Abstract
Glutathione is an essential metabolite protecting cells against oxidative stress and aging. Here, we show that endogenously synthesized glutathione undergoes intercellular cycling during growth to stationary phase. Genome-wide screening identified ∼270 yeast deletion mutants that overexcrete glutathione, predominantly in the reduced form, and identified a surprising set of functions important for glutathione homeostasis. The highest excretors were affected in late endosome/vacuolar functions. Other functions identified included nitrogen/carbon source signaling, mitochondrial electron transport, ubiquitin/proteasomal processes, transcriptional regulation, ion transport and the cellular integrity pathway. For many mutants the availability of branched chain amino acids and extracellular pH influenced both glutathione homeostasis and cell viability. For all mutants tested, the onset of glutathione excretion occurred when intracellular concentration exceeded the maximal level found in the parental strain; however, in some mutants prolonged excretion led to substantial depletion of intracellular glutathione. These results significantly contribute to understanding mechanisms affecting glutathione homeostasis in eukaryotes and may provide insight into the underlying cause of glutathione depletion in degenerative processes such as Parkinson's disease. The important implications of these data for use of the yeast deletion collection for the study of other phenomena also are discussed.
INTRODUCTION
The crucial role of the essential metabolite glutathione (l-γ-glutamylcysteinylglycine) in cellular responses to oxidative stress and metabolism has been well documented (Carmel-Harel and Storz, 2000; Cnubben et al., 2001). Its effectiveness as a cellular redox buffer is due to its low (E′0 = -240 mV) redox potential and its high (1-10 mM) cellular concentration, which are maintained via strict regulation of glutathione biosynthesis and NADPH-dependent glutathione reductase activity (Meister and Anderson, 1983; Schafer and Buettner, 2001). Glutathione also is thought to act as a redox buffer in the endoplasmic reticulum (ER) where the reduced form (GSH) competes for the oxidizing equivalents derived from the disulfide bond-forming machinery, thereby minimizing the genesis of a hyperoxidizing environment (Cuozzo and Kaiser, 1999; Bass et al., 2004). Intracellular glutathione depletion may be attributed to several factors, including increased degradation, conjugation, oxidation, efflux/excretion, and/or decreased synthesis. In mammalian cells, abnormal glutathione homeostasis, particularly glutathione depletion, occurs after exposure to many chemicals/drugs and plays a role in certain degenerative diseases (e.g., Parkinson's), cell aging, and apoptosis (Nestelbacher et al., 2000; Schulz et al., 2000) (Hammond et al., 2001; Lou et al., 2003). For example, exposure of T24 bladder carcinoma cell lines to the flavoprotein inhibitor diphenyleneiodonium leads to heightened efflux of reduced glutathione and Fas-mediated apoptosis (Pullar and Hampton, 2002). Alternatively in hepatic cells efflux of glutathione disulfide (GSSG) constitutes the primary mechanism for turnover of the hepatic glutathione pool (Bartoli and Sies, 1978; Ookhtens et al., 1985; Masuda et al., 1993).
An association between altered calcium and glutathione homeostasis has been reported. Glutathione is reported to modulate the activity of, and serves as a transportable substrate for, ryanodine receptor Ca2+ channels (Balshaw et al., 2001; Banhegyi et al., 2003). Elevated glutathione efflux is associated with ischemic reperfusion (Shivakumar et al., 1995) and occurs after exposure of hippocampus cells to N-methyl-d-aspartate (Wallin et al., 1999), situations where Ca2+ homeostasis is disrupted. Interestingly, exposure of astrocyte and neuronal cells to the neurotoxic peptide β amyloid also leads to calcium-dependent glutathione depletion and pronounced cell death (Abramov et al., 2003).
Saccharomyces cerevisiae cells disrupted for glutathione biosynthesis exhibit reduced tolerance to a wide range of stress conditions (Izawa et al., 1995; Turton et al., 1997; Grant et al., 1998; Maris et al., 2000) and undergo apoptosis at a high rate relative to the parental cells (Madeo et al., 1999). Conversely, exogenous administration of glutathione rescues the accelerated aging of S. cerevisiae cells in elevated oxygen (Nestelbacher et al., 2000). Glutathione is known to protect mitochondrial macromolecules from the deleterious effects of reactive oxygen species (Meister, 1995; Sugiyama et al., 2000; Lee et al., 2001). In S. cerevisiae, glutathione also serves as a starvation source of nitrogen (Mehdi and Penninckx, 1997) and sulfur (Miyake et al., 1999). Its use/degradation necessitates vacuolar compartmentalization and cleavage of the γ-glutamyl linkage via the specific action of γ-glutamyltranspeptidase (Mehdi et al., 2001). In S. cerevisiae, glutathione excretion was shown to be stimulated by heterologus expression of the bovine pancreatic trypsin inhibitor or overexpression of the resident-ER chaperone Kar2 (Bannister and Wittrup, 2000).
Although there have been many studies on regulation of the glutathione biosynthetic pathway, particularly in response to stress (reviewed in Soltaninassab et al., 2000; Penninckx, 2002), there is limited understanding of how complex interconnected cellular networks function to influence glutathione homeostasis. In addition, the processes that influence glutathione transport into, around and out of cells have not been studied in terms of the role that these phenomena play in the maintenance of cellular glutathione homeostasis. This study used the power of the S. cerevisiae genome-wide deletion library to determine the cellular processes influencing glutathione homeostasis, by using extracellular glutathione as a marker of its aberrant intracellular metabolism and/or retention. This study also provides an insight into the fundamental nature of a broad range of mutants present in this library and has serious implications for interpreting data obtained after screening of this valuable resource.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
The S. cerevisiae strains used in this study are derivatives of BY4743 MATa/Matα ura3?0/ura3?0 leu2?0/leu2?0 his3?1/his3?1 met15?0/MET15 LYS2/lys2?0), which were homozygous for the relevant gene deletion. The construction of the yeast genome deletion library was described previously (Winzeler et al., 1999). Strains were grown in YPD medium [2% (wt/vol) d-glucose, 2% (wt/vol) bacteriological peptone, 1% (wt/vol) yeast extract] or SD medium [0.17% (wt/vol) yeast nitrogen base without amino acids, 0.5% (wt/vol) ammonium sulfate, 2% (wt/vol) d-glucose] supplemented unless indicated with leucine (131 mg l-1), isoleucine (65.6 mg l-1), valine (58.6 mg l-1), histidine (209 mg l-1), uracil (22.4 mg l-1), and adenine (13.5 mg l-1). Where specified leucine, isoleucine, and valine where added at twofold and fourfold higher concentration. Where required, media were solidified by the addition of 2% (wt/vol) agar. Respiratory capacity was assessed by observing growth on YPG medium [as for YPD medium except glucose was replaced with 3% (wt/vol) glycerol] after 2 d at 30°C. Dithiothreitol (DTT) sensitivity was assessed by spotting 5 μl of stationary phase cells on YPD medium containing 12 mM DTT. Relative growth was compared with that of the parental strain after 2 d and 3 d at 30°C.
Determination of Glutathione Levels
Glutathione levels were determined using the microtiter-based method described previously (Vandeputte et al., 1994; Grant et al., 1998). Cells were harvested at the appropriate growth phase via centrifugation (1 min; 800 × g). Extracellular glutathione was measured directly from the resulting supernatant. For estimation of intracellular glutathione, cells were washed twice with ice-cold SD medium (lacking glucose or amino acids), resuspended in ice-cold 1.3% (wt/vol) 5-sulfosalicylic acid, and broken with glass beads by using a minibead beater (Biospec Products, Bartlesville, OK) for 60 s at 4°C followed by incubation on ice (15 min). The resulting mixture was clarified by centrifugation (10 min, 13,000 × g at 4°C), and glutathione levels were measured in the resulting supernatant. For quantification of oxidized glutathione, samples (including GSSG standards) were pretreated with 5% (vol/vol) 2-vinylpyridine for 1 h at room temperature before analysis. Glutathione levels are expressed as nanomoles per A600, where A600 of 1 corresponded to a cell density of 2.5 × 107 cells ml-1.
Genome-wide Screening of Mutations Leading to Increased Glutathione Excretion
Cells were pregrown in 96-well plates to stationary phase in YPD medium and inoculated using a 96 pin replicator in SD medium, followed by incubation at 30°C with shaking at 500 rpm for 2 d. Deletion mutants found to excrete twofold or greater glutathione were regrown in triplicate as described above, except cells were grown in 24-well plates and extracellular glutathione concentration was determined and normalized to cell density (A600). Cells were judged to “overexcrete” glutathione if they produced more than twofold that of the parental strain. For kinetic analysis of changes in intra- and extracellular glutathione during the various stages of growth, cells were inoculated (A600 of 0.01) in SD medium and incubated at 30°C. Samples were taken at the intervals shown and glutathione quantified according to the above-mentioned procedure.
Determination of the Cellular Capacity for Use of Glutathione as a Sole Nitrogen Source
Cells pregrown in YPD medium were inoculated (initial A600 of 0.01) in SD medium lacking ammonium sulfate and incubated at 30°C for 1 d. Under these conditions cell growth usually ceased at A600 of 0.5-1.0. These cells were reinoculated in SD medium lacking ammonium sulfate, supplemented with either no supplement, 5 mM ammonium sulfate, 1 mM GSH, 5 mM GSH, or 5 mM ammonium sulfate supplemented with either 1 or 5 mM GSH (controls) and incubated at 30°C for 2 d.
Generation of Respiratory Petite Cells
Rho0 petite cells lacking a mitochondrial genome were generated as described previously (Fox et al., 1991). Briefly, exponentially growing cells were incubated in YPD medium containing 20 μg ml-1 ethidium bromide for 1 d and spread on YPD medium to form single colonies (48 h). Colonies were replica plated on YPG and YPD medium to identify petite isolates. Loss of respiratory function in these petites was confirmed by their inability to reduce 2,3,5-triphenyltetrazolium chloride (TTC). Then, 0.5% (wt/vol) TTC was dissolved in premelted 48°C agar [1.2% (wt/vol)] and overlaid on the yeast colonies. Reduction of TTC (colorless) requires the activity of the respiratory chain and leads to production of an insoluble red pigment (Slater, 1967).
