Abstract
A fraction of the nuclear estrogen receptor α (ERα) is localized to the plasma membrane region of 17β-estradiol (E2) target cells. We previously reported that ERα is a palmitoylated protein. To gain insight into the molecular mechanism of ERα residence at the plasma membrane, we tested both the role of palmitoylation and the impact of E2 stimulation on ERα membrane localization. The cancer cell lines expressing transfected or endogenous human ERα (HeLa and HepG2, respectively) or the ERα nonpalmitoylable Cys447Ala mutant transfected in HeLa cells were used as experimental models. We found that palmitoylation of ERα enacts ERα association with the plasma membrane, interaction with the membrane protein caveolin-1, and nongenomic activities, including activation of signaling pathways and cell proliferation (i.e., ERK and AKT activation, cyclin D1 promoter activity, DNA synthesis). Moreover, E2 reduces both ERα palmitoylation and its interaction with caveolin-1, in a time- and dose-dependent manner. These data point to the physiological role of ERα palmitoylation in the receptor localization to the cell membrane and in the regulation of the E2-induced cell proliferation.
INTRODUCTION
The sex steroid 17β-estradiol (E2) acts by binding to its nuclear receptors (i.e., ERα and ERβ) that then transactivate target genes. In addition, E2 induces rapid, nongenomic actions involving plasma membrane-associated signaling that require a membrane ER (Coleman and Smith, 2001; Kelly and Levin, 2001; Jakacka et al., 2002; Marino et al., 2002). Although different structural and functional properties have been reported for the membrane-associated ER by comparison with nuclear ERα and ERβ (Ropero et al., 2002; Toran-Allerand et al., 2002; Deecher et al., 2003), immunocytochemical studies revealed the presence of a significant fraction of nuclear ER also on the plasma membrane (Pappas et al., 1995; Norfleet et al., 1999; Dan et al., 2003; Razandi et al., 2003; Arvanitis et al., 2004; Song et al., 2004). In addition, a single mRNA originates a similarly sized nuclear and membrane ER in ERα-transfected Chinese hamster ovary and HeLa cells (Razandi et al., 1999; Marino et al., 2002, 2003). Thus, ERα localizes to both the nucleus and the plasma membrane. Moreover, the membrane ERα is emerging as the primary endogenous mediator of E2 rapid responses important in cell proliferation (Marino et al., 1998, 2002; Castoria et al., 1999, 2001; Razandi et al., 1999, 2000; Lobenhofer et al., 2000; Acconcia et al., 2004a; Fernando and Wimalasena, 2004).
Debate is open regarding the structural bases and the mechanisms for ERα maintenance at and translocation to the plasma membrane. ERα does not display any intrinsic trans-membrane domain (Song et al., 2004); thus, ERα interaction with specific membrane proteins have been proposed to explain its membrane localization (Chambliss and Shaul, 2002; Chambliss et al., 2002; Migliaccio et al., 2002; Razandi et al., 2002, 2003; Toran-Allerand et al., 2002; Arvanitis et al., 2004). In particular, the Ser522 residue has been reported as necessary for ERα interaction with caveolin-1. However, the expression of ERα-Ser522Ala mutant in Chinese hamster ovary cells decreased only 60% of ERα on the membrane, extracellular signal-regulated kinase (ERK) activation, and cyclin D1 gene transcription (Razandi et al., 2003). Thus, the direct ERα:caveolin-1 interaction could not be the sole molecular mechanism for ERα membrane association. Furthermore, because the protein:protein interaction occurs only after some minutes of E2 stimulation (5-15 min) (Song et al., 2004), this mechanism does not justify the receptor presence at the plasma membrane of unstimulated target cells reported previously (Pappas et al., 1995; Norfleet et al., 1999; Razandi et al., 1999; Levin, 2001; Dan et al., 2003). Very recently, we reported that human wild-type ERα underwent palmitoyl acyl transferase (PAT)-dependent S-palmitoylation. Moreover, a point mutation of the ERα-Cys447 residue to Ala completely impaired receptor palmitoylation and the E2-induced rapid activation of the ERK/mitogen-activated protein kinase (MAPK) signaling pathway (Acconcia et al., 2004b), although this mutant binds E2 with the same affinity than the wild-type ERα (Katzenellenbogen et al., 1993; Neff et al., 1994). This observation raises the intriguing hypothesis that the palmitoylation of ERα could be the major determinant for ERα residence at the plasma membrane.
Here, we examine the role of palmitoylation in localizing ERα to the plasma membrane and in promoting the ERα: caveolin-1 interaction, as well as the role of E2 in regulating ERα palmitoylation. Our results indicate that ERα palmitoylation is required for ERα:protein interaction (i.e., caveolin-1) and for the receptor localization to and maintenance at the plasma membrane. As a consequence, ERα palmitoylation enables rapid E2 signaling important for cell proliferation.
MATERIALS AND METHODS
Reagents
17β-Estradiol, gentamicin, penicillin and other antibiotics, GenElute plasmid maxiprep kit, DMEM, RPMI 1640 medium (without phenol red), charcoal-stripped fetal calf serum, and the PAT inhibitor 2-bromohexadecanoic acid (2-bromopalmitate; 2-Br) (IC50 of ∼4 μM; Varner et al., 2003) were purchased from Sigma-Aldrich (St. Louis, MO). The estrogen receptor inhibitor ICI 182,780 was obtained from Tocris Cookson (Ballwin, MO). 9,10-[3H]Palmitic acid (specific activity 57 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Methyl-1-[3H]thymidine (specific activity 81 Ci/mmol) was purchased from Amersham Biosciences UK (Little Chalfont, Buxkinghamshire, United Kingdom). Lipofectamine reagent was obtained from Invitrogen (Carlsbad, CA). The luciferase kit was obtained from Promega (Madison, WI). Bradford protein assay was obtained from Bio-Rad (Hercules, CA). The monoclonal, anti-phospho-ERK, anti-ERα D12 (N terminus), anti-AKT, and anti-β-actin as well as the polyclonal anti-ERK, anti-caveolin-1, and anti-ERα MC20 (C terminus) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal anti-ERα (full-length) antibody was purchased from Novocastra (Newcastle, United Kingdom). The polyclonal anti-phospho-AKT antibody was purchased from New England Biolabs (Beverly, MA). CDP-Star, chemiluminescence reagent for Western blot was obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). All the other products were from Sigma-Aldrich. Analytical or reagent grade products, without further purification, were used.
