Abstract
Antimicrobial stewardship is recognized as a key component to stop the current European spread of antimicrobial resistance. It has also become evident that antimicrobial resistance is a problem that cannot be tackled by single institutions or physicians. Prevention of antimicrobial resistance needs rigorous actions at ward level, institution level, national level and at supra-national levels. Countries can learn from each other and possibly transplant best practices across borders to prevent antimicrobial resistance. The aim of this study is to highlight some of the success stories of proven cost-effective interventions, and to describe the actions that have been taken, the outcomes that have been found, and the difficulties that have been met. In some cases we came across substantial scope for real-life cost savings. Although the best approach to effectively hinder the spread of antimicrobial resistance remains unclear and may vary significantly among settings, several EU-wide examples demonstrate that cost-effective antimicrobial stewardship is possible. Such examples can encourage others to implement (the most cost-effective) elements in their system.
Key words: Antimicrobial resistance, Antimicrobial stewardship, Cost-effectiveness, Sustainable healthcare
Competing interest statement
Conflict of interest: the authors declare no potential conflict of interest.
Introduction
Antimicrobial resistance is a worldwide and growing public healthcare problem.1-3 The recently published Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations shows that increasing antimicrobial resistance leads to substantial clinical burden in terms of morbidity, mortality, and lower quality of life.4 Today, resistant bacteria cause in Europe and the US only 50,000 deaths per year. However, if adequate use of antibiotics will not improve, it has been estimated that in the year 2050 approximately 10 million people will die as a consequence of antimicrobial resistance. The economic burden will also be substantial, in particular due to additional hospitalizations, expensive secondary treatment options, and productivity losses at the labor market. Yet, despite the severe consequences of inappropriate antibiotic use and the alarming future projections, antibiotic use has for example increased with 4-5% last year in Dutch hospital care.5 A more intense focus on appropriate use of antibiotics is necessary to keep healthcare systems sustainable.6
The World Health Organization defines appropriate use of antibiotics as the cost-effective use of antibiotics which maximizes clinical therapeutic effect while minimizing both drug-related toxicity and the development of antimicrobial resistance.6 The objective of antimicrobial resistance policies is to ensure patient safety and to keep future bacterial infections treatable. Antimicrobial resistance policies addresses two main mechanisms. First, preventing the spread of (resistant) micro-organisms by effective infection control. Second, reducing inadequate prescription and inadequate use of antibiotics. To achieve maximum benefit, these antimicrobial policies should be implemented in curative care (primary care and hospital care), and also in long-term care (nursing and residential homes).
Several regulators and institutions at the forefront have shown to limit the spread of antimicrobial resistance effectively by interventions such as antimicrobial stewardship teams, public health campaigns, governmental regulations, improved diagnostics, and interventions to improve adherence to infection prevention guidelines. Moreover, some of them also claimed substantial cost-effectiveness of their antimicrobial policy as well as absolute cost savings. Effective EU-wide implementation and sustained use of cost-effective antimicrobial policies can consequently lead to improved safety and quality of care while contributing to more sustainable healthcare.
The objective of this article is to provide examples of infection prevention and adequate use of antibiotics that have proven to be both clinically effective and of economic value. We describe the content of these examples and provide insight into the cost-effectiveness.
Materials and Methods
We searched for proven cost-effective examples of Dutch antimicrobial policies and infection control policies. After selecting Dutch examples, an inventory was made of additional promising EU-wide examples. First, we created a long-lost of promising cases based on expert opinion and the personal networks of the organization committee of the EU Antimicrobial Resistance One Health Ministerial Conference (February 2016, Amsterdam, the Netherlands) and the research team members. From this long-lost only cases with proven cost-effectiveness were selected for the shortlist. The final selection of all examples was also based on the quality of examples and the type of antimicrobial policy (to describe a wide spread of cost-effective policies, implemented across healthcare settings). Thus, this sample – if broadly implemented – holds the promise for substantial improvement across entire health systems. The EU-wide examples were primarily selected to create a magazine as input for the EU Antimicrobial Resistance One Health Ministerial Conference. The cases in the current research article are drawn from the magazine. If the EU-wide institutions were willing to participate (all were), an inventory was made of the following topics: i) the content of the antimicrobial policy; ii) the costs of the antimicrobial policy; and iii) the effectiveness of the antimicrobial policy. If available, we used peer-reviewed literature for the data inventory (high quality evidence). If not available, we used non-peer-reviewed literature, such as research reports, conference presentations, or personal communication with study authors (lower quality evidence).
