Abstract
Osteoblasts are highly coupled by gap junctions formed by connexin43. Overexpression of connexin45 in osteoblasts results in decreased chemical and electrical coupling and reduces gene transcription from connexin response elements (CxREs) in the osteocalcin and collagen Iα1 promoters. Here, we demonstrate that transcription from the gap junction-dependent osteocalcin CxRE is regulated by extracellular signal-regulated protein kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) cascades. Overexpression of a constitutively active mitogen-activated protein kinase kinase (MEK), Raf, or Ras can increase transcription more than twofold of the CxRE, whereas inhibition of MEK or PI3K can decrease transcription threefold from the osteocalcin CxRE. Importantly, disruption of gap junctional communication by overexpression of connexin45 or treatment with pharmacological inhibitors of gap junctions results in reduced Raf, ERK, and Akt activation. The consequence of attenuated gap junction-dependent signal cascade activation is a decrease in Sp1 phosphorylation by ERK, resulting in decreased Sp1 recruitment to the CxRE and inhibited gene transcription. These data establish that ERK/PI3K signaling is required for the optimal elaboration of transcription from the osteocalcin CxRE, and that disruption of gap junctional communication attenuates the ability of cells to respond to an extracellular cue, presumably by limiting the propagation of second messengers among adjacent cells by connexin43-gap junctions.
INTRODUCTION
Osteoblasts communicate with each other and with osteocytes by gap junctions formed between juxtaposed cells. Osteoblasts express primarily connexin43 (Cx43), a gap junction protein that assembles to form channels with permeability that favors negatively charged ions and molecules, up to ∼1.2-kDa molecular mass. Genetic studies in mice and humans have demonstrated the importance of Cx43 for bone development and turnover. Ablation of the Cx43 gene in mice results in a delay in endochondral and intramembranous bone formation, due at least in part to a cell autonomous osteoblast deficiency (Lecanda et al., 2000). Accordingly, calvarial osteoblasts isolated from Cx43 null mice demonstrate a delay in expression of many phenotypic markers of osteogenesis, including the down-regulation of expression of several genes, including osteocalcin, bone sialoprotein, and type I collagen (Lecanda et al., 2000). Recently, 24 separate point mutations in the human Cx43 gene have been shown to result in the autosomal dominant disorder oculodentodigital dysplasia (ODDD). (Paznekas et al., 2003; Kjaer et al., 2004; Richardson et al., 2004) ODDD results in abnormalities in craniofacial elements, limbs, and dentition.
Despite the accumulating data on the importance of Cx43 in bone biology, the mechanisms by which gap junctions regulate bone cell function remain uncertain. We hypothesize that gap junctions function to equalize or amplify signals among adjacent cells to coordinate cell activity in response to a variety of cues. Consistent with this hypothesis, it has been reported that disruption of gap junctional communication affects osteoblast gene transcription. Disruption of gap junctional coupling in osteoblasts results in down-regulation of markers of osteogenesis, including osteocalcin and bone sialoprotein (Lecanda et al., 1998; Li et al., 1999; Schiller et al., 2001a,d; Stains et al., 2003). ROS17/2.8 rat osteosarcoma cells are highly coupled by gap junctions formed exclusively by Cx43 (Steinberg et al., 1994). It has been demonstrated that overexpression of connexin45 (Cx45), which forms gap junctions with smaller size and opposite charge permeability than Cx43, in these cells results in a drastic decrease in chemical and electrical coupling of the resulting heteromeric gap junctions, indicating that in a mixed Cx43/Cx45 environment the biophysical properties of Cx45 prevail. Thus, Cx45 acts as a partial dominant negative connexin for Cx43 function (Koval et al., 1995; Li et al., 1999; Martinez et al., 2002). Using this model of Cx45 overexpression to inhibit the gap junctional communication provided by Cx43, we and others have observed close similarities with osteoblasts derived from Cx43 null mice, including down-regulation of osteocalcin and bone sialoprotein transcription (Lecanda et al., 1998; Li et al., 1999; Stains et al., 2003).
Recently, we have exploited the partial dominant negative action of Cx45 on Cx43 function to elucidate the molecular mechanisms by which gap junctional communication modulates gene transcription in osteoblasts (Stains et al., 2003). We identified a minimal element (-70 to -57 relative to transcription start) in the proximal promoter of the osteocalcin (OC) gene that is sensitive to gap junctional communication. We termed this unique transcriptional element a connexin response element (CxRE). We identified the complex binding the OC CxRE as containing the ubiquitous transcription factors Sp1 and Sp3 and demonstrated that Sp1 can activate transcription from this element, whereas Sp3 represses transcription. Finally, we demonstrated that gap junctional communication regulates transcription by altering the recruitment of Sp1/Sp3 to the promoter. Under well coupled conditions, the transactivator Sp1 is phosphorylated and preferentially recruited to the CxRE. Disruption of Cx43-gap junctions by overexpression of Cx45 leads to the preferential recruitment of the repressor Sp3. We hypothesize that second messengers passing through Cx43-gap junctions activate signal transduction pathways leading to the recruitment of Sp1.
In the present study, we undertake a molecular approach to define the pathways that link the gap junction channel at the plasma membrane to the transcription factors binding the CxRE in the nucleus. By defining the molecular details of how Cx43-gap junctions modulate cell function, we hope to gain insights into their biological role.
MATERIALS AND METHODS
Cell Culture and Reagents
ROS17/2.8 cells were cultured in α-minimal essential medium + 10% fetal bovine serum (FBS), as reported previously (Stains et al., 2003). Growth arrested cells were obtained by deprivation of serum for 24 h. Pretreatment with inhibitors were performed for 20 min before stimulation with 10% FBS. Routine molecular biology reagents and chemicals were from Promega (Madison, WI) and Sigma-Aldrich (St. Louis, MO), respectively. Antibodies against signal pathway components were from Cell Signaling Technology (Beverly, MA). Anti-GAPDH antibodies were from Chemicon International (Temecula, CA). Anti-Sp1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Sp1 (Thr739) antibodies were a kind gift from Gilles Pages (Centre Antoine Lacassagne, Nice, France). The constitutively active pFC-MEK1 was from Stratagene (La Jolla, CA). The constitutively active RAF (pCMV RafCAAX) and RAS (pCMV RasV12) and the signal pathway-specific reporters (pAP1-LUC, pCRE-Luc and pSRE-Luc) were from BD Biosciences Clontech (Palo Alto, CA).
Plasmid Constructs
The OC CxRE-RSVLUC luciferase reporter construct contains a single copy of the osteocalcin CxRE cloned upstream of a Rous sarcoma virus minimal promoter and has been described previously (Stains et al., 2003). The RSV minimal promoter has been described by others (Boudreaux and Towler, 1996). The OC CxRE spans the -70 to -57 region (relative to the transcriptional start site) of the rat osteocalcin proximal promoter. The pcDNA-cCx45 plasmid was generated by subcloning the chicken Cx45 (cCx45) coding sequence from the pSFFV-cCx45 (provided by Dr. Thomas Steinberg, Washington University, St. Louis, MO) into the EcoRI site of pcDNA3.