Cell Culture and DNA Synthesis
HepG2 and HeLa cells were routinely grown in 5% CO2 in modified, phenol red-free RPMI 1640 or DMEM media containing 10% (vol/vol) charcoal-stripped fetal calf serum, l-glutamine (2 mM), gentamicin (10 μg/ml), and penicillin (100 U/ml). Cells were passaged every 2 d. DNA synthesis was assayed by incubating subconfluent cells (70-80%) with methyl-1-[3H]thymidine (final concentration, 1 μCi/ml). Cells were simultaneously treated with either E2 [final concentration, 10 nM in ethanol/phosphate-buffered saline (PBS) 1:10 (vol/vol)] or vehicle [ethanol/PBS 1:10 (vol/vol)]. Methyl-1-[3H]thymidine incorporation was assayed 1 h after E2 administration as reported previously (Marino et al., 2001a). When indicated, the PAT inhibitor 2-Br was added 30 min before E2 administration (final concentration 10 μM).
Plasmids
The reporter plasmid pXP2-D1-2966-luciferase (pD1), the empty vector (pCMV5), the expression vector pCR3.1-β-galactosidase, wild-type human ERα pSG5-HE0, pSG5-Cys447Ala (human ERα point mutant Cys447 residue to Ala), and pKCR2-HE14 (N-terminal deletion mutant of HE0 lacking A/B and DNA binding domains Δ1-281) have been described previously (Herbert et al., 1994; Marino et al., 2001b, 2002; Acconcia et al., 2004b). A luciferase dose-response curve showed that the maximum effect was obtained when 1.0 μg of plasmids was transfected together with 1.0 μg of pCR3.1-β-galactosidase to normalize for transfection efficiency (∼55-65%). Plasmids were purified for transfection using the GenElute plasmid maxiprep kit according to the manufacturer's instructions.
Transfection and Luciferase Assay
HeLa cells were grown to ∼70% confluence and then transfected using Lipofectamine reagent according to the manufacturer's instructions. Six hours after transfection, the medium was changed, and 24 h after the cells were stimulated with 10 nM E2 for 6 h. The cell lysis procedure as well as the subsequent measurement of luciferase gene expression was performed using the luciferase kit according to the manufacturer's instructions with an PerkinElmer Life and Analytical Sciences (Bad Wildbad, Germany) luminometer. When indicated, the PAT inhibitor 2-Br (final concentration 10 μM) was added 30 min before E2 administration.
Cell Labeling with [3H]Palmitate and Immunoprecipitation
Forty-two hours after transfection with either plasmid containing empty, wild-type ERα or the ERα point mutant, HeLa cells and untransfected HepG2 cells were incubated with 0.5 mCi/ml [3H]palmitate at 37°C for different times ranging between 0 and 240 min. Where indicated, HepG2 and HeLa cells were stimulated with different concentrations of E2 (1, 10, and 100 nM) at different times (10, 60, and 240 min) in the presence of [3H]palmitate. Cells were then washed in ice-cold PBS, harvested by scraping, and lysed in 50 μl of lysis buffer [10 mM Tris, pH 7.5, 1 mM EDTA, 0.5 mM EGTA, 10 mM NaCl, 1% (vol/vol) Triton X-100, and 1% (wt/vol) sodium cholate] containing protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 5 μg/ml aprotinin). The cell lysates were then clarified by centrifugation and immunoprecipitated as described previously (Han et al., 2002). Briefly, equal amounts of soluble cell extracts were incubated with either 2 μg of caveolin-1 antibody or different anti-ERα antibodies. The optimal signal was obtained using 1 μg of anti-ERα D12 (N terminus) together with 1 μg of either anti-ERα AER311 (region 495-595), anti-ERα MC20 (C terminus), or anti-ERα (full-length ERα). The lysates and antibodies were incubated for 90 min at 4°C, and then 20 μl of protein A-agarose was added for 30 min at 4°C. After centrifugation (50,000 × g for 15 min), the supernatant, and the immunoprecipitated proteins were separated in 7-10% SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose and then probed overnight at 4°C with anti-caveolin-1 or anti-ERα MC20 antibodies. The nitrocellulose was stripped and then probed with anti-caveolin-1 or anti-ERα MC20 antibodies. In some experiments, the radioactivity present in immunoprecipitated proteins and in the supernatant was monitored by counting with a Canberra Packard (Milan, Italy) liquid beta-counter.
Confocal Miscroscopy Studies
Wild-type ERα or ERα-Cys447Ala mutant was transiently expressed in HeLa cells grown on coverslips. To visualize only the cytosolic and the plasma membrane distribution of wild-type ERα and ERα-Cys447Ala mutant, HeLa cells were freeze-thawed before fixation in 4% (vol/vol) p-formaldehyde (Mardones and Gonzalez, 2003) and then incubated with MC-20 rabbit polyclonal antibody diluted 1:200 in PBS. After washing, cells were incubated with anti-rabbit fluorescein isothiocyanate-conjugated antibody (Sigma-Aldrich) diluted 1:50 in PBS containing 10 mg/ml bovine serum albumin (BSA). Finally, the slides were mounted in PBS medium containing 80% glycerol and 50 mg/ml 1,4-diazabicyclo[2.2.2]octane (Sigma-Aldrich). Evaluation of antibody specificity was carried out either by omitting the primary antibody or by using nonspecific sera. The confocal imaging was performed on a Radiance 2000 confocal laser scanning microscope (Bio-Rad), equipped with a Nikon 40×, 1.4 numerical aperture objective and with Krypton ion lasers.
