Abstract
We previously reported that lipid rafts are involved in long-chain fatty acid (LCFA) uptake in 3T3-L1 adipocytes. The present data show that LCFA uptake does not depend on caveolae endocytosis because expression of a dominant negative mutant of dynamin had no effect on uptake of [3H]oleic acid, whereas it effectively prevented endocytosis of cholera toxin. Isolation of detergent-resistant membranes (DRMs) from 3T3-L1 cell homogenates revealed that FAT/CD36 was expressed in both DRMs and detergent-soluble membranes (DSMs), whereas FATP1 and FATP4 were present only in DSMs but not DRMs. Disruption of lipid rafts by cyclodextrin and specific inhibition of FAT/CD36 by sulfo-N-succinimidyl oleate (SSO) significantly decreased uptake of [3H]oleic acid, but simultaneous treatment had no additional or synergistic effects, suggesting that both treatments target the same mechanism. Indeed, subcellular fractionation demonstrated that plasma membrane fatty acid translocase (FAT/CD36) is exclusively located in lipid rafts, whereas intracellular FAT/CD36 cofractionated with DSMs. Binding assays confirmed that [3H]SSO predominantly binds to FAT/CD36 within plasma membrane DRMs. In conclusion, our data strongly suggest that FAT/CD36 mediates raft-dependent LCFA uptake. Plasma membrane lipid rafts might control LCFA uptake by regulating surface availability of FAT/CD36.
INTRODUCTION
Adipocytes are the primary site for lipid storage and mobilization and, as such, one of their major roles is the uptake and release of long-chain fatty acids (LCFAs). The permeation of LCFAs across the adipocyte plasma membrane relies on a high-affinity, low-capacity carrier-facilitated transport system (Abumrad et al., 1981). Several proteins in the adipocyte plasma membrane have been implicated in fatty acid transport or binding such as plasma membrane fatty acid binding protein (FABPpm) (Stremmel et al., 1986), fatty acid transport protein (FATP) (Schaffer and Lodish, 1994), caveolin-1 (Trigatti et al., 1999), and fatty acid translocase (FAT/CD36) (Abumrad et al., 1993). The important role of FAT/CD36 for LCFA uptake in adipocytes has been extensively studied. When overexpressed in cultured fibroblasts FAT/CD36 increases saturable, high-affinity LCFA uptake (Ibrahimi et al., 1996). Moreover, FAT/CD36 knockout mice have increased serum fasting levels of nonesterified free fatty acids and show reduced uptake of oleate in isolated adipocytes (Febbraio et al., 1999).
Very recent findings indicate that specialized microdomains of the plasma membrane, termed lipid rafts, are essential for regulating LCFA uptake (Kolleck et al., 2002; Pohl et al., 2002, 2004; Razani et al., 2002; Ring et al., 2002; Vistisen et al., 2004). Rafts are membrane domains that are enriched in sphingolipids and cholesterol and form a liquid-ordered subdomain that contains a select set of membrane proteins (reviewed in Parton and Simons, 1995). On the basis of their particular properties as detergent-resistant membranes (DRMs), lipid rafts can be isolated from cell lysates (Parton and Simons, 1995). Caveolae are a specialized subset of rafts forming characteristic flask-shaped invaginations. Caveolin-1 is the major structural protein of caveolae and can bind fatty acids saturably and with high affinity (Trigatti et al., 1999). Whereas rafts are found in all cell types, caveolae are only found in some cell types among which adipocytes express a particularly high number of caveolae. Estimates have been made that from 15 to 30% of the adipocyte plasma membrane surface are caveolae (Fan et al., 1983). Caveolae participate in a large number of important cellular functions. These include the organization of numerous transmembrane signaling complexes in many cell types, the regulation of cellular cholesterol homeostasis, and formation of endocytic vesicles (Tran et al., 1987; reviewed in Parton 2003). It is known that albumin, which functions as a carrier for fatty acids, can be endocytosed by caveolae (Schubert et al., 2001). The endocytotic internalization of caveolae requires the GTPase dynamin II (Henley et al., 1998; Orth et al., 2002). Expression of a dominant negative dynamin mutant results in loss of endocytosis by caveolae and clathrin-coated pits (Orth et al., 2002).
Recently we showed that in 3T3-L1 adipocytes, lipid rafts are involved in binding and uptake of LCFAs. However, the mechanism of lipid raft-mediated LCFA uptake remained elusive. Theoretically, lipid raft-dependent LCFA uptake could rely either on receptor-mediated endocytosis or on facilitated transport by a fatty acid binding protein located in plasma membrane lipid rafts. In the present study, we investigated both of these hypotheses. First we prevented caveolae endocytosis by transient expression of the dynamin II mutant K44A (Orth et al., 2002) to investigate a potential involvement of lipid raft endocytosis in LCFA uptake. Subsequently, we characterized the distribution of FATP1 and FATP4, the major representatives of the FATP family in adipose tissue (Stahl, 2004), and FAT/CD36 in raft and nonraft membrane domains of whole cell lysates and plasma membrane fractions. FAT/CD36 has previously been shown to be localized in caveolae (Souto et al., 2003; Pohl et al., 2004); however, it is unclear whether these are plasma membrane caveolae involved in LCFA uptake. The functional role of FAT/CD36 associated with lipid rafts was studied using sulfo-N-succinimidyl oleate (SSO), an oleic acid derivative that specifically binds to and cross-links FAT/CD36 on the plasma membrane, resulting in an arrest of the transport function of this protein (reviewed by Coort et al., 2002). FATP1 and FABPpm function are not affected by SSO (Van Nieuwenhoven et al., 1995). Furthermore, SSO does not permeate the plasma membrane and thus does not directly affect intracellular transport processes (reviewed by Coort et al., 2002).
