binding responses in the WITS cohort (Fig. 3C).
FIG 3.
Maternal plasma linear V3-specific IgG binding responses and MTCT risk against peptides with single amino acid residue substitutions. (A) Magnitudes of maternal plasma linear V3-specific IgG binding responses to V3.B wild type, V3.B F317A A319K D322R, V3.B D322R, V3.B A319K, and V3.B F317A. The V3-specific MAb CH22 was used as a positive control (mean MFIs at 5 μg/ml, 13,817, 25,241, 26,639, and 3,710 for V3.B F317A A319K D322A, V3.B D322A, V3.B A319K, and V3.B F317A, respectively) in NT (blue) and T (red) women. Normal human serum was used as a negative control (mean MFI at 1:500 dilution, 100). (B) Odds ratio plot of V3.B wild-type, V3.B triple-mutant, and V3.B single-mutant peptides. As the analysis was designed as a secondary, immune correlate analysis, significant raw P values are indicated by asterisks (P < 0.05). (C) Predicted structural locations of amino acid targets of maternal IgG responses associated with reduced MTCT risk. The HIV Env V3 loop with amino acid residue positions F317, A319, and D322, which are targeted by maternal plasma binding and neutralizing IgG responses associated with reduced MTCT risk, is shown in red. The V3 structure was generated using PyMOL.
IgG subclass of maternal V3-specific IgG responses and MTCT risk.
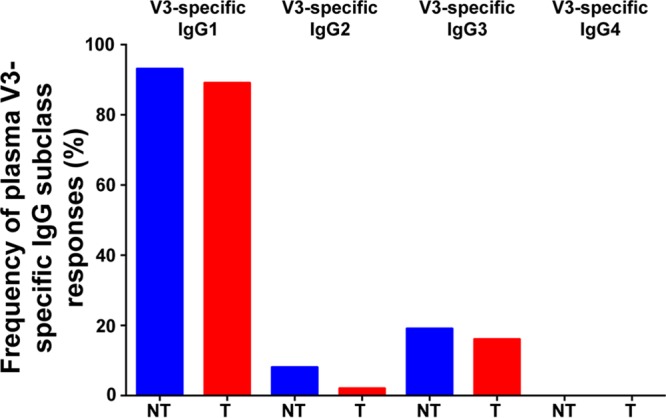
A retrospective analysis of the moderately efficacious RV144 vaccine trial revealed that HIV-1 risk in vaccine recipients was associated with anti-V1V2-specific IgG3 subclass responses and distinguished this vaccine regimen from a nonefficacious vaccine (31, 32). While the specific mechanism by which IgG3 subclass responses mediate reduced HIV-1 transmission risk is unclear, the importance of IgG3 subclass responses in vertical HIV transmission settings remains unexplored. Therefore, to determine whether V3-specific IgG subclass responses differed in frequency between nontransmitting and transmitting women in the WITS cohort, we measured maternal plasma V3-directed IgG subclass responses against a scaffolded gp70MNV3 protein. Maternal V3-specific IgG1 subclass binding responses were detectable at the highest frequency of all IgG subclasses and in the majority of nontransmitting and transmitting women (93% of nontransmitters and 89% of transmitters) (Fig. 4). The V3-specific IgG3 subclass responses were also detectable at similar frequencies in nontransmitting and transmitting women (19% of nontransmitters and 16% of transmitters). Similarly, V3 IgG2-specific responses were detectable in only 8% of nontransmitters and 2% of transmitters, and there was no statistical difference in the frequencies of IgG2 subclass responses. V3-specific IgG4 subclass responses were not detectable in any women from the cohort. Thus, no IgG subclass response solely contributes to the potentially protective V3-specific IgG response that was associated with reduced MTCT risk.
FIG 4.
Maternal V3-specific IgG subclass response frequencies in nontransmitting and transmitting mothers.
Maternal V3-specific IgG contributes to tier 1 virus-neutralizing responses associated with reduced MTCT risk.
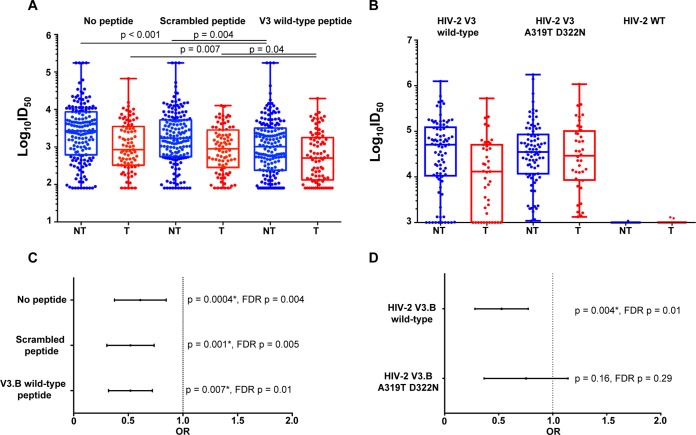
We previously reported that maternal V3-specific binding, tier 1 virus neutralization, and CD4 binding site-blocking responses strongly correlated with one another and were colinear in their abilities to predict MTCT risk in the cohort (22). We therefore sought to assess the degree to which maternal V3-specific IgG antibodies contribute to the potentially protective tier 1 virus-neutralizing responses (22). To assess the contribution of maternal plasma linear V3-specific IgG to tier 1 HIV-1 neutralization, we performed V3 peptide competition neutralization assays with the tier 1 clade B variant SF162. Maternal V3-specific IgG neutralizing responses against SF162 remained predictive of reduced MTCT risk in the presence of the V3.B peptide, a scrambled peptide, and no peptide (Table 1). However, maternal SF162 neutralizing responses in both nontransmitting and transmitting mothers were reduced in the presence of the V3.B peptide compared to both the scrambled- and no-peptide conditions, and the reduction in the 50% infective dose (ID50) in the presence of a V3 wild-type (WT) peptide was statistically significant (Fig. 5A), suggesting that maternal V3-specific neutralizing antibodies that target linear V3 epitopes partially mediate the tier 1 virus neutralization response. However, antibodies directed against Env epitopes other than V3, such as those directed against the CD4 binding site, also likely contribute to the tier 1 virus neutralization phenotype that was associated with reduced MTCT risk.
