Abstract
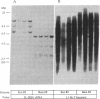
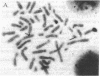
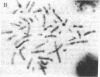
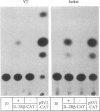
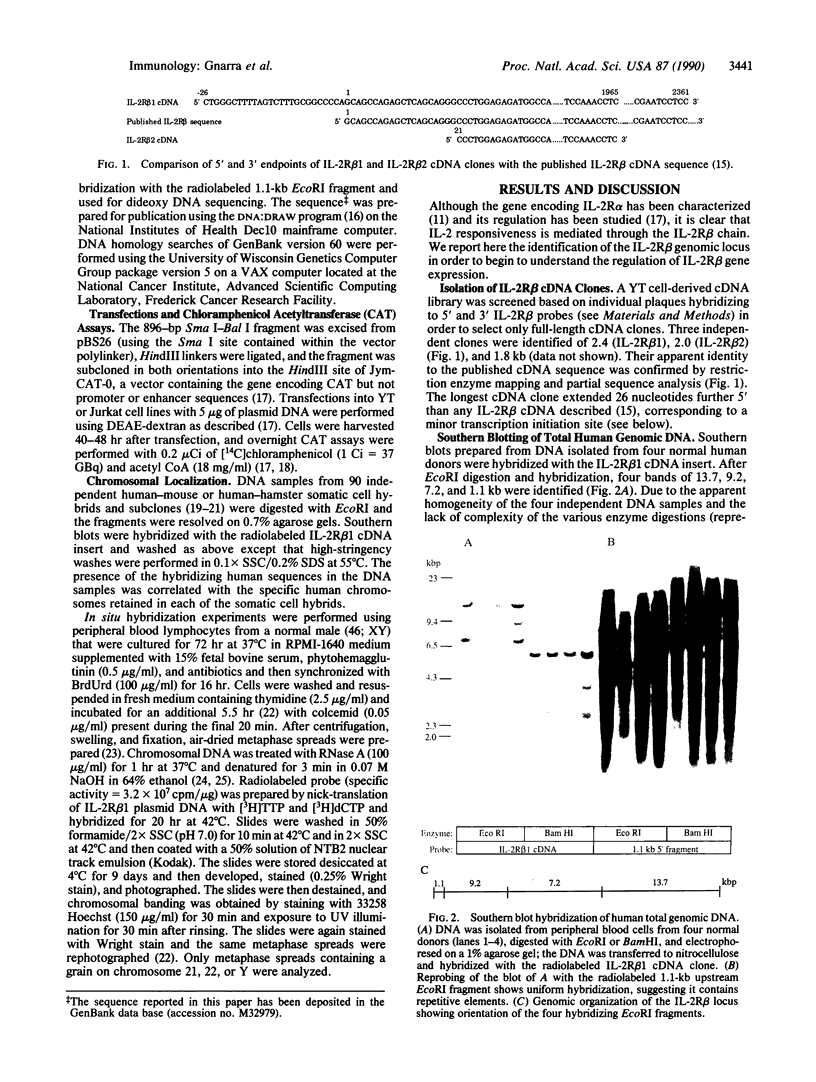
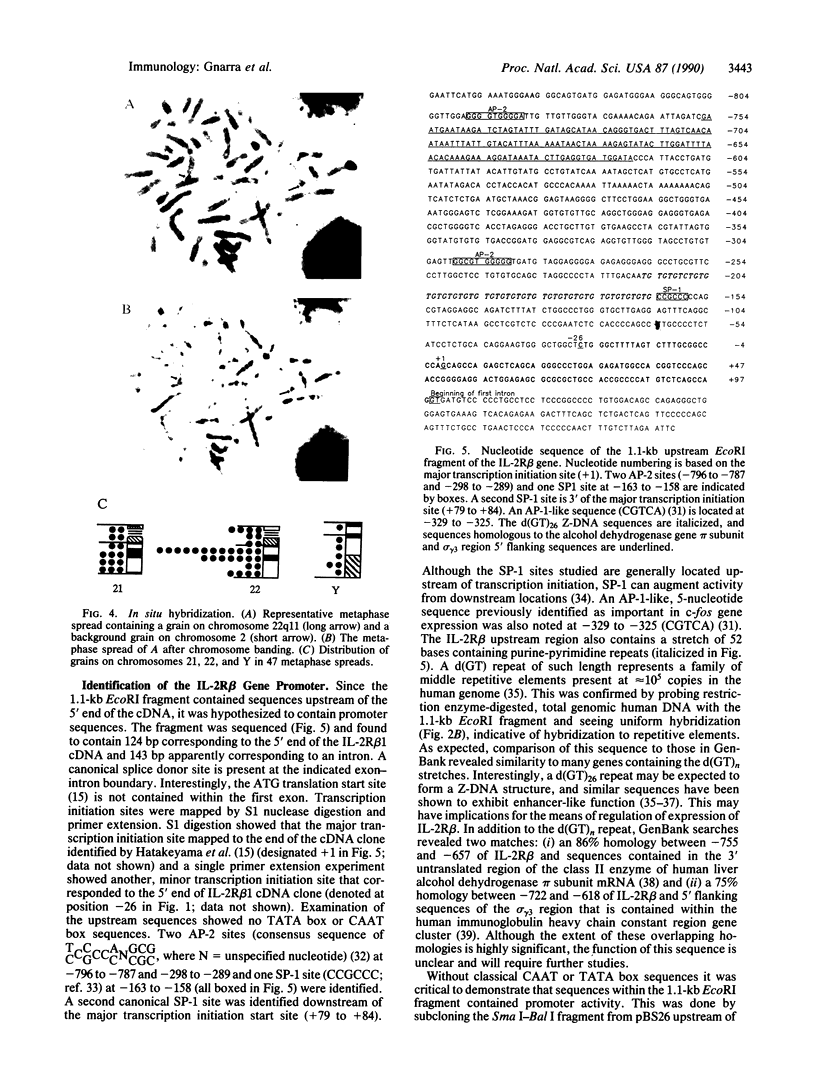
Interleukin 2 (IL-2) binds to and stimulates activated T cells through high-affinity IL-2 receptors (IL-2Rs). Such receptors represent a complex consisting of at least two proteins, the 55-kDa IL-2R alpha chain and the 70-kDa IL-2R beta chain. The low-affinity, IL-2R alpha chain cannot by itself transduce a mitogenic signal, whereas IL-2 stimulates resting lymphocytes through the intermediate-affinity, IL-2R beta receptor. We report here identification of the genomic locus for IL-2R beta. The exons are contained on four EcoRI fragments of 1.1, 9.2, 7.2, and 13.7 kilobases. The 1.1-kilobase EcoRI fragment lies at the 5'-most end of the genomic locus and contains promoter sequences. The promoter contains no TATA box-like elements but does contain the d(GT)n class of middle repetitive elements, which may play an interesting regulatory role. The IL-2R beta gene is localized to chromosome 22q11.2-q12, a region that is the locus for several lymphoid neoplasias.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahori Y., Handa H., Imai K., Abe M., Kameyama K., Hibiya M., Yasui H., Okamura K., Naito M., Matsuoka H. Sigma region located between C mu and C delta genes of human immunoglobulin heavy chain: possible involvement of tRNA-like structure in RNA splicing. Nucleic Acids Res. 1988 Oct 25;16(20):9497–9511. doi: 10.1093/nar/16.20.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. T., Walls J. D., Reifel-Miller A. E., Grinnell B. W. E1A-induced enhancer activity of the poly(dG-dT).poly(dA-dC) element (GT element) and interactions with a GT-specific nuclear factor. Mol Cell Biol. 1989 Nov;9(11):5248–5253. doi: 10.1128/mcb.9.11.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt B., Burns J., Flannery D., McGee J. O. Direct visualization of single copy genes on banded metaphase chromosomes by nonisotopic in situ hybridization. Nucleic Acids Res. 1988 May 11;16(9):3951–3961. doi: 10.1093/nar/16.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Tigges M. A., Minie M. E., Koshland M. E. A model system for peptide hormone action in differentiation: interleukin 2 induces a B lymphoma to transcribe the J chain gene. Cell. 1986 Nov 21;47(4):609–617. doi: 10.1016/0092-8674(86)90625-2. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J. The human interleukin-2 receptor. Annu Rev Immunol. 1986;4:69–95. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Robb R. J., Roth J. A., Neckers L. M., Lachman L. B., Wilson D. J., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983 Oct 1;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hög J. O., von Bahr-Lindström H., Hedén L. O., Holmquist B., Larsson K., Hempel J., Vallee B. L., Jörnvall H. Structure of the class II enzyme of human liver alcohol dehydrogenase: combined cDNA and protein sequence determination of the pi subunit. Biochemistry. 