MATERIALS AND METHODS
[3H]Oleic acid was purchased from Biotrend (Cologne, Germany). Fatty acid-free bovine serum albumin (BSA) (fraction V), cyclodextrin, dexamethasone, phloretin, EGTA, leupeptin, pepstatin, sucrose, EDTA, chymostatin, HOSu(SO3)Na, dicyclohexylcarbodiimide, N,N-dimethylformamide (DMF), and nonradiolabeled oleic acid were purchased from Sigma-Aldrich (St. Louis, MO). Ultima-Gold scintillation fluid was purchased from Packard (Groningen, The Netherlands). Dimethyl sulfoxide (DMSO) was from Merck (Darmstadt, Germany).
Tr3-Isobutyl-1-methylxanthin was from Calbiochem (Bad Soden, Germany). The mouse antibody against the human transferrin receptor was from Zymed Laboratories (South San Francisco, CA). The antibody against caveolin-1 was from BD Transduction Laboratories (Heidelberg, Germany), and anti-Na,K-ATPase and anti-calreticulin were from Novus Biological (Littleton). Rabbit antibodies against mouse gp27/gp26 were a kind gift of Dr. M. Dominguez (Department of Anatomy and Cell Biology, McGill University, Montreal, Quebec, Canada).
Primary polyclonal antibodies against mouse FAT/CD36 were generated using the synthetic peptide SYKGKRNLSYWPSYC to which a cysteine residue had been added before coupling to keyhole limpet hemocyanin. The antiserum was produced by injection of guinea pigs (Peptide Specialty Laboratories, Heidelberg, Germany).
Cell Culture
3T3-L1 fibroblasts were obtained from the American Type Culture Collection (Rockville, MD) and cultured in DMEM supplemented with 10% (vol/vol) fetal calf serum (FCS; Invitrogen, Carlsbad, CA), 2 mM l-glutamine, 100 U/l penicillin, and 100 μg/l streptomycin at 37°C in 10% CO2 and passaged at ∼70% confluence. Confluent fibroblasts were induced to differentiate 1 d after reaching confluence by addition of DMEM containing 10% (vol/vol) FCS, 5 μg/ml insulin, 0.25 mM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 100 ng/ml d-biotin. After 72 h, the medium was replaced with fresh FCS and DMEM containing insulin. Adipocytes were refed with FCS and DMEM every 72 h and used for experiments 8–10 d postdifferentiation.
Synthesis of Sulfosuccinimidyl-Oleate
The synthesis of unlabeled and radiolabeled sulfosuccinimidyl derivatives of oleate (SSOs) was performed as described by Harmon et al. (1991). Oleate (0.25 mmol), HOSu(SO3)Na (0.25 mmol), and dicyclohexylcarbodiimide (DCC) (0.275 mmol) were dissolved in 0.5 ml of dry DMF and stirred at room temperature overnight. For synthesis of radiolabeled SSO, trace amounts of [3H]oleic acid were initially added to the nonradioactive oleic acid. The precipitated dicyclohexylurea was removed by filtration. The filtrate was transferred to 3°C for 4 h. Eight volumes of ethyl acetate was added, and the precipitated product was collected by filtration under nitrogen in a Glove Bag and then stored in a vacuumed desiccator over phosphorus pentoxide. Stock solutions of SSO were made dissolving the compounds in DMSO.
Treatment with [3H]SSO
Treatment of 3T3-L1 cells was performed exactly as described by Abumrad et al. (1991). Briefly, on day 10 after differentiation 3T3-L1 cells were released from dishes and washed three times with cold Krebs-Ringer-HEPES (KRH) buffer and checked for viability. Preparations were used only if >90% of cells excluded trypan blue. Cells were suspended [30% (vol/vol)] in KRH buffer containing 0.2% fatty acid-free bovine serum albumin and glucose (2 mM). Afterward, aliquots of SSO stock solution were added to the adipocyte cell suspension to a final concentration of 400 μmol of SSO. To avoid toxicity of DMSO the concentration of DMSO was always kept <0.05%. The cells were incubated in a metabolic shaker at 37°C for 30 min. At the end of the incubation, the cells were washed three times with KRH buffer containing 0.2% fatty acid-free BSA to remove any unbound sulfosuccinimidyl-oleate. The cells were either resuspended for assay of oleate transport or used for plasma membrane isolation experiments or isolation of detergent resistant membranes.
