Abstract
Ypt/Rab GTPases control various aspects of vesicle formation and targeting via their diverse effectors. We report a new role for these GTPases in protein recycling through a novel effector. The F-box protein Rcy1, which mediates plasma membrane recycling, is identified here as a downstream effector of the Ypt31/32 GTPase pair because it binds active GTP-bound Ypt31/32 and colocalizes with these GTPases on late Golgi and endosomes. Furthermore, Ypt31/32 regulates the polarized localization and half-life of Rcy1. This suggests that Ypt/Rabs can regulate the protein level of their effectors, in addition to the established ways by which they control their effectors. We show that like Rcy1, Ypt31/32 regulate the coupled phosphorylation and recycling of the plasma membrane v-SNARE Snc1. Moreover, Ypt31/32 and Rcy1 regulate the recycling of the furin-homolog Kex2 to the Golgi. Therefore, Ypt31/32 and Rcy1 mediate endosome-to-Golgi transport, because this is the only step shared by Snc1 and Kex2. Finally, we show that Rcy1 physically interacts with Snc1. Based on this result and because F-box proteins serve as adaptors between specific substrates and ubiquitin ligases, we propose that Ypt31/32 GTPases regulate the function of Rcy1 in the phosphorylation and/or ubiquitination of proteins that recycle through the Golgi.
INTRODUCTION
Transport of proteins through intracellular membrane-bound compartments is a highly regulated process. In this process, cargo-carrying vesicles bud from one compartment and fuse with the next (Rothman, 1994). GTPases that belong to the Ypt/Rab family are key regulators of the various steps of the exporting (exocytic) and importing (endocytic) pathways (Segev, 2001c; Stenmark and Olkkonen, 2001). Like all other GTPases, Ypt/Rabs cycle between GTP-and GDP-bound forms. When in the GTP-bound form they interact with their downstream effectors. Through these effectors, Ypt/Rabs regulate the various aspects of vesicular transport: vesicle formation, motility and attachment, and membrane remodeling and fusion (Segev, 2001b). A single Ypt/Rab GTPase can interact with multiple effectors and thereby control numerous functions that involve one compartment (Pfeffer, 2001; Munro, 2002). Two modes by which Ypt/Rabs affect the function of their effectors have been suggested: recruitment of effectors to specific membranes and regulation of effector activity (Carroll et al., 2001; Segev, 2001a,b). In addition, we suggest here that Ypt/Rabs modulate effector protein stability.
The Ypt31/32 GTPases function as an essential pair. Neither is required for cell viability, but together they provide an essential function: exit from the yeast trans-Golgi (Jedd et al., 1997). Here, we show that this pair regulates another vesicular transport step that involves the trans-Golgi, that of recycling proteins through this compartment. This second function requires a new Ypt31/32 effector, Rcy1. Rcy1 is an F-box protein that was shown to be involved in the recycling of the v-SNARE Snc1 from endosomes to the plasma membrane (PM) (Galan et al., 2001). However, this study does not identify the transport step that is mediated by Rcy1: endosome to PM, endosome to Golgi, or Golgi to PM. We show here that Ypt31/32 and Rcy1 are required for recycling not only of Snc1 to the PM but also of Kex2, a trans-Golgi resident protein, to the Golgi. Based on these findings, we suggest that the step regulated by Ypt31/32 and Rcy1 is the transport step shared by recycling to the PM or the Golgi, namely, endosome to Golgi. Recycling of PM proteins is also a transport step regulated by the closest mammalian Ypt31/32 homolog, Rab11 (Ullrich et al., 1996; Green et al., 1997; Ren et al., 1998; Prekeris et al., 2000). This implies functional conservation between these homologous GTPases.
In addition, we show here that Rcy1 interacts with Snc1, and together with Ypt31/32, increases the phosphorylation level of Snc1, which correlates with the latter's recycling to the PM (Galan et al., 2001). F-box proteins are known to be components of ubiquitin–ligase complexes and serve as the selective adaptors that bind specific substrates (Kipreos and Pagano, 2000; Jackson and Eldridge, 2002). Through its N-terminal F-box domain, Rcy1 interacts with Skp1, a subunit of the Skp1-cullin-F-box protein (SCF) ubiquitin–ligase complex. However, Rcy1 does not seem to interact with the other subunits of the SCF complex (Galan et al., 2001). Therefore, if Rcy1 is involved in ubiquitination, it must be done through a non-SCF complex. Together, our results suggest that Ypt31/32 GTPases act through their effector, Rcy1, to mark proteins for recycling through the Golgi by phosphorylation and perhaps by SCF-independent ubiquitination. If true, this would expand the repertoire of Ypt/Rab effector functions to include protein sorting and perhaps posttranslational modification.
MATERIALS AND METHODS
Strains, Plasmids, and Reagents
Yeast strains used in this study are wild type (NSY125), ypt31Δ/32ts (NSY348), ypt31Δ/32ts pep4Δ (NSY355), rcy1Δ (NSY657, ura3–52; or NSY818, leu2), and rcy1Δ pep4Δ (NSY819). Green fluorescent protein (GFP)-Snc1 and GFP-Snc1-pem were integrated into the chromosome in wild type (NSY729), ypt31Δ/32ts (NSY733), and rcy1Δ (NSY737), as described previously (Lewis et al., 2000). Ypt31 (NSY938) was tagged on the chromosome at the N terminus with enhanced green fluorescent protein (EGFP) as described previously (Prein et al., 2000), by using a plasmid obtained from B. Prein (Technical University of Graz, Graz, Austria). Kex2 was tagged on the chromosome at the C terminus with yellow fluorescent protein (YFP) by using a polymerase chain reaction (PCR) product from the Kex2-YFP from the Yeast Resource Center (http://depts.washington.edu/~yeastrc/index.html) and homologous recombination. Strains containing Kex2-YFP are as follows: wild-type (NSY970), pep4Δ (NSY971), ypt31Δ/32ts (NSY972), and ypt31Δ/32ts pep4Δ (NSY973). PEP4 was deleted using the hygromycin B resistance gene hphMX3 (Goldstein and McCusker, 1999). Strains expressing red fluorescent protein (RFP)-tagged compartmental markers were described previously (Huh et al., 2003). The strain expressing Sec7-DsRed-p-tdimer (G19) was obtained from B. Glick (University of Chicago, Chicago, IL). The wild-type strain NSY 958 (MHY753) was used for colocalization of Ypt31 and GFP-Rcy1 with the DsRed-FYVE plasmid.
The following plasmids were used in this study: GFP-Rcy1 (pNS586) was constructed by cloning the PCR product of the Rcy1 open reading frame (ORF) into a GAL1 promoter-driven GFP vector (pTS408) (Carminati and Stearns, 1997). GST-Rcy1 (pNS705) was obtained from the yeast GST-ORF collection (Zhu et al., 2001). Kex2-HA expressing plasmid (URA and LEU marked) was obtained from S. Nothwehr (Nothwehr et al., 1995). The 2 μ plasmid expressing DsRed-FYVE domain under the MET3 promoter was obtained from S. Emr (Odorizzi et al., 1998). Strains and plasmids used in the two-hybrid assay are described in the next section.
Antibodies used in this study are anti-Ypt31/32 (Jedd et al., 1997), anti-glutathione S-transferase (GST) (Molecular Probes, Eugene, OR, for Western blot analysis, and Upstate Biotechnology, Lake Placid, NY, for immunoprecipitation), anti-Snc1 (Protopopov et al., 1993), anti-G6PDH (Sigma-Aldrich, St. Louis, MO), anti-HA for Kex2 (Covance, Berkeley, CA), anti-Emp47 (Schroder et al., 1995), and anti-GFP (BD Biosciences Clontech, Palo Alto, CA).
Yeast Two-Hybrid Screen and Assays
The two-hybrid screen in which Rcy1 was identified as a Ypt32 interactor used PJ69-4a cells expressing the Ypt32-C221S C222S (Ypt32-SS) from a GAL4-BD (pGBDU) vector (James et al., 1996). The cells were transformed with a cDNA library expressing yeast genes from the GAL4-AD (pACT2) plasmids (Harper et al., 1993). Transformants that grew on SD-HIS + 3 mM 3AT plates were isolated as potential Ypt32 interactors. They were tested for their ability to confer growth under more stringent conditions, SD–Ade, and their identity was determined by sequencing. The isolated plasmid containing the C-terminal half of RCY1 in pACT2 is pNS572.
The two-hybrid mating assay used MATa (PJ69-4a) cells expressing Rcy1 from a GAL4-AD (pACT2) plasmid and MATα (SL3004) cells expressing Ypts from GAL4-BD (pGBDU) plasmids. The full-length Rcy1 in the GAL4-AD vector was constructed by cloning the Rcy1 PCR fragment into the NotI and SacI sites of pACT2 plasmid (pNS640). The SS forms of Ypt1, Ypt6, Ypt31, Ypt32, and Sec4, analogous to Ypt32-C221S C222S, were cloned by PCR to the GAL4-BD vector (pNS 299, 293, 287, 288, and 294, respectively). The nucleotide-restricted forms of Ypt32-SS—Q72L, S27N, and N126I—were cloned by PCR into the GAL4-BD vector (pNS289, 290, and 291, respectively). Media used for the two-hybrid assays included SD-Ura-Leu for diploid mating control and SD-Ura-Leu-His + 3–5 mM 3AT or SD-Ura-Leu-Ade for testing interaction. The β-galactosidase liquid assays were done as described previously (Current Protocols in Molecular Biology, www.mrw.interscience.wiley.com/cp/cpmb/articles/mb1306/frame.html).