Transfections and Luciferase Reporter Assays
Transfections were performed as described previously (Stains et al., 2003, 2004). Briefly, ROS17/2.8 cells were seeded at high density (2 × 105 cells/well) in 24-well plates. Sixteen hours later, the cells were transfected with the appropriate plasmid by using LipofectAMINE reagent according to manufacturer's directions. Reporter plasmid (0.5 μg/well) and pcDNA-cCx45 expression plasmid (0.25 μg/well) were used in each transfection. Total DNA content was kept constant in all experiments by addition of pcDNA. After 48 h, the cells were rinsed in phosphate-buffered saline (PBS) and lysed in 1× passive lysis buffer. Luciferase activity was monitored using an Optocomp luminometer. Transfection efficiency was monitored by cotransfection with a β-galactosidase reporter. All experiments were performed in triplicate and repeated three to five times.
Western Blotting and Immunoprecipitations
Whole cell extracts from ROS17/2.8 cells were prepared using modified radioimmunoprecipitation assay buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mM EGTA, 1 mM EDTA, 2 mM sodium vanadate; 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS plus protease inhibitors) containing protease and phosphatase inhibitors. Insoluble material was pelleted and the supernatants were electrophoresed on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked in 5% nonfat dry milk, probed with the indicated primary antibodies, and detected with the appropriate horseradish peroxidase-conjugated antibodies (1:5000) and enhanced chemiluminescence detection reagents. Densitometry was performed on immunoblots by using a UVP EpiChem3 gel documentation system and Labworks 4.0 software. Immunoprecipitations were performed using 500 μg of whole cell extract and 3 μg of anti-phospho-threonine antibodies. Immunoprecipitated material was collected using protein A/G plus. Washed immunoprecipitated material was eluted in SDS sample buffer, immunoblotted, and probed with anti-Sp1 antibodies.
Formaldehyde Cross-linking and Chromatin Immunoprecipitation (ChIP)
Formaldehyde ChIP assay was performed as described previously (Stains et al., 2003). Briefly, ROS17/2.8 were transiently transfected with pcDNA or pcDNA-cCx45 by using Lipofectamine. The cells were then treated with vehicle (0.1% dimethyl sulfoxide [DMSO]) or 10 μM U0126 overnight. The following day, the cells were rinsed twice in PBS, and cross-linked in 1% formaldehyde at room temperature for 10 min. Cells were collected, lysed, and the fixed chromatin was sonicated 10 times for 10 s each. Sheared genomic DNA was electrophoresed on an agarose gel to ensure complete shearing of the DNA. The cross-linked chromatin was diluted with immunoprecipitation (IP) buffer (20 mM Tris, pH 8, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100), precleared with protein A/G Plus, and then immunoprecipitated with 3 μg of rabbit anti-Sp1 antibodies. The immunoprecipitated material was then washed and eluted as described by Shang et al. (2002). Protein cross-links were reversed by heating overnight at 65°C. DNA was purified using QIAquick polymerase chain reaction (PCR) purification kit. For PCR, 1 μl from a 50-μl extraction was used per reaction. Titration of PCR conditions was performed to ensure experiments were performed in a linear range of amplification. PCR primers used OC Promoter-F, CCAATTAGTCCTGGCAGCAT; OC Promoter-R, TTGCTGTGTGGGACTTGTCT; COL1A1 Promoter-F, TGGACTCCTTTCCCTTCCTT; and COL1A1 Promoter-R, GAACCCTGCCTCTTGGAGA.
RESULTS
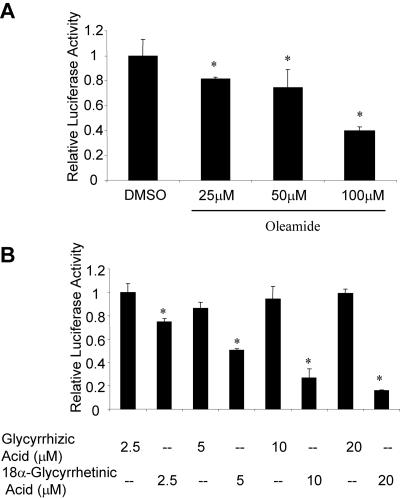
Previously, we reported that overexpression of exogenous Cx45 in an endogenous Cx43 background results in diminished gap junctional communication leading to repressed gene transcription from CxREs (Lecanda et al., 1998; Stains et al., 2003). To prove that this effect was due to loss of Cx43 function and not gain of Cx45 function, we assessed the ability of pharmacological inhibitors of gap junctions to inhibit CxRE-driven gene transcription. Treatment of cells with the gap junction inhibitor oleamide reduced transcription of the OC CxRE to levels similar to that observed in Cx45 overexpression (Figure 1A). This effect was dose dependent, with the best levels of inhibition observed at 100 μM. This concentration of oleamide is consistent with the levels required to disrupt gap junctional coupling (Schiller et al., 2001c; Subauste et al., 2001; Krutovskikh et al., 2002; Ransjo et al., 2003). Similarly, the gap junction inhibitor 18α-glycyrrehetinic acid exhibited dose-dependent inhibition of CxRE-mediated gene transcription (Figure 1B). In contrast, the inactive analog glycyrrhizic acid did not affect transcription. These data support the notion that the effect of Cx45 overexpression on gene transcription is caused by loss of Cx43 function.
Figure 1.
Disruption of gap junctional communication leads to a decrease in transcription from the OC CxRE. ROS17/2.8 cells were transfected with the OC CxRE-RSVLUC luciferase reporter and treated for 16 h with the gap junction inhibitors. (A) Treatment of the cells with the gap junction inhibitor oleamide decreased transcription from the OC CxRE, as determined by luciferase activity. The most effective dose of oleamide was 100 μM. DMSO was used as a vehicle control (0.1%). (B) Treatment of the cells with the gap junction inhibitor 18α-glycyrrhetinic acid decreased transcription from the OC CxRE in a dose-dependent manner, as determined by luciferase activity. The inactive analog glycyrrhizic acid did not affect CxRE-mediated transcription. Data are presented as mean ± SD from a representative experiment performed in triplicate. Asterisk indicates significant difference (p < 0.05) compared with control.
Extracellular Signal-regulated Protein Kinase (ERK) Controls Transcription from the OC CxRE
We hypothesized that disruption of Cx43-gap junctions limits the capacity to propagate signal molecules among coupled cells, resulting in attenuated activation of signal transduction cascades. We directed our focus on the ERK mitogen-activated protein kinase (MAPK) cascade, which has been shown to regulate phosphorylation and transcriptional activity of Sp1 (Merchant et al., 1999; Milanini-Mongiat et al., 2002). Because we had demonstrated previously that the alteration in gene transcription caused by perturbation of gap junctional communication is caused by posttranslational modification of Sp1, we hypothesized that ERK signaling is a likely candidate for modulating Sp1 recruitment to CxREs (Stains et al., 2003).