Results
In this section, we describe proven cost-effective examples of a sample of interventions that are directed at reduction of infections with microorganisms, more adequate use of antibiotics, a reduction of antimicrobial resistance rates, or a combination of these. We have described both examples of horizontal approaches at national or hospital level and vertical approaches (more narrow-based programs) at the level of an individual hospital ward or primary care practice.
Horizontal approaches at hospital level
Antimicrobial stewardship teams: lower- and high-quality evidence
To control the spread of antimicrobial resistance, antimicrobial stewardship teams are mandatory for every Dutch hospital. Antimicrobial stewardship teams have the purpose to stimulate adequate antibiotic use, which should result in a reduction of antimicrobial resistance rates, less use of last resort antibiotics, and shorter length of hospital stay. Stewardship teams will therefore enhance quality of care, while also yielding (large) cost-savings.7
Two Dutch hospitals proved cost-effectiveness of their antimicrobial stewardship team. The Canisius-Wilhelmina Hospital in Nijmegen estimated a €40,000 hospital-wide cost-saving over a 12-month period after introduction of the antimicrobial stewardship team.8 The team consists of an internist-infectivologist, a microbiologist, a pharmacist, and an IT specialist. Key elements of the Canisius-Wilhelmina Hospital stewardship team are antimicrobial vigilance alerts (daily monitoring of used antibiotics), audit-feedback, and a IV-oral switch program. Implementation of the antimicrobial stewardship team resulted in 25% less prescription of last resort antibiotics, modification of more than half of all antibiotic prescriptions, and a 1-day earlier IV-oral switch.
The University Medical Centre Groningen (UMCG, the Netherlands) also proved substantial cost-effectiveness of their antimicrobial stewardship program. In addition to the standard routines, the UMCG program holds an important and unique element: the face-to-face day 2 case audit. The aim of this audit is to streamline therapy as early as possible. The hospital pharmacist sends an automatic e-mail alert to all stewardship members 48 hours after start of antibiotic therapy. This then triggers a case audit: a stewardship member visits the ward to discuss the patient’s therapy with the bedside physician. Together, they decide on further treatment (e.g., IV-oral switch and dosage), based on diagnostics and local guidelines. The applicability of this therapy will be discussed again after thirty days of treatment. These face-to-face consultations are used to create an effective learning moment.
The clinical effectiveness and cost-effectiveness of this antimicrobial stewardship team were studied at a urology ward.9,10 Hospital costs from patients in this effectiveness study were compared with a historical cohort from the same ward: there was a statistically and clinically significant reduction in the number of antibiotic prescriptions (Figure 1). In addition, patients switched significantly earlier from IV to oral therapy, had a shorter length of hospital stay, and required less nursing time on the ICU. During the programs’ first year, the study group spent almost €70,000 less than the historical cohort, while the total costs for the intervention were about €27,000 in this period. One-off costs for setting up the program (stewardship meetings and the development of the pharmacy e-alert program) were €17,000. Structural cost for running this program (case audits, stewardship meetings, and maintenance of the pharmacy e-alert program) totaled about €10,000. This economic evaluation not only strongly indicates cost-effectiveness of the antimicrobial stewardship (a possible return on investment of 700%), it also shows that a financial break-even point can be reached within a few months. It should however be emphasized that these results only hold for patients without severe underlying comorbidity. Nevertheless, given that both lower antibiotic use and lower length of stay predict increasing quality of care, this stewardship team seems to have a major impact and may lead to positive investment returns, even shortly after implementation (Figure 2). Antimicrobial stewardship is also highly relevant in long-term care. Given the large variation in prevalence of resistant bacteria in long-term care institutions, it is relevant to monitor prevalence of resistant bacteria closely.11 Sweden has developed a nationwide web-based tool for data collection and feedback on bacterial infections and antibiotic consumption in long-term care.12 Web-based reporting makes it easy for staff to register the infections, the prescribed antibiotics and the risk factors. It also makes it possible to evaluate prescribing of antibiotics in relation to various infection symptoms and how the presence of certain risk factors relate to the prevalence of infections. Since data can be easily extracted from the reporting module, all participating facilities can get instant feedback on their own results. Evidence on (cost-)effectiveness of this web-based tool in long-term care is not yet available, but given the major impact of infections on patient safety in long-term care, this seems a promising way to approach this problem.