Western Blot and Fractionation Analyses of Yeast Cell Lysate
Wild-type and mutant yeast cells expressing Kex2-HA, GFP-Rcy1, GST-Rcy1, or GFP-Snc1 were grown at 26°C to mid-log phase (OD600 = 0.5–0.8) in synthetic medium (SM): SM-Ura or SM-Leu or SM-Leu-Ura supplemented with 2% glucose or 2% raffinose when induction was needed. Expression of GFP-Rcy1 was induced by 2% galactose for 5 h. When a shift to 37° was required, the cells were split and grown either at 26 or at 37°C for the indicated times. Typically, 4–8 OD units of cells were harvested at 3000 rpm and lysed by glass beads in Laemmli buffer or buffer 88 (Jones et al., 1998) at 4°C. Protein lysates were separated from unbroken cells and glass beads by centrifugation. Whole cell lysates were then subjected to 10–15% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and subjected to Western blot analysis using appropriate antibodies. Protein determination was done by RC/DC Lowry-based or Bio-Rad assay (Bio-Rad, Hercules, CA). Quantification of the bands was done using AlphaEase FC and Alpha-Imager (Alpha Innotech, San Leandro, CA). For fractionation analysis, cell lysates were centrifuged at 13,000 × g for 30 min to generate pellet P13 and supernatant fractions. P13 fractions were analyzed on iodixanol density gradients (Optiprep; Axis-Shield PoC AS, Oslo, Norway) according to recommended protocol from company.
Pulse-Chase Analysis
Pulse-chase analysis has been described previously by W. Tansey (http://tanseylab.cshl.edu/protocols.htmlü with the following modifications. Yeast cells were grown at 26°C in SM-Ura-Leu supplemented with 2% raffinose to mid-log phase, induced with 2% galactose, and shifted to 37°C for 2 h as mentioned above. Cells were then pulsed with 35S-Translabel in SM-Cys-Leu-Met-Ura supplemented with 2% galactose for 30 min at 37°C. Cells were chased with 1% Met/Cys and 100 μg/ml cycloheximide (www.mbb.yale.edu/fl/m_hochstrasser/protocols/P-C_IPprotocol.htm) for the indicated times. GST-Rcy1 and G6PDH were immunoprecipitated onto 50% Protein A (Zymed Laboratories, South San Francisco, CA) and protein G (Santa Cruz Biotechnology, Santa Cruz, CA) bead slurry with anti-GST and anti-G6PDH, respectively. Beads were resuspended in Laemmli buffer and boiled to elute proteins, which were then run on 10% SDS-PAGE. After gels were dried and exposed to a PhosphorImager plate, the quantification of bands was conducted using ImageQuant (Amersham Biosciences, Piscataway, NJ).
Coprecipitation from Yeast Cell Lysates
Cells containing GST or GST-Rcy1 expressed under the GAL1 promoter were induced with 2% galactose for 4 h at 30°C. The GST pull-down was done as described previously (Martzen et al., 1999) with the following modifications. Cells were harvested by centrifugation at 3000 rpm and washed once with ice-cold water. The cells were then broken with glass beads using the following extraction buffer: 50 mM Tris-Cl, pH 7.5, 1 mM EDTA, 4 mM MgCl2, 10% glycerol, 100 mM NaCl, and protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN). Lysates were incubated with a 50% glutathione bead slurry (Amersham Biosciences) for 4 h at 4°C with constant agitation. Beads were then washed with wash buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 4 mM MgCl2, 10% glycerol, and 0.1% Triton X-100). Beads were resuspended in Laemmli buffer and boiled to elute proteins. GST-Rcy1, Ypt31, and Snc1 were detected by Western blot analysis with the appropriate antibodies.
Fluorescence Microscopy
Direct fluorescence microscopy was used for localization of GFP-, RFP-, DsRed-, or YFP-tagged proteins in live cells. Cells expressing GFP-Rcy1 under the GAL1–10 promoter were grown to OD 0.1–0.2 in SD-Ura medium supplemented with 2% raffinose at 26°C. GFP-Rcy1 protein expression was induced by 2% galactose for 4 h. To polarize the cells, α-factor was added to a final concentration of 10 μg/ml, and cells were grown at 26°C for 2 h. Cells expressing GFP-Snc1 or GFP-Snc1-pem were grown in SD-Ura medium supplemented with 2% glucose to mid-log phase at 26°C. The cells were then split and continued to grow at 26°C or were shifted to 37°C for 90 min. Live cells were immobilized on slides by concanavalin A (Zheng et al., 1998) (Lewis et al., 2000) and observed under fluorescein isothiocyanate (FITC) optics. Indirect immunofluorescence microscopy and anti-Ypt31/32 antibodies were used for the localization of Ypt31/32 protein as described previously (Jedd et al., 1997).
For colocalization of Ypt31/32 and GFP-Rcy1, fixed cells were stained with rabbit anti-Ypt31/32 antibodies and secondary Texas Red-conjugated anti-rabbit antibody. The Ypt31/32 staining was viewed by the Texas Red filter. GFP-Rcy1 and EGFP-Ypt31 was detected using the FITC filter. RFP/DsRed-tagged compartmental markers were detected using a Texas Red filter. Actin was stained with rhodamine-phalloidin (Molecular Probes) as described previously (Pringle et al., 1989). FM4-64 (Molecular Probes) marking of endosomes and the vacuolar limiting membrane has been described previously (Vida and Emr, 1995). Pictures of cell sections (0.5 μm) were taken using a deconvolution Axioscope (Carl Zeiss, Thornwood, NY) microscope, and one section of a Z-series is shown. Cell fluorescence was quantified using Photoshop 7.0 as described previously (Blackwell et al., 2003), except that total cell fluorescence per cell area was used.
RESULTS
Rcy1 Interacts with Active Ypt31/32 GTPases
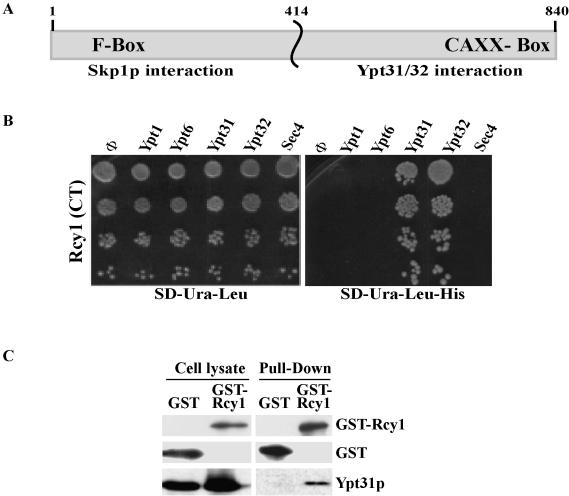
The C-terminal half of Rcy1 was identified as a Ypt32-GTPase interactor in a two-hybrid screen in which Ypt32-C221S C222S (Ypt32-SS) was used as a bait (see Materials and Methods). This form of the Ypt cannot be modified by lipid (Khosravi-Far et al., 1991), and therefore it is more likely to localize to the nucleus where the two-hybrid interaction occurs. The C-terminal half of Rcy1, from amino acid 414 to the end (amino acid 840), Rcy1-(414-C) (Figure 1A), was one of the strongest Ypt32 interators isolated in our screen (it was isolated once; 5 × 105 transformants were tested) and allowed growth on SD-His + 3AT as well as on SD-Ade media (Figures 1B and 2A).
Figure 1.
The C-terminal domain of Rcy1 specifically interacts with Ypt31/32 GTPases. (A) Schematic diagram of RCY1 domains. The N-terminal domain contains an F-box and interacts with Skp1 (Galan et al., 2001), whereas the C-terminal domain contains a CAAX box that has been implicated in membrane localization (Galan et al., 2001) and interacts with Ypt31/32 (shown here). (B) The C-terminal domain of Rcy1p (Rcy1-CT) interacts specifically with Ypt31 and Ypt32 in the yeast two-hybrid mating assay. Yeast MATa cells expressing Rcy1 from a GAL4-AD (LEU2) vector were mated with MATα cells expressing the different exocytic Ypts from GAL4-BD (URA3) plasmids: Ypt1, Ypt6, Ypt31, Ypt32, and Sec4. The SD-Ura-Leu medium (left) supports growth of all diploid cells, whereas growth on the SD-Ura-Leu-His medium (right) requires two-hybrid interaction. (C) Rcy1 coprecipitates with Ypt31/32 from yeast cell lysates. Lysates from yeast cells expressing GST (NSY794) or GST-Rcy1 (NSY624) under the GAL1 promoter were prepared. Glutathione beads were used to pull down GST or GST-Rcy1. Cell lysates (two left lanes, 25% of the cell lysate used for pull-down) and GST-pull down fraction (two right lanes) were analyzed by Western blot. GST proteins were detected using an affinity-purified polyclonal anti-GST antibody, and Ypt31/32 proteins were detected using a polyclonal anti-Ypt31/32 antibody. Results shown in this figure are representative of at least two experiments.
