GRAPHICAL ABSTRACT
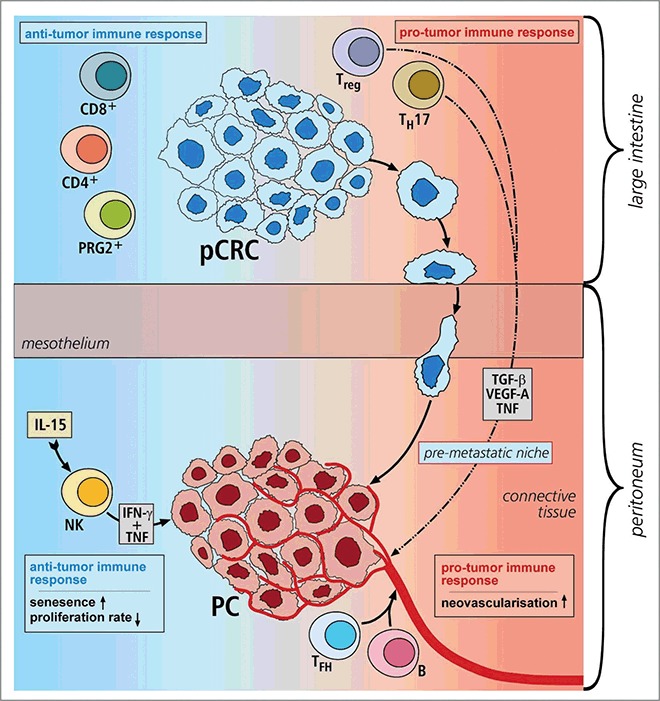
Illustration 1. Overview of immune response in primary CRC and PC.
KEYWORDS: Peritoneal carcinomatosis, proliferation, senescence, tumor milieu, TH1, TH17
ABSTRACT
Background: Peritoneal carcinomatosis (PC) is a terminal evolution from primary colorectal cancer (pCRC) associated with poor patient survival. Impact of the immune cell infiltrate on PC pathogenesis is unknown. Therefore, we characterized the immunological tumor microenvironment regarding proliferation, senescence and neovascularization. Methods: Formalin-fixed and paraffin-embedded (FFPE) tissue of PC and pCRC was examined by immunohistochemistry. Cells infiltrating resected tissue were isolated and analyzed by flow cytometry. PCR arrays detected the expression of genes relevant for helper T (TH) cell responses, like TH1, TH2 and TH17 response. Results: PC tumor cells demonstrate significantly lower proliferation rates than pCRC, but show significantly more senescence. PC is surrounded by significantly increased numbers of cytotoxic active Natural Killer (NK) cells, follicular helper T cells (TFH) and B cells, whereas pCRC shows more CD4+ TH cells, CD8+ cytotoxic T (TC) cells, eosinophilic granulocytes, TH17 and regulatory T (Treg) cells. PC is characterized by significantly increased interferon-γ (IFNγ), an upregulation of tumor necrosis factor (TNF) and the NK cell-regulating cytokine interleukin-15 (IL-15). An upregulation of angiogenesis-related genes, like vascular endothelial growth factor-A (VEGF-A), leads to severe neovascularization in PC. Correlations of PC results reveal that elevated numbers of interleukin-17 (IL-17) positive cells are associated with high cancer cell proliferation, whereas high numbers of IFNγ positive cells correlate with more tumor cells in senescence. Conclusion: The cellular immune reaction is modified during metastasis, inducing senescence in PC tumor cells. Immune surveillance in PC is facilitated by NK cells and high levels of IFNγ and TNF. Counteracting this effect, TFH and B cells combined with VEGF-A enhancement promote neovascularization in PC (Illustration 1).
During metastasis from primary CRC to PC the immune cell infiltrate changes, accompanied by the induction of senescence in PC cancer cells (marked red): In pCRC, the antitumor immune response is facilitated by CD4+TH cells, CD8+TC cells and PRG2+ eosinophilic granulocytes. The premetastatic niche development is promoted by Treg cells and TH17 cells producing systemic factors like VEGF-A, TGF-β and TNF. Along with TFH and B cells, as with a pro-tumor immune response, they support metastatic formation and lead to severe neovascularization in PC. This is counterbalanced by the IL-15-induced activation and proliferation of NK cells. The secreted cytokines IFNγ and TNF mediate immunosurveillance.
Abbreviations
- ACTB
β-actin
- AEC
3-amino-9-ethylcarbazole
- B, B cells; CCL11
chemokine ligand 11
- CD
cluster of differentiation
- CDK
cyclin dependent kinase
- CDKN2A
cyclin-dependent kinase Inhibitor 2A
- CI
confidence interval
- CRS
cytoreductive surgery
- CXCR5
CXC chemokine receptor 5
- DAB
3, 3′-diaminobenzidinetetrahydrochlorhydrate
- DNA
DNA
- EGF
epidermal growth factor
- FACS
fluorescence activated cell sorting
- FCS
fetal calf serum
- FFPE
formalin-fixed and paraffin-embedded
- Foxp3
forkhead box P3
- H3K9me3
tri-methyl-histone H3
- HBSS
Hank's Balanced Salt Solution
- HIPEC
hyperthermic intraperitoneal chemotherapy
- HPF
high-power field
- HRP
horseradish peroxidase
- IFNγ
interferon-γ
- IL
interleukin
- ILC
innate lymphoid cells
- IL-15, interleukin-15; Ki-67
Kiel-67
- mRNA
mRNA
- MHC
major histocompatibility complex
- NK cells
natural killer cells
- PC
peritoneal carcinomatosis
- PCNA
proliferating cell nuclear antigen
- PCR
polymerase chain reaction
- pCRC
primary colorectal cancer
- PRG2
proteoglycan 2
- p21Cip1
cyclin-dependent kinase inhibitor 1
- RNA
ribonucleic acid
- RPMI
Roswell Park Memorial Institute
- RUNX1
runt-related transcription factor 1
- SD
standard deviation
- STAT
signal transducers and activators of transcription
- TC cells
cytotoxic T cells
- TFH cells
follicular helper T cells
- TGF-β
transforming growth factor-β
- TH cells
helper T cells
- TNF
tumor necrosis factor
- Treg cells
regulatory T cells
- UICC
union internationale contre le cancer
- VEGF-A
vascular endothelial growth factor-A
Introduction
Peritoneal carcinomatosis (PC) is a frequent terminal evolution from primary colorectal cancer (pCRC).1 Current multimodal therapies contain the combination of cytoreductive surgery, hyperthermic intraperitoneal chemotherapy and systemic chemotherapy, but cannot provide consistent long-term survival.2-4 Currently, no marker exists to predict which patients benefit from these aggressive treatments.