Assay for [3H]Oleic Acid Transport
The [3H]oleic acid uptake assays were performed as described previously (Stremmel et al., 1986) using confluent 3T3-L1 cell monolayers. Briefly, trace amounts of [3H]oleic acid mixed with 173 μmol/l nonradioactive oleic acid were dissolved in a defatted BSA solution (173 μmol/l) at a ratio of 1:1. 2 ml of the oleate/BSA solution was incubated with each 3T3-L1 cell monolayer in a 5-cm Ø culture dish at 37°C. The uptake was stopped by removal of the solution followed by addition of 5 ml of an ice-cold stop solution containing 0.5% (wt/vol) albumin and 200 μM phloretin. The stop solution was discharged after 2 min, and the culture dishes were washed by dipping them six times in ice-cold incubation buffer. NaOH (2 mol/l) was added to lyse the cells, and aliquots of the lysate were used for protein and radioactivity determination. Radioactivity was determined after the addition of 10 ml of Ultima-Gold in a 1217 Rackbeta liquid scintillation counter (Amerhsam Biosciences AB, Uppsala, Sweden).
Transfection of the Dynamin II Mutant K44A
Dynamin II K44A in pCMV5 was provided by S. Schmid (Scripps Research Institute, La Jolla, CA). Five days after differentiation of 3T3-L1 cells, transient transfection using the FuGene 6 Transfection reagent (Roche Diagnostics, Indianapolis, IN) was performed with 2 μg of the expression plasmid according to the instructions of the manufacturer (Roche Diagnostics, Basel, Switzerland). Transfections were performed 2 d before functional assays.
Functional Assays
Two days after transient transfection, caveolae-mediated endocytosis was assayed by monitoring the internalization of rhodamine-labeled cholera toxin (List Biological Laboratories, Campbell, CA). Cells were rinsed with phosphate-buffered saline (PBS) and then chilled to 4°C and incubated 15 min in DMEM containing 4 μg/ml rhodamine-cholera toxin B. Afterward, cells were rinsed four times with 4°C PBS to remove unbound toxin. These cells then were incubated ∼2.5 h in DMEM at 37°C to allow internalization. Afterward cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at 4°C. Cell cores were stained with 4,6-diamidino-2-phenylindole (DAPI) (List Biological Laboratories) 1:100 in PBS.
Fractionation of Total Membranes by Discontinuous OptiPrep Step Gradient
Fractionation of the membranes was performed using the OptiPrep gradient by AXIS-Shield (AXIS-Shield, Oslo, Norway) according to the instructions of the manufacturer. Briefly, cells were washed once in PBS to remove the culture medium and then once in the homogenization buffer (25 mM sucrose, 0.5 mM EDTA, 10 mM Tris). Afterward, cells were scraped in 3 ml of homogenization buffer and centrifuged at 1000 × g for 5 min. The pellet was resolved in 2 ml of homogenization buffer and cells were lysed by 10 passages through a fine syringe needle followed by treatment with a tight-fitting Dounce homogenizator. The homogenate was centrifuged at 1000 × g for 10 min to obtain a postnuclear supernatant. The supernatant was centrifuged at 100,000 × g for 40 min and resuspended in 1 ml of homogenization buffer containing 25% (wt/vol) iodixnol. The cell suspension was loaded on a nine-step OptiPrep gradient, which consisted of 25, 22, 19, 16, 13, 10, 7, 4, and 1% (wt/vol) iodixanol. Centrifugation was done in Beckman SW 41Ti rotor at 200,000 × g for 3 h at 4°C. Eighteen fractions were collected from the bottom of each centrifuge tube. A quarter of each fraction was analyzed with SDS-PAGE and Western blotting.
Isolation of Detergent-resistant Membranes
Detergent extraction with 3-[(3-cholamidopropyl)dimethylammonio]propane-sulfonate (CHAPS) was performed as described previously (Fiedler et al., 1993). 3T3-L1 cells were rinsed with ice-cold PBS and scraped on ice into 300 μl of 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 3 mM EDTA (TNE) buffer containing leupeptin, pepstatin, chymostatin, and antipain (each at 25 μg/ml). Cells were homogenized 15 times through a 22-gauge needle followed by 10 strokes with a tight-fitting Dounce homogenizer. The lysate was centrifuged for 5 min at 3000 rpm to obtain a postnuclear supernatant, which was subjected to extraction with 20 mM CHAPS in TNE buffer on ice. The extracts were adjusted to 40% sucrose and overlaid with a discontinuous sucrose gradient (6 ml of 30% sucrose in TNE or 2 ml of TNE without sucrose). The gradients were centrifuged at 200, 000 × g in a Beckman SW41 rotor for 16–22 h at 4°C. Fractions (1 ml each) were obtained and used for liquid scintillation counting and Western blotting.
Western Blot Analysis
For determination of the respective proteins, aliquots of membrane fractions were separated with SDS-PAGE and Western blotting, as we have described previously (Pohl et al., 2004). The origin of the antibodies is specified in Materials and Methods. Antibody binding was visualized using the enhanced chemiluminescence reagents (Amersham Biosciences). Immunoreactive bands on autoradiography films were scanned (Epson GT 9600; Epson, Tokyo, Japan) and quantified using Raytest image software (Raytest, Straubenhardt, Germany).