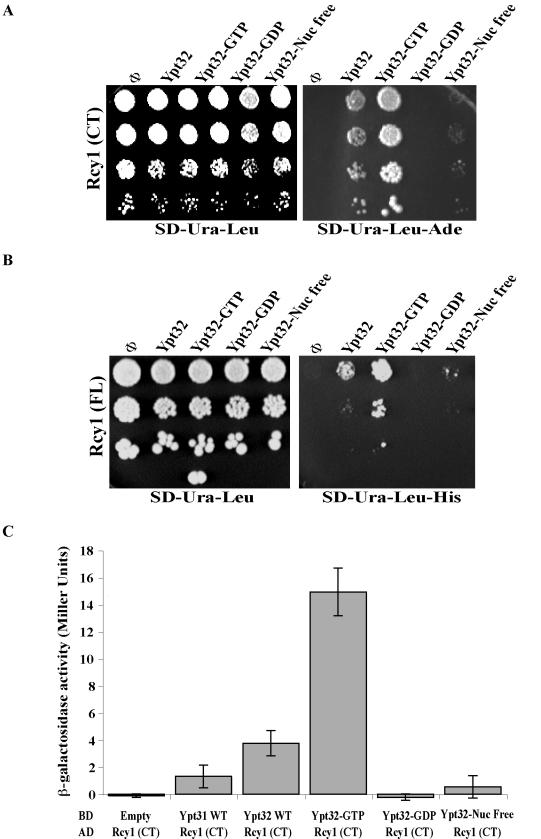
Figure 2.
Rcy1p interacts specifically with the GTP-bound form of Ypt31/32 GTPases. The C-terminal domain of Rcy1 (Rcy1-CT) (A) full-length Rcy1 (Rcy1-FL) (B) specifically interact with activated Ypt32 in the yeast two-hybrid mating plate assay. Yeast MATa cells expressing Rcy1 from the GAL4-AD (LEU2) vector were mated with MATα cells expressing the various nucleotide-bound forms of Ypt32 from GAL4-BD (URA3) plasmids: wild type, Q72L (GTP), S27D (GDP), and N126I (nucleotide free). Growth of the diploid cells on SD-Ura-Leu is shown on the left, whereas interaction is shown on the right as growth on SD-Ura-Leu-Ade in A, and SD-Ura-Leu-His in B (results shown in A and B are representative of at least two experiments). (C) The C-terminal domain of Rcy1 (Rcy1-CT) specifically interacts with Ypt32-GTP in the yeast two-hybrid quantitative assay. Diploid cells expressing GAL4-AD-Rcy1-CT and GAL4-BD-Ypts, Ypt31, and different nucleotide-bound forms of Ypt32 from A were tested for the strength of interactions by using a β-galactosidase liquid assay. The results represent two independent experiments; the error bars represent SD.
To determine whether the interaction of Rcy1 is specific to Ypt32, the plasmid identified in the screen was used for further two-hybrid analyses. MATa cells expressing Rcy1-(414-C) from the GAL4-activation domain plasmid were mated with MATα cells expressing the various Ypts from the GAL4-binding domain plasmid (see Materials and Methods). Rcy1-(414-C) interacts with both Ypt31 and Ypt32, but not with the other yeast exocytic Ypts—Ypt1, Sec4, and Ypt6 — even under the least stringent condition (SD-His) (Figure 1B). These results indicate that Rcy1-(414-C) interacts specifically with the Ypt31/32 pair.
To determine whether Rcy1 interacts preferentially with one of the nucleotide-bound forms of the Ypt32 GTPase, various Ypt32 mutants that are predicted to be restricted for nucleotide cycling were used in the two-hybrid assay. The strength of the interactions between Rcy1-(414-C) and wild type, GTP (Q72L), GDP (S27N), or nucleotide-free (N126I) forms of Ypt32 (Olkkonen and Stenmark, 1997) was compared. All these Ypt32 forms also contain the SS modification at the C terminus of Ypt32. Rcy1-(414-C) interacts most strongly with the GTP-bound form of Ypt32 as shown both by growth under the most stringent conditions (SD-Ade) and by β-galactosidase assays. This interaction is even stronger than the interaction with wild-type Ypt32 or Ypt31. Rcy1-(414-C) shows marginal interaction with the nucleotide-free mutant form but not with the GDP-bound form (Figure 2, A and C). These results indicate that Rcy1-(414-C) interacts preferentially with the GTP-bound form of Ypt32. Because specific interaction with the GTP-bound form of a GTPase is typical of all GTPase effectors, the two-hybrid results suggested that Rcy1 is an effector of Ypt31/32 GTPases.
Rcy1-(414-C) also interacts with wild-type Ypt32-C221 C222 (Ypt32-CC), but to a lesser extent than with the Ypt32-SS variant (our unpublished data). The Ypt-CC form can be modified by lipids and localizes to exocytic organelles. The lower level of interaction with the CC form is probably due to a smaller amount of the Ypt32-CC available for the two-hybrid interaction in the nucleus. This result indicates that Rcy1-(414-C) can interact with the lipid modified Ypt32. However, this lipid modification is not required for the interaction.
Two-hybrid analysis using full-length Rcy1 also shows interaction with wild-type and GTP-bound Ypt32, albeit to a lesser extent than that seen with only the C-terminal half of Rcy1 (Figure 2B). The strength of this interaction is similar to the interaction of Ypt32 with another effector, Sec2 (Ortiz et al., 2002). Both can be seen on SD-His plates (but not SD-Ade) and are below the level of detection of the β-galactosidase assay (our unpublished data). The full-length Rcy1, but not its C-terminal half, interacts with Skp1, as expected (Galan et al., 2001; our unpublished data). The weaker twohybrid interaction of Ypt32 with full-length Rcy1, than Ypt32 interaction with Rcy1-(414-C), might be due to a smaller level of the Rcy1 protein. Alternatively, other protein(s) might be involved in facilitating the interaction of the full-length Rcy1 with Ypt31/32.
An independent approach was used to test the interaction of Rcy1 with Ypt31/32. In a coprecipitation experiment, GST-tagged Rcy1 was used to determine whether full-length Rcy1 interacts with Ypt31/32 GTPases in yeast cells. The presence of Rcy1 and Ypt31/32 in yeast cell lysates and on glutathione-agarose beads was determined by Western blot analysis. As shown in Figure 1C, Ypt31/32 proteins are present in the crude lysate, and they coprecipitate with GST-Rcy1, but not with GST alone. These results show that full-length Rcy1 and Ypt31/32 GTPases interact with each other in vivo. This interaction occurs through the C-terminal half of Rcy1 and the GTP-bound form of Ypt31/32, as indicated by the two-hybrid analyses.
Ypt31/32 and Rcy1 Colocalize in Golgi and Endosomes
If Rcy1 does function as a Ypt31/32 effector, it should colocalize with this GTPase pair. Previous results suggest that the cellular localization of Rcy1 and Ypt31/32 is similar. Specifically, they were independently shown to exhibit a polarized localization to the growing bud (Jedd et al., 1997; Galan et al., 2001). However, because a number of transport organelles have been shown to be polarized to the bud, including the Golgi apparatus and trans-Golgi-derived vesicles (Byers, 1981; Segev et al., 1988) (Preuss et al., 1992), a more direct test of colocalization was needed. Fluorescence microscopy was used to determine whether Rcy1 and Ypt31/32 colocalize to the same organelle. Rcy1, tagged with GFP at its N terminus, was shown to be functional by complementation of the cold sensitivity of the rcy1Δ (Galan et al., 2001; our unpublished data). Cells were fixed and GFP-Rcy1 localization was determined (green). Ypt31/32 localization was visualized by immunofluorescence by using anti-Ypt31/32 antibodies (red). There is extensive overlap between the localization of Rcy1 and Ypt31/32 (Figure 3A). The result shows that the majority of Ypt31/32 and Rcy1 resides in the same compartment. The fact that this compartment seems punctate and is polarized to the growing bud suggests that it is either Golgi or endosomes. This colocalization is consistent with the supposition that Rcy1 is an interactor of Ypt31/32 GTPases.
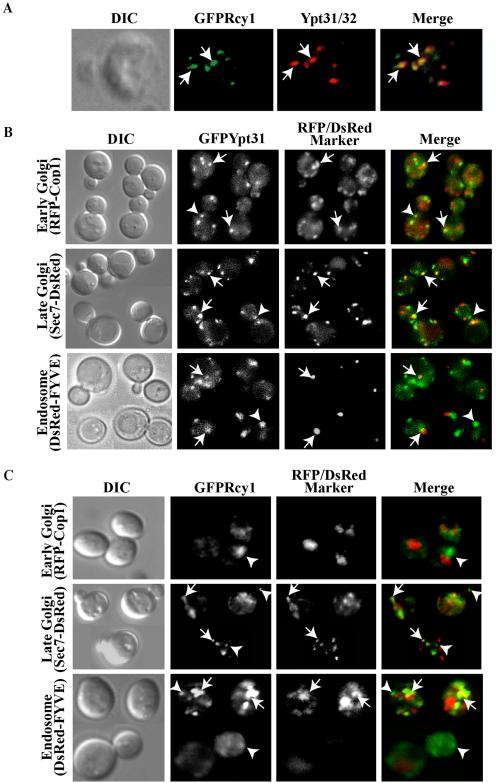
Figure 3.
