Abstract
Background:
This review article is important for allergists/immunologists and otolaryngologists. It discussed chronic rhinosinusitis, epidemiology, pathogenesis, innate adaptive immunology, nuclear factor–kappa B related to inflammation, sepsis, complement, reactive oxygen species, asthma, sinusitis, elderly pathogenesis, oxidative stress, depression, seasonal variation, vitamin D, genetic susceptibility and sepsis, hereditary angioedema related to trauma and stress.
Objective:
The objective of this review is to link chronic rhinosinusitis, epidemiology, innate and adaptive immunology, NF-kappa B related to inflammation, sepsis, complement, reactive oxygen species, asthma and sinusitis.
Methods:
A literature search was conducted from several articles, prospective studies, recent reviews and earlier reports. A synergistic relationship develops between activation of the innate immune system and the loss of organ barrier functions. Many complex factors, such as genetics, physical agents, mediators in the development of organ failure both in asthma, sinusitis, stress, depression and trauma, leading to posttraumatic organ failure. Asthma and sepsis, a common condition encountered in hospital environments remains an important cause of death at intensive care units where allergists/immunologists and otolaryngologists are frequently consulted. The patient's immune surveillance could fail to eliminate the pathogen, allowing it to spread and there is a proinflammatory mediator release with inappropriate activation.
Conclusion:
This review discussed chronic rhinosinusitis, sinusitis related to trauma, the innate and adaptive immunology, NF-kappa B related to inflammation, sepsis, complement, inflammation, reactive oxygen species, asthma pathogenesis, and asthma in the elderly, oxidative stress, depression, seasonal variation and vitamin D, cytokines, genetic susceptibility related to sepsis, hereditary angioedema related to trauma and stress.
Keywords: Sinusitis, innate and adaptive immunology, sepsis, asthma, trauma, inflammation
The purpose of this review was to link chronic rhinosinusitis (CRS), epidemiology, innate and adaptive immunology, nuclear factor–kappa B (NF-κB) related to inflammation, sepsis, complement, reactive oxygen species (ROS), asthma, and sinusitis for allergists immunologists and otolaryngologists. Pathogenesis in the elderly, oxidative stress, depression, seasonal variation and vitamin D, trauma and cytokines, genetic susceptibility related to sepsis, hereditary angioedema (HAE) related to trauma and stress also were covered.
CRS is a multifactorial disease with impaired ostial patency, mucociliary impairment, allergy, bacterial or fungal infection, an immunocompromised state, and environmental and genetic factors thought to be associated with risk factors. CRS has been defined as persistent symptomatic inflammation of the nasal and sinus mucosa that results from the interaction of multiple host and environmental factors.1 On epidemiologic grounds, some association has been found between CRS prevalence and air pollution, active cigarette smoking, secondhand smoke exposure, perennial allergic rhinitis, and gastroesophageal reflux.2
CRS is broadly classified into two groups: CRS with nasal polyposis and CRS without nasal polyposis. Many definitions of CRS with nasal polyposis exist, and estimates of its prevalence vary.3 Estimated prevalence ranges widely, from 2% to 16%. It is more common in female subjects, ages 18–64 years, and in southern and midwestern regions of the United States. CRS is more prevalent in patients with comorbid diseases, such as asthma, chronic obstructive pulmonary disease, and environmental allergies. Few studies examined patient ethnicity, socioeconomic status, geographic location, and cultural factors in the CRS populations.
EPIDEMIOLOGY
This article provides an overview of the epidemiology, racial variations, and economic burden of CRS.4 Despite active research in tertiary care settings, an understanding of CRS etiology, its natural history, and geographic and racial variation requires thorough data entry at the level of primary care settings, emergency departments, and otolaryngologists. Only by the use of validated universal definitions for CRS, well-defined methodology in epidemiologic research, and accurate entry of disease variables will it be possible to obtain epidemiologic data on the natural evolution of the disease and its socioeconomic factors, and thereby make advances toward disease prevention.4 A recent article listed the presentation of available literature on CRS epidemiology rhinosinusitis: epidemiology and cost.4 Understanding CRS allows us to characterize and differentiate the heterogeneous pathology of chronic sinonasal inflammation based on histopathology, inflammatory pattern, cytokine profile, and remodeling processes. Epidemiologic studies are urgently needed to fill the immense gaps in knowledge, and a fundamental reconsideration of CRS as a chronic episodic disease may provide a critical framework with which future studies can conceptualize this epidemic, which silently claims a heavy individual and societal toll.5
An article reviewed insights that surround the etiology and pathogenesis of CRS and highlighted the increasing recognition of host-mediated mechanisms in driving mucosal inflammation, and it published differences between epithelium from patients with CRS and normal controls, and classified several broad categories.6 Alterations were reported in the various components of the epithelial innate immune system, including epithelial-expressed pattern-recognition receptors and the levels of antimicrobial innate immune effector molecules. Other studies demonstrated differences in the proteins involved in maintaining epithelial barrier integrity. Recent studies showed that, in CRS, epithelial-derived cytokines, chemokines, and inducible surface proteins are involved in recruiting and activating cells of the adaptive immune system.6
The sinonasal epithelium provides a mechanical and innate immune barrier to a diverse array of environmental agents. This barrier also plays a key role in regulating the acquired mucosal immune response in the nose. Results of recent studies indicated that defects in this barrier may foster the development of chronic sinonasal inflammation in response to environmental agents and pathogenic or commensal organisms. The ability to dissect and analyze defects in the inflammatory response in rhinosinusitis may help identify novel targets for drug development.6