Electrophoresis and Immunoblotting
After treatment, cells were lysed and solubilized in 0.125 M Tris, pH 6.8, containing 10% (wt/vol) SDS, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml leupeptin, and then the cell lysates were boiled for 2 min. Total proteins were quantified using the Bradford protein assay (Bradford, 1976). Solubilized proteins (20 μg) were resolved by 10% SDS-PAGE at 100 V for 1 h at 24°C and then electrophoretically transferred to nitrocellulose for 45 min at 100 V and 4°C. The nitrocellulose was treated with 3% (wt/vol) BSA in 138 mM NaCl, 25 mM Tris, pH 8.0, at 24°C for 1 h and then probed overnight at 4°C with either anti-phospho-ERK or anti-phospho-AKT antibodies. The nitrocellulose was stripped by restore western blot stripping buffer (Pierce Chemical, Rockford, IL) for 10 min at room temperature and then probed with either anti-ERK or anti-AKT and anti-β-actin antibodies. Antibody reaction was visualized with chemiluminescence Western blotting detection reagent (Amersham Biosciences UK). Where indicated, the PAT inhibitor 2-Br (10 μM) was added 30 min before E2 administration.
RESULTS
Palmitoylation Is Required for ERα Localization to the Plasma Membrane and Its Interaction with Caveolin-1
We previously demonstrated that ERα was a palmitoylated protein and that the ERα-Cys447 to Ala point mutation impaired palmitoylation of the receptor. This lipid modification was necessary for the induction of nongenomic ERK/MAPK signal transduction pathway (Acconcia et al., 2004b). Based on these findings, we postulated that palmitoylation of ERα be important for the localization of this receptor to the membrane.
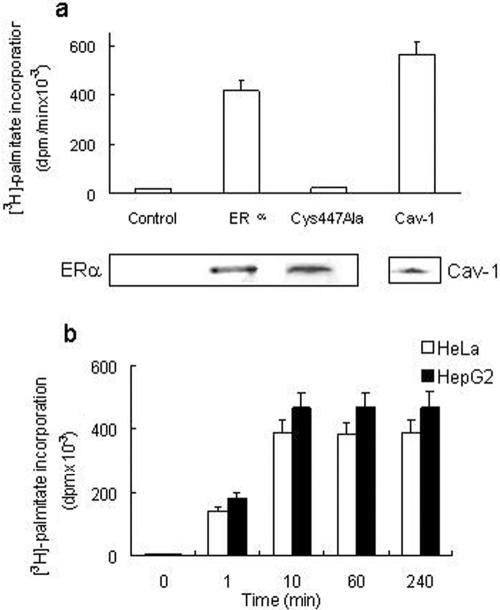
We first verified the occurrence of ERα palmitoylation in human cancer cells containing transfected (HeLa) or endogenous (HepG2) receptor. HeLa cells were transiently transfected with either an empty vector, a wild-type ERα-encoding vector or the ERα missense mutant in which Cys447 was changed to Ala by site-directed mutagenesis (Acconcia et al., 2004b), and then incubated with [3H]palmitate for 4 h at 37°C. After ERα immunoprecipitation, the radioactivity present in both the immunoprecipitate and the supernatant was determined. As expected, the cell transfected with the empty vector (control) did not immunoprecipitate ERα or incorporate [3H]palmitate (Figure 1a). ERα was successfully immunoprecipitated in wild-type ERα-transfected HeLa cells, as revealed by [3H]palmitate incorporation in the 67-kDa band corresponding to ERα (Figure 1a). The ERα-Cys447Ala mutant containing cells immunoprecipitate ERα but failed to incorporate [3H]palmitate (Figure 1a). ERα was not detected in the supernatant fractions (our unpublished data). In addition, the radioactivity present in the palmitoylated caveolin-1 (22-kDa band; Resh, 1999) immunoprecipitated from untransfected HeLa cells was detected as positive control (cav-1) (Figure 1a). ERα palmitoylation was essentially complete within 10 min and remained constant >240 min (Figure 1b). No difference in [3H]palmitate incorporation occurred between ERα-transfected HeLa cells and HepG2 cells (Figure 1b).
Figure 1.
ERα palmityolation. (a) [3H]Palmitate incorporation into the empty vector (control) or wild-type ERα or ERα-Cys447Ala mutant-transfected HeLa cells and into immunoprecipitated caveolin-1 (Cav-1) of untransfected HeLa cells has been evaluated after 4 h of palmitate labeling (top). Western blot analysis of the corresponding 67-kDa band of immunoprecipitated ERα or the 22-kDa band of immunoprecipitated caveolin-1 (bottom). (b) Time course of [3H]palmitate incorporation into immunoprecipitated ERα in ERα-transfected HeLa and in HepG2 cells. Data are the means of four independent experiments ± SD.
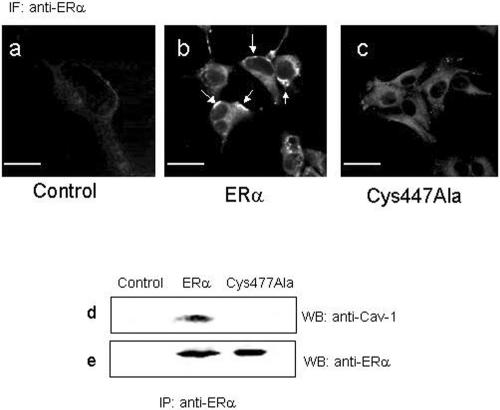
To visualize the cytosolic and/or plasma membrane localization of wild-type and ERα-Cys447Ala mutant, transfected HeLa cells were studied by immunofluorescence staining (Figure 2, a-c). By using the anti-ERα MC20 antibody, a major population of membrane-associated ERα receptor was clearly present in HeLa cells expressing wild-type ERα, whereas a weak cytosolic staining and no membrane-bound receptor could be detected in HeLa cells expressing the ERα-Cys447Ala mutant (Figure 2, compare b with c). Moreover, no ERα was detected in HeLa cells that had been transfected with the empty vector (Figure 2a, control). We conclude that Cys447 is important for surface membrane localization.
Figure 2.
Subcellular localization of ERα and ERα-Cys447Ala mutant and ERα:caveolin-1 interaction. Confocal microscopy analysis of empty vector (a; control), wild-type ERα (b), and ERα-Cys447Ala mutant (c)-transfected HeLa cells by using anti-ERα MC20 antibody on freeze-thawed HeLa cells. White arrows indicate the membrane localization of wild-type ERα. In a, fluorescence signal is superimposed to laser pseudophase contrast images of the cells. Bar, 10 μm. Empty vector (control) or wild-type ERα or ERα-Cys447Ala mutant-transfected HeLa cells were subjected to ERα immunoprecipitation with anti-ERα MC20 antibody followed by Western blot with anti-caveolin-1 antibody (d) or by Western blot with anti-ERα MC20 antibody (e).