PC develops from tumor propagation per continuitatem or due to contamination during resection of the primary tumor. Neoplastic cells spread transcoelomic, attach to the mesothelial surface and invade the peritoneal surface to become vascularized.5 Especially the postoperative inflammation and the immunological modifications involved in wound healing seem to aggravate the implantation of tumor cells.6
“Tumor-promoting inflammation” and “avoiding immune destruction” are emerging hallmarks of cancer7 and the link between tumor-associated inflammation and tumor progression is recognized.8 In pCRC, a distinct immune response is involved in tumor development and progression.9 The production of pro-inflammatory cytokines potentially leads to mutations in oncogenes and tumor suppressor genes (Adenomatous polyposis coli (APC) gene, tumor suppressor p53 and Kirsten rat sarcoma viral oncogene (KRAS)).10 Immunosurveillance is executed by cytotoxic T (TC) cells, natural killer (NK) cells and helper T (TH) cells.10 In this regard, coordinated TH1 and TC lymphocyte infiltration in pCRC is associated with favorable clinical outcome,11,12 whereas infiltration with TH17 cells is linked to poor prognosis.13
The impact of immune cells on pCRC tumorigenesis and prognosis is recognized, but unclear for PC of colorectal origin. Therefore, we characterized the microenvironment of PC compared with pCRC. This study demonstrates that the cellular immune reaction is modified during peritoneal metastasis, inducing senescence in PC tumor cells.
Results
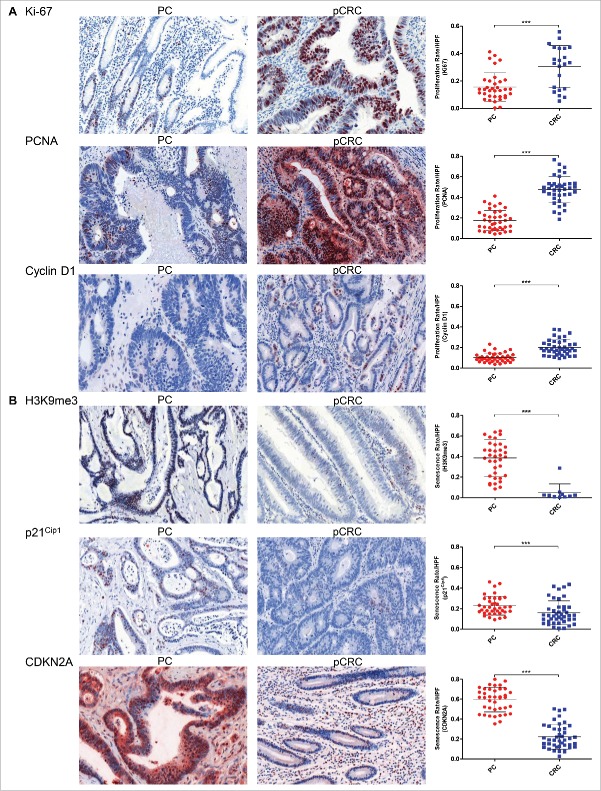
Cancer cells of peritoneal carcinomatosis show less proliferation and increased senescence
Since the impact of the tumor microenvironment on PC tumorigenesis is unclear, we determined proliferation and senescence by immunohistochemical staining. To evaluate proliferation rates (PR) Ki-67, proliferating cell nuclear antigen (PCNA) and cyclin D1 were used as markers. Senescence rates (SR) were determined by the senescence markers tri-methyl-histone H3 (H3K9me3), cyclin-dependent kinase inhibitor 1 (p21Cip1) and cyclin-dependent kinase Inhibitor 2A (CDKN2A), which codes for the protein p16INK4A. With PC patients having a short-life expectancy, we expected highly proliferating cancer cells, which are often a feature of aggressive cancers.14 Surprisingly, our results revealed the contrary, where tumor cells in PC demonstrate significantly lower PR than tumor cells in pCRC, but show significantly higher numbers of senescent cells (Table 1) (Figs. 1A and B; for 10× magnification of all immunohistochemical staining see Supporting Figs. 1A–O). A positive correlation between the two markers for proliferation Ki-67 and cyclin D1 emphasizes the concordance of these results (p = 0.034; n = 34). These findings indicate that the tumor microenvironment in the peritoneum facilitates senescence in cancer cells after metastasis of pCRC to PC has occurred.
Table 1.
Significantly lower proliferation and significantly higher senescence rates in PC cancer cells compared with pCRC.
| PC (Rate/HPF) |
pCRC (Rate/HPF) |
||||||
|---|---|---|---|---|---|---|---|
| Cell Division Rate | Mean ± SD | 95% CI | n | Mean ± SD | 95% CI | n | p |
| Proliferation | |||||||
| Ki-67 | 0.16 ± 0.10 | 0.12–0.19 | 35 | 0.30 ± 0.15 | 0.24–0.37 | 23 | 0.0006 |
| PCNA | 0.17 ± 0.10 | 0.14–0.21 | 39 | 0.48 ± 0.13 | 0.43 ± 0.52 | 39 | <0.0001 |
| Cyclin D1 | 0.10 ± 0.04 | 0.09–0.12 | 39 | 0.20 ± 0.07 | 0.18–0.22 | 41 | <0.0001 |
| Senescence | |||||||
| H3K9me3 | 0.39 ± 0.18 | 0.33–0.45 | 37 | 0.05 ± 0.09 | 0–0.11 | 10 | <0.0001 |
| p21Cip1 | 0.23 ± 0.09 | 0.20–0.26 | 39 | 0.16 ± 0.12 | 0.12–0.20 | 42 | 0.0005 |
| CDKN2A | 0.60 ± 0.12 | 0.56–0.64 | 40 | 0.22 ± 0.12 | 0.18–0.26 | 42 | <0.0001 |
Abbreviations: PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; Rate, ratio of positive stained cancer cells divided by all cancer cells; HPF, high-power field; SD, standard deviation; CI, confidence interval; Ki67, PCNA and cyclin D1 as proliferation markers; H3K9me3, p21Cip1 and CDKN2A as senescence markers.
Figure 1.
High senescence and low proliferation rates of tumor cells in peritoneal carcinomatosis. (A) Immunohistochemical staining for Ki-67, PCNA and cyclin D1 reveal significantly lower tumor cell proliferation rates in PC (n = 35 (Ki-67), n = 39 (PCNA), n = 39 (cyclin D1)) compared with pCRC (n = 23 (Ki-67), n = 39 (PCNA), n = 41 (cyclin D1)). (B) H3K9me3, p21Cip1 and CDKN2A staining reveal a significantly higher tumor cell senescence rates in PC (n = 37 (H3K9me3), n = 39 (p21Cip1), n = 40 (CDKN2A)) compared with pCRC (n = 10 (H3K9me3), n = 42 (p21Cip1), n = 42 (CDKN2A)). Proliferation rates and senescence rates are defined by the ratio of positive stained cancer cells to all cancer cells per HPF. Cells were counted in five HPF per patient (20 × magnification; Mean and SD are marked in graph; ***p ≤ 0 .001); PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; HPF, high-power field.