1987 Apr 7;26(7):1926–1932. doi: 10.1021/bi00381a021. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Deregulation of interleukin-2 receptor gene expression in HTLV-I-induced adult T-cell leukemia. Science. 1985 Jun 7;228(4704):1215–1217. doi: 10.1126/science.2988127. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Battey J., Hollis G. F., Swan D. C., Siebenlist U., Leder P. Localization of human variable and constant region immunoglobulin heavy chain genes on subtelomeric band q32 of chromosome 14. Nucleic Acids Res. 1982 Dec 20;10(24):8155–8170. doi: 10.1093/nar/10.24.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Hieter P. A., Hollis G. F., Swan D., Otey M. C., Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982 May 1;155(5):1480–1490. doi: 10.1084/jem.155.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Swan D. C., Tronick S. R., Gol R., Klimanis D., Moore D. E., Aaronson S. A. Regional chromosomal localization of N-ras, K-ras-1, K-ras-2 and myb oncogenes in human cells. Nucleic Acids Res. 1983 Dec 10;11(23):8221–8236. doi: 10.1093/nar/11.23.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Ray A., Sassone-Corsi P., Sehgal P. B. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol. 1989 Dec;9(12):5537–5547. doi: 10.1128/mcb.9.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Seigel L. J., Harper M. E., Wong-Staal F., Gallo R. C., Nash W. G., O'Brien S. J. Gene for T-cell growth factor: location on human chromosome 4q and feline chromosome B1. Science. 1984 Jan 13;223(4632):175–178. doi: 10.1126/science.6318318. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. Automated preparation of DNA sequences for publication. Nucleic Acids Res. 1986 Jan 10;14(1):65–73. doi: 10.1093/nar/14.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sharon M., Siegel J. P., Tosato G., Yodoi J., Gerrard T. L., Leonard W. J. The human interleukin 2 receptor beta chain (p70). Direct identification, partial purification, and patterns of expression on peripheral blood mononuclear cells. J Exp Med. 1988 Mar 1;167(3):1265–1270. doi: 10.1084/jem.167.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Singh L., Purdom I. F., Jones K. W. Effect of different denaturing agents on the detectability of specific DNA sequences of various base compositions by in situ hybridisation. Chromosoma. 1977 Apr 20;60(4):377–389. doi: 10.1007/BF00292860. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J. M., Kaneko Y., Mitelman F. Report of the committee on structural chromosome changes in neoplasia. Cytogenet Cell Genet. 1989;51(1-4):533–562. doi: 10.1159/000132807. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]