FIG 5.
Maternal plasma linear V3-specific neutralizing responses and MTCT risk. (A) Maternal neutralizing responses to HIV-1 SF162 in nontransmitting and transmitting women in the presence of no peptide, a scrambled peptide, and a V3.B wild-type linear peptide, with P values from a Mann-Whitney test. (B) Maternal plasma linear V3-specific neutralizing responses against HIV-2 V3.B wild type and a HIV-2 V3.B A319T D322N mutant. The V3-specific MAb CH22 was used as a positive control, and normal human serum was used as a negative control. (C) Odds ratio plot of maternal tier 1 virus-neutralizing responses to HIV-1 SF162 in the presence of no peptide and scrambled and V3.B wild-type peptide and MTCT risk. (D) Odds ratio plot of maternal V3-specific neutralizing responses against HIV-2 V3.B wild-type and HIV-2 V3.B A319T D322N chimeric viruses and MTCT risk. As the analysis was designed as a secondary, immune correlate analysis, significant raw P values are indicated by asterisks (P < 0.05).
Mapping the fine specificity of the V3-specific neutralizing antibodies associated with reduced MTCT risk.
Amino acid residue positions 319 and 322 are located in the C-terminal V3 region, which we found to be important for maternal plasma V3-specific IgG binding responses (Fig. 2). To probe the fine specificity of maternal V3-mediated neutralization of maternal tier 1 virus-neutralizing responses that were associated with reduced MTCT risk in the WITS cohort (22), we used highly sensitive HIV-2/HIV-1 V3 chimeric viruses, which detect only V3-directed HIV-1-neutralizing responses (33). We measured maternal V3-specific IgG neutralizing responses against HIV-2/HIV-1 V3.B wild type, HIV-2/HIV-1 V3.B A319T D322N chimeric viruses, and an HIV-2 wild-type control in a TZM-bl cell neutralization assay. As expected, neither HIV-1 Env-specific nor V3-specific IgG polyclonal responses from nontransmitting or transmitting women neutralized the HIV-2 WT control virus (Fig. 5). However, maternal V3-specific neutralizing responses to HIV-2 V3.B wild-type chimeric virus were highly predictive of reduced risk of MTCT (OR = 0.49; CI = 0.30 to 0.79; P = 0.004) in the peripartum cohort. Interestingly, the association with the isolated V3-specific neutralization of HIV-2 V3.B was completely abrogated when amino acid residues A319 and D322 in the C-terminal region of the V3 loop were mutated to A319T and D322N in the chimeric virus (OR = 0.69; CI = 0.40 to 1.17; P = 0.16). These results suggest that the V3 loop is an important target of potentially protective neutralizing responses in nontransmitting women and that V3-specific neutralizing responses depend on amino acid residues A319 and D322 in the V3 loop (Fig. 3C). Thus, maternal V3-specific neutralizing antibodies that target amino acid residues A319 and D322 are predictive of reduced risk of MTCT of HIV. While potentially protective maternal V3-specific IgG binding responses predictive of reduced MTCT risk were dependent on amino acid residue F317, the HIV-2 V3.B F317A chimeric virus did not achieve the infectivity threshold of 10 times the cell control background relative luminescent units (RLU) for use in a TZM-bl cell neutralization assay.
The role of maternal V3-specific IgG in coreceptor usage of autologous maternal circulating viruses.
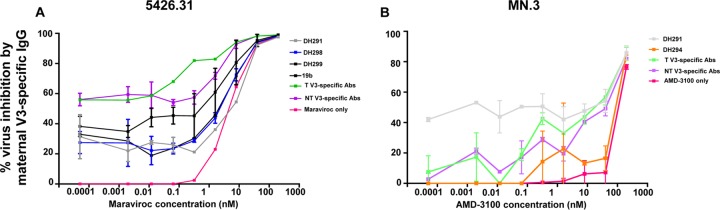
The V3 loop has been described as a determinant of virus tropism through modulation of coreceptor interaction (34); thus, V3-specific maternal antibodies could block MTCT by interfering with coreceptor binding. To explore this potential alternative mechanism of V3-specific IgG in virus transmission, we investigated whether V3-specific IgG antibodies impact the virus coreceptor usage of autologous maternal viruses isolated from a nontransmitting mother (22). We utilized V3-specific IgG monoclonal antibodies (MAbs) isolated from a nontransmitting mother and plasma V3-specific IgG antibodies purified from a nontransmitting and a transmitting mother from the WITS cohort to assess their impacts on the susceptibility of a circulating virus variant from a nontransmitting mother to the CCR5 inhibitor maraviroc. Maternal V3-specific IgG monoclonal antibodies DH291, DH298, DH299, and 19b; a heterologous positive control specific for the C-terminal region (22); and polyclonal V3-specific Abs isolated from a nontransmitting and a transmitting mother, when present at subneutralizing concentrations of 5 μg/ml, altered the susceptibility of the maternal autologous virus to low levels of the CCR5 inhibitor (Fig. 6A). Furthermore, maternal V3-specific IgG antibodies DH291 and DH294 and polyclonal V3-specific antibodies isolated from a nontransmitting and a transmitting mother could similarly modulate coreceptor inhibition of a CXCR4-tropic virus, MN.3, when we tested them at subneutralizing concentrations of 1 μg/ml. Altogether, these results suggest that maternal V3-specific IgG could block autologous maternal virus transmission through an alternative nonneutralizing pathway of altering viral interactions with coreceptors expressed on infant cells.
FIG 6.