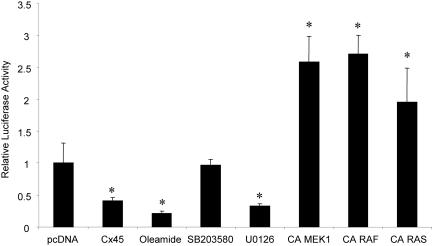
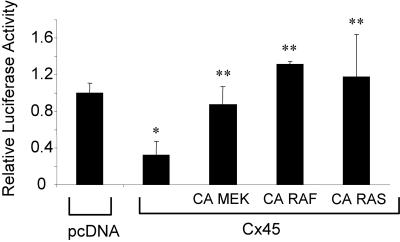
To assess the influence of ERK signaling on the OC CxRE, we used a promoter-luciferase reporter system containing a single copy of the OC CxRE placed upstream of an RSV minimal promoter (OC CxRE-RSVLUC). ROS17/2.8 cells were transiently cotransfected with the OC CxRE-RSVLUC reporter and pcDNA-cCx45 or pcDNA. As expected from our previous data, overexpression of Cx45 reduced transcription from the OC CxRE 2.5-fold, and treatment with 100 μM oleamide reduced transcription 4.8-fold (Figure 2). Whereas treatment with the p38 MAPK inhibitor SB203580 had no effect on transcription, cell exposure to 10 μM U0126, an inhibitor of mitogen-activated protein kinase kinase (MEK)1/2, which is upstream of ERK, reduced transcription to levels similar to that obtained from disruption of gap junctions (Figure 2). The role of ERK cascade signaling in regulating CxRE transcription was confirmed by overexpression of constitutively active MEK (pFC-MEK1), Raf (Raf-CAAX), and Ras (Ras V12) constructs in luciferase reporter assays. All three constructs supported at least a twofold increase in CxRE transcription, demonstrating that the Ras > Raf > MEK > ERK cascade can modulate transcription from the OC CxRE.
Figure 2.
ERK activity modulates transcription from the OC CxRE. ROS17/2.8 cells were transiently cotransfected with pcDNA-cCx45 or pcDNA and OC CxRE-RSVLUC reporter plasmid. Cells were treated with 100 μM oleamide, 10 μM SB203580, 10 μM U0126, or vehicle (0.1% DMSO) for 16 h. At 48 h posttransfection, the cells were assayed for luciferase activity. In some instances, cells were cotransfected with constitutively active (CA) MEK, Raf, and Ras. Transcription from the OC CxRE reporter was reduced two- to threefold by overexpression of cCx45, treatment with 100 μM oleamide (gap junction inhibitor) and with 10 μM U0126 (MEK inhibitor), but not by 10 μM SB203580 (p38 inhibitor). In contrast, transcription was increased nearly two- to threefold by expression of the CA MEK, CA Raf, and CA Ras. Data are presented as mean ± SD from a representative experiment performed in triplicate. Asterisk indicates significant difference (p < 0.05) compared with pcDNA control.
ERK Signaling Is Regulated by Gap Junctional Communication
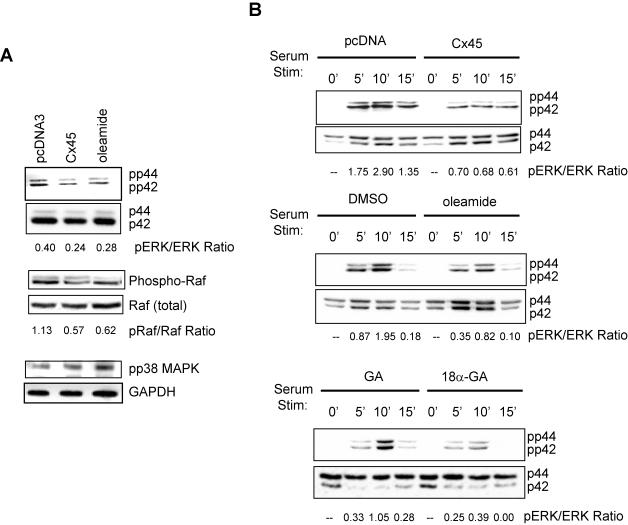
We next examined whether ERK activity is affected by disruption of Cx43-mediated gap junctional communication. First, we assessed the “basal” activation of ERK in ROS17/2.8 cells maintained in 10% FBS. Cells were transiently transfected with either pcDNA-cCx45 or pcDNA, or treated with 100 μM oleamide. As shown in Figure 3A, Western blotting with anti-phospho-ERK antibodies revealed a nearly two-fold reduction in active ERK when gap junctions communications was perturbed either by overexpression of a dominant connexin (i.e., Cx45) or by treatment with the gap junction inhibitor oleamide. Total ERK in the cell extract was unaffected. Likewise, the levels of activated Raf were diminished twofold when gap junctional communication was attenuated. In contrast, the activation of p38 MAPK was slightly increased under the same conditions, as assessed using an anti-phospho-p38 MAPK antibody.
Figure 3.
ERK but not p38 MAPK signaling is attenuated by disruption of gap junctional communication. (A) ROS17/2.8 cells were transiently transfected with pcDNA-cCx45 or pcDNA. Cells were treated with 100 μM oleamide for 16 h, where indicated. Forty-eight hours posttransfection, whole cell extracts were prepared, electrophoresed, and immunoblotted by using phospho-ERK, total ERK, phospho-Raf, total Raf, phospho-p38 MAPK, and GAPDH antibodies. Overexpression of cCx45 or treatment with oleamide markedly reduced phosphorylated, but not total ERK and Raf abundance compared with mock-transfected cells. The abundance of phosphorylated p38 MAPK was slightly increased by Cx45 or oleamide. (B) ROS17/2.8 cells were transfected with pcDNA-cCx45 or pcDNA. At 24 h posttransfection, the cells were serum deprived overnight. The following day, the cells were stimulated with 10% FBS for 5, 10, or 20 min, after pretreatment for 20 min with either 100 μM oleamide, 0.1% DMSO, 5 μM 18α-glycyrrhetinic acid (18α-GA), or 5 μM glycyrrhizic acid (GA). The cells were subsequently lysed, electrophoresed, and immunoblotted using anti-phospho-ERK antibodies. Total ERK was used to normalize load amounts. The activation of ERK was markedly reduced in gap junction communication disrupted cells after 5, 10, and 20 min of serum stimulation. The ratio of phosphorylated to total protein is shown beneath each blot.