RESULTS
Endogenously Synthesized Glutathione Undergoes Intra/Intercellular Cycling
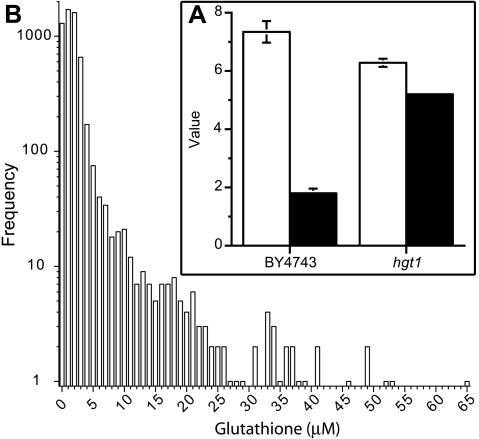
While studying a glutathione-hyperexcreting strain (GHS4), we noted that wild-type yeast cells also excrete low levels of endogenously synthesized glutathione, although the levels are generally very low (Figure 1A). In the absence of knowledge of the mechanism of glutathione export, we postulated that a mutation affecting the uptake of glutathione should lead to its increased accumulation in the extracellular medium. HGT1 encodes the sole GSH uptake transporter in S. cerevisiae (Bourbouloux et al., 2000). In the hgt1 deletion mutant, extracellular glutathione overaccumulated threefold relative to wild-type parental strain BY4743 (Figure 1A), indicating that during growth to stationary phase yeast cells excrete and reimport endogenously synthesized glutathione. Analysis of the intra- and extracellular glutathione pool during growth of the wild-type strain showed that glutathione excretion commenced at a distinct stage during exit from exponential phase coinciding with when the intracellular glutathione level reached a maximum (Figure 2). This indicates that onset of efflux is influenced by cytoplasmic glutathione concentration.
Figure 1.
Disruption of HGT1, encoding a high-affinity glutathione uptake transporter, and a broad range of other genes leads to overaccumulation of extracellular glutathione in stationary phase cultures of S. cerevisiae. (A; inset) The parental strain (BY4743) and hgt1 mutant were grown to stationary phase (2 d) in SD medium, and final cell yield (open bars; A600) and extracellular glutathione (closed bars; μmol/A600) were quantified. (B) Frequency distribution of extracellular glutathione accumulated by each of the deletion mutants analyzed in this study. Deletion mutants pregrown in YPD medium (3 d) were diluted (1/10) and inoculated in SD medium by using a 96 pin replicator (initial A600 of <0.01). Cells were incubated at 30°C for 3 d (without agitation), and extracellular glutathione was quantified. High-range glutathione overexcretion occurred predominantly after deletion of genes encoding functions associated with the late endosome and vacuole (under these conditions the parental strain excreted 1-2 μM glutathione).
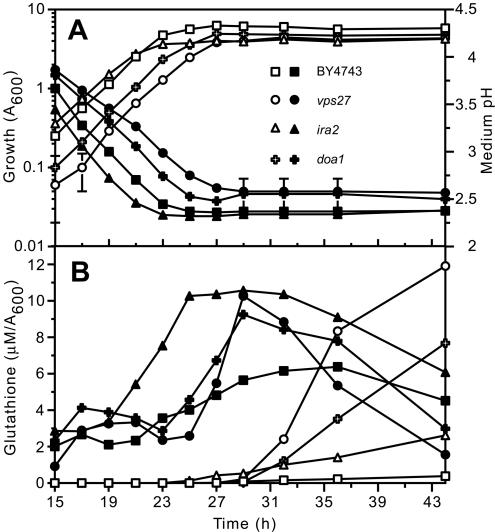
Figure 2.
Influence of growth phase on glutathione levels in the parental strain (BY4743) and representative glutathione-overexcreting mutants. The parent (squares) and representative glutathione-overexcreting mutants vps27 (circles), ira2 (triangles), and doa1 (crosses) were inoculated (A600 of 0.01) in SD medium and incubated at 30°C. (A) Growth (open symbols; A600) and medium pH (closed symbols; initial pH of medium was 4.5) and (B) intracellular glutathione (closed symbols; μM/A600) and extracellular glutathione (open symbols; μM/A600) were quantified at intervals between 15 and 44 h postinoculation as indicated.
Detailed analysis of strain GHS4 showed that the altered glutathione metabolism exhibited by these cells was not due to decreased uptake of the tripeptide, as determined by the accumulation of radiolabeled glutathione (our unpublished data). This raised the possibility that certain mutations could give rise to increased glutathione excretion by influencing glutathione synthesis, transport/excretion, and/or degradation. Because excreted glutathione is derived from the intracellular pool, extracellular glutathione provides a useful marker to study the genetic and environmental/nutritional factors that influence glutathione metabolism and its efflux from the cell.
Genome-wide Analysis of Mutations That Lead to Glutathione Overexcretion
Genome-wide screening of the available S. cerevisiae deletion strains (Winzeler et al., 1999) was used to identify cellular processes involved in glutathione homeostasis. Mutant strains pregrown in rich medium were inoculated and grown in SD medium and at stationary phase extracellular glutathione was quantified. A surprisingly large number of the deletion mutants (276 of 4659 strains) overexcreted glutathione (2- to 37-fold) into the medium. Figure 1B shows the frequency distribution of the level of glutathione excreted by all of the mutants examined from which it is evident that there was a wide range of levels excreted.
Deletion strains were grouped according to the gene ontology descriptions of function of the encoded gene product in the Saccharomyces Genome Database (www.yeastgenome.org). Most of the deletions were of genes that fell into nine defined functional groups, as indicated in Table 1 (Figure 3). A comprehensive list is available as supporting material (Supplementary Tables 1-10). These functional groups included mitochondrial electron transport, nitrogen metabolism, protein sorting (mainly via the late endosomal pathway/vacuolar function), RAS/protein kinase A pathway, transcription regulation, translation/protein synthesis, cell wall/integrity, ubiquitin-related functions, and ion homeostasis. These data gave a clear and novel insight into the cellular functions and metabolic and regulatory networks that influence glutathione homeostasis. These processes are for the most part conserved between yeast and higher eukaryotes.
Table 1.
Cellular processes influencing glutathione homeostasis identified by glutathione overexcretion mutants
| Functional group/cellular process | No. of genes | Glutathionea excretion | Examples of mutants |
|---|---|---|---|
| Mitochondrial electron transport chain | 66 | 2-25 | mrs1, cox6, qcr7 |
| RAS/PKA; carbon signaling | 2 | 22-25 | ira2, pde2 |
| Nitrogen signaling amino acid metabolism | 5 | 3-24 | ure2, npr2, gdh1 |
| Secretory pathway; vacuolar protein sorting | 66 | 2-37 | vps2, pep3, pep12 |
| Translation | 10 | 2-11 | tif2, egd1, rpl21a |
| Ubiquitin/proteasome related | 10 | 2-35 | ubp6, ump1, doa1 |
| Cell integrity; cytoskeleton; inositol metabolism | 16 | 2-21 | mpk1, ino1, ecm25 |
| Ion homeostasis | 13 | 2-11 | ptk2, nhx1, vma2 |
| Transcription; chromatin remodeling | 16 | 2-11 | gcn5, snf5, paf1 |
| Miscellaneous | 20 | 2-24 | kcs1, akr1, slx8 |
| Genes of unknown function | 46 | 2-13 | prm7, yim2 |
Fold glutathione excreted relative to the parental strain in stationary phase
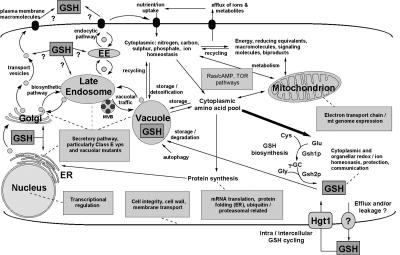
Figure 3.
Overview of the cellular processes influencing cellular glutathione homeostasis leading to glutathione overaccumulation. The figure depicts the glutathione biosynthetic pathway, its position in cellular metabolism, and known and putative transport routes, some of which may mediate glutathione efflux. Boxed annotations indicate some of the cellular processes that are essential to prevent abnormal glutathione homeostasis.
RAS/Protein Kinase A Pathway and Nitrogen Metabolism Signaling Affect Glutathione Homeostasis
Glutathione homeostasis depends on the RAS/protein kinase A (PKA) and target of rapamycin (TOR) signaling pathways (Table 1; Supplementary Table 1). The RAS/PKA pathway plays an integral role in cellular metabolism regulating proliferation in response to nutrient status and respiratory metabolism (Thevelein and de Winde, 1999). Deletion of IRA2 and PDE2, encoding negative regulators of the RAS/PKA pathway, led to extensive glutathione overexcretion (22- to 25-fold; Table 1).
The TOR signaling pathway plays an integral role in the control of nitrogen assimilation (Magasanik and Kaiser, 2002). As part of the TOR complex, Ure2 is a main regulator acting to repress genes involved in the use of poor nitrogen sources in the presence of rich ones; ure2 mutants fail to regulate nitrogen assimilation and metabolism appropriately (ter Schure et al., 2000; Magasanik and Kaiser, 2002). Deletion of URE2 led to extensive glutathione overexcretion (24-fold; Table 1). Additionally, a number of other mutants previously shown to influence cellular nitrogen metabolism (npr2, gdh1, and pro1) were identified as glutathione overexcretors.