Metastasis of primary colorectal cancer to peritoneal carcinomatosis is accompanied by distinct changes in the immune cell infiltrate
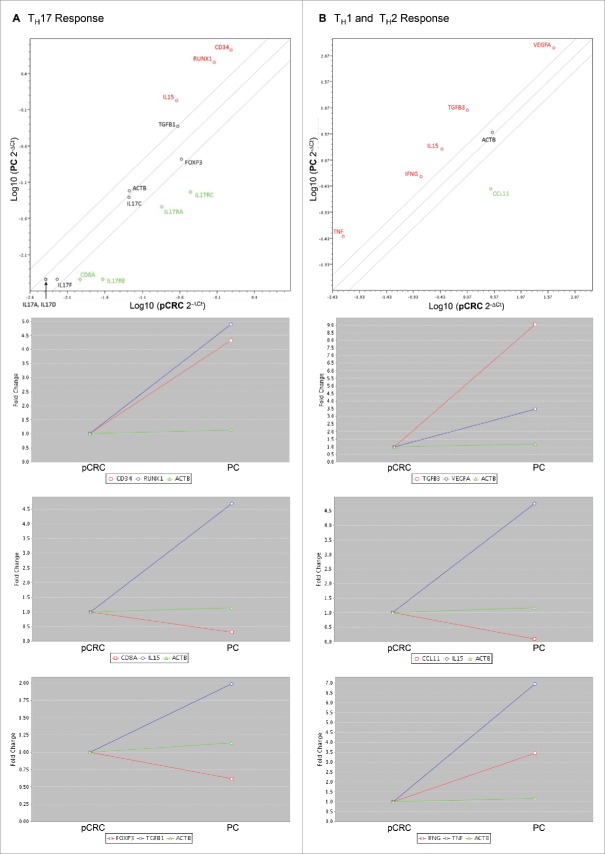
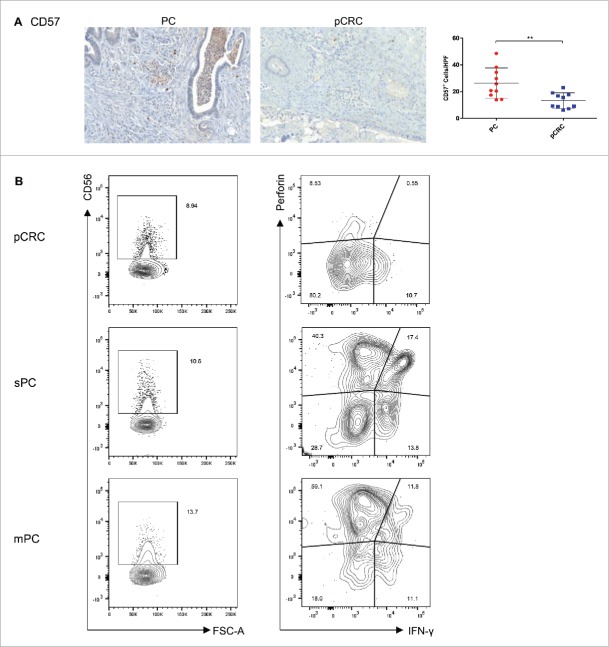
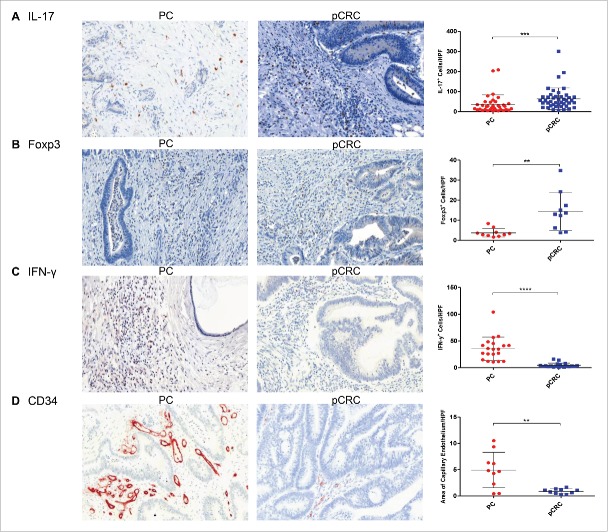
To determine the immune cell infiltrate of the tumor microenvironment, human samples of PC and pCRC were stained for cluster of differentiation 4 (CD4+), CD8+, CD20, CD79a, CD138, CXC chemokine receptor 5 (CXCR5) and proteoglycan 2 (PRG2) by immunohistochemistry. The PC tumor microenvironment is dominated by significantly higher numbers of CXCR5+ follicular helper T (TFH) cells, CD20+ B cells and a tendency to more CD79a+ and CD138+ plasma cells in comparison with pCRC. Whereas pCRC shows significantly higher levels of CD4+ TH cells, and also elevated levels of CD8+ TC cells and PRG2+ eosinophilic granulocytes compared with PC (Table 2) (Figs. 2A–F). Fluorescence activated cell sorting (FACS) analyses of CD4+ and CD8+ expression support these findings (Supporting Fig. 2A). To verify these results polymerase chain reaction (PCR) arrays were performed (Figs. 3A and B; for summary of all PCR array results see Supporting Figs. 3A–D). Concordant with our immunohistochemistry results arrays revealed a downregulation of the TC cell marker CD8A and chemokine ligand 11 (CCL11) in PC, compared with pCRC. CCL11 is able to activate eosinophils and induce eosinophilic chemotaxis. In addition, the arrays reveal strong upregulation of interleukin-15 (IL-15) in PC, compared with pCRC. IL-15 regulates NK cell activation and proliferation. In this regard, via immunohistochemistry, we were able to show that the area surrounding PC is dominated by significantly higher numbers of CD57+ NK cells, compared with pCRC (Table 2) (Fig. 4A).
Table 2.