According to our hypothesis, we anticipated that Cx43-gap junctions potentiate signaling among cells in response to an extracellular cue by propagating second messengers to adjacent cells. Thus, cells with disrupted gap junctional communication would have an attenuated response to an extracellular cue. To test this hypothesis, we analyzed the ability of cells to activate ERK after stimulation with serum in well coupled cells and in cells with diminished gap junctional communication. Growth-arrested cells were either transfected with pcDNA-cCx45 or treated with the gap junction inhibitors oleamide (100 μM) or 18α-glycyrrhetinic acid (5 μM). Cells transfected with pcDNA or treated with DMSO (0.1%) or the inactive analog glycyrrhizic acid (5 μM) served as the respective controls. Consistent with the results on “basal” ERK activation, immunoblotting with anti-phospho ERK antibodies revealed that well coupled cells had a two- to threefold higher activation of ERK in response to 10% FBS at 5, 10, or 15 min after stimulation (Figure 3B). Importantly, the activation of ERK under the well coupled conditions used in the control experiments reveal the total activation of ERK by both extracellular ligand-receptor-mediated activation and gap junctional propagation of the signal. The ERK activation observed under conditions of disrupted gap junctional communication reflects only the extracellular ligand-receptor-mediated activation. By inference, these data demonstrate that gap junctional communication provided by Cx43 accounts for 50-60% of the total cellular response to an extracellular cue, because ERK activation in response to serum stimulation is attenuated more than twofold by disruption of Cx43-gap junctions.
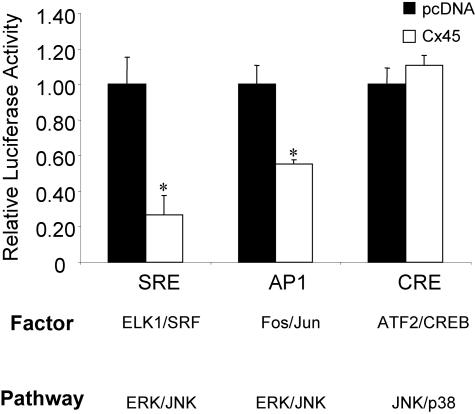
To validate that ERK activity is decreased as a consequence of Cx45 overexpression, a series of signal pathway-specific reporter constructs were used in cCx45 cotransfection experiments, including a serum response element luciferase reporter (SRE-LUC), constituting an Elk1/Srf cognate, and from an AP1-binding reporter (AP1-LUC), containing a Fos/Jun cognate, which are responsive to changes in ERK activity, as well as a p38 MAPK/c-Jun NH2-terminal kinase-responsive cAMP-response element reporter (CRE-LUC). Consistent with the immunoblotting data, transcription from both the ERK-sensitive SRE- and AP1-LUC was markedly decreased in Cx45 overexpressing cells. In contrast, transcription from the p38-responsive CRE-LUC was weakly increased by Cx45 overexpression (Figure 4). Thus, disruption of Cx43-gap junctions by cCx45 expression seems to specifically affect the ERK signaling pathway, rather than Sp1 activity directly, because transcription from an AP1 and SREs, which do not involve Sp1, also were down-regulated by cCx45 expression.
Figure 4.
Transcription from ERK but not p38 signal pathway-specific reporters is repressed by Cx45 overexpression. ROS 17/2.8 cells were cotransfected with the signal pathway-specific reporters SRE-LUC, AP1-LUC, or CRE-LUC along with either pcDNA or cCx45. At 48 h posttransfection, the cells were assayed for luciferase activity. Activity of the SRE- and AP1-LUC reporters, which are regulated by the ERK signaling cascade, was significantly reduced when cCx45 was expressed compared with pcDNA-transfected cells. In contrast, the p38 MAPK regulated CRE-LUC reporter was unaffected by cCx45 overexpression. Data are presented as mean ± SD from a representative experiment performed in triplicate. Asterisk indicates significant difference (p < 0.05) compared with pcDNA control.
Activation of the ERK Cascade Rescues the Gap Junctional Regulation of CxRE-mediated Transcription
Having shown a role for ERK signaling in Cx43-gap junction-regulated gene transcription, we next determined whether activation of the ERK signaling cascade could rescue the transcriptional defect observed when Cx43 gap junctions are disrupted. Thus, we used constitutively active upstream transducers of the ERK cascade in cotransfection experiments with pcDNA-cCx45 and the OC CxRE-RSVLUC reporter. Overexpression of constitutively active MEK1, Raf, and Ras all rescue the transcriptional repression caused by overexpression of cCx45 on the OC CxRE (Figure 5). These data directly implicate the Ras > Raf > MEK > ERK signaling cascade in connexin modulation of transcriptional activity.
Figure 5.
Overexpression of constitutively active (CA) MEK, Raf, or Ras “rescues” the transcriptional deficiency caused by Cx45 overexpression. (A) ROS 17/2.8 cells were cotransfected with pcDNA-cCx45 or pcDNA, and OC CxRE-RSVLUC in the presence or absence of CA MEK, CA Raf, or CA Ras. The transcriptional repression from the OC CxRE caused by Cx45 overexpression was “rescued” by all three constitutively active mutants. Data are presented as mean ± SD from a representative experiment performed in triplicate. Asterisk indicates significant difference (p < 0.05) compared with pcDNA control. Double asterisks indicate significant difference (p < 0.05) compared with pcDNA-cCx45 control.
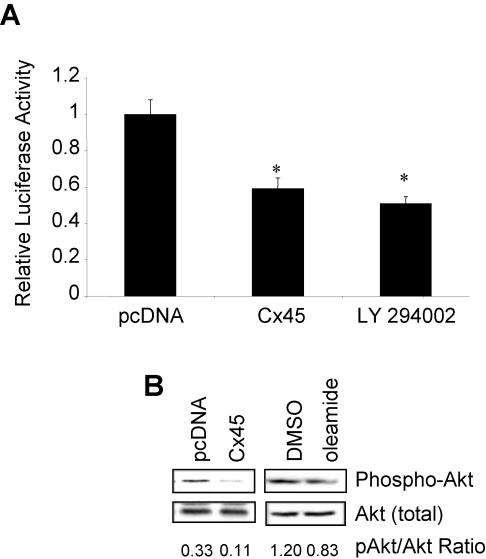
However, cell exposure to the PI3K inhibitor LY294002 also decreased OC CxRE transcriptional activity by 50% (Figure 6A). Because PI3K can act either upstream or downstream of Ras and can activate other signaling pathways, such as Akt, we then tested whether overexpression of cCx45 affected PI3K > Akt signaling. Intriguingly, overexpression of cCx45 also attenuated Akt phosphorylation without affecting total Akt present in the cells (Figure 6B), suggesting that overexpression of Cx45 in a Cx43 background regulates PI3K activity, with consequent attenuation of activity of downstream effectors, Akt and the Ras > ERK pathways.
Figure 6.