RESULTS
Inhibition of Endocytosis by Transient Expression of the Dynamin II Mutant K44A
To investigate whether lipid raft-mediated LCFA uptake in 3T3-L1 adipocytes is mediated by an endocytotic transport process, we transiently expressed the dominant negative dynamin II mutant K44A linked to green fluorescent protein (GFP). Twenty-four hours after transfection, ∼60% of cells expressed GFP. To confirm the functional inhibition of caveolae-mediated endocytosis by K44A in 3T3L1 adipocytes, we showed that the uptake of rhodamine-labeled cholera toxin B was inhibited by expression of K44A (Figure 1). Cholera toxin B is a glycosphingolipid-binding ligand known to be internalized by caveolae-mediated endocytosis (Parton 1994; Lencer et al., 1999). Instead of accumulating within intracellular compartments, the toxin remained concentrated at the surface of dynamin II-GFP–positive cells. However, when cells were transiently transfected and incubated with [3H]oleic acid for 5 min, there was no effect on [3H]oleic acid uptake compared with controls (10.4 ± 1.2 mmol oleate/mg protein in controls compared with 11.3 ± 1.2 mmol oleate/mg protein in transfected cells). Thus, under our experimental conditions endocytosis was not essential for LCFA uptake to occur.
Figure 1.
Inhibition of cholera toxin uptake by the dominant negative dynamin II mutant K44A. (A) Rhodamine-labeled cholera toxin. (B) GFP-labeled K44A. (C) Merged image showing that cells transiently expressing K44A do not efficiently take up cholera toxin, which remains at the cell surface (40× magnification).
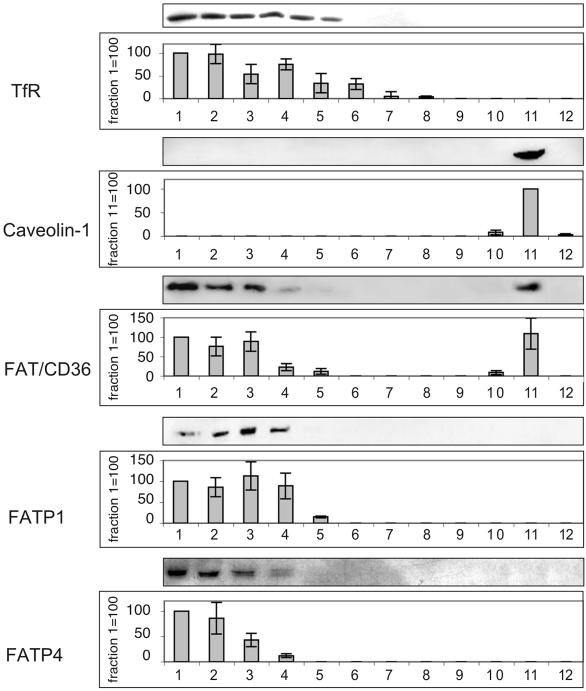
Expression of FATP1, FATP4, and FAT/CD36 in DRMs
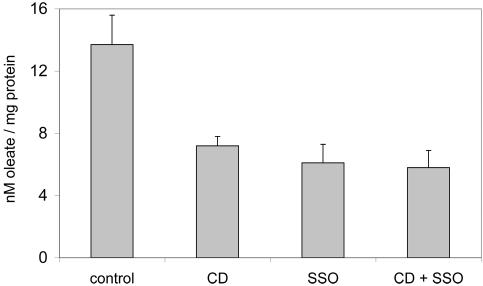
To investigate the presence of known LCFA binding proteins in lipid rafts, detergent lysates were subjected to centrifugation on sucrose density gradients. Due to their insolubility, lipid rafts localize to the upper (low-density) fractions. As shown in Figure 2, caveolin-1 was exclusively located in fraction 11 (DRM fraction), whereas the transferrin receptor (a marker protein of detergent-soluble membranes) was present in only the lower fractions (DSM fractions). FAT/CD36 was expressed in caveolin-1-enriched DRMs as well as in nonlipid raft fractions. In contrast, FATP1 and FATP4 were exclusively expressed in DSMs (Figure 2). To further elucidate the functional significance of FAT/CD36 expression in lipid rafts, we inhibited FAT/CD36 function and lipid raft function by treatment with SSO and by cyclodextrin, respectively. Both treatments resulted in a significant decrease of oleate uptake (Figure 3). However, combining both treatments did not show any additional effect on oleate incorporation. These results suggest that both treatments inhibit the same pathway of LCFA uptake.
Figure 2.
Isolation of a lipid raft-enriched membrane fraction. CHAPS-insoluble lipid rafts (DRMs) were separated from soluble membranes (DSMs) on sucrose gradients and immunoblotted with antibodies to the transferrin receptor (TfR), caveolin-1, FAT/CD36, FATP1, and FATP4. Representative blots are shown. For quantification of the respective protein a given fraction (indicated on the y-axis) has been set to 100, and the other fractions were expressed relative to this fraction. Values are means ± SD of three independent experiments.
Figure 3.
Effects of cyclodextrin and SSO on [3H]oleate uptake. Pretreatment of cells with β cyclodextrin (10 mM for 30 min at 37°C) or SSO (400 μM for 30 min at 37°C) resulted in a significant reduction in the rate of [3H]oleate uptake over the course of 5 min. However, combining both treatments had no additional effect on [3H]oleate uptake. The asterisks indicate statistical significance (p < 0.05). Values are means ± SD of three independent experiments.