CRS is the second most common chronic medical condition in the United States and represents a group of disorders characterized by inflammation of the nasal mucosa and paranasal sinuses. CRS with or without nasal polyps is defined as inflammation of the nose characterized by two or more symptoms, either nasal blockage, obstruction, congestion, or nasal discharge with or without facial pain and/or pressure; and/or with or without reduction or loss of smell.7
Patients who undergo endoscopic sinus surgery (ESS) are at risk of complications due to the close proximity of the sinuses to the orbit and anterior skull base. A prospective study evaluated the complications of ESS and identified patient characteristics that are risk factors for the complications.8 The study was conducted with 706 patients who underwent ESS for CRS, and patients completed preoperative examinations that included computed tomography; endoscopic observation for nasal polyps; and tests for comorbidities, including asthma and vascular disease.8 Perioperative complications were evaluated based on information provided by the surgeons. Multivariate analysis was performed to identify patient characteristics that were risk factors for complications. Perioperative complications occurred in 41 patients; a major complication, cerebrospinal fluid leakage, occurred in one patient; and minor complications occurred in 40 patients, with the most common being intraoperative hemorrhage. Multivariate analysis indicated that the presence of asthma and the total polyp score correlated significantly with the occurrence of complications.8 Thus, the risk factors for perioperative complications were asthma and the polyp score; the surgeon should confirm whether the patient has lower airway disease, especially asthma, before operating and also should determine the grade of nasal polyps.8
Current literature related to asthma diagnosis, epidemiology, pathogenesis, and treatment linked with rhinosinusitis is important. Asthma is very heterogeneous; new theories and treatments are emerging. Asthma is a growing epidemic among children and adults in the United States. The severity of asthma is caused by many factors, such as a lack of education, poor early recognition, decreased symptom awareness, improper medications, and phenotypic changes. Genetic variation, innate immune genes, those involved in tissue remodeling and arachidonic acid metabolism, and inflammatory mediators might contribute to the pathogenesis of CRS linked with asthma.9 This extensive review addressed concepts of the burden of asthma and sinusitis, altered innate immunity, adaptive immunity, asthma remodeling, the airway epithelium, the role of airway smooth-muscle cells, united allergic airway, genetics (an integral part in asthma), and CRS.9 The role of vitamin D in both asthma and CRS in the elderly and in pediatric populations, various treatment options, exhaled nitric oxide, the link with CRS and biofilms, CRS phenotypes, and the treatment of asthma and CRS were also briefly addressed.9
Microbial biofilms have been implicated in the pathogenesis of CRS with nasal polyposis Although biofilms are characterized by an extremely high resistance against chemical and physical agents, low-frequency ultrasound treatment has been suspected to be an efficient and safe method for biofilm disruption.10 CRS is a highly prevalent disease in the adult and pediatric populations, and causes significant burden, and the management is considered one of the most costly public health conditions; comorbidities include asthma, aspirin sensitivity, and nasal polyposis.11 Staphylococcus aureus biofilms and exotoxins that act as superantigens have been implicated as playing an important pathologic role in the incidence, maintenance, and ongoing burden of CRS. A better understanding of the interplay between bacterial factors, host factors, and the environment will facilitate better management of this disease. This literature review focused on these factors and highlighted current research in this field.11 Eradicating the bacteria without the biofilm leaves the potential for future infections, and another generation of bacterial cells can inhabit the biofilm, which allows infection to persist, likely even more virulent because time allows for more-resistant mutations.11
Midface fractures commonly occur after trauma to the face and may cause changes in the normal sinus outflow system. A study examined the incidence of rhinosinusitis after midface fractures and reported that patients who experienced a midface fracture had a much higher risk of developing CRS that negatively affected their long-term quality of life. These patients should be monitored with long-term follow-up and treated appropriately.12
SINUSITIS RELATED TO TRAUMA
Trauma to the anterior pituitary by a metallic foreign body from the right nostril to the sella, responsible for panhypopituitarism and sinusitis, has been reported.13 The direct trauma to the pituitary gland by a metallic foreign body is exceptional, and this report of neglected panhypopituitarism, discovered 31 years after injury with a pair of scissors, was found. Given the age of the trauma, the patient refusing any surgery, and the surgical risks of a possible extraction of the metal object, abstention was required.13
Changes in maxillary sinus mucosa of patients with odontogenic maxillary sinusitis associated with the presence of a foreign body, depending on its duration, can develop, and foreign bodies present in the maxillary sinuses cause marked structural reorganization of the mucous membrane, usually with the predominance of hypertrophic and polypous changes.14 The effect of evaluation of repeated debridement after ESS was reported.4 The researchers believed that excessive debridement may induce more surgical trauma and cause more facial pain to patients, and, in terms of subjective recovery and health care costs, the appropriate extending of postoperative management time and decreasing intervention frequencies will not affect the therapeutic effect of endoscopic surgery for chronic sinusitis.15
INNATE AND ADAPTIVE IMMUNE SYSTEM IN HEALTH AND DISEASE
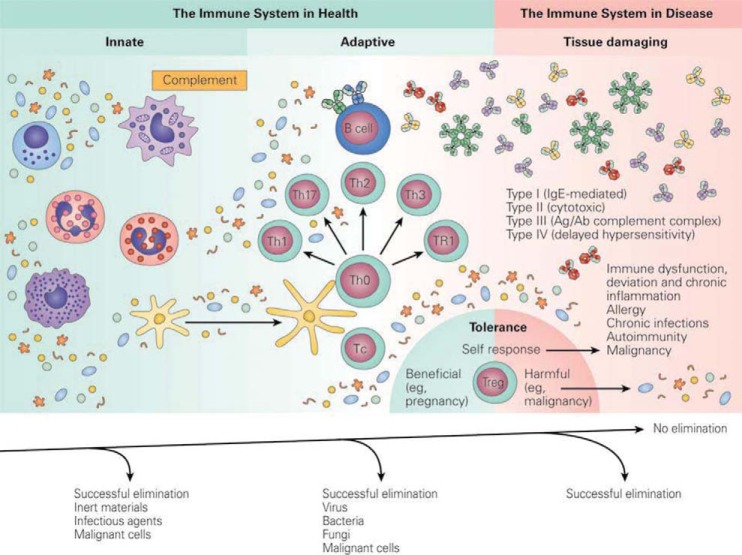
The innate system can occur within a few hours of contact with a pathogen that includes complement and other cells (Fig. 1). Neutrophils, T cells, their subdivisions, and tissue damage with immune dysfunction, deviation, and chronic inflammation can occur.16 The adaptive immune response occurs within a few days and generates immune memory. Microbial mechanisms triggered by innate immunity can control most infections before the adaptive response, and can result in harmful effects, and an uncontrolled inflammatory response that is initiated against infection is seen in severe sepsis and its complications.16
Figure 1.