Mutants Affected in Respiratory Chain Function Overexcrete Glutathione
A large number of mutants affected in mitochondrial functions overexcreted glutathione (Table 1); however, the spectrum of these mutants showed that the loss of the mitochondrial electron transport chain and not the loss of respiratory ATP production per se led to glutathione efflux. Although at least 341 nuclear genes are required for respiratory growth by S. cerevisiae (Dimmer et al., 2002), the deletion of only those encoding components of the electron transport chain or its synthesis led to glutathione overexcretion (generally 6- to 11-fold parental level; Supplementary Table 2). This also included genes involved with maintenance of the mitochondrial genome. Because this genome encodes a limited number of proteins, including subunits of the electron transport chain complexes III, IV, and the F1F0-ATPase, the loss of mitochondrial DNA (mtDNA) also should result in altered glutathione homeostasis. An isogenic ρ0 petite lacking mtDNA was therefore generated and found to overexcrete glutathione into the medium fourfold relative to the parent. Interestingly, deletion of YPR004c, encoding a putative electron transfer flavoprotein that localizes to the mitochondria, where it forms part of a large supramolecular complex (Grandier-Vazeille et al., 2001; Ohlmeier et al., 2004), led to 25-fold glutathione overexcretion but did not affect respiratory competence.
Some of the nonmitochondrial deletions caused poor or no growth on medium containing a sole nonfermentable carbon source (glycerol; see supplementary data). In these cases, it could not be determined whether the resulting glutathione excretion was due to the loss of the normal function attributed to that protein or the resulting mitochondrial dysfunction that ensued.
Mutants Affected in Secretory Pathway/Vacuolar Function
Surprisingly, a major set of 75 mutants included those affected in specific components of the secretory pathway. These were involved in normal functioning of the ER, Golgi, and vacuole, and the plasma membrane-to-vacuole and Golgi-to-vacuole endosomal pathways (Jones et al., 1997; Kaiser et al., 1997). Very prominent among these was a group of 16 mutants affected in class E vacuolar protein sorting (Raymond et al., 1992), and these included the highest excretors of glutathione (up to 37-fold; vps2).
The late endosome plays a key role in regulating plasma and endosomal membrane components, including transporters, by ensuring the appropriate sorting of material (particularly monoubiquitinated proteins) into multivesicular bodies before delivery to the vacuole for proteolysis (Lemmon and Traub, 2000; Katzmann et al., 2002). Additionally, a large number of resident vacuolar proteins transit from the Golgi to the vacuole via the late endosome. Disruption of the class E subset of vacuolar protein sorting genes (VPS2, 4, 20, 22-25, 27, 28, 31, 32, 36, 37, 44, 60, and DID2) leads to the formation of an enlarged prevacuolar or “class E” compartment (Raymond et al., 1992). These are required for appropriate sorting of proteins at the late endosome (Katzmann et al., 2002). The data indicated the essential role of the class E VPS genes with respect to the maintenance of glutathione homeostasis. Disruption of NHX1/VPS44, encoding a Na+/H+ antiporter of the endo-membrane system, led to formation of an enlarged prevacuolar compartment; however, the severity of this phenotype is lower relative to other class E vps mutants (Bowers et al., 2000). Consistent with this observation, the level of glutathione excreted by the nhx1 mutant was lower than that by the other class E vps mutants.
Because vps2 cells, which excreted the highest level of glutathione, were defective in both endosomal protein sorting and respiratory growth, there may be an additive effect of disrupting these processes on glutathione metabolism. Glutathione excretion by respiratory petite vps27 cells was therefore compared with that of the isogenic grande vps27 cells. Petite vps27 cells excreted 30% more glutathione relative to grande vps27 cells, indicating that disruption of these cellular processes had an additive effect, suggesting they may act via different mechanisms.
Deletion of a number of genes, including those encoding SNAREs (pep12, gos1), PI3P binding proteins (pep7, fab1, vps27) and GTP-signaling (ypt7, ccz1), which affect various aspects of Golgi-to-vacuole endosomal sorting; endosometo-Golgi retrieval (vps35 or mutants affecting the SacIIVps53-Luv1 complex); and vacuolar biogenesis, inheritance, and morphology (pep7, pep3, vps33), led to considerable glutathione overexcretion (2- to 28-fold). Appropriate endosomal/vacuolar function is therefore essential for maintenance of glutathione homeostasis.
These mutations may affect glutathione homeostasis via disruption of vacuolar glutathione storage/degradation. In S. cerevisiae, glutathione degradation is restricted to the vacuolar lumen where the γ-glutamyl linkage is degraded by γ-glutamyltranspeptidases (Penninckx, 2002). Vacuolar glutathione transport/degradation may therefore be important for regulating glutathione turnover and some of the glutathione-overexcreting strains could be defective in glutathione degradation. Mutants were therefore tested for their ability to grow on glutathione as a sole nitrogen source. They were depleted of an assimilable nitrogen source and inoculated in medium containing glutathione (1 or 5 mM) as a sole nitrogen source and growth assessed after 2 d. This would identify mutants defective in uptake and/or vacuolar transport/degradation of GSH. Interestingly, deletion of the high affinity GSH uptake transporter (hgt1) did not prevent utilization of GSH as a sole nitrogen source, indicating that other transport mechanisms (possibly low affinity uptake via endocytosis) could adequately facilitate GSH uptake when present at 1 mM in the growth medium. These data indicate that it is unlikely that any single deletion mutant was unable to use GSH due to defective transport across the plasma membrane.
Of the 276 glutathione-overexcreting strains, 54 (see supplementary data) exhibited reduced capacity for using GSH as a sole nitrogen source (underlined loci; Supplementary Tables 1-10). These included strains disrupted in vacuolar biogenesis/protein sorting/function (pep3, pep7, pep12), maintenance of cell integrity (mpk1, bck1), spindle pole body formation (spc72, ynl225c), transcription activation/chromatin remodeling (hfi1, gcn5, sin3), nitrogen metabolism (ure2), and RAS/PKA pathway function (ira2). Because many of these genes encode functions not normally associated with GSH uptake their deletion may alter the kinetics of vacuolar glutathione transport and/or its subsequent degradation. Surprisingly, although deletion of components of the ECSRT-II and III complexes, Did2p or Vps4p prevented glutathione utilization, disruption of Vps27p, Vps60p, Vps24p, or components of the ESCRT-I complex (Katzmann et al., 2002) did not have this effect. Because all of class E vps mutants overexcreted glutathione it is unlikely that defective glutathione degradation is the primary cause of altered glutathione homeostasis in these cells.
Endoplasmic Reticulum/Golgi Function Influences Glutathione Homeostasis
Endoplasmic reticulum and Golgi dysfunction also led to glutathione overexcretion, although to a lesser extent than mutants affected in the late endosome and vacuole function. This included components of the Sec34/Sec35 complex, glycosyltransferases, and Pmr1, a Golgi-localized Ca2+/Mn2+-ATPase that influences N-linked glycosylation (Kaiser et al., 1997). Genes associated with the unfolded protein response also were included. Cells cope with the accumulation of misfolded proteins in the ER and/or general ER stress by inducing a transcriptional response termed the unfolded protein response (Kaufman, 1999; Mori, 2000). Deletion of a number of genes up-regulated by the unfolded protein response, including VPS23, LUV1, VPS35, and EUG1 (Travers et al., 2000) led to glutathione overexcretion.
Membranes, Cell Wall, Inositol Metabolism, Ion Homeostasis, and Transcription
Deletion of genes involved with inositol biosynthesis (INO1, INO4), cell integrity (BCK1, MPK1), cell wall structure/biosynthesis (CWH36, ECM25), and microtubule/spindle body formation caused glutathione overexcretion (2- to 21-fold). These defects may lead to either increased leakage of glutathione or effects on its transport. Because glutathione is anionic at cellular pH its transport across cell membranes would be affected by membrane potential and/or the movement of other ions. Deletion of a number of proteins involved in the maintenance of membrane potential and ion transport (pmp3, ptk2, nhx1, vma2, pmr1), across one or more cellular membranes, led to glutathione excretion. These included ones altered in transport of calcium and monovalent cations (H+, K+, Na+).
Appropriate regulation of transcription is also essential for maintenance of glutathione homeostasis, and glutathione overexcretion followed the deletion of a number proteins involved with SAGA, ADA, RSC, or SWI/SNF complexes, which modulate transcription via interactions with RNA polymerase II or through chromatin remodeling (Sudarsanam and Winston, 2000; Fry and Peterson, 2001; Muchardt and Yaniv, 2001).
Analysis of the Redox State of Excreted Glutathione
For the parental strain and most mutants, glutathione was excreted predominantly in the reduced form with the ratio of GSH:GSSG generally in the range ∼25-50:1 (stationary phase; see Supplementary Table 11). For most strains, the redox state of extracellular glutathione was comparable with that of the intracellular fraction, indicating that glutathione was transported/leaked either directly across the plasma membrane or from the cytosol into a cellular compartment capable of communicating with the extracellular medium. There were some notable exceptions. Mutants affected in normal functioning of the mitochondrial electron transport chain constituted the largest group of strains that excreted a low ratio of GSH:GSSG (8:1), whereas the pmr1 mutant excreted the lowest ratio of GSH:GSSG (2:1). Hypergeometric distribution analysis (Tavazoie et al., 1999) of strains excreting the highest absolute levels of GSSG (top 100 of 276) confirmed that this population was enriched for strains defective in mitochondrial respiratory function (p < 10-14). Interestingly, in all mutants tested elevated GSSG excretion was not associated with intracellular overaccumulation of GSSG (GSH:GSSG ∼25-50:1). Presumably, in these mutants the GSSG was generated in a specific compartment, such as the ER, which is known to contain a low (∼3:1) ratio of GSH:GSSG (Hwang et al., 1992; Bass et al., 2004), before excretion.