Ypt31/32 colocalize with Rcy1, and they both reside on late Golgi and endosomes. (A) GFP-Rcy1 and Ypt31/32 colocalize. Wild-type cells (NSY125) expressing GFP-Rcy1 were grown at 26°C and synchronized using α-factor (see Materials and Methods). The cells were fixed and Ypt31/32 localization was viewed by indirect immunofluorescence microscopy by using affinity-purified anti-Ypt31/32 antibodies (red). GFP-Rcy1 was viewed using an FITC filter (green). (B) Ypt31 colocalizes with Golgi and endosomal markers. Cells expressing EGFP-Ypt31 and RFP-Cop1, Sec7-DsRED, or DsRED-FYVE were viewed by direct fluorescence microscopy. EGFP-Ypt31was viewed using an FITC filter (green) and RFP- or DsRed-tagged markers were viewed using a Texas Red filter (red). (C) Rcy1 colocalizes with late Golgi and endosomal markers. Cells expressing GFP-Rcy1 and RFP-Cop1, Sec7-DsRed, or DsRED-FYVE were viewed by direct fluorescence microscopy. GFP-Rcy1 was viewed using an FITC filter (green) and RFP- or DsRed-tagged markers were viewed using a Texas Red filter (red). The images in A, B, and C were deconvolved using a Zeiss Axioscope microscope. The merged image is shown on the right, and the contour of the cells is shown in the differential interference contrast (DIC) image. Arrows point to regions of colocalization, whereas arrowheads point to regions of Ypt31/32 or Rcy1 (green) that do not colocalize with the compartmental markers (red).
To determine the identity of the compartment(s) on which Ypt31/32 and Rcy1 reside, their colocalization with known Golgi and endosomal markers was determined by fluorescence microscopy. Ypt31 colocalize with an early Golgi marker, Cop1, but to a greater extent with a late Golgi marker, Sec7. They also colocalize with an endosomal marker, PI(3)P-FYVE domain (Figure 3B). GFP-Rcy1 colocalizes with a late but not early Golgi marker, as well as with an endosomal marker (Figure 3C). These results indicate that both Ypt31/32 and Rcy1 reside on the trans-Golgi and endosomes.
Ypt31/32 Regulate the Protein Level and Cellular Distribution of Rcy1
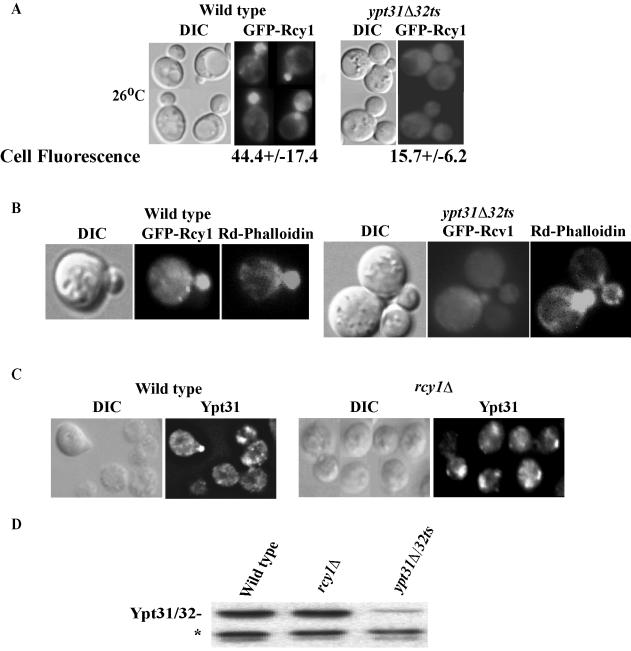
The C-terminal half of Rcy1 has been implicated in the protein's membrane localization (Galan et al., 2001). Because Ypt31/32 interact with this domain, and Ypt/Rabs are known to affect membrane recruitment of their effectors, we wished to determine whether Ypt31/32 affect the cellular localization of Rcy1. The localization of GFP-Rcy1 was followed by fluorescence microscopy in wild-type and ypt31Δ/32ts mutant cells. In wild-type cells, the localization of Rcy1 is mostly polarized to small buds in agreement to what was shown previously (Galan et al., 2001). In contrast, Rcy1 is not polarized to the buds of ypt31Δ/32ts mutant cells, even at the permissive temperature for this mutant (26°C). Rather, it is dispersed all over the cell (Figure 4A). At 37°C, the localization is similar (our unpublished data). In addition, the GFP-Rcy1 fluorescence is ∼3 times less intense in ypt31Δ/32ts mutant cells than in wild-type cells (Figure 4A, fluorescence panels). The polarized cellular localization of Rcy1 is dependent on the actin cytoskeleton (Galan et al., 2001). To determine whether the Rcy1 polarization defect in ypt31Δ/32ts mutant cells is an indirect result of a defect in the actin cytoskeleton, actin cellular distribution was tested in ypt31Δ/32ts mutant cells at the permissive temperature. Under these conditions, Rcy1 localization is still defective, but there is no effect on actin polarization (Figure 4B). Therefore, the defect in Rcy1 polarization in ypt31Δ/32ts mutant cells is independent of the actin cytoskeleton.
Figure 4.
Ypt31/32 affect the localization and the protein level of Rcy1 but not vice versa. (A) Ypt31/32 affect the localization of Rcy1. GFP-Rcy1 was expressed in wild-type (NSY125) and ypt31Δ/32ts mutant (NSY348) cells growing at 26°C. Cells were synchronized by incubation with α-factor (see Materials and Methods for details). GFP-Rcy1 protein localization was then examined by direct fluorescence microscopy. Bottom, cell fluorescence was quantified in Photoshop 7.0. Eleven random cells were quantified for each strain; ± represents the SEM. (B) Actin polarization is normal in ypt31Δ/32ts cells. Cells expressing GFP-Rcy1 were grown at 26°C, fixed, and stained with rhodamine-phalloidin, and then cells were examined by direct fluorescence microscopy. GFP-Rcy1 was viewed using an FITC filter and actin staining was viewed using a Texas Red filter. (C)Ypt31 localization is not disrupted in rcy1Δ mutants. Exponentially growing wild-type (NSY125) and rcy1Δ (NSY657) cells were fixed and Ypt31/32 localization was determined using affinity-purified anti-Ypt31p antibody and indirect immunofluorescence microscopy. In panels A–C, the contour of the cells is shown in the DIC image. (D) The level of Ypt31/32 is not affected by Rcy1. Protein level of Ypt31/32, in wild-type (NSY125), rcy1Δ (NSY657), and ypt31Δ/32ts (NSY348) cells was detected by Western blot analysis by using affinity-purified anti-Ypt31/32 antibody. Equal loading was confirmed by Ponceau S staining and a cross-reacting band that is present in ypt31Δ cells (*).
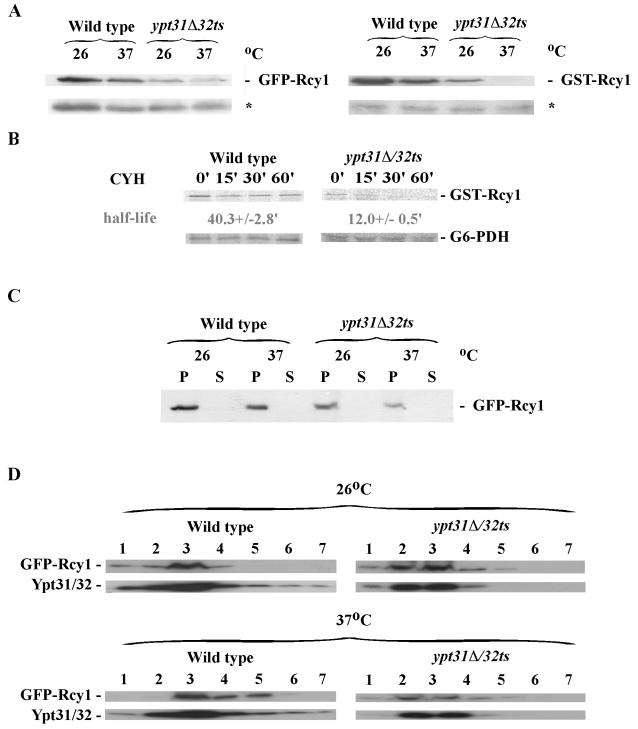
To determine whether the decreased GFP-Rcy1 fluorescence in the ypt31Δ/32ts mutant cells was due to a reduction in its protein level, we used immunoblot analysis. Western blots of whole-cell lysates probed with anti-GFP antibodies revealed that the level of GFP-Rcy1 protein is significantly reduced in ypt31Δ/32ts mutant cells, even at 26°C. Similar results were obtained with GST-Rcy1 (Figure 5A). To determine whether the diffused GFP-Rcy1 fluorescence seen in ypt31Δ/32ts mutant cells is caused by a defect in membrane attachment, cell fractionation analysis was performed. Figure 5C shows that GFP-Rcy1 protein is found in the P13 fraction of cell lysates derived from both the wild-type and mutant cells. Both GFP-Rcy1 and Ypt32 proteins, which are present in the P13 fraction from wild-type and ypt31Δ/32ts mutant cells, are found with membranes at the top of iodixanol density gradients (as opposed to forming aggregates, Figure 5D). Therefore, the lack of Rcy1 bud polarization in ypt31Δ/32ts cannot be attributed to lack of Rcy1 attachment to membranes. Instead, it might reflect defective polarization of the Rcy1-containing membranes to the bud.
Figure 5.