Altered CCR5 and CXRC4 coreceptor inhibitor sensitivity of HIV-1 in the presence of maternal V3-specific antibodies. (A) Maternal V3-specific IgG antibodies isolated from a nontransmitting and a transmitting mother tested at subneutralizing concentrations (5 μg/ml) against an autologous circulating virus (5426.31) in the presence of serially diluted maraviroc. (B) Maternal V3-specific IgG antibodies from a nontransmitting and a transmitting mother tested at subneutralizing concentrations against MN.3 in the presence of serially diluted AMD-3100. The error bars indicate the standard deviation.
DISCUSSION
Our limited understanding of mechanistic immune correlates of protection against MTCT of HIV remains a major challenge in developing safe and effective immune strategies to prevent pediatric HIV. Yet, our recent humoral immune correlate analysis of clade B HIV-infected women demonstrated that maternal V3-specific IgG binding and tier 1 virus-neutralizing responses were both predictive of reduced MTCT risk in clade B HIV-infected women (22). This result was surprising, as HIV Env-specific responses that target the V3 loop predominantly mediate neutralization of tier 1 virus variants, yet infant transmitted/founder HIV-1 variants uniformly resemble the more difficult to neutralize tier 2-like viruses. The V3 loop of gp120 has been extensively described as an important target of HIV Env-specific cross-clade neutralizing antibodies (35, 36). In fact, the V3 loop has been previously described as the PND on the HIV Env glycoprotein spike and is a major target of autologous virus-neutralizing antibodies (23) (Fig. 1). V3-specific antibodies are relatively easy to induce by vaccination but have been shown to neutralize only easy-to-neutralize T cell-adapted strains and not clinically relevant strains (37, 38). However, it was recently shown in the moderately efficacious RV144 vaccine efficacy trial that vaccine-elicited antibody responses mediated immune pressure in breakthrough viruses at V3 amino acid residue position F317 (27, 28). Interestingly, we determined that maternal V3-specific binding responses predictive of reduced MTCT risk in the WITS cohort were similarly dependent on V3 amino acid residue F317. Together, these studies suggest that amino acid position 317 may be important for the binding of protective antibody responses in more than one transmission setting. These findings also suggest that V3-specific IgG responses may be important in reducing HIV transmission risk.
Maternal V3-specific IgG responses associated with reduced MTCT risk were previously reported (22), and in this study, we demonstrate that amino acids localized to the C-terminal region in the V3 loop are important for this association (Fig. 2). Importantly, the association was observed in measures of humoral immunity by different assays (BAMA, ELISA, and neutralization), which demonstrates the robustness of this finding. Despite the repeatable association of maternal V3-specific IgG antibody responses and reduced MTCT risk, it should be noted that the protective effect of maternal V3-specific IgG binding responses against reduced MTCT risk was modest in both the multiplex BAMA and ELISAs. Furthermore, a caveat to our findings is that while maternal V3-specific IgG binding responses to the V3.B K305Q I307T H308T mutant were still predictive of reduced MTCT risk by BAMA, the association between maternal V3-specific IgG binding responses and reduced MTCT risk as measured by ELISA only trended toward significance (Fig. 2 and Table 1). The differences in the association of maternal V3-specific IgG binding to the V3.B K305Q I307T H308T mutant and reduced MTCT risk as measured by BAMA and ELISA may be explained by sensitivity differences in these assays.
Anti-V1V2-specific IgG3 subclass responses were associated with decreased HIV-1 risk in vaccinees from the moderately protective RV144 vaccine efficacy trial (31). Here, we tested maternal V3-specific IgG3 subclass responses and found they were detected at similarly low frequencies in nontransmitting and transmitting women. Maternal V3-specific IgG2 subclass responses were also detected at similarly low frequencies in nontransmitting compared to transmitting women. As expected, maternal V3-specific IgG1 subclass responses were most frequently detected in both nontransmitting and transmitting women and at similar frequencies within these groups (Fig. 4). A potential explanation for the lack of association between V3-specific IgG subclass and reduced transmission risk is that the scaffolded gp70 MNV3 protein differs from that of wild-type V3.B at amino acid residues in the C-terminal region, which we have demonstrated to be important for the binding of potentially protective V3-specific IgG responses. Therefore, the V3-specific IgG subclass, as measured by our current assay, does not seem to be a major determinant of MTCT of HIV.
We previously identified maternal tier 1 virus-neutralizing responses as predictive of reduced MTCT risk in the WITS cohort (22). Interestingly, in this study, maternal plasma neutralization of the HIV tier 1 virus variant SF162 in the presence of a V3.B wild-type peptide was still predictive of reduced MTCT risk, suggesting that other protective antibody responses directed against epitopes other than the V3 loop may mediate neutralization (Fig. 5A). It is also possible that potentially protective maternal V3-specific IgG responses are mediated by nonlinear-epitope-targeting antibodies and may not be inhibited by the linear V3.B wild-type peptide. We therefore measured the isolated contributions of maternal V3-specific neutralizing responses to tier 1 virus neutralization using sensitive HIV-2/HIV-1 V3 chimeric viruses. Interestingly, the isolated maternal V3-specific neutralizing response to the HIV-2 V3 wild-type chimeric virus was highly predictive of reduced MTCT risk, suggesting that the protective mechanism of V3-specific IgG responses is virus neutralization (Fig. 5B). However, the insertion of two nonconsensus amino acid residues at positions 319 and 322 abrogated the association of the V3-specific neutralization and reduced MTCT risk, suggesting these amino acid residues are critical for V3-specific IgG-mediated neutralization. Amino acid residue positions 319 and 322 are in close proximity in V3 and also in the C-terminal region of the V3 crown. Therefore, residues 319 and 322 may encompass a single binding and neutralizing epitope of protective maternal antibody responses (Fig. 3C). However, it should be noted that due to the inability to produce an infectious HIV-2 V3 F317A chimeric virus, we cannot rule out this C-terminal amino acid residue as the target of potentially protective maternal V3-specific neutralizing responses associated with reduced MTCT risk. Another caveat to our study is that key amino acid residues that mediate the binding of potentially protective maternal V3-specific responses can vary across viral clades. Therefore, it remains unclear if maternal V3-specific responses that target amino acid residues F317, A319, and D322 mediate decreased MTCT risk of non-clade B HIV infection. It should also be noted that maternal V3-specific neutralizing response inhibition by a linear V3.B wild-type peptide with F317, A319, and D322 amino acid residues did not remove the association between tier 1 virus neutralization and reduced MTCT risk. However, the association of maternal V3-specific neutralizing responses and reduced MTCT risk was completely removed by the insertion of nonconsensus residues into the C-terminal region of HIV-2 V3.B chimeric virus. The observed differences in the inhibition of potentially protective maternal V3-specific responses could perhaps be due to differences in the neutralization sensitivities of HIV-1 tier 1 isolates and chimeric HIV-2 V3 viruses and to differences in conformation and structure between linear V3 peptides and the V3 loop on chimeric viruses. Altogether, these findings suggest that the potentially protective maternal neutralizing responses in the WITS cohort target the C-terminal domain in the V3 loop.