Figure 1.
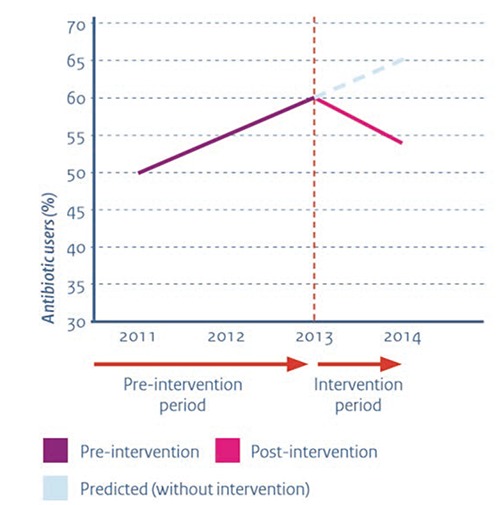
Antibiotic use on a urology department before and after implementation of the antimicrobial stewardship team.
Figure 2.
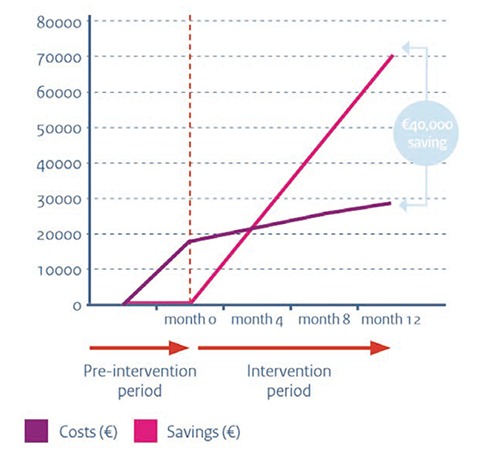
Costs and savings of the antimicrobial stewardship team.
Horizontal approaches at national level
Public health campaigns – lower- and high-quality evidence
Public health campaigns can raise awareness on responsible antibiotic use. In France, for example the overall antibiotic consumption fell by 10.7% (as compared to a pre-intervention period) during a nationwide mass media campaign that lasted from 2002 until 2010 – called microorganisms in question.13 The campaign used a range of tools, including TV and radio spots, information booklets, and leaflets. Examples of common slogans used were antibiotics are not automatic, an antibiotic cannot heal, and antibiotics, if you use them incorrectly, they will be less strong. It should however be highlighted that the observed decrease might have been caused by other initiatives in France or elsewhere or by the introduction of a S. pneumonia vaccine during the study period.
Poland celebrates an Antibiotic Awareness Day each year on the 18th of November to raise awareness for the threat posed by antimicrobial resistance, as well as the need for appropriate use of antibiotics.14 Different stakeholders are engaged: scientific societies, outpatient clinics, hospitals, schools, government bodies, public transport, television, radio, the press. Some information about antimicrobial resistance and the use of antibiotics are disseminated in the form of posters, leaflets, articles in scientific journals, educational materials for schools, radio broadcasts, advertising spots on TV and short texts on webpages. Scientific conferences are also organized. In 2009, a questionnaire study revealed that about 30% of the people who were informed about the rational use of antibiotics stated that they adapted their antibiotic use.14 The Antibiotic Awareness Day therefore seems a proven way to change prescribing behavior. Finally, in Malta, a nation-wide approach using educational and motivational interventions led to more responsible use of antibiotics. After this awareness campaign a nation-wide reduction in the proportion of respondents who stated that they had obtained antibiotics without a doctor’s prescription came to the fore.12
Governmental regulations on package size: lower quality evidence
In Spain, antibiotic consumption was significantly decreased after adjusting the package size of oral antibiotics.12 In September 2011, an expert working group was convened with the aim of reviewing the size of the package for oral antibiotics. This panel included a GP, a pediatrician, a pharmacist, and government representatives. All oral antibiotic packages were reviewed and a proposal was made to adjust their size to the most common dosage and duration of treatment according to clinical practices. From May 2012 to April 2014, there was a transitory period of coexistence of the old and adjusted packages. Over 900 packages were adjusted. According to the ECDC ESAC-Net surveillance data, this had a significant impact on the antibiotic consumption in terms of number of packages per thousand inhabitants and per day.12
Vertical approaches at hospital ward level
Prevention of surgical infections with S. aureus: high-quality evidence
Reductions in use of antibiotics can also be capitalized by a reduction of infections. S. aureus is worldwide the most common hospital-acquired infection.15 Infection rates are increasing due to the widespread dissemination of methicillin-resistant S. aureus (MRSA). Infection with MRSA is associated with substantial morbidity and mortality.16 In 2008, there were an estimated 380,000 infections in EU hospitals. MRSA accounts for 5400 attributable deaths and for more than 1 million in-hospital days. The attributable hospital costs caused by MRSA are considerable, reaching approximately €380 million annually. Reducing the number of S. aureus transmissions therefore offers interesting opportunities.