Significant differences in the immune cell infiltrate and the effector cytokines of PC compared with pCRC.
| PC (Cells/HPF) |
pCRC (Cells/HPF) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Immune Cells | Markers | Mean ± SD | 95% CI | n | Mean ± SD | 95% CI | n | p | |
| TFH cells | CXCR5 | 53 ± 18 | 41–66 | 10 | 21 ± 10 | 13–29 | 9 | 0.0009 | |
| B cells | CD20 | 51 ± 32 | 27–74 | 10 | 14 ± 11 | 6–22 | 10 | 0.0029 | |
| Plasma cells | CD79a | 63 ± 43 | 32–94 | 10 | 31 ± 28 | 11–51 | 10 | 0.0544 | |
| CD138 | 45 ± 39 | 16–73 | 10 | 21 ± 20 | 7–35 | 10 | 0.0887 | ||
| TH cells | CD4 | 55 ± 24 | 38–72 | 10 | 286 ± 144 | 175–397 | 9 | <0.0001 | |
| TC cells | CD8 | 77 ± 45 | 44–109 | 10 | 124 ± 88 | 61–187 | 10 | 0.2150 | |
| Eosinophils | PRG2 | 0 ± 2 | 0–0 | 10 | 1 ± 1 | 0–2 | 10 | 0.0019 | |
| NK cells | CD57 | 26 ± 11 | 18–34 | 10 | 13 ± 6 | 9–17 | 10 | 0.0068 | |
| Treg |
Foxp3 |
4 ± 2 |
2–5 |
10 |
14 ± 9 |
8–21 |
10 |
0.0013 |
|
| Cytokines | IL-17 | 37 ± 47 | 21–52 | 36 | 64 ± 54 | 47–81 | 43 | 0.0002 | |
| IFNγ | 35 ± 22 | 25–46 | 20 | 4 ± 4 | 3–6 | 20 | <0.0001 | ||
Abbreviations: PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; HPF, high-power field; SD, standard deviation; CI, confidence interval; TFH, follicular helper T cells; TH, helper T cells; TC, cytotoxic T cells; NK, natural killer cells; Treg, regulatory T cells; IL-17, interleukin-17; IFNγ, interferonγ.
Figure 2.
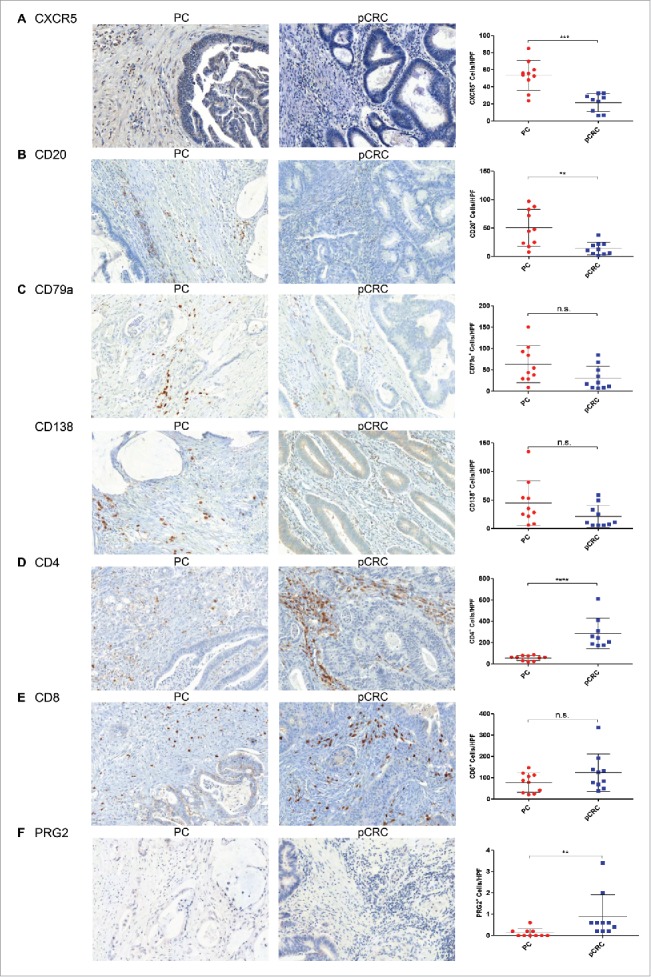
Distinct changes in the immune cell infiltrate accompany the metastasis of primary colorectal cancer to peritoneal carcinomatosis. (A) Immunohistochemical staining of PC shows significantly higher levels of CXCR5+TFH cells (n = 10) compared with pCRC (n = 9). (B) Significantly higher numbers of CD20+B cells (n = 10) are detected in PC in comparison with pCRC (n = 10). (C) PC demonstrates a tendency to higher levels of CD79a+ (n = 10) and CD138+ (n = 10) plasma cells in comparison with pCRC (n = 10). (D) Significantly higher levels of CD4+TH cells (n = 9) appear in pCRC compared with PC (n = 10). (E) pCRC reveals a tendency to elevated numbers of CD8+TC cells (n = 10) compared with PC (n = 10). (F) Samples of pCRC show significantly higher numbers of PRG2+ eosinophilic granulocytes (n = 10) compared with samples of PC (n = 10). Cells were counted in five HPF per patient (20× magnification; Mean and SD are marked in graph; n.s. p > 0.05; **p ≤ 0 .01; ***p ≤ 0 .001; ****p ≤ 0 .0001); PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; HPF, high-power field.
Figure 3.
Upregulation of genes encoding for senescence inducing cytokines and neovascularization promoting factors in peritoneal carcinomatosis. (A) PCR array for the human TH17 response demonstrates an upregulation of genes encoding for IL-15, TGF-β1, CD34 and RUNX1, a downregulation of genes encoding for CD8A, Foxp3 and IL-17 receptors A, C and E in the group of PC compared with the group of pCRC, as well as the same amount of IL-17A, C, D and F in both groups. (B) PCR array for human TH1 and TH2 response shows an upregulation of genes encoding for IL-15, IFNγ, TNF, TGF-β3 and VEGF-A in the group of PC compared with the group of pCRC, along with a downregulation of CCL11. Scatter plot and multigroup plot show fold change, which is the normalized (2−ΔCt) gene expression in PC divided by the normalized gene expression in pCRC. Upregulation is marked red; downregulation is marked green. Beta-actin (ACTB) functions as housekeeping gene.
Figure 4.
Peritoneal carcinomatosis reveals higher levels of cytotoxic active NK cells compared with primary colorectal cancer. (A) Significantly elevated numbers of CD57+NK cells (n = 10) were detected by immunohistochemistry in samples of PC in comparison with samples of pCRC (n = 10). Cells were counted in five HPF per patient (20 × magnification; Mean and SD are marked in graph; **p ≤ 0 .01); PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; HPF, high-power field. (B) Flow cytometry analyses of CD56+, CD56+Perforin+, CD56+IFNγ+ and CD56+CD107a+ cells reveal higher expressions in synchronous and metachronous PC compared with pCRC. Dotplots show T cells pre-gated on CD45+ and CD3+ cells.
To investigate how cytotoxicity evolves regarding spatiotemporal changes of the tumor microenvironment, FACS analyses of NK cells in different tumor stages were performed. Primary CRC without metastasis (pCRC), primary CRC with synchronous PC by time of diagnosis (sPC), and metachronous PC (mPC) were tested as different entities. First, overall increased numbers of CD56+ NK cells in sPC and mPC, compared with pCRC, are detectable (Fig. 4B). Regarding cytotoxicity of NK cells, augmented perforin+ and interferon-γ (IFNγ)-secreting CD56+ cells were located in sPC and mPC. Additionally, NK cells in sPC and mPC showed expression of the degranulation marker CD107a (Supporting Fig. 2C). These findings demonstrate substantially more cytotoxic active NK cells are present in synchronous and metachronous PC, compared with primary CRC.