The immune system in health and disease (Source: Ref. 16).
Complement is part of the innate immune system and an important effector mechanism of humoral immunity. A schematic representation of the classic complement cascade that shows the initiation of the pathway by binding of the C1q component of C1 binding to the Fc region of an immunoglobulin G antibody molecule bound to an antigen on the surface of a target cell is shown in Fig. 2.17 The schematic representation of the alternative pathway may be formed by one of the other pathways or by nonspecific proteolytic cleavage of C3, binds with factor B, which is analogous to C2 (Fig. 3), along with the late steps of complement activation and the membrane attack complex.17
Figure 2.
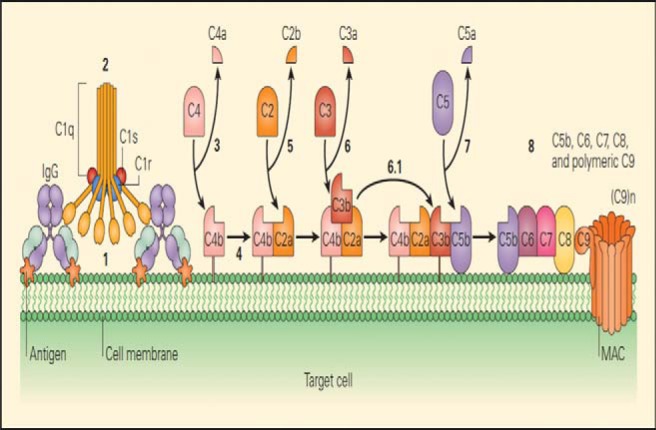
Schematic representation of the classical complement cascade, showing the initiation of the pathway by binding of the C1q component of C1 binding to the Fc region of an immunoglobulin G (IgG) antibody molecule bound to an antigen on the surface of a target cell. The numbers in bold indicate the sequential steps that are involved in the activation of each of the components, leading to the final lytic event carried out by the membrane attack complex (MAC). Some sources now use a revised nomenclature for the fragment of C2, in which C2a is the small fragment that diffuse away, and C2b is the larger fragment that binds with C4b and acts in the convertase, C4b2b (Source: Refs. 16 and 17).
Figure 3.
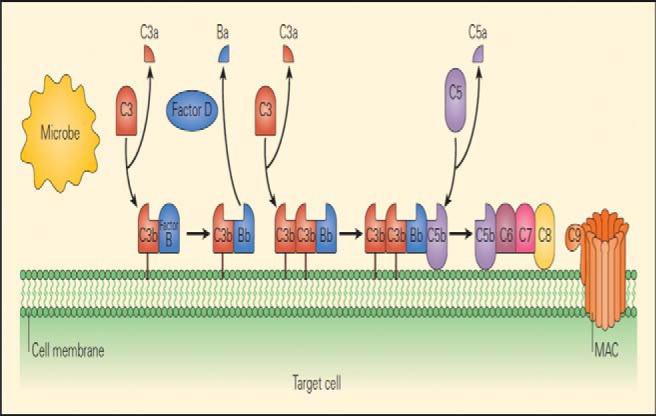
Schematic representation of the alternative pathway, showing the initiation of the pathway when C3b, which may be formed by one of the other pathways or by nonspecific proteolytic cleavage of C3, binds with factor B, which is analogous to C2. When bound to C3b, B acquires a conformation that allows it to be cleaved by the protease D, after which the subsequent steps of the pathway are similar to those of the other two pathways (Source: Refs. 16 and 17).
ROLE OF NF-κB RELATED TO INFLAMMATION
Activated neutrophils can upregulate the expression of many genes, in particular, those that encode cytokines and chemokines, and subsequently release the corresponding proteins. Many of these genes depend on the activation of transcription factors, such as NF-κB, for inducible expression.18 NF-κB activation may underlie the action of proinflammatory stimuli toward human neutrophil gene expression and add a new facet to our understanding of neutrophil biology.18 Systemic inflammation subsequent to polytrauma can be connected to polymorphonuclear neutrophils (PMN) dysregulation characterized by reduced NF-κB translocation and cytokine expression. NF-κB activation, as well as its downstream regulation of interleukin (IL) 8 expression in PMN, can follow major trauma.19 NF-κB translocation was significantly increased on admission and reduced within 6 hours, whereas it increased in the survivors' group. Thus, a concomitant initial increase in transcriptional NF-κB activity and IL-8 messenger RNA expression was observed in the early posttraumatic period, which preceded the downregulation of the innate immune system.19
The IL-8 concentration in the pulmonary fluid of patients with a thoracic trauma is seen as an indicator for the occurrence of acute respiratory distress syndrome (ARDS) because increased levels correlate with the incidence.20 Discriminating between ARDS and other similar diseases is critically important; however, only a few biomarkers are currently available for diagnostic purposes.21 Predicting the severity, response to therapy, or outcome of the illness is also important for developing treatment strategies for each patient. In parallel with progress in understanding the pathophysiology of ARDS, various humoral factors induced by inflammation and molecules derived from activated cells or injured tissues have been shown as potential biomarkers that may be applied in clinical practice.21 In a review, the current understanding of the basic pathophysiology of ARDS and associated candidate biomarkers are discussed.21
Cigarette smoke–induced airway inflammation plays a role in the pathogenesis of airway inflammation. Dong et al.22 reviewed resolvin-D1, which ameliorates inflammatory responses in lung injury, and asthma and inhibited IL-8 and hydrogen peroxide production induced by cigarette smoke extract (CSE) attenuating NF-κB activation. Resolvin-D1–attenuated cigarette smoke extract triggered inhibitor of NK-κB degradation and NF-κB/p65 activation dose dependently, and inhibited NF-κB DNA binding activity and inhibited cigarette smoke extract induced IL-8 and hydrogen peroxide production.22
NF-κB also plays an important role in asthma and SLE, including B-cell development, signaling, and cytokines, which play a crucial role in the pathogenesis of SLE and T-cell development.23 The roles of dendritic cells, which can promote tolerance or immunity to antigens, of polymorphisms and of NF-κB linked with SLE were discussed.23
INFLAMMATION, INNATE IMMUNITY, AND REACTIVE OXYGEN SPECIES