Ypt31/32 control the protein stability of Rcy1. (A) The level of Rcy1 protein is reduced in ypt31Δ32ts mutant cells. Wild-type (NSY125) and ypt31Δ/32ts mutant (NSY348) cells expressing GFP-Rcy1 (left) or GST-Rcy1 (right) were grown at 26°C and GFP- or GST-Rcy1 expression was induced for 5 h. After 4 h, half of each culture was shifted to 37°C for 1 h. Cell lysates were prepared and tested by Western blot analysis by using anti-GFP or anti-GST antibodies. Total cell lysate (100 μg, as determined by Bio-Rad assay) was loaded on each lane. Equal loading was confirmed by Ponceau S staining and a cross-reacting band that is present in cells containing the empty vector (*). (B) GST-Rcy1 is unstable in ypt31Δ/32ts mutant cells. Wild-type and mutant cells expressing GST-Rcy1 were grown as in A, except that cultures were shifted to 37°C 3 h after the induction of GST-Rcy1 expression. Cells were pulsed at 37°C with 35S-Translabel for 30 min, chased for the indicated times, and samples were analyzed as described in Materials and Methods. The half-life of GST-Rcy1 is indicated; ± represents the SEM. (C) GFP-Rcy1 protein is found in the P13 pellet fraction of wild-type and ypt31Δ/32ts mutant cells. Cell lysates were centrifuged at 13,000 × g for 30 min to generate pellet (P) and supernatant (S) fractions that were tested by Western blot analysis by using anti-GFP antibody. The results shown in this figure are representative of three experiments. (D) Rcy1 and Ypt31/32 in the P13 fraction are associated with membranes. P13 fractions from part C were analyzed on iodixanol density gradients; gradient fractions were collected and tested by Western blot analysis. Membranes fractionate to the top of the gradient. The results shown in this figure are representative of at least two independent experiments.
A decrease in the steady-state level of a protein can result from an increase in its turnover rate. To test this possibility, the turnover rate of GST-Rcy1 in wild-type and ypt31Δ/32ts mutant cells was compared using pulse-chase (Figure 5B) and cycloheximide-chase analyses (our unpublished data). The half-life of Rcy1 is ∼3 times shorter in ypt31Δ/32ts mutant cells than in wild type. These results indicate that Ypt31/32 regulate the level of Rcy1 by affecting its stability.
Ypt/Rabs are expected to affect their effectors and not vice versa. If Rcy1 is a Ypt31/32 effector, deletion of RCY1 should not affect the localization of Ypt31/32. To confirm this, the localization of Ypt31/32 in wild-type and rcy1Δ cells was determined using indirect immunofluorescence microscopy and anti-Ypt31/32 antibodies. We observed no significant differences in the localization (Figure 4C) or the protein level (Figure 4D) of Ypt31/32 in wild-type and rcy1Δ cells. Together, these results indicate that Ypt31/32 affect the level and overall cellular localization of Rcy1, whereas Rcy1 does not have such effects on Ypt31/32. These data further support the idea that Rcy1 is a downstream effector of Ypt31/32 GTPases.
Ypt31/32 and Rcy1 Regulate Recycling through the Golgi
The Ypt31/32 essential pair was previously shown to be required for exit from the Golgi (Benli et al., 1996; Jedd et al., 1997). On the other hand, Rcy1 is not essential for cell viability and was shown to have a role in recycling of GFP-Snc1 to the plasma membrane (Wiederkehr et al., 2000; Galan et al., 2001). However, the specific Rcy1-mediated transport step in recycling to the PM (i.e., endosome to PM, endosome to Golgi, or Golgi to PM) was not identified. We and others have suggested that Ypt/Rabs are compartment specific but can regulate more than one transport step involving that compartment (Jedd et al., 1995; Gerrard et al., 2000; Segev, 2001c). If Rcy1 is a downstream effector of Ypt31/32, the proteins should have a common function that is not essential for cell viability and involves the Golgi. Such a step might be recycling through the Golgi. Therefore, we asked whether Ypt31/32 and Rcy1 affect the recycling of the Golgi resident protein Kex2 and whether Ypt31/32, like Rcy1, affect the Golgi-to-PM recycling of the v-SNARE Snc1.
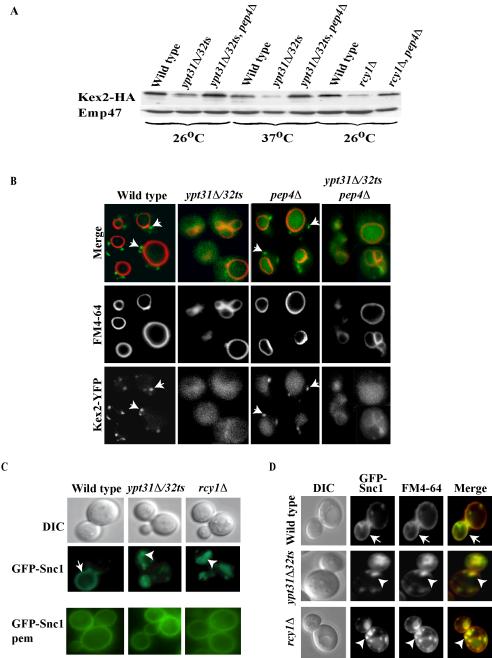
Kex2 is a furin homolog that resides in the Golgi. This protein was shown to cycle between the trans-Golgi and early endosomes. Proteins implicated in this cycling are generally not essential for cell viability (Brickner and Fuller, 1997) (Nothwehr et al., 1996; Luo and Chang, 1997). Failure in Golgi retention or recycling from early endosomes results in transport of Kex2 to the vacuole, where it is degraded by vacuolar proteases (Wilcox et al., 1992). The stability of hemagglutinin (HA)-tagged Kex2 was followed by Western blot analysis. The level of Kex2 is significantly reduced in ypt31Δ/32ts (26°C, and more pronounced at 37°C) and rcy1Δ, relative to wild-type cells. This reduction can be suppressed by the deletion of PEP4 (Figure 6A), which encodes a vacuolar protease that regulates the activation of the other vacuolar proteases (Jones et al., 1982). This Kex2 stability analysis suggests that in ypt31/32 and rcy1 mutant cells, Kex2 is not recycled effectively from early endosomes to the Golgi, but instead is mislocalized to the vacuole.
Figure 6.
Ypt31/32 and Rcy1 play a role in the recycling of the Kex2 and Snc1 proteins. (A) Ypt31/32 and Rcy1 affect Kex2 stability. Wild-type (NSY125), ypt31Δ/32ts (NSY348), ypt31Δ/32ts pep4Δ (NSY355), rcy1Δ (NSY818), and rcy1Δ pep4Δ (NSY819) cells expressing HA-tagged Kex2 protein were grown entirely at 26°C or shifted to 37°C for 90 min before harvest as indicated. Cell lysates were prepared and Kex2 protein level was measured by Western blot analysis by using anti-HA antibodies. Total protein determination and Emp47 were used as loading and blotting controls. Typical results representing five experiments are shown. (B) Microscopic assay showing that Ypt31/32 GTPases are important for the recycling of Kex2 to the Golgi. Wild-type (NSY970), pep4Δ (NSY971), ypt31Δ/32ts (NSY972), and ypt31Δ/32ts pep4Δ (NSY973) cells expressing endogenous Kex2 tagged with YFP on the chromosome were grown at 26°C. FM4-64 was internalized for 30 min to mark the vacuolar membrane. Intracellular localization was determined by direct fluorescence using an FITC filter for Kex2-YFP and Texas Red filter for FM4-64. Kex2-YFP in the Golgi is seen as green dots outside the vacuolar ring (arrows) in wild-type but not mutant cells. (C) Ypt31/32 and Rcy1 are involved in Snc1 recycling to the plasma membrane. Wild-type (NSY729), ypt31Δ/32ts (NSY733), and rcy1Δ (NSY737) cells expressing GFP-tagged Snc1 protein were grown at 26°C. GFP-Snc1 intracellular localization was determined by direct fluorescence by using an FITC filter. GFP-Snc1-pem, which cannot be internalized, is shown at the bottom. This form of Snc1 localizes to the PM in wild-type and mutant cells. (D) Snc1 localizes to early endosomes in ypt31Δ/32ts and rcy1Δ mutant cells. Cells were grown and treated as in C, except that early endosomes were marked by FM4-64 internalized for 5 min (some FM4-64 is still on the plasma membrane after such a short pulse). In C and D, arrows indicate PM staining, and arrowheads point to internalized GFP-Snc1; the contour of the cells is shown in the DIC image.
The mislocalization of Kex2 in ypt31Δ/32ts mutant cells was tested directly using a novel live cell microscopy assay for Kex2-YFP (Figure 6B). This assay shows directly the block in Kex2 retention or recycling, as opposed to inferring the block from the reduction in Kex2 stability in the steadystate assay, which is an indirect measure of its retention or recycling. In wild-type cells, Kex2-YFP is present in Golgi punctae, and in pep4Δ cells, it also accumulates in the vacuole. In mutant cells, Kex2-YFP is not seen in the Golgi punctae, but instead it is found in the vacuole (more pronounced in the ypt31Δ/32ts pep4Δ mutant).
The recycling of GFP-Snc1 was followed by fluorescence microscopy. In wild-type cells, this marker is localized mainly to the plasma membrane of the growing bud (Lewis et al., 2000). In ypt31Δ/32ts mutant cells, and as was previously shown for rcy1Δ cells (Galan et al., 2001), GFP-Snc1 is mostly internalized even at the permissive temperature (Figure 6C). To verify that GFP-Snc1 can reach the PM in mutant cells, GFP-Snc1-pem was used. This mutant form of Snc1 can reach the PM, but it cannot be internalized (Lewis et al., 2000). GFP-Snc1-pem is localized to the PM in wild-type as well as in ypt31Δ/32ts and rcy1Δ mutant cells (Figure 6C, bottom). These observations indicate that Ypt31/32 and Rcy1 are involved in the recycling of Snc1 from an intracellular compartment to the plasma membrane. This compartment is probably the early endosome, based on the complete colocalization of the internalized Snc1 with the fluorescent dye FM4-64 (Figure 6D), which stains early endosomes after a short incubation (Vida and Emr, 1995). Together, these results show a role for Ypt31/32 and Rcy1 in the recycling of two proteins that recycle through the Golgi, Kex2 and Snc1.