The control of S. aureus transmission traditionally focused on preventing cross-infection between patients. However, it has been shown that most S. aureus infections originate from patients’ own flora (i.e., presence in the nose).17 Approximately 20% of the healthy population carries S. aureus and is therefore considered a risk factor for subsequent infection to various patient groups. Removal of S. aureus from the nose before surgery has shown to prevent infections. The Amphia Hospital Breda in the Netherlands developed a non-invasive screen-and-treat strategy to eradicate nasal S. aureus bacteria. The strategy entails rapid identification of S. aureus carriers by means of screening (a real-time polymerase-chainreaction assay), followed by treatment with mupirocon nasal ointment and chlorhexidine soap.
Jan Kluytmans and colleagues were the first to study the effectiveness of the screen-and-treat strategy in a double-blind, randomized, controlled trial design.18 The study in five Dutch hospitals proved statistically and clinically significant reduction in S. aureus infections during surgery. The rate of S. aureus infection was 3.4% (17 of 504 patients) in the screen-and-treat strategy group, compared to 7.7% (32 of 413 patients) in the placebo group. The results of this trial provide solid evidence for this preventive intervention: the risk of hospital associated S. aureus infections was reduced by nearly 60%. Moreover, a Cochrane review conducted by researchers from the same study group confirmed effectiveness.19
The screen-and-treat strategy is also highly cost-effective.20 The group performed an economic evaluation in which all hospital costs made during the 12 months after (cardiothoracic or orthopedic) surgery were taken into account. The mean total costs for a screened-and-treated patient undergoing surgery were considerably lower than costs for a placebo-treated patient (€8600 vs. €10,500, Figure 3). The reduction in infections led to a shorter total hospital stay (on average two days) and less nursing time at the ICU. Given that screening is relatively cheap (around €20), and treating even cheaper (€5 for the mupirocon nasal ointment and €5 for the chlorhexidine soap), the financial investments are almost negligible.
Figure 3.
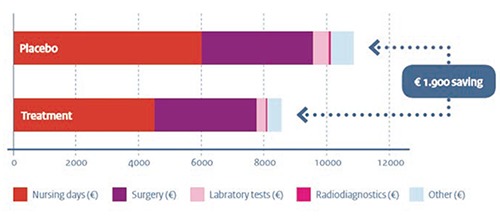
Mean hospital costs per patient for placebo and treatment group.
The results of this study show clear benefit of the screen-and-treat strategy in cardiothoracic and orthopedic surgery. A total of €400,000 per thousand surgeries could be saved, based on the nasal S. aureus carriage rate of 20%. Worldwide millions of surgical procedures are performed each year. Huge numbers of patients would therefore benefit from this screen-and-treat strategy, accompanied by large savings.
Diagnostic tests in primary care - high quality evidence
A final example for reducing antibiotic use is the reduction of unnecessary prescriptions in primary care. Major drivers of such unnecessary prescribing are diagnostic uncertainty and patient expectations.21 An intervention that has shown to address both predictors effectively is the C-reactive protein (CRP) Point of Care Test, in combination with communication skills training.