The cell-regulated innate immune system often contributes to posttraumatic multiple organ dysfunctions. With a cell fraction of 50–60% of all leukocytes, polymorphonuclear leukocytes have been shown to be key effector cells within the innate inflammatory immune reaction, and immunologic abnormalities can provoke multiple organ failure in severely injured patients in two forms, which follow a biphasic pattern.24 The first phase is caused by the immune system during a systemic inflammatory response, and, in the second phase, the patient is more susceptible for sepsis due to host defense failure or immune paralysis.24 Polymorphonuclear phagocytes and monocytes are the main effector cells of the innate immune system that are involved in organ failure and are controlled by cytokines, chemokines, complement factors, and specific tissue signals.25 Major torso trauma can prime and activate polymorphonuclear neutrophils (PMN) within 3–6 hours after injury. Postinjury priming of PMNs may create an early vulnerable window, during which a second event activates exuberant PMN cytotoxic superoxide anion O2 release, which renders the injured patient at high risk for multiple organ failure.26
The impact of trauma on neutrophil function was evaluated by Hazeldine et al.27 Trauma-induced changes occur related to neutrophil biology, such as posttraumatic complications in multiple organ failure and ARDS. This area is gaining considerable interest is the manipulation of neutrophil function as a means by which to potentially improve patient outcome.27
Neutrophils play an essential role in the body's innate immune response to infection, and these phagocytic cells possess an impressive array of microbicidal weapons that can be brought to bear on an invading pathogen, including a variety of toxic oxygen radical species and proteolytic enzymes.28 Quinones undergo highly regulated redox reactions in the mitochondria, Golgi apparatus, plasma membrane, and endoplasmic reticulum. Important consequences are the production of and protection against ROS. The mechanisms by which the inflammatory response and ROS production occur is also be discussed.28
ASTHMA AND OXIDATIVE STRESS
Oxidative stress occurs as a result of inflammation but also from environmental exposure to air pollution.29 There is an important role of oxidative stress, and therapeutic interventions that decrease exposure to environmental ROS or that augment endogenous antioxidant defenses might be beneficial as adjunctive therapies in patients with asthma.30 Jiang et al.31 investigated the molecular redox mechanisms in allergic asthma and provided a basis for further investigation of oxidative stress in allergic asthma and the signaling involved in its pathogenesis. The researchers indicate that oxidative stress byproduct represents a novel method by which asthma disease severity can be monitored to attenuate levels of ROS and alleviate oxidative stress–induced airway inflammation in patients with asthma.31
There is a need for interventional studies that provide evidence of a link between oxidative stress and depression. Patients with asthma and with different degrees of severity have been observed. An increase in processes of lipid peroxidation, depression of antioxidant protection, increase in oxide nitrogen metabolites in blood serum, and condensate of exhaled air were detected. These pathologic changes may be considered as a manifestation of system oxidative stress more expressed in the bronchi.32 Asthma and major depressive disorder co-occur at higher rates than expected, and alterations in the immune, autonomic nervous, and other key systems are apparent and may contribute to this increased risk of co-occurrence.33 High rates of major depressive disorder in asthma may result from the stress of chronic illness, the medications used to treat, or a combination of the two. The high level of co-occurrence may also reflect dysregulation of certain stress-sensitive biologic processes that contribute to the pathophysiology of both conditions.
Frieri et al.34 recently reviewed asthma, stress, and depression in women. This review described major clinical implications of stress, anxiety, and depression, and associated hormonal changes.34 Screening for depression and cognitive decline is particularly important in the geriatric population and in women because early intervention can significantly improve outcomes. Encouraging patients to participate in their own health care is an important goal. These interventions can impact quality of life and lead to more-effective management. The approach to asthma in the elderly population is summarized in Fig. 4.
Figure 4.
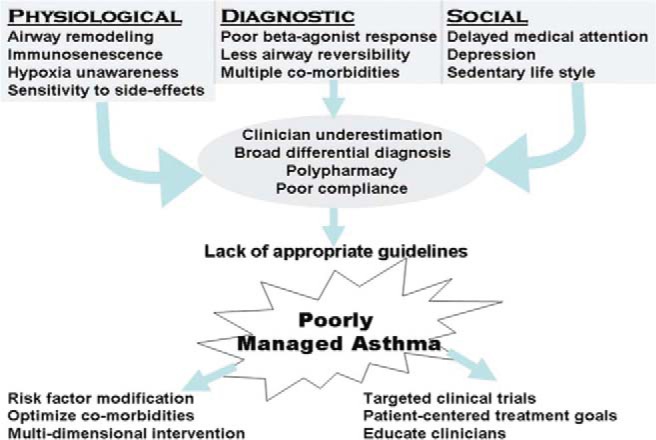
General algorithm, summarizing the multiple issues that lead to poorly managed asthma in the geriatric population. The goals of a multidisciplinary approach are stated to help guide treatment (Source: Ref. 41).
Di Marco et al.35 investigated the correlation between patients' characteristics, including anxiety and depression, and the level of asthma control evaluated by the asthma control test.35 Patients with poorly controlled asthma were more frequently women, older, obese, and with a worse pulmonary function, with more anxiety, and/or more depression. The presence of anxiety and depression was associated with a higher health care utilization. A better understanding of this association may have major clinical implications, mainly in patients with poorly controlled asthma in whom the presence of anxiety and depression should be investigated.35 Wang et al.36 reported the relationship between anxiety and depression, and stated that only anxiety is associated with worse asthma control and that a significant interaction effect of depression and anxiety accounted for lower asthma-related quality-of-life scores.