Snc1 Phosphorylation Is Regulated by Ypt31/32
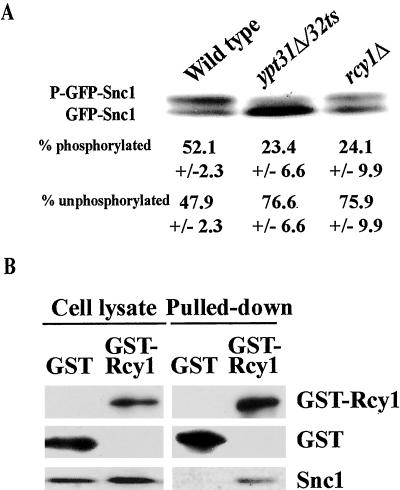
The phosphorylation state of Snc1 has been shown to correlate with its recycling. Specifically, Snc1 is phosphorylated when on the plasma membrane, but the unphosphorylated form is predominant when inside the cell. The two forms can be separated by gel electrophoresis. The abundance of the higher molecular weight phosphorylated Snc1 was previously shown to be decreased in rcy1Δ cells (Galan et al., 2001). An even lower level is observed in ypt31Δ/32ts mutant cells (Figure 7A). These results suggest that Ypt31/32, like Rcy1, regulate the phosphorylation state of Snc1.
Figure 7.
Rcy1 interacts with Snc1 and together with Ypt31/32 affects the level of Snc1 phosphorylation. (A) Ypt31/32 and Rcy1 affect the phosphorylation state of Snc1. Wild-type and mutant cells expressing GFP-Snc1 were grown at 26°C. Cell lysates were prepared and assayed by Western blot analysis by using anti-Snc1 antibodies. The higher molecular weight band is the phosphorylated form of Snc1, whereas the lower molecular weight band is the unphosphorylated form (Galan et al., 2001). The bands were quantified using an Alpha Imager, and the percentage of phosphorylated Snc1 averaged from three independent experiments is indicated; ± represents the SEM. (B) Rcy1 interacts with Snc1. Cell lysates were prepared from wild-type cells expressing GST (NSY784) or GST-Rcy1 (NSY624) as described in Materials and Methods. Cell lysates (two left lanes, 25% of the cell lysate used for pull-down) and GST-pull down fraction (two right lanes) were assayed by Western blot analysis. GST proteins were detected using a polyclonal anti-GST antibody, and Snc1 protein was detected using a polyclonal anti-Snc1 antibody. The results shown are representative of two experiments. Snc1 occurs as two bands in A and one band in B because the separation of the two bands requires electrophoresis in a high percent gel (15%) for a long period (overnight at 4°C), which was not done in B.
Rcy1 Interacts with Its Recycling Substrate Snc1
Rcy1 contains an F-box domain and has been shown to interact with Skp1. Skp1 is a subunit of the ubiquitin ligase SCF complex, although Rcy1 does not seem to be part of this specific complex (Galan et al., 2001). F-box proteins have been shown to physically interact with protein substrates that are modified by ubiquitin ligases (Kipreos and Pagano, 2000; Jackson and Eldridge, 2002). Because Rcy1 is required for the efficient phosphorylation and recycling of Snc1, we wished to determine whether Rcy1 physically interacts with Snc1. GST-Rcy1 was pulled down from yeast cell lysates, and the presence of Snc1 was tested by immunoblot analysis. As shown in Figure 7B, Snc1 is detected in the GST-Rcy1 pull-down, but not in the GST control. The physical interaction between the F-box protein Rcy1 and Snc1, whose PM recycling is dependent on Rcy1, together with the fact that F-box proteins are known to interact with substrates of ubiquitin ligases for posttranslational modification, suggest that Snc1 serves as a substrate for an Rcy1-dependent protein modification.
DISCUSSION
Results shown here point to four new aspects of Ypt31/32 and Rcy1 function. First, we show that Ypt31/32 are upstream regulators of Rcy1. Second, we suggest regulation of protein level as a new way by which Ypt/Rab GTPases influence their effectors. Third, we determine the intracellular localization of Ypt31/32 and Rcy1, and the transport step mediated by them. Finally, we show direct interaction of Rcy1 with Snc1, whose transport is mediated by Rcy1. This last aspect allows us to propose a model for the mechanism of action of Rcy1. This model suggests a novel paradigm for Ypt/Rab GTPase function, in marking of proteins for recycling.
Rcy1 Is a New Effector of Ypt31/32 GTPases
We show here that Rcy1 is a novel Ypt31/32 effector that mediates the Ypt31/32 GTPases function in recycling through the Golgi. Ypt/Rab effectors are defined as proteins that interact with the active, GTP-bound form of Ypt/Rabs and function downstream of these GTPases. Rcy1 meets these criteria. First, it interacts preferentially with the active GTP-bound form of Ypt31/32, and the interaction is specific to this GTPase pair. Second, it colocalizes with these GTPases. Third, Ypt31/32 affect the level and the localization of Rcy1, but not vice versa, indicating that Rcy1 functions downstream of Ypt31/32. Fourth, Ypt31/32 and Rcy1 share a function in regulating the recycling of proteins through the Golgi.
A connection between Rcy1 and another Ypt has been recently reported based on genetic evidence: overexpression of the active form of Ypt6, or deletion of its proposed GAP, Gyp2, suppresses the recycling defect of rcy1Δ cells (Lafourcade et al., 2003). These genetic interactions imply that Ypt6 plays a role in a pathway parallel to that of the Ypt31/32–Rcy1 module. Alternatively, these data are consistent with the existence of a GTPase cascade in which Ypt6 functions downstream of the Ypt31/32–Rcy1 module.
Ypt31/32 GTPases Affect Rcy1 Protein Level
Two mechanisms have been proposed to explain how Ypt/Rab GTPases regulate their effectors: recruitment to specific membranes and control of downstream effector interactions (Carroll et al., 2001; Pfeffer, 2001; Segev, 2001a,2001b). Here, we propose a novel mechanism by which Ypt/Rabs regulate their effectors: regulation of effector protein stability. This suggestion is based on the observation that in ypt31/32 mutant cells the level and the stability of Rcy1 protein are lower than in wild-type cells. Three independent ways were used to show that the level of Rcy1 is significantly reduced in ypt31/32 mutant cells: cellular fluorescence, Western blot, and pulse-chase analyses. The half-life of Rcy1 is 3 times shorter in ypt31/32 mutant cells than in wild-type cells, as determined by steady-state and pulse-chase analyses.
Rcy1 has a C-terminal CAAX motif that is essential for its proper cellular localization and function (Galan et al., 2001). Proteins with CAAX motifs are usually prenylated and this lipid modification is required for their membrane attachment (Del Villar et al., 1996). Prenylated proteins, like the Ypt/Rabs, can cycle between membranes and the cytosol (Segev, 2001c; Munro, 2002). However, it is not known whether this is true for Rcy1. The modulation of effector protein level could be linked to recruitment to membranes if the soluble effector is more prone to degradation. In this scenario, Ypt31/32 control Rcy1 protein level by recruiting it to membranes where it is stabilized. The decrease in Rcy1 protein level in ypt31/32 mutant cells in this model results from a defect in the recruitment of Rcy1 to the correct membranes and degradation of the soluble Rcy1. Alternatively, it is possible that the modulation of effector protein level by Ypt/Rabs is not coupled with membrane recruitment, but that it is more direct.
In addition to the lower level of Rcy1, the intracellular localization pattern of the remaining Rcy1 is abnormal in ypt31/32 mutant cells. Specifically, it is not polarized to the growing bud, but instead it is distributed throughout the whole cell. However, we suggest that this lack of polarization does not reflect a defect in Rcy1 recruitment to membranes because Rcy1 is found in the membrane fraction even in ypt31/32 mutant cells. The lack of Rcy1 polarization probably results indirectly from a defect in the polarization of the Rcy1-containing compartment, Golgi, and/or endosomes in ypt31/32 mutant cells. However, the Rcy1 polarization defect might not be linked to Ypt31/32-Rcy1–mediated recycling through the Golgi, because Ypt31/32 GTPases have at least one other function: exit of PM-destined vesicles from the trans-Golgi. Although actin is required for Rcy1 polarization (Galan et al., 2001), the polarization defect in ypt31/32 mutant cells is not due to a defect in actin polarization.