The PI3K/Akt pathways also regulated transcription from the OC CxRE and are regulated by gap junctional communication. (A) ROS 17/2.8 cells were cotransfected with pcDNA-cCx45 or pcDNA, and OC CxRE-RSVLUC. Twenty-four hours posttransfection the cultures were treated with 10 μM LY294002, an inhibitor of PI3K, or vehicle (0.1% DMSO). At 48 h posttransfection, the cells were harvested and assayed for luciferase activity. Treatment with the PI3K inhibitor decreased transcription from the OC CxRE twofold. Data are presented as mean of triplicate samples ± SD from a representative experiment. Asterisk indicates significant difference (p < 0.05) compared with pcDNA control. (B) ROS17/2.8 cells were transiently transfected with pcDNA-cCx45 or pcDNA, or treated with 0.1% DMSO or 100 μM oleamide. Whole cell extracts were prepared, electrophoresed, and immunoblotted using phospho-Akt antibodies. The blot was then stripped and reprobed using a total-Akt antibody. Disruption of Cx43-gap junctional communication reduced the abundance of the activated phospho-Akt.
ERK Signaling Is Required for the Phosphorylation and Recruitment of Sp1 to the CxRE
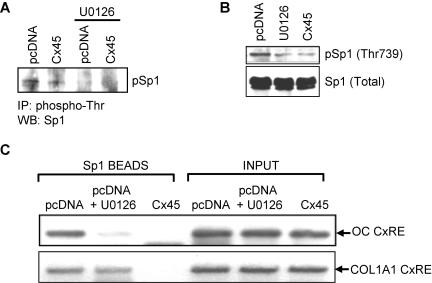
It has been reported that Sp1 is a target of phosphorylation by ERK signaling on threonine 453 and 739 (Milanini-Mongiat et al., 2002) and that phosphorylation of Sp1 by ERK leads to increased DNA binding (Merchant et al., 1999). We have previously demonstrated that Sp1 is phosphorylated in ROS 17/2.8 cells, but when Cx43-gap junctions are disrupted by Cx45 overexpression the level of threonine phosphorylation is dramatically reduced (Stains et al., 2003). To assess whether ERK activity is responsible for the decrease in Sp1 phosphorylation induced by Cx45 overexpression, we performed immunoprecipitations by using anti-phosphothreonine antibodies in cells transfected with either pcDNA-cCx45 or empty vector, and treated with 10 μM U0126 or vehicle. The immunoprecipitated material was then immunoblotted with anti-Sp1 antibodies. As shown in Figure 7A, phosphorylation of Sp1 was reduced by Cx45 overexpression and treatment of the cells with 10 μM U0126. These results were validated using an anti-phospho Sp1 (Thr739) antibody (generous gift of Dr. Gilles Pages). The threonine phosphorylation of Sp1 was markedly reduced in 10 μM U0126-treated or Cx45-overexpressing cells compared with those transfected with pcDNA, whereas the abundance of total Sp1 was unaffected (Figure 7B).
Figure 7.
Disruption of the ERK signaling cascade results in decreased threonine phosphorylation of Sp1 and recruitment to the CxRE. (A) ROS17/2.8 cells were transiently transfected with pcDNA-cCx45 or pcDNA. After 24 h, cells were treated with 10 μM U0126 or 0.1% dimethyl sulfoxide. Forty-eight hours posttransfection, whole cell extracts were prepared. Immunoprecipitations were performed on 500 μg of precleared extract using anti-phospho-threonine antibodies. The immunoprecipitated material was electrophoresed and immublotted with anti-Sp1 antibodies. Overexpression of Cx45 and treatment with U0126 decreased phosphorylation of Sp1. (B) ROS17/2.8 cells were treated as described above. Whole cell extracts were prepared, electrophoresed, and immunoblotted using phospho-Sp1 (Thr739) antibodies. The blot was then stripped and reprobed using a total-Sp1 antibody. Overexpression of Cx45 or treatment with U0126 reduced the abundance of the phosphorylated Sp1. (C) Chromatin immunoprecipitations were performed under these conditions as described in Materials and Methods. The ability of Sp1 antibodies to immunoprecipitate the osteocalcin promoter (top) was markedly reduced by treatment of the cells with 10 μM U0126 or by overexpression of Cx45. Similar results were achieved with the collagen I α1 gene promoter (bottom). The primers used for these studies span the CxREs in both of these promoters.
The biological relevance of Sp1 phosphorylation by ERK was determined by chromatin immunoprecipitations. We examined whether interference with ERK activity alters Sp1 occupancy of both the OC CxRE and the collagen I α1 CxREs. As previously shown, Sp1 was detected to bind both the OC and collagen I α1 CxREs in pcDNA-transfected cells, and the abundance of Sp1 on the CxRE was markedly reduced when the cells were transfected with pcDNA-cCx45 (Figure 7C). Importantly, cell exposure to 10 μM U0126 greatly reduces the recruitment of Sp1 to both promoters, confirming the notion that ERK signaling increases recruitment of Sp1 to CxREs. Thus, signaling via ERK, which is modulated by gap junctional communication, is required for threonine phosphorylation of Sp1. As a result of this posttranslational modification, recruitment of Sp1 to the CxRE is enhanced and gene transcription is up-regulated, establishing the ERK cascade as a molecular link between Cx43 at the plasma membrane and CxREs in the nucleus.
DISCUSSION
The cell-to-cell diffusion of signal molecules through gap junctions within a cell network allows a coordinated action of cells in a population. Accordingly, disruption of such coordinated activity may result in cell dysfunction. Indeed, connexin mutations have been associated with a number of congenital disorders of various tissues, for example Charcot-Marie-Tooth disease (Bergoffen et al., 1993), nonsyndromic deafness (Kelsell et al., 1997), oculodentodigital dysplasia (Paznekas et al., 2003; Richardson et al., 2004), as well as neoplasia (Pitts et al., 1988; Cesen-Cummings et al., 1998). There is now ample evidence that one mechanism by which gap junctions affect cellular function is via regulating gene transcription (Philippe et al., 1994; Vozzi et al., 1995; Nicholson et al., 2001; Flachon et al., 2002; Fritz et al., 2002; Huang et al., 2002; Kojima et al., 2002). In previous work, we have demonstrated such a role for Cx43 in bone. Either dominant (Cx45 overexpression) or recessive (gene deletion) disruption of Cx43 function results in an altered expression of osteoblastic genes (Lecanda et al., 1998, 2000; Stains et al., 2003). We have attributed these transcriptional changes to connexin modulation of recruitment of transcription factors Sp1/Sp3 to CxREs in at least two osteoblast promoters (Stains et al., 2003).