CHRONIC SINUSITIS AND ASTHMA PATHOGENESIS IN THE ELDERLY
A high prevalence of hyposmia and anosmia can occur, especially in the elderly, associated with chronic sinusitis. Several different pathophysiologic processes, such as head trauma, aging, autoimmunity, and toxic exposures, can contribute to smell impairment, with distinct implications concerning prognosis and possible treatment. As allergists, we are most likely to see this symptom in patients with CRS, and this now appears to be due more to the mucosal inflammation than to physical airway obstruction. Several different pathophysiologic processes, such as head trauma, aging, autoimmunity, and toxic exposures, can contribute to smell impairment, with distinct implications concerning prognosis and possible treatment.37
Grainge and Davies38 reviewed epithelial injury and repair in airway diseases and considered three important groups of environmental stimuli, such as environmental pollution, acetaminophen, viruses, including rhinovirus; agents that cause barrier disruption (e.g., house-dust mite allergens). The pathology associated with each stimulus was considered, and potential future treatments that arise from research on their effects were presented.38 Frieri39 reviewed airway remodeling, various inflammatory cells, the role of angiogenesis, vascular endothelial cell growth factor, respiratory syncytial virus, and other biomarkers, including exhaled nitric oxide. Control of allergic asthma by using immunomodulation and other strategies were addressed.
Follenweider and Lambertino40 reviewed the epidemiology of asthma in the United States. In 2009 there were 2.1 million asthma-related emergency department visits. Emergency department visits lend an opportunity for providers to identify and intervene in the care of patients whose asthma is poorly controlled. 40 Asthma is associated with significant morbidity and mortality in the geriatric population, and the diagnosis is frequently missed. Factors that contribute to this include respiratory changes caused by aging, immunosenescence, a lack of symptoms, polypharmacy, clinician unawareness, and a lack of evidence-based guidelines for diagnosis and management.40 Madeo et al.41 addressed age-related changes, pathophysiology, and the role of allergy in elderly patients with asthma. Asthma in the elderly and in younger populations were compared, and a broad set of goals to guide future management driven by a multidiscipline approach was discussed.41 Age-related changes in pulmonary function, immunity, and perception of symptoms has rendered the management of asthma in the elderly particularly challenging. Managing these patients is further complicated by the unique issues of this population, which include multiple comorbidities, including chronic sinusitis.41
Nassef et al.42 reviewed how fracture risk influences our decision making in asthma care. The researchers stated the importance of monitoring measures of control in the treatment of asthma and equal vigilance in monitoring long-term adverse effects, e.g., increased fracture risk, of the medications used to achieve that control essential to the total care of patients with asthma.42
ROLE OF VITAMIN D IN CHRONIC SINUSITIS AND FRACTURES
A large number of human, animal, and in vitro studies have indicated that vitamin D3 plays a critical role in inflammatory airway diseases such as asthma, CRS, and allergic rhinitis. Vitamin D3 acts on a broad range of immune cells involved in the pathogenesis of these diseases, including T cells, dendritic cells, macrophages, and B cells. In addition, vitamin D3 can also regulate the functions of a number of nonimmune cells, including epithelial cells, fibroblasts, and smooth-muscle cells.43
Bird et al.44 reviewed the association between seasonal variation in vitamin D, postural sway, and falls, and stated the risk and whether postural sway varies seasonally and is associated with serum vitamin D and falls. Vitamin D levels varied seasonally, peaking in summer. 44 The incidence of falls and injurious falls were lower in spring, with the highest fall rate at the end of autumn. Postural sway remained stable across the year, whereas vitamin D varied seasonally. This study provided important evidence for clinicians and researchers and provided interventions that measured balance outcomes across seasons. Frieri and Valluri 45 reviewed, in an earlier study, vitamin D deficiency as a risk factor for allergic disorders and immune mechanisms. Vitamin D deficiency has been extensively reviewed. Vitamin D deficiency was related to the immune system dysregulation and was positively correlated with the prevalence of allergies, and, after adjusting the model for age, sex, race, smoking, alcohol, and educational status, the odds ratio still remained significant. There was also a positive correlation with allergy subtypes, such as prevalence of rashes, sneezing, and sinus infections, with low vitamin D.45
TRAUMA AND CYTOKINES
Severe trauma can lead to immediate hyperinflammatory responses with neutrophil activation, continuous interleukin secretion related to IL-6, which can induce upregulation of further major anti-inflammatory cytokines mediators, e.g., IL-1046 which can markedly inhibit lymphocyte and phagocytic functions, essential for an adequate immune response to invading microbes.46
A recent murine study evaluated the role of intraalveolar tumor necrosis factor (TNF) α and IL-6 in a combination of skin burn and smoke inhalation injuries because this combined trauma is associated with an increased morbidity and mortality compared with either of these traumas alone.47 The differences between the combined and singular traumas indicated that TNF-α plays a role in the immunologic hyporesponsiveness of the lung and, therefore, in the systemic pathophysiologic pathway that often leads to patient mortality. An inverse correlation between TNF-α and IL-6, both classic markers of inflammation, in the intraalveolar space was observed.48
SEPSIS
Measurements of thioredoxin (Trx), macrophage migration inhibitory factor (MIF), IL-6, IL-8, IL-10, and procalcitonin (PCT) in plasma from patients with systemic inflammatory stress syndrome (SIRS) and/or sepsis, neutropenic sepsis, healthy volunteers, and patients before esophagectomy were evaluated.49 Plasma levels of Trx, MIF, IL-6, IL-8, IL-10, and PCT were raised in patients with SIRS and/or sepsis. Trx and MIF differed from cytokines and PCT in that levels were significantly lower in patients with neutropenia compared with the main SIRS and/or sepsis group. IL-8 and PCT levels were significantly greater in the group of patients with neutropenia. The link between MIF and Trx highlighted in this study has implications for future investigations into the pathogenesis of SIRS and/or sepsis.49