The C-terminal region in the V3 loop encompasses amino acid residues that are predictive of coreceptor usage in CCR5- and CXCR4-tropic viruses (39–41). In fact, coreceptor preference for CXCR4 can be predicted by the presence of acidic amino acid residues at position 322, whereas the absence of charged amino acid residues at position 322 is generally predictive of CCR5-tropic viruses (40). However, maternal clade B CCR5-tropic viruses predominantly initiate infection of the infant (transmitted/founder viruses) (42). Circulating autologous CXCR4-tropic viruses could potentially be blocked by maternal protective V3-specific IgG binding and neutralizing responses and enable only coreceptor binding of CCR5-tropic strains. Interestingly, maternal V3-specific IgG antibodies mediated coreceptor binding inhibition of a maternal circulating virus, 5426.31, and reference strain MN.3, suggesting that maternal autologous V3-specific IgG may affect viral interactions with the CCR5 and CXCR4 coreceptors on infant CD4+ T cells (Fig. 6). This finding suggests that V3-specific IgG may utilize more than one mechanism (neutralization and modulation of coreceptor usage) to reduce MTCT risk. However, the ability of V3-specific IgG to modulate coreceptor binding inhibition was examined in only one nontransmitting and one transmitting mother and should be explored in a larger population of HIV-infected pregnant mothers.
In the setting of pregnancy, the infant is passively immunized with maternal IgG that is raised against the viruses to which the infant is exposed. Maternal HIV Env-specific IgG responses may select vertically transmitted viruses that escape maternal IgG through mutations of specific amino acid residues in the envelope spike. Importantly, we previously reported that viruses isolated from a nontransmitting mother were sensitive to neutralization by autologous V3-specific IgG monoclonal antibodies. There was also evidence of immune escape from this V3-specific autologous virus-neutralizing response by specific compensatory mutations within the envelope yet outside the V3 loop. Mutations at amino acid residue positions N188 and V200 within the C2 region mediated maternal autologous virus neutralization sensitivity to V3-specific IgG responses, potentially by making the V3 loop accessible to neutralizing antibodies (22). Other studies have also reported Env amino acid mutations outside V3 that increase V3 exposure to V3-specific neutralizing antibodies (43). In this study, we identified amino acid mutations within the V3 loop C-terminal region that completely remove the association between maternal protective V3-specific responses and reduced MTCT risk. Therefore, mutations both outside and within the V3 loop may be important to enable accessibility to V3-specific binding and neutralizing antibodies.
In this study, we have demonstrated that maternal V3-specific responses specific for the C-terminal region are associated with reduced MTCT risk. These V3-specific responses may mediate reduced MTCT risk through the following mechanisms: (i) maternal autologous virus neutralization and/or (ii) modulation of coreceptor inhibition of maternal autologous viruses. Importantly, the elicitation of these easy-to-induce, weakly neutralizing antibodies is readily achievable by existing immunogens, which can safely and effectively raise serum levels of V3-specific IgG neutralizing antibodies in humans (44). Therefore, immunization regimens aimed at temporarily raising the levels of maternal V3-specific IgG and autologous-virus-neutralizing responses in HIV-infected pregnant women may perhaps be achieved through “original antigenic sin” by vaccination with heterologous clade V3 immunogens. The identification of amino acid residue positions F317, A319, and D322 within the C-terminal region could serve as a benchmark for assessing the potential immunogenicity and efficacy of maternal HIV immunization strategies targeting the V3 loop, with the goal of developing an immunization strategy that can synergize with ART to eliminate MTCT and achieve an HIV-free generation.
MATERIALS AND METHODS
Study cohort.
Maternal plasma samples were obtained from the WITS cohort, an observational cohort that was followed in North America before ART was the standard of clinical care. This study enrolled ART-naive HIV-infected pregnant women from 1988 to 2007. Transmitting mothers (n = 83) were selected from the WITS cohort by the following criteria: no ART treatment during pregnancy, detectable plasma viral loads (>50 copies/ml plasma), and a nonheparin plasma sample available between the end of the second trimester (≥25 weeks of gestation) and 2 months postpartum. Transmitting mothers were matched in a 1:2 ratio (case to control) to nontransmitting mothers (n = 165) using propensity score matching for known covariates of transmission risk—maternal viral load, peripheral CD4+ T cell count at the time closest to delivery, delivery mode (vaginal versus caesarian section), and infant gestational age (as determined by estimated delivery date)—as described previously (22). Importantly, comparison of the clinical characteristics of matched transmitting and nontransmitting mothers did not reveal significant differences in maternal viral loads, peripheral CD4+ T cell counts, infant gestational ages, or delivery modes (22).
Ethics statement.
Approval was obtained from the institutional review board at Duke University to utilize deidentified maternal plasma samples from the cohort.
Binding antibody multiplex assay.