In this report, we demonstrate that the type of gap junctional communication provided by Cx43 is essential for optimal activation of signal transduction through ERK. We hypothesize that the cellular response to an extracellular cue is twofold. There is a “primary” response of the cells to an extracellular stimulus (e.g., growth factors, such as serum used in this study) that is mediated by the classical extracellular ligand-receptor activation. This leads to activation of signal transduction and generation of second messengers. When gap junctions are present, propagation of these second messengers to adjacent cells by gap junctions leads to a “secondary” response. This gap junction-dependent secondary response is mediated at least in part by activation of the ERK signal transduction cascade. Importantly, our data demonstrate that the gap junction component of cellular response to an extracellular cue accounts for as much as 50% of the total response of a population of cells. Such a model for the role of gap junctions, particularly those composed of Cx43, in coordinating homogeneous signaling among cells in a local population has been recently reported (Lin et al., 2004). Furthermore, the ability of Cx43 gap junctions to propagate second messengers has been well documented (Boitano et al., 1992; Goldberg et al., 1998; Kam et al., 1998; Niessen et al., 2000). The advantages of using gap junctions to propagate a signal are twofold. First, gap junctions permit a uniform response to an extracellular signal in cells that may have heterogeneous expression level of receptors or limited access to the extracellular ligand (e.g., osteocytes). Second, gap junctions permit a rapid and specific transfer of signals among adjacent cells, coordinating the function of local populations of cells, and allowing only coupled cells to respond.
In addition to the ERK pathway, we have evidence that the PI3K and Akt pathway may be likewise affected by disruption of Cx43-gap junctions. In some contexts, PI3K has been shown to act upstream of ERK (Karnitz et al., 1995; Parrizas et al., 1997). In other tissues, the PI3K pathway acts independent of ERK. Therefore, it is not clear whether the effect of Cx45 overexpression on both Akt and ERK represents divergence of signaling from PI3K activation, or cross talk among the two arms of cellular signaling by second messengers traversing the gap junction.
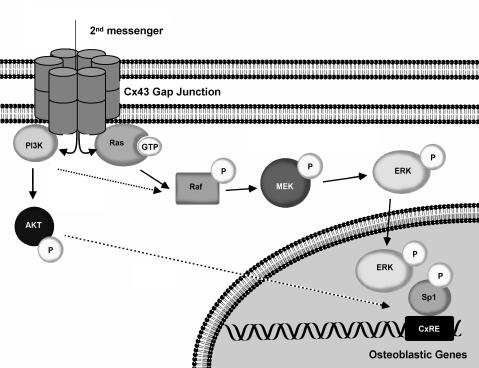
We propose a model in which second messengers, activated by a primary cell response to an extracellular cue, are propagated by Cx43-gap junctions. We term this the gap junction-dependent secondary response. On traversing the gap junctional pore, the second messenger activates Ras > Raf > MEK > ERK and PI3K > Akt signaling. The activated ERK is subsequently translocated to the nucleus where it phosphorylates Sp1 leading to its preferential recruitment to the CxRE, leading to robust gene transcription and a potentiation of the primary response. When the permeability of gap junctions is decreased by the formation of Cx43/Cx45 heteromeric channels or pharmacological inhibition, activation of the Ras > ERK pathway is markedly decreased. This results in hypophosphorylation of Sp1 and a concomitant failure to recruit Sp1 to the promoter and diminished gene transcription (Figure 8). Whether PI3K/AKT signaling acts in a parallel or linear manner with respect to ERK signaling remains to be determined.
Figure 8.
Model of gap junctional modulation of gene transcription. Gap junctional communication permits the intercellular propagation of second messengers that activate the ERK/PI3K signal cascades. These signals converge on nuclear function by regulating the recruitment of the transactivator Sp1 to the CxRE in the promoter, leading to robust transcription. When gap junctional communication is disrupted the cellular response is attenuated, due to the failure to propagate signals among cells. Putative steps are highlighted by dotted lines.
Interestingly, Ras and PI3K are recruited to the inner leaflet of the plasma membrane. We hypothesize that Ras and/or PI3K interact either directly or indirectly with connexins, contributing to the assembly of a signaling complex in association with the gap junction plaque. Such a spatial arrangement would provide for efficient signaling as second messengers are trafficked through the gap junctional channels. Indeed, numerous signal proteins have been demonstrated in various cell types to interact with Cx43, including c-src (Giepmans et al., 2001; Toyofuku et al., 2001), protein kinase C (PKC) α and ε (Doble et al., 2000; Bowling et al., 2001; Schulz et al., 2003), and p38 MAPK (Schulz et al., 2003). Furthermore, many of these interactions are connexin-isotype specific (Herve et al., 2004). Cx43 is also a target for ERK MAPK, PKC, PI3K and src phosphorylation, indicating at least a transient physical association (Lampe and Lau, 2000, 2004; Cruciani and Mikalsen, 2002). Additionally, signaling via Cx43 hemichannels has been implicated in ERK activation in a noncanonical manner to provide antiapoptotic signals to osteoblasts (Plotkin et al., 2002).
The notion that gap junctions are required for optimal responsiveness of bone cells to extracellular cues has been demonstrated in various settings. Using an antisense RNA approach to decrease Cx43 expression, gap junctional communication has been shown to be required for a full osteoblastic response to parathyroid hormone (Van der Molen et al., 1996). Similar results were obtained using chemical inhibitors of gap junctions (Schiller et al., 2001b). In chondrocytes, inhibitors of gap junctional communication were demonstrated to block chondrogenic differentiation induced by bone morphogenetic protein-2 (Zhang et al., 2002). It is noteworthy that both of these extracellular ligands can activate the ERK cascade in osteoblasts (Lou et al., 2000; Swarthout et al., 2001; Lai and Cheng, 2002; Xiao et al., 2002). Furthermore, ERK activity is important for osteogenesis (Lai et al., 2001) and osteoblast-specific gene transcription (Xiao et al., 2002; Zhao et al., 2002; Qiao et al., 2004). In fact, it is likely that the delayed mineralization and cell autonomous osteoblast dysfunction observed in the genetically ablated Cx43 null mice (Lecanda et al., 2000) is a result of decrease hormonal responsiveness, manifested at least in part as diminished ERK activation.
In conclusion, we have demonstrated that when Cx43 function is attenuated either by Cx45 overexpression or treatment with a gap junction inhibitor, the activity of ERK is markedly decreased in response to extracellular cues (e.g., serum stimulation). The consequences of which is altered transcription factor recruitment to CxREs and attenuated gene transcription. These data demonstrate that Cx43-gap junctions are important for the elaboration of an “optimal” response of a population of cells. By understanding the mechanisms of how gap junctions propagate signals, we gain critical insight into the biologically relevant molecules that pass through them.
Acknowledgments
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01 AR41255 to R.C.; and T32 AR07033 to J.S.), and by funds from the Barnes-Jewish Hospital Foundation, St. Louis, MO.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-04-0339. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0339.