Sepsis and its sequelae are the leading causes of death among the critically ill in noncoronary intensive care units (ICU), and, currently, treatment for sepsis is largely supportive, based on fluid resuscitation and administration of vasopressors and antibiotics. A greater understanding of the pathophysiology of SIRS and/or sepsis could help identify other effective pharmacotherapies.50 The only pharmacotherapy shown to reduce mortality in sepsis, in a randomized clinical trial, was activated protein C.50
GENETIC SUSCEPTIBILITY RELATED TO SEPSIS
Sepsis is a clinical syndrome that occurs in patients after infection or injury and is a leading cause of morbidity and mortality. CD86 polymorphisms have been associated with susceptibility to pneumonia-induced sepsis and may affect gene expression in monocytes. CD86 (B7–2) is expressed on various immune cells and can play a critical role in immune responses. Genetic polymorphisms in the CD86 gene may affect the development of sepsis.51 Polymorphisms in the CD86 gene have diverse effects on the pathogenesis of pneumonia-induced sepsis, in which rs17281995G/C may increase the risk of the disease by interfering with the gene expression of CD86 in monocytes, and CD86 polymorphism affects pneumonia-induced sepsis by decreasing gene expression in monocytes.52
Genetic variants of the A disintegrin and metalloproteinase 10 (ADAM10) gene have been shown to be associated with susceptibility to several inflammatory-related diseases, and the clinical relationship in the development of sepsis was reviewed in 440 patients with sepsis. The rs653765 CC genotype in severe sepsis showed a higher ADAM10 level compared with healthy groups, and the rs653765 CC polymorphism had a strong impact on the production of the ADAM10 substrates CX3CL1, IL-6R and TNF-α, and ADAM10 might be clinically important and play a critical role in the pathogenesis of the development of sepsis, with potentially important therapeutic implications.53
Sepsis and septic shock frequently cause the admission or complicate the clinical course of critically ill patients admitted in the ICU. Genetic variations that disrupt the immune sensing of infectious organisms could affect the ability of the immune system to respond to infection and may influence both the genetic predisposition to infection and the diversity of the clinical presentation of sepsis.54 The aim of a recent study was to uncover possible associations between common functional immune gene polymorphisms (of both innate and adaptive immunity) and ICU-acquired sepsis and mortality. Thus, a role that host immune genetic variations may play in the susceptibility to ICU-acquired sepsis and ICU mortality.54
Injury due to trauma can induce immune function changes, which lead to both proinflammatory activation known as SIRS and an anti-inflammatory reaction known as compensatory anti-inflammatory response syndrome. SIRS with proven infection is referred to as sepsis; however, clinically, it is often difficult to isolate the microbial inoculum, which makes the differential diagnosis between SIRS and sepsis difficult. This differential diagnosis, in turn, is crucial for further therapeutic decisions: is an antimicrobial therapy and aggressive search for a septic focus with all its adverse effects necessary, or is a focused symptomatic therapy of the SIRS the adequate treatment concept?55 In multiple trauma situations, both syndromes can develop simultaneously, recently described as mixed antagonist response syndrome.
Genetic susceptibility can influence immune dysregulation and can affect sepsis outcomes, as listed by Cardoso et al.56 The primary goal of their study was to evaluate the genetic susceptibility that affects sepsis outcome in 72 patients with sepsis admitted to the ICU.56 Seven polymorphisms were genotyped in key inflammatory response genes in sepsis, including TNF-α, IL-1β, IL-10, IL-8, Toll-like receptor 4, CXCR1, and CXCR2. The primary finding showed that patients who were homozygous for the major A allele in IL-10 rs1800896 had almost a five times higher chance of developing septic shock compared with heterozygotes.56 Analysis of these data supports the hypothesis that molecular testing has clinical usefulness to improve sepsis prognostic models; therefore, enrichment of the ICU portfolio by including these biomarkers will aid in the early identification of patients with sepsis and who may develop septic shock.56
The majority of trauma victims end up being managed, at least initially, in the emergency department of health care facilities. The initial management of these patients is often challenging, requiring precise interpretation of symptoms and signs by specialized and experienced personnel, the utilization of high technology imaging modalities for an accurate diagnosis, timely and appropriate resuscitation measures, frequent monitoring of response, and timely consultation with the appropriate specialist.57 The high death rate in this previous study is multifactorial. The factors responsible for late presentation at the definitive care center are multiple. Thus, justify the fact that the public needs to be aware of the fact of high velocity trauma of today's world is beyond the comprehension of alternative practitioners.57
According to a recent study, there is limited literature on early unplanned hospital readmissions after acute traumatic injury, especially at suburban facilities.58 A retrospective review of the trauma registry at a suburban, state-designated, level-I academic trauma center, Stony Brook University School of Medicine, from July 2009 to June 2012, was performed for all admitted (≥24 hours) adult (ages ≥18 years) trauma patients who were discharged alive, including unplanned readmissions within 30 days of discharge.58 On multivariate analysis, only major comorbidities, hospital length of stay, abdominal abbreviated injury score ≥ 3 and discharge to a skilled nursing facility or a subacute facility were significant predictors. Index admission to surgical services was associated with a significantly lower readmission risk. The researchers stated that trauma patients are infrequently readmitted, the index admission to a surgical service reduces the risk of readmission, and earlier medical follow-up should be considered.58
HAE, TRAUMA, AND STRESS