In summary, cellular immune reactions are modified in the process of metastasis. Tumor immunity changes from a TH and TC cell-dominated antitumor immune response in pCRC to a B and TFH cell-mediated pro-tumor immune response in PC, counterbalanced by cytotoxic effects executed by NK cells.
After metastatic formation tumor immune surveillance is facilitated by high levels of IFNγ and TNF
Previous studies show that the balance of IL-17 and IFNγ affects tumor activity of colitis-associated CRC.15,16 To identify cytokines potentially influencing cancer cell division rates, human samples of PC and pCRC were stained for IFNγ, IL-17A and forkhead box P3 (Foxp3). Foxp3 functions as a transcription factor of regulatory T (Treg) cells, which play a role in CRC pathogenesis and metastasis.17,18 IL-17 promotes tumorigenesis, whereas IFNγ acts against tumors.
Primary CRC reveals significantly higher levels of IL-17+TH17 cells and Foxp3+Treg cells than PC (Table 2) (Figs. 5A and B). FACS analyses of CD3+CD4+Foxp3+ lymphocyte expression (Supporting Fig. 2B) and PCR array results (Fig. 3A) support these findings. In contrast, PC shows significantly increased numbers of IFNγ+ cells, compared with pCRC (Table 2) (Fig. 5C). We verified these results with PCR arrays (Figs. 3A and B), demonstrating an upregulation of genes encoding for IFNγ and tumor necrosis factor (TNF) in PC. The different IL-17 subtypes (IL17-A, -C, -D, -F) are present at the same levels in both groups, but their receptors (IL-17R) are upregulated in pCRC compared with PC.
Figure 5.
Composition of the tumor microenvironment changes during metastasis from primary colorectal cancer to peritoneal carcinomatosis. (A) Immunohistochemical staining shows significantly higher numbers of IL-17A+TH17 cells in pCRC (n = 43) compared with PC (n = 36). (B) Significantly higher levels of Foxp3+Treg cells (n = 10) were found in pCRC compared with PC (n = 10). (C) Significantly elevated numbers of IFNγ+ cells (n = 20) were detected in PC in comparison with pCRC (n = 20). (D) Significantly more capillary endothelium was found in samples of PC (n = 10) compared with samples of pCRC (n = 10). Cells were counted and area of capillary endothelium was measured in five HPF per patient (20× magnification; Mean and SD are marked in graph; **p ≤ 0 .01; ***p ≤ 0 .001; ****p ≤ 0 .0001); PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; HPF, high-power field.
In the presence of TNF, IFNγ leads to growth arrest by inducing senescence in tumor cells.19 In support of this idea, we were able to show a significant positive correlation between patients with high levels of IFNγ and increased numbers of senescent cells (p = 0.035; n = 35).
Therefore, TH17 and Treg cells at the primary tumor site (pCRC) seem to support tumor progression and metastasis. After metastatic formation has occurred (PC), local tumor immune surveillance is facilitated by high levels of IFNγ and TNF.
Severe neovascularization characterizes the peritoneal carcinomatosis tumor microenvironment
Tumor metastasis and invasiveness is associated with neovascularization induced by hypoxia. The above mentioned PCR arrays reveal an upregulation of angiogenesis-related genes like vascular endothelial growth factor-A (VEGF-A), CD34, runt-related transcription factor 1 (RUNX1), transforming growth factor-β1 (TGF-β1) and TGF-β3 in samples of PC (Figs. 3A and B). TGF-β mediates neovascularisation and influences the balance of TH17 and Treg cells. RUNX1 regulates the differentiation of haematopoietic stem cells into mature blood cells. VEGF-A increases vascular permeability and induces angiogenesis. CD34 is expressed on capillary endothelium and haematopoietic precursors. To prove that PC is more vascularized than pCRC, human samples were stained for CD34. PC reveals significantly more angiogenesis than pCRC (Table 3) (Fig. 5D). These results demonstrate that PC tumor cells and the surrounding immune infiltrate stimulate the production of angiogenetic factors, resulting in severe neovascularization.
Table 3.
Significantly more angiogenesis in PC compared with pCRC.
| PC (Sq Inch/HPF) |
pCRC (Sq Inch/HPF) |
||||||
|---|---|---|---|---|---|---|---|
| Angiogenesis | Mean ± SD | 95% CI | n | Mean ± SD | 95% CI | n | p |
| CD34 | 4.9 ± 3.4 | 2.5–7.3 | 10 | 0.9 ± 0.5 | 0.5–1.5 | 10 | 0.0089 |
Abbreviations: PC, peritoneal carcinomatosis; pCRC, primary colorectal cancer; Sq Inch, area of capillary endothelium in square inches; HPF, high-power field; SD, standard deviation; CI, confidence interval.
Tumor cell division rates of peritoneal carcinomatosis are influenced by the balance of IFNγ and IL-17
To investigate how the unique immunological tumor microenvironment of PC impacts tumor progression, immunohistochemical results were correlated with tumor cell senescence and proliferation. Remarkably, elevated IL-17 levels in PC show a significant positive correlation with increased tumor cell PR (p = 0.04; n = 34), whereas high levels of IFNγ demonstrate a significant positive correlation with elevated levels of senescent cells (p = 0.035; n = 35). Furthermore, statistical analyses of PC samples revealed a negative correlation between the cell-cycle inhibitor and marker for senescence CDKN2A and the marker for capillary endothelium CD34 (p = 0.026; n = 9). This suggests, depending on the tumor microenvironment, either severe neovascularization or senescent tumor cells dominate PC. Proliferation and SR were independent of PC extent, as neither Ki-67 nor H3K9me3 correlated with patient peritoneal cancer index. Therefore, the balance of IL-17 and IFNγ influences tumor cell division rates and tumor progression, depending on the most predominant cytokine.
Discussion
It is known that the pathogenesis and prognosis of pCRC are influenced by the associated immune cell infiltrate,10,11,13 but this is unclear for PC of colorectal origin. Our current studies were conducted to characterize the immunological tumor microenvironment of PC and its impact on cancer cell proliferation and neovascularisation. With PC associated with short life expectancy, one would suspect highly proliferating cells being a feature of an aggressive tumor. Surprisingly, our results demonstrate the contrary, where cancer cells in PC are senescent and show proliferation arrest compared with pCRC.