The ability of wild-type ERα and of ERα-Cys447Ala mutant to bind the scaffolding plasma membrane protein caveolin-1 was examined by coimmunoprecipitation of caveolin-1 and ERα. This was accomplished in HeLa cells transiently transfected with empty, ERα, or ERα-Cys447Ala expression vectors. No association between caveolin-1 and ERα occurred in cells transfected with the empty vector (control), confirming the lack of any ER isoforms in HeLa cells (Figure 2, d and e, lane control). In ERα-expressing HeLa cells, a 22-kDa band, corresponding to caveolin-1, and a 67-kDa band, corresponding to ERα, were present (Figure 2, d and e, lane ERα). On the other hand, ERα-Cys447Ala mutant-expressing cells failed to interact with caveolin-1 because ERα could be immunoprecipitated, but coimmunoprecipitation of caveolin-1 could not be observed (Figure 2, d and e, lane Cys447Ala). These data are consistent with the role of palmitoylation in ERα localization to the plasma membrane and in the receptor association with caveolin-1.
ERα Palmitoylation Is Negatively Modulated by E2
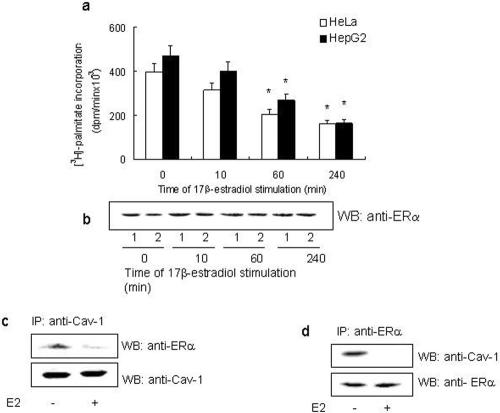
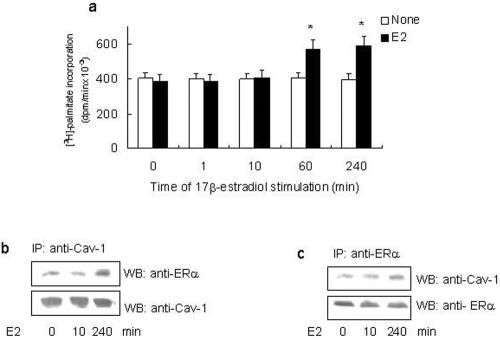
To assess the ability of E2 to modulate ERα palmitoylation, HeLa cells transfected with the ERα expression vector and HepG2 cells were incubated with [3H]palmitate for 4 h in the presence of different E2 concentrations. Physiological E2 concentration (10 nM) decreased by half the amount of [3H]palmitate incorporated in wild-type ERα; similar results were obtained at higher E2 concentrations (i.e., 100 nM), whereas a lower hormone concentration (i.e., 1 nM) was ineffective (our unpublished data). The time course of 10 nM E2 stimulation in cells containing transfected (HeLa) and endogenous (HepG2) ERα showed that 60 min of E2 stimulation induced the decrease of [3H]palmitate incorporation (Figure 3a) without any change in the protein level (Figure 3b). The E2-induced reduction in [3H]palmitate incorporation in both cell lines was completely prevented by pretreating the cells with the ERα inhibitor ICI 182,780 (1 μM; our unpublished data). In addition to 48% reduction of [3H]palmitate incorporation in ERα at 60 min, we found that E2 induced a >90% reduction in the ability of ERα to form a complex with caveolin-1 (Figure 3, c and d). It is important to note that although E2 reduces palmitoylation of ERα and complex formation of ERα with caveolin-1, it had no effect on the cellular protein level of either proteins (Figure 3, c and d). We further asked whether the A/B and C domains of ERα contributed in E2-induced down-regulation of both ERα palmitoylation and its association to caveolin-1. Surprisingly, from 60 min until 240 min, E2 induced a 40% increase in both receptor palmitoylation (Figure 4a) and receptor association with caveolin-1 (Figure 4, b and c) in HeLa cells transfected with ERα deletion mutant lacking the A/B and C domains (HE14).
Figure 3.
E2 effect on ERα palmityolation and ERα association with caveolin-1. Time course (a) and the corresponding Western blot analysis (b) of E2-stimulated ERα-transfected HeLa (open bars; 1) and HepG2 (filled bars; 2) cells. [3H]palmitate incorporation in immunoprecipitated ERα was performed at 4 h. Data are the means of four independent experiments ± SD. *p < 0.001, compared with unstimulated samples (0) were determined by using Student's t test. Wild-type ERα-transfected HeLa cells were subjected to caveolin-1 immunoprecipitation (c) or ERα immunoprecipitation (d) with anti-caveolin-1 or anti-ERα MC20 antibodies followed by Western blot with anti-caveolin-1 or with anti-ERα MC20 antibodies.
Figure 4.
E2 effect on A/B and C domains deleted ERα mutant (HE14) palmityolation and association with caveolin-1. (a) Time course of E2-stimulated ERα-HE14 mutant-transfected HeLa cells. [3H]Palmitate incorporation in immunoprecipitated ERα-HE14 mutant was performed at 4 h in the absence (open bars) or presence (filled bars) of 10 nM E2. Data are the means of four independent experiments ± SD. *p < 0.001, compared with unstimulated samples (open bars), were determined by using Student's t test. ERα-HE14 mutant-transfected HeLa cells were subjected to caveolin-1 immunoprecipitation (b) or ERα immunoprecipitation (c) with anti-caveolin-1 or anti-ERα MC20 antibodies followed by Western blot with anti-caveolin-1 or with anti-ERα MC20 antibodies.