HAE is important for allergists/immunologists, surgeons, and otolaryngologists. HAE is a rare autosomal dominant disease most commonly associated with defects in C1 esterase inhibitor (C1-INH) and manifests as recurrent episodes of edema in various body locations, atypical symptoms (e.g., ascites), ARDS, and hypovolemic shock have also been reported. Attacks can be triggered by stress and physical trauma. HAE disease severity is highly variable and may be influenced by polymorphisms in other genes and in other factors, such as hormones, trauma, stress, and infection. Frazer-Abel and Giclas59 reviewed the importance of laboratory testing in the diagnosis of HAE, which has increased with the advent of new treatments.59 HAE is subdivided into types that can be differentiated only by laboratory testing and measurement of C1q levels, and testing for anti–C1-INH autoantibodies can help differentiate acquired angioedema from HAE. Diagnostic testing for the third hereditary form, called estrogen-dependent HAE, HAE with normal C1-INH, or HAE type III, still presents challenges, and definitive testing may have to wait until there is a more-complete understanding of this mixed group of patients and genetic analysis of C1-INH and other proteins involved in HAE.59
Pham et al.60 recently reported on successful use of daily intravenous infusion of C1 esterase inhibitor concentrate in the treatment of a patient with HAE and ascites, hypovolemic shock, sepsis, and renal and respiratory failure. Due to the potential severity of attacks, allergists and otolaryngologists must be knowledgeable about the recognition and treatment of laryngeal angioedema. HAE can also be associated with some inflammatory and autoimmune disorders, e.g., systemic lupus erythematosus. C1-INH has been used in some patients with myocardial infarction, ischemic stroke, sepsis, or capillary leak syndrome, and off-label supratherapeutic doses without evidence of a thrombogenic effect. Thromboembolic events reported with C1-INH use are rare, and patients with HAE who experienced such events often have underlying thromboembolic risk factors.61
CONCLUSION
This review article was a literature search and discussion of CRS, sinusitis related to trauma, the innate and adaptive immunology NF-κB related to inflammation, sepsis, complement, inflammation, ROS, asthma pathogenesis and asthma in the elderly, oxidative stress, depression, seasonal variation and vitamin D, cytokines, genetic susceptibility related to sepsis, HAE related to trauma, and stress. Traumatic injury from blunt objects and falls is also related to asthma, stress, inflammation, anxiety, and depression, which can occur both in traumatic sinusitis and patients with asthma. Stress management both in trauma and asthma is based on the notion that stress causes an immune imbalance in susceptible individuals. These various topics were clearly linked in this review.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am J Rhinol 22:549–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamilos DL. Chronic rhinosinusitis: Epidemiology and medical management. J Allergy Clin Immunol 128:693–707; quiz 708–709, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy 27:473–478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: Epidemiology and cost. Allergy Asthma Proc 34:328–334, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Tan BK, Kern RC, Schleimer RP, Schwartz BS. Chronic rhinosinusitis: The unrecognized epidemic. Am J Respir Crit Care Med 188:1275–1277, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 18:21–26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Settipane RA, Peters AT, Chandra R. Chronic rhinosinusitis. Chapter 4. Am J Rhinol Allergy 27(suppl. 1):S11–S15, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Asaka D, Nakayama T, Hama T, et al. Risk factors for complications of endoscopic sinus surgery for chronic rhinosinusitis. Am J Rhinol Allergy 26:61–64, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Frieri M. Asthma linked with rhinosinusitis: An extensive review. Allergy Rhinol (Providence) 5:41–49,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karosi T, Sziklai I, Csomor P. Low-frequency ultrasound for biofilm disruption in chronic rhinosinusitis with nasal polyposis: In vitro pilot study. Laryngoscope 123:17–23, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Madeo J, Frieri M. Bacterial biofilms and chronic rhinosinusitis. Allergy Asthma Proc 34:335–341, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Yelverton JC, Jackson P, Schmidt RS. Chronic rhinosinusitis in patients requiring surgical repair of a midface fracture. Ear Nose Throat J 93:E26–E28, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Lakouichmi M, Baïzri H, Mouhsine A, et al. An unusual intracranial metallic foreign bodies and panhypopituitarism. Pan Afr Med J 19:33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vishniakov VV, Makarova NV, Pashovkina OV. Changes in the mucous membrane of the patients with chronic maxillary sinusitis caused by foreign bodies [in Russian with English abstract]. Vestn Otorinolaringol (1):12–14, 2014. [PubMed] [Google Scholar]
- 15. Shi L, Feng Y, Cui W, et al. Effect evaluation of repeated debridement after endoscopic sinus surgery. Int J Clin Exp Med 8:928–933, 2015. [PMC free article] [PubMed] [Google Scholar]
- 16. Bellanti JA. The Immune System in Health and Disease. Immunology IV. ICARE Press, 2012. [Google Scholar]
- 17. Berger M. Complement. Chapter 4. In Immunology IV. Clinical Applications in Health and Disease. Bellanti JA. (Ed). Bethesda: I Care Press, 101–129, 2012. [Google Scholar]
- 18. McDonald PP, Bald A, Cassatella MA. Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood 89:3421–3433, 1997. [PubMed] [Google Scholar]
- 19. Stegmaier JC, Kirchhoff C, Bogner V. Dynamics of neutrophilic NF-kB translocation in relation to IL-8 mRNA expression after major trauma. Inflamm Res 57:547–554, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouros D, Alexandrakis MG, Antoniou KM, et al. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for acute respiratory distress syndrome. BMC Pulm Med 4:6, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care 2:32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong J, Zhang M, Liao Z, et al. Resolvin-D1 inhibits interleukin-8 and hydrogen peroxide production induced by cigarette smoke extract in 16HBE cells via attenuating NF-κB activation. Chin Med J (Engl) 127:511–517, 2014. [PubMed] [Google Scholar]
- 23. Zubair A, Frieri M. NF-κB and systemic lupus erythematosus: Examining the link. J Nephrol 26:953–959, 2013. [DOI] [PubMed] [Google Scholar]
- 24. DeLong WG, Jr, Born CT. Cytokines in patients with polytrauma. Clin Orthop Relat Res (422):57–65, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Hietbrink F, Koenderman L, Rijkers GT, Leenen L. Trauma: The role of the innate immune system. World J Emerg Surg 1:15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Botha AJ, Moore FA, Moore EE, et al. Postinjury neutrophil priming and activation: An early vulnerable window. Surgery 118:358–364, 1995. [DOI] [PubMed] [Google Scholar]