Cell proliferation is a biological process to maintain tissue homeostasis. We assessed proliferation activity in PC and CRC by proliferation markers (Ki-67, PCNA and cyclin D1).20 The Ki-67 antigen is expressed during the late G1, S, G2 and M phase of the cell cycle, whereas resting cells (G0 phase) lack Ki-67 expression.21 PCNA is expressed during the DNA synthesis and is a cofactor for the DNA polymerase, which is elevated during the G1/S phase of the cell cycle.22 Cyclin D1 is a regulatory subunit of cyclin-dependent kinases 4 (CDK4) and CDK6, whose activity is required for cell cycle G1/S transition.23,24 Each of the proliferation markers demonstrated a significant proliferation arrest in PC compared with CRC, suggesting that the cell cycle is delayed in PC by the action of several control stages. A low number of proliferating cells, as found in PC, suggests growth arrest in cancer cells, probably due to cellular senescence.
Senescent cells are metabolically active, but in growth arrest, usually with a G1 DNA content, and fail to initiate DNA replication. This is caused by the expression of cell-cycle inhibitors, like cyclin-dependent kinase inhibitors p21Cip1 and p16INK4a 25. A pivotal hallmark of senescence is senescence-associated heterochromatin foci (SAHF). SAHF regions are enriched in chromatin repression marks like trimethylation of lysine 9 in histone 3 (H3K9me3).26,27 H3K9me3 plays a key role in cancer immune surveillance by T cells and has been described as responsible for immune evasion and progression in colon carcinoma.28 CDKN2A codes for the protein p16INK4A, which inhibits CDK4 and CDK6 and thereby blocks transition from G1 to S-phase.29,30 p16INK4a also has been shown to have a causative role in SAHF formation.31 p21Cip1 mediates the p53-dependent cell cycle G1 phase arrest in response to a variety of stress stimuli by inhibiting the activity of CDK2 or CDK4 complexes.32 All these senescence markers (H3k9me3, CDKN2A and p21Cip1) demonstrated significantly higher SR in PC compared with CRC. Senescent tumor cells have been shown to be subject to enhanced immunosurveillance.33 In summary, this set of data suggests that PC cells have evolved senescence as tumor escape mechanism leading to evasion from chemotherapeutic treatment.
The tumor microenvironment of PC is characterized by significantly increased numbers of NK cells. NK cells play a crucial role in tumor immunosurveillance, where they control tumor growth, dissemination and recurrence34 by releasing cytotoxic granzymes, IFNγ, TNF-α and the pore-forming protein perforin, inducing programmed cell death.35 By secreting IFNγ, the infiltrating NK cells in PC are highly cytotoxic active and appear simultaneously with high levels of TNF. Braumüller et al. identified that the combined action of the TH1-derived IFNγ and TNF directly induces permanent growth arrest in cancer cells.19 Therefore, NK cell induced senescence is a potential mechanism of immune cell-facilitated tumor surveillance in PC.
Furthermore, our findings potentially explain why current chemotherapeutic agents used in PC treatment, particularly targeting rapidly dividing cells, are not as effective as they are in pCRC. We therefore suggest that the Ki-67 index in resected tissues from exploratory laparotomy could serve as proliferation marker to determine which of the advanced tumor patients would likely benefit from cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy. In breast cancer, immunohistochemical assessment of Ki-67 has successfully been used for the prognosis and prediction of chemotherapeutic responsiveness.36
We also investigated how the immune cell infiltrate changes during the progression from pCRC to PC. Bindea et al. emphasize that immune reactions evolve with tumor progression.37 T cell subpopulations are highly expressed at early CRC stages. In contrast, the density of B, TFH and antigen-presenting cells increases with late tumor stages. With PC being defined as UICC stage IV of CRC it shows high levels of TFH, B and plasma cells, but significantly fewer CD4+TH and CD8+TC cells compared with pCRC, consistent with the study of Bindea et al. TFH cells support the differentiation of antigen-specific B cells into memory and plasma cells. Increased TFH cell activity is involved in lymphoma development.38 B cells promote tumor progression via STAT3 regulated-angiogenesis.39
Looking at immune responses facilitating metastasis, IL-17+ TH17 cells and Foxp3+ Treg cells are significantly higher in pCRC. TH17 cell recruitment to the primary tumor site inhibits antitumor immunity and enhances metastasis.18,40 Supporting this theory, IL-17 deficiency prevents metastasis in colon cancer models.41 Treg cells suppress NK and CD8+TC cells, increasing tumor growth, invasion and migration from the primary tumor site.42 Additional studies are needed to investigate the impact of intestinal permeability and bacterial translocation on peritoneal metastasis, considering this is known in colorectal cancer and late stages of liver disease.43,44
Primary tumors effect distant sites to become pre-metastatic niches by producing systemic factors like VEGF-A, TGF-β and TNF.45 PC being rich in VEGF-A, TGF-β and TNF indicates a successful pre-metastatic niche development that has progressed to metastatic formation. This is mediated by the lack of TH1 cells in PC, supporting epithelial tumor seeding,46 the abundance of TNF, promoting tumor growth in experimental models of colitis-associated cancer,47 and by TGF-β, facilitating tumor growth, invasion and metastasis in advanced stages of CRC.48 All of these factors, and the mentioned high levels of B and TFH cells, support neovascularization.
Tumor angiogenesis is mediated by several key angiogenic mediators, such as VEGF-A, and is crucial for the growth and dissemination of tumor cells. VEGF-A is induced by hypoxia and is a target of the TGF-β signaling pathway.49 Consistent with this mechanism our results show significantly more neovascularization in the PC microenvironment. Additionally, a negative correlation between CD34 enrichment and high SR was observed. This is congruent with a study of Foersch et al., demonstrating, that the lack of VEGFR2 signaling in colorectal cancer cells is accompanied by an upregulation of various markers for cellular senescence.50
Therefore, VEGF-A antibodies are a promising approach to treat PC patients. Monoclonal antibodies against VEGF- (Bevacizumab) and EGF-receptors (Cetuximab) are currently being used in metastatic CRC, depending on molecular markers (KRAS and BRAF mutations),51 and have already shown a benefit in long-term survival.52,53 Further studies are needed to determine, if blocking angiogenic mediators could improve long-term survival, especially for the subgroup of PC patients, who show an extended level of neovascularization.
In summary (Illustration 1), our studies reveal that metastasis of primary CRC to PC is accompanied by changes in the immune cell infiltrate inducing senescence. Angiogenesis-related factors promote extensive neovascularization. The balance between anti- and pro-tumor effects influences cancer cell division rates.
Methods
Patients
FFPE samples of patients (n = 38) who had tumor surgery on PC of colorectal origin between 2004 and 2008 were analyzed. The control group consisted of patients (n = 45) who had tumor surgery between 2004 and 2011 on pCRC without a diagnosed metastasis. FACS analysis was performed with samples of patients (n = 8) treated with tumor surgery in 2014. The study was conducted at the Department of Surgery, University Medical Center Regensburg, Germany, according to the principles of Helsinki and approved by the Local Ethics Committee (No. 14-101-0014).