ERα Palmitoylation Is Necessary for Nongenomic Activities
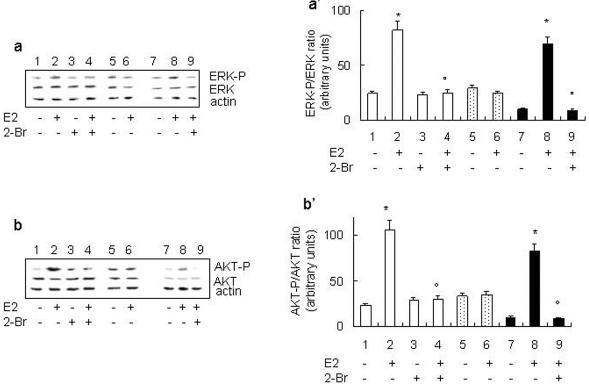
In HepG2 cells, the rapid E2-induced activation of both the ERK/MAPK and phosphoinositide-3-kinase (PI3K)/AKT pathways is sufficient and necessary for E2-induced cell cycle progression (i.e., DNA synthesis and transcription of the cyclin D1 gene) (Marino et al., 2002, 2003). We determined next whether ERα palmitoylation could impact on these rapid nongenomic ERα activities. E2 induced ERK (Figure 5, a and a′) and AKT (Figure 5, b and b′) phosphorylation in ERα-transfected HeLa and HepG2 cells. If the cells were pretreated with the PAT inhibitor 2-Br, the E2-induced activation of signaling kinases was completely blocked without affecting the basal level of phosphorylation. In agreement with our data mentioned above, E2 was ineffective in inducing ERK and AKT phosphorylation in ERα-Cys447Ala mutant-transfected HeLa cells. After reprobing the membranes using total ERK and AKT antibodies to recognize the nonphosphorylated forms of these proteins, we found that E2-induced phosphorylation of signaling proteins occurred in the absence of changes in their expression levels.
Figure 5.
Effect of ERα palmitoylation on E2-induced ERK and AKT phosphorylation. Western blot analysis of ERK phosphorylation in ERα (lanes 1-4) or ERα-Cys447Ala mutant (lanes 5 and 6)-transfected HeLa and HepG2 (lanes 7-9) cells were performed on unstimulated (-) and stimulated (+) cells for 10 min with E2 (10 nM) in the presence or absence of 10 μM PAT inhibitor 2-Br (30-min pretreatment). The same filter was reprobed with anti-total ERK and anti-actin antibodies. (a) Typical Western blot. (a′) Densitometric analysis of four different experiments. Data are the means ± SD. p < 0.001, compared with unstimulated samples (*) or with E2-stimulated samples (°) were determined by using Student's t test. Western blot analysis of AKT phosphorylation in ERα (lanes 1-4) or ERα-Cys447Ala mutant (lanes 5 and 6)-transfected HeLa and HepG2 (lanes 7-9) cells were performed on unstimulated (-) and stimulated (+) cells for 30 min with E2 (10 nM) in the presence or absence of 10 μM PAT inhibitor 2-Br (30-min pretreatment). The same filter was reprobed with anti-total AKT and anti-actin antibodies. (b) Typical Western blot. (b′) Densitometric analysis of four different experiments. Data are the means ± SD. p < 0.001, compared with unstimulated samples (*) or with E2-stimulated samples (°) were determined by using Student's t test.
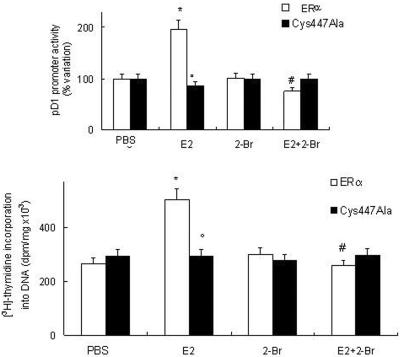
We have recently demonstrated that the ER-dependent cyclin D1 transcription and DNA synthesis are some of the downstream targets of E2-induced ERK and AKT activation (Marino et al., 2002, 2003). Accordingly, in ERα transfected cells, E2 induced cyclin D1 promoter activity and [3H]thymidine incorporation into DNA (Figure 6). In contrast, neither the cyclin D1 promoter activity (Figure 6a) nor [3H]thymidine incorporation into DNA (Figure 6b) was affected by E2 administration to the ERα-Cys447Ala mutant-transfected cells. In addition, the pretreatment of HeLa cells with the PAT inhibitor 2-Br prevented nongenomic E2-evoked effects (Figure 6). Notably, no changes in cyclin D1 promoter basal transcription activity and DNA synthesis were detected after the treatment with the PAT inhibitor 2-Br alone. Thus, palmitoylated ERα mediates the E2-induced activation of ERK, AKT, cyclin D1 promoter activity, and DNA synthesis. These findings demonstrate a critical role of palmitoylation in ERα-mediated cell proliferation.
Figure 6.
Effect of ERα palmitoylation on E2-induced cyclin D1 promoter activity and DNA synthesis. (a) Luciferase assay detection on HeLa cells cotransfected with pXP2-D1-2966-luciferase and wild-type ERα or ERα-Cys447Ala mutant expression vectors. After transfection, cells were treated with vehicle (PBS) or E2 (10 nM) in the presence or absence of 10 μM PAT inhibitor 2-Br (30-min pretreatment). (b) [3H]Thymidine incorporation into DNA in ERα or ERα-Cys447Ala mutant-transfected HeLa cells treated with vehicle (PBS) or E2 (10 nM) in the presence or in absence of 10 μM PAT inhibitor 2-Br (30-min pretreatment). Data are the means ± SD of four independent experiments. *p < 0.001, compared with respective control values (PBS); °p < 0.001, compared with ERα-transfected cells stimulated with E2 values; and #p < 0.001, compared with respective E2 values, were determined by using Student's t test.
DISCUSSION
The mechanism(s) underling the mitogenic role played by E2 in different target tissues is now better understood based on the studies reported by different laboratories, including our own. These studies designate a strict relationship between E2-induced nongenomic functions and cell proliferation (Marino et al., 1998, 2001a,b, 2002, 2003; Castoria et al., 1999, 2001; Razandi et al., 1999, 2000; Lobenhofer et al., 2000; Acconcia et al., 2004a; Fernando and Wimalasena, 2004). The activation of ERK/MAPK, PI3K/AKT, and protein kinase C, rapidly generated after E2 binding to ERα, are all defined as necessary and sufficient for E2-induced cell cycle-regulating genes (e.g., cyclin D1) and the G1-to-S phase progression in different cell lines. These actions are thought to require a plasma membrane ERα.