- 27. Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury 45:1824–1833, 2014. [DOI] [PubMed] [Google Scholar]
- 28. Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: Role of oxidants as priming agents. Antioxid Redox Signal 4:69–83, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Madeo J, Zubair A, Marianne F. A review on the role of quinones in renal disorders. Springerplus 2:139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Syed S, Zubair A, Frieri M. Immune response to nanomaterials: Implications for medicine and literature review. Curr Allergy Asthma Rep 13:50–57, 2013. [DOI] [PubMed] [Google Scholar]
- 31. Jiang L, Diaz PT, Best TM, et al. Molecular characterization of redox mechanisms in allergic asthma. Ann Allergy Asthma Immunol 113:137–142, 2014. [DOI] [PubMed] [Google Scholar]
- 32. Pobed'onna HP. Antioxidant protection, metabolites of nitrogen oxide on the forming of oxidative stress in patients with bronchial asthma [in Ukrainian with English abstract]. Lik Sprava Jul-Sep:36–40, 2005. [PubMed] [Google Scholar]
- 33. Van Lieshout RJ, Bienenstock J, MacQueen GM. A review of candidate pathways underlying the association between asthma and major depressive disorder. Psychosom Med 71:187–195, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Frieri M, O'Connor M, Nassef M. Asthma, stress and depression in women. Allergy Asthma Proc 36:256–261, 2015. [DOI] [PubMed] [Google Scholar]
- 35. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med 104:22–28, 2010. [DOI] [PubMed] [Google Scholar]
- 36. Wang G, Wang L, Szczepaniak WS, et al. Psychological status in uncontrolled asthma is not related to airway hyperresponsiveness. J Asthma 47:93–99, 2010. [DOI] [PubMed] [Google Scholar]
- 37. Gaines AD. Anosmia and hyposmia. Allergy Asthma Proc 31:185–189, 2010. [DOI] [PubMed] [Google Scholar]
- 38. Grainge CL, Davies DE. Epithelial injury and repair in airways diseases. Chest 144:1906–1912, 2013. [DOI] [PubMed] [Google Scholar]
- 39. Frieri M. Advances in the understanding of allergic asthma. Allergy Asthma Proc 28:614–619, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Follenweider LM, Lambertino A. Epidemiology of asthma in the United States. Nurs Clin North Am 48:1–10, 2013. [DOI] [PubMed] [Google Scholar]
- 41. Madeo J, Li Z, Frieri M. Asthma in the geriatric population. Allergy Asthma Proc 34:427–433, 2013. [DOI] [PubMed] [Google Scholar]
- 42. Nassef M, Temprano J, Frieri M, Rossman S. Should fracture risk influence our decisionmaking in asthma care. Ann Allergy Asthma Immunol 106:164–167, 2011. [DOI] [PubMed] [Google Scholar]
- 43. Yawn J, Lawrence LA, Carroll WW, Mulligan JK. Vitamin D for the treatment of respiratory diseases: Is it the end or just the beginning? J Steroid Biochem Mol Biol 148:326–337, 2015. [DOI] [PubMed] [Google Scholar]
- 44. Bird ML, Hill KD, Robertson I, et al. The association between seasonal variation in vitamin D, postural sway, and falls risk: An observational cohort study. J Aging Res 2013:751310, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frieri M, Valluri A. Vitamin D deficiency as a risk factor for allergic disorders and immune mechanisms. Allergy Asthma Proc 32:438–444, 2011. [DOI] [PubMed] [Google Scholar]
- 46. Bouros D, Alexandrakis MG, Antoniou KM, et al. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for acute respiratory distress syndrome. BMC Pulm Med 4:6, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma 42:863–870; discussion 870–871, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Stromps JP, Fuchs P, Demir E, et al. Intraalveolar TNF-α in combined burn and inhalation injury compared with intraalveolar interleukin-6. J Burn Care Res 36:e55–e61, 2015. [DOI] [PubMed] [Google Scholar]
- 49. Leaver SK, MacCallum NS, Pingle V, et al. Increased plasma thioredoxin levels in patients with sepsis: Positive association with macrophage migration inhibitory factor. Intensive Care Med 36:336–341, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bernard GR, Ely EW, Wright TJ, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med 29:2051–2059, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Wang C, Gui Q, Zhang K. Functional polymorphisms in CD86 gene are associated with susceptibility to pneumonia-induced sepsis. APMIS 123:433–438, 2015. [DOI] [PubMed] [Google Scholar]
- 52. Song H, Tang L, Xu M, et al. CD86 polymorphism affects pneumonia-induced sepsis by decreasing gene expression in monocytes. Inflammation 38:879–885, 2015. [DOI] [PubMed] [Google Scholar]
- 53. Cui L, Gao Y, Xie Y, et al. An ADAM10 promoter polymorphism is a functional variant in severe sepsis patients and confers susceptibility to the development of sepsis. Crit Care 19:73, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kompoti M, Michopoulos A, Michalia M, et al. Genetic polymorphisms of innate and adaptive immunity as predictors of outcome in critically ill patients. Immunobiology 220:414–421, 2015. [DOI] [PubMed] [Google Scholar]
- 55. Novotny AR, Reim D, Assfalg V, et al. Mixed antagonist response and sepsis severity dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology 217:616–621, 2012. [DOI] [PubMed] [Google Scholar]
- 56. Cardoso CP, de Oliveira AJ, Botoni FA, et al. Interleukin-10 rs2227307 and CXCR2 rs1126579 polymorphisms modulate the predisposition to septic shock. Mem Inst Oswaldo Cruz 110:453–460, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ugare GU, Bassey IE, Udosen JE, et al. Trauma death in a resource constrained setting: Mechanisms and contributory factors, the result of analysing 147 cases. Niger J Clin Pract 17:397–402, 2014. [DOI] [PubMed] [Google Scholar]
- 58. Copertino LM, McCormack JE, Rutigliano DN, et al. Early unplanned hospital readmission after acute traumatic injury: The experience at a state-designated level-I trauma center. Am J Surg 209:268–273, 2015. [DOI] [PubMed] [Google Scholar]
- 59. Frazer-Abel A, Giclas PC. Update on laboratory tests for the diagnosis and differentiation of hereditary angioedema and acquired angioedema. Allergy Asthma Proc 32(suppl 1.):S17–S21, 2011. [DOI] [PubMed] [Google Scholar]
- 60. Pham H, Santucci S, Yang WH. Successful use of daily intravenous infusion of C1 esterase inhibitor concentrate in the treatment of a hereditary angioedema patient with ascites, hypovolemic shock, sepsis, renal and respiratory failure. Allergy Asthma Clin Immunol 10:62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crowther M, Bauer KA, Kaplan AP. The thrombogenicity of C1 esterase inhibitor (human): Review of the evidence. Allergy Asthma Proc 35:444–453, 2014. [DOI] [PubMed] [Google Scholar]