Ypt31/32 and Rcy1 Regulate Recycling through the Golgi
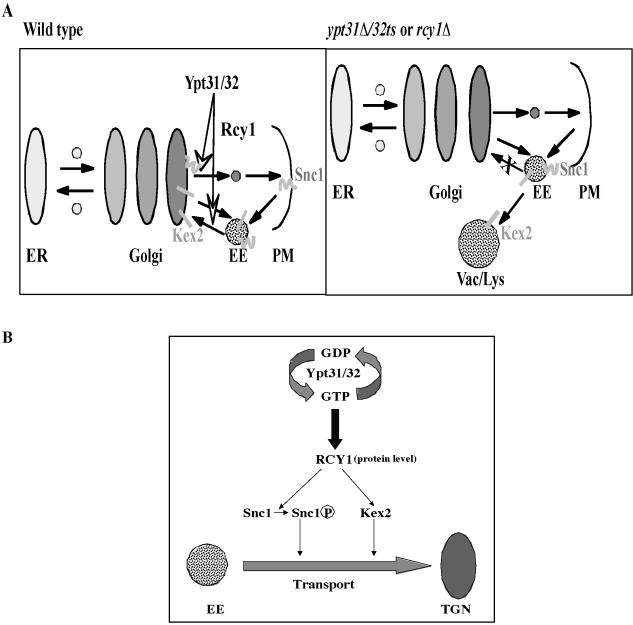
We previously suggested that a single Ypt/Rab GTPase can regulate multiple transport steps that involve a specific compartment (Jedd et al., 1995; Segev, 2001c). Ypt31/32 GTPases have been shown to be required for exit of trans-Golgi vesicles (Jedd et al., 1997). Here, we suggest that, together with their effector Rcy1, they play an additional role that also involves the Golgi: sorting or marking proteins that are recycled through the Golgi. This suggestion is based on the observations that the recycling of two proteins is defective in ypt31/32 and rcy1 mutant cells: the Golgi resident protein Kex2 and the trans-Golgi-to-PM v-SNARE Snc1. Kex2 cycles between the Golgi and early endosomes. When the retention or the recycling of Kex2 is blocked, it is transported to the vacuole where it is rapidly degraded (Wilcox et al., 1992; Holthuis et al., 1998; Spelbrink and Nothwehr, 1999). The Snc1/2 pair is required for fusion of trans-Golgi–derived vesicles with the PM (Protopopov et al., 1993), and it is transported back from early endosomes, probably through the trans-Golgi (Lewis et al., 2000). Because Ypt31/32 and Rcy1 affect the recycling of both Kex2 and Snc1, the simplest explanation is that they regulate a transport step that is common for the recycling of the two markers, which is endosome to Golgi (Figure 8A).
Figure 8.
Proposed role of Ypt31/32 GTPases in the regulation of protein recycling through the Golgi. (A) Model of the transport step regulated by Ypt31/32 and Rcy1. We suggest that Ypt31/32 and its effector Rcy1 regulate endosome-to-Golgi recycling. In wild-type cells (left), Kex2 cycles between the trans-Golgi and early endosomes, whereas the v-SNARE Snc1 is endocytosed into early endosomes and is transported back to the PM via the trans-Golgi. In ypt31Δ/32ts and rcy1Δ mutant cells (right), endosome-to-Golgi transport is blocked. In these cells, Kex2 is transported to the vacuole where it is degraded, whereas most of the Snc1 accumulates in early endosomes. (B) Model of the mechanism by which the Ypt31/32 GTPases and their effector Rcy1 regulate the sorting and marking of proteins that are destined to be transported through the Golgi. In this model, Ypt31/32 GTPases, in their active form, bind their effector Rcy1 and modulate its protein level. They may also regulate its membrane recruitment and/or activity. The Rcy1 F-box protein, in turn, binds the recycling proteins Snc1 and Kex2 and mediates their marking for recycling by phosphorylation. This phosphorylation might be followed by ubiquitination via a non-SCF complex.
An alternative explanation for our results is that, together, Ypt31/32 and Rcy1 regulate two independent transport steps: Golgi to endosome for Kex2 and endosome to Golgi for Snc1. This explanation is based on the suggestion that transport of Kex2 to the vacuole in certain mutants can be triggered by increased Golgi-to-endosome transport, if the function of the regulator is to prevent rapid exit from the Golgi (Ha et al., 2001). Regardless of the transport step regulated by Ypt31/32 and Rcy1, they seem to have a role in the sorting, and probably the marking, of recycling proteins. This latter idea is based on the findings that Rcy1 interacts with Snc1 and that Ypt31/32 and Rcy1 affect Snc1's phosphorylation state (see below).
In agreement with a role for Ypt31/32 and Rcy1 in transport between late Golgi and endosomes, the two proteins reside in these two compartments, as determined by their partial colocalization with known compartmental markers. However, it is still unclear whether Ypt31/32 and Rcy1 function in protein sorting in both these compartments, or only in one of them. We favor the possibility that Ypt31/32 and Rcy1 function in the late Golgi because Ypt31/32 have another role in transport from this compartment, formation of trans-Golgi-to-PM vesicles, and Ypt/Rab GTPases are thought to be compartment specific (Pfeffer, 2001; Segev, 2001c). In such a case, the Ypt31/32–Rcy1 role in protein recycling to the Golgi will be executed at the Golgi, for example, by marking proteins for recycling before they leave the Golgi (see below).
Mechanism of Ypt31/32 and Rcy1 Action
Ypt/Rab GTPases regulate all the known aspects of vesicle transport. These processes range from vesicle formation, motility, and attachment to membrane remodeling and fusion (Segev, 2001b). Here, we propose a function for Ypt/Rab GTPases that is not in their known repertoire. This novel function is regulation of the sorting, and perhaps the marking, of proteins destined for recycling through the Golgi. This idea is based on the fact that Rcy1 is an F-box protein, and proteins that belong to this group couple the interaction of specific substrates with ubiquitin ligases. Rcy1 interacts with at least one known component of the SCF-ubiquitin-ligase Skp1. Two pieces of evidence support the Rcy1-mediated protein-marking idea. First, Rcy1 interacts with the substrate Snc1, as shown by the GST-pull-down experiment (Figure 7B). Second, both Ypt31/32 and Rcy1 affect the phosphorylation state of the recycling proteins (Figure 7A). The phosphorylation state of Snc1 correlates with its recycling: the phosphorylated form is found on the PM, whereas the nonphosphorylated form is found inside the cell (not on the PM) (Galan et al., 2001). Together, our results suggest that Ypt31/32 act through their effector Rcy1 to regulate the level of Snc1 phosphorylation and thereby affect the recycling of Snc1 from an intracellular compartment to the plasma membrane (Figure 8B).
How do Ypt31/32 and Rcy1 mark proteins for recycling through the Golgi? One possibility is that the recycling proteins are marked by phosphorylation. Both the yeast Snc1 and the mammalian Kex2 homolog furin are phosphorylated. The phosphorylation of Snc1 is correlated with its recycling, and furin phosphorylation is known to influence its recycling (Galan et al., 2001) (Jones et al., 1995). The phosphorylation state of Snc1 is dependent on Ypt31/32 and Rcy1, as shown by the reduced level of phosphorylated GFP-Snc1 in ypt31/32 or rcy1 mutant cells. This suggests that Ypt31/32 and Rcy1 are required for efficient phosphorylation, or inhibition of dephosphorylation, and thereby mark proteins for endosome-to-Golgi transport.
An alternative idea is based on the facts that F-box proteins are known to be involved in ubiquitination of proteins and that phosphorylation can be a prerequisite for ubiquitination (Hicke et al., 1998). Snc1 was recently identified as a ubiquitinated protein in a large-scale yeast proteomic screen (Peng et al., 2003). Ubiquitination usually leads to degradation of proteins either by the proteosome or by transport to the lysosome/vacuole (Hicke, 2001; Weissman, 2001). However, a number of cases are known for ubiquitination that does not lead to degradation (Hoege et al., 2002; Ostendorff et al., 2002; Sun and Allis, 2002). Rcy1 was shown to interact with Skp1, but not in the context of the known ubiquitin ligase complex SCF (Galan et al., 2001). Nevertheless, it is possible that a non-SCF complex to which Rcy1 binds is involved in ubiquitination. In this case, ubiquitination of proteins by this complex would lead to their recycling rather than to their degradation. If ubiquitination were involved, it would constitute an extension of the role for ubiquitin-based recycling of PM proteins to include recycling of proteins to the Golgi. Regardless of whether the recycling proteins are marked by phosphorylation and/or ubiquitination, this implies a novel role for Ypt/Rab GTPases in the regulation of cargo sorting and marking. Further studies are required for the elucidation of the mechanism by which Ypt31/32 and Rcy1 mediate marking of proteins for recycling.
Acknowledgments
We thank S. Elledge for the two-hybrid cDNA library and plasmids; P. James, S. Lemon, S. Fields, and T. Hazbun for two-hybrid strains and plasmids; YeastResource Center for plasmids and strains; M. Snyder for the GST-ORF collection; J. Gerst for anti-Snc1 antibody; H. Riezman for anti-Emp47 antibody; M. Lewis and H. Pelham for GFP-Snc1 plasmids; S. Nothwehr for Kex2-HA plasmids; E. O'Shea for the RFP-tagged compartmental marker strains; B. Prein for the EGFP plasmid; S. Emr for the DsRed-FYVE plasmid; B. Glick and C. Reinke for the Sec7-DsRed strain; and M. Hochstrasser for the wild-type yeast strain MHY753. We thank N. Dejesus for technical help with yeast-two hybrid assays; T. Orenic for help with the deconvolution microscope; and D. Stone, C. Palfrey, and A. Serachitopol for critical reading of the manuscript. This research was supported by grant GM-45444 from the National Institutes of Health to N. S.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–03–0258. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0258.