Maternal plasma V3-specific binding responses were measured against a multiclade panel of consensus V3 peptides designed by Bette Korber (45): V3.B (NNTRKSIHIGPGRAFYATGDIIGDIRQAHC), V3.M (NNTRKSIHIGPGQAFYATGDIIGDIRQAHC), V3.C (NNTRKSIRIGPGQTFYATGDIIGDIRQAHC), V3.A (NNTRKSVRIGPGQAFYATGDIIGDIRQAHC), V3.CRF2 (NNTRKSVRIGPGQTFYATGDIIGDIRQAHC), V3.D (NNTRQSTHIGPGQALYTT-RIIGDIRQAHC), and V3.CRF1 (NNTRTSITIGPGQVFYRTGDIIGDIRKAYC). The V3.B K305Q/I307T/H308T mutant (NNTRQSTTIGPGRAFYATGDIIGDIRQAHC), V3.B F317L/A319T/D322R mutant (NNTRKSIHIGPGRALYTT-RIIGDIRQAHC), V3.B K305A (NNTRASIHIGPGRAFYATGDIIGDIRQAHC), V3.B I307A (NNTRKSAHIGPGRAFYATGDIIGDIRQAHC), V3.B H308A (NNTRKSIAIGPGRAFYATGDIIGDIRQAHC), V3.B F317A (NNTRKSIHIGPGRAAYATGDIIGDIRQAHC), V3.B A319K (NNTRKSIHIGPGRAFYKTGDIIGDIRQAHC), V3.B D322A (NNTRKSIHIGPGRAFYATGAIIGDIRQAHC), and V3.B F317A/A319K/D322A (NNTRKSIHIGPGRAAYKTGAIIGDIRQAHC) peptides were purchased from CPC Scientific (Sunnyvale, CA). The V3 mutant peptides were named in accordance with the HIV HXB2 amino acid standard numbering scheme. BAMA were performed as described previously (46). Briefly, a total of 5 × 106 carboxylated fluorescent beads (Luminex Corp., Austin, TX) were covalently coupled to 25 μg of V3 consensus clade and mutant peptides. Maternal plasma was tested at a 1:2,500 dilution against V3.B, V3.M, and V3.B F317L A319T D322R mutant peptides and at a 1:250 dilution against V3.C, V3.A, V3.CRF2, V3.B, and V3.B K305Q I307T H308T mutant peptides. Maternal plasma was tested at a 1:25 dilution against V3.D and V3.CRF1 peptides. Finally, maternal plasma was tested at a 1:1,000 dilution against V3.B K305A, V3.B I307A, V3.B H308A, V3.B F317A, V3.B A319K, V3.B D322A, and V3.B F317A A319K D322A peptides. Maternal plasma V3-specific IgG was detected with a mouse anti-human IgG (Southern Biotech, Birmingham, AL) phycoerythrin-conjugated antibody at 4 μg/ml as described previously (46). Antibody measurements were acquired using a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA). CH22, a V3-specific monoclonal IgG (47), was used as a positive control. Normal human serum was used a negative control. All mean fluorescence intensity (MFI) values were adjusted by subtracting the blank-bead MFI. A panel of 30 human HIV-seronegative samples was used to determine a binding (MFI) cutoff against each antigen for each of the tested dilutions.
Env V3-specific IgG subclass BAMA.
To measure V3-specific IgG subclass responses, maternal samples diluted 1:40 were tested against a scaffolded V3 loop protein, gp70MNV3. IgG subclass biotinylated antibody detectors were used to measure subclass specificity. IgG1-biotin (BD Pharmingen; clone G17-1), IgG2-biotin (BD Pharmingen; G18-21), IgG3-biotin (Calbiochem; clone:HP6047), and IgG4-biotin (BD Pharmingen; clone G17-4) were detected at 4 μg/ml in separate assays for each IgG subclass. The plates were washed, and streptavidin-conjugated phycoerythrin antibody diluted 1:100 was added to the wells. A V3-specific MAb, CH22 (47), was included as a positive control, and normal human serum was used as the negative control. All MFI values were adjusted by subtracting the blank-bead MFI.
V3 linear peptide ELISA.
Binding of maternal IgG to V3.B wild type, a V3.B K305Q I307T H308T mutant, and a V3.B/D F317L A319T D322R mutant was measured by plate-based ELISA. High-binding 384-well ELISA plates (Corning) were coated with 2 μg/ml of V3 peptides overnight at 4°C and then blocked with SuperBlock (4% whey protein, 15% goat serum, and 0.5% Tween 20 diluted in 1× phosphate-buffered saline [PBS]). Maternal plasma V3-specific IgG was tested at a 1:100 starting dilution in 12 serial 3-fold dilutions. The V3-specific MAb CH22 (47) was used as the positive control, and the respiratory syncytial virus (RSV)-specific monoclonal antibody pavilizumab (Synagis) was used as the negative control. An anti-horseradish peroxidase (HRP)-conjugated goat anti-human IgG antibody (Sigma-Aldrich) was used at 1:10,000 dilution, followed by addition of SureBlue reserve TMB substrate (KPL, Gaithersburg, MD). Reactions were stopped by stop solution (KLP, Gaithersburg, MD). Optical densities were detected at 450 nm. The log10 50% effective concentration (EC50) and log10 area under the concentration-time curve (AUC) were calculated for maternal V3-specific IgG binding responses against each V3.B, V3.B mutant, and V3.B/D mutant.
Peptide competition neutralization assays.
Maternal plasma samples at 1:80 final dilution were incubated with 50 μg/ml of either an SF162 V3 peptide (NNTRKSITIGPGRAFYATGDIIGDIRQAH) or a scrambled peptide (HTGKYTYPTNIAIRGRGNKFRNKKI) or with no peptide for 1 h at 37°C. Preabsorbed maternal samples were then used to measure neutralization of the tier 1 HIV-1 SF162 in a TZM-bl cell-based neutralization assay, as described previously (48). Briefly, neutralizing activity was measured after 48 h by a reduction in luciferase gene expression. Maternal plasma neutralizing activity was reported as 50% reduction in RLU compared to the virus control after subtraction of the background RLU from cell control wells.
Neutralization assay against wild-type HIV-2 V3.B and HIV-2 V3.B A319T and D322N chimeras.