The idea that surface membrane ER is identical to the nuclear ERα seems now very plausible as deduced by using antibodies raised against multiple epitopes of the nuclear receptor (Pappas et al., 1995; Norfleet et al., 1999; Levin, 2001; Dan et al., 2003). Moreover, the ERα presence on the human cell plasma membrane and the ERα role in the cellular effects of E2 are increasingly accepted (Razandi et al., 1999; Simoncini et al., 2000; Kousteni et al., 2001; Wise et al., 2001).
Despite clear progress, many questions are still unanswered such as the structural bases and the mechanism(s) for ERα localization to and maintenance at the plasma membrane. This issue has been addressed in a few papers that indicate that the ERα membrane localization could result from receptor interaction with specific membrane proteins and/or lipid modifications (Chambliss and Shaul, 2002; Chambliss et al., 2002; Razandi et al., 2002; Li et al., 2003; Acconcia et al., 2004b; Arvanitis et al., 2004). In particular, in the region of plasma membrane, ERα can form a third-party protein (i.e., Shc/ERα/insulin-like growth factor-1 [IGF-1] receptor) and/or associates in a ternary complex to the membrane tyrosine kinase Src and p85 (the catalytic subunit of PI3K), causing Src/Ras/ERK and PI3K/AKT pathway activation and DNA synthesis (Castoria et al., 2001; Migliaccio et al., 2002; Song et al., 2004). This association occurs only after some minutes of E2 stimulation (5-15 min), suggesting a recruitment of ERα from the cytosol, but does not clarify the receptor presence at the plasma membrane reported in several papers (Pappas et al., 1995; Norfleet et al., 1999; Razandi et al., 1999; Levin, 2001; Dan et al., 2003). Recently, the Ser522 residue within the E domain of ERα has been reported to be critical in linking the receptor to the cell membrane through an interaction with caveolin-1 (Razandi et al., 2003). However, the expression of ERα-Ser522Ala mutant in Chinese hamster ovary cells decreased only 60% of ERα on the membrane, ERK activation, and cyclin D1 gene transcription, suggesting that Ser522 is not the sole residue for ERα membrane association. We recently identified the ERα posttranslational modification with palmitate (Acconcia et al., 2004b), and here, for the first time, we demonstrate that palmitoylation is a determinant for ERα residence at the plasma membrane caveolae. In fact, we prove that ERα localization to the membrane is dependent on ERα palmitoylation because the unpalmitoylable ERα-Cys477Ala mutant, which maintains an intact Ser522 residue, is unable to localize to the membrane, to interact with caveolin-1, and to generate the E2-induced rapid membrane-starting signal pathways important in cell proliferation. Thus, present data describe a new model in which Cys447 residue in the E domain of the ERα assures the receptor localization to and maintenance at the plasma membrane caveolae. Moreover, the A/B and C domains of ERα do not contribute to membrane ER localization; in fact, HE14 ERα deletion mutant, which contains only E and F domains, still undergoes to palmitoylation and associates to caveolin-1.
One of the main findings in this study is the time- and concentration-dependent negative regulation of wild-type ERα palmitoylation exerted by E2. Because palmitoylation is a rapid reversible chemical modification (Bijlmakers and Marsh, 2003), it is most likely that the membrane-bound full-length receptor undergoes to the E2-induced depalmitoylation. Moreover, the E2 binding to the soluble, cytosolic, receptor, enacting ERα structural modifications (Brzozowski et al., 1997), could impair the PAT action (i.e., PAT:ERα recognition). In fact, it has been reported that the Cys447 residue is not yet able to react with the iodoacetic acid a cysteine-reacting reagent after E2 binding (Hegy et al., 1996), suggesting that the Cys447 residue could be buried into the protein matrix. In addition, E2 also decreases the ERα:caveolin-1 complex further sustaining the pivotal role played by palmitoylation in ERα membrane localization. On the other hand, the deletion of A/B and C domains of the ERα may impair the E2-induced structural modifications of cytosolic ERα, thus enhancing its palmitoylation (40%). In addition these domains may facilitate the E2-dependent depalmitoylation and dissociation from caveolin-1 of the membrane bound ERα. As a consequence, the A/B and C domains render both soluble and membrane-bound receptor competent to E2 regulation.
The ERα palmitoylation is necessary for E2-induced ERK/MAPK and PI3K/AKT pathway activation as well as for cyclin D1 transcription and DNA synthesis as demonstrated by the effect of the PAT inhibitor 2-Br and the nonresponsive behavior of the ERα-Cys447Ala mutant. The palmitoylation of ERα barely influences ERα genomic activities (i.e., estrogen responsive element-containing gene transcription) (Acconcia et al., 2004b). Similar results were obtained in HepG2 cells, which express endogenous ERα. In fact, ERα palmitoylation was detected and regulated by E2 in the HepG2 cell line. Moreover, inhibition of the PAT activity by 2-Br prevents ERK/MAPK and PI3K/AKT activation. Our findings indicate a critical requirement of palmitoylation for the ERα plasma membrane localization and the initiation of the nongenomic events important to E2-dependent G1-S phase progression (i.e., cyclin D1 transcription and DNA synthesis).
In conclusion, palmitoylation enables ERα to reside at the plasma membrane and to interact with caveolin-1. On E2 binding, the ERα may undergo depalmitoylation and dissociate from caveolin-1. Thus, ERα could be relocated by docking to other partner proteins (i.e., Shc/IGF-1 receptor; Src/p85) (Castoria et al., 2001; Migliaccio et al., 2002; Song et al., 2002, 2004). As a consequence, the nongenomic signals could be generated (i.e., ERK/MAPK, PI3K/AKT). Thus, a cycle of inactive/active receptor may occur in the proximity of the plasma membrane as reported for the ERα nuclear pool (Reid et al., 2003). In the future, electron microscopic strategies (e.g., double labeling of ERα and caveolin-1, association/release of ERα from membrane in the presence of E2) will substantiate this action mechanism. However, from now on palmitoylation can be regarded as a physiological regulatory device enabling ERα to initiate E2-induced cell proliferation and provides a new potential target in the treatment of E2-related cancers.