The CRP Point of Care Test is a highly accurate diagnostic tool to differentiate between presumably viral and bacterial infections, such as acute bronchitis and pneumonia. A low outcome of the CRP test result reassures the GP that other diagnostics and antibiotic treatment are unnecessary. CRP tests can be done swiftly in everyday general practice by using a finger prick blood sample. Results are available in a few minutes.
The effectiveness of this CRP Point of Care Test in combination with enhanced communication training was recently investigated in the Netherlands.22 The effectiveness was studied in a large-scale, pragmatic, randomized trial with a 1-month follow-up period. The combined intervention resulted in a statistically and clinically significant reduction in the number of antibiotic prescriptions (a prescription rate of 23% compared to 68% in the control group). Assuming nation-wide implementation in the Netherlands, the researchers claimed that between 150,000 and 240,000 antibiotic prescriptions could be saved annually. Importantly, patients’ recovery and satisfaction were similar in both study groups, despite the substantial reduction in antibiotic prescribing.
The economic evaluation showed that already one month after running the program, cost-savings exceed the initial investments.23 Patients in the intervention group required less additional diagnostics (e.g., chest X-ray and spirometry), used less antibiotics, and visited the GP less often than control group patients (accounting for an average cost-saving of €22 per patient). Given the low intervention costs (€15 per patient) and the fact that the CRP test can be performed in just three minutes, the feasibility and financial investments cannot be hurdles for further implementation.
Transferability and cost-effectiveness of CRP Point of Care Test was studied in four other EU countries (United Kingdom, Poland, Spain, and Belgium).24 GPs across nations were trained online to interpret CRP tests adequately and to communicate these effectively. The study proved transferability between these countries: the antibiotic prescribing rate in the intervention groups was significantly lower (33%) than in the control groups (48%), despite differences across European primary care settings. Hence, the CRP Point of Care Test is an example that interventions designed in one country can be transferred to other countries with different health systems and prescribing rates.
Discussion
The objective of the present paper was to provide insight into the costs and potential health and financial gains associated with infection control and policies to reduce the spread of antimicrobial resistance. The content of several European proven cost-effective examples were described. Despite some methodological limitations in some of the studies, the evidence suggests that several cost-effective approaches are available to reduce the spread of antimicrobial resistance efficiently. In addition, these examples show that not only multifaceted and complex approaches are cost-effective (e.g., antimicrobial stewardship teams), but also smaller and more feasible approaches with a high potential implementation can be cost-effective (e.g., the CRP Point of Care Test and the screen-and-treat strategy). EU-wide implementation of (the most effective components of) the proven cost-effective examples described in this report may lead to a reduction of infections with microorganisms, more adequate use of antimicrobials, and a reduction of antimicrobial resistance rates. These outcomes will contribute to improved patient safety and patient health, leading to more sustainable healthcare.
The cost-effectiveness of infection control and adequate use of antibiotics can be further enhanced through regional cooperation. Healthcare institutions are generally seen as the source of antimicrobial resistance. However, the high connectivity of healthcare networks (e.g., the transfer of patients between departments; or the transfer of patients from curative to long-term care, and vice versa) will impact the effectiveness of infection control strategies and adequate use of antibiotics. Healthcare institutions should therefore cooperate regionally and internationally to fight antimicrobial resistance successfully. Especially in areas with high levels of cross-region and cross-border patient referrals, since the weakest link in the healthcare chain determines the effectiveness of prevention. Intensive cooperation across departments, institutions, nations, and sectors is also in line with the One Health Approach.25
In 2016, Jim O’Neill estimated that over 40 billion USD is needed over the coming decade to take global action on antimicrobial resistance.26 Almost half of this amount (16 billion USD) is needed to promote the development of new antibiotics to treat resistant patients in urgent need of antibiotics. Although it is extremely important to invest in extending the pipeline of new antibiotics using market incentives such as market entry rewards, we should not neglect the importance of early prevention. The examples in this report suggest that this is possible in a cost-effective way and might even generate substantial savings for providers that spearhead these possibilities.