Kinetic Analysis of Intra-/Extracellular Glutathione Homeostasis during Growth
Representative mutants from the above-mentioned groups were selected for analysis of the changes in intra- and extracellular glutathione during the various stages of growth in batch culture. Glutathione excretion by the parental strain began as cells approached stationary phase (Figure 2). Of the other mutants tested, all excreted glutathione in this phase, except for those in the RAS/PKA pathway (e.g., ira2; Figure 2) mutants, which did so during exponential fermentative growth. Importantly, all mutants began excreting glutathione when the intracellular level in the mutant exceeded the maximal concentration found in the parental strain (the maximum intracellular level in the mutants was about twofold that of the parental strain). In many mutant strains, this led to total synthesis of a considerable excess of glutathione (intra + extracellular glutathione). One possible explanation of these data is that cells exert strict control over the cytoplasmic level of glutathione and that “excess” glutathione is excreted to maintain the internal concentration at an optimal level. Glutathione is distributed between the cytosol, the vacuole, and other compartments such as the endoplasmic reticulum (and possibly other compartments of the secretory pathway) and mitochondria; therefore, intracellular glutathione accumulation reflects elevated levels in at least one of these compartments. Importantly, in the vps27 mutant glutathione efflux did not cease when the intracellular level fell below that found in the parental strain (i.e., the maximal level, which was observed in stationary phase cells), and this led to substantial depletion of intracellular glutathione as cells entered stationary phase (Figure 2). In the ino1 and ure2 mutants depletion of glutathione in stationary phase was even more pronounced. Altered kinetics of glutathione homeostasis, particularly depletion of glutathione in stationary phase, indicated that plasma membrane permeability could be affected in some of the glutathione-overexcreting mutants.
Glutathione-overexcreting Mutants Exhibit Increased Propidium Iodide (PI) Staining
To determine whether a change in membrane permeability was associated with aberrant glutathione homeostasis, the proportion of PI-positive staining cells was quantified in stationary phase (Deere et al., 1998). PI fluorescently stains nucleic acid; however, its diffusion into intact cells is low (Deere et al., 1998). Incubation of glutathione-overexcreting mutants with PI therefore would indicate whether the membrane permeability and/or viability of these strains was affected, relative to parental cells. All glutathione-overexcreting mutants tested exhibited increased positive staining to propidium iodide in stationary phase (48 h), relative to the parental strain (∼10% positive-stained cells). These strains included the ino1 (94%; 14-fold GSH), vps36 (41%; 26-fold GSH), ure2 (66%; 24-fold GSH), doa1 (55%; 25-fold GSH), ubp6 (52%; 18-fold GSH), npr2 (80%; 6-fold), pde2 (78%; 22-fold GSH), ira2 (77%; 25-fold GSH), and mrpl11 (49%; 8-fold GSH) mutants. The level of glutathione excreted by each strain also is provided. These values are expressed in fold extracellular glutathione (GSH) relative to the parental strain.
Some glutathione efflux is therefore probably the result of increased membrane permeability or cell death. However, this can only account for a proportion of glutathione efflux because across the mutants, there was a lack of correlation between the extent of PI staining and the amount of extracellular glutathione. For some mutants (e.g., vps36, pmr1), there were relatively low levels of PI staining (<40%) but high levels of extracellular glutathione, although this was less common than the reverse situation of low glutathione and high PI staining. Moreover in Figure 2B it is clear that the vps27 mutant and other mutants tested all overaccumulated intracellular glutathione before its detection in the medium. For the pmr1 and mitochondrial mutants, there was a very different ratio of oxidized to reduced glutathione in the extracellular medium compared with the intracellular pools, for most of the other mutants the redox state of glutathione in the extracellular medium indicated that it is likely to have originated in the cytosol, and this may be consistent with the mutation affecting the permeability of the plasma membrane.
These aspects of increased permeability in many of the mutants show that the data obtained need to be interpreted with caution. However the data do provide useful information about glutathione homeostasis because intracellular overaccumulation of glutathione precedes its excretion or loss across the membrane, and it is not merely membrane leakage that leads to overaccumulation of glutathione in the medium. In fact, for many mutants the total amount of glutathione (intra- and extracellular) greatly exceeds that produced by the wild-type. In Figure 1, it is also clear that for some mutants tested in more detail (ira2, pde2, doa1), there is no eventual intracellular depletion of glutathione that would be consistent with straight-forward loss of membrane integrity. However, for others (ure2, ino1, vps27), there was depletion in late stationary phase.
Because onset of abnormal glutathione homeostasis occurred at a distinct point in the growth phase, this raised the possibility that a change in culture conditions (environmental stress or nutrient availability) was closely associated with overaccumulation. The following sections address the key environmental factors that were found to influence this homeostasis.
Glutathione Homeostasis Is Affected by the Relative Availability of Branched Chain Amino Acids (BCCAs)
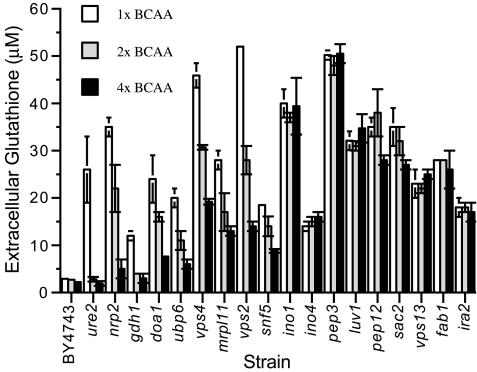
In the media used in these experiments, the availability of leucine was a factor limiting growth of the strains that are leucine auxotrophs (apparent from the data in Figure 5). Because leucine supplementation can suppress synthesis of other BCAAs, isoleucine and valine also were added (Dickinson, 1999). BCAA limitation was a key factor leading to overaccumulation of extracellular glutathione in a broad range of mutants; however, the magnitude of this response was strain-dependent. For example, glutathione homeostasis of the ure2 mutant was strongly influenced by the relative availability of BCAA in the growth media (Figure 4; for complete data set, see Supplementary Table 11) because addition of excess BCAAs decreased extracellular glutathione to the parental level in stationary phase. The mutants strongly influenced by BCAA availability included those affected in nitrogen metabolism (npr2, gdh1) and many of the class E vps mutants (Figure 4). For these mutants, growth in elevated BCAAs reduced accumulation of extracellular glutathione. In contrast, glutathione overexcretion by a number of mutants, including ino1, pep3, and ira2, was unaffected/less affected by the relative availability of BCAAs (Figure 4).
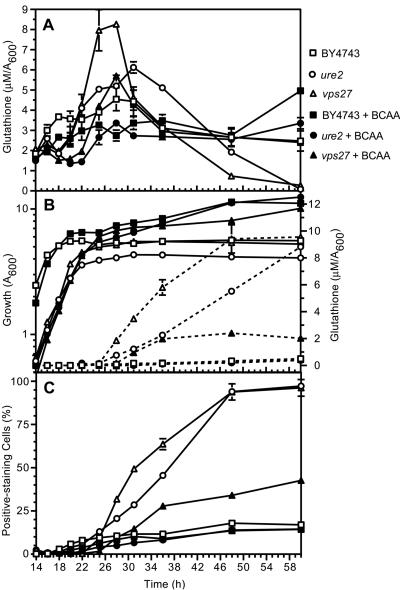
Figure 5.
Effect of the relative availability of branched chain amino acids on intra- and extracellular glutathione, and propidium iodide staining during growth of the parent (BY4743) and ure2 and vps27 mutants. The parent (BY4743; squares), ure2 (circles), and vps27 (triangles) strains were inoculated (A600 of 0.01) in SD medium supplemented with 1 time (open symbols) or 4 times (closed symbols) branched chain amino acids (see Figure 4 for concentrations). (A) Intracellular glutathione. (B) Growth (solid lines; left axis) and extracellular glutathione (dashed lines; right axis). (C) Propidium iodide staining were quantified between 14 and 60 h.
Figure 4.
Influence of the relative availability of BCAAs on extracellular glutathione accumulation by selected deletion mutants. The parent (BY4743) and deletion mutants shown were inoculated (initial A600 of 0.01) in SD medium supplemented with leucine, isoleucine, and valine: 1 time, 131, 66, and 59 mg l-1 (open bars); 2 times, 262, 112, and 118 mg l-1 (shaded bars); 4 times, 524, 264, and 236 mg l-1 (closed bars), respectively, and grown to stationary phase (2 d; 30°C) at which time extracellular glutathione was quantified.
To understand the association between BCAA limitation, glutathione homeostasis, and altered plasma membrane permeability, a more detailed analysis was performed. The parental strain and the ure2 and vps27 mutants were grown in SD medium supplemented with 1 or 4 times BCAAs (see Materials and Methods for concentrations). Culture density, intra- and extracellular glutathione, and PI staining were determined from exponential to stationary phase (14-60 h). The data in Figure 5B indicate that BCAA limitation led to a reduction in the growth of the parental strain and this was closely associated with onset of increased intracellular glutathione overaccumulation (Figure 5A). In the parental strain, BCAA limitation did not affect the degree of glutathione efflux (Figure 5B) nor the proportion of cells that stained with PI (Figure 5C). These trends differed with the ure2 and vps27 mutants (Figure 5, A-C), where BCAA-limitation (indicated by the reduction in growth rate) was closely associated with an increased proportion of PI-staining cells; a transient yet substantial overaccumulation of intracellular glutathione; and heightened glutathione efflux, which led to depletion of the intracellular pool (Figure 5, A-C). In both strains, these abnormal trends in glutathione homeostasis were abrogated by increased BCAA supplementation. The data indicate that the “BCAA-responsive” glutathione-overexcreting mutants are unable to respond appropriately to BCAA limitation, and this leads to increased permeability of the plasma membrane.
Because increased BCAA supplementation could not abolish the abnormal glutathione homeostasis of the vps27 mutant, and many other mutants (Figure 4), this indicated that a change in another environmental parameter, another stress, may be more closely associated with these changes. Because mutants affected in maintenance of membrane potential or transport of H+/K+/Na+ (pmp3, ptk2, vma2, nhx1) overaccumulated extracellular glutathione, cation homeostasis also influences glutathione homeostasis. The role of these cations in the abnormal glutathione homeostasis exhibited by the mutants in this study was investigated.