Acknowledgments
We thank Prof. Rakesh Kumar (Department of Molecular and Cellular Oncology, M.D. Anderson Cancer Center, University of Texas, Houston, TX) and Prof. Hynda Kleinman (Craniofacial and Developmental Biology, National Institutes of Health, Bethesda, MD) for the helpful and critical discussions. The generous gift of antiserum anti-ERα AR311 from Dr. Ciro Abbondanza (Dipartimento di Patologia Generale, Seconda Università degli Studi di Napoli, Napoli, Italy) and of human ERα-H14 mutant expression vector from Prof. Pierre Chambon (Institut de Génétique et Biologie Moléculaire et Cellulaire, Strasbourg, France) are gratefully acknowledged. We express gratitude to Drs. Spartaco Santi and Massimo Riccio (Consiglio Nazionale delle Ricerche, Bologna, Italy) for helpful advices in performing confocal microscopy analysis. The editorial assistance of Peter DeMuro also is acknowledged. This work was supported by grants from 2004 Università “Roma Tre” and Fondo per gli Investimenti della Ricerca di Base 2001 to M.M. V.T. was supported by a grant from Istituto Superiore di Sanità (1%), Rome, Italy.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-07-0547. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0547.
Abbreviations used: 2-Br, 2-bromohexadecanoic acid, 2-bromopalmitate; E2, 17β-estradiol; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; IGF-1, insulin-like growth factor-1; PAT, palmitoyl acyl transferase; PI3K, phosphoinositide-3-kinase.
References
- Acconcia, F., Ascenzi, P., Fabozzi, G., Visca, P., and Marino, M. (2004b). S-Palmitoylation modulates human estrogen receptor-α functions. Biochem. Biophys. Res. Commun. 316, 878-883. [DOI] [PubMed] [Google Scholar]
- Acconcia, F., Totta, P., Ogawa, S., Cardillo, I., Inoue, S., Leone, S., Trentalance, A., Muramatsu, M., and Marino, M. (2004a). Survival versus apoptotic 17β-estradiol effect: role of ERα and ERβ activated non-genomic signalling. J. Cell. Physiol. Published online at www.interscience.wiley.com/DOI10.1002/5CP.20219. [DOI] [PubMed]
- Arvanitis, D. N., Wang, H., Bagshaw, R. D., Callahan, J. W., and Boggs, J. M. (2004). Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J. Neurosci. Res. 75, 603-613. [DOI] [PubMed] [Google Scholar]
- Bijlmakers, M. J., and Marsh, M. (2003). The on-off story of protein palmitoylation. Trends Cell Biol. 13, 32-42. [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 772, 248-254. [DOI] [PubMed] [Google Scholar]
- Brzozowski, A. M., Pike, A.C.W., Dauter, Z., Hubbard, R. E., Bonn, T., Engstrom, O., Homan, L., Greene, G. F., Gustafsson, J.-Å., and Carlquist, M. (1997) Molecular basis of agonism and antagoism in the oestrogen receptor. Nature 389, 753-758. [DOI] [PubMed] [Google Scholar]
- Castoria, G., Barone, M. V., Di Domenico, M., Bilancio, A., Ametrano, D., Migliaccio, A., and Auricchio, F. (1999). Non-trascriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 18, 2500-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria, G., Migliaccio, A., Bilancio, A., Di Domenico, M., de Falco, A., Lombardi, M., Fiorentino, R., Varricchio, L., Barone, M. V., and Auricchio, F. (2001). PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 20, 6050-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss, K. L., and Shaul, P. W. (2002). Rapid activation of endothelial NO synthase by estrogen: evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids 67, 413-419. [DOI] [PubMed] [Google Scholar]
- Chambliss, K .L., Yuhanna, I. S., Anderson, R. G., Mendelsohn, M. E., and Shaul, P. W. (2002). ERβ has nongenomic action in caveolae. Mol. Endocrinol. 16, 938-946. [DOI] [PubMed] [Google Scholar]
- Coleman, K. M., and Smith, C. L. (2001). Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front. Biosci. 6, D1379-D1391. [DOI] [PubMed] [Google Scholar]
- Dan, P., Cheung, J.C., Scriven, D. R., and Moore, E. D. (2003). Epitope-dependent localization of estrogen receptor-α, but not -β, in en face arterial endothelium. Am. J. Physiol. 284, H1295-H1306. [DOI] [PubMed] [Google Scholar]
- Deecher, D. C., Swiggard, P., Frail, D. E., and O'Connor, L. T. (2003). Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12). Endocrine 22, 211-223. [DOI] [PubMed] [Google Scholar]
- Fernando, R. I., and Wimalasena, J. (2004). Estradiol abrogates apoptosis in breast cancer cells through inactivation of BAD: Ras dependent non-genomic pathways requiring signaling through ERK and AKT. Mol. Biol. Cell 15, 3266-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. M., Kim, Y., Lee, J. S., Lee, C. S., Lee, B. D., Ohba, M., Kuroki, T., Suh, P.-G., and Ryu, S. H. (2002). Localization of phospholipase D1 to caveolin-enriched membrane via palmitoylation: implications for epidermal growth factor signalling. Mol. Biol. Cell 13, 3976-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegy, G. B., Shackleton, C. H., Carlquist, M., Bonn, T., Engstrom, O., Sjoholm, P., and Witkowska, H. E. (1996). Carboxymethylation of the human estrogen receptor ligand-binding domain-estradiol complex: HPLC/ESMS peptide mapping shows that cysteine 447 does not react with iodoacetic acid. Steroids 61, 367-373. [DOI] [PubMed] [Google Scholar]
- Herbert, B., Truss, M., Beato, M., and Müller, R. (1994). Inducibile regulatory elements in the human cyclin D1 promoter. Oncogene 9, 1295-1304. [PubMed] [Google Scholar]
- Jakacka, M., Ito, M., Martinson, F., Ishikawa, T., Lee, E. J., and Jameson, J. L. (2002). An estrogen receptor ERα deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol. Endocrinol. 16, 2188-2201. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen, B. S., Bhardwaj, B., Fang, H., Ince, B. A., Pakdel, F., Reese, J. C., Schodin. D. and Wrenn, C. K. (1993) Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J. Steroid. Biochem. Mol. Biol. 47, 39-48. [DOI] [PubMed] [Google Scholar]