[3H]SSO Binds to FAT/CD36 Located in DRMs
To investigate whether lipid raft FAT/CD36 binds LCFA, 3T3-L1 adipocytes were treated with [3H]SSO for 30 min followed by extraction of DRMs by sucrose density gradient centrifugation. Scintillation counting of the fractions showed the major peak of [3H]SSO cofractionated with DRMs and some [3H]SSO was found in the DSM fractions as well (Figure 4A). SDS-PAGE demonstrated that within the DRM fraction, [3H]SSO was specifically bound to FAT/CD36. The 88-kDa band representing FAT/CD36 (as confirmed by Western Blotting) was excised from the gel and assessed for the presence of [3H]SSO. Figure 4B shows that ∼80% of the total [3H]SSO content in the DRM fraction were associated with the FAT/CD36 band, whereas only 20% were found in the rest of the gel. In contrast, [3H]SSO in the DSM fractions was not predominantly associated with FAT/CD36. Because specific binding of SSO in live cells is limited to FAT/CD36 on the plasma membrane, the presence of [3H]SSO in the DSM fractions probably reflects nonspecific interactions with the plasma membrane. As opposed to live cells, [3H]SSO incubated with total cell homogenates mainly associated with FAT/CD36 in the DSM fractions (Figure 4C). This pattern is due to binding of [3H]SSO to previously intracellular FAT/CD36 in DSM and parallels the quantitative distribution of FAT/CD36 in DSMs and DRMs.
Figure 4.
Binding of [3H]SSO to lipid raft-enriched membrane fractions and FAT/CD36. After incubating live 3T3-L1 adipocytes (A and B) or total cell homogenates (C) with 400 μM [3H]SSO for 30 min, the reaction was stopped by an ice-cold solution containing 0.5% (wt/vol) albumin and 200 μM phloretin. Afterward, CHAPS-insoluble lipid rafts were separated from soluble membranes on sucrose gradients. (A) Binding of [3H]SSO to DRMs of live cells. When radioactivity of DRM and DSM fractions was determined the major peak of [3H]SSO cofractionated with DRMs. (B) Binding of [3H]SSO to FAT/CD36 in DRM and DSM fractions of live cells. One hundred microliters of each CHAPS fraction was separated by 12% SDS-PAGE, and the 88-kDa band representing FAT/CD36 (as confirmed by Western blotting) was excised from the gel and assessed for radioactivity. The figure shows [3H]SSO content associated with the FAT/CD36 band (closed squares) and the rest of the gel (open diamonds). (C) Binding of [3H]SSO to FAT/CD36 in DRM and DSM fractions of cell homogenates. As opposed to live cells, [3H]SSO incubated with total cell homogenates mainly associated with FAT/CD36 bands (closed squares) in DSM fractions. However, there was also modest radioactivity that was not associated with FAT/CD36 (open diamonds). Values in A, B, and C are means ± SD of five independent experiments.
Together, these results show that at the plasma membrane level [3H]SSO is predominantly bound to FAT/CD36 located in DRMs, whereas there is only very little binding to FAT/CD36 in DSMs. However, in total cell lysates the majority of FAT/CD36 is located in DSMs, suggesting that this might represent an intracellular FAT/CD36 pool that normally does not get into contact with extracellular SSO. Alternatively, FAT/CD36 located in DSMs on the plasma membrane level might not bind SSO efficiently. To address these open questions, we studied the subcellular distribution of FAT/CD36 by membrane fractionation.
Differential Subcellular Distribution of FAT/CD36, FATP1, FATP4, and Caveolin-1
To investigate the subcellular distribution of FAT/CD36, FATP1, FATP4, and caveolin-1 in 3T3-L1 adipocytes, we fractionated cell homogenates by a discontinuous iodixanol OptiPrep step gradient. We used antibodies against Na-K-ATPase, gp26/27, and calreticulin as markers for the plasma membrane, Golgi-network, and endoplasmic reticulum (ER), respectively. Figure 5A shows that fractions containing plasma membrane (nos. 1–7), Golgi network (nos. 7–14), and ER (nos. 14–18) could be separated. FAT/CD36 was present in the plasma membrane and Golgi fractions (Figure 5B). In contrast, FATP1 and FATP4 were expressed on the plasma membrane and in the ER. A large pool of caveolin-1 was present in the plasma membrane and Golgi fractions and a somewhat lower amount in the ER fractions as well.
Figure 5.
Membrane fractionation of 3T3-L1 cell homogenates. (A) Expression of marker proteins for plasma membrane (anti-Na,K-ATPase), Golgi network (gp 26/27), and ER (calreticulin). (B) Expression of proteins involved in LCFA uptake in membrane fractions. Fractionation was performed using a discontinuous OptiPrep step gradient, and equal amounts of protein were separated by SDS-PAGE, blotted, and probed with antibodies against the indicated proteins. Signals were quantified by densitometry, and protein content was expressed relative to a given fraction (indicated on the y-axis) that has been set to 100. Values are means ± SD of three independent experiments. (C) Binding of [3H]SSO to membrane fractions. Membrane fractionation was performed after incubation of 3T3-L1 adipocytes for 30 min with 400 μM [3H]SSO. Values are means ± SD of five independent experiments.
Using this assay, we also confirmed that [3H]SSO did not tether significantly to any intracellular membranes when incubated with live cells (Figure 5C). The failure of [3H]SSO to recognize FAT/CD36 in DSMs of live cells but not homogenates (Figure 4, B and C) indicates that FAT/CD36 in DSMs is restricted to the intracellular compartment.