Glutathione Efflux Is Strongly Influenced by Extracellular pH
During growth in abundant glucose, S. cerevisiae cells acidify the extracellular medium (Figure 2). This process facilitates secondary uptake of a broad range of metabolites and serves to maintain intracellular pH at an appropriate level (Serrano et al., 1986). The critical importance of effective H+ extrusion is demonstrated by the essential nature of the plasma membrane Pma1p H+-ATPase (Serrano et al., 1986), which is very abundant (Chang and Fink, 1995; Ghaemmaghami et al., 2003).
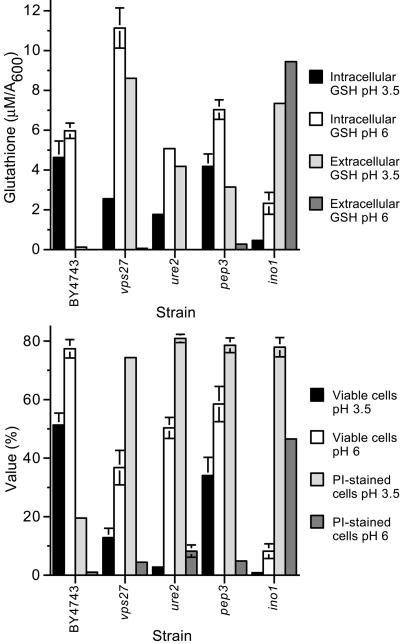
To study the role of H+ stress in glutathione efflux, the parental strain and glutathione-overexcreting mutants were grown in medium buffered to pH 3.5 or 6, and stationary phase extracellular glutathione was quantified. For most strains, glutathione overexcretion was prevented or substantially reduced after growth at pH 6, relative to pH 3.5 (Supplementary Table 11). Examples of mutants where glutathione efflux was less affected by extracellular pH include the ino1 and ino4 mutants. These data indicate that H+ influx/stress is a major “driving force” leading to intracellular glutathione overaccumulation, its efflux, and depletion. The association between H+ stress and glutathione homeostasis was studied in more detail in several mutants (Figure 6, A and B). Under these conditions, growth of cells in medium buffered to pH 6, relative to pH 3.5, led to reduced glutathione efflux (except for ino1 cells) and minimized depletion of the intracellular glutathione pool (Figure 6A). Indeed, at pH 6 the vps27 mutant overaccumulated intracellular glutathione in stationary phase. It also was noted that the stationary phase viability of the glutathione-overexcreting mutants was lower than that of the parental strain, when cells were grown at pH 3.5 (Figure 6B). In this respect, the loss of cell viability (colony-forming units), membrane permeability (PI staining), and magnitude of intracellular glutathione depletion seemed to correlate (Figure 6B). Interestingly, at pH 3.5, extracellular glutathione, cell viability, and PI staining did not correlate to the same extent. Extracellular glutathione accumulated by the vps27 mutant was twofold that of the ure2 mutant, indicating that disruption of the late endosome to vacuolar protein sorting pathway led to higher quantities of extracellular glutathione relative to the other mutants tested, in a manner that could not be accounted for by greater loss of cell viability or permeability alone. These phenotypes were rescued by increasing external pH. H+ stress therefore provided the key stimulus leading to increased membrane permeability and altered glutathione homeostasis under these conditions. From Figure 2, it is evident that low external pH, per se, had little effect on glutathione homeostasis in these mutants (∼15-23 h; except ira2); however, BCAA-limitation (∼25-45 h) exacerbated the deleterious effects of H+ stress.
Figure 6.
Effect of external pH on glutathione homeostasis, cell viability, and cell permeability in stationary phase. The parent (BY4743), vps27, ure2, pep3, and ino1 strains were inoculated (A600 of 0.01) in SD medium buffered (25 mM piperazine-N,N-bis(3-propanesulfonic acid)/[2-(N-morpholino)ethane sulfonic acid]) to pH 3.5 or 6.0 and grown to stationary phase (48 h). Intracellular and extracellular glutathione (A) and cell viability (colony-forming units; %) and propidium iodide staining (%) (B) were quantified.
Leucine limitation of S. cerevisiae alters vacuolar morphology, causing cells to form a single large vacuole, whereas leucine repletion leads to accumulation of several fragmented vacuoles (Cakar et al., 2000). It was therefore interesting to note that many mutants that excreted glutathione in a manner less dependent on availability of BCAAs were affected in processes associated with vacuolar function/morphology, including pep3, vps33 (class C vps genes), and luv1 (Bryant and Stevens, 1998; Conboy and Cyert, 2000; Peterson and Emr, 2001). Because H+ stress was associated with increased efflux and the vacuole is known to play an important role in pH regulation (Jones et al., 1997), this indicated that some of these mutants may excrete glutathione in the absence of BCAA limitation due to a higher sensitivity to H+ stress or due to the presence of a general leaky phenotype (ino1, ino4). The former seems unlikely because viability of the pep3 mutant (“BCAA unresponsive”) was not affected by low external pH to the same degree as the vps27 and ure2 mutants, which exhibited highly BCAA-responsive glutathione excretion. Although pep3 cells exhibit a pronounced vacuole fragmentation phenotype (Raymond et al., 1992), and because the vacuole is a key glutathione storage organelle (Penninckx, 2002), these cells still overaccumulated intracellular “whole cell” glutathione during growth in SD medium (our unpublished data). The BCAA-unresponsive mutants might therefore excrete glutathione due to severe disruption of vacuolar function resulting in overaccumulation of cytosolic GSH, relative to the parental strain and the BCAA-responsive mutants. This raised questions on the impact of these mutations and of this potential overaccumulation of cytosolic glutathione on the cellular tolerance to reductive stress.
The Role of Glutathione Metabolism in the Cellular Tolerance to Reductive Stress
The reducing agent DTT has been used to subject S. cerevisiae cells to reductive stress (Cuozzo and Kaiser, 1999; Trotter and Grant, 2002). Consequently, the tolerance of the glutathione-overexcreting mutants was determined by spotting stationary phase cells on medium containing DTT, and growth relative to the parental strain was determined after 2 and 3 d. Sixty-five of the 276 mutants exhibited reduced tolerance to DTT (see supplementary material). A strong correlation was noted between decreased capacity for utilization of GSH as a sole nitrogen source and hypersensitivity to DTT (33 of the 54 strains that could not use GSH as a sole nitrogen source also were sensitive to DTT). Of the 276 glutathione-overexcreting mutants only four (egd1, pmr1, ubp6, doa1) that could use GSH as a sole nitrogen source were found to be DTT hypersensitive. Surprisingly, many of the BCAA-unresponsive mutants, including pep3, pep7, pep12, luv1, and fab1, exhibited the highest sensitivity to DTT with no detectable growth observed after 3 d. This observation is consistent with the hypothesis that vacuolar function is important for DTT tolerance. Vacuolar dysfunction may affect detoxication of DTT and/or lead to overaccumulation of cytosolic glutathione, thereby exacerbating the reductive stress caused by DTT.
These data indicate that the late endosomal pathway for trafficking from the endosome to the vacuole is critical for relief of reductive stress and that this is strongly correlated with the ability of the cell to use glutathione as a sole nitrogen source, which requires its degradation in the vacuole. The class E vps mutants displayed two different phenotypes in terms of DTT tolerance. Those affected in the initial steps of the pathway (Katzmann et al., 2002) identified as the Vps27 and ESCRT-I complex components (Vps23, 28, 37) were not sensitive to DTT, whereas those affected in ESCRT-II/III and Vps4 functions were very sensitive to DTT. This reflected the ability of the mutants to use GSH as a sole nitrogen source. Of the class E vps mutants, vps22 was the most sensitive to DTT. Given that this strain also excreted glutathione in a BCAA-unresponsive manner, this gene product may play a more direct role in vacuolar function. Although all class E components are required for sorting of monoubiquitinated cargo at the late endosome and the maturation of this organelle into multivesicular bodies, only the ESCRT-II/III complexes are required for vacuolar transport and/or degradation of glutathione and tolerance to reductive stress. Because glutathione degradation occurs in the vacuole it is not yet possible to discriminate whether relief of reductive stress depends on GSH transport to the vacuole or its degradation because mutations affecting the endosomal pathway also would affect the localization or activity of putative vacuolar membrane glutathione transporters.
DISCUSSION
The S. cerevisiae gene deletion library (Winzeler et al., 1999) has been used effectively to study a broad range of issues, including: the cellular response to Na+ (Warringer et al., 2003), oxidative stress (Thorpe et al., 2004), K1 killer toxin (Page et al., 2003), bleomycin (Aouida et al., 2004), amiodarone (Gupta et al., 2003), UV radiation (Birrell et al., 2001), general cell biology (Bonangelino et al., 2002; Dimmer et al., 2002; Seeley et al., 2002; Lagorce et al., 2003), and modeling of degenerative processes (Outeiro and Lindquist, 2003; Willingham et al., 2003).
Here, we show that reduced glutathione produced endogenously by S. cerevisiae is cycled at a low rate between the cytoplasm and the extracellular medium. Screening of the yeast deletion library (Winzeler et al., 1999) identified a surprisingly large number of genes (276) and cellular processes required for maintaining appropriate glutathione homeostasis. These provide a detailed and novel insight into physiological processes affecting cellular glutathione homeostasis.
