Abstract
May-Thurner syndrome (MTS) also known as Cockett’s syndrome is a rare condition responsible for 2%-3% of all cases of deep venous thrombosis (DVT). The thrombosis results from mechanical compression of the left common iliac vein against the body of the fifth lumbar vertebra by the right common iliac artery. Repetitive hyperplasia of the venous wall by compression results in spur formation that in turn causes venous flow obstruction and results in the DVT. Our case is a young female who had acute extensive proximal DVT due to MTS that was successfully managed using mechanical thrombectomy with a venous stent. MTS although a rare entity should be suspected especially in young patients with unilateral DVT with extensive clots especially on left lower extremity without any antecedent risk factors.
Key words: May-Thurner syndrome, left lower extremity deep vein thrombosis, Trellis® procedure, venous mechanical thrombectomy, left iliac vein stenting
Introduction
May-Thurner syndrome (MTS) known as iliac vein compression syndrome refers to compression of the left common iliac vein (LCIV) by a right common iliac artery (RCIA) against the body of the fifth lumbar vertebra. MTS can present with unilateral DVT, venous stasis ulcers, skin discoloration and pulmonary embolism. Among anatomically acquired risk factors of DVT, LCIV compression by RCIA is a common pattern seen in 22% of the normal population.1 It is responsible for 2% to 3% of all lower extremity DVT and 18%-49% of left sided lower extremity DVT.2 It is more common in young, otherwise healthy women between ages 20-50 years of age.
Case Report
A 36-year-old African American female presented to the emergency room (ER) with a four-day history of left leg pain and burning sensation. She was previously evaluated in the ER four days prior to this visit for the left lower extremity pain, was given steroids and pain medications and given a tentative diagnosis of sciatica. However, her symptoms progressed and she noticed an increased swelling of the left lower extremity when compared to the right, which prompted her to visit the ER. She had a prior history of back pain, pyelonephritis, four cesarean sections and bilateral tubal ligation. There was no history of prolonged immobilization, prolonged travel or oral contraceptive use and no recent trauma to her leg. There was no prior personal and family history of blood clots. She did, however, smoke a half pack per day cigarette for ten years.
On presentation, vitals included a blood pressure of 107/61 mmHg, pulse 71 beats/min, respiratory rate of 18/min, temperature 98.7°F and oxygen saturation of 98% on ambient air. She had unremarkable cardiac, respiratory and abdominal examination. She had a swollen left lower extremity extending up to the thigh, tender and erythematous left lower leg at calf with bilateral positive dorsal pedal pulses. Laboratory data including complete blood count, complete metabolic panel, coagulation profile (international normalized ratio, prothrombin time, and white blood cell count with neutrophil predominance without bands). An x-ray of the lumbar spine showed decreased lordosis but no fracture, destructive lesion, or significant degenerative lesions. A lower extremity Doppler ultrasound revealed occlusive acute deep venous thrombosis in the left common femoral, deep femoral veins and non-occlusive venous thrombosis in the left distal greater saphenous and popliteal veins.
Anticoagulation therapy with intravenous heparin was initiated. As the clot burden was very high, interventional radiology (IR) guided clot lysis via Trellis® procedure was done. A computed tomographic (CT) scan of the chest with intravenous contrast revealed a right lower lobe segmental pulmonary embolus and normal pulmonary parenchyma. A contrast CT scan of the abdomen and pelvis to demonstrate anatomy and pelvic clot burden was performed which revealed a completely occluded left common iliac vein and its branches. The right common iliac artery appeared to compress the origin of the left common iliac vein, which is consistent with MTS (Figure 1).
Figure 1.
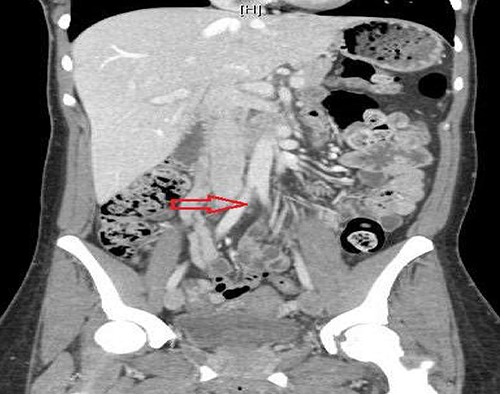
Computed tomographic scan abdomen pelvis contrast demonstrating compression of the left common iliac vein by the right common iliac artery.