HIV-2 WT and HIV-2 HIV-1 V3 A319T D322N chimeric viruses were obtained as gifts from George Shaw. HIV-2 HIV-1 V3.B wild-type chimeric virus was generated by site-directed mutagenesis (33). We performed site-directed mutagenesis using a QuikChange II XL site-directed mutagenesis kit according to the manufacturer's instructions. Chimeric viruses were sequenced to ensure the desired mutations were present. To produce infectious molecular clones, 12 μg of viral plasmid was transfected with FuGene (Promega, Madison, WI) at a 1:4 volume ratio in 293T cells, and viruses were titrated in TZM-bl cells as described previously (49). Infectious viruses with infectivity of at least 10 times the cell control background RLU were used in the TZM-bl cell neutralization assay. HIV-2 WT, HIV-2 V3.B A319T D322N, and HIV-2 V3.B wild type were tested in the neutralization assay. Chimeric HIV-2 V3.B F317A was successfully mutagenized but did not meet the infectivity threshold of 10 times the cell background. Maternal plasma was tested for neutralization against the HIV-2 WT, HIV-2 V3.B WT, and HIV-2 V3.B A319T D322N chimeric viruses as described above at a starting dilution of 1:1,000 in a TZM-bl cell line. DH295, a V3-specific IgG tier 1 virus-neutralizing antibody isolated from a nontransmitting mother from the WITS cohort, was included as the positive control. Normal human serum was included as the negative control.
V3-specific IgG modulation of coreceptor usage assays.
The CCR5 inhibitor maraviroc (NIH AIDS reagent program; number 11580) and the CXCR4 inhibitor bicyclam JM-2987 (AMD-3100; NIH AIDS reagent program; number 8128) were diluted 5-fold, starting at a 1-μM concentration, and added to TZM-bl cells. Dilutions of maraviroc and bicyclam were separately added to TZM-bl cells and incubated for 1 h at 37°C. Maternal V3-specific IgG antibodies isolated from a nontransmitting mother (DH291, DH294, DH298, and DH299) and a positive-control heterologous V3-specific IgG MAb (19b) were tested at a nonneutralizing concentration of 5 μg/ml against a virus isolated from the same nontransmitting mother (5426.31). We also selected a nontransmitting and a transmitting mother to isolate plasma V3-specific IgG antibodies and tested their abilities to modulate virus coreceptor usage at subneutralizing concentrations against the CCR5-tropic nontransmitting maternal virus 5426.31 and the CXCR4-tropic virus MN.3. Briefly, maternal undiluted plasma was incubated with Bio-V3.B wild-type peptide conjugated to streptavidin-magnetic beads (NEB; S1420S) at 4 mg/ml overnight at 37°C in rotation. Maternal polyclonal V3-specific antibodies were separated from plasma with magnetic beads; eluted with 0.1 M glycine, pH 2.9; and buffer exchanged with 1× PBS in 100,000 Da Amicon Ultra-0.5 ml centrifugal filters (EMD Millipore; UFC510008) at 2,500 × g. Maternal V3-specific IgG MAbs and purified antibodies were incubated with MN.3 virus at subneutralizing concentrations of 1 μg/ml for 1 h at 37°C and then added to TZM-bl cells in the presence of diluted maraviroc or bicyclam. Virus infectivity was measured after 48 h by measuring luciferase activity with Bright-Glo (Promega, Madison, WI) in the presence and absence of V3-specific MAbs and maraviroc.
Statistical methods.
The statistical analysis plan was finalized prior to data analysis. A multivariable logistic regression model was used, with transmission status as the dependent variable, as described previously (22). In our primary analysis, we measured maternal V3-specific IgG responses against a panel of nine V3 peptides listed in Table 1. Maternal plasma V3-specific IgG responses against each of these V3 peptides were measured and analyzed in a multivariable logistic regression model, with transmission status as the dependent variable. To correct for multiple comparisons in our primary analysis, we applied an FDR (q value; P value) with a significance threshold of a P value of <0.05 and q value of <0.2. In a secondary analysis, we measured maternal V3-specific IgG binding and neutralizing responses against V3 mutant peptides and viruses that were designed based on results from our primary analysis. All primary outcome P values were adjusted for multiple-comparison corrections and were used to interpret results from the primary analysis. Multiple-comparison P values are listed as FDR P values. All secondary-analysis P values are listed as raw P values. Raw P values were used to interpret results from secondary analyses. Significant raw P values are marked with asterisks. All regression analyses were adjusted for the following known covariates of risk of MTCT of HIV: maternal plasma viral load at the time of delivery, peripheral CD4+ T cell count, infant gestational age, and delivery mode. A Mann-Whitney-Wilcoxon signed-rank test was used to compare the inhibition (50% infective dose [ID50]) in a TZM-bl cell neutralization assay under no-peptide, scrambled-peptide, and V3 peptide conditions.
ACKNOWLEDGMENTS
We acknowledge Robert Parks, Amit Kumar, and Judith Lucas for technical support. We also thank George Shaw for providing the HIV-2/HIV-1 V3 chimeric virus plasmids and Shaunna Shen for providing the V3 clade consensus peptides.
We report no commercial or financial conflicts of interest.
This research was supported by the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518); a small grant to Genevieve G. Fouda; and NIH grant R01 (1R01AI122909) to Sallie R. Permar. David R. Martinez is supported by an American Society for Microbiology Robert D. Watkins Graduate Research Fellowship.