This prediction was indeed confirmed by isolation of DRMs from plasma membrane fractions and intracellular membrane fractions of 3T3-L1 adipocytes (Figure 6). Whereas FAT/CD36 expression at the plasma membrane was restricted to DRMs intracellular FAT/CD36 was expressed predominantly in DSMs.
Figure 6.
Isolation of a lipid raft-enriched membrane fraction from plasma membrane fractions (A) and nonplasma membrane fractions (B). Representative blots are shown. For quantification of FAT/CD36 a given fraction (indicated on the y-axis) has been set to 100, and the other fractions were expressed relative to this fraction. Values are means ± SD of three independent experiments. Whereas FAT/CD36 at the plasma membrane level was exclusively located within DRMs (A), intracellular FAT/CD36 was expressed predominantly in DSMs with only minor amounts cofractioning with DRMs (B).
DISCUSSION
A number of recent studies in cell lines and caveolin-1 knockout mice have suggested that lipid rafts/caveolae and their marker protein, caveolin-1, are involved in the cellular uptake of fatty acids (Pohl et al., 2002, 2004; Trigatti et al., 1999, Razani et al., 2002; Ring et al., 2002). However, the mechanism of lipid raft-mediated LCFA uptake remains unclear. This study demonstrates for the first time that in the plasma membrane of 3T3-L1 adipocytes FAT/CD36 is exclusively located in lipid rafts, indicating that lipid raft-mediated fatty acid uptake depends on FAT/CD36 action.
The functional significance of this finding is underlined by the demonstration of [3H]SSO binding to FAT/CD36 in plasma membrane DRMs. Inhibition of FAT/CD36 function by SSO or disruption of lipid rafts by cyclodextrin decreased uptake of LCFA by a similar degree but had no synergistic effects, indicating that both agents inhibit a common pathway of LCFA uptake. Furthermore, the intracellular pool of FAT/CD36 cofractionating with the Golgi apparatus was not associated with lipid rafts. We and others have previously reported the expression of FAT/CD36 in lipid rafts of adipocytes (Souto et al., 2003; Pohl et al., 2004). Immunogold labeling and electron microscopy of the adipocyte plasma membrane confirmed that caveolae have a limited protein composition with caveolins, semicarbazide-sensitive amine oxidase, and FAT/CD36 being their major proteinaceous constituents (Souto et al., 2003). Our finding of FAT/CD36 expression in both the plasma membrane and intracellularly contrasts with a recent report by Stahl et al. (2002) describing abundant expression of FAT/CD36 on the adipocyte plasma membrane but no intracellular pools visualized by confocal laser scanning microscopy. However, in myocytes (Bonen et al., 1999, 2000; Luiken et al., 2002) and COS-7 cells (Frank et al., 2002) FAT/CD36 is present on the plasma membrane and inside cells as found by us in 3T3-L1 adipocytes.
The subcellular localization of FATP1 and FATP4 was addressed by only a few investigators (Schaffer and Lodish 1994; Stahl et al., 2002). Stahl et al. (2002) used differential centrifugation to demonstrate expression of FATP1 and FATP4 at the plasma membrane and in intracellular microsome fractions of adipocytes. In our study, we found FATP1 and FATP4 in DSMs of the plasma membrane and the ER fraction of adipocytes, but not in DRM. Caveolin-1 was highly expressed at the plasma membrane level but also was found in abundance in Golgi fractions and to a lesser extent in the ER. This finding is consistent with previous work by Furuchi and Anderson (1998) that demonstrated caveolin-1 expression at the same locations.
Together, our results strongly suggest that LCFA uptake requires binding to FAT/CD36 located in lipid rafts. Because caveolae/lipid rafts are involved in endocytosis of different solutes (Tran et al., 1987; Schubert et al., 2001), it is conceivable that after binding of LCFA to FAT/CD36 budding of caveolae from the plasma membrane is involved in the mechanism of LCFA incorporation. However, inhibition of endocytosis by transient overexpression of a mutant dynamin II resulted in decreased incorporation of rhodamine-labeled cholera toxin, but it had no effect on uptake of radiolabeled oleate. These results indicate that endocytosis is of minor importance for direct LCFA uptake in our experimental setting.