This research also provided an interesting perspective on a subset of mutants present in the deletion library that have implications for use of this excellent resource. Many of the glutathione-overexcreting mutants exhibited altered permeability of the plasma membrane to one or more molecules. Evidently, many of these mutants are affected in their ability to maintain H+ homeostasis, cope with H+ stress, or respond appropriately to amino acid limitation, and these factors were associated with abnormal glutathione homeostasis. Disturbances to H+ homeostasis could result from a change in the activity, abundance, and/or localization of key proteins involved in the maintenance of H+ homeostasis, including Pma1, a highly abundant plasma membrane H+-ATPase (Chang and Fink, 1995; Ghaemmaghami et al., 2003). Mutations affecting transcription, translation, cell proliferation, and secretory pathway function also could disrupt the cell's capacity to maintain ion homeostasis and/or influence its integrity.
Although these factors have important implications for glutathione homeostasis, particularly loss of glutathione from cells due to increased leakage, secretion, or excretion, these facts are also worth considering when using the mutant collection (Winzeler et al., 1999) to study cellular responses to toxic agents/stress. Care is needed to establish whether the reduced fitness of a mutant after exposure to a particular stress is due to the toxicity of the agent or in some manner associated with disruption of a cellular process that is already substantially affected (e.g., plasma membrane integrity or H+/glutathione homeostasis). The proportion of viable cells and intracellular glutathione level during these tests must be considered, especially if strains are pregrown to stationary phase before assessing stress tolerance. Despite the above-mentioned caveat, careful consideration of the data has extended considerably knowledge of the cellular processes influencing glutathione homeostasis.
It was surprising that mutants affected in secretory pathway function, specifically in the late endosome-to-vacuole pathway, were the highest excretors of glutathione. It is unlikely that glutathione efflux is mediated via Golgi-to-plasma membrane vesicular transport for several reasons. First, in actively growing wild-type cells glutathione efflux is low, under conditions in which a considerable quantity of material is delivered to the plasma membrane from the Golgi (Kaiser et al., 1997), and this trafficking would not be expected to increase dramatically in the glutathione overexcreting mutants. Second, the soluble vacuolar hydrolase carboxypeptidase Y (CPY) is transported to the vacuolar lumen via the late endosome protein-sorting pathway (Kaiser et al., 1997). Disruption of this process leads to elevated excretion of CPY, the extent of which depends on the gene affected (Raymond et al., 1992; Bonangelino et al., 2002). Importantly, in the former screen (Raymond et al., 1992) class E vps mutants excreted low levels of CPY relative to other vps mutants, particularly the class B vps mutants, which excreted only low levels of glutathione. Additionally, a number of mutations not directly involved with secretory pathway protein sorting also lead to moderate-to-high CPY excretion (Bonangelino et al., 2002), but do not cause glutathione overexcretion. These data are not consistent with the hypothesis that glutathione overexcretion by class E vps mutants arose from diversion of trans-Golgi vesicles en route to the late endosome, to the plasma membrane.
Of the mutants tested, the trend in increased membrane permeability closely followed the trend in intracellular glutathione overaccumulation; however, appearance of glutathione in the extracellular medium was delayed. Because intracellular glutathione overaccumulation preceded onset of efflux and intracellular depletion, these data are not consistent with complete loss of cell integrity and “simple” leakage of glutathione from the cell, in the early stages of stationary phase. If this were the case, then glutathione would not be expected to overaccumulate in the intracellular fraction before its detection in the extracellular medium unless altered membrane permeability was caused by intracellular glutathione overaccumulation (discussed below). The data indicate that increased accumulation of intracellular glutathione occurred in concert with a gradual change in plasma membrane permeability. This overaccumulation is consistent with loss of glutathione from the cytosol to a subcellular compartment followed by its repletion in the cytosolic fraction through heightened biosynthesis. Repletion of intracellular glutathione under these conditions would have required some degree of metabolic activity, particularly during the period of intracellular glutathione overaccumulation, and this response occurred despite the onset of PI staining. Glutathione might normally traffic to the vacuole via the late endosome pathway, after transport of cytosolic glutathione across the limiting membrane of the late endosome. Disruption of late endosome to vacuole trafficking could then lead to excretion of glutathione via endosome to plasma membrane vesicular trafficking. This model would account for the glutathione concentration in the intracellular fraction before its efflux.
The two key characteristics of glutathione efflux were that extracellular glutathione accumulated predominantly in the reduced form via a mechanism strongly influenced by extracellular pH. Cellular H+ homeostasis is influenced by the flux of other cations across cellular membranes, particularly K+ and Na+ (Rodriguez-Navarro, 2000; Serrano and Rodriguez-Navarro, 2001; Yenush et al., 2002). Altered K+/Na+ homeostasis and/or membrane potential therefore also may affect glutathione compartmentalization and/or efflux. These observations are important because they indicate that movement/transport of glutathione, which constitutes a highly abundant anionic species at cytosolic pH, is affected by cellular ion homeostasis. Glutathione concentration in to these compartments, in an analogous manner to the chloride ion (Weisz, 2003), could provide a counterbalancing charge to cation flux thereby influencing the kinetics of cation transport or vice versa. In this model, overaccumulation and depletion of intracellular glutathione are in principle part of the same phenomenon i.e., loss of glutathione from the cytosolic pool in to a compartment. Whether repletion or depletion of the cytosolic pool occurs depends on the capacity of the cell to replace glutathione via synthesis. This hypothesis is supported by the observation that glutathione and glutathione S-conjugate transport to the vacuole is affected by vacuolar membrane potential (Li et al., 1996; Mehdi et al., 2001).
In vps27 cells, the late endosome “adopts” some functions of the vacuole. These cells accumulate an acidic late endosome, which is juxtaposed to a vacuole with a pH neutral lumen (Jones et al., 1997). This aspect of H+ homeostasis in class E vps mutants also could influence the kinetics of “anionic” glutathione transport into the endosomal system. Perhaps a glutathione transporter that is normally localized to the vacuolar membrane, or a cation transporter, accumulates on the late endosome membrane in class E (or other) vps mutants, facilitating hyperaccumulation of glutathione in the late endosome. Disruption of vps36 was previously shown to affect trafficking of a mutant form of Pma1 (Luo and Chang, 2000). Although the H+ v-ATPase is known to overaccumulate on the late endosomes of class E vps mutants (Raymond et al., 1992), and H+ flux through this pump may influence glutathione flux, deletion of genes encoding components of the v-ATPase also led to increased accumulation of extracellular glutathione. Overaccumulation of intracellular glutathione in vps27 cells at pH 6 indicates that the tripeptide may be concentrated in the endosomal system (or another cellular compartment) under these conditions; however, decreasing external pH may be required to stimulate endosome to plasma membrane vesicular transport, promoting glutathione efflux. Interestingly, in sea urchin eggs exocytosis leads to release of lumenal H+ in to the extracellular medium, and low external pH has an inhibitory effect on endocytosis (Smith et al., 2002). At present, the effect of external pH on the kinetics of endosome to plasma membrane vesicular transport does not seem to have been studied.
Overaccumulation of extracellular glutathione also could be due to direct outward leakage across the plasma membrane, although intracellular glutathione would have occurred at a time when it was “lost” from the cell at heightened levels. In some mutants, intracellular glutathione overaccumulation could have contributed to the loss of membrane integrity, and the subsequent depletion of glutathione from cells. In support of this idea disruption of key negative modulators of the RAS/protein kinase A carbon signaling (pde2, ira2) and TOR signaling (ure2) pathways overaccumulated intracellular glutathione. Inappropriate regulation of plasma (or other) membrane transport also could promote glutathione oversynthesis. Glutathione is proposed to place a load on the ER disulphide bond-forming machinery, where selective transport of reduced glutathione in to the ER lumen (Banhegyi et al., 2003) is thought to prevent the genesis of hyperoxidizing conditions in this compartment (Cuozzo and Kaiser, 1999). Interestingly, overexpression of GSH1 (encoding γ-glutamylcysteine synthetase) in wild-type cells elevates intracellular glutathione (Grant et al., 1997) but does not affect extracellular glutathione levels (our unpublished data). Disruption of certain cellular processes could lead to increased glutathione production as well as reduce the cells capacity to tolerate excess intracellular glutathione, leading to plasma membrane permeabilization.
BCAA limitation-associated membrane permeabilization could result from a reduced ability to regulate or maintain plasma membrane proteins, an inability to modulate cell proliferation in response to nutrient limitation, or heightened glutathione synthesis due to diversion of amino acid flux to glutathione biosynthesis. Synthesis of glutamate, a key molecule in nitrogen metabolism, is stimulated in response to leucine limitation (Dickinson, 1999). Importantly, mutants affected in nitrogen metabolism, including ure2, encoding a key negative regulator of the TOR signaling pathway, exhibited the most responsive BCAA limitation-associated changes in glutathione homeostasis and membrane permeability, highlighting the association between nitrogen metabolism and glutathione homeostasis. Leucine limitation of wild-type yeast cells leads to a change in vacuolar morphology from several fragmented vacuoles (leucine replete cells) to a single large organellar structure (leucine starved cells; Cakar et al., 2000). Because leucine limitation also leads to overaccumulation of intracellular glutathione, this change in vacuolar morphology may occur, in part, to facilitate increased storage/turnover of excess glutathione synthesis resulting from the cellular response to leucine limitation. The BCAA-unresponsive nature of glutathione excretion by the class C vps mutants, which exhibit a severe fragmented vacuole phenotype (Raymond et al., 1992), could result from overaccumulation of cytosolic glutathione in the absence of BCAA limitation.