Abbreviations used: CMV, cytomegalovirus; Cx43, connexin43; Cx45, connexin45; CRE, cAMP response element-binding protein response element; CxRE, connexin response element; ERK, extracellular signal-regulated kinase; FBS, fetal bovine serum; MAPK, mitogen-activated protein kinase; OC, osteocalcin; ODDD, oculodentodigital dysplasia; PI3K, phosphatidylinositol 3-kinase; RSV, Rous sarcoma virus; SRE, serum response element.
References
- Bergoffen, J., Scherer, S. S., Wang, S., Oronzi Scott, M., Bone, L. J., Paul, D. L., Chen, K., Lensch, M. W., Chance, P. F., and Fischbeck, K. H. (1993). Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262, 2039-2042. [DOI] [PubMed] [Google Scholar]
- Boitano, S., Dirksen, E. R., and Sanderson, M. J. (1992). Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258, 292-295. [DOI] [PubMed] [Google Scholar]
- Boudreaux, J. M. and Towler, D. A. (1996). Synergistic induction of osteocalcin gene expression. J. Biol. Chem. 271, 7508-7515. [DOI] [PubMed] [Google Scholar]
- Bowling, N., Huang, X., Sandusky, G. E., Fouts, R. L., Mintze, K., Esterman, M., Allen, P. D., Maddi, R., McCall, E., and Vlahos, C. J. (2001). PKC-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. J. Mol. Cell. Cardiol. 33, 789-798. [DOI] [PubMed] [Google Scholar]
- Cesen-Cummings, K., Fernstrom, M. J., Malkinson, A. M., and Ruch, R. J. (1998). Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis 19, 61-67. [DOI] [PubMed] [Google Scholar]
- Cruciani, V. and Mikalsen, S. O. (2002). Connexins, gap junctional intercellular communication and kinases. Biol. Cell 94, 433-443. [DOI] [PubMed] [Google Scholar]
- Doble, B. W., Ping, P., and Kardami, E. (2000). The epsilon subtype of PKC is required for cardiomyocyte connexin-43 phosphorylation. Circ. Res 86, 293-301. [DOI] [PubMed] [Google Scholar]
- Flachon, V., Tonoli, H., Selmi-Ruby, S., Durand, C., Rabilloud, R., Rousset, B., and Munari-Silem, Y. (2002). Thyroid cell proliferation in response to forced expression of gap junction proteins. Eur. J. Cell Biol. 81, 243-252. [DOI] [PubMed] [Google Scholar]
- Fritz, S., Kunz, L., Dimitrijevic, N., Grunert, R., Heiss, C., and Mayerhofer, A. (2002). Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J. Clin. Endocrinol. Metab. 87, 1362-1367. [DOI] [PubMed] [Google Scholar]
- Giepmans, B. N., Hengeveld, T., Postma, F. R., and Moolenaar, W. H. (2001). Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J. Biol. Chem. 276, 8544-8549. [DOI] [PubMed] [Google Scholar]
- Goldberg, G. S., Lampe, P. D., Sheedy, D., Stewart, C. C., Nicholson, B. J., and Naus, C. C. (1998). Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp. Cell Res. 239, 82-92. [DOI] [PubMed] [Google Scholar]
- Herve, J. C., Bourmeyster, N., and Sarrouilhe, D. (2004). Diversity in protein-protein interactions of connexins: emerging roles. Biochim. Biophys. Acta 1662, 22-41. [DOI] [PubMed] [Google Scholar]
- Huang, R., Lin, Y., Wang, C. C., Gano, J., Lin, B., Shi, Q., Boynton, A., Burke, J., and Huang, R. P. (2002). Connexin 43 suppresses human glioblastoma cell growth by down-regulation of monocyte chemotactic protein 1, as discovered using protein array technology. Cancer Res. 62, 2806-2812. [PubMed] [Google Scholar]
- Kam, Y., Kim, D. Y., Koo, S. K., and Joe, C. O. (1998). Transfer of second messengers through gap junction connexin 43 channels reconstituted in liposomes. Biochim. Biophys. Acta 1372, 384-388. [DOI] [PubMed] [Google Scholar]
- Karnitz, L. M., Burns, L. A., Sutor, S. L., Blenis, J., and Abraham, R. T. (1995). Interleukin-2 triggers a novel phosphatidylinositol 3-kinase-dependent MEK activation pathway. Mol. Cell. Biol. 15, 3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell, D. P., Dunlop, J., Stevens, H. P., Lench, N. J., Liang, J. N., Parry, G., Mueller, R. F., and Leigh, I. M. (1997). Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80-83. [DOI] [PubMed] [Google Scholar]
- Kjaer, K. W., Hansen, L., Eiberg, H., Leicht, P., Opitz, J. M., and Tommerup, N. (2004). Novel Connexin 43 (GJA1) mutation causes oculo-dento-digital dysplasia with curly hair. Am. J. Med. Genet. 127A, 152-157. [DOI] [PubMed] [Google Scholar]
- Kojima, T., Spray, D. C., Kokai, Y., Chiba, H., Mochizuki, Y., and Sawada, N. (2002). Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp. Cell Res. 276, 40-51. [DOI] [PubMed] [Google Scholar]
- Koval, M., Geist, S. T., Westphale, E. M., Kemendy, A. E., Civitelli, R., Beyer, E. C., and Steinberg, T. H. (1995). Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J. Cell Biol. 130, 987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutovskikh, V. A., Piccoli, C., Yamasaki, H., and Yamasaki, H. (2002). Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 21, 1989-1999. [DOI] [PubMed] [Google Scholar]
- Lai, C.-F., Chaudhary, L., Fausto, A., Halstead, L. R., Ory, D. S., Avioli, L. V., and Cheng, S. L. (2001). Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem. 276, 14443-14450. [DOI] [PubMed] [Google Scholar]
- Lai, C. F. and Cheng, S. L. (2002). Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J. Biol. Chem. 277, 15514-15522. [DOI] [PubMed] [Google Scholar]
- Lampe, P. D. and Lau, A. F. (2000). Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 384, 205-215. [DOI] [PubMed] [Google Scholar]
- Lampe, P. D. and Lau, A. F. (2004). The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 36, 1171-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda, F., Towler, D. A., Ziambaras, K., Cheng, S.-L., Koval, M., Steinberg, T. H., and Civitelli, R. (1998). Gap junctional communication modulates gene expression in osteoblastic cells. Mol. Biol. Cell 9, 2249-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda, F., Warlow, P. M., Sheikh, S., Furlan, F., Steinberg, T. H., and Civitelli, R. (2000). Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 151, 931-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., Zhou, Z., Yellowley, C. E., and Donahue, H. J. (1999). Inhibiting gap junctional intercellular communication alters expression of differentiation markers in osteoblastic cells. Bone 25, 661-666. [DOI] [PubMed] [Google Scholar]
- Lin, G. C., Rurangirwa, J. K., Koval, M., and Steinberg, T. H. (2004). Gap junctional communication modulates agonist-induced calcium oscillations in transfected HeLa cells. J. Cell Sci. 117, 881-887. [DOI] [PubMed] [Google Scholar]