REFERENCES
- 1.UNAIDS. 2015. Progress Report on the Global Plan: towards the elimination of new HIV infections among children and keeping their mothers alive. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. 2008. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS 22:973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 3.Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, Rekacewicz C, Newell ML, Delfraissy JF, Cunningham-Schrader B, Mirochnick M, Sullivan JL. 2002. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 288:189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 4.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, Jimenez E, O'Neill E, Bazin B, Delfraissy J-F, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J, f Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 331:1173–1180. [DOI] [PubMed] [Google Scholar]
- 5.Suthar AB, Hoos D, Beqiri A, Lorenz-Dehne K, McClure C, Duncombe C. 2013. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta-analysis. Bull World Health Organ 91:46–56. doi: 10.2471/BLT.12.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thea DM, Steketee RW, Pliner V, Bornschlegel K, Brown T, Orloff S, Matheson PB, Abrams EJ, Bamji M, Lambert G, Schoenbaum EA, Thomas PA, Heagarty M, Kalish ML. 1997. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS 11:437–444. [DOI] [PubMed] [Google Scholar]
- 7.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, O'Sullivan MJ, Van Dyke RB, Jimenez E, Rouzioux C, Flynn PM, Sullivan JL. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 335:1621–1629. [DOI] [PubMed] [Google Scholar]
- 8.Dickover RE, Garratty EM, Herman SA, Sim MS, Plaeger S, Boyer PJ, Keller M, Deveikis A, Stiehm ER, Bryson YJ. 1996. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission. Effect of maternal zidovudine treatment on viral load. JAMA 275:599–605. [PubMed] [Google Scholar]
- 9.European Mode of Delivery Collaboration. 1999. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet 353:1035–1039. doi: 10.1016/S0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos LM, Lippi J, Rutherford GW, Duarte GS, Martins NG, Santos VS, Gurgel RQ. 2013. Maternal risk factors for HIV infection in infants in northeastern Brazil. Int J Infect Dis 17:e913–. doi: 10.1016/j.ijid.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. 2010. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 51:1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund J, Glezen WP, Piedra PA. 1998. Maternal immunization against viral disease. Vaccine 16:1456–1463. doi: 10.1016/S0264-410X(98)00108-X. [DOI] [PubMed] [Google Scholar]
- 13.Safrit JT, Ruprecht R, Ferrantelli F, Xu W, Kitabwalla M, Van Rompay K, Marthas M, Haigwood N, Mascola JR, Luzuriaga K, Jones SA, Mathieson BJ, Newell ML. 2004. Immunoprophylaxis to prevent mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr 35:169–177. doi: 10.1097/00126334-200402010-00012. [DOI] [PubMed] [Google Scholar]
- 14.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. 2012. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braibant M, Barin F. 2013. The role of neutralizing antibodies in prevention of HIV-1 infection: what can we learn from the mother-to-child transmission context? Retrovirology 10:103. doi: 10.1186/1742-4690-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omenda MM, Milligan C, Odem-Davis K, Nduati R, Richardson BA, Lynch J, John-Stewart G, Overbaugh J. 2013. Evidence for efficient vertical transfer of maternal HIV-1 envelope-specific neutralizing antibodies but no association of such antibodies with reduced infant infection. J Acquir Immune Defic Syndr 64:163–166. doi: 10.1097/QAI.0b013e31829f6e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi P, Moschese V, Broliden PA, Fundaró C, Quinti I, Plebani A, Giaquinto C, Tovo PA, Ljunggren K, Rosen J. 1989. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with the uninfected status of children born to seropositive mothers. Proc Natl Acad Sci U S A 86:8055–8058. doi: 10.1073/pnas.86.20.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markham RB, Coberly J, Ruff AJ, Hoover D, Gomez J, Holt E, Desormeaux J, Boulos R, Quinn TC, Halsey NA. 1994. Maternal IgG1 and IgA antibody to V3 loop consensus sequence and maternal-infant HIV-1 transmission. Lancet 343:390–391. doi: 10.1016/S0140-6736(94)91225-4. [DOI] [PubMed] [Google Scholar]
- 19.Ugen KE, Goedert JJ, Boyer J, Refaeli Y, Frank I, Williams WV, Willoughby A, Landesman S, Mendez H, Rubinstein A. 1992. Vertical transmission of human immunodeficiency virus (HIV) infection. Reactivity of maternal sera with glycoprotein 120 and 41 peptides from HIV type 1. J Clin Invest 89:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JB, Nduati R, Blish CA, Richardson BA, Mabuka JM, Jalalian-Lechak Z, John-Stewart G, Overbaugh J. 2011. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J Virol 85:5252–5261. doi: 10.1128/JVI.02216-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaillon A, Wack T, Braibant M, Mandelbrot L, Blanche S, Warszawski J, Barin F. 2012. The breadth and titer of maternal HIV-1-specific heterologous neutralizing antibodies are not associated with a lower rate of mother-to-child transmission of HIV-1. J Virol 86:10540–10546. doi: 10.1128/JVI.00518-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, Jaeger FH, Pollara J, Martelli A, Liebl BE, Lloyd K, Yates NL, Overman RG, Shen X, Whitaker K, Chen H, Pritchett J, Solomon E, Friberg E, Marshall DJ, Whitesides JF, Gurley TC, Von Holle T, Martinez DR, Cai F, Kumar A, Xia SM, Lu X, Louzao R, Wilkes S, Datta S, Sarzotti-Kelsoe M, Liao HX, Ferrari G, Alam SM, Montefiori DC, Denny TN, Moody MA, Tomaras GD, Gao F, Haynes BF. 2015. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest 125:2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moody MA, Gao F, Gurley TC, Amos JD, Kumar A, Hora B, Marshall DJ, Whitesides JF, Xia SM, Parks R, Lloyd KE, Hwang KK, Lu X, Bonsignori M, Finzi A, Vandergrift NA, Alam SM, Ferrari G, Shen X, Tomaras GD, Kamanga G, Cohen MS, Sam NE, Kapiga S, Gray ES, Tumba NL, Morris L, Zolla-Pazner S, Gorny MK, Mascola JR, Hahn BH, Shaw GM, Sodroski JG, Liao HX, Montefiori DC, Hraber PT, Korber BT, Haynes BF. 2015. Strain-specific V3 and CD4 binding site autologous HIV-1 neutralizing antibodies select neutralization-resistant viruses. Cell Host Microbe 18:354–362. doi: 10.1016/j.chom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders CJ, McCaffrey RA, Zharkikh I, Kraft Z, Malenbaum SE, Burke B, Cheng-Mayer C, Stamatatos L. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J Virol 79:9069–9080. doi: 10.1128/JVI.79.14.9069-9080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shioda T, Levy JA, Cheng-Mayer C. 1992. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 89:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anonymous. 2016. Correction: comprehensive sieve analysis of breakthrough HIV-1 sequences in the RV144 vaccine efficacy trial. PLoS Comput Biol 12:e1004733. doi: 10.1371/journal.pcbi.1004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolla-Pazner S, Edlefsen PT, Rolland M, Kong XP, de Camp A, Gottardo R, Williams C, Tovanabutra S, Sharpe-Cohen S, Mullins JI, de Souza MS, Karasavvas N, Nitayaphan S, Rerks-Ngarm S, Pitisuttihum P, Kaewkungwal J, O'Connell RJ, Robb ML, Michael NL, Kim JH, Gilbert P. 2014. Vaccine-induced human antibodies specific for the third variable region of HIV-1 gp120 impose immune pressure on infecting viruses. EBioMedicine 1:37–45. doi: 10.1016/j.ebiom.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang GY, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O'Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GB, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schon A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, McDermott AB, Mascola JR, Kwong PD. 2015. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22:522–531. doi: 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foley BLT, Apetrei C, Hahn B, Mizrachi I, Mullins J, Rambaut A, Wolinsky S, Korber B ed. 2013. HIV sequence compendium, LA-UR 13–26007. Theoretical Biology and Biophysics Group T-6, Los Alamos National Laboratory, Los Alamos, NM, USA. [Google Scholar]
- 31.Yates NL, Liao HX, Fong Y, de Camp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 33.Davis KL, Bibollet-Ruche F, Li H, Decker JM, Kutsch O, Morris L, Salomon A, Pinter A, Hoxie JA, Hahn BH, Kwong PD, Shaw GM. 2009. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1 V3-specific antibodies in human plasma. J Virol 83:1240–1259. doi: 10.1128/JVI.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cormier EG, Dragic T. 2002. The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J Virol 76:8953–8957. doi: 10.1128/JVI.76.17.8953-8957.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eda Y, Takizawa M, Murakami T, Maeda H, Kimachi K, Yonemura H, Koyanagi S, Shiosaki K, Higuchi H, Makizumi K, Nakashima T, Osatomi K, Tokiyoshi S, Matsushita S, Yamamoto N, Honda M. 2006. Sequential immunization with V3 peptides from primary human immunodeficiency virus type 1 produces cross-neutralizing antibodies against primary isolates with a matching narrow-neutralization sequence motif. J Virol 80:5552–5562. doi: 10.1128/JVI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, Zolla-Pazner S, Kong XP. 2010. Conserved structural elements in the V3 crown of HIV-1 gp120. Nat Struct Mol Biol 17:955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 37.Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol 68:6006–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spenlehauer C, Saragosti S, Fleury HJ, Kirn A, Aubertin AM, Moog C. 1998. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol 72:9855–9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cashin K, Sterjovski J, Harvey KL, Ramsland PA, Churchill MJ, Gorry PR. 2014. Covariance of charged amino acids at positions 322 and 440 of HIV-1 Env contributes to coreceptor specificity of subtype B viruses, and can be used to improve the performance of V3 sequence-based coreceptor usage prediction algorithms. PLoS One 9:e109771. doi: 10.1371/journal.pone.0109771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resch W, Hoffman N, Swanstrom R. 2001. Improved success of phenotype prediction of the human immunodeficiency virus type 1 from envelope variable loop 3 sequence using neural networks. Virology 288:51–62. doi: 10.1006/viro.2001.1087. [DOI] [PubMed] [Google Scholar]
- 41.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishko M, Somasundaran M, Brewster F, Sullivan JL, Clapham PR, Luzuriaga K. 2011. Genotypic and functional properties of early infant HIV-1 envelopes. Retrovirology 8:67. doi: 10.1186/1742-4690-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zolla-Pazner S, Cohen SS, Boyd D, Kong XP, Seaman M, Nussenzweig M, Klein F, Overbaugh J, Totrov M. 2015. Structure/function studies involving the V3 region of the HIV-1 envelope delineate multiple factors that affect neutralization sensitivity. J Virol 90:636–649. doi: 10.1128/JVI.01645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartlett JA, Wasserman SS, Hicks CB, Dodge RT, Weinhold KJ, Tacket CO, Ketter N, Wittek AE, Palker TJ, Haynes BF. 1998. Safety and immunogenicity of an HLA-based HIV envelope polyvalent synthetic peptide immunogen. DATRI 010 Study Group. Division of AIDS Treatment Research Initiative AIDS 12:1291–1300. [DOI] [PubMed] [Google Scholar]
- 45.Shen X, Duffy R, Howington R, Cope A, Sadagopal S, Park H, Pal R, Kwa S, Ding S, Yang OO, Fouda GG, Le Grand R, Bolton D, Esteban M, Phogat S, Roederer M, Amara RR, Picker LJ, Seder RA, McElrath MJ, Barnett S, Permar SR, Shattock R, DeVico AL, Felber BK, Pavlakis GN, Pantaleo G, Korber BT, Montefiori DC, Tomaras GD. 2015. Vaccine-induced linear epitope-specific antibodies to simian immunodeficiency virus SIVmac239 envelope are distinct from those induced to the human immunodeficiency virus type 1 envelope in nonhuman primates. J Virol 89:8643–8650. doi: 10.1128/JVI.03635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SSA, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin C, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fowler MG, Qin M, Fiscus SA, Currier JS, Makanani B, Martinson F, Chipato T, Browning R, Shapiro D, Mofenson L. 2015. Promise: efficacy and safety of 2 strategies to prevent perinatal HIV transmission, abstr 31LB, p 93 Abstr Conf Retrovir Opportun Infect, Seattle, Washington, 23 to 26 February 2015. [Google Scholar]