This paper has two important limitations. First, the focus of the paper was to give an overview of useful stewardship initiatives at different levels of healthcare. The chosen method of citing only a few specifically selected proven cost-effective cases contains an unavoidable selection bias in itself. Nevertheless, the cases show that efficacious prevention at local, national and international level can go hand in hand, not only with cost-effective care, but (sometimes) also with substantial cost-savings. This may help organizations in healthcare to build a business case and invite them to start a stewardship initiative. Second, the results of some cases have not been published in research reports or (peer-reviewed) research articles. These results were retrieved from lower quality evidence, such as oral conference presentations, personal communication with authors, and from (scientific) magazines, which makes it hard to compare the evidence.
Several key lessons can be learned from the presented examples. These lessons may help to design successful and cost-effective policies to reduce the spread of antimicrobial resistance. First, the importance of awareness about the relevance and benefits of cost-effective policies. Second, the continuous surveillance and feedback in an open dialogue culture. Third, sustained implementation of cost-effective approaches across Europe.
Raising awareness about adequate use of antibiotics
The first step for successful implementation and change is to raise awareness.27 Awareness of appropriate antibiotic use can for example be effectively raised through public health campaigns. A range of tools can be used, including TV and radio spots, information booklets/leaflets for patients and healthcare institutions, press releases, and awareness days. Studies in France, Poland, and Malta have shown that public health campaigns improve knowledge and awareness about appropriate antibiotic use dramatically. Moreover, these campaigns have led to significant decreases in antibiotic use. Although the cost-effectiveness of public health campaigns has not been studied, the (self-reported) degrees of reduction in antibiotic use suggests that these campaigns are valuable strategies to effectively raise awareness for a large audience.
Continuous surveillance and feedback on prescribing behavior
Dutch antimicrobial stewardship teams decreased antimicrobial resistance in curative care with very good value for money. Effective surveillance and feedback by these teams have shown to lead to more adequate prescribing of antibiotics, fewer infections, shorter length of hospital stay, and less antimicrobial resistance. Key in this respect is the combination of closely monitoring of healthcare professionals’ behavior (prescribing of antibiotics and adherence to infection control guidelines) and tailored (oral or written) feedback on behavior. Preconditions are clear professional responsibilities in combination with an open dialogue culture, based on openness and trust.
Sustained implementation of cost-effective approaches across Europe
A precondition for long-term effects of these policies is a sustained implementation (also by avoiding and overcoming of resistance to change) of cost-effective approaches. Most examples in this report have proven to be cost-effective in their study setting; one example (CRP Point of Care Test) has also proven to be transferable to other EU countries. Our cost-effective data are from Dutch studies, but the beneficiary effects might be even higher in the majority of European countries that encounter more difficulties with antimicrobial prescriptions.
Conclusions
Given the rapid EU-wide spread of antimicrobial resistance and the limited pipeline of new antibiotics, cost-effective interventions in the areas of public health and care provision are urgently needed to improve infection prevention and antimicrobial prescribing. We have presented the cost-effectiveness of several antimicrobial policies that have shown to improve antimicrobial prescribing and infection control successfully and efficiently. Healthcare institutions and professionals can benefit from the presented studies by implementing (the most effective elements of) antimicrobial policies. Policymakers can redesign (parts of) their health system in such a way that these policies are more sought for by their agents. A sustained implementation strategy holds the promise to improve patient outcomes, while leading to substantial cost-savings. This clearly adds to the sustainability of EU-wide healthcare.
Acknowledgements
The authors would like to thank the researchers who conducted the studies described in this paper for their collaboration and contribution to this paper: Jan Kluytmans (Amphia Hospital Breda); Jochen Cals (Department of Family Medicine, Maastricht University); Alex Friedrich, Bahnu Sinha, and Jan-Willem Dik (University Medical Centre Groningen); and Tom Sprong (Canisius-Wilhelmina Hospital Nijmegen). We also want to thank Marlies Hulscher for helpful comments on earlier versions of this paper.
References
- 1.Smith R, Coast J. The true costs of antimicrobial resistance. BMJ 2013;346:f1493. [DOI] [PubMed] [Google Scholar]
- 2.Hawkey PM. The growing burden of antimicrobial resistance. J Antimicrob Chemother 2008;62:i1-i9. [DOI] [PubMed] [Google Scholar]
- 3.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Exp Rev Anti Infect Ther 2008;6:751-63. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Available from: http://www.amr-review.org/. Retrieved on 10th September 2015.