- Lou, J., Tu, Y., Li, S., and Manske, P. R. (2000). Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem. Biophys. Res. Commun. 268, 757-762. [DOI] [PubMed] [Google Scholar]
- Martinez, A. D., Hayrapetyan, V., Moreno, A. P., and Beyer, E. C. (2002). Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ. Res. 90, 1100-1107. [DOI] [PubMed] [Google Scholar]
- Merchant, J. L., Du, M., and Todisco, A. (1999). Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem. Biophys. Acta 254, 454-461. [DOI] [PubMed] [Google Scholar]
- Milanini-Mongiat, J., Pouyssegur, J., and Pages, G. (2002). Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J. Biol. Chem. 277, 20631-20639. [DOI] [PubMed] [Google Scholar]
- Nicholson, S. M., Gomes, D., de Nechaud, B., and Bruzzone, R. (2001). Altered gene expression in Schwann cells of connexin32 knockout animals. J. Nucl. Med. 66, 23-36. [DOI] [PubMed] [Google Scholar]
- Niessen, H., Harz, H., Bedner, P., Kramer, K., and Willecke, K. (2000). Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J. Cell Sci. 113, 1365-1372. [DOI] [PubMed] [Google Scholar]
- Parrizas, M., Saltiel, A. R., and LeRoith, D. (1997). Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 272, 154-161. [DOI] [PubMed] [Google Scholar]
- Paznekas, W. A., et al. (2003). Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe, J., Pacheco, I., and Meda, P. (1994). Insulin gene transcription is rapidly inhibited by decreasing glucose concentration in rat islet cells. Diabetes 43, 523-528. [DOI] [PubMed] [Google Scholar]
- Pitts, J. D., Finbow, M. E., and Kam, E. (1988). Junctional communication and cellular differentiation. Br. J. Cancer 9, 52-57. [PMC free article] [PubMed] [Google Scholar]
- Plotkin, L. I., Manolagas, S. C., and Bellido, T. (2002). Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 277, 8648-8657. [DOI] [PubMed] [Google Scholar]
- Qiao, M., Shapiro, P., Kumar, R., and Passaniti, A. (2004). Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a PI3K/ERK-dependent and Akt-independent signaling pathway. J. Biol. Chem. 279, 42709-42718. [DOI] [PubMed] [Google Scholar]
- Ransjo, M., Sahli, J., and Lie, A. (2003). Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem. Biophys. Res. Commun. 303, 1179-1185. [DOI] [PubMed] [Google Scholar]
- Richardson, R., Donnai, D., Meire, F., and Dixon, M. J. (2004). Expression of Gja1 correlates with the phenotype observed in oculodentodigital syndrome/type III syndactyly. J Med. Genet. 41, 60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, P. C., D'Ippolito, G., Balkan, W., Roos, B. A., and Howard, G. A. (2001a). Gap junctional communication is required for the maturation process of osteoblastic cells in culture. Bone 28, 362-369. [DOI] [PubMed] [Google Scholar]
- Schiller, P. C., D'Ippolito, G., Balkan, W., Roos, B. A., and Howard, G. A. (2001b). Gap-junctional communication mediates parathyroid hormone stimulation of mineralization in osteoblastic cultures. Bone 28, 38-44. [DOI] [PubMed] [Google Scholar]
- Schiller, P. C., D'Ippolito, G., Brambilla, R., Roos, B. A., and Howard, G. A. (2001c). Inhibition of gap-junctional communication induces the trans-differentiation of osteoblasts to an adipocytic phenotype in vitro. J. Biol. Chem. 276, 14133-14138. [DOI] [PubMed] [Google Scholar]
- Schiller, P. C., D'Ippolito, G., Brambilla, R., Roos, B. A., and Howard, G. A. (2001d). Inhibition of gap-junctional communication induces the trans-differentiation of osteoblasts to an adipocytic phenotype in vitro. J. Biol. Chem. 276, 14133-14138. [DOI] [PubMed] [Google Scholar]
- Schulz, R., Gres, P., Skyschally, A., Duschin, A., Belosjorow, S., Konietzka, I., and Heusch, G. (2003). Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 17, 1355-1357. [DOI] [PubMed] [Google Scholar]
- Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A., and Brown, M. (2002). Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843-852. [DOI] [PubMed] [Google Scholar]
- Stains, J. P., Lecanda, F., Screen, J., Towler, D. A., and Civitelli, R. (2003). Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J. Biol. Chem. 278, 24377-24387. [DOI] [PubMed] [Google Scholar]
- Stains, J. P., Lecanda, F., Towler, D. A., and Civitelli, R. (2004). Heterogeneous nuclear ribonucleoprotein K represses transcription from a cytosine-thymidine-rich element in the osteocalcin promoter. Biochem. J. Published online ahead of press, September 10, 2004, DOI 20040680. [DOI] [PMC free article] [PubMed]
- Steinberg, T. H., et al. (1994). Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J 13, 744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste, M. C., List, B., Guan, X., Hahn, K. M., Lerner, R., and Gilula, N. B. (2001). A catalytic antibody produces fluorescent tracers of gap junction communication in living cells. J. Biol. Chem. 276, 49164-49168. [DOI] [PubMed] [Google Scholar]
- Swarthout, J. T., Doggett, T. A., Lemker, J. L., and Partridge, N. C. (2001). Stimulation of extracellular signal-regulated kinases and proliferation in rat osteoblastic cells by parathyroid hormone is PKC-dependent. J. Biol. Chem. 276, 7586-7592. [DOI] [PubMed] [Google Scholar]
- Toyofuku, T., Akamatsu, Y., Zhang, H., Kuzuya, T., Tada, M., and Hori, M. (2001). c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 276, 1780-1788. [DOI] [PubMed] [Google Scholar]
- Van der Molen, M. A., Rubin, C. T., McLeod, K. J., McCauley, L. K., and Donahue, H. J. (1996). Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J. Biol. Chem. 271, 12165-12171. [DOI] [PubMed] [Google Scholar]
- Vozzi, C., Ulrich, S., Charollais, A., Philippe, J., Orci, L., and Meda, P. (1995). Adequate connexin-mediated coupling is required for proper insulin production. J. Cell Biol. 131, 1561-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, G., Gopalakrishnan, R., Jiang, D., Reith, E., Benson, M. D., and Franceschi, R. T. (2002). Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 17, 101-110. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Green, C., and Stott, N. S. (2002). Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J. Cell Physiol. 193, 233-243. [DOI] [PubMed] [Google Scholar]
- Zhao, M., Xiao, G., Berry, J. E., Franceschi, R. T., Reddi, A., and Somerman, M. J. (2002). Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J. Bone Miner. Res. 17, 1441-1451. [DOI] [PubMed] [Google Scholar]