Immunohistochemistry
FFPE samples were stained with anti-human antibodies for CXCR5 (Sigma-Aldrich, HPA042432), IFNγ (Abcam, ab25101), PRG2 (Sigma-Aldrich, HPA038515), Ki-67 (Abcam, ab16667), H3K9me3 (Cell Signaling, #9733S), PCNA (Cell Signaling, #13110), p21Cip1 (Cell Signaling, #2947), cyclin D1 (Cell Signaling, #2978), CDKN2A (Abcam, ab54210), CD34 (Ventana, 790-2927), Foxp3 (eBioscience, 14-4776) and IL-17 (R&D Systems, AF-317-NA). Immunohistochemical staining for CD4+, CD8+, CD57, CD20, CD79a and CD138 were provided by the Department of Pathology, University Medical Center Regensburg. For quantifying positive cell staining five high-power fields (HPFs; 20× magnification) were counted per slide by two independent examiners.
To define proliferation and SR each tumor slide was manually scanned with a microscope at 20× magnification. We captured five areas per slide (five HPFs) with each showing a characteristic staining of the whole slide. Negative and positive stained tumor cells were counted via ImageJ (National Institutes of Health, USA) cell counter function. For determination of the PR, we analyzed the proliferation markers Ki-67, PCNA and cyclin D1. The PR is defined as: For determination of the SR, we analyzed the senescence markers H3K9me3, p21Cip1 and CDKN2A. The SR is defined as:
RNA isolation and PCR array
RNA was extracted from FFPE samples using the RNeasy FFPE Kit (Qiagen, Hilden, Germany), as described by the manufacturer. A pool of 10 PC samples was compared with a pool of 10 pCRC samples. Expression of genes relevant for TH1, TH2 and TH17 cell responses were determined with RT2 Profiler PCR Arrays (Qiagen) using RT2 SYBR Green qPCR Mastermix (Qiagen) and the LightCycler 480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany).
Flow cytometry
Single cell suspensions of tumors were stained with anti-human antibodies specific for CD45 (BD, 560777), CD3 (Miltenyi Biotec, 130-094-363), CD4 (Miltenyi Biotec, 130-094-153 and 130-094-963), CD8 (Miltenyi Biotec, 130-096-561), CD56 (Beckman Coulter, IM2474), CD107a (Miltenyi Biotec, 130-095-520), IFNγ (eBioscience, 11-7319-82), Foxp3 (eBioscience, 25-1178-42) and perforin (eBioscience, 25-9994-42). Analyses were performed using a FACSCanto II flow cytometer (BD).
Statistical analysis
Normal distributed data was evaluated by standard two-tailed Student's t-tests. Wilcoxon-Mann–Whitney tests were used for data not showing a normal distribution. Pearson's correlation, with bivariate analysis, was used for correlation analysis. Statistics were evaluated with SPSS Statistics software (SPSS GmbH Software, Munich, Germany). Graph Pad Prism was used to calculate standard deviation (SD) and 95% confidence interval (CI) of experimental data sets. p-values ≤ 0 .05 are considered marginally significant and labeled with an asterisk (*).
For further information regarding methods see Supporting Material.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Tatjana Schifferstein, Marcus Kielmanowicz and Manuela Kovács-Sautter provided skillful technical support. We thank all members of the laboratory of Chronic Immunopathology for critical and helpful discussions.
Funding
This project was supported by grant Fi1526/4–1 and Fi1526/5–1 from the Deutsche Forschungsgemeinschaft and by the Regensburg Center of Interventional Immunology with salaries for laboratory assistance and analysis. The authors gratefully acknowledge this support.
Author contributions
C.T.S., S.B., R.K., S.F.F. planned, performed and analyzed the experiments, C.T.S., S.B., R.K., S.F.F. interpreted the experiments, C.T.S., R.K., S.F.F. wrote the manuscript, S.B., P.R. contributed to the experimental design, G.G., P.P. supervised the surgical sampling, E.K.G, H.J.S. made helpful suggestions.
References
- 1.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012; 99:699-705; PMID:22287157; http://dx.doi.org/ 10.1002/bjs.8679 [DOI] [PubMed] [Google Scholar]
- 2.Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, van Ramshorst B, van Ginkel RJ, Havenga K, Bremers AJ et al.. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol 2013; 20:4224-30; PMID:23897008; http://dx.doi.org/ 10.1245/s10434-013-3145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zani S, Papalezova K, Stinnett S, Tyler D, Hsu D, Blazer DG 3rd. Modest advances in survival for patients with colorectal-associated peritoneal carcinomatosis in the era of modern chemotherapy. J Surg Oncol 2013; 107:307-11; PMID:22811275; http://dx.doi.org/ 10.1002/jso.23222 [DOI] [PubMed] [Google Scholar]
- 4.Aoyagi T, Terracina KP, Raza A, Takabe K. Current treatment options for colon cancer peritoneal carcinomatosis. World J Gastroenterol 2014; 20:12493-500; PMID:25253949; http://dx.doi.org/17262739 10.3748/wjg.v20.i35.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol 2007; 95:93-6; PMID:17262739; http://dx.doi.org/ 10.1002/jso.20676 [DOI] [PubMed] [Google Scholar]
- 6.Ceelen WP, Bracke ME. Peritoneal minimal residual disease in colorectal cancer: mechanisms, prevention, and treatment. Lancet Oncol 2009; 10:72-9; PMID:19111247; http://dx.doi.org/ 10.1016/S1470-2045(08)70335-8 [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Fichtner-Feigl S, Kesselring R, Strober W. Chronic inflammation and the development of malignancy in the GI tract. Trends Immunol 2015; 36:451-9; PMID:26194796; http://dx.doi.org/ 10.1016/j.it.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:12490959; http://dx.doi.org/ 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010; 138:2101-14 e5; PMID:20420949; http://dx.doi.org/ 10.1053/j.gastro.2010.01.058 [DOI] [PubMed] [Google Scholar]
- 11.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 13.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F et al.. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263-71; PMID:21303976; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2907 [DOI] [PubMed] [Google Scholar]
- 14.Ishida H, Miwa H, Tatsuta M, Masutani S, Imamura H, Shimizu J, Ezumi K, Kato H, Kawasaki T, Furukawa H et al.. Ki-67 and CEA expression as prognostic markers in Dukes' C colorectal cancer. Cancer Lett 2004; 207:109-15; PMID:15050740; http://dx.doi.org/ 10.1016/j.canlet.2003.10.032 [DOI] [PubMed] [Google Scholar]
- 15.Jauch D, Martin M, Schiechl G, Kesselring R, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut 2011; 60:1678-86; PMID:21948944; http://dx.doi.org/ 10.1136/gutjnl-2011-300612 [DOI] [PubMed] [Google Scholar]
- 16.Martin M, Kesselring RK, Saidou B, Brunner SM, Schiechl G, Mouris VF, Wege AK, Rümmele P, Schlitt HJ, Geissler EK et al.. RORgammat hematopoietic cells are necessary for tumor cell proliferation during colitis-associated tumorigenesis in mice. Eur J Immunol 2015; 45:1667-79. [DOI] [PubMed] [Google Scholar]
- 17.Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M, von Smolinski D, Kasper S, Sparwasser T, Gruber AD et al.. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res 2014; 74:4258-69; PMID:24906621; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3065 [DOI] [PubMed] [Google Scholar]