Exposure of yeast cells to DTT leads to ER stress and activation of an unfolded protein response (Kaufman, 1999). Overaccumulation of intracellular glutathione is proposed to contribute to the DTT hypersensitivity of the yeast thioredoxins mutants (Trotter and Grant, 2002). The data presented here indicate the role of the vacuole in DTT tolerance and intra/extracellular glutathione homeostasis. Although DTT detoxification could be affected in the vacuolar mutants disruption of vacuolar glutathione storage/degradation could exacerbate the deleterious effects of DTT. The differential responses of the class E vps mutants to DTT indicates that although these proteins all play an important role in sorting of cargo in to multivesicular bodies (Raymond et al., 1992), they also affect other aspects of protein sorting in a distinct manner. In general, disruption of processes downstream of ESCRT-I function (Katzmann et al., 2002) led to defective growth on glutathione as a sole nitrogen source and hypersensitivity to DTT, the exception being the vps24 mutant. Using these distinct phenotypical differences the roles of Did2 and Vps60 in late endosome protein sorting, which are reported to contain ESCRT-III-like motifs (Katzmann et al., 2002), also could be hypothesized. From this study, the did2 and vps60 mutants exhibited phenotypes similar to class E vps mutants affected in ESCRT-II/III and ESCRT-I complex processes, respectively. Of the class E vps genes only disruption of VPS24, 28, and 60 are reported to reduce the fitness of cells expressing α-synuclein (Willingham et al., 2003). Although the relevance of this restricted group is unclear, these mutants belong to the subset of strains that exhibited normal DTT tolerance. A better understanding of the nature of the difference in DTT tolerance exhibited by the class E vps mutants may provide additional insight in to the effects of α-synuclein expression on yeast cell biology and of the role of this protein in Parkinson's disease.
Although regulation of glutathione biosynthesis has been studied extensively, there is little known of the way complex interconnected cellular networks influence intra- and intercellular glutathione homeostasis. This genome-wide approach has identified many genes not previously linked to the maintenance of glutathione homeostasis, which is affected by a range of cellular processes that influence its synthesis, degradation, and transport/leakage. This study provides insight in to how genetic and/or environmental factors influence glutathione homeostasis, and these findings may be relevant to our understanding of glutathione homeostasis in higher eukaryotes and how changes in certain cellular processes, including ion homeostasis, may contribute to glutathione depletion and cell degeneration. For example abnormal glutathione metabolism, altered calcium homeostasis, and endoplasmic reticulum, proteasomal, and mitochondrial dysfunction have all been associated with the pathology or Parkinson's disease and/or apoptosis (Schulz et al., 2000; McNaught and Olanow, 2003; Verkhratsky and Toescu, 2003; Tretter et al., 2004). Here, we found that disruption of any one of these processes is sufficient to lead to pronounced changes in glutathione homeostasis.
Supplementary Material
Acknowledgments
We thank Michael Breitenbach and Glenn Wheeler for helpful discussions. This research was supported by grants from the Australian Research Council, the Millennium Award from the Ramaciotti Foundations, and an Australia Postgraduate Award to G.G.P.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-07-0560. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0560.
Abbreviations used: BCAA, branched chain amino acid; vps, vacuolar protein sorting.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Abramov, A. Y., Canevari, L., and Duchen, M. R. (2003). Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J. Neurosci. 23, 5088-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouida, M., Page, N., Leduc, A., Peter, M., and Ramotar, D. (2004). A genome-wide screen in Saccharomyces cerevisiae reveals altered transport as a mechanism of resistance to the anticancer drug bleomycin. Cancer Res. 64, 1102-1109. [DOI] [PubMed] [Google Scholar]
- Balshaw, D. M., Xu, L., Yamaguchi, N., Pasek, D. A., and Meissner, G. (2001). Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 276, 20144-20153. [DOI] [PubMed] [Google Scholar]
- Banhegyi, G., Csala, M., Nagy, G., Sorrentino, V., Fulceri, R., and Benedetti, A. (2003). Evidence for the transport of glutathione through ryanodine receptor channel type 1. Biochem. J. 376, 807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister, S. J., and Wittrup, K. D. (2000). Glutathione excretion in response to heterologous protein secretion in Saccharomyces cerevisiae. Biotechnol. Bioeng. 68, 389-395. [DOI] [PubMed] [Google Scholar]
- Bartoli, G. M., and Sies, H. (1978). Reduced and oxidized glutathione efflux from liver. FEBS Lett. 86, 89-91. [DOI] [PubMed] [Google Scholar]
- Bass, R., Ruddock, L. W., Klappa, P., and Freedman, R. B. (2004). A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J. Biol. Chem. 279, 5257-5262. [DOI] [PubMed] [Google Scholar]
- Birrell, G. W., Giaever, G., Chu, A. M., Davis, R. W., and Brown, J. M. (2001). A genome-wide screen in Saccharomyces cerevisiae for genes affecting UV radiation sensitivity. Proc. Natl. Acad. Sci. USA 98, 12608-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino, C. J., Chavez, E. M., and Bonifacino, J. S. (2002). Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2486-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbouloux, A., Shahi, P., Chakladar, A., Delrot, S., and Bachhawat, A. K. (2000). Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275, 13259-13265. [DOI] [PubMed] [Google Scholar]
- Bowers, K., Levi, B. P., Patel, F. I., and Stevens, T. H. (2000). The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 11, 4277-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N. J., and Stevens, T. H. (1998). Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. Mol. Biol. Rev. 62, 230-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakar, Z. P., Sauer, U., Bailey, J. E., Muller, M., Stolz, M., Wallimann, T., and Schlattner, U. (2000). Vacuolar morphology and cell cycle distribution are modified by leucine limitation in auxotrophic Saccharomyces cerevisiae. Biol. Cell 92, 629-637. [DOI] [PubMed] [Google Scholar]
- Carmel-Harel, O., and Storz, G. (2000). Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54, 439-461. [DOI] [PubMed] [Google Scholar]
- Chang, A., and Fink, G. R. (1995). Targeting of the yeast plasma membrane H+-ATPase: a novel gene AST1 prevents mislocalization of mutant ATPase to the vacuole. J. Cell Biol. 128, 39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnubben, N.H.P., Rietjens, I., Wortelboer, H., van Zanden, J., and van Bladeren, P. J. (2001). The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharmacol. 10, 141-152. [DOI] [PubMed] [Google Scholar]
- Conboy, M. J., and Cyert, M. S. (2000). Luv1p/Rki1p/Tcs3p/Vps54p, a yeast protein that localizes to the late Golgi and early endosome, is required for normal vacuolar morphology. Mol. Biol. Cell 11, 2429-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuozzo, J. W., and Kaiser, C. A. (1999). Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1, 130-135. [DOI] [PubMed] [Google Scholar]
- Deere, D., Shen, J., Vesey, G., Bell, P., Bissinger, P., and Veal, D. (1998). Flow cytometry and cell sorting for yeast viability assessment and cell selection. Yeast 14, 147-160. [DOI] [PubMed] [Google Scholar]
- Dickinson, R. (1999). Nitrogen metabolism. In: The Metabolism and Molecular Physiology of Saccharomyces cerevisiae, ed. R. Dickinson and M. Schweizer, Padstow: Taylors & Francis, 57-72.
- Dimmer, K. S., Fritz, S., Fuchs, F., Messerschmitt, M., Weinbach, N., Neupert, W., and Westermann, B. (2002). Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, T. D., Folley, L. S., Mulero, J. J., McMullin, T. W., Thorsness, P. E., Hedin, L. O., and Costanzo, M. C. (1991). Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 194, 149-165. [DOI] [PubMed] [Google Scholar]
- Fry, C. J., and Peterson, C. L. (2001). Chromatin remodeling enzymes: who's on first? Curr. Biol. 11, R185-R197. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., and Weissman, J. S. (2003). Global analysis of protein expression in yeast. Nature 425, 737-741. [DOI] [PubMed] [Google Scholar]
- Grandier-Vazeille, X., Bathany, K., Chaignepain, S., Camougrand, N., Manon, S., and Schmitter, J. M. (2001). Yeast mitochondrial dehydrogenases are associated in a supramolecular complex. Biochemistry 40, 9758-9769. [DOI] [PubMed] [Google Scholar]
- Grant, C. M., MacIver, F. H., and Dawes, I. W. (1997). Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol. Biol. Cell 8, 1699-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, C. M., Perrone, G., and Dawes, I. W. (1998). Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 253, 893-898. [DOI] [PubMed] [Google Scholar]
- Gupta, S. S., Ton, V. K., Beaudry, V., Rulli, S., Cunningham, K., and Rao, R. (2003). Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 278, 28831-28839. [DOI] [PubMed] [Google Scholar]
- Hammond, C. L., Lee, T. K., and Ballatori, N. (2001). Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J. Hepatol. 34, 946-954. [DOI] [PubMed] [Google Scholar]
- Hwang, C., Sinskey, A. J., and Lodish, H. F. (1992). Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257, 1496-1502. [DOI] [PubMed] [Google Scholar]
- Izawa, S., Inoue, Y., and Kimura, A. (1995). Oxidative stress response in yeast: effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 368, 73-76. [DOI] [PubMed] [Google Scholar]
- Jones, E., Webb, G., and Hiller, M. (1997). Biogenesis and function of the yeast vacuole. In: The Molecular and Cellular Biology of the Yeast Saccharomyces cerevisiae: Cell Cycle and Cell Biology, ed. J. Pringle, J. Broach, and E. Jones, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 363-470.
- Kaiser, C. A., Gimeno, R., and Shaywitz, D. (1997). Protein secretion, membrane biogenesis, and endocytosis. In: The Molecular and Cellular Biology of the Yeast Saccharomyces cerevisiae: Cell Cycle and Cell Biology, ed. J. Pringle, J. Broach, and E. Jones, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 91-228.