Function of FAT/CD36 is crucial for LCFA uptake in adipocytes. This study gives evidence that lipid rafts are necessary to target FAT/CD36 to the plasma membrane and therefore provide an explanation for our previous finding that disruption of lipid rafts results in inhibition of LCFA uptake in adipocytes (Pohl et al., 2004). We suggest that lipid rafts are involved in LCFA uptake by regulating FAT/CD36 expression and function at the plasma membrane level. This putative mechanism might also involve caveolin-1. Frank et al. (2002) reported that in COS-7 cells FAT/CD36 was targeted to the plasma membrane only when cotransfected with caveolin-1. Furthermore, coexpression of FAT/CD36 with caveolin-1 shifts the cellular distribution of FAT/CD36 from the Golgi to the plasma membrane (Frank et al., 2002). These findings are in line with the results of the present study and indicate that caveolin-1 might induce targeting of FAT/CD36 to caveolae at the plasma membrane level. Therefore, malfunction of FAT/CD36 might be the underlying mechanism for severely increased free fatty acid levels and resistance to diet-induced obesity in caveolin-1 knockout mice (Razani et al., 2002). However, it is important to emphasize that isolation of DRMs does not distinguish caveolae from noncaveolar rafts. Hence, FAT/CD36 is localized in rafts, but it remains to be determined whether it is in caveolae. Contradictory results as to whether CD36 is present in caveolar rafts or not have been reported. Souto et al. (2003) immunopurified caveolin-1 from adipocytes and found FAT/CD36 copurified with caveolin-1. Zeng et al. (2003) suggested that in Chinese hamster ovary cells FAT/CD36 is localized in lipid rafts but not in caveolae. Only little is known about the regulation of FAT/CD36 function, but several lines of evidence point toward a translocation mechanism for increasing LCFA uptake by FAT/CD36. In muscle cells, the distribution of FAT/CD36 between intracellular membranes and the plasma membrane plays a role for the regulation of this protein's function in LCFA uptake (Bonen et al., 1999, 2000; Luiken et al., 2002). A very recent report by Vistisen et al. (2004) suggested that in sarcolemma, FAT/CD36 colocalizes with the muscle-specific caveolae marker protein caveolin-3, indicating that caveolae may regulate cellular fatty acid uptake by FAT/CD36. This mechanism could hold true for adipocytes as well, because we found that FAT/CD36 in 3T3-L1 adipocytes is present in both an intracellular pool and at the plasma membrane level where it is associated with a caveolin-1–positive lipid raft fraction.
In summary, we have shown that at the cell surface FAT/CD36 is exclusively located within lipid rafts, whereas intracellularly FAT/CD36 is found in nonlipid raft membranes cofractionating with the Golgi apparatus. Thus, lipid raft microdomains might control fatty acid uptake by regulating FAT/CD36 surface availability. We propose a model in which FAT/CD36 recycles from intracellular nonlipid raft domains to lipid raft regions of the plasma membrane. Caveolin-1 might target FAT/CD36 to the plasma membrane. However, the mechanism of action and regulation of FAT/CD36 as well as the other proteins involved in LCFA uptake is not well understood and is the subject of ongoing investigations by a number of groups.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (STR216/11-1) and by a Dietmar Hopp Stiftung research grant to W.S.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–07–0616. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0616.
Abbreviations used: CD, cyclodextrin; DRM, detergent-resistant membrane; DSM, detergent-soluble membrane; ER, endoplasmic reticulum; LCFA, long-chain fatty acid; FABPpm, plasma membrane fatty acid binding protein; FATP, fatty acid transport protein; FAT/CD36, fatty acid translocase; SSO, sulfo-N-succinimidyl oleate.
References
- Abumrad, N. A., Perkins, R. C., Park, J. H., and Park, C. R. (1981). Mechanism of long chain fatty acid permeation in the isolated adipocyte. J. Biol. Chem. 256, 9183-9191. [PubMed] [Google Scholar]
- Abumrad, N. A., Forest, C. C., Regen, D. M., and Sanders, S. (1991). Increase in membrane uptake of long-chain fatty acids early during preadipocyte differentiation. Proc. Natl. Acad. Sci. USA 88, 6008-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumrad, N. A., El-Maghrabi, M. R., Amri, E. Z., Lopez, E., and Grimaldi, P. A. (1993). Cloning of rat adipocyte membrane protein implicated in binding or transport of long chain fatty acids that is induced during preadipocyte differentiation: homology with human CD 36, J. Biol. Chem. 268, 17665-17668. [PubMed] [Google Scholar]
- Bonen, A., Dyck, D. J., Ibrahimi, A., and Abumrad, N. A. (1999). Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am. J. Physiol. 276, 642-649. [DOI] [PubMed] [Google Scholar]
- Bonen, A., Luiken, J. J., Arumugam, Y., Glatz, J. F., and Tandon, N. N. (2000). Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J. Biol. Chem. 275, 14501-14508. [DOI] [PubMed] [Google Scholar]
- Coort, S. L., Willems, J., Coumans, W. A., van der Vusse, G. J., Bonen, A., Glatz, J. F., and Luiken, J. J. (2002). Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol. Cell. Biochem. 239, 213-219. [PubMed] [Google Scholar]
- Fan, J. Y., Carpentier, J. L., van Obberghen, E., Grunfeld, C., Gorden, P., and Orci, L. (1983). Morphological changes of the 3T3–L1 fibroblast plasma membrane upon differentiation to the adipocyte form. J. Cell Sci. 61, 219-230. [DOI] [PubMed] [Google Scholar]