- 18.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15:73-86; PMID:25614318; http://dx.doi.org/ 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al.. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361-5; PMID:23376950; http://dx.doi.org/ 10.1038/nature11824 [DOI] [PubMed] [Google Scholar]
- 20.Jurikova M, Danihel L, Polak S, Varga I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta histochemica 2016; 118:544-52; PMID:27246286; http://dx.doi.org/ 10.1016/j.acthis.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sanchez-Acuna G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Medicina oral, patologia oral y cirugia bucal 2013; 18:e174-9; PMID:23229269; http://dx.doi.org/ 10.4317/medoral.18573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzinska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res 2009; 29:3049-52; PMID:19661314 [PubMed] [Google Scholar]
- 23.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 1993; 7:812-21; PMID:8491378; http://dx.doi.org/ 10.1101/gad.7.5.812 [DOI] [PubMed] [Google Scholar]
- 24.Dai Y, Wilson G, Huang B, Peng M, Teng G, Zhang D, Zhang R, Ebert MP, Chen J, Wong BC et al.. Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis 2014; 5:e1170; PMID:24722295; http://dx.doi.org/ 10.1038/cddis.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8:729-40; PMID:17667954; http://dx.doi.org/ 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 26.Bernardes de Jesus B, Blasco MA. Assessing cell and organ senescence biomarkers. Circ Res 2012; 111:97-109; PMID:22723221; http://dx.doi.org/ 10.1161/CIRCRESAHA.111.247866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012; 13:343-57; PMID:22473383; http://dx.doi.org/ 10.1038/nrg3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paschall AV, Yang D, Lu C, Choi JH, Li X, Liu F, Figueroa M, Oberlies NH, Pearce C, Bollag WB et al.. H3K9 trimethylation silences fas expression to confer colon carcinoma immune escape and 5-fluorouracil chemoresistance. J Immunol 2015; 195:1868-82; PMID:26136424; http://dx.doi.org/ 10.4049/jimmunol.1402243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 2006; 8:1291-7; PMID:17028578; http://dx.doi.org/ 10.1038/ncb1491 [DOI] [PubMed] [Google Scholar]
- 30.Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell Cycle 2011; 10:2497-503; PMID:21775818; http://dx.doi.org/ 10.4161/cc.10.15.16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Micco R, Sulli G, Dobreva M, Liontos M, Botrugno OA, Gargiulo G, dal Zuffo R, Matti V, d'Ario G, Montani E et al.. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol 2011; 13:292-302; PMID:21336312; http://dx.doi.org/ 10.1038/ncb2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 2005; 65:3980-5; PMID:15899785; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3995 [DOI] [PubMed] [Google Scholar]
- 33.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A et al.. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547-51; PMID:22080947; http://dx.doi.org/ 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- 34.Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: What we know and perspectives. World J Gastroenterol 2014; 20:3738-50; PMID:24833840; http://dx.doi.org/23192659 10.3748/wjg.v20.i14.3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vacca P, Martini S, Garelli V, Passalacqua G, Moretta L, Mingari MC. NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term IL-2 activation. Eur J Immunol 2013; 43:550-61; PMID:23192659; http://dx.doi.org/ 10.1002/eji.201242783 [DOI] [PubMed] [Google Scholar]
- 36.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T et al.. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011; 103:1656-64; PMID:21960707; http://dx.doi.org/ 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al.. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/ 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 38.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol 2013; 13:412-26; PMID:23681096; http://dx.doi.org/ 10.1038/nri3447 [DOI] [PubMed] [Google Scholar]
- 39.Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W, Hoon DS, Wakabayashi M, Forman S, Yu H. B cells promote tumor progression via STAT3 regulated-angiogenesis. PloS one 2013; 8:e64159; PMID:23734190; http://dx.doi.org/ 10.1371/journal.pone.0064159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesselring R, Jauch D, Fichtner-Feigl S. Interleukin 21 impairs tumor immunosurveillance of colitis-associated colorectal cancer. OncoImmunology 2012; 1:537-8; PMID:22754778; http://dx.doi.org/ 10.4161/onci.19407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 2010; 10:248-56; PMID:20336152; http://dx.doi.org/ 10.1038/nri2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 2006; 176:1582-7; PMID:16424187; http://dx.doi.org/ 10.4049/jimmunol.176.3.1582 [DOI] [PubMed] [Google Scholar]
- 43.Kesselring R, Glaesner J, Hiergeist A, Naschberger E, Neumann H, Brunner SM, Wege AK, Seebauer C, Köhl G, Merkl S et al.. IRAK-M expression in tumor cells supports colorectal cancer progression through reduction of antimicrobial defense and stabilization of STAT3. Cancer Cell 2016; 29:684-96; PMID:27150039; http://dx.doi.org/ 10.1016/j.ccell.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015; 421:44-53; PMID:25595554; http://dx.doi.org/ 10.1016/j.jim.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014; 16:717-27; PMID:25082194; http://dx.doi.org/ 10.1038/ncb3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ et al.. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410:1107-11; PMID:11323675; http://dx.doi.org/ 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 47.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S et al.. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Investig 2008; 118:560-70; PMID:18219394; http://dx.doi.org/ 10.1172/jci32453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res 2009; 19:89-102; PMID:19050696; http://dx.doi.org/ 10.1038/cr.2008.316 [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem 2001; 276:38527-35; PMID:11486006; http://dx.doi.org/ 10.1074/jbc.M104536200 [DOI] [PubMed] [Google Scholar]
- 50.Foersch S, Sperka T, Lindner C, Taut A, Rudolph KL, Breier G et al.. VEGFR2 Signaling Prevents Colorectal Cancer Cell Senescence to Promote Tumorigenesis in Mice With Colitis. Gastroenterology 2015; 149:177-89 e10; PMID:25797700; http://dx.doi.org/ 10.1053/j.gastro.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 51.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S et al.. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29:2011-9; PMID:21502544; http://dx.doi.org/ 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 52.Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC, Zhu DX et al.. Anti-EGFR and anti-VEGF agents: Important targeted therapies of colorectal liver metastases. World J Gastroenterol 2014; 20:4263-75; PMID:24764664; http://dx.doi.org/25666296 10.3748/wjg.v20.i15.4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer 2015; 14:81-90; PMID:25666296; http://dx.doi.org/ 10.1016/j.clcc.2014.12.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.