She underwent IR guided infrarenal inferior vena cava filter placement and Trellis® procedure which failed to lyse clots with Alteplase® infusion. Post-lysis imaging revealed significant residual thrombus along the course of the lysis catheter involving the left common iliac, left common femoral and left superficial femoral veins. Minimal to no flow through the native left iliac vein was observed. Next day, successful left lower extremity venous mechanical thrombectomy with stenting of the left iliac vein had to be done. Post-stent venogram revealed restored central flow to the iliofemoral venous system (Figure 2A and B). Later, the patient was discharged on oral anticoagulants. IVC filter was removed at 12 months’ follow-up visit and there has been no recurrence of DVT.
Figure 2.
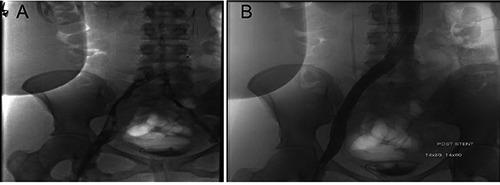
A) Post-Trellis® venogram revealed residual thrombus along the course of the lysis catheter; B) Post-stent venogram revealed restored central flow to the iliofemoral venous system.
Discussion
In 1851, Virchow first noticed the predominance of left-sided iliofemoral DVT over right-sided DVT.3 More than 50 years later, McMurrich4 studied 107 cadavers (between 1906 and 1908) and observed the same phenomenon with the left common iliac vein being obstructed in 29.9% as compared to the right common iliac vein being obstructed only in 2.8% of subjects and suggested a congenital etiology for this predominance. In 1943, Ehrich and Krumbhaar5 postulated an acquired cause for left common iliac vein obstruction. They studied 412 cadavers including infants and fetuses and found that vascular thickening causing obstruction was present only in 1.5% of infants/stillborn and only 2.3% of juveniles as compared to 20.5% in adults. May and Thurner, in 1957, studied 430 cadavers and found focal intimal vascular thickening and septate formation in 22% of subjects. They called this thickening a spur. This finding was essentially absent in the fetal group. They suggested that compression of LCIV by RCIA leads to chronic, repetitive microtrauma that in turn causes venous endothelial injury. It results in collagen and elastin deposition in the vein causing the formation of webs and spurs.1 Until now, only cadavers were studied. Thomas and Cockett, in 1965, observed that patients with spurs could remain asymptomatic due to the development of collaterals bypassing the narrowed left common iliac vein.6 In 2010, it was established that the only significant reason for left-sided DVT being 3 to 8 times more common than right-sided DVT is May-Thurner syndrome.7 Although compression of LCIV by the RCIA is a common pattern, it may be missed due to the presence of other easily identifiable risk factors for DVT like prolonged travel, OCP use, family history of DVT or pregnancy. Due to the frequency of this anatomic pattern, MTS should be considered in young to middleaged females with idiopathic left iliofemoral DVT.8
Most of the patients with MTS are asymptomatic. When symptomatic, it can present acutely with pain in the leg, unilateral leg swelling, DVT and pulmonary embolism.9 However, pulmonary embolism is a rare finding due to the protection offered by the left common iliac vein narrowing. It can trap large emboli, analogous to an IVC filter. Chan KT et al.10 showed that ≥70% compression of iliocaval veins could reduce the risk of symptomatic pulmonary embolism by 80%. Patients can also present with chronic signs and symptoms such as venous ulcerations, skin changes including pigmentation, varicose veins, and recurrent superficial venous thrombophlebitis.11 There is some clinical association of MTS with patent foramen ovale and cryptogenic stroke12 and since the left common iliac vein is compressed against the fifth lumbar vertebra, it is more common in patients with scoliosis.13
Diagnosis of MTS can be made using several imaging techniques, but the initial evaluation starts with a venous Doppler ultrasound to diagnose DVT. Although it is very accurate to diagnose DVT in the lower extremity, visualization of a clot so high at the level of the left common femoral vein is very difficult to detect using ultrasound and its use is also limited by body habitus and bowel gases. Therefore, visualization of common iliac and external iliac veins using ultrasound was only 47% and 79% respectively.8
In contrast to Doppler ultrasound, both CT venography, and magnetic resonance (MR) Venography are highly sensitive and specific imaging modalities that not only help to visualize the clot accurately but can also determine the degree of stenosis in the vessel. CT images may show bony artifacts in the pelvis. MR angiography, on the other hand, has the advantage of being able to better differentiate intraluminal thrombus from contrast mixing and provides a better estimate of the degree of collateral flow especially with the recent advent of blood pool, gadolinium-based contrast agents.9 Since extensive pelvic collateral venous drainage bypassing the narrowed LCIV is a hallmark of MTS, MR angiography is the gold standard for diagnosing this syndrome.14
Anticoagulation is the treatment of choice for DVT, but it is not very effective for MTS when used alone.7 The mainstay of treatment for MTS includes endovascular thrombolysis with or without prophylactic IVC filter placement followed by angioplasty/stenting of the left common iliac vein.15,16 Endovascular thrombolysis is a catheter-directed introduction of tissue plasminogen activator (TPA). TPA is used to expand the intraluminal space thus making enough room for subsequent stent placement.17 Earlier practices suggested the placement of retrievable IVC filters before catheter-directed thrombolysis to prevent possible fatal PE events related to the procedure especially in cases of extensive DVT.18 It was found that patients with MTS, rarely present with a PE as venous spurs appear to afford some protection against PE.19
Thus, IVC filter placement is of questionable utility in MTS patients except in patients without occlusions who cannot receive anticoagulant treatment. However, the Society for Vascular Surgery (SVS) 2012 guidelines does not recommend IVC placement prophylactically given the risks and complications associated with this procedure.20 It is important to do angioplasty with stent placement after endovascular procedure since the patency rate is very low without subsequent stent placement.
By doing thrombectomy, angioplasty and stenting together, one-year stent patency rate is 79% to 83%.9,21 Long-term anticoagulation for at least 6 months and compression stockings are required to prevent recurrent DVT due to May-Thurner syndrome.9
Conclusions
May-Thurner syndrome although a rare entity should be suspected especially in young patients with unilateral DVT with extensive clots especially on left lower extremity without any antecedent risk factors.
References
- 1.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419-27. [DOI] [PubMed] [Google Scholar]
- 2.Oguzkurt L, Ozkan U, Tercan F, Koc Z. Ultrasonographic diagnosis of iliac vein compression (May-Thurner) syndrome. Diagnostic and interventional radiology (Ankara, Turkey) 2007;13:152-5. [PubMed] [Google Scholar]
- 3.R. V. Ueber die Erweiterung kleinerer Gefäfse. Virchows Archiv. 1851;3:427-62. [Google Scholar]
- 4.McMurrich JP. The occurence of congenital adhesions in the common iliac veins their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908;135:342-5. [Google Scholar]
- 5.Ehrich WE. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943;26: 737-50. [Google Scholar]
- 6.Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg 1965;52:816-21. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon RK, Stead LG. Acute deep vein thrombus due to May-Thurner syndrome. Am J Emerg Med 2010;28:254.e3-4. [DOI] [PubMed] [Google Scholar]
- 8.Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vase Surg 2013;27:984-95. [DOI] [PubMed] [Google Scholar]
- 9.Butros SR, Liu R, Oliveira GR, et al. Venous compression syndromes: clinical features, imaging findings and management. Br J Radiol 2013;86: 20130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KT, Popat RA, Sze DY, et al. Common iliac vein stenosis and risk of symptomatic pulmonary embolism: an inverse correlation. J Vasc Intervent Radiol 2011;22:133-41. [DOI] [PubMed] [Google Scholar]
- 11.Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010;22:223-30. [DOI] [PubMed] [Google Scholar]
- 12.Kiernan TJ, Yan BP, Cubeddu RJ, et al. May-Thurner syndrome in patients with cryptogenic stroke and patent foramen ovale: an important clinical association. Stroke 2009;40:1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oteros Fernandez R, Bravo Rodriguez F, Delgado Acosta F, Gonzalez Barrios I. [May-Thurner syndrome and surgery for scoliosis]. Radiologia 2008;50:245-7. [DOI] [PubMed] [Google Scholar]
- 14.McDermott S, Oliveira G, Erguí E, et al. May-Thurner syndrome: can it be diagnosed by a single MR venography study? Diagn Interv Radiol (Ankara, Turkey) 2013;19:44-8. [DOI] [PubMed] [Google Scholar]
- 15.Moudgill N, Hager E, Gonsalves C, et al. May-Thurner syndrome: case report and review of the literature involving modern endovascular therapy. Vascular 2009;17:330-5. [DOI] [PubMed] [Google Scholar]
- 16.Liew A, Douketis J. Catheter-directed thrombolysis for extensive iliofemoral deep vein thrombosis: review of literature and ongoing trials. Exp Rev Cardiovasc Ther 2015:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Brazeau NF, Harvey HB, Pinto EG, et al. May-Thurner syndrome: diagnosis and management. VASA Zeitschr Gefasskrank 2013;42:96-105. [DOI] [PubMed] [Google Scholar]
- 18.Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol 2003;14:425-40. [DOI] [PubMed] [Google Scholar]
- 19.Hager ES, Yuo T, Tahara R, et al. Outcomes of endovascular intervention for May-Thurner syndrome. J Vasc Surg 2013;1:270-5. [DOI] [PubMed] [Google Scholar]
- 20.Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012;55:1449-62. [DOI] [PubMed] [Google Scholar]
- 21.Alirhayim Z, El Atrache M, Rocco N, Drake S. Symptomatic ileofemoral deep vein thrombosis due to May-Thurner syndrome. BMJ Case Rep 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