- Kelly, M. J., and Levin, E. R. (2001). Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 12, 152-156. [DOI] [PubMed] [Google Scholar]
- Kousteni, S., et al. (2001). Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719-790. [PubMed] [Google Scholar]
- Levin, E. R. (2001). Cell localization, physiology, and nongenomic actions of estrogen receptors. J. Appl. Physiol. 91, 1860-1867. [DOI] [PubMed] [Google Scholar]
- Li, L., Haynes, M. P., and Bender, J. R. (2003). Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc. Natl. Acad. Sci. USA 100, 4807-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobenhofer, E. K., Huper, G., Iglehart, J. D., and Marks, J. R. (2000). Inhibition of mitogen-activated protein kinase and phosphatidylinositol 3-kinase activity in MCF-7 cells prevents estrogen-induced mitogenesis. Cell Growth Differ. 11, 99-110. [PubMed] [Google Scholar]
- Mardones, G., and Gonzalez, A. (2003). Selective plasma membrane permeabilization by freeze-thawing and immunofluorescence epitope access to determine the topology of intracellular membrane proteins. J. Immunol. Methods 275, 169-177. [DOI] [PubMed] [Google Scholar]
- Marino, M., Pallottini, V., and Trentalance, A. (1998). Estrogens cause rapid activation of IP3-PKC-α signal transduction pathway in HEPG2 cells. Biochem. Biophys. Res. Commun. 245, 254-258. [DOI] [PubMed] [Google Scholar]
- Marino, M., Distefano, E., Pallottini, V., Caporali, S., Ceracchi, G., and Trentalance, A. (2001a). β-Estradiol stimulation of DNA synthesis requires different PKC isoforms in HepG2 and MCF7 cells. J. Cell Physiol. 188, 170-177. [DOI] [PubMed] [Google Scholar]
- Marino, M., Distefano, E., Trentalance, A., and Smith, C. L. (2001b). Estradiol-induced IP3 mediate the estrogen receptor activity expressed in human cells. Mol. Cell. Endocrinol. 182, 19-26. [DOI] [PubMed] [Google Scholar]
- Marino, M., Acconcia, F., Bresciani, F., Weisz, A., and Trentalance, A. (2002). Distinct non-genomic signal transduction pathways controlled by 17β-estradiol regulate DNA synthesis and cyclin D1 gene transcription in HepG2 cells. Mol. Biol. Cell 13, 3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, M., Acconcia, F., and Trentalance, A. (2003). Biphasic estradiol-induced AKT-phosphorylation is modulated by PTEN via MAP kinase in HepG2 cells. Mol. Biol. Cell 14, 2583-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio, A., Castoria, G., Di Domenico, M., de Falco, A., Bilancio, A., and Auricchio, F. (2002). Src is an initial target of sex steroid hormone action. Ann. N.Y. Acad. Sci. 963, 185-190. [DOI] [PubMed] [Google Scholar]
- Neff, S., Sadowski, C., and Miksicek, R. J. (1994). Mutational analysis of cysteine residues within the hormone-binding domain of the human estrogen receptor identifies mutants that are defective in both DNA-binding and subcellular distribution. Mol. Endocrinol. 8, 1215-1223. [DOI] [PubMed] [Google Scholar]
- Norfleet, A. M., Thomas, M. L., Gametchu, B., and Watson, C. S. (1999). Estrogen receptor-α detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology 140, 3805-3814. [DOI] [PubMed] [Google Scholar]
- Pappas, T. C., Gametchu, B., and Watson, C. S. (1995). Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 9, 404-410. [DOI] [PubMed] [Google Scholar]
- Razandi, M., Pedram, A., Greene, G. L., and Levin, E. R. (1999). Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 13, 307-319. [DOI] [PubMed] [Google Scholar]
- Razandi, M., Pedram, A., and Levin, E. R. (2000). Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol. Endocrinol. 14, 1434-1447. [DOI] [PubMed] [Google Scholar]
- Razandi, M., Oh, P., Pedram, A., Schnitzer, J., and Levin, E. R. (2002). ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol. 16, 100-115. [DOI] [PubMed] [Google Scholar]
- Razandi, M., Alton, G., Pedram, A., Ghonshani, S., Webb, P., and Levin, E. R. (2003). Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol. Cell. Biol. 23, 1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, G., Hubner, M. R., Metivier, R., Brand, H., Denger, S., Manu, D., Beaudouin, J., Ellenberg, J., and Gannon, F. (2003). Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol. Cell 11, 695-707. [DOI] [PubMed] [Google Scholar]
- Resh, M. D. (1999). Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451, 1-16. [DOI] [PubMed] [Google Scholar]
- Ropero, A. B., Soria, B., and Nadal, A. (2002). A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol. Endocrinol. 16, 497-505. [DOI] [PubMed] [Google Scholar]
- Simoncini, T., Hafezi-Moghadam, A., Brazil, D. P., Ley, K., Chin, W. W., and Liao, J. K. (2000). Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, R. X., McPherson, R. A., Adam, L., Bao, Y., Shupnik, M., Kumar, R., and Santen, R. J. (2002). Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol. Endocrinol. 16, 116-127. [DOI] [PubMed] [Google Scholar]
- Song, R. X., Barnes, C. J., Zhang, Z., Bao, Y., Kumar, R., and Santen, R. J. (2004). The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc. Natl. Acad. Sci. USA 101, 2076-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand, C. D., Guan, X., MacLusky, N. J., Horvath, T. L., Diano, S., Singh, M., Connolly, E. S., Jr., Nethrapalli, I. S., and Tinnikov, A. A. (2002). ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 22, 8391-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner, A. S., Ducker, C. E., Xia, Z., Zhuang, Y., De Vos, M. L., and Smith, C. D. (2003). Characterization of human palmitoyl-acyl transferase activity using peptides that mimic distinct palmitoylation motifs. Biochem. J. 373, 91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, P. M., Dubal, D. B., Wilson, M. E., Rau, S. W., and Bottner, M. (2001). Neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinology 142, 969-973. [DOI] [PubMed] [Google Scholar]