- Febbraio, M., Abumrad, N. A., Hajjar, D. P., Sharma, K., Cheng, W., Pearce, S. F., and Silverstein, R. L. (1999). A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274, 19055-19062. [DOI] [PubMed] [Google Scholar]
- Fiedler, K., Kobayashi, T., Kurzchalia, T. V., and Simons, K. (1993). Glycosphingolipid enriched, detergent-insoluble complexes in protein sorting epithelial cells. Biochemistry 32, 6365-6373. [DOI] [PubMed] [Google Scholar]
- Frank, P. G., Marcel, Y. L., Connelly, M. A., Lublin, D. M., Franklin, V., Williams, D. L., and Lisanti, M. P. (2002). Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. Biochemistry 41, 11931-11940. [DOI] [PubMed] [Google Scholar]
- Furuchi, T., and Anderson, R. G. (1998). Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J. Biol. Chem. 273, 21099-21104. [DOI] [PubMed] [Google Scholar]
- Harmon, C. M., Luce, P., Beth, A. H., and Abumrad, N. A. (1991). Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J. Membr. Biol. 121, 261-268. [DOI] [PubMed] [Google Scholar]
- Henley, J. R., Krueger, E.W.A., Oswald, B. J., and McNiven, M. A. (1998). Dynamin-mediated internalization of caveolae. J. Cell Biol. 41, 85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi, A., Sfeir, Z., Magharaie, H., Amri, E. Z., Grimaldi, P., and Abumrad, N. A. (1996). Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc. Natl. Acad. Sci. USA 93, 2646-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolleck, I., Guthmann, F., Ladhoff, A. M., Tandon, N. N., Schlame, M., and Rüstow, B. (2002). Cellular cholesterol stimulates uptake of palmitate by redistribution of fatty acid translocase in type II pneumocytes. Biochemistry 41, 6369-6375. [DOI] [PubMed] [Google Scholar]
- Lencer, W. I., Hirst, T. R., and Holmes, R. K. (1999). Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450, 177-190. [DOI] [PubMed] [Google Scholar]
- Luiken, J. J., Dyck, D. J., Han, X. X., Tandon, N. N., Arumugam, Y., Glatz, J. F., and Bonen, A. (2002). Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282, 491-495. [DOI] [PubMed] [Google Scholar]
- Orth, J. D., Krueger, E. W., Cao, H., and McNiven, M. A. (2002). The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. USA 99, 167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, R. G., and Simons, K. (1995). Digging into caveolae. Science 269, 1398-1399. [DOI] [PubMed] [Google Scholar]
- Parton, R. G. (1994). Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155-166. [DOI] [PubMed] [Google Scholar]
- Parton, R. G. (2003). Caveolae–from ultrastructure to molecular mechanisms. Nat. Rev. Mol. Cell. Biol. 4, 162-167. [DOI] [PubMed] [Google Scholar]
- Pohl, J., Ring, A., Ehehalt, R., Schulze-Bergkamen, H., Schad, A., Verkade, P., and Stremmel, W. (2004). Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry, 43, 4179-4187. [DOI] [PubMed] [Google Scholar]
- Pohl, J., Ring, A., and Stremmel, W. (2002). Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J. Lipid Res. 43, 1390-1399. [DOI] [PubMed] [Google Scholar]
- Razani, B., et al. (2002). Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277, 8635-8647. [DOI] [PubMed] [Google Scholar]
- Ring, A., Pohl, J., Völkl, A., and Stremmel, W. (2002). Evidence for vesicles that mediate long-chain fatty acid uptake by human microvascular endothelial cells. J. Lipid Res. 43, 2095-2104. [DOI] [PubMed] [Google Scholar]
- Schaffer, J. E., and Lodish, H. F. (1994). Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79, 427-436. [DOI] [PubMed] [Google Scholar]
- Schubert, W., Frank, P. G., Razani, B., Park, D. S., Chow, C. W., and Lisanti, M. P. (2001). Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619-48622. [DOI] [PubMed] [Google Scholar]
- Souto, R. P., Vallega, G., Wharton, J., Vinten, J., Tranum-Jensen, J., and Pilch, P. F. (2003). Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J. Biol. Chem. 278, 18321-18329. [DOI] [PubMed] [Google Scholar]
- Stahl, A., Evans, J. G., Pattel, S., Hirsch, D., and Lodish, H. F. (2002). Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev. Cell. 2, 477-488. [DOI] [PubMed] [Google Scholar]
- Stahl, A. (2004). A current review of fatty acid transport proteins (SLC27). Pflugers Arch. 447, 722-727. [DOI] [PubMed] [Google Scholar]
- Stremmel, W., Strohmeyer, G., and Berk, P. D. (1986). Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc. Natl. Acad. Sci. USA 83, 3584-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, D., Carpentier, J. L., Sawano, F., Gorden, P., and Orci, L. (1987). Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc. Natl. Acad. Sci. USA 84, 7957-7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigatti, B. L., Anderson, R.G.W., and Gerber, G. E. (1999). Identification of caveolin-1 as a fatty acid binding protein. Biochem. Biophys. Res. Commun. 255, 34-39. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhoven, F. A., Verstijnen, C. P., Abumrad, N. A., Willemsen, P. H., Van Eys, G. J., Van der Vusse, G. J., and Glatz, J. F. (1995). Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem. Biophys. Res. Commun. 207, 747-752. [DOI] [PubMed] [Google Scholar]
- Vistisen, B., Roepstorff, K., Roepstorff, C., Bonen, A., van Deurs, B., and Kiens, B. (2004). Sarcolemmal FAT/CD36 in human skeletal muscle colocalizes with caveolin-3 and is more abundant in type 1 than in type 2 fibers. J. Lipid Res. 45, 603-609. [DOI] [PubMed] [Google Scholar]
- Zeng, Y., Tao, N., Chung, K. N., Heuser, J. E., and Lublin, D. M. (2003). Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J. Biol. Chem. 278, 45931-45936. [DOI] [PubMed] [Google Scholar]