- Katzmann, D. J., Odorizzi, G., and Emr, S. D. (2002). Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 3, 893-905. [DOI] [PubMed] [Google Scholar]
- Kaufman, R. J. (1999). Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211-1233. [DOI] [PubMed] [Google Scholar]
- Lagorce, A., Hauser, N. C., Labourdette, D., Rodriguez, C., Martin-Yken, H., Arroyo, J., Hoheisel, J. D., and Francois, J. (2003). Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278, 20345-20357. [DOI] [PubMed] [Google Scholar]
- Lee, J. C., Straffon, M. J., Jang, T. Y., Higgins, V. J., Grant, C. M., and Dawes, I. W. (2001). The essential and ancillary role of glutathione in Saccharomyces cerevisiae analysed using a grande gsh1 disruptant strain. FEMS Yeast Res. 1, 57-65. [DOI] [PubMed] [Google Scholar]
- Lemmon, S. K., and Traub, L. M. (2000). Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol. 12, 457-466. [DOI] [PubMed] [Google Scholar]
- Li, Z. S., Szczypka, M., Lu, Y. P., Thiele, D. J., and Rea, P. A. (1996). The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 271, 6509-6517. [DOI] [PubMed] [Google Scholar]
- Lou, H., Ookhtens, M., Stolz, A., and Kaplowitz, N. (2003). Chelerythrine stimulates GSH transport by rat Mrp2 (Abcc2) expressed in canine kidney cells. Am. J. Physiol. 285, G1335-G1344. [DOI] [PubMed] [Google Scholar]
- Luo, W., and Chang, A. (2000). An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol. Biol. Cell 11, 579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., Frohlich, E., Ligr, M., Grey, M., Sigrist, S. J., Wolf, D. H., and Frohlich, K. U. (1999). Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145, 757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik, B., and Kaiser, C. A. (2002). Nitrogen regulation in Saccharomyces cerevisiae. Gene 290, 1-18. [DOI] [PubMed] [Google Scholar]
- Maris, A. F., Kern, A. L., Picada, J. N., Boccardi, F., Brendel, M., and Henriques, J. A. (2000). Glutathione, but not transcription factor Yap1, is required for carbon source-dependent resistance to oxidative stress in Saccharomyces cerevisiae. Curr. Genet. 37, 175-182. [DOI] [PubMed] [Google Scholar]
- Masuda, Y., Ozaki, M., and Aoki, S. (1993). K+-driven sinusoidal efflux of glutathione disulfide under oxidative stress in the perfused rat liver. FEBS Lett. 334, 109-113. [DOI] [PubMed] [Google Scholar]
- McNaught, K. S., and Olanow, C. W. (2003). Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson's disease. Ann. Neurol. 53 (suppl 3), S73-S84; discussion, S84-S76. [DOI] [PubMed] [Google Scholar]
- Mehdi, K., and Penninckx, M. J. (1997). An important role for glutathione and gamma-glutamyltranspeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae. Microbiology 143 (Pt 6), 1885-1889. [DOI] [PubMed] [Google Scholar]
- Mehdi, K., Thierie, J., and Penninckx, M. J. (2001). gamma-Glutamyl transpeptidase in the yeast Saccharomyces cerevisiae and its role in the vacuolar transport and metabolism of glutathione. Biochem. J. 359, 631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, A. (1995). Mitochondrial changes associated with glutathione deficiency. Biochim. Biophys. Acta 1271, 35-42. [DOI] [PubMed] [Google Scholar]
- Meister, A., and Anderson, M. E. (1983). Glutathione. Annu. Rev. Biochem. 52, 711-760. [DOI] [PubMed] [Google Scholar]
- Miyake, T., Sammoto, H., Kanayama, M., Tomochika, K., Shinoda, S., and Ono, B. (1999). Role of the sulphate assimilation pathway in utilization of glutathione as a sulphur source by Saccharomyces cerevisiae. Yeast 15, 1449-1457. [DOI] [PubMed] [Google Scholar]
- Mori, K. (2000). Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101, 451-454. [DOI] [PubMed] [Google Scholar]
- Muchardt, C., and Yaniv, M. (2001). When the SWI/SNF complex remodels the cell cycle. Oncogene 20, 3067-3075. [DOI] [PubMed] [Google Scholar]
- Nestelbacher, R., Laun, P., Vondrakova, D., Pichova, A., Schuller, C., and Breitenbach, M. (2000). The influence of oxygen toxicity on yeast mother cell-specific aging. Exp. Gerontol. 35, 63-70. [DOI] [PubMed] [Google Scholar]
- Ohlmeier, S., Kastaniotis, A. J., Hiltunen, J. K., and Bergmann, U. (2004). The yeast mitochondrial proteome, a study of fermentative and respiratory growth. J. Biol. Chem. 279, 3956-3979. [DOI] [PubMed] [Google Scholar]
- Ookhtens, M., Hobdy, K., Corvasce, M. C., Aw, T. Y., and Kaplowitz, N. (1985). Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J. Clin. Investig. 75, 258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro, T. F., and Lindquist, S. (2003). Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302, 1772-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, N., et al. (2003). A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics 163, 875-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx, M. J. (2002). An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2, 295-305. [DOI] [PubMed] [Google Scholar]
- Peterson, M. R., and Emr, S. D. (2001). The class C Vps complex functions at multiple stages of the vacuolar transport pathway. Traffic 2, 476-486. [DOI] [PubMed] [Google Scholar]
- Pullar, J. M., and Hampton, M. B. (2002). Diphenyleneiodonium triggers the efflux of glutathione from cultured cells. J. Biol. Chem. 277, 19402-19407. [DOI] [PubMed] [Google Scholar]
- Raymond, C. K., Howald-Stevenson, I., Vater, C. A., and Stevens, T. H. (1992). Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro, A. (2000). Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469, 1-30. [DOI] [PubMed] [Google Scholar]
- Schafer, F. Q., and Buettner, G. R. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Rad. Biol. Med. 30, 1191-1212. [DOI] [PubMed] [Google Scholar]
- Schulz, J. B., Lindenau, J., Seyfried, J., and Dichgans, J. (2000). Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 267, 4904-4911. [DOI] [PubMed] [Google Scholar]
- Seeley, E. S., Kato, M., Margolis, N., Wickner, W., and Eitzen, G. (2002). Genomic analysis of homotypic vacuole fusion. Mol. Biol. Cell 13, 782-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, R., Kielland-Brandt, M. C., and Fink, G. R. (1986). Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 319, 689-693. [DOI] [PubMed] [Google Scholar]
- Serrano, R., and Rodriguez-Navarro, A. (2001). Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 13, 399-404. [DOI] [PubMed] [Google Scholar]
- Shivakumar, B. R., Kolluri, S. V., and Ravindranath, V. (1995). Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J. Pharmacol. Exp. Ther. 274, 1167-1173. [PubMed] [Google Scholar]
- Slater, E. (1967). Application of inhibitors and uncouplers for a study of oxidative phosphorylation. Methods Enzymol. 10, 48-57. [Google Scholar]
- Smith, R. M., Baibakov, B., Lambert, N. A., and Vogel, S. S. (2002). Low pH inhibits compensatory endocytosis at a step between depolarization and calcium influx. Traffic 3, 397-406. [DOI] [PubMed] [Google Scholar]
- Soltaninassab, S. R., Sekhar, K. R., Meredith, M. J., and Freeman, M. L. (2000). Multi-faceted regulation of gamma-glutamylcysteine synthetase. J. Cell. Physiol. 182, 163-170. [DOI] [PubMed] [Google Scholar]
- Sudarsanam, P., and Winston, F. (2000). The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 16, 345-351. [DOI] [PubMed] [Google Scholar]
- Sugiyama, K., Izawa, S., and Inoue, Y. (2000). The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae. J. Biol. Chem. 275, 15535-15540. [DOI] [PubMed] [Google Scholar]
- Tavazoie, S., Hughes, J. D., Campbell, M. J., Cho, R. J., and Church, G. M. (1999). Systematic determination of genetic network architecture. Nat. Genet. 22, 281-285. [DOI] [PubMed] [Google Scholar]
- ter Schure, E. G., van Riel, N. A., and Verrips, C. T. (2000). The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 24, 67-83. [DOI] [PubMed] [Google Scholar]
- Thevelein, J. M., and de Winde, J. H. (1999). Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33, 904-918. [DOI] [PubMed] [Google Scholar]
- Thorpe, G. W., Fong, C. S., Alic, N., Higgins, V. J., and Dawes, I. W. (2004). Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 101, 6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers, K. J., Patil, C. K., Wodicka, L., Lockhart, D. J., Weissman, J. S., and Walter, P. (2000). Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249-258. [DOI] [PubMed] [Google Scholar]
- Tretter, L., Sipos, I., and Adam-Vizi, V. (2004). Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem. Res. 29, 569-577. [DOI] [PubMed] [Google Scholar]
- Trotter, E. M., and Grant, C. M. (2002). Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 43, 869-878. [DOI] [PubMed] [Google Scholar]
- Turton, H. E., Dawes, I. W., and Grant, C. M. (1997). Saccharomyces cerevisiae exhibits a yAP-1-mediated adaptive response to malondialdehyde. J. Bacteriol. 179, 1096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte, C., Guizon, I., Genestie-Denis, I., Vannier, B., and Lorenzon, G. (1994). A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol. Toxicol. 10, 415-421. [DOI] [PubMed] [Google Scholar]
- Verkhratsky, A., and Toescu, E. C. (2003). Endoplasmic reticulum Ca2+ homeostasis and neuronal death. J. Cell. Mol. Med. 7, 351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin, C., Weber, S. G., and Sandberg, M. (1999). Glutathione efflux induced by NMDA and kainate: implications in neurotoxicity? J. Neurochem. 73, 1566-1572. [DOI] [PubMed] [Google Scholar]
- Warringer, J., Ericson, E., Fernandez, L., Nerman, O., and Blomberg, A. (2003). High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl. Acad. Sci. USA 100, 15724-15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz, O. A. (2003). Organelle acidification and disease. Traffic 4, 57-64. [DOI] [PubMed] [Google Scholar]
- Willingham, S., Outeiro, T. F., DeVit, M. J., Lindquist, S. L., and Muchowski, P. J. (2003). Yeast genes that enhance the toxicity of a mutant Huntingtin fragment or alpha-synuclein. Science 302, 1769-1772. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A., et al. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901-906. [DOI] [PubMed] [Google Scholar]
- Yenush, L., Mulet, J. M., Arino, J., and Serrano, R. (2002). The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21, 920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.