References
- Benli, M., Doring, F., Robinson, D. G., Yang, X., and Gallwitz, D. (1996). Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15, 6460-6475. [PMC free article] [PubMed] [Google Scholar]
- Blackwell, E., Halatek, I. M., Kim, H. J., Ellicott, A. T., Obukhov, A. A., and Stone, D. E. (2003). Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol. Cell. Biol. 23, 1135-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner, J. H., and Fuller, R. S. (1997). SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J. Cell Biol. 139, 23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers, B. (1981). Cytology of the yeast life cycle. In: The Molecular Biology of the Yeast Saccharomyces, ed. E.J.J.N. Strathern, and J. Broach, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 59-96.
- Carminati, J. L., and Stearns, T. (1997). Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, K. S., Hanna, J., Simon, I., Krise, J., Barbero, P., and Pfeffer, S. R. (2001). Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292, 1373-1376. [DOI] [PubMed] [Google Scholar]
- Del Villar, K., Dorin, D., Sattler, I., Urano, J., Poullet, P., Robinson, N., Mitsuzawa, H., and Tamanoi, F. (1996). C-terminal motifs found in Ras-superfamily G-proteins: CAAX and C-seven motifs. Biochem. Soc. Trans. 24, 709-713. [DOI] [PubMed] [Google Scholar]
- Galan, J. M., Wiederkehr, A., Seol, J. H., Haguenauer-Tsapis, R., Deshaies, R. J., Riezman, H., and Peter, M. (2001). Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 21, 3105-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard, S. R., Bryant, N. J., and Stevens, T. H. (2000). VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol. Biol. Cell 11, 613-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. L., and McCusker, J. H. (1999). Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541-1553. [DOI] [PubMed] [Google Scholar]
- Green, E. G., Ramm, E., Riley, N. M., Spiro, D. J., Goldenring, J. R., and Wessling-Resnick, M. (1997). Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem. Biophys. Res. Commun. 239, 612-616. [DOI] [PubMed] [Google Scholar]
- Ha, S. A., Bunch, J. T., Hama, H., DeWald, D. B., and Nothwehr, S. F. (2001). A novel mechanism for localizing membrane proteins to yeast trans-Golgi network requires function of synaptojanin-like protein. Mol. Biol. Cell 12, 3175-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K., and Elledge, S. J. (1993). The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805-816. [DOI] [PubMed] [Google Scholar]
- Hicke, L. (2001). Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2, 195-201. [DOI] [PubMed] [Google Scholar]
- Hicke, L., Zanolari, B., and Riezman, H. (1998). Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J. Cell Biol. 141, 349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege, C., Pfander, B., Moldovan, G. L., Pyrowolakis, G., and Jentsch, S. (2002). RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135-141. [DOI] [PubMed] [Google Scholar]
- Holthuis, J. C., Nichols, B. J., Dhruvakumar, S., and Pelham, H. R. (1998). Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17, 113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, W. K., Falvo, J. V., Gerke, L. C., Carroll, A. S., Howson, R. W., Weissman, J. S., and O'Shea, E. K. (2003). Global analysis of protein localization in budding yeast. Nature 425, 686-691. [DOI] [PubMed] [Google Scholar]
- Jackson, P. K., and Eldridge, A. G. (2002). The SCF ubiquitin ligase: an extended look. Mol. Cell 9, 923-925. [DOI] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E. A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., Mulholland, J., and Segev, N. (1997). Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137, 563-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., Richardson, C., Litt, R., and Segev, N. (1995). The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J. Cell Biol. 131, 583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B. G., Thomas, L., Molloy, S. S., Thulin, C. D., Fry, M. D., Walsh, K. A., and Thomas, G. (1995). Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14, 5869-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. W., Zubenko, G. S., and Parker, R. R. (1982). PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics 102, 665-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S., Richardson, C. J., Litt, R. J., and Segev, N. (1998). Identification of regulators for Ypt1 GTPase nucleotide cycling. Mol. Biol. Cell 9, 2819-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far, R., Lutz, R. J., Cox, A. D., Conroy, L., Bourne, J. R., Sinensky, M., Balch, W. E., Buss, J. E., and Der, C. J. (1991). Isoprenoid modification of Rab proteins terminating in CC or CXC motifs. Proc. Natl. Acad. Sci. USA 88, 6264-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos, E. T., and Pagano, M. (2000). The F-box protein family. Genome. Biol. Available online at http://genomebiology.com/2000/1/5/REVIEWS/3002. Accessed November 23, 2004. [DOI] [PMC free article] [PubMed]
- Lafourcade, C., Galan, J. M., and Peter, M. (2003). Opposite roles of the F-box protein Rcy1p and the GTPase-activating protein Gyp2p during recycling of internalized proteins in yeast. Genetics 164, 469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M. J., Nichols, B. J., Prescianotto-Baschong, C., Riezman, H., and Pelham, H. R. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, W., and Chang, A. (1997). Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J. Cell Biol. 138, 731-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzen, M. R., McCraith, S. M., Spinelli, S. L., Torres, F. M., Fields, S., Grayhack, E. J., and Phizicky, E. M. (1999). A biochemical genomics approach for identifying genes by the activity of their products. Science 286, 1153-1155. [DOI] [PubMed] [Google Scholar]
- Munro, S. (2002). Organelle identity and the targeting of peripheral membrane proteins. Curr. Opin. Cell Biol. 14, 506-514. [DOI] [PubMed] [Google Scholar]
- Nothwehr, S. F., Bryant, N. J., and Stevens, T. H. (1996). The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol. Cell. Biol. 16, 2700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Conibear, E., and Stevens, T. H. (1995). Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol. 129, 35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., Babst, M., and Emr, S. D. (1998). Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847-858. [DOI] [PubMed] [Google Scholar]
- Olkkonen, V. M., and Stenmark, H. (1997). Role of Rab GTPases in membrane traffic. Int. Rev. Cytol. 176, 1-85. [DOI] [PubMed] [Google Scholar]
- Ortiz, D., Medkova, M., Walch-Solimena, C., and Novick, P. (2002). Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157, 1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorff, H. P., Peirano, R. I., Peters, M. A., Schluter, A., Bossenz, M., Scheffner, M., and Bach, I. (2002). Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature 416, 99-103. [DOI] [PubMed] [Google Scholar]
- Peng, J., Schwartz, D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., Roelofs, J., Finley, D., and Gygi, S. P. (2003). A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 21, 921-926. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487-491. [DOI] [PubMed] [Google Scholar]
- Prein, B., Natter, K., and Kohlwein, S. D. (2000). A novel strategy for constructing N-terminal chromosomal fusions to green fluorescent protein in the yeast Saccharomyces cerevisiae. FEBS Lett. 485, 29-34. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., and Scheller, R. H. (2000). A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell 6, 1437-1448. [DOI] [PubMed] [Google Scholar]
- Preuss, D., Mulholland, J., Franzusoff, A., Segev, N., and Botstein, D. (1992). Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol. Biol. Cell 3, 789-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J. R., Preston, R. A., Adams, A. E., Stearns, T., Drubin, D. G., Haarer, B. K., and Jones, E. W. (1989). Fluorescence microscopy methods for yeast. Methods Cell Biol. 31, 357-435. [DOI] [PubMed] [Google Scholar]
- Protopopov, V., Govindan, B., Novick, P., and Gerst, J. E. (1993). Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell 74, 855-861. [DOI] [PubMed] [Google Scholar]
- Ren, M., Xu, G., Zeng, J., De Lemos-Chiarandini, C., Adesnik, M., and Sabatini, D. D. (1998). Hydrolysis of GTP on Rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95, 6187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. E. (1994). Mechanisms of intracellular protein transport. Nature 372, 55-63. [DOI] [PubMed] [Google Scholar]
- Schroder, S., Schimmoller, F., Singer-Kruger, B., and Riezman, H. (1995). The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in alpha-COP. J. Cell Biol. 131, 895-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev, N. (2001a). Cell biology. A TIP about Rabs. Science 292, 1313-1314. [DOI] [PubMed] [Google Scholar]
- Segev, N. (2001b). Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13, 500-511. [DOI] [PubMed] [Google Scholar]
- Segev, N. (2001c). Ypt/Rab GTPases: regulators of protein trafficking. Sci. Available online at http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/100/re11. Accessed November 23, 2004.
- Segev, N., Mulholland, J., and Botstein, D. (1988). The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell 52, 915-924. [DOI] [PubMed] [Google Scholar]
- Spelbrink, R. G., and Nothwehr, S. F. (1999). The yeast GRD20 gene is required for protein sorting in the trans-Golgi network/endosomal system and for polarization of the actin cytoskeleton. Mol. Biol. Cell 10, 4263-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., and Olkkonen, V. M. (2001). The Rab GTPase family. Genome. Available online at http://genomebiology.com/2001/2/5/REVIEWS/3007. Accessed November 23, 2004. [DOI] [PMC free article] [PubMed]
- Sun, Z. W., and Allis, C. D. (2002). Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418, 104-108. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida, T. A., and Emr, S. D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128, 779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, A. M. (2001). Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell. Biol. 2, 169-178. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., Avaro, S., Prescianotto-Baschong, C., Haguenauer-Tsapis, R., and Riezman, H. (2000). The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149, 397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, C. A., Redding, K., Wright, R., and Fuller, R. S. (1992). Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol. Biol. Cell 3, 1353-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., Wu, J. N., Schober, W., Lewis, D. E., and Vida, T. (1998). Isolation of yeast mutants defective for localization of vacuolar vital dyes. Proc. Natl. Acad. Sci. USA 95, 11721-11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., et al. (2001). Global analysis of protein activities using proteome chips. Science 293, 2101-2105. [DOI] [PubMed] [Google Scholar]