- 5.de Greef SC, Mouton JW, Schoffelen AF. NethMap 2016: consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands. Report published by the National Institute for Public Health and the Environment, the Netherlands. Available from: http://www.rivm.nl/dsresource?objectid=rivmp:320165&type=org&disposition=inline&ns_nc=1. Retrieved on 27th July 2016.
- 6.World Health Organization. WHO Global Strategy for Containment of Antimicrobial Resistance. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 7.Scheetz MH, Bolon MK, Postelnick M, et al. Cost-effectiveness analysis of an antimicrobial stewardship team on bloodstream infections: a probabilistic analysis. J Antimicrob Chemother 2009;63:816-25. [DOI] [PubMed] [Google Scholar]
- 8.Sprong T. Clinical intelligence in daily practice of the Canisius-Wilhelmina Ziekenhuis A-team. Oral presentation at the University Medical Centre Groningen, Groningen, the Netherlands: 2015. [Google Scholar]
- 9.Dik JW, Hendrix R, Lo-Ten-Foe JR, et al. Automatic 2-day intervention by a multidisciplinary antimicrobial stewardship-team leads to multiple positive effects. Front Microbiol 2015;6:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dik JW, Hendrix R, Friedrich AW, et al. Cost-minimization model of a multidisciplinary antibiotic stewardship team based on a successful implementation on a urology ward of an academic hospital. PLoS One 2015;10:e0126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoef L, Roukens M, de Greeff S, et al. Carriage of antimicrobial-resistant commensal bacteria in Dutch long-term care facilities. J Antimicrob Chemother 2016. Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 12.The Netherlands EU Presidency 2016. AMR Next: EU Antimicrobial Resistance One Health ministerial conference 2016. Available from https://english.eu2016.nl/documents/publications/2016/02/10/amr-next. Retrieved on 10th June 2016
- 13.Sabuncu E, David J, Bernède-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS One 2009;6:e1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazínska B, Hryniewicz W. European antibiotic awareness day educational campaign – has it changed public attitudes to antibiotic use in Poland? Pol Merkur Lekarski. 2010;29:296-303. [PubMed] [Google Scholar]
- 15.Kluytmans JA, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control/European Medicines Agency (ECDC/EMEA). Joint technical report the bacterial challenge: time to react. Stockholm: ECDC/EMEA; 2009. Available from: http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf.Retrieved on 7th July 2016. [Google Scholar]
- 17.Kluytmans JA, Mouton J, Yzerman E, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis 1995;17:216-9. [DOI] [PubMed] [Google Scholar]
- 18.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. New Engl J Med 2010;362:9-17. [DOI] [PubMed] [Google Scholar]
- 19.van Rijen MM, Bonten M, Wenzel R, Kluytmans JA. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2011;2:CD006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijen MM, Bode LG, Baak DA, et al. Reduced costs for Staphylococcus aureus carriers treated prophylactically with mupirocin and chlorhexidine in cardiothoracic and orthopaedic surgery. PLoS One. 2012;7:e43065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coenen S, Michiels B, van Royen P, et al. Antibiotics for coughing in general practice: a questionnaire study to quantify and condense the reasons for prescribing. BMC Fam Pract 2002;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cals JW, Butler CC, Hopstaken RM, et al. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomized trial. BMJ 2009;338:b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cals JW, Ament AJ, Hood K, et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. J Eval Clin Pract 2011;17:1059-69 [DOI] [PubMed] [Google Scholar]
- 24.Little P, Stuart B, Francis N, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomized, factorial, controlled trial. Lancet 2013;382:1175-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammie SL, Hughes JM. Antimicrobial resistance, food safety, and One Health: the need for convergence. Ann Rev of Food Sci Technol 2016;7:287-312. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. from http://www.amr-review.org/. Retrieved on 7th July 2016.
- 27.Grol R, Wensing M. Implementatie: effectieve verbetering van de patiëntenzorg. Amsterdam, Reed Business; 2011